合成生物学 ›› 2021, Vol. 2 ›› Issue (2): 287-301.DOI: 10.12211/2096-8280.2020-077
• 特约评述 • 上一篇
酿酒酵母适应性实验室进化工具的最新进展
李祎1, 林振泉2, 刘子鹤1
- 1.北京化工大学生命科学与技术学院,北京 100029
2.北京化工大学北京软物质科学与工程高精尖创新中心,北京 100029
-
收稿日期:2020-09-16修回日期:2021-02-14出版日期:2021-04-30发布日期:2021-04-30 -
通讯作者:林振泉,刘子鹤 -
作者简介:李祎 (1994—),男,硕士研究生,研究方向为合成生物学和天然产物。E-mail:2019210667@mail.buct.edu.cn林振泉 (1987—),男,博士后,研究方向为代谢工程、合成生物学和生物基化学品。E-mail:linzq2@126. com刘子鹤 (1984—),女,副教授,硕士生导师,研究方向为代谢工程和合成生物学。E-mail:zihe@mail.buct.edu.cn -
基金资助:国家重点研发计划(2018YFA0900100);中国博士后科学基金第67批面上项目(2020M670115)
Advances in yeast based adaptive laboratory evolution
LI Yi1, LIN Zhenquan2, LIU Zihe1
- 1.College of Life Science and Technology,Beijing University of Chemical Technology,Beijing 100029,China
2.Beijing Advanced Innovation Center for Soft Matter Science and Engineering,Beijing University of Chemical Technology,Beijing 100029,China
-
Received:2020-09-16Revised:2021-02-14Online:2021-04-30Published:2021-04-30 -
Contact:LIN Zhenquan, LIU Zihe
摘要:
酿酒酵母作为重要的模式生物和微生物细胞工厂已广泛用于代谢工程、系统生物学和合成生物学研究。由于微生物系统代谢和调控网络的复杂性,通过人工理性设计及基因扰动难以获得鲁棒性较好的表型,因此进化工程在构建稳定的微生物细胞工厂中发挥着重要作用。微生物适应性实验室进化工程通过模拟自然进化过程,构建大量遗传多样性的突变库,经过筛选可快速获得稳定性状的菌株。随着合成生物学和系统生物学的发展,研究人员开发了多种基因组规模的多位点快速进化工具,并结合计算机辅助进化系统实现了自动化连续进化技术。本综述着重介绍在酿酒酵母中基因组规模的多位点快速进化,包括合成型酿酒酵母基因组重排(SCRaMbLE)、寡核苷酸介导的多位点进化工具(YOGE和eMAGE)和基于CRISPR系统的多位点编辑(CHAnGE、MAGESTIC、Target-AID和yEvolvR)等基因组规模的进化策略的研究;以及自动化连续进化技术(eVOLVER、ICE和ACE等)。利用合成生物学方法通过在基因组水平进行多位点快速编辑或连续进化以产生具有遗传多样性的细胞群体,并在特定的筛选条件下对目标突变株进行富集,进而解析其基因型表型关系,可实现在短期内快速高效识别适应性进化的关键因素和协同作用。最后展望了实验室适应性进化工程与计算机辅助设计、自动化技术有机结合的机遇和在高通量筛选方向的挑战。
中图分类号:
引用本文
李祎, 林振泉, 刘子鹤. 酿酒酵母适应性实验室进化工具的最新进展[J]. 合成生物学, 2021, 2(2): 287-301.
LI Yi, LIN Zhenquan, LIU Zihe. Advances in yeast based adaptive laboratory evolution[J]. Synthetic Biology Journal, 2021, 2(2): 287-301.
| 分类 | 进化策略 | 进化原理 | 优点 | 缺点 | 应用 |
|---|---|---|---|---|---|
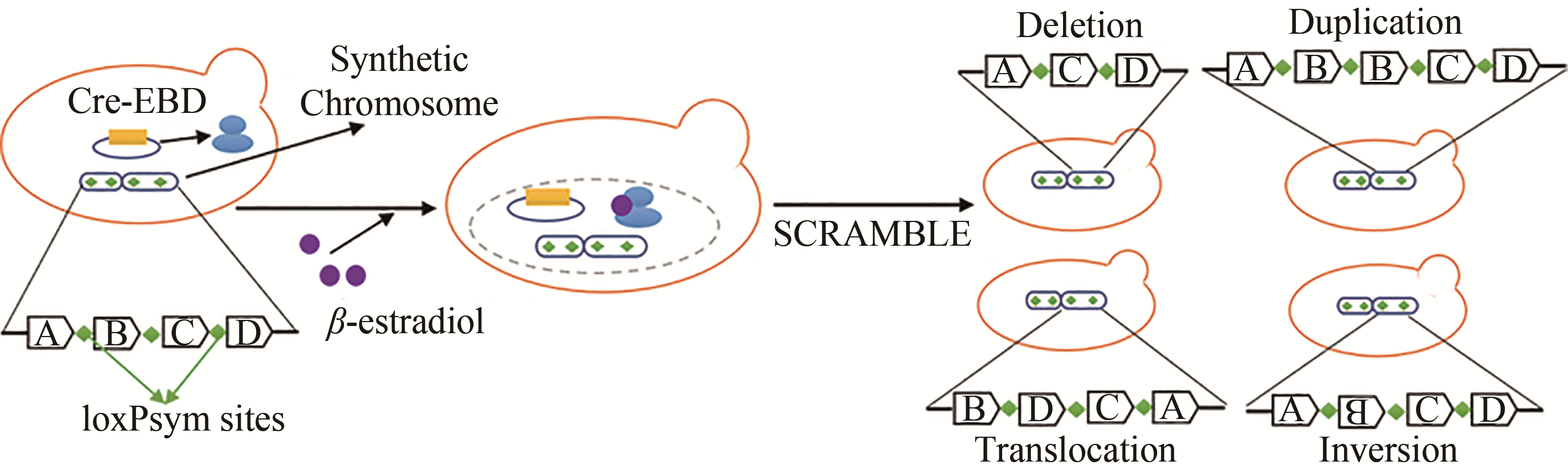
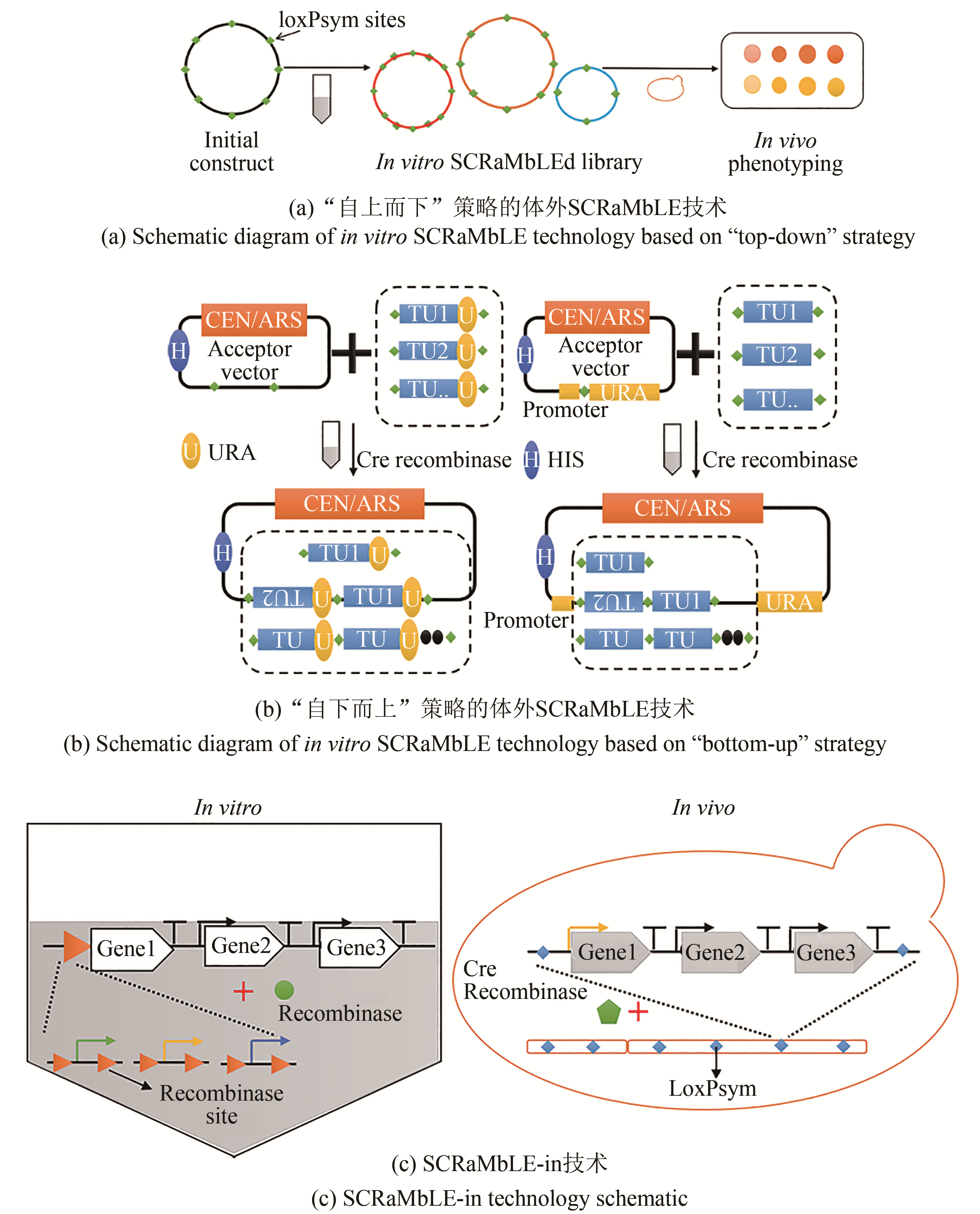
| 重组酶介导的进化工程 | SCRaMbLE | 通过基因组重构,加入多个loxPsym位点,并利用Cre重组酶特异性识别loxPsym位点并介导位点之间重组 | 可同时实现缺失、倒置、重复和异位等重组事件 | 需提前构建具有多个loxPsym位点的基因组序列 | 提高紫堇素和青霉素的生物合成[ |
| SCRaMbLE-in | 快速优化宿主以有效提高异源途径的表达;是SCRaMbLE技术的继承和发展 | 提高β-胡萝卜素的产量[ | |||
| 基于寡核苷酸的多位点进化工具 | eMAGE | 突变的ssODN与复制叉的后随链结合并整合到基因组上引入突变点 | 较高的等位基因替换效率(40%) | 突变效率随单链DNA寡核苷酸突变序列的增加而降低 | 提高类胡萝卜素的产量[ |
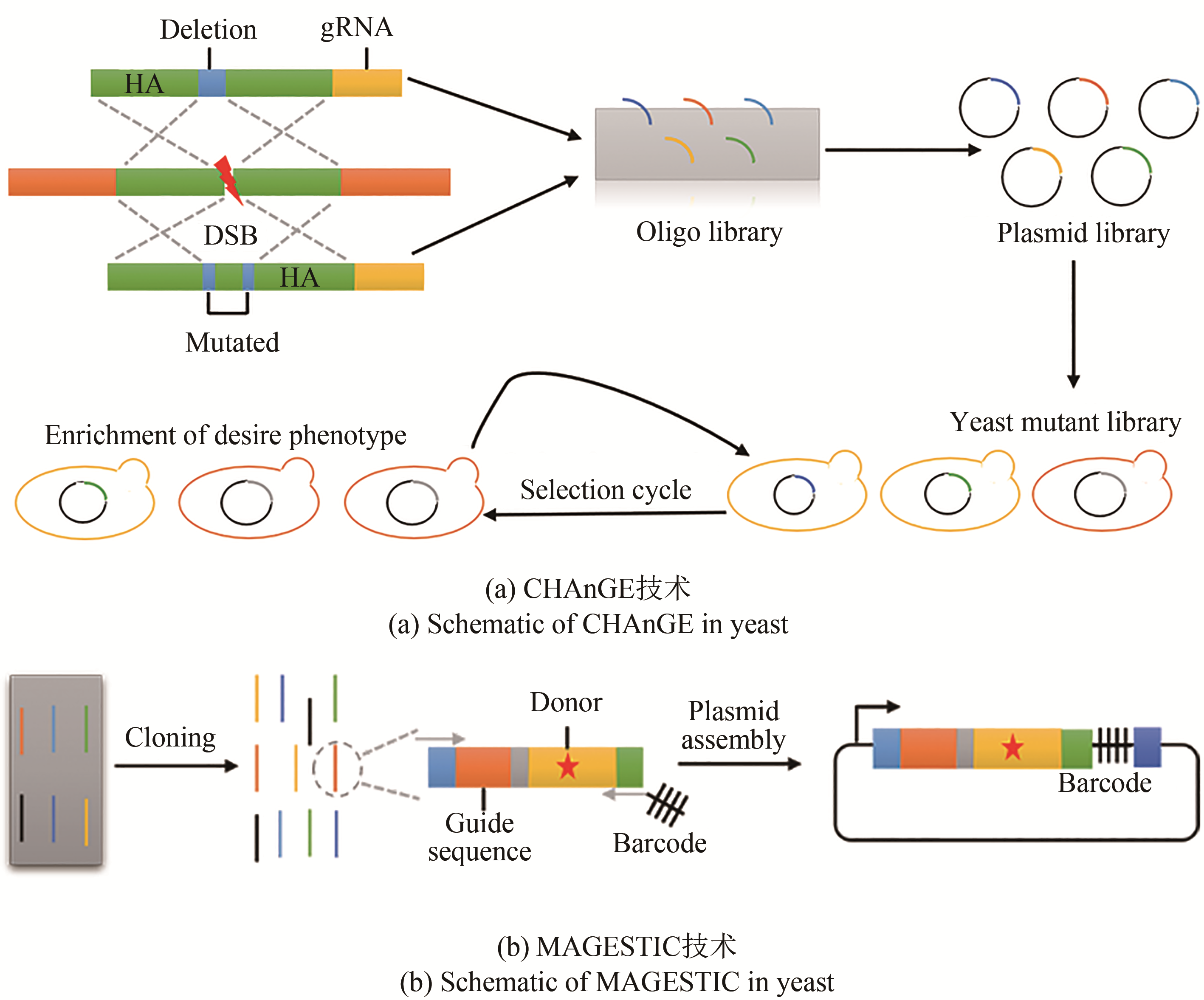
| CRISPR 系统介导的多位点编辑 | CHAnGE | 通过gRNA介导的同源修复促进了基因组编辑的精确性和有效性 | 通过对gRNA的测序进行突变位点的靶向定位 | 依赖于gRNA和供体DNA的协同,突变效率随着片段长度的增加而降低 | 提高酿酒酵母对糠醛和乙酸的耐受性[ |
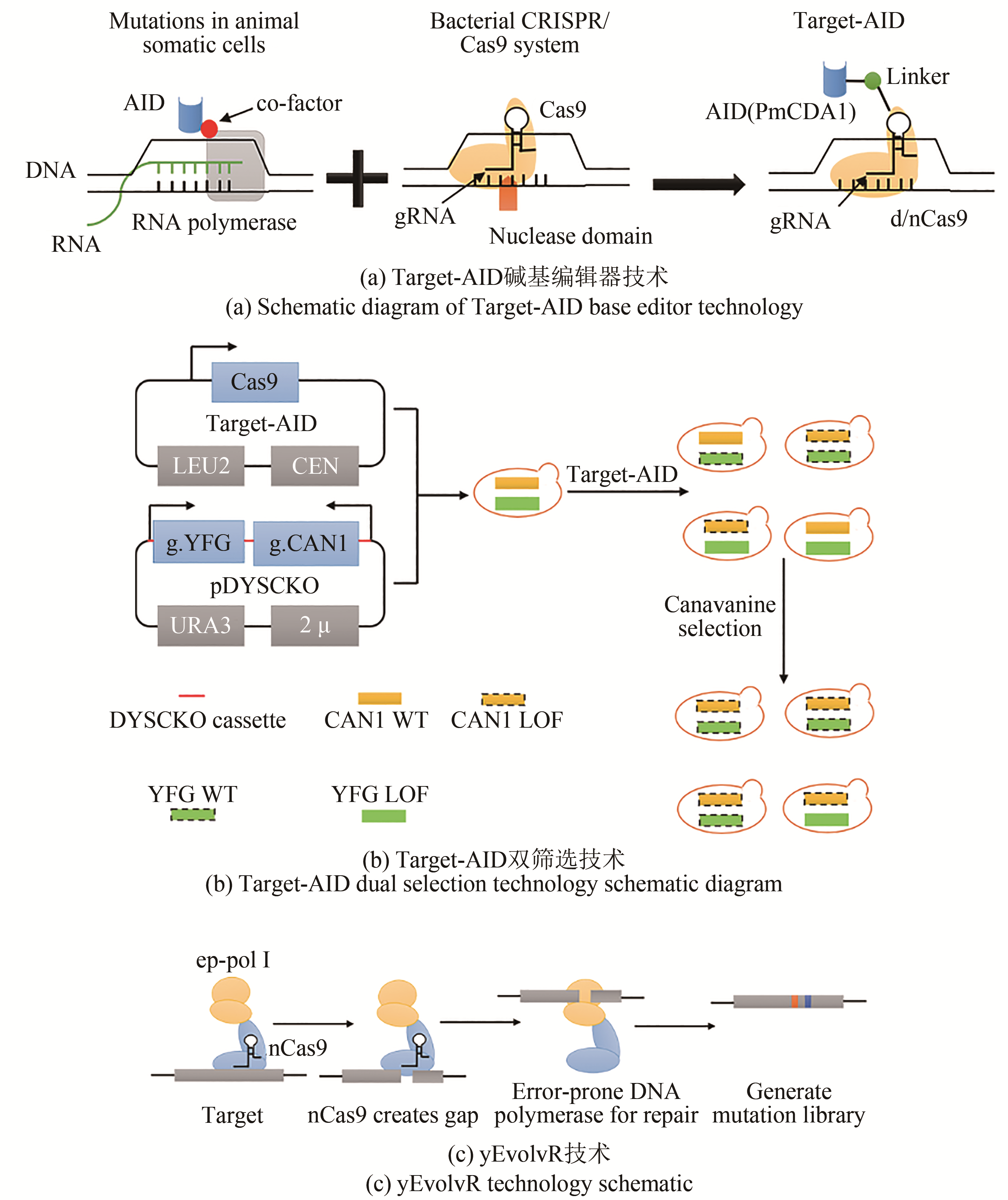
| Targeted-AID | 将胞嘧啶脱氨酶与CRISPR/Cas9系统融合,实现基因组DNA上胞嘧啶的靶向编辑 | 可在目标位点进行精确的碱基编辑 | 突变率较低 | 对ADE1和CAN1基因进行同时编辑突变率达到31%[ | |
| yEvolvR | 由nCas9-gRNA引导的在目标基因座上形成缺口,PolⅠ-5M以低保真度合成新链并切割置换的链 | 在切割位点上游20 bp到下游40 bp的区域内进行编辑;靶标突变率提高12 434倍 | 酿酒酵母整体突变率较低 | 提高内源基因的多样性,即还原URA3*中无义突变的同时使CAN1失活[ |
表1 酿酒酵母中基因组的多位点快速进化工具
Tab. 1 Multi-site rapid evolution tools for genome in S. cerevisiae
| 分类 | 进化策略 | 进化原理 | 优点 | 缺点 | 应用 |
|---|---|---|---|---|---|
| 重组酶介导的进化工程 | SCRaMbLE | 通过基因组重构,加入多个loxPsym位点,并利用Cre重组酶特异性识别loxPsym位点并介导位点之间重组 | 可同时实现缺失、倒置、重复和异位等重组事件 | 需提前构建具有多个loxPsym位点的基因组序列 | 提高紫堇素和青霉素的生物合成[ |
| SCRaMbLE-in | 快速优化宿主以有效提高异源途径的表达;是SCRaMbLE技术的继承和发展 | 提高β-胡萝卜素的产量[ | |||
| 基于寡核苷酸的多位点进化工具 | eMAGE | 突变的ssODN与复制叉的后随链结合并整合到基因组上引入突变点 | 较高的等位基因替换效率(40%) | 突变效率随单链DNA寡核苷酸突变序列的增加而降低 | 提高类胡萝卜素的产量[ |
| CRISPR 系统介导的多位点编辑 | CHAnGE | 通过gRNA介导的同源修复促进了基因组编辑的精确性和有效性 | 通过对gRNA的测序进行突变位点的靶向定位 | 依赖于gRNA和供体DNA的协同,突变效率随着片段长度的增加而降低 | 提高酿酒酵母对糠醛和乙酸的耐受性[ |
| Targeted-AID | 将胞嘧啶脱氨酶与CRISPR/Cas9系统融合,实现基因组DNA上胞嘧啶的靶向编辑 | 可在目标位点进行精确的碱基编辑 | 突变率较低 | 对ADE1和CAN1基因进行同时编辑突变率达到31%[ | |
| yEvolvR | 由nCas9-gRNA引导的在目标基因座上形成缺口,PolⅠ-5M以低保真度合成新链并切割置换的链 | 在切割位点上游20 bp到下游40 bp的区域内进行编辑;靶标突变率提高12 434倍 | 酿酒酵母整体突变率较低 | 提高内源基因的多样性,即还原URA3*中无义突变的同时使CAN1失活[ |
| 进化策略 | 进化原理 | 优点 | 缺点 | 应用 |
|---|---|---|---|---|
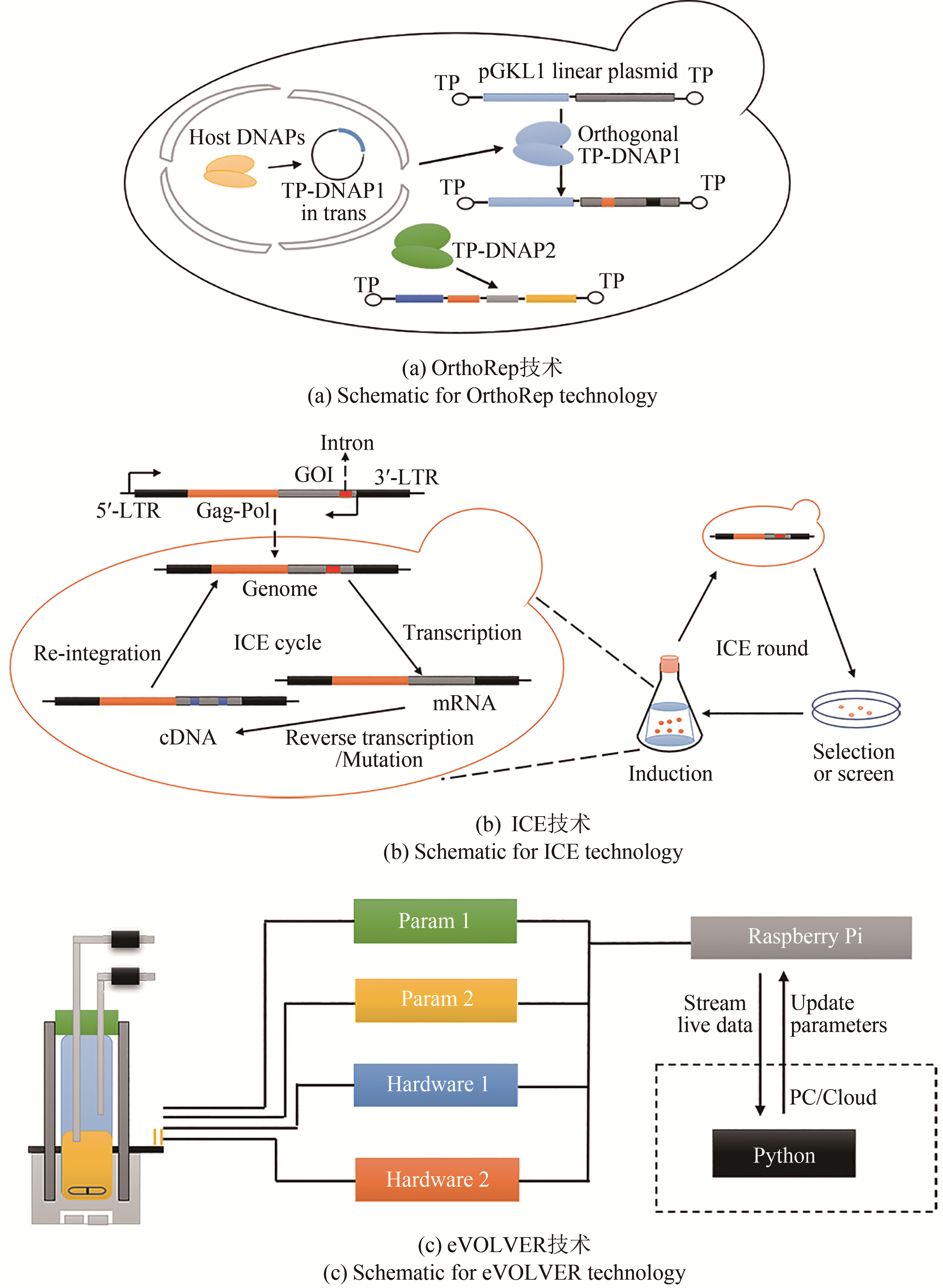
| OrthoRep | 利用游离质粒上的复制机制和宿主DNA复制的正交性,提高游离载体DNA聚合酶的突变率 | 可在不改变宿主基因组突变率的情况下,对正交质粒上的目标基因进行突变 | 需要特殊设计正交DNA复制系统并将其整合到酵母 | 提高酿酒酵母对乙胺嘧啶的耐受性[ |
| ICE | 高突变率的Ty1逆转录酶将目标基因的mRNA逆转录为cDNA,Ty1整合酶再将其整合至Ty1识别位点以进行下一轮突变 | ICE技术可以扩展到含有LTR逆转录转座子的真核生物中 | 突变效率受到同源重组的限制 | 提高酿酒酵母对1-丁醇的耐受性[ |
| eVOLVER | 通过实时控制反应器中各种培养参数,从而获得种群的进化参数图 | 对培养程序的自主编程,培养参数的实时监控 | 复杂的系统组装和应用计算机语言设计培养程序 | 构建了在温度和盐度二维应力梯度下酿酒酵母遗传变异的适应性图[ |
| ACE | 将OrthoRep和eVOLVER系统相结合 | 进化速度快、可伸缩性强 | 效率受体内OrthoRep系统的突变率和体外eVOLVER系统复杂组装的双重影响 | 提高酿酒酵母对3 mol/L乙胺嘧啶的耐受性[ |
表2 用于酿酒酵母的自动化连续进化工具
Tab. 2 Automated continuous evolution tools for S. cerevisiae
| 进化策略 | 进化原理 | 优点 | 缺点 | 应用 |
|---|---|---|---|---|
| OrthoRep | 利用游离质粒上的复制机制和宿主DNA复制的正交性,提高游离载体DNA聚合酶的突变率 | 可在不改变宿主基因组突变率的情况下,对正交质粒上的目标基因进行突变 | 需要特殊设计正交DNA复制系统并将其整合到酵母 | 提高酿酒酵母对乙胺嘧啶的耐受性[ |
| ICE | 高突变率的Ty1逆转录酶将目标基因的mRNA逆转录为cDNA,Ty1整合酶再将其整合至Ty1识别位点以进行下一轮突变 | ICE技术可以扩展到含有LTR逆转录转座子的真核生物中 | 突变效率受到同源重组的限制 | 提高酿酒酵母对1-丁醇的耐受性[ |
| eVOLVER | 通过实时控制反应器中各种培养参数,从而获得种群的进化参数图 | 对培养程序的自主编程,培养参数的实时监控 | 复杂的系统组装和应用计算机语言设计培养程序 | 构建了在温度和盐度二维应力梯度下酿酒酵母遗传变异的适应性图[ |
| ACE | 将OrthoRep和eVOLVER系统相结合 | 进化速度快、可伸缩性强 | 效率受体内OrthoRep系统的突变率和体外eVOLVER系统复杂组装的双重影响 | 提高酿酒酵母对3 mol/L乙胺嘧啶的耐受性[ |
| 1 | D' OELSNITZ S, ELLINGTON A. Continuous directed evolution for strain and protein engineering [J]. Current Opinion in Biotechnology, 2018, 53: 158-163. |
| 2 | CSÖRGŐ B, NYERGES A, PÁL C. Targeted mutagenesis of multiple chromosomal regions in microbes [J]. Current Opinion in Microbiology, 2020, 57: 22-30. |
| 3 | WANG Zhikun, GAO Cuijuan, WANG Qian, et al. Production of pyruvate in Saccharomyces cerevisiae through adaptive evolution and rational cofactor metabolic engineering [J]. Biochemical Engineering Journal, 2012, 67: 126-131. |
| 4 | REYES L H, GOMEZ J M, KAO K C. Improving carotenoids production in yeast via adaptive laboratory evolution [J]. Metabolic Engineering, 2014, 21(1): 26-33. |
| 5 | DRAGOSITS M, MATTANOVICH D. Adaptive laboratory evolution-principles and applications for biotechnology [J]. Microbial Cell Factories, 2013, 12(1): 64. |
| 6 | STRUCKO T, ZIRNGIBL K, PEREIRA F, et al. Laboratory evolution reveals regulatory and metabolic trade-offs of glycerol utilization in Saccharomyces cerevisiae [J]. Metabolic Engineering, 2018, 47: 73-82. |
| 7 | GIBSON B, DAHABIEH M, KROGERUS K, et al. Adaptive laboratory evolution of ale and lager yeasts for improved brewing efficiency and beer quality [J]. Annual Review of Food Science and Technology, 2020, 11(1): 23-44. |
| 8 | KAWAI K, KANESAKI Y, YOSHIKAWA H, et al. Identification of metabolic engineering targets for improving glycerol assimilation ability of Saccharomyces cerevisiae based on adaptive laboratory evolution and transcriptome analysis [J]. Journal of Bioscience and Bioengineering, 2019, 128(2): 162-169. |
| 9 | CASPETA L, CHEN Yun, GHIACI P, et al. Altered sterol composition renders yeast thermotolerant [J]. Science, 2014, 346(6205): 75-78. |
| 10 | PEREIRA R, WEI Yongjun, MOHAMED E, et al. Adaptive laboratory evolution of tolerance to dicarboxylic acids in Saccharomyces cerevisiae [J]. Metabolic Engineering, 2019, 56: 130-141. |
| 11 | ZHU Guoxing, YIN Nannan, LUO Qiuling, et al. Enhancement of sphingolipids synthesis improves osmotic tolerance of Saccharomyces cerevisiae [J]. Applied and Environmental Microbiology, 2020, 86(8): 2911-2919. |
| 12 | ZHANG Chao, QIN Jiufu, DAI Yiwei, et al. Atmospheric and room temperature plasma (ARTP) mutagenesis enables xylitol over-production with yeast Candida tropicalis [J]. Journal of Biotechnology, 2019, 296: 7-13. |
| 13 | EL-HUSSIENY N I, BAKRI M M, GANASH M, et al. Chemical mutagenesis of Saccharomyces cerevisiae for enhancing bioethanol production with fermentation at very high sugar concentration [J]. BioResources, 2020, 15(1): 16. |
| 14 | ZHANG Chaolei, SHEN Hongwei, ZHANG Xibin, et al. Combined mutagenesis of Rhodosporidium toruloides for improved production of carotenoids and lipids [J]. Biotechnology Letters, 2016, 38(10): 1733-1738. |
| 15 | YIN Nannan, ZHU Guoxing, LUO Qiuling, et al. Engineering of membrane phospholipid component enhances salt stress tolerance in Saccharomyces cerevisiae [J]. Biotechnology and Bioengineering, 2020, 117(3): 710-720. |
| 16 | VOORDECKERS K, COLDING C, GRASSO L, et al. Ethanol exposure increases mutation rate through error-prone polymerases [J]. Nature Communications, 2020, 11(1): 3664. |
| 17 | YAMADA R, KASHIHARA T, OGINO H. Improvement of lipid production by the oleaginous yeast Rhodosporidium toruloides through UV mutagenesis [J]. World Journal of Microbiology and Biotechnology, 2017, 33(5): 99. |
| 18 | GUO Minrui, CHENG Shaobo, CHEN Guogang, et al. Improvement of lipid production in oleaginous yeast Rhodosporidium toruloides by ultraviolet mutagenesis [J]. Engineering in Life Sciences, 2019, 19(8): 548-556. |
| 19 | PARK Won-Kun, YANG Ji-Won, KIM Hyun-Soo. Identification of novel genes responsible for salt tolerance by transposon mutagenesis in Saccharomyces cerevisiae [J]. Journal of Industrial Microbiology & Biotechnology, 2015, 42(4): 567-575. |
| 20 | KUMAR A, SERINGHAUS M, BIERY M, et al. Large-scale mutagenesis of the yeast genome using a Tn7-derived multipurpose transposon [J]. Genome Research, 2004, 14(10A): 1975-1986. |
| 21 | NI Haiying, LAPLAZA J M, JEFFRIES T W. Transposon mutagenesis to improve the growth of recombinant Saccharomyces cerevisiae on D-xylose [J]. Applied and Environmental Microbiology, 2007, 73(7): 2061-2066. |
| 22 | PINEL D, COLATRIANO D, JIANG Heng, et al. Deconstructing the genetic basis of spent sulphite liquor tolerance using deep sequencing of genome-shuffled yeast [J]. Biotechnology for Biofuels, 2015, 8(1): 53. |
| 23 | YIN Hua, MA Yanlin, DENG Yang, et al. Genome shuffling of Saccharomyces cerevisiae for enhanced glutathione yield and relative gene expression analysis using fluorescent quantitation reverse transcription polymerase chain reaction [J]. Journal of Microbiological Methods, 2016, 127: 188-192. |
| 24 | MAGOCHA T A, ZABED H, YANG Miaomiao, et al. Improvement of industrially important microbial strains by genome shuffling: current status and future prospects [J]. Bioresource Technology, 2018, 257: 281-289. |
| 25 | SNOEK T, NICOLINO M P, BREMT S VAN DEN, et al. Large-scale robot-assisted genome shuffling yields industrial Saccharomyces cerevisiae yeasts with increased ethanol tolerance [J]. Biotechnology for Biofuels, 2015, 8(1): 32. |
| 26 | CIRINO P C, MAYER K M, UMENO D. Generating mutant libraries using error-prone PCR [M]// ARNOLD F H, GEORGIOU G. Directed Evolution Library Creation: Methods and Protocols. Totowa: Humana Press Inc, 2003: 3-9. |
| 27 | TANG Zizhong, JIN Weiqiong, SUN Rong, et al. Improved thermostability and enzyme activity of a recombinant phyA mutant phytase from Aspergillus niger N25 by directed evolution and site-directed mutagenesis [J]. Enzyme and Microbial Technology, 2018, 108: 74-81. |
| 28 | GŁÓD D. Modification of fatty acid selectivity of Candida antarctica lipase A by error-prone PCR [J]. Biotechnology Letters, 2017, 39(5): 767-773. |
| 29 | MARKEL U, ESSANI K D, BESIRLIOGLU V, et al. Advances in ultrahigh-throughput screening for directed enzyme evolution [J]. Chemical Society Reviews, 2020, 49(1): 233-262. |
| 30 | GOFFEAU A, BARRELL B G, BUSSEY H, et al. Life with 6000 Genes [J]. Science, 1996, 274(5287): 546. |
| 31 | PORRO D, GASSER B, FOSSATI T, et al. Production of recombinant proteins and metabolites in yeasts [J]. Applied Microbiology and Biotechnology, 2011, 89(4): 939-948. |
| 32 | NEVOIGT E. Progress in metabolic engineering of Saccharomyces cerevisiae [J]. Microbiology and Molecular Biology Reviews, 2008, 72(3): 379-412. |
| 33 | SHEN Yue, STRACQUADANIO G, WANG Yun, et al. SCRaMbLE generates designed combinatorial stochastic diversity in synthetic chromosomes [J]. Genome Research, 2015, 26(1): 36-49. |
| 34 | HAMA S, KIHARA M, NODA H, et al. Development of cell recycle technology incorporating nutrient supplementation for lignocellulosic ethanol fermentation using industrial yeast Saccharomyces cerevisiae [J]. Biochemical Engineering Journal, 2018, 137: 23-29. |
| 35 | ZAHOOR A, KÜTTNER F T F, BLANK L M, et al. Evaluation of pyruvate decarboxylase-negative Saccharomyces cerevisiae strains for the production of succinic acid [J]. Engineering in Life Sciences, 2019, 19(10): 711-720. |
| 36 | SUÁSTEGUI M, Chiam Yu NG, CHOWDHURY A, et al. Multilevel engineering of the upstream module of aromatic amino acid biosynthesis in Saccharomyces cerevisiae for high production of polymer and drug precursors [J]. Metabolic Engineering, 2017, 42: 134-44. |
| 37 | MEADOWS A L, HAWKINS K M, TSEGAYE Y, et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production [J]. Nature, 2016, 537(7622): 694-697. |
| 38 | PROMDONKOY P, SIRIPONG W, DOWNES J J, et al. Systematic improvement of isobutanol production from D-xylose in engineered Saccharomyces cerevisiae [J]. AMB Express, 2019, 9(1): 160. |
| 39 | JIANG Shuangying, SI Tong, DAI Junbiao. Whole-genome regulation for yeast metabolic engineering [J]. Small Methods, 2020, 4(2): 1900640. |
| 40 | DYMOND J S, RICHARDSON S M, COOMBES C E, et al. Synthetic chromosome arms function in yeast and generate phenotypic diversity by design [J]. Nature, 2011, 477(7365): 471-476. |
| 41 | LIU Wei, LUO Zhouqing, WANG Yun, et al. Rapid pathway prototyping and engineering using in vitro and in vivo synthetic genome SCRaMbLE-in methods [J]. Nature Communications, 2018, 9(1): 1936. |
| 42 | JONES S. MAGE in yeast is a go [J]. Nature Biotechnology, 2017, 35(12): 1147-1148. |
| 43 | BAO Zehua, HAMEDIRAD M, XUE Pu, et al. Genome-scale engineering of Saccharomyces cerevisiae with single-nucleotide precision [J]. Nature Biotechnology, 2018, 36(6): 505-508. |
| 44 | NISHIDA K, ARAZOE T, YACHIE N, et al. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems [J]. Science, 2016, 353: 6305. |
| 45 | TOU C J, SCHAFFER D V, DUEBER J E. Targeted diversification in the S. cerevisiae genome with CRISPR-guided DNA polymerase I [J]. ACS Synthetic Biology, 2020, 9(7): 1911-1916. |
| 46 | DYMOND J, BOEKE J. The Saccharomyces cerevisiae SCRaMbLE system and genome minimization [J]. Bioengineered, 2012, 3(3): 170-173. |
| 47 | ZHANG Weimin, MITCHELL L A, BADER J S, et al. Synthetic genomes [J]. Annual Review of Biochemistry, 2020, 89(1): 77-101. |
| 48 | PRETORIUS I S, BOEKE J D. Yeast 2.0-connecting the dots in the construction of the world's first functional synthetic eukaryotic genome [J]. FEMS Yeast Research, 2018, 18(4): 32. |
| 49 | NAGY A. Cre recombinase: The universal reagent for genome tailoring [J]. Genesis, 2000, 26(2): 99-109. |
| 50 | STEENSELS J, GORKOVSKIY A, VERSTREPEN K J. SCRaMbLEing to understand and exploit structural variation in genomes [J]. Nature Communications, 2018, 9(1): 1937. |
| 51 | JIA Bin, WU Yi, LI Bingzhi, et al. Precise control of SCRaMbLE in synthetic haploid and diploid yeast [J]. Nature Communications, 2018, 9(1): 1933. |
| 52 | HOCHREIN L, MITCHELL L A, SCHULZ K, et al. L-SCRaMbLE as a tool for light-controlled Cre-mediated recombination in yeast [J]. Nature Communications, 2018, 9(1): 1931. |
| 53 | LUO Zhouqing, WANG Lihui, WANG Yun, et al. Identifying and characterizing SCRaMbLEd synthetic yeast using ReSCuES [J]. Nature Communications, 2018, 9(1): 1930. |
| 54 | WU Yi, ZHU Ruiying, MITCHELL L A, et al. In vitro DNA SCRaMbLE [J]. Nature Communications, 2018, 9(1): 1935. |
| 55 | WANG Juan, XIE Zexiong, MA Yuan, et al. Ring synthetic chromosome V SCRaMbLE [J]. Nature Communications, 2018, 9(1): 3783. |
| 56 | BLOUNT B A, GOWERS G O F, HO J C H, et al. Rapid host strain improvement by in vivo rearrangement of a synthetic yeast chromosome [J]. Nature Communications, 2018, 9(1): 1932. |
| 57 | SHEN M J, WU Yi, YANG Kun, et al. Heterozygous diploid and interspecies SCRaMbLEing [J]. Nature Communications, 2018, 9(1): 1934. |
| 58 | MA Lu, LI Yunxiang, CHEN Xinyu, et al. SCRaMbLE generates evolved yeasts with increased alkali tolerance [J]. Microbial Cell Factories, 2019, 18(1): 52. |
| 59 | LI Yunxiang, WU Yi, MA Lu, et al. Loss of heterozygosity by SCRaMbLEing [J]. Science China Life Sciences, 2019, 62(03): 381-393. |
| 60 | AUXILLOS J Y, GARCIA-RUIZ E, JONES S, et al. Multiplex genome engineering for optimizing bioproduction in Saccharomyces cerevisiae [J]. Biochemistry, 2019, 58(11): 1492-1500. |
| 61 | DICARLO J, CONLEY A, PENTTILÄ M, et al. Yeast oligo-mediated genome engineering (YOGE) [J]. ACS Synthetic Biology, 2013, 2(12): 741-749. |
| 62 | BARBIERI E M, MUIR P, AKHUETIE-ONI B O, et al. Precise editing at DNA replication forks enables multiplex genome engineering in eukaryotes [J]. Cell, 2017, 171(6): 1453-1467. |
| 63 | LIEBER M. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway [J]. Annual Review of Biochemistry, 2010, 79: 181-211. |
| 64 | RONDA C, MAURY J, JAKOČIU̅NAS T, et al. CrEdit: CRISPR mediated multi-loci gene integration in Saccharomyces cerevisiae [J]. Microbial Cell Factories, 2015, 14(1): 97. |
| 65 | ANDREW A H, JESSICA M W, MAX G S, et al. Efficient multiplexed integration of synergistic alleles and metabolic pathways in yeasts via CRISPR-Cas [J]. Cell Systems, 2015, 1(1): 88-96. |
| 66 | BAO Zehua, XIAO Han, LIANG Jing, et al. Homology-integrated CRISPR-Cas (HI-CRISPR) system for one-step multigene disruption in Saccharomyces cerevisiae [J]. ACS Synthetic Biology, 2015, 4(5): 585-594. |
| 67 | HILLE F, RICHTER H, WONG Shi Pey, et al. The biology of CRISPR-Cas: backward and forward [J]. Cell, 2018, 172(6): 1239-1259. |
| 68 | ROY K R, SMITH J D, VONESCH S C, et al. Multiplexed precision genome editing with trackable genomic barcodes in yeast [J]. Nature Biotechnology, 2018, 36(6): 512-520. |
| 69 | KOMOR A C, KIM Y B, PACKER M S, et al. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage [J]. Nature, 2016, 533(7603): 420-424. |
| 70 | KUSCU C, ADLI M. CRISPR-Cas9-AID base editor is a powerful gain-of-function screening tool [J]. Nature Methods, 2016, 13(12): 983-984. |
| 71 | DESPRÉS P, DUBÉ A, NIELLY-THIBAULT L, et al. Double selection enhances the efficiency of Target-AID and Cas9-based genome editing in yeast [J]. Genes Genomes Genetics, 2018, 8(10): 3163-3171. |
| 72 | DESPRÉS P C, DUBÉ A K, SEKI M, et al. Perturbing proteomes at single residue resolution using base editing [J]. Nature Communications, 2020, 11(1): 1871. |
| 73 | HALPERIN S O, TOU C J, WONG E B, et al. CRISPR-guided DNA polymerases enable diversification of all nucleotides in a tunable window [J]. Nature, 2018, 560(7717): 248-252. |
| 74 | ZHONG Ziwei, LIU Chang C. Probing pathways of adaptation with continuous evolution [J]. Current Opinion in Systems Biology, 2019, 14: 18-24. |
| 75 | JANG Sungho, KIM Minsun, HWANG Jaeseong, et al. Tools and systems for evolutionary engineering of biomolecules and microorganisms [J]. Journal of Industrial Microbiology & Biotechnology, 2019, 46(9): 1313-1326. |
| 76 | ARZUMANYAN G A, GABRIEL K N, RAVIKUMAR A, et al. Mutually orthogonal DNA replication systems in vivo [J]. ACS Synthetic Biology, 2018, 7(7): 1722-1729. |
| 77 | RAVIKUMAR A, ARRIETA A, LIU Chang C. An orthogonal DNA replication system in yeast [J]. Nature Chemical Biology, 2014, 10(3): 175-177. |
| 78 | CROOK N, ABATEMARCO J, SUN Jie, et al. In vivo continuous evolution of genes and pathways in yeast [J]. Nature Communications, 2016, 7(1): 13051. |
| 79 | HEINS Z J, MANCUSO C P, KIRIAKOV S, et al. Designing automated, high-throughput, continuous cell growth experiments using eVOLVER [J]. Journal of Visualized Experiments, 2019, 147: 59652. |
| 80 | ZHONG Ziwei, WONG B G, RAVIKUMAR A, et al. Automated continuous evolution of proteins in vivo [J]. ACS Synthetic Biology, 2020, 9(6): 1270-1276. |
| 81 | RIX G, WATKINS-DULANEY E J, ALMHJELL P J, et al. Scalable, continuous evolution for the generation of diverse enzyme variants encompassing promiscuous activities [J]. Nature Communications, 2020, 11(1): 5644. |
| 82 | RAVIKUMAR A, ARZUMANYAN G A, OBADI M K A, et al. Scalable continuous evolution of genes at mutation rates above genomic error thresholds [J]. Cell, 2018, 175(7): 1946-1957. |
| 83 | JAVANPOUR A A, LIU Chang C. Genetic compatibility and extensibility of orthogonal replication [J]. ACS Synthetic Biology, 2019, 8(6): 1249-1256. |
| 84 | MOSER F, BROERS N J, HARTMANS S, et al. Genetic circuit performance under conditions relevant for industrial bioreactors [J]. ACS Synthetic Biology, 2012, 1(11): 555-564. |
| 85 | HOPE E A, AMOROSI C J, MILLER A W, et al. Experimental evolution reveals favored adaptive routes to cell aggregation in yeast [J]. Genetics, 2017, 206(2): 1153. |
| 86 | WONG B G, MANCUSO C P, KIRIAKOV S, et al. Precise, automated control of conditions for high-throughput growth of yeast and bacteria with eVOLVER [J]. Nature Biotechnology, 2018, 36(7): 614-623. |
| 87 | TAN Zhenglin, ZHENG Xiang, WU Yinan, et al. In vivo continuous evolution of metabolic pathways for chemical production [J]. Microbial Cell Factories, 2019, 18(1): 82. |
| 88 | CAO Mingfeng, TRAN V G, ZHAO Huimin. Unlocking nature's biosynthetic potential by directed genome evolution [J]. Current Opinion in Biotechnology, 2020, 66: 95-104. |
| 89 | DIKICIOGLU D, PIR P, OLIVER S G. Predicting complex phenotype-genotype interactions to enable yeast engineering: Saccharomyces cerevisiae as a model organism and a cell factory [J]. Biotechnology Journal, 2013, 8(9): 1017-1034. |
| 90 | CARRASCO-LÓPEZ C, GARCÍA-ECHAURI S A, KICHUK T, et al. Optogenetics and biosensors set the stage for metabolic cybergenetics [J]. Current Opinion in Biotechnology, 2020, 65: 296-309. |
| 91 | MITCHLER M M, GARCIA J M, MONTERO N E, et al. Transcription factor-based biosensors: a molecular-guided approach for natural product engineering [J]. Current Opinion in Biotechnology, 2021, 69: 172-181. |
| 92 | 夏思杨, 江丽红, 蔡谨, 等. 酿酒酵母基因组进化的研究进展[J]. 合成生物学, 2020, 1(5): 556-569. |
| XIA Siyang, JIANG Lihong, CAI Jin, et al. Advances in genome evolution of Saccharomyces cerevisiae [J]. Synthetic Biology Journal, 2020, 1(5): 556-569. | |
| 93 | KIM N M, SINNOTT R W, SANDOVAL N R. Transcription factor-based biosensors and inducible systems in non-model bacteria: current progress and future directions [J]. Current Opinion in Biotechnology, 2020, 64: 39-46. |
| 94 | LEAVELL M D, SINGH A H, KAUFMANN-MALAGA B B. High-throughput screening for improved microbial cell factories, perspective and promise [J]. Current Opinion in Biotechnology, 2020, 62: 22-28. |
| 95 | PRANGEMEIER T, F-X LEHR, SCHOEMAN R M, et al. Microfluidic platforms for the dynamic characterisation of synthetic circuitry [J]. Current Opinion in Biotechnology, 2020, 63: 167-176. |
| 96 | ZHANG Jianzhi, CHEN Yongcan, FU Lihao, et al. Accelerating strain engineering in biofuel research via build and test automation of synthetic biology [J]. Current Opinion in Biotechnology, 2021, 67: 88-98. |
| [1] | 潘颖佳, 夏思杨, 董昌, 蔡谨, 连佳长. 基因增变器驱动的酿酒酵母基因组连续进化[J]. 合成生物学, 2023, 4(1): 225-240. |
| [2] | 何博, 付宗恒, 吴毅, 赵广荣. 哺乳动物合成基因组学研究进展[J]. 合成生物学, 2022, 3(1): 78-97. |
| [3] | 孙文涛, 张昕哲, 万盛通, 王茹雯, 李春. Ⅱ型细胞色素P450酶氧化β-香树脂醇的选择性调控研究[J]. 合成生物学, 2021, 2(5): 804-814. |
| [4] | 李晓东, 杨成帅, 王平平, 严兴, 周志华. 构建酿酒酵母细胞工厂从头合成倍半萜类化合物α-新丁香三环烯和β-石竹烯[J]. 合成生物学, 2021, 2(5): 792-803. |
| [5] | 盛月, 张根林. 酵母终止子工程:从机理探索到人工设计[J]. 合成生物学, 2020, 1(6): 709-721. |
| [6] | 夏思杨, 江丽红, 蔡谨, 黄磊, 徐志南, 连佳长. 酿酒酵母基因组进化的研究进展[J]. 合成生物学, 2020, 1(5): 556-569. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||