合成生物学 ›› 2021, Vol. 2 ›› Issue (1): 33-45.DOI: 10.12211/2096-8280.2020-064
从药物多肽到蛋白质全合成:酶促拼接的方法原理与前沿应用
杨新宇1,2, 朱彤1,2, 李瑞峰1,2, 吴边1
- 1.中国科学院微生物研究所,中国科学院微生物生理与代谢工程重点实验室,微生物资源前期开发国家重点实验室,北京 100101
2.中国科学院大学生命科学学院,北京 100049
-
收稿日期:2020-05-18修回日期:2021-01-06出版日期:2021-02-28发布日期:2021-03-12 -
通讯作者:吴边 -
作者简介:杨新宇(1994—),男,硕士研究生,研究方向为酶促蛋白质化学合成与修饰。E-mail:yangxy@im.ac.cn
吴边(1982—),男,博士生导师,研究员,研究方向为微生物催化元件的深度挖掘、机理解析、合成设计。致力于将蛋白质计算机设计前沿方法引入酶工程的研究,改造复杂生物大分子结构,优化催化元件性能,并在此基础上构建重要药物前体的生物合成途径。E-mail:wub@im.ac.cn -
基金资助:国家重点研发计划(2018YFA0901600);国家自然科学基金优秀青年科学基金(31822002);国家自然科学基金面上项目(31870055)
Enzymatic ligation technologies for the synthesis of pharmaceutical peptides and proteins
YANG Xinyu1,2, ZHU Tong1,2, LI Ruifeng1,2, WU Bian1
- 1.CAS Key Laboratory of Microbial Physiological and Metabolic Engineering,State Key Laboratory of Microbial Resources,Institute of Microbiology,Chinese Academy of Sciences,Beijing 100101,China
2.University of Chinese Academy of Sciences,Beijing 100049,China
-
Received:2020-05-18Revised:2021-01-06Online:2021-02-28Published:2021-03-12 -
Contact:WU Bian
摘要:
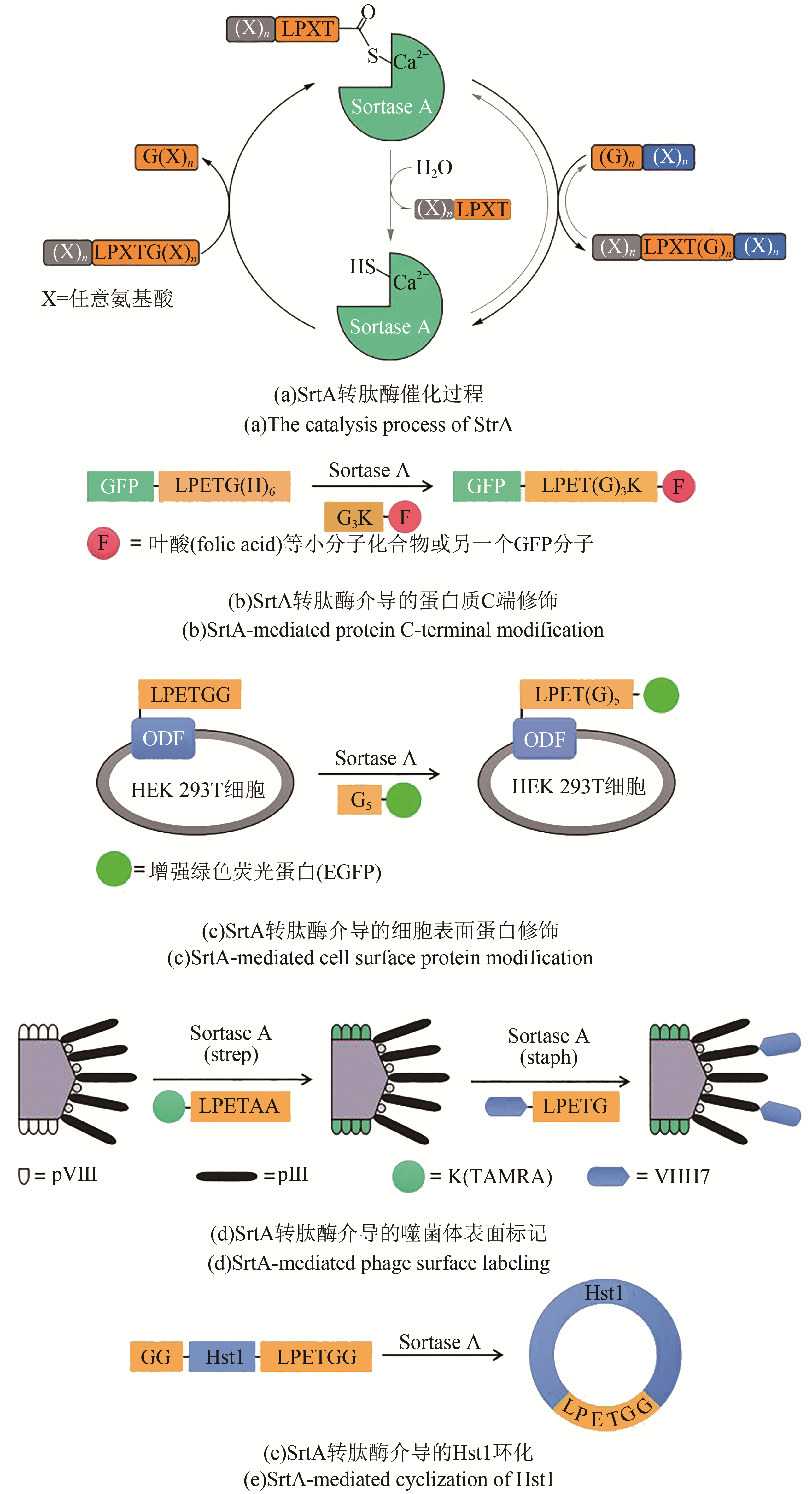
蛋白质是生命活动的基础功能元件,其化学合成与定点修饰已成为合成生物学领域探索复杂生物大分子“结构-功能”关系的重要前沿方向。近年来,以多肽固相合成与特异性拼接为核心的蛋白质合成和修饰技术蓬勃发展,打破了生命合成系统仅能使用天然及少数非天然氨基酸的瓶颈,为制备含有数百个氨基酸残基的非天然蛋白质提供了技术平台,让原子水平的蛋白质人工设计成为现实。作为一类广受关注的多肽拼接策略,基于天然或人工改造多肽连接酶的技术方法不仅在基础研究领域拓展了人们对蛋白质这一生命核心元件的理解,还在工业领域崭露头角,被应用于多种多肽类药物的生产。针对蛋白质合成领域中酶促多肽拼接技术平台,本文介绍了Sortase A转肽酶、Butelase 1转肽酶以及Subtilisin人工连接酶的来源以及催化过程,探讨了各自的优势以及局限性,并综述了三种酶在蛋白质修饰、蛋白质合成、多肽药物环化等方面的应用。通过计算机辅助设计、定向进化等技术对转肽酶、连接酶进行改造来提升其在底物谱、催化活性等方面的特性,将化学方法与酶促方法联用来建立多样的生物大分子从头设计与合成路线是目前的主要发展趋势。
中图分类号:
引用本文
杨新宇, 朱彤, 李瑞峰, 吴边. 从药物多肽到蛋白质全合成:酶促拼接的方法原理与前沿应用[J]. 合成生物学, 2021, 2(1): 33-45.
YANG Xinyu, ZHU Tong, LI Ruifeng, WU Bian. Enzymatic ligation technologies for the synthesis of pharmaceutical peptides and proteins[J]. Synthetic Biology Journal, 2021, 2(1): 33-45.
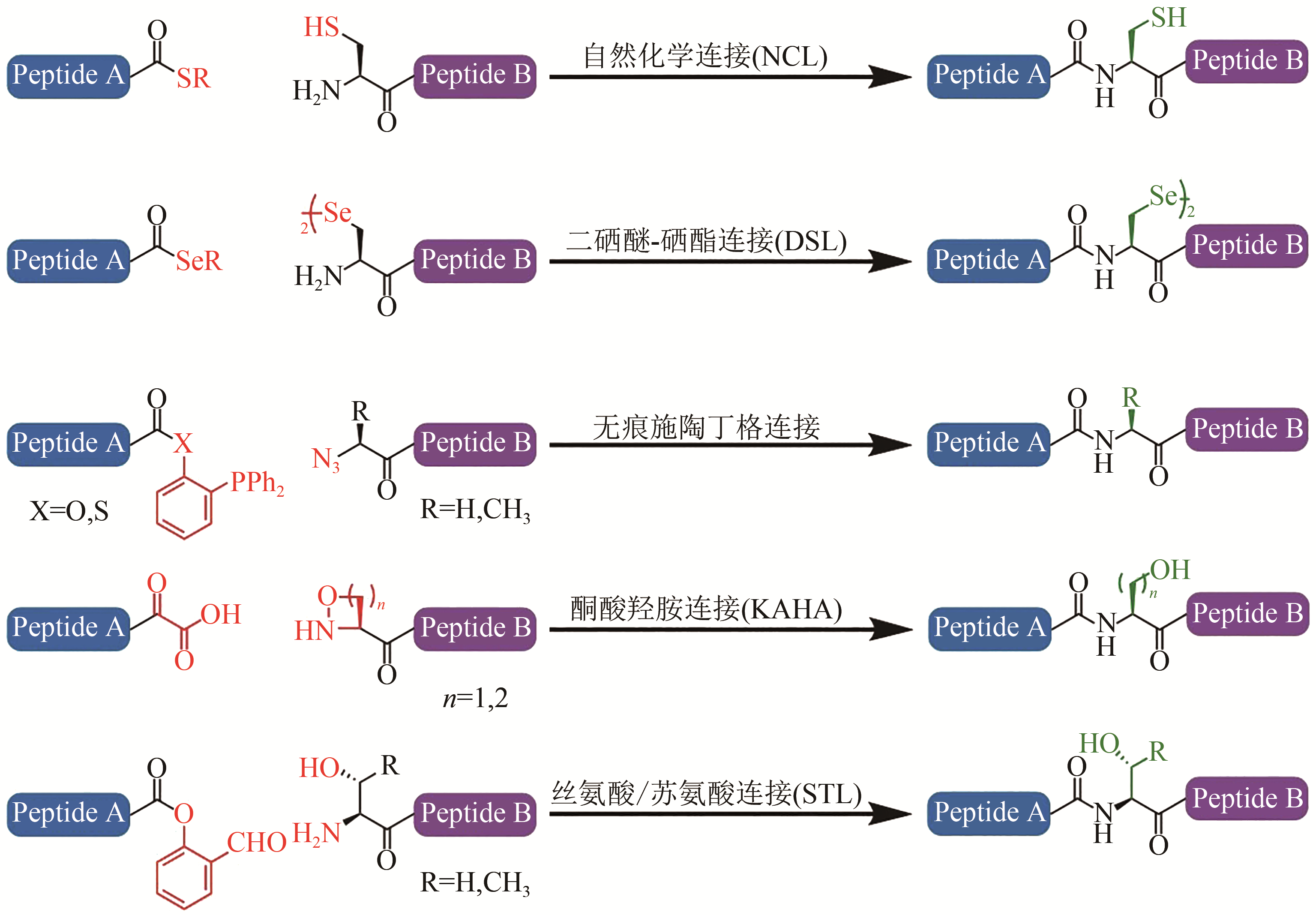
图1 主要化学连接方法[13-17](红色标记的基团参与选择性多肽捕获反应,绿色标记的基团代表连接“疤痕”或序列限制)
Fig. 1 Chemical ligation methods[13-17](Red groups participate the chemoselective capture reactions, and green groups represent ‘scar’ or sequence restrictions)
| 项目 | SrtA转肽酶 | Butelase 1转肽酶 | Subtilisin人工连接酶 | NCL | |
|---|---|---|---|---|---|
| 最初来源物种 | 金黄色葡萄球菌Staphylococcus aureus | 蝶豆 Clitoria ternatea | 解淀粉芽孢杆菌 Bacillus amyloliquefaciens | —— | |
| 酶的获取与产量[ | SrtA转肽酶可通过商业渠道获得或大肠杆菌重组表达 >40 mg/L | Butelase 1转肽酶通过植物材料提取 约5 mg/kg | Omniligase-1可通过商业渠道获得或枯草芽孢杆菌重组表达 >500 mg/L | —— | |
| 底物活化 | 底物无需活化 | 底物无需活化 | 多肽C端活化为 氧酯或硫酯 | 多肽C端活化为 硫酯 | |
连接 位点 序列 | C端 | LPXT-G | N/D-HV | X4X3X2X1—,X2外均避免Pro,X4偏好疏水&芳香族氨基酸 | 无限制 |
| N端 | (G) n | —X1X2,其中X1避免Pro和 酸性氨基酸,X2一般为ILVC | —X1’X2’均避免Pro | Cys | |
| 连接“疤痕” | LPXT(G) n | 一个Asn/Asp残基 | 无痕(X4X3X2X1 X1’X2’) | 一个Cys残基 | |
应用 范围 | 多肽首 尾环化 | 短肽(大于19个氨基酸残基)和蛋白质皆可环化 | 短肽(大于9个氨基酸残基)和蛋白质皆可环化 | 环化多肽一般不超过40个残基 | 环化多肽一般不超过40个残基 |
蛋白末 端修饰 | 蛋白质N端与C端皆可 | 蛋白质N端与C端皆可 | 大多用于蛋白质N端修饰鲜有C端修饰 | 蛋白质N端与C端皆可 | |
蛋白质 全合成 | 尚无应用报道 | 尚无应用报道 | 可以应用 | 广泛应用 | |
表1 三类酶促连接策略以及NCL方法的比较
Tab. 1 Comparison between NCL method and three enzymatic strategies
| 项目 | SrtA转肽酶 | Butelase 1转肽酶 | Subtilisin人工连接酶 | NCL | |
|---|---|---|---|---|---|
| 最初来源物种 | 金黄色葡萄球菌Staphylococcus aureus | 蝶豆 Clitoria ternatea | 解淀粉芽孢杆菌 Bacillus amyloliquefaciens | —— | |
| 酶的获取与产量[ | SrtA转肽酶可通过商业渠道获得或大肠杆菌重组表达 >40 mg/L | Butelase 1转肽酶通过植物材料提取 约5 mg/kg | Omniligase-1可通过商业渠道获得或枯草芽孢杆菌重组表达 >500 mg/L | —— | |
| 底物活化 | 底物无需活化 | 底物无需活化 | 多肽C端活化为 氧酯或硫酯 | 多肽C端活化为 硫酯 | |
连接 位点 序列 | C端 | LPXT-G | N/D-HV | X4X3X2X1—,X2外均避免Pro,X4偏好疏水&芳香族氨基酸 | 无限制 |
| N端 | (G) n | —X1X2,其中X1避免Pro和 酸性氨基酸,X2一般为ILVC | —X1’X2’均避免Pro | Cys | |
| 连接“疤痕” | LPXT(G) n | 一个Asn/Asp残基 | 无痕(X4X3X2X1 X1’X2’) | 一个Cys残基 | |
应用 范围 | 多肽首 尾环化 | 短肽(大于19个氨基酸残基)和蛋白质皆可环化 | 短肽(大于9个氨基酸残基)和蛋白质皆可环化 | 环化多肽一般不超过40个残基 | 环化多肽一般不超过40个残基 |
蛋白末 端修饰 | 蛋白质N端与C端皆可 | 蛋白质N端与C端皆可 | 大多用于蛋白质N端修饰鲜有C端修饰 | 蛋白质N端与C端皆可 | |
蛋白质 全合成 | 尚无应用报道 | 尚无应用报道 | 可以应用 | 广泛应用 | |
| 1 | LAU J L, DUNN M K. Therapeutic peptides: historical perspectives, current development trends, and future directions [J]. Bioorganic & Medicinal Chemistry, 2018, 26(10): 2700-2707. |
| 2 | XIAO Y, JIE M, LI B, et al. Peptide-based treatment: a promising cancer therapy [J]. Journal of Immunology Research, 2015,2015: 761820. |
| 3 | VADEVOO S M P, GURUNG S, KHAN F, et al. Peptide-based targeted therapeutics and apoptosis imaging probes for cancer therapy [J]. Archives of Pharmacal Research, 2019, 42(2): 150-158. |
| 4 | TOPLAK A, NUIJENS T, QUAEDFLIEG P J L M, et al. Peptiligase, an enzyme for efficient chemoenzymatic peptide synthesis and cyclization in water [J]. Advanced Synthesis & Catalysis, 2016, 358: 2140-2147. |
| 5 | ZORZI A, DEYLE K, HEINIS C. Cyclic peptide therapeutics: past, present and future [J]. Current Opinion in Chemical Biology, 2017, 38: 24-29. |
| 6 | CHOW H Y, ZHANG Y, MATHESON E, et al. Ligation technologies for the synthesis of cyclic peptides [J]. Chemical Reviews, 2019, 119(17): 9971-10001. |
| 7 | WEEKS A M, WELLS J A. Subtiligase-catalyzed peptide ligation [J]. Chemical Reviews, 2020, 120(6): 3127-3160. |
| 8 | HOUEN G. Peptide antibodies[M]. New York: Humana Press, 2015: 33-50. |
| 9 | BEHRENDT R, WHITE P, OFFER J. Advances in Fmoc solid-phase peptide synthesis [J]. Journal of Peptide Science, 2016, 22(1): 4-27. |
| 10 | SCHMIDT M, TOPLAK A, QUAEDFLIEG P J L M, et al. Enzyme-mediated ligation technologies for peptides and proteins [J]. Current Opinion in Chemical Biology, 2017, 38: 1-7. |
| 11 | ZHANG Y, PARK K, SUAZO K F, et al. Recent progress in enzymatic protein labelling techniques and their applications [J]. Chemical Society Reviews, 2018, 47(24): 9106-9136. |
| 12 | AGOURIDAS V, MAHDI O E, DIEMER V, et al. Native chemical ligation and extended methods: mechanisms, catalysis, scope, and limitations [J]. Chemical Reviews, 2019, 119(12): 7328-7443. |
| 13 | CONIBEAR A C, WATSON E E, PAYNE R J, et al. Native chemical ligation in protein synthesis and semi-synthesis [J]. Chemical Society Reviews, 2018, 47(24):9046-9068. |
| 14 | TAM A, SOELLNER M B, RAINES R T. Water-soluble phosphinothiols for traceless Staudinger ligation and integration with expressed protein ligation [J]. Journal of the American Chemical Society, 2007, 129(37): 11421-11430. |
| 15 | PUSTERLA I, BODE J W. The mechanism of the a-ketoacid-hydroxylamine amide-forming ligation [J]. Angewandte Chemie International Edition, 2012, 51: 513-516. |
| 16 | ZHANG Y F, XU C, LAM H Y, et al. Protein chemical synthesis by serine and threonine ligation [J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(17): 6657-6662. |
| 17 | KULKARNI S S, WATSON E E, PREMDJEE B, et al. Diselenide-selenoester ligation for chemical protein synthesis [J]. Nature Protocols, 2019, 14: 2229-2257. |
| 18 | DAWSON P E, MUIR T W, CLARK-LEWIS I, et al. Synthesis of proteins by native chemical ligation [J]. Science, 1994, 266(5186):776-779. |
| 19 | ZHENG J, TANG S, QI Y, et al. Chemical synthesis of proteins using peptide hydrazides as thioester surrogates [J]. Nature Protocols, 2013, 8: 2483-2495. |
| 20 | FLOOD D T, HINTZEN J C J, BIRD M J, et al. Leveraging the Knorr pyrazole synthesis for the facile generation of thioester surrogates for use in native chemical ligation [J]. Angewandte Chemie International Edition, 2018, 57: 11634-11639. |
| 21 | MUIR T W, SONDHI D, COLE P A. Expressed protein ligation: a general method for protein engineering [J]. Proceedings of the National Academy of Sciences of the United States of America, 1998, 95(12): 6705-6710. |
| 22 | MALINS L R, PAYNE R J. Recent extensions to native chemical ligation for the chemical synthesis of peptides and proteins [J]. Current Opinion in Chemical Biology, 2014, 22: 70-78. |
| 23 | VILA-PERELLÓ M, LIU Z, SHAH N H, et al. Streamlined expressed protein ligation using split inteins [J]. Journal of the American Chemical Society, 2013, 135: 286-292. |
| 24 | MAZMANIAN S K, LIU G, TON-THAT H, et al. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall [J]. Science, 1999, 285(5428): 760-763. |
| 25 | ATONS J M, CHEW G L, GUIMARAES C P, et al. Site-specific N-and C-terminal labeling of a single polypeptide using sortases of different specificity [J]. Journal of the American Chemical Society, 2009, 131(31): 10800-10801. |
| 26 | NUIJENS T, TOPLAK A, SCHMIDT M, et al. Natural occurring and engineered enzymes for peptide ligation and cyclization [J]. Frontiers in Chemistry, 2019, 7: 829. |
| 27 | MAO H, HART S A, SCHINK A, et al. Sortase-mediated protein ligation: a new method for protein engineering [J]. Journal of the American Chemical Society, 2004, 126(9): 2670-2671. |
| 28 | TANAKA T, YAMAMOTO T, TSUKIJI S, et al. Site-specific protein modification on living cells catalyzed by sortase [J]. ChemBioChem, 2008, 9: 802-807. |
| 29 | YAMAMOTO T, NAGAMUNE T. Expansion of the sortase-mediated labeling method for site-specific N-terminal labeling of cell surface proteins on living cells [J]. Chemical Communications, 2009, 9: 1022-1024. |
| 30 | HESS G T, CRAGNOLINI J J, POPP M W, et al. M13 bacteriophage display framework that allows sortase-mediated modification of surface-accessible phage proteins [J]. Bioconjugate Chemistry, 2012, 23(7): 1478-1487. |
| 31 | VAN'T HOF W, MAŇÁSKOVÁ S H, VEERMAN E C I, et al. Sortase-mediated backbone cyclization of proteins and peptides [J]. Biological Chemistry, 2015, 396(4): 283-293. |
| 32 | BOLSCHER J G M, OUDHOFF M J, NAZMI K, et al. Sortase A as a tool for high-yield histatin cyclization [J]. The FASEB Journal, 2011, 25(8): 2650-2658. |
| 33 | STANGER K, MAURER T, KALUARACHCHI H, et al. Backbone cyclization of a recombinant cystine-knot peptide by engineered Sortase A [J]. FEBS Letters, 2014, 588(23): 4487-4496. |
| 34 | ZHANG J, YAMAGUCHI S, NAGAMUNE T. Sortase A-mediated synthesis of ligand-grafted cyclized peptides for modulating a model protein-protein interaction [J]. Biotechnology Journal, 2015, 10: 1499-1505. |
| 35 | SCHMOHL L, SCHWARZER D. Sortase-mediated ligations for the site-specific modification of proteins [J]. Current Opinion in Chemical Biology, 2014, 22: 122-128. |
| 36 | SCHMIDT M, TOPLAK A, QUAEDFLIEG P J L M, et al. Enzyme-catalyzed peptide cyclization [J]. Drug Discovery Today. Technologies, 2017, 26: 11-16. |
| 37 | THOMPSON R E, STEVENS A J, MUIR T W. Protein engineering through tandem transamidation [J]. Nature Chemistry, 2019, 11: 737-743. |
| 38 | LI Y, LI Y, PAN M, et al. Irreversible site-specific hydrazinolysis of proteins by use of sortase [J]. Angewandte Chemie International Edition, 2014, 53: 2198-2202. |
| 39 | NGUYEN G K T, WANG S J, QIU Y B, et al. Butelase 1 is an Asx-specific ligase enabling peptide macrocyclization and synthesis [J]. Nature Chemical Biology, 2014, 10(9): 732-738. |
| 40 | NGUYEN G K T, KAM A, LOO S, et al. Butelase 1: a versatile ligase for peptide and protein macrocyclization [J]. Journal of the American Chemical Society, 2015, 137(49): 15398-15401. |
| 41 | NGUYEN G K T, QIU Y B, CAO Y, et al. Butelase-mediated cyclization and ligation of peptides and proteins [J]. Nature Protocols, 2016, 11(10): 1977-1988. |
| 42 | HEMU X, QIU Y B, NGUYEN G K T, et al. Total synthesis of circular bacteriocins by Butelase 1 [J]. Journal of the American Chemical Society, 2016, 138(22): 6968-6971. |
| 43 | HEMU X, ZHANG X H, TAM J P. Ligase-controlled cyclo-oligomerization of peptides [J]. Organic Letters, 2019, 21(7): 2029-2032. |
| 44 | NGUYEN G K T, CAO Y, WANG W, et al. Site-specific N-terminal labeling of peptides and proteins using butelase 1 and thiodepsipeptide [J]. Angewandte Chemie International Edition, 2015, 54: 15694-15698. |
| 45 | BI X, YIN J, NGUYEN G K T, et al. Enzymatic engineering of live bacterial cell surfaces using butelase 1 [J]. Angewandte Chemie International Edition, 2017, 56: 7822-7825. |
| 46 | CAO Y, NGUYEN G K T, CHUAH S, et al. Butelase-mediated ligation as an efficient bioconjugation method for the synthesis of peptide dendrimers [J]. Bioconjugate Chemistry, 2016, 27(11): 2592-2596. |
| 47 | CAO Y, NGUYEN G K T, TAM J P, et al. Butelase-mediated synthesis of protein thioesters and its application for tandem chemoenzymatic ligation [J]. Chemical Communications, 2015, 51(97): 17289-17292. |
| 48 | NUIJENS T, SCHMIDT M. Butelase 1-mediated ligation of peptides and proteins [M]. New York: Humana Press, 2019: 83-109. |
| 49 | HARRIS K S, DUREK T, KAAS Q, et al. Efficient backbone cyclization of linear peptides by a recombinant asparaginyl endopeptidase [J]. Nature Communications, 2015, 6: 10199. |
| 50 | YANG R, WONG Y H, NGUYEN G K T, et al. Engineering a catalytically efficient recombinant protein ligase [J]. Journal of the American Chemical Society, 2017, 139: 5351-5358. |
| 51 | POLGARF L, BENDER M L. The reactivity of thiol-subtilisin, an enzyme containing a synthetic functional group [J]. Biochemistry, 1967, 6(2): 610-620. |
| 52 | NAKATSUKA T, SASAKI T, KAISER E T. Peptide segment coupling catalyzed by the semisynthetic enzyme thiolsubtilisin [J]. Journal of the American Chemical Society, 1987, 109(12): 3808-3810. |
| 53 | ABRAHMSÉN L, TOM J, BURNIER J, et al. Engineering subtilisin and its substrates for efficient ligation of peptide bonds in aqueous solution [J]. Biochemistry, 1991, 30(17): 4151-4159. |
| 54 | CHANG T K, JACKSON D Y, BURNIER J P, et al. Subtiligase: a tool for semisynthesis of proteins [J]. Proceedings of the National Academy of Sciences of the United States of America, 1994, 91(26): 12544-12548. |
| 55 | ATWELL S, WELLS J A. Selection for improved subtiligases by phage display [J]. Proceedings of the National Academy of Sciences of the United States of America, 1999, 96(17): 9497-9502. |
| 56 | WEEKS A M, WELLS J A. Engineering peptide ligase specificity by proteomic identification of ligation sites [J]. Nature Chemical Biology, 2018, 14(1): 50-57. |
| 57 | JACKSON D Y, BURNIER J, QUAN C, et al. A designed peptide ligase for total synthesis of ribonuclease a with unnatural catalytic residues [J]. Science, 1994, 266(5183): 243-247. |
| 58 | JACKSON D Y, BUMIER J P, WELLS J A. Enzymatic cyclization of linear peptide esters using subtiligase [J]. Journal of the American Chemical Society, 1995, 117: 819-820. |
| 59 | HENAGER S H, CHU N, CHEN Z, et al. Enzyme-catalyzed expressed protein ligation[J]. Nature Methods, 2016, 13(11): 925-927. |
| 60 | MAHRUS S, TRINIDAD J C, BARKAN D T, et al. Global sequencing of proteolytic cleavage sites in apoptosis by specific labeling of protein N termini [J]. Cell, 2008, 134: 866-876. |
| 61 | NUIJENS T, TOPLAK A, QUAEDFLIEG P J L M, et al. Engineering a diverse ligase toolbox for peptide segment condensation [J]. Advanced Synthesis & Catalysis, 2016, 358(24): 4041-4048. |
| 62 | SCHMIDT M, TOPLAK A, QUAEDFLIEG P J L M, et al. Omniligase-1: a powerful tool for peptide head-to-tail cyclization [J]. Advanced Synthesis & Catalysis, 2017, 359: 2050-2055. |
| 63 | PAWLAS J, NUIJENS T, PERSSON B, et al. Sustainable, cost-efficient manufacturing of therapeutic peptides using chemo-enzymatic peptide synthesis (CEPS) [J]. Green Chemistry, 2019, 21: 6451-6467. |
| 64 | SCHMIDT M, HUANG Y, OLIVEIRA E F T, et al. Efficient enzymatic cyclization of disulfide-rich peptides using peptiligases [J]. ChemBioChem, 2019, 20(12): 1524-1529. |
| 65 | RICHELLE G J J, SCHMIDT M, IPPEL H, et al. A one-pot "triple-c'' multicyclization methodology for the synthesis of highly constrained isomerically pure tetracyclic peptides [J]. ChemBioChem, 2018, 19(18): 1934-1938. |
| 66 | STREEFKERK D E, SCHMIDT M, IPPEL J H, et al. Synthesis of constrained tetracyclic peptides by consecutive CEPS, CLIPS, and oxime ligation [J]. Organic Letters, 2019, 21: 2095-2100. |
| 67 | SCHMIDT M, TOPLAK A, ROZEBOOM H J, et al. Design of a substrate-tailored peptiligase variant for the efficient synthesis of thymosin-α1 [J]. Organic & Biomolecular Chemistry, 2018, 16: 609-618. |
| 68 | HENNINOT A, COLLINS J C, NUSS J M. The current state of peptide drug discovery: back to the future? [J]. Journal of Medicinal Chemistry, 2018, 61: 1382-1414. |
| 69 | HUANG P, BOYKEN S E, BAKER D. The coming of age of de novo protein design [J]. Nature, 2016, 537: 320-327. |
| 70 | MILLS J H, KHARE S D, BOLDUC J M, et al. Computational design of an unnatural amino acid dependent metalloprotein with atomic level accuracy [J]. Journal of the American Chemical Society, 2013, 135(36): 13393-13399. |
| [1] | 后佳琦, 姜楠, 马莲菊, 卢元. 无细胞蛋白质合成:从基础研究到工程应用[J]. 合成生物学, 2022, 3(3): 465-486. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||