合成生物学 ›› 2021, Vol. 2 ›› Issue (4): 482-496.DOI: 10.12211/2096-8280.2021-051
生物药产业蓬勃发展早期的奠基性系统开发——缅怀王义翘教授的开创性研究和成就
谢良志1,2
- 1.北京市蛋白和抗体研发及制备工程技术研究中心,北京神州细胞生物技术集团股份公司,北京 100176
2.单克隆抗体上游研发技术北京市重点实验室,北京义翘神州科技股份有限公司,北京 100176
-
收稿日期:2021-04-26修回日期:2021-08-02出版日期:2021-08-31发布日期:2021-09-10 -
作者简介:谢良志 (1966—),男,博士,教授,主要从事生物化工、动物细胞培养、大分子生物药、基因工程疫苗、抗病毒中和抗体药物研发、临床研究和大规模生产及管理。E-mail:liangzhi@yahoo.com
Contributions to the booming biologics industrialization —a dedicated pioneer: Professor. Daniel I. C. Wang
XIE Liangzhi1,2
- 1.Beijing Protein and Antibody R&D Engineering Center,Sinocelltech Ltd. ,Beijing 100176,China
2.Beijing Antibody Research Key Laboratory,Sino Biological Inc. ,Beijing 100176,China
-
Received:2021-04-26Revised:2021-08-02Online:2021-08-31Published:2021-09-10
摘要:
以单克隆抗体药物及重组蛋白为代表的生物药在肿瘤、自身免疫性疾病、病毒感染等多个重大疾病的预防及治疗领域发挥了重要作用,已成为全球制药行业的主攻方向之一。动物细胞培养技术和工艺放大是生物药产业化的核心技术,存在巨大挑战,在20世纪90年代初仍被认为是一个很难破解的“黑箱”,是制约行业发展的关键瓶颈之一。自60年代开始,王义翘教授领导的实验室和美国麻省理工学院生物技术工程中心(Biotechnology Process Engineering Center, MIT-BPEC)对动物细胞培养技术进行了全方位系统的研究和探索,涵盖了从贴壁细胞的微载体培养到CHO细胞的无血清悬浮培养,从批次培养、连续培养、灌流培养到高密度流加培养过程中的关键技术和工程问题,包括细胞代谢有毒副产品的控制、高密度流加培养工艺的过程控制、蛋白质糖基化多样性质量分析和控制、搅拌和鼓泡导致的细胞损伤等关键瓶颈。王义翘教授团队数十年的前瞻性研究和多学科交叉合作在动物细胞代谢调控、数十种营养物质的化学计量平衡配比、无血清培养基的开发、有毒代谢副产物的控制、大分子生物药的糖基化多样性分析和质量控制、生物反应器的设计和工艺放大等一系列关键技术的研发方面取得了突破性的前沿成果,为90年代后高密度流加培养工艺在生物技术产品大规模商业化生产中的广泛应用奠定了坚实的基础,为推动全球生物制药技术和产品的蓬勃发展做出了不可磨灭的伟大贡献。本文作者结合90年代初期在王义翘教授实验室学习期间的亲身经历,试图从自己熟悉的专业领域总结王教授团队在生物药产业化起步阶段针对动物细胞培养的关键技术攻关方面取得的重要成果,从一个侧面概述王教授1965—1995三十年间所开展的前沿性研究对全球生物制药此后近30年来的蓬勃发展所做出的奠基性工作和杰出贡献。谨以此文致敬和缅怀恩师王义翘教授!
中图分类号:
引用本文
谢良志. 生物药产业蓬勃发展早期的奠基性系统开发——缅怀王义翘教授的开创性研究和成就[J]. 合成生物学, 2021, 2(4): 482-496.
XIE Liangzhi. Contributions to the booming biologics industrialization —a dedicated pioneer: Professor. Daniel I. C. Wang[J]. Synthetic Biology Journal, 2021, 2(4): 482-496.
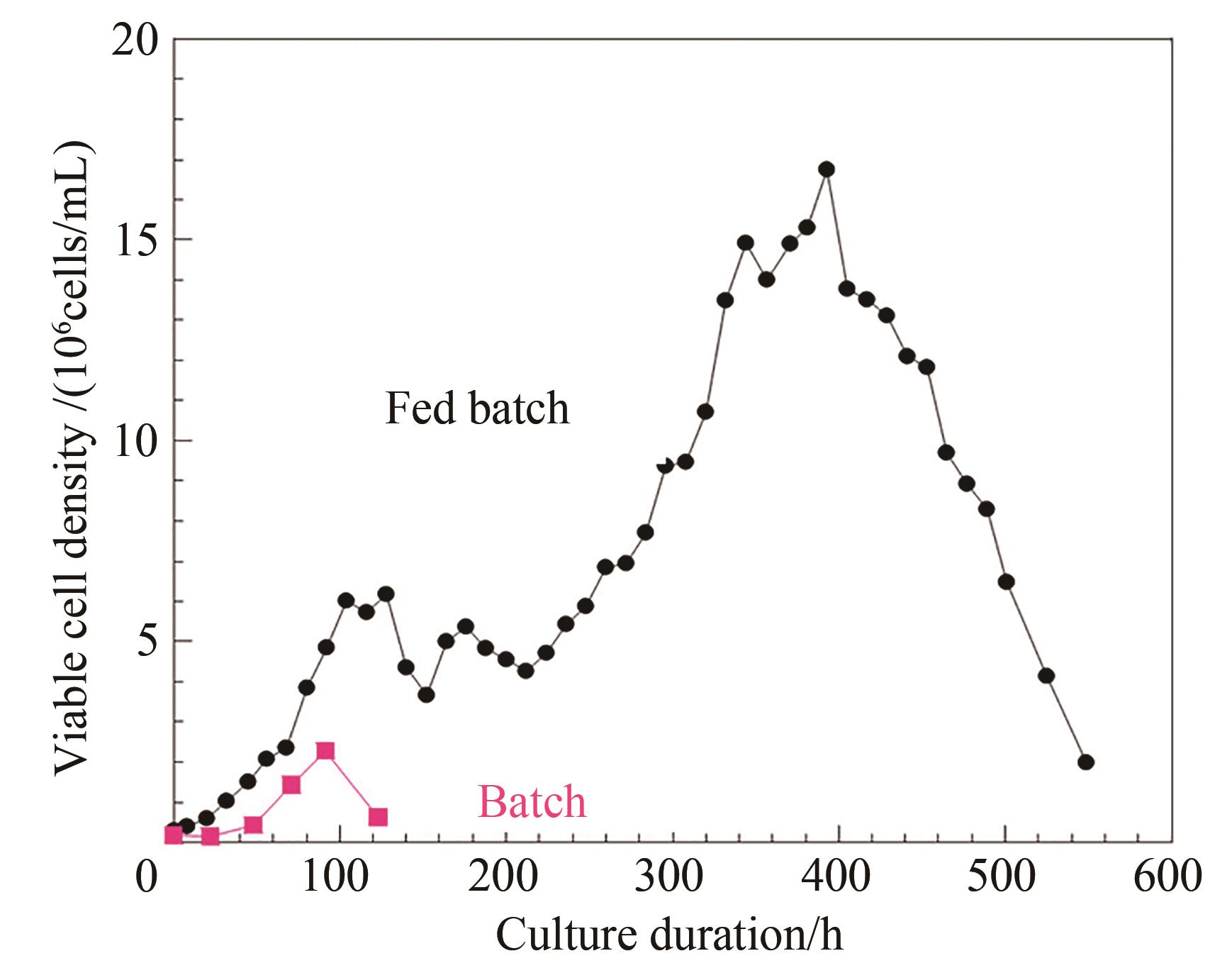
图2 化学计量平衡和加料控制的流加培养工艺中活细胞密度与批次培养对比
Fig. 2 Comparison of the viable cell density in the perfusion culture developed on stoichiometric balance and feed control to that observed in the batch process
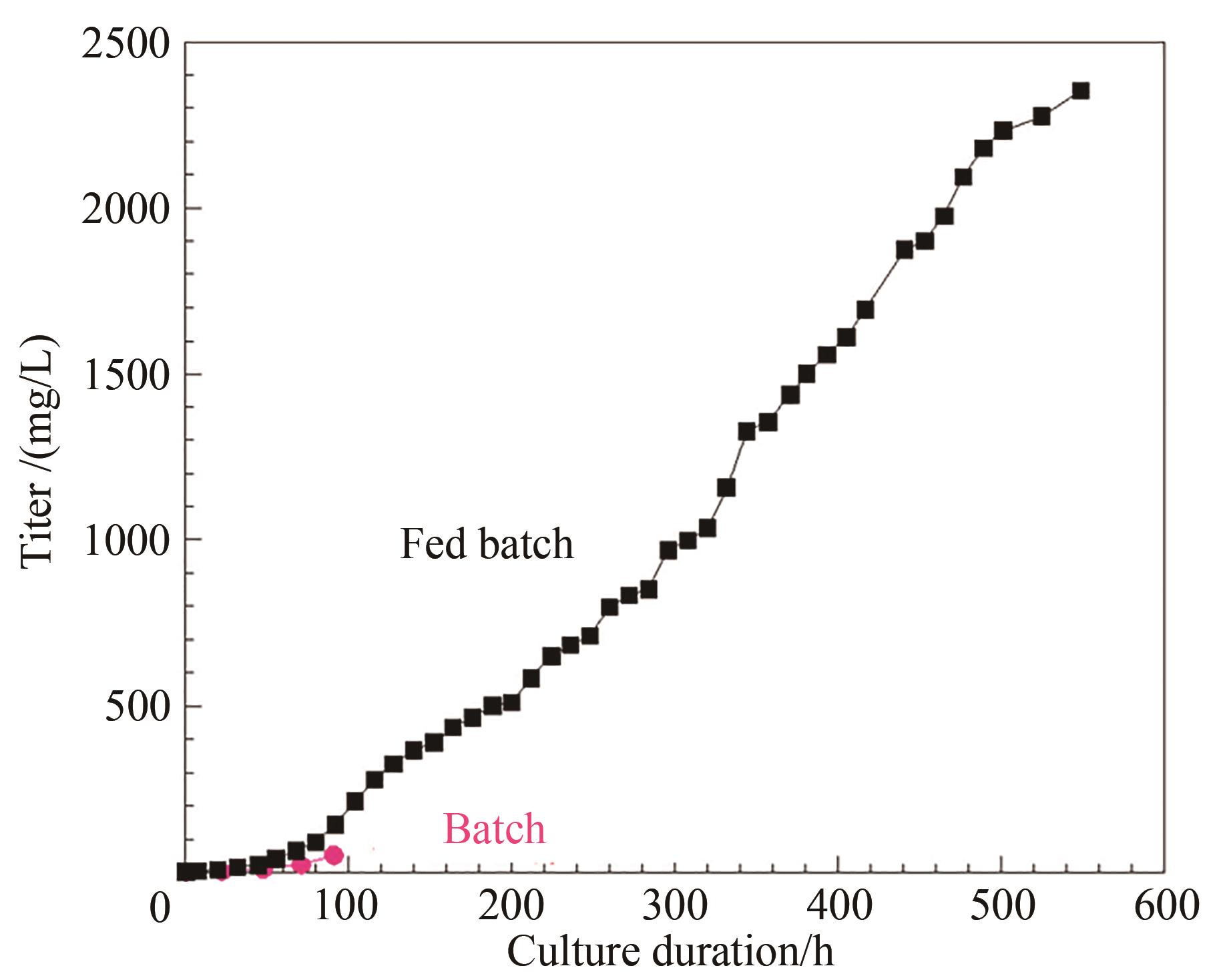
图3 化学计量平衡和加料控制的流加培养工艺中抗体产量与批次培养对比
Fig. 3 Comparison of the antibody yield in the perfusion culture developed on stoichiometric balance and feed control with that of the batch process
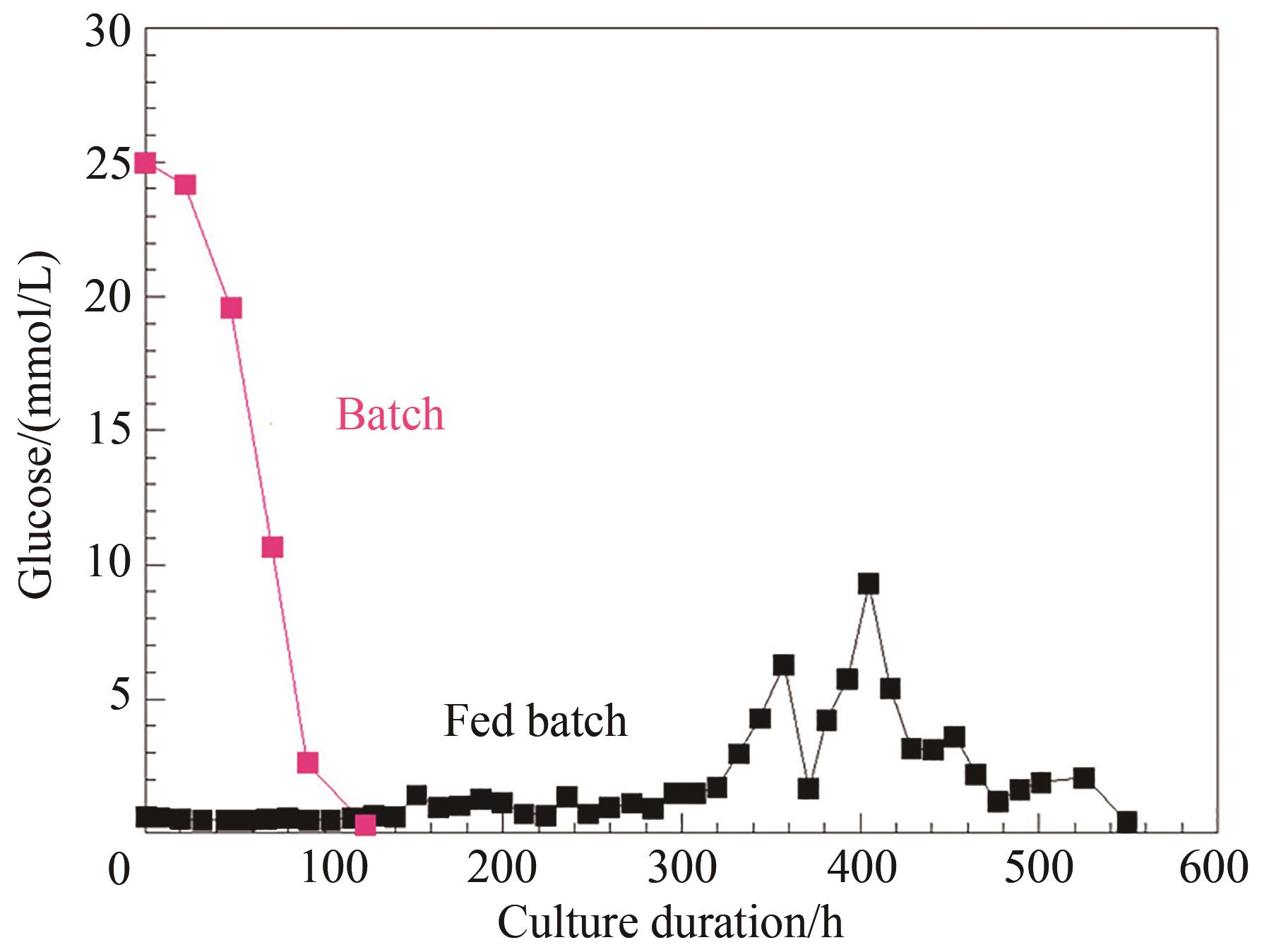
图4 化学计量平衡和加料控制的流加培养工艺中葡萄糖控制情况与批次培养对比
Fig. 4 Comparison of the glucose control index of the perfusion culture developed on stoichiometric balance and feed control with that of the batch process
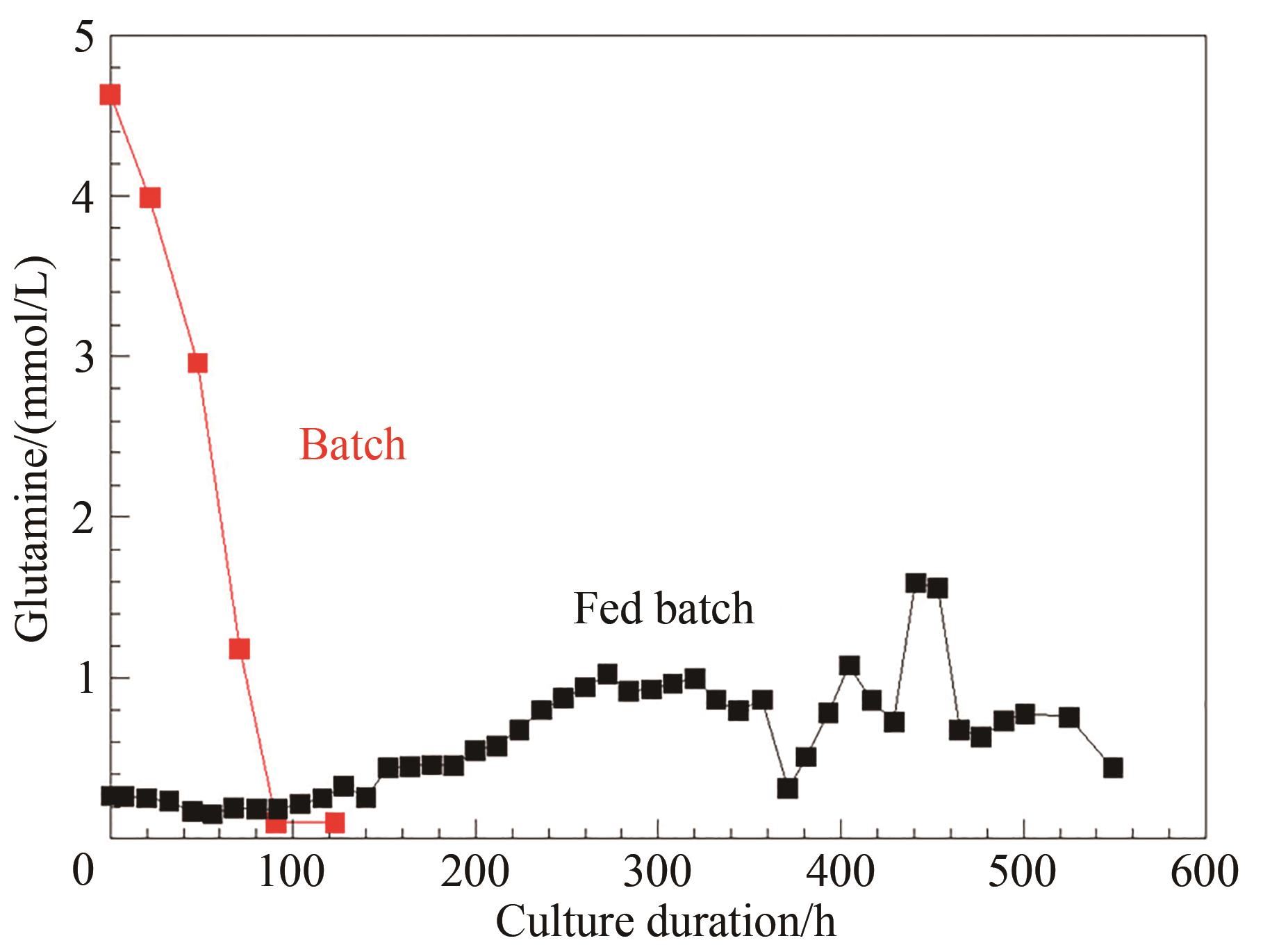
图5 化学计量平衡和加料控制的流加培养工艺中谷氨酰胺控制情况与批次培养对比
Fig. 5 Comparison of the glutamine control for the perfusion culture developed on stoichiometric balance and feed control with that of the batch process
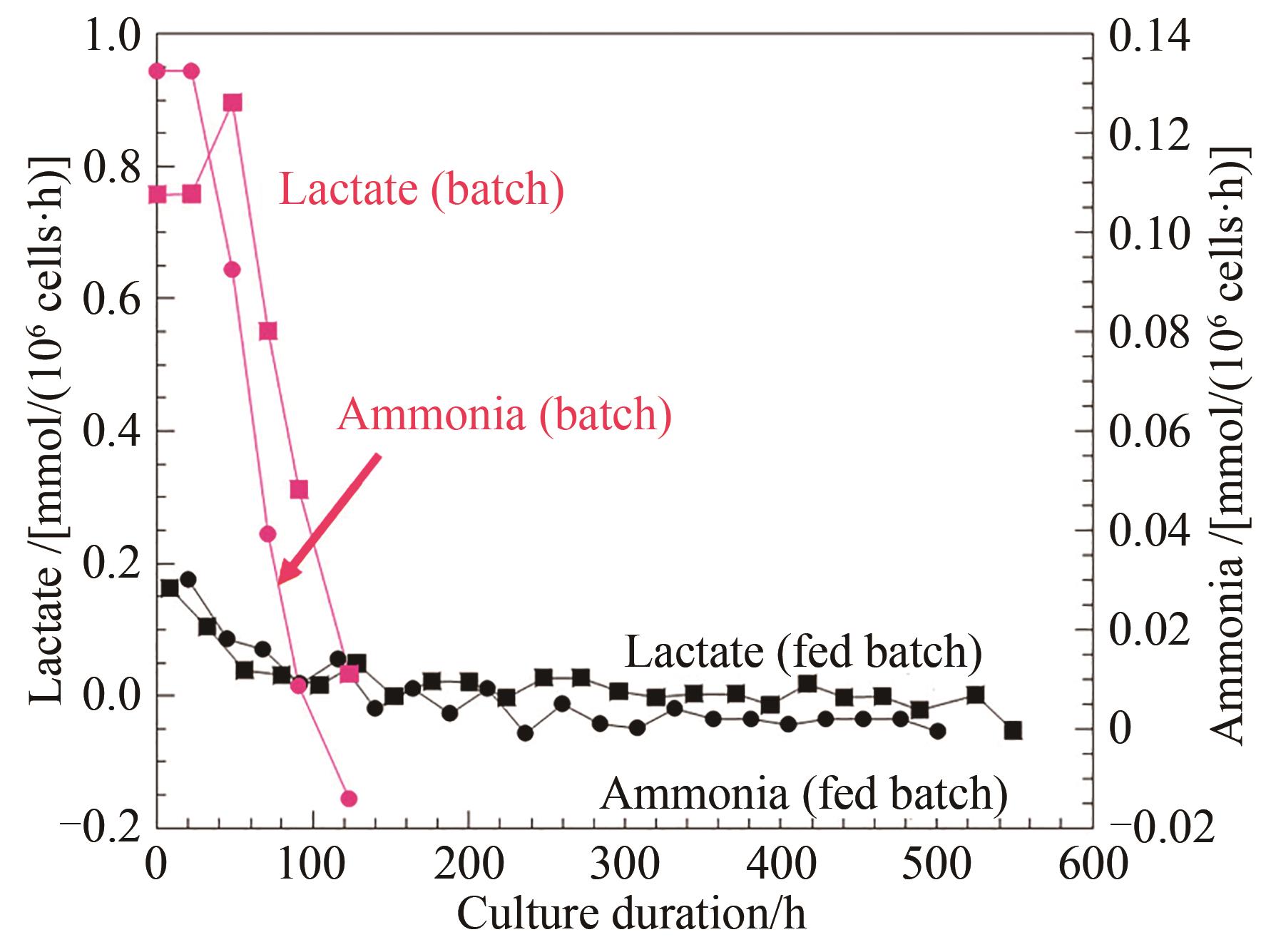
图6 化学计量平衡和加料控制的流加培养工艺中副产物乳酸和氨产生速率与批次培养对比
Fig. 6 Comparison of the byproduct lactic acid and ammonia production for the perfusion developed on stoichiometric balance and feed control with those of the batch process
| 68 | LJUNGGREN J, HAGGSTROM L. Glutamine limited fed-batch culture reduces ammonium ion production in animal cells[J]. Biotechnology Letters, 1990, 12: 705-710. |
| 69 | LJUNGGREN J, HAGGSTROM L. Glutamine limited fed-batch culture reduces the overflow metabolism of amino acids in myeloma cells[J]. Cytotechnology, 1992, 8: 45-56. |
| 70 | MILLER W M, WILKE C R, BLANCH H W. The transient responses of hybridoma cells to nutrient additions in continuous culture: II. glutamine pulse and step changes[J]. Biotechnology and Bioengineering, 1989, 33: 487-499. |
| 71 | LAZO P A. Amino acids and glucose utilization by different metabolic pathways[J]. European Journal of Biochemistry, 1981, 117: 19-25. |
| 72 | LUAN Y T, MUTHARASAN R, MAGEE W E. Effect of various glucose/glutamine ratios on hybridoma growth, viability, and monoclonal antibody formation[J]. Biotechnology Letters, 1987, 9: 535-538. |
| 73 | LJUNGGREN J, HAGGSTROM L. Catabolic control of hybridoma cells by glucose and glutamine limited fed batch cultures[J]. Biotechnology and Bioengineering, 1994, 44: 808-818. |
| 74 | LUAN Y T, MUTHARASAN R, MAGEE W E. Factors governing lactic acid formation in long term cultivation of hybridoma cells[J]. Biotechnology Letters, 1987, 9: 751-756. |
| 75 | LUAN Y T, MUTHARASAN R, MAGEE W E. Strategies to extend the longevity of hybridomas in culture and promote yield of monoclonal antibodies[J]. Biotechnology Letters, 1987c, 9: 691-696. |
| 76 | MILLER W M, BLANCH H W, WILKE C R. A kinetics analysis of hybridoma growth and metabolism in batch and continuous suspension culture: effect of nutrient concentration, dilution rate, and pH[J]. Biotechnology and Bioengineering, 1988, 32: 947-965. |
| 77 | MILLER W M, WILKE C R and BLANCH H W. Transient responses of hybridoma cells to nutrient additions in continuous culture: 1. glucose pulse and step changes[J]. Biotechnology & Bioengineering, 1989, 33: 477-486. |
| 78 | XIE L Z. Stoichiometric medium design and nutritional control in fed-batch cultivation of animal cells[D]. Cambridge, MA: Massachusetts Institute of Technology, 1997. |
| 79 | XIE L Z, WANG D I C. High density and high monoclonal antibody production through medium design and rational control in a bioreactor[J]. Biotechnology and Bioengineering, 1996, 51:725-729. |
| 80 | XIE L Z, WANG D I C. Integrated approaches to the design of media and feeding strategies for fed-batch cultures of animal cells[J]. Trends in Biotechnology, 1997, 15:109-113. |
| 81 | CHEN K Q, LIU Q, XIE L Z, et al. Engineering of mammalian cell line for reduction of lactate formation and high monoclonal antibody production[J]. Biotechnology and Bioengineering, 2001, 72:55-61. |
| 1 | TRAFTON A. Daniel Wang Institute Professor and pioneer in biochemical engineering, dies at 84.[N/OL]. MIT News, 2020-09-02. . |
| 2 | Baltimore JAFFE R., Harbison, Wang elevated to ranks of Institute Professors[N/OL]. MIT News, 1995-06-07. . |
| 3 | HATTON T A. DANIEL I.C. Wang: A tribute to an inspirational leader and colleague[J]. Biotechnology and Bioengineering, 2006, 95: 262-269. |
| 4 | ELLIS G. MIT’s Daniel I. C. Wang, co-founder of AIChE’s society for biological engineering, dies at 84[N/OL]. AIChE Biochemical Engineer ChEnected News, 2020-09-08. . |
| 5 | PRESTON L, HATCH R. Role of NSF engineering research centers in development of the field of bioengineering[EB/OL]. 5-E(a) Emerging Fields Catalyzed by ERCs. 2020, . |
| 6 | FISCHER S. Brain storm: David Berry profile[N/OL]. Published on Boston Magazine, 2011-04-20, . |
| 7 | ZHANG Y H P. Biofuel researcher wins biotechnology award[EB/OL]. Virginia Tech Daily Articles. [2010-01-04], . |
| 8 | CHUCK A S, DESHPANDE R R, DISTLER A R, et al. Commercial production of recombinant erythropoietins[M]//MOLINEUX G, FOOTE M A, ELLIOTT S G. Erythropoietins and erythropoiesis. Switzerland: Birkhauser Verlag 2003. |
| 9 | BOEDEKER B G. Production processes of licensed recombinant factor Ⅷ preparations[J]. Semin Thromb Hemost, 2001, 27:385-394. |
| 10 | AFEYAN N, COONEY C L. Professor Daniel I.C. Wang: a legacy of education, innovation, publication, and leadership[J]. Biotechnology and Bioengineering, 2006, 95: 206-217. |
| 11 | A L van WEZEL. Growth of cell strains and primary cells on microcarriers in homogeneous culture[J]. Nature, 1967, 216:64-65. |
| 12 | A L van WEZEL. Microcarrier cultures of animal cells[M]// KRUSE P F and PATTERSON M R. Tissue culture, methods and applications. New York: Academic Press, 1973: 372-377. |
| 82 | BIBILA T A, RANUCCI C S, GLAZOMITSKY K, et al. Monoclonal antibody process development using medium concentrates[J]. Biotechnology Progress, 1994, 10: 87-96. |
| 83 | BIBILA T A, ROBINSON D K. In pursuit of the optimal fed-batch process for monoclonal antibody production[J]. Biotechnology Progress, 1995, 11: 1-13. |
| 84 | BUSHELL M E, BELL S L, SCOTT M F, et al. Enhancement of monoclonal antibody yield by hybridoma fed-batch culture, resulting in extended maintenance of viable cell population[J]. Biotechnology and Bioengineering, 1994, 44: 1099-1106. |
| 85 | JO E C, PARK H J, PARK J M, et al. Balanced nutrient fortification enables high-density hybridoma cell culture in batch culture[J]. Biotechnology and Bioengineering, 1990, 36: 717-722. |
| 86 | JO E, PARK H, KIM D, et al. Repeated fed-batch culture of hybridoma cells in nutrient-fortified high density medium[J]. Biotechnology and Bioengineering, 1993, 42: 1229-1237. |
| 87 | ZHOU W, REHM J, HU W S. High viable cell concentration fed-batch cultures of hybridoma cells through on-line nutrient feeding[J]. Biotechnology and Bioengineering, 1995, 46: 579-587. |
| 88 | TREMBLAY M D, PERRIER M, CHAVARIE C, et al. Optimization of fed-batch culture of hybridoma cells using dynamic programming: single and multi feed cases[J]. Bioprocess Engineering, 1992, 7: 229-234. |
| 89 | XIE L Z, WANG D I C. Stoichiometric analysis of animal cell growth and its application in medium design[J]. Biotechnology and Bioengineering, 1994, 43:1164-1174. |
| 90 | WOHLPART D, KIRWAN D and GAINER J. Effects of cell density and glucose and glutamine levels on the respiration rates of hybridoma cells[J]. Biotechnology and Bioengineering, 1990, 36: 630-63. |
| 91 | ZENG A P, DECKWER W D. Mathematical modeling and analysis of glucose and glutamine utilization and regulation in cultures of continuous mammalian cells[J]. Biotechnology and Bioengineering, 1995, 47: 334-346. |
| 92 | ZIELKE H R, OZAND P T, TILDON J T, et al. Reciprocal regulation of glucose and glutamine utilization by human diploid fibroblasts[J]. Journal of Cellular Physiology, 1978, 95: 41-48. |
| 93 | XIE L Z, WANG D I C. Fed-batch cultivation of animal cells using different medium design concepts and feeding strategies[J]. Biotechnology and Bioengineering, 1994, 43:1175-1189. |
| 94 | XIE L Z, WANG D I C. Applications of improved stoichiometric model in medium design and fed-batch cultivation of animal cells in bioreactor[J]. Cytotechnology, 1994, 15: 17-29 |
| 95 | XIE L Z, NYBERG G, GU X, et al. Gamma interferon production and quality in stoichiometric fed-batch cultures of Chinese hamster ovary cell under serum-free conditions[J]. Biotechnology and Bioengineering, 1997, 56:577-582. |
| 13 | van HEMERT P, KILBURN D G, van WEZEL A L. Homogeneous cultivation of animal cells for the production of virus and virus products[J]. Biotechnology and Bioengineering, 1969, 11:875-885. |
| 14 | HORNG C, McLIMANS W. Primary suspension culture of calf anterior pituitary cells on microcarrier surface[J]. Biotechnology and Bioengineering, 1975, 17:713-732. |
| 15 | SPIER R E, WHITESIDE J P. The production of foot-and-mouth disease virus from BHK 21 C13 cells grown on the surface of glass spheres[J]. Biotechnology and Bioengineering, 1976,18: 649-657. |
| 16 | LEVINE D W, WANG J S, WANG D I C, et al. Microcarrier cell culture: New method for research scale application[J]. Somatic Cell Genetics, 1977, 3:149. |
| 17 | LEVINE D W, WANG D I C, THILLY W G. Optimization of growth surface parameters in microcarrier cell culture[J]. Biotechnology and Bioengineering, 1979a, 21:821. |
| 18 | LEVINE D W, THILLY W G, WANG D I C. Parameters affecting cell growth on reduced charge microcarriers[J]. Developments in Biological Standardization, 1979b, 42:159. |
| 19 | GIARD D J, THILLY W G, WANG D I C, et al. Virus production with a newly developed microcarrier system[J]. Applied and Environmental Microbiology, 1977, 34:668. |
| 20 | GIARD D J, LOEB D H, THILLY W G, et al. Human interferon production with diploid fibroblast cells grown on microcarriers[J]. Biotechnology and Bioengineering, 1979, 21:433. |
| 21 | GIARD D J, FLEISCHAKER R J, SINSKEY A J, et al. Large-scale production of human fibroblast interferon[J]. Developments in Industrial Microbiology, 1981, 22:299. |
| 22 | HU W S, MEIER J, WANG D I C. A mechanistic analysis of the inoculums requirement for the cultivation of mammalian cells on microcarriers[J]. Biotechnology and Bioengineering, 1984, 27:585. |
| 23 | HU W S, WANG D I C. Serial propagation of mammalian cells on microcarriers[J]. Biotechnology and Bioengineering, 1985, 27:1466. |
| 24 | HU W S, WANG D I C. Mammalian cell culture technology: a review from an engineering perspective[M]//THILLY W G. Mammalian cell technology.Stoneham, MA: Butterworth, 1986: 167. |
| 25 | HU W S, MEIER J, WANG D I C. Use of surface aerator to improve oxygen transfer and cell growth in cell culture[J]. Biotechnology and Bioengineering, 1986, 28:122. |
| 26 | HU W S, WANG D I C. Selection of microcarrier diameter or the cultivation of mammalian cells on microcarriers[J]. Biotechnology and Bioengineering, 1987, 30:548. |
| 27 | CROUGHAN M S, WANG D I C. Growth and death in over-agitated microcarrier cell culture[J]. Biotechnology and Bioengineering, 1989, 33:731. |
| 28 | CROUGHAN M S, WANG D I C. Hydrodynamic effects on animal cells in microcarrier bioreactors[M]//HO C S, WANG D I C. Animal cell bioreactor.Stoneham, MA: Butterworth-Heinemann Press, 1991: 213. |
| 96 | CHIOU T W, HSIEH Y C, HO C S. High density culture of insect cells using rational medium design and feeding strategy[J]. Bioprocess Engineering, 2000, 22: 483-491 |
| 97 | XIE L Z, WANG D I C. Material balance studies on animal cell culture metabolism using a stoichiometrically based reaction network[J]. Biotechnology and Bioengineering, 1996, 52:579-590. |
| 98 | XIE L Z, WANG D I C. Energy metabolism and ATP balance in animal cell cultivation using a stoichiometrically based reaction network[J]. Biotechnology and Bioengineering, 1996, 52:591-601. |
| 99 | GOETZE A M, LIU Y D, ZHANG Z, et al. High-mannose glycans on the Fc region of therapeutic IgG antibodies increase serum clearance in humans[J]. Glycobiology, 2011, 21: 949-959 |
| 100 | LUO C, CHEN S, XU N, et al. Glycoengineering of pertuzumab and its impact on the pharmacokinetic/pharmacodynamic properties[J]. Scientific Reports. 2017, 7: 46347 |
| 101 | LIU L. Antibody glycosylation and its impact on the pharmacokinetics and pharmacodynamics of monoclonal antibodies and Fc-fusion proteins[J]. Journal of Pharmaceutical Sciences, 2015, 104: 1866-1884 |
| 102 | REUSCH D, TEJADA M L. Fc glycans of therapeutic antibodies as critical quality attributes[J]. Glycobiology, 2015, 25: 1325-1334. |
| 103 | SHINKAWA T, NAKAMURA K, YAMANE N, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity[J]. Journal of Biological Chemistry, 2003, 278:3466-3473. |
| 104 | SHIELDS R L, LAI J, KECK R, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fc RIII and antibody-dependent cellular toxicity[J]. Journal of Biological Chemistry, 2002, 277: 26733-26740. |
| 105 | HARMON B J, GU X, WANG D I C. Rapid monitoring of site-specific glycosylation microheterogeneity of recombinant human interferon-gamm[J]. Analytical Chemistry, 1996, 68:1465-147 |
| 106 | GU X J, XIE L, HARMON B J, et al. Influence of primatone RL supplementation and sialyation of recombinant human interferon gamma produced by Chinese hamster ovary culture using serum-free media[J]. Biotechnology and Bioengineering, 1997, 56:353-360. |
| 107 | GU X J, HARMON B J, WANG D I C. Site-and-branch-specific sialyation of recombinant interferon-gamma in Chinese hamster ovary cell culture[J]. Biotechnology and Bioengineering, 1997a, 55:390-398. |
| 108 | GU X J, HARMON B J, WANG D I C. Monitoring and characterization of glycoprotein quality in animal cell cultures[M]// GALINDOE, RAMIREZ O T. Advances in bioprocessing engineering II. In advances in bioprocess engineering II. Netherlands: Kluwer Academic Publisher, 1998: 1-24. |
| 109 | GU X J. Characterization and improvement of interferon-[gamma] glycosylation in Chinese hamster ovary cell culture[D]. Cambridge, MA: Massachusetts Institute of Technology, 1997. |
| 110 | NYBERG G B, BALCARCEL R R, FOLLSTAD B D, et al. Metabolism of peptide amino acids by Chinese hamster ovary cells grown in a complex medium[J]. Biotechnology and Bioengineering, 1998, 62:324-335. |
| 29 | CROUGHAN M S, J-F HAMEL, WANG D I C. Hydrodynamic effects on animal cells grown in microcarrier cultures[J]. Biotechnology and Bioengineering, 1987, 29:130. |
| 30 | CROUGHAN M S, J-F HAMEL, WANG D I C. Effects of microcarrier concentration on animal cell culture[J]. Biotechnology and Bioengineering, 1988, 32:975. |
| 31 | CROUGHAN M S, SAYRE E, WANG D I C. Viscous reduction of turbulent damage in animal cell culture[J]. Biotechnology and Bioengineering, 1989, 33:862. |
| 32 | PERRY S D, WANG D I C. Fiber bed reactor design for animal cell culture[J]. Biotechnology and Bioengineering, 1989, 34:1. |
| 33 | CHIOU T W, MURAKAMI S, WANG D I C. A fiber-bed bioreactor for anchorage-dependent animal cell culture. part I: Bioreactor design and operations[J]. Biotechnology and Bioengineering, 1991, 37:755. |
| 34 | CHIOU T W. A modified airlift fiber-bed bioreactor for animal cell culture[D]. Cambridge, MA: Massachusetts Institute of Technology, 1992. |
| 35 | MURAKAMI S, CHIOU T W, WANG D I C. A fiber-bed bioreactor for anchorage-dependent animal cell culture. Part II: Scale-up potentials[J]. Biotechnology and Bioengineering, 1991, 37:762. |
| 36 | WANG D I C, SCHARER J, HUMPHREY A E. Kinetics of death of bacterial spores at elevated temperatures[J]. Applied Microbiology, 1964, 12:451. |
| 37 | WANG D I C, HUMPHREY A E, EAGLETON L C. Measurement of the kinetics of biological systems at elevated temperatures utilizing flow techniques[J]. Biotechnology and Bioengineering, 1964, 6:367. |
| 38 | WANG D I C, OCHOA A. Measurements on the interfacial areas of hydrocarbons in yeast fermentations and relationships to specific growth rates[J]. Biotechnology and Bioengineering, 1972, 14:345. |
| 39 | COONEY C L, WANG D I C, MATELES R I. Measurement of heat evolution during fermentation[J]. Biotechnology and Bioengineering, 1969, 11:269. |
| 40 | COONEY C L, WANG D I C, MATELES R I. Growth of enterobacter aerogenes under dual limitation in a chemostat[J]. Biotechnology and Bioengineering, 1976, 18:189. |
| 41 | COONEY C L, WANG H Y, WANG D I C. Computer-aided material balance for prediction of fermentation parameter[J]. Biotechnology and Bioengineering, 1977, 19:55. |
| 42 | WANG H Y, COONEY C L, WANG D I C. Computer-aided baker's yeast fermentation[J]. Biotechnology and Bioengineering, 1977, 19:69. |
| 43 | KENNEDY M J, WANG D I C, STEPHANOPOULOS G N. Estimating cell concentration in the presence of suspended solid: light scatter technique[J]. Biotechnology and Bioengineering, 1992, 40:875. |
| 44 | KENNEDY M J, THAKUR M S, WANG D I C, et al. Techniques for the estimation of cell concentration in the presence of suspended solids: a review[J]. Biotechnology Progress, 1992, 8:375. |
| 45 | LASKO D, WANG D I C. In situ fermentation monitoring with recombinant firefly luciferase[J]. Biotechnology and Bioengineering, 1993, 42:30-36. |
| 46 | LASKO D R, WANG D I C. On-line monitoring of intracellular ATP concentration in Escherichia coli fermentations[J]. Biotechnology and Bioengineering, 1996, 52:364-372. |
| 47 | AUNINS J G, WOODSON B A, HALE T K, et al. Effect of paddle impeller geometry on power input and mass transfer in small-scale animal cell culture vessels[J]. Biotechnology and Bioengineering, 1989, 34:1127. |
| 48 | NEWLAND M, KAMAL M N, GREENFIELD P F, et al. Ammonia inhibition of hybridomas propagated in batch, fed-batch, and continuous culture[J]. Biotechnology and Bioengineering, 1994, 43: 434-438. |
| 49 | DOYLE C AND BUTLER M. The effect of pH on the toxicity of ammonia to a murine hybridoma[J]. Journal of Biotechnology, 1990, 15: 91-100. |
| 50 | HANSEN H A, EMBORG C. Influence of ammonium on growth, metabolism, and productivity of a continuous suspension Chinese ovary cell culture[J]. Biotechnology Progress, 1994, 10: 121-124. |
| 51 | HASSELL T, GLEAVE S AND BUTLER M. Growth inhibition in animal cell culture the effect of lactate and ammonia[J]. Applied Biochemistry and Biotechnology, 1991, 30: 29-41. |
| 52 | OMASA T, HIGASHIYAMA K, SHIOYA S, et al. Effects of lactate concentration on hybridoma culture in lactate-controlled fed-batch operation[J]. Biotechnology and Bioengineering, 1992, 39: 556-564. |
| 53 | BORYS M C, LINZER D I H, PAPOUTSAKIS E T. Ammonia affects the glycosylation patterns of recombinant mouse placental lactogen-I by Chinese hamster ovary cells in a pH-dependent mammer[J]. Biotechnology and Bioengineering, 1994, 43: 505-514. |
| 54 | ANDERSEN D C, GOOCHEE C F. The effect of ammonia on the O-linked glycosylation of granulocyte colony-stimulated factor produced by Chinese hamster ovary cells[J]. Biotechnology and Bioengineering, 1995, 47: 96-105. |
| 55 | GLACKEN M W, FLEISCHAKER R J, SINSKEY A J. Reduction of waste product excretion via nutrient control: possible strategies for maximizing product and cell yields on serum in cultures of mammalian cells[J]. Biotechnology and Bioengineering, 1986, 28: 1376-1389. |
| 56 | GLACKEN M W. Development of mathematical descriptions of mammalian cell culture kinetics for the optimization of fed-batch bioreactors[D]. Cambridge, MA: Massachusetts Institute of Technology, 1987. |
| 57 | CHANG D Y S, WANG D I C. In situ removal of ammonia and lactate from suspension hybridoma cultures through electrical means[J]. Biotechnology and Bioengineering, 1995, 47:308-318. |
| 58 | CHANG D Y S, WANG D I C. Nutrient enrichment and in situ removal of ammonia and lactate through electrical means for suspension hybridoma cultures[J]. Biotechnology and Bioengineering, 1995, 47:319–326. |
| 59 | KATUNUMA N, HUZINO A, TOMINO I. Organ specific control of glutamine metabolism[M]//Weber G. Advances in enzyme regulation: vol 5. Bristol: Pergamon Press, 1967, 55-69. |
| 60 | KATUNUMA N, KATSUNUMA T, TOWATARI T, et al. Regulatory mechanisms of glutamine catabolism[M]//PRUSINER S and STADTMAN E R. The Enzymes of Glutamine Metabolism. New York: Academic Press, 1973: 227-258. |
| 61 | KOVACEVIC Z. The pathway of glutamine and glutamate oxidation in isolated mitochondria from mammalian cells[J]. Biochemical Journal, 1971, 125: 757-763. |
| 62 | LANKS K W. Glutamine is responsible for stimulating glycolysis by L929 cells[J]. Journal of cellular physiology, 1986, 126: 319-321. |
| 63 | LANKS K W AND LI P W. End products of glucose and glutamine metabolism by cultured cell lines[J]. Journal of cellular physiology, 1988, 135: 151-155. |
| 64 | LIN A, AGRAWAL P. Glutamine decomposition in DMEM: effect of pH and serum concentration[J]. Biotechnology Letters, 1988, 10: 695-698. |
| 65 | TRITSCH G L, MOORE G E. Spontaneous decomposition of glutamine in cell culture media[J]. Experimental Cell Research, 1962, 28: 360-364. |
| 66 | ZIELKE H R, ZIEKLE C L, OZAND P T. Glutamine: a major energy source for cultured mammalian cells[J]. Federation Proceedings, 1984, 43: 121-125. |
| 67 | KUROKAWA H, PARK Y S, LIJIMA S, et al. Growth characteristics in fed-batch culture of hybridoma cells with control of glucose and glutamine concentrations[J]. Biotechnology and Bioengineering, 1994, 44: 95-103. |
| 111 | NYBERG G B, BALCARCEL R R, FOLLSTAD B D, et al. Metabolic effects on recombinant interferon-g glycosylation in continuous culture of Chinese hamster ovary cells[J]. Biotechnology and Bioengineering, 1998, 63:336-347. |
| 112 | AUGENSTEIN D C, SINSKEY A J and WANG D I C. Effect of shear on the death of two strains of mammalian tissue cells[J]. Biotechnology and Bioengineering, 1971, 13: 409-418. |
| 113 | CROUGHAN M S. Hydrodynamic effects of animal cells in microcarrier bioreactors[D]. Cambridge, MA: Massachusetts Institute of Technology,1988. |
| 114 | ORTON D R. Quantitative and mechanistic effects of bubble aeration on animal cells in culture[D]. Cambridge, MA: Massachusetts Institute of Technology, 1993. |
| 115 | MEIER S J. Modeling sparging related death in animal cell bioreactors[D]. Cambridge, MA: Massachusetts Institute of Technology, 1998. |
| 116 | MEIER S J, HATTON T A, WANG D I C. Cell death from bursting bubbles: role of cell attachment to rising bubbles in sparged reactors[J]. Biotechnology and Bioengineering, 1999, 62:468-478. |
| 117 | MA N, CHALMERS J J, AUNINS J G, et al. Quantitative studies of cell-bubble interactions and cell damage at different pluronic F-68 and cell concentrations[J]. Biotechnology Progress, 2004, 20:1183-1191. |
| [1] | 邵明威, 孙思勉, 杨时茂, 陈国强. 基于极端微生物的生物制造[J]. 合成生物学, 2024, 5(6): 1419-1436. |
| [2] | 陈国强, 谭丹. 重编程微生物底盘用于PHA材料的定制化低成本生物合成[J]. 合成生物学, 2024, 5(5): 1211-1226. |
| [3] | 陈江楠, 陈潇宁, 刘心怡, 万薇, 章义鑫, 张自豪, 郑逸飞, 郑陶然, 王宣, 王子瑜, 闫煦, 张旭, 吴赴清, 陈国强. 基于工程化盐单胞菌的下一代工业生物技术[J]. 合成生物学, 2020, 1(5): 516-527. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||

