• •
“EKylation”策略改造真菌抗冻蛋白及机制解析
崔忠信1,2, 王怡1, 张雷1,2, 齐海山1,2
- 1.天津大学,合成生物与生物制造学院,天津 300072
2.天津大学,合成生物技术全国重点实验室,教育部合成生物学前沿科学中心,天津 300072
-
收稿日期:2025-03-19修回日期:2025-07-18出版日期:2025-08-01 -
通讯作者:张雷,齐海山 -
作者简介:崔忠信 (1999—),男,博士在读,研究方向为功能蛋白的智能设计张雷 (1980—),男,博士,教授。研究方向为合成生物学、生物医学工程、生物材料与细胞冷冻保存技术。齐海山 (1986—),男,博士,副教授。研究方向为功能蛋白合成生物技术、细胞工厂智能创建。
Engineering fungal antifreeze proteins through ‘EKylation’ and mechanism analysis
CUI Zhongxin1,2, WANG Yi1, ZHANG Lei1,2, QI Haishan1,2
- 1.School of Synthetic Biology and Biomanufacturing,Tianjin University,Tianjin,China.
2.State Key Laboratory of Synthetic Biology,Frontiers Science Center for Synthetic Biology,Tianjin University,Tianjin,China.
-
Received:2025-03-19Revised:2025-07-18Online:2025-08-01 -
Contact:ZHANG Lei, QI Haishan
摘要:
抗冻蛋白(Antifreeze proteins, AFPs)是一类通过非依数性机制降低冰点并有效抑制冰晶生长的生物大分子,广泛分布于极地鱼类、昆虫及耐寒微生物等生物体内以维持其低温适应性,在食品冷冻保存、低温医学及低温生物技术等领域具有广泛应用,开发高活性AFPs具有重要研究价值。本研究利用“EKylation”策略对雪腐病核瑚菌(Typhula ishikariensis)来源的AFPs(RCSB ID:5B5H)开展分子改造研究。基于两性离子多肽的电荷可调特性与蛋白质稳定化机制,将两性离子多肽(EK)20定点偶联至5B5H的N端结构域,经两性离子改造的重组蛋白5B5H-EK高级结构保持稳定,热滞活性较野生型提升27.8%。分子动力学模拟进一步揭示5B5H-EK的冰晶结合面并未发生改变,且具有更强的抑制冰晶生长能力和冰晶结合能力,促使水分子形成短程有序的"类冰水"结构。该研究为理性设计高效抗冻蛋白及环境适应性抗冻材料提供了新策略。
中图分类号:
引用本文
崔忠信, 王怡, 张雷, 齐海山. “EKylation”策略改造真菌抗冻蛋白及机制解析[J]. 合成生物学, DOI: 10.12211/2096-8280.2025-019.
CUI Zhongxin, WANG Yi, ZHANG Lei, QI Haishan. Engineering fungal antifreeze proteins through ‘EKylation’ and mechanism analysis[J]. Synthetic Biology Journal, DOI: 10.12211/2096-8280.2025-019.
| 蛋白名称 | 5′端引物序列 | 3′端引物序列 |
|---|---|---|
| 5B5H | TGGGCAGCAGCCATCATCAT | TGGTGGTGGTGGTGCTC |
| 5B5H-EK | GTGGGCAGCAGCCATCATCAT | TGGTGGTGGTGGTGCTCT |
表1 引物序列
Table 1 Primer sequence
| 蛋白名称 | 5′端引物序列 | 3′端引物序列 |
|---|---|---|
| 5B5H | TGGGCAGCAGCCATCATCAT | TGGTGGTGGTGGTGCTC |
| 5B5H-EK | GTGGGCAGCAGCCATCATCAT | TGGTGGTGGTGGTGCTCT |
| 种类 | 序列 |
|---|---|
| 5B5H | AGPTAVPLGTAGNYAILASAGVSTVPQSVITGAVGLSPAAATFLTGFSLTMSSTGTFSTSTQVTGQLTAADYGTPTPSILTTAIGDMGTAYVNAATRSGPNFLEIYTGALGGKILPPGLYKWTSPVGASADFTIIGTSTDTWIFQIAGTLGLAAGKKIILAGGAQAKNIVWVVAGAVSIEAGAKFEGVILAKTAVTLKTGSSLNGRILSQTAVALQKATVVQK |
| 5B5H-EK | EKEKEKEKEKEKEKEKEKEKEKEKEKEKEKEKEKEKEKEKAGPTAVPLGTAGNYAILASAGVSTVPQSVITGAVGLSPAAATFLTGFSLTMSSTGTFSTSTQVTGQLTAADYGTPTPSILTTAIGDMGTAYVNAATRSGPNFLEIYTGALGGKILPPGLYKWTSPVGASADFTIIGTSTDTWIFQIAGTLGLAAGKKIILAGGAQAKNIVWVVAGAVSIEAGAKFEGVILAKTAVTLKTGSSLNGRILSQTAVALQKATVVQK |
表2 5B5H和5B5H-EK氨基酸序列
Table 2 the amino acid sequence of 5B5H and 5B5H-EK
| 种类 | 序列 |
|---|---|
| 5B5H | AGPTAVPLGTAGNYAILASAGVSTVPQSVITGAVGLSPAAATFLTGFSLTMSSTGTFSTSTQVTGQLTAADYGTPTPSILTTAIGDMGTAYVNAATRSGPNFLEIYTGALGGKILPPGLYKWTSPVGASADFTIIGTSTDTWIFQIAGTLGLAAGKKIILAGGAQAKNIVWVVAGAVSIEAGAKFEGVILAKTAVTLKTGSSLNGRILSQTAVALQKATVVQK |
| 5B5H-EK | EKEKEKEKEKEKEKEKEKEKEKEKEKEKEKEKEKEKEKEKAGPTAVPLGTAGNYAILASAGVSTVPQSVITGAVGLSPAAATFLTGFSLTMSSTGTFSTSTQVTGQLTAADYGTPTPSILTTAIGDMGTAYVNAATRSGPNFLEIYTGALGGKILPPGLYKWTSPVGASADFTIIGTSTDTWIFQIAGTLGLAAGKKIILAGGAQAKNIVWVVAGAVSIEAGAKFEGVILAKTAVTLKTGSSLNGRILSQTAVALQKATVVQK |
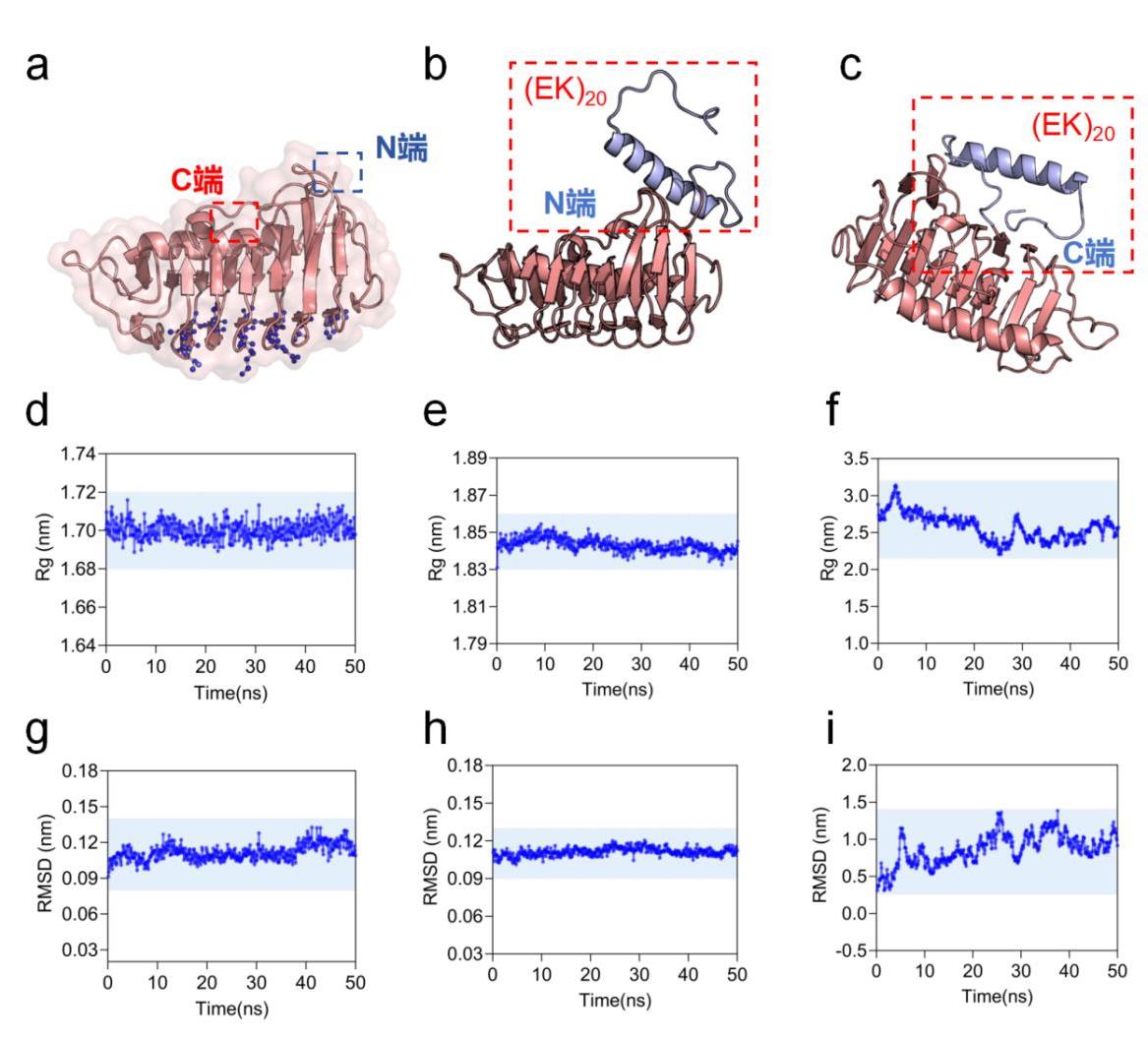
图1 改造前后5B5H抗冻蛋白结构稳定性解析(a)5B5H改造位点探究(b)5B5H(N端修饰)空间结构(c)5B5H(C端修饰)空间结构(d)5B5H水环境Rg模拟结果(e)5B5H(N端修饰)水环境Rg模拟结果(f)5B5H(C端修饰)水环境Rg模拟结果(g)5B5H水环境RMSD模拟结果(h)5B5H(N端修饰)水环境RMSD模拟结果(i)5B5H(C端修饰)水环境RMSD模拟结果
Fig. 1 Conformation resolution of EK, 5B5H and 5B5H-EK(a) 5B5H modification site exploration (b) 5B5H (N-terminal modification) spatial structure (c) 5B5H (C-terminal modification) spatial structure (d) 5B5H aqueous environment Rg simulation results (e) 5B5H (N-terminal modification) aqueous environment Rg simulation results (f) 5B5H (C-terminal modification) aqueous environment Rg simulation results (g) 5B5H aqueous environment RMSD simulation results (h) 5B5H (N-terminal modification) aqueous environment RMSD simulation results (i) 5B5H (C-terminal modification) aqueous environment RMSD simulation results
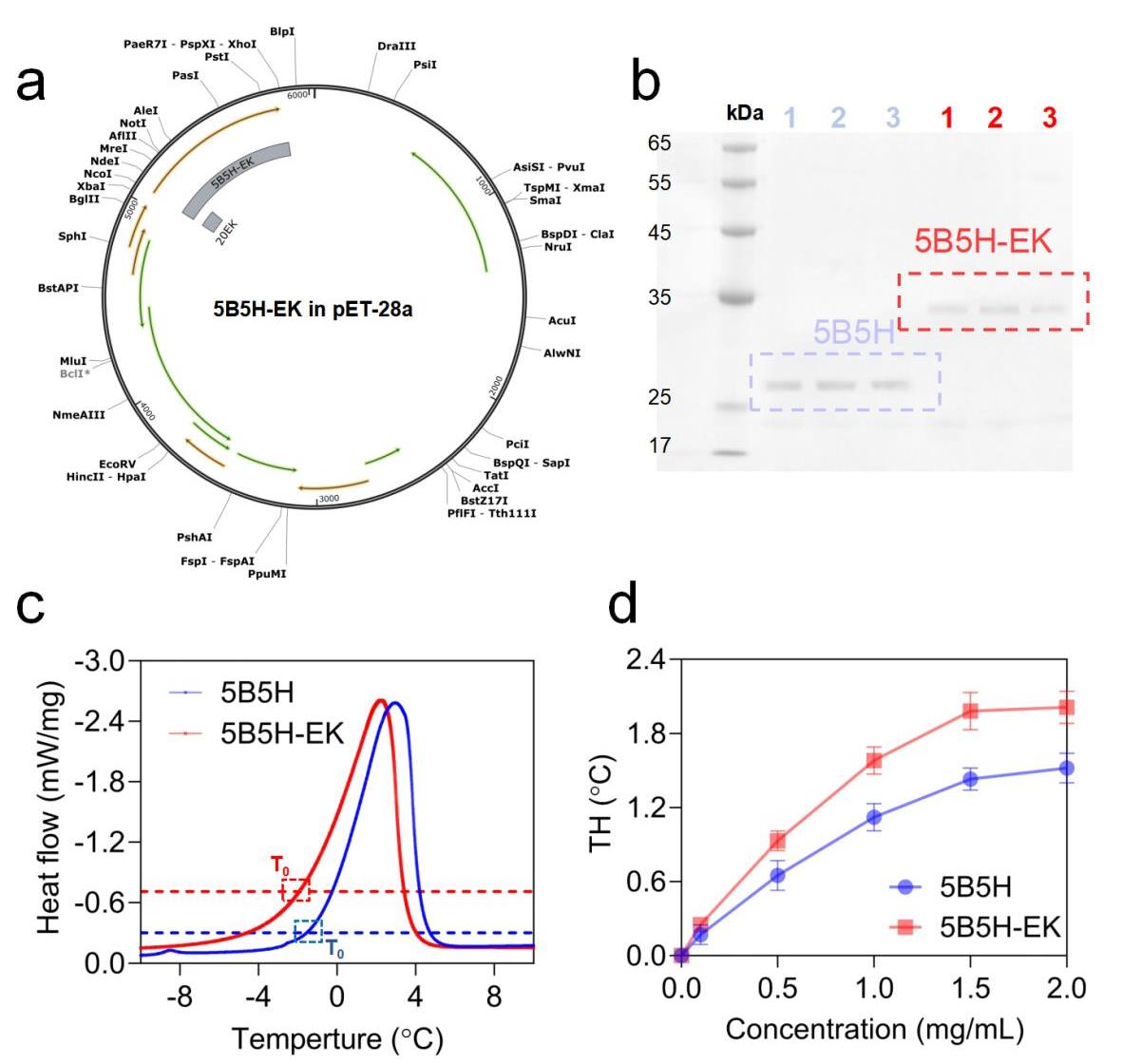
图2 5B5H和5B5H-EK的发酵,纯化和热滞活性测量(a) 5B5H-EK质粒图谱(b) 5B5H和5B5H-EK蛋白纯化结果,其中1,2,3泳道分别为同一种蛋白的平行样品 (c) 5B5H和5B5H-EK在2mg/mL下的DSC测试数据(d)5B5H和5B5H-EK在不同浓度下的热滞活性
Fig. 2 Fermentation, purification and heat-lag activity measurements of 5B5H and 5B5H-EK(a) Plasmid of 5B5H-EK (b) Purification results of 5B5H and 5B5H-EK proteins (c) DSC results of 5B5H and 5B5H-EK at 2 mg/mL (d) Thermal hysteresis activities of 5B5H and 5B5H-EK at different concentrations
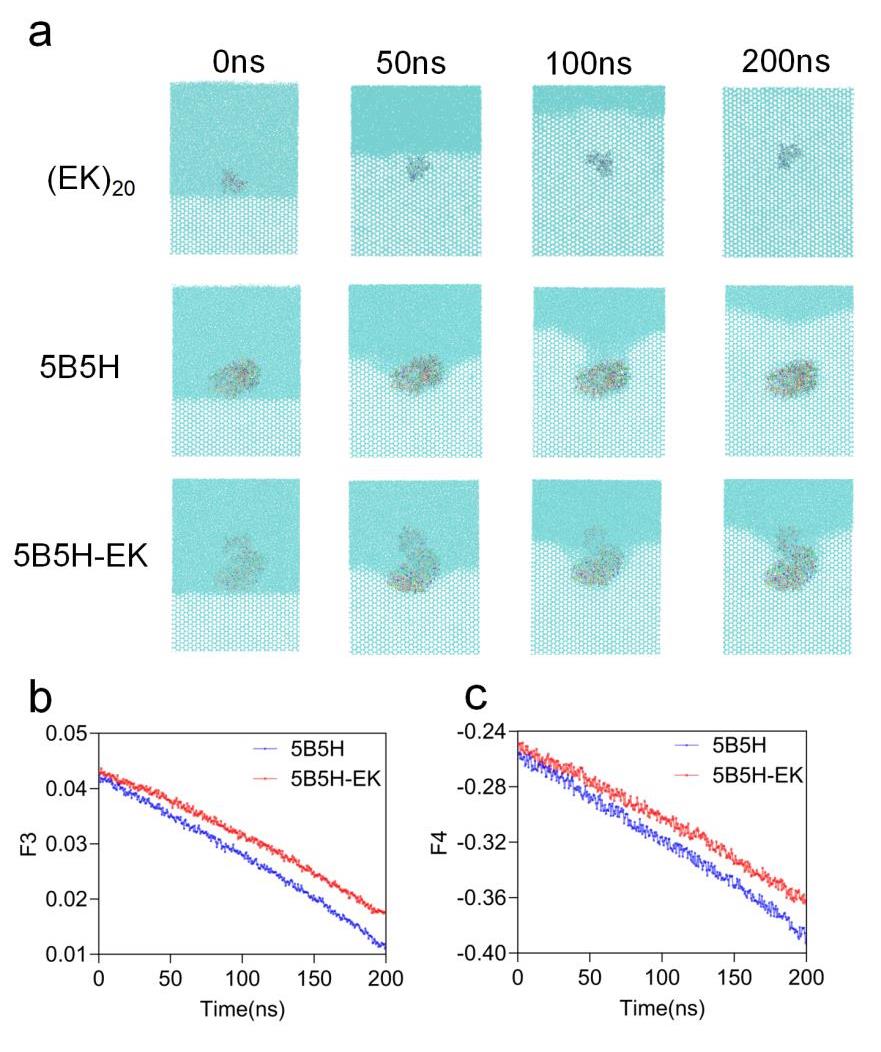
图3 5B5H和5B5H-EK冰水环境模拟结果(a) EK,5B5H和5B5H-EK的冰水环境抑制冰晶生长结果(b)5B5H和5B5H-EK冰水环境整体的F3 (c)5B5H和5B5H-EK冰水环境整体的F4
Fig. 3 5B5H and 5B5H-EK ice-water environment simulation results(a) Ice-water environment inhibition of ice crystal growth results of EK,5B5H and 5B5H-EK (b) F3 of 5B5H and 5B5H-EK in entire ice environment (c) F4 of 5B5H and 5B5H-EK in entire ice environment
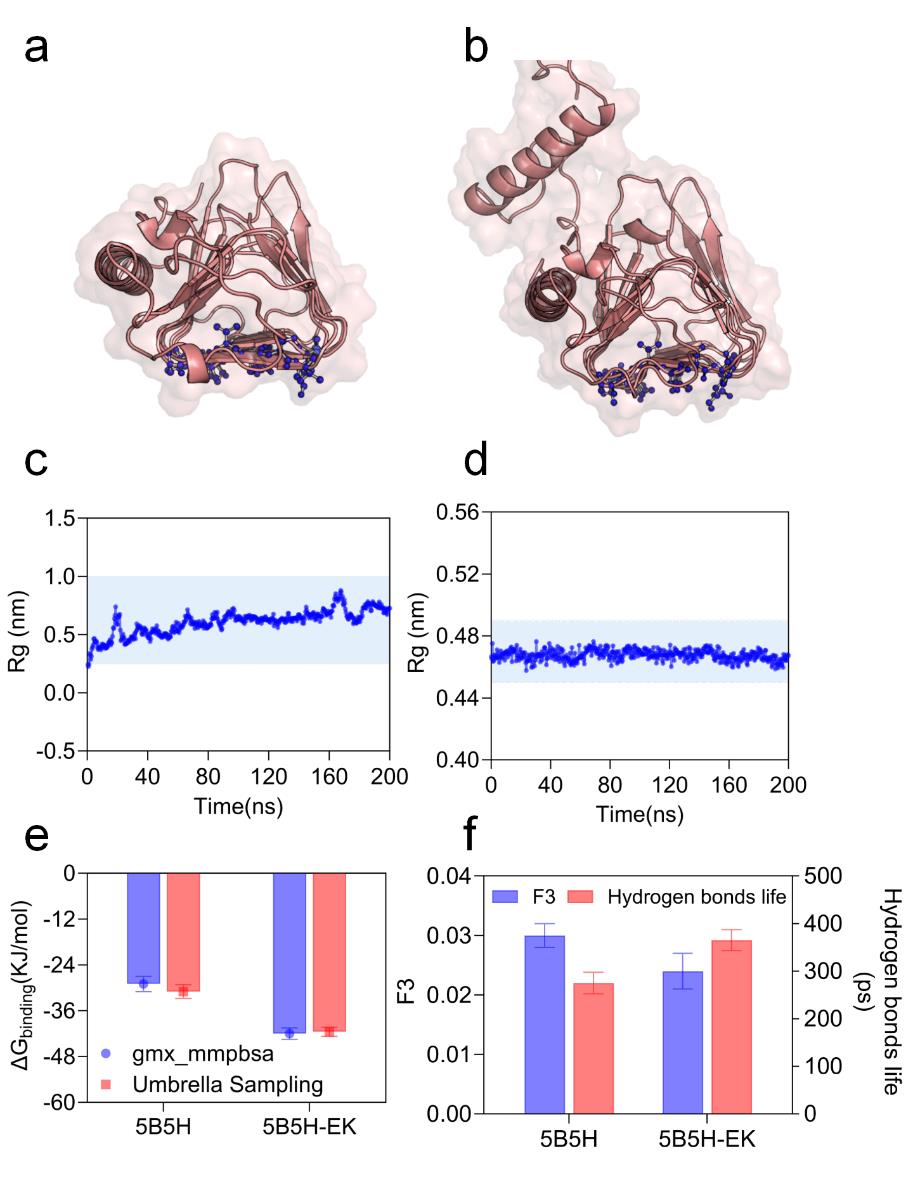
图4 5B5H和5B5H-EK冰晶结合面解析(a)5B5H的冰晶结合面空间构象(b)5B5H-EK的冰晶结合面空间构象(c)5B5H冰晶结合面稳定性(d)5B5H-EK冰晶结合面稳定性(e)5B5H和5B5H-EK与冰晶结合能 (f) 5B5H和5B5H-EK冰晶结合面周围水动力学
Fig. 4 5B5H and 5B5H-EK ice-crystal bonding surface resolution(a) Conformation of 5B5H's IBF (b) Conformation of 5B5H-EK's IBF (c) 5B5H ice-crystal bonding surface stability (d) 5B5H-EK ice-crystal bonding surface stability (e) 5B5H and 5B5H-EK with ice-crystal bonding energies (f) Hydrodynamics around the ice-crystal bonding surface of 5B5H and 5B5H-EK
| [1] | Yu H, Zheng H, Liu Y, Yang Q, Li W, Zhang Y, Fu F. Antifreeze protein from Ammopiptanthus nanus functions in temperature-stress through domain A. Sci Rep. 2021;11(1):8458. |
| [2] | Sreter JA, Foxall TL, Varga K. Intracellular and Extracellular Antifreeze Protein Significantly Improves Mammalian Cell Cryopreservation. Biomolecules. 2022;12(5):669. |
| [3] | Li X, Wang L, Yin C, Lin J, Wu Y, Chen D, Qiu C, Jia B, Huang J, Jiang X, Yang L, Liu L. Antifreeze protein from Anatolia polita (ApAFP914) improved outcome of vitrified in vitro sheep embryos. Cryobiology. 2020; 93:109-114. |
| [4] | Hudait A, Moberg DR, Qiu Y, Odendahl N, Paesani F, Molinero V. Preordering of water is not needed for ice recognition by hyperactive antifreeze proteins. Proc Natl Acad Sci U S A. 2018;115(33):8266-8271. |
| [5] | Zielkiewicz J. Solvation of molecules from the family of "domain of unknown function" 3494 and their ability to bind to ice. J Chem Phys. 2024;161(16):165101. |
| [6] | Chakraborty S, Jana B. Optimum Number of Anchored Clathrate Water and Its Instantaneous Fluctuations Dictate Ice Plane Recognition Specificities of Insect Antifreeze Protein. J Phys Chem B. 2018;122(12):3056-3067. |
| [7] | Drori R, Stevens CA. Divergent Mechanisms of Ice Growth Inhibition by Antifreeze Proteins. Methods Mol Biol. 2024;2730:169-181. |
| [8] | Kar RK, Bhunia A. Biophysical and biochemical aspects of antifreeze proteins: Using computational tools to extract atomistic information. Prog Biophys Mol Biol. 2015;119(2):194-204. |
| [9] | Chakraborty S, Jana B. Molecular Insight into the Adsorption of Spruce Budworm Antifreeze Protein to an Ice Surface: A Clathrate-Mediated Recognition Mechanism. Langmuir. 2017;33(28):7202-7214. |
| [10] | Grabowska J, Kuffel A, Zielkiewicz J. Molecular dynamics study on the role of solvation water in the adsorption of hyperactive AFP to the ice surface. Phys Chem Chem Phys. 2018;20(39):25365-25376. |
| [11] | Ekpo MD, Xie J, Hu Y, Liu X, Liu F, Xiang J, Zhao R, Wang B, Tan S. Antifreeze Proteins: Novel Applications and Navigation towards Their Clinical Application in Cryobanking. Int J Mol Sci. 2022;23(5):2639. |
| [12] | Elliott GD, Wang S, Fuller BJ. Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology. 2017;76:74-91. |
| [13] | Xiang H, Yang X, Ke L, Hu Y. The properties, biotechnologies, and applications of antifreeze proteins. Int J Biol Macromol. 2020;153:661-675. |
| [14] | Kasahara K, Waku T, Wilson PW, Tonooka T, Hagiwara Y. The Inhibition of Icing and Frosting on Glass Surfaces by the Coating of Polyethylene Glycol and Polypeptide Mimicking Antifreeze Protein. Biomolecules, 2020;10(2):259. |
| [15] | Gao, Y, Qi, H, Fan, D, Yang, J, Zhang, L. Beetle and mussel-inspired chimeric protein for fabricating anti-icing coating. Colloids and surfaces. B, Biointerfaces, 2022;210:112252. |
| [16] | Liu Z, Yang W, Wei H, Deng S, Yu X, Huang T. The mechanisms and applications of cryoprotectants in aquatic products: An overview. Food Chem. 2023;408:135202. |
| [17] | Kim HJ, Lee JH, Hur YB, Lee CW, Park SH, Koo BW. Marine Antifreeze Proteins: Structure, Function, and Application to Cryopreservation as a Potential Cryoprotectant. Mar Drugs. 2017;15(2):27. |
| [18] | Diao H, Lin S, Li D, Li S, Feng Q, Sun N. Control on moisture distribution and protein changes of Antarctic krill meat by antifreeze protein during multiple freeze-thaw cycles. J Food Sci. 2022;87(10):4440-4452. |
| [19] | Ekpo, MD, Xie, J, Hu, Y, Liu, X, Liu, F, Xiang, J, Zhao, R, Wang, B, Tan, S. Antifreeze Proteins: Novel Applications and Navigation towards Their Clinical Application in Cryobanking. Int. J. Mol. Sci. 2022;23(5): 2639. |
| [20] | Elliott GD, Wang S, Fuller BJ. Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology. 2017;76:74-91. |
| [21] | Kim HJ, Lee JH, Hur YB, Lee CW, Park SH, Koo BW. Marine Antifreeze Proteins: Structure, Function, and Application to Cryopreservation as a Potential Cryoprotectant. Mar Drugs. 2017;15(2):27. |
| [22] | Radziwon K, Weeks AM. Protein engineering for selective proteomics. Curr Opin Chem Biol. 2021;60:10-19. |
| [23] | Samineni L, Acharya B, Behera H, Oh H, Kumar M, Chowdhury R. Protein engineering of pores for separation, sensing, and sequencing. Cell Syst. 2023;14(8):676-691. |
| [24] | Lutz S, Iamurri SM. Protein Engineering: Past, Present, and Future. Methods Mol Biol. 2018;1685:1-12. |
| [25] | Rennella E, Sahtoe D D, Baker D, Kay L E. Exploiting conformational dynamics to modulate the function of designed proteins [J]. Proc Natl Acad Sci, 2023; 120(18):e2303149120. |
| [26] | Yang Q F, Tang C. On the necessity of an integrative approach to understand protein structural dynamics [J]. J. Zhejiang Univ. Sci. B, 2019;20(6):496-502. |
| [27] | Marshall C B, Daley M E, Sykes B D, Davies P L. Enhancing the activity of a beta-helical antifreeze protein by the engineered addition of coils [J]. Biochemistry, 2004; 43(37):11637-46. |
| [28] | Keefe AJ, Jiang S. Poly(zwitterionic)protein conjugates offer increased stability without sacrificing binding affinity or bioactivity. Nat Chem. 2011;4(1):59-63. |
| [29] | Cui Z, Wang Y, Zhang L, Qi H. Zwitterionic Peptides: From Mechanism, Design Strategies to Applications. ACS Appl Mater Interfaces. 2024;16(42):56497-56518. |
| [30] | Liu EJ, Sinclair A, Keefe AJ, et al. EKylation: Addition of an Alternating-Charge Peptide Stabilizes Proteins. Biomacromolecules. 2015;16(10):3357-3361. |
| [31] | Liu EJ, Jiang S. Expressing a Monomeric Organophosphate Hydrolase as an EK Fusion Protein. Bioconjug Chem. 2018;29(11):3686-3690. |
| [32] | Yuan Z, Li B, Niu L, et al. Zwitterionic Peptide Cloak Mimics Protein Surfaces for Protein Protection. Angew Chem Int Ed Engl. 2020;59(50):22378-22381. |
| [33] | Shao Q. Effect of conjugated (EK)10 peptide on structural and dynamic properties of ubiquitin protein: a molecular dynamics simulation study. J Mater Chem B. 2020;8(31):6934-6943. |
| [34] | Teng J, Liu Y, Shen Z, Lv W, Chen Y. Molecular simulation of zwitterionic polypeptides on protecting glucagon-like peptide-1 (GLP-1). Int J Biol Macromol. 2021;174:519-526. |
| [35] | Biswas AD, Barone V, Daidone I. High Water Density at Non-Ice-Binding Surfaces Contributes to the Hyperactivity of Antifreeze Proteins. J Phys Chem Lett. 2021;12(36):8777-8783.[36]FaragH, PetersB. Engulfment Avalanches and Thermal Hysteresis for Antifreeze Proteins on Supercooled Ice. J Phys Chem B. 2023;127(24):5422-5431. |
| [37] | Kim EJ, Lee JH, Lee SG, Han SJ. Improving thermal hysteresis activity of antifreeze protein from recombinant Pichia pastoris by removal of N-glycosylation. Prep Biochem Biotechnol. 2017;47(3):299-304. |
| [38] | Kim DE, Chivian D, Baker D. Protein structure prediction and analysis using the Robetta server. Nucleic Acids Res. 2004;32(Web Server issue):W526- W531. |
| [39] | Chivian D, Kim DE, Malmström L, et al. Automated prediction of CASP-5 structures using the Robetta server. Proteins. 2003;53 :524-533. |
| [40] | Park H, Kim DE, Ovchinnikov S, Baker D, DiMaio F. Automatic structure prediction of oligomeric assemblies using Robetta in CASP12. Proteins. 2018;86(Suppl 1):283-291. |
| [41] | Páll S, Zhmurov A, Bauer P, et al. Heterogeneous parallelization and acceleration of molecular dynamics simulations in GROMACS. J Chem Phys. 2020;153(13):134110. |
| [42] | Hess B, Kutzner C, van der Spoel D, Lindahl E. GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation. J Chem Theory Comput. 2008;4(3):435-447. |
| [43] | Linse JB, Hub JS. Three- and four-site models for heavy water: SPC/E-HW, TIP3P-HW, and TIP4P/2005-HW. J Chem Phys. 2021;154(19):194501. |
| [44] | Bore SL, Piaggi PM, Car R, Paesani F. Phase diagram of the TIP4P/Ice water model by enhanced sampling simulations. J Chem Phys. 2022;157(5):054504. |
| [45] | Ngo ST, Pham MQ. Umbrella Sampling-Based Method to Compute Ligand-Binding Affinity. Methods Mol Biol. 2022;2385:313-323. |
| [46] | Sharpee TO, Destexhe A, Kawato M, et al. 25th Annual Computational Neuroscience Meeting: CNS-2016. BMC Neurosci. 2016;17(Suppl 1):54. |
| [47] | Kozuch DJ, Stillinger FH, Debenedetti PG. Combined molecular dynamics and neural network method for predicting protein antifreeze activity. Proc Natl Acad Sci U S A. 2018;115(52):13252-13257. |
| [48] | Park H, Kim DE, Ovchinnikov S, Baker D, DiMaio F. Automatic structure prediction of oligomeric assemblies using Robetta in CASP12. Proteins. 2018;86(Suppl 1):283-291. |
| [49] | Azzaz F, Fantini J. The epigenetic dimension of protein structure. Biomol Concepts. 2022;13(1):55-60. |
| [50] | Cheng J, Hanada Y, Miura A, Tsuda S, Kondo H. Hydrophobic ice-binding sites confer hyperactivity of an antifreeze protein from a snow mold fungus. Biochem J. 2016;473(21):4011-4026. |
| [51] | Wang, Y, Miyazaki, R, Saitou, S, Hirasaka, K, Takeshita, S, Tachibana, K, Taniyama, S. The effect of ice crystals formations on the flesh quality of frozen horse mackerel (Trachurus japonicus). J Texture Stud. 2018;49(5):485-491. |
| [52] | Feng S, Yi J, Wu X, Ma Y, Bi J. Effects of cell morphology on the textural attributes of fruit cubes in freeze-drying: Apples, strawberries, and mangoes as examples. J Texture Stud. 2023;54(5):775-786. |
| [53] | Schaudinn C, Tautz C, Laue M. Thin polyester filters as versatile sample substrates for high-pressure freezing of bacterial biofilms, suspended microorganisms and adherent eukaryotic cells. J Microsc. 2019;274(2):92-101. |
| [54] | Lee J, Hitzenberger M, Rieger M, Kern NR, Zacharias M, Im W. CHARMM-GUI supports the Amber force fields. J Chem Phys. 2020;153(3):035103. |
| [55] | Kognole AA, Lee J, Park SJ, Jo S, Chatterjee P, Lemkul JA, Huang J, MacKerell AD, Jr Im W. CHARMM-GUI Drude prepper for molecular dynamics simulation using the classical Drude polarizable force field. J Comput Chem. 2022;43(5):359-375. |
| [56] | McMullen P, Fang L, Qiao Q, Shao Q, Jiang S. Impacts of a Zwitterionic Peptide on its Fusion Protein. Bioconjug Chem. 2022;33(8):1485-1493. |
| [57] | Li C, Xia Y, Liu C, Huang R, Qi W, He Z, Su R. Lubricin-Inspired Loop Zwitterionic Peptide for Fabrication of Superior Antifouling Surfaces. ACS Appl Mater Interfaces. 2021;13(35):41978-41986. |
| [58] | Prajakta N, Bappa G, Subhadip D, Sudip R, Rajnish K,Molecular dynamics study on growth of carbon dioxide and methane hydrate from a seed crystal, Chin. J. Chem. Eng, 2019; 27(9): 2074-2080. |
| [59] | Dalal P, Sönnichsen FD. Source of the ice-binding specificity of antifreeze protein type I. J Chem Inf Comput Sci. 2000;40(5):1276-1284. |
| [60] | Jung W, Campbell RL, Gwak Y, Kim JI, Davies PL, Jin E. New Cysteine-Rich Ice-Binding Protein Secreted from Antarctic Microalga, Chloromonas sp. PLoS One. 2016;11(4):e0154056. |
| [61] | Hudait A, Qiu Y, Odendahl N, Molinero V. Hydrogen-Bonding and Hydrophobic Groups Contribute Equally to the Binding of Hyperactive Antifreeze and Ice-Nucleating Proteins to Ice. J Am Chem Soc. 2019;141(19):7887-7898. |
| [62] | Hudait A, Moberg DR, Qiu Y, Odendahl N, Paesani F, Molinero V. Preordering of water is not needed for ice recognition by hyperactive antifreeze proteins. Proc Natl Acad Sci U S A. 2018;115(33):8266-8271. |
| [1] | SIM Byuri, 赵一雷. 预反应态模型浅析:催化活性和近过渡态分子模拟[J]. 合成生物学, 2022, 3(3): 567-586. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
