合成生物学 ›› 2024, Vol. 5 ›› Issue (4): 867-882.DOI: 10.12211/2096-8280.2024-065
骨骼肌芯片及其在生物医学领域的研究进展
王达庆1, 陶婷婷2, 张旭2, 李洪敬1
- 1.大连医科大学附属第一医院,辽宁 大连 116011
2.中国科学院大连化学物理研究所,辽宁 大连 116023
-
收稿日期:2024-08-22修回日期:2024-08-28出版日期:2024-08-31发布日期:2024-09-19 -
通讯作者:张旭,李洪敬 -
作者简介:王达庆 (1997—),男,骨科学博士研究生。研究方向为关节外科与运动医学。-mail:wangdaqing2020@gmail.com张旭 (1986—),男,博士,副研究员。研究方向为基于器官芯片的心血管疾病研究。E-mail:zhangxu6@dicp.ac.cn李洪敬 (1968—),男,医学博士,教授,主任医师,博士生导师。研究方向为关节外科及运动医学。E-mail:lhj68430@163.com -
基金资助:国家自然科学基金(82102229);大连市“登峰计划”科研项目(DF2023004)
Advances in skeletal muscle-on-a-chip for biomedical research
WANG Daqing1, TAO Tingting2, ZHANG Xu2, LI Hongjing1
- 1.The First Affiliated Hospital of Dalian Medical University,Dalian 116011,Liaoning,China
2.Dalian Institute of Chemical Physics,Chinese Academy of Sciences,Dalian 116023,Liaoning,China
-
Received:2024-08-22Revised:2024-08-28Online:2024-08-31Published:2024-09-19 -
Contact:ZHANG Xu, LI Hongjing
摘要:
骨骼肌作为人体最丰富的组织之一,是人体运动功能的主要承担者,并且在能量代谢、免疫调节和衰老过程中发挥重要作用。骨骼肌所处的微环境结构复杂,包括多种细胞类型、独特的三维结构以及力学特征。因此,建立高仿生的骨骼肌模型具有一定的挑战性。器官芯片可以精确地模拟人体组织的关键结构和功能特性,从而为骨骼肌模型的建立提供了一种新的途径。本文综述了目前骨骼肌芯片的构建及其在疾病建模、药物评价与再生医学等生物医学研究中的应用。依据人体骨骼肌组织微环境的特点,重点介绍了构建骨骼肌芯片的关键要素,包括动态培养环境、机械刺激、电刺激、血管化与神经化,以及其他工程策略包括各向异性支架的制备与两端锚定的策略等。目前的骨骼肌芯片在细胞来源及功能等方面仍存在一定的局限性。未来通过与基因编辑、生物传感等技术相结合,骨骼肌芯片有望在生物医学研究领域发挥更重要的作用。
中图分类号:
引用本文
王达庆, 陶婷婷, 张旭, 李洪敬. 骨骼肌芯片及其在生物医学领域的研究进展[J]. 合成生物学, 2024, 5(4): 867-882.
WANG Daqing, TAO Tingting, ZHANG Xu, LI Hongjing. Advances in skeletal muscle-on-a-chip for biomedical research[J]. Synthetic Biology Journal, 2024, 5(4): 867-882.
| 细胞来源 | 优势 | 局限性 | 应用 |
|---|---|---|---|
| 原代细胞[ | 生理相关性 能够保留患者特定的疾病病理特征 | 增殖能力有限 获取较困难 存在个体差异 | 研究肌肉发育、疾病机制和评估治疗干预措施的理想材料 个性化医疗 |
| 细胞系[ | 易于获取 增殖速度快 生理一致性 | 缺乏生理相关性 人源细胞系缺乏 | 初步的机制研究与药物筛选 高通量研究 |
| 干细胞[ | 无限增殖 易于操控 | 分化效率不确定 | 肌肉发育、遗传性肌肉疾病研究 再生医学 |
表1 用于构建骨骼肌芯片的细胞来源
Table 1 Cell sources for skeletal muscle-on-a-chip
| 细胞来源 | 优势 | 局限性 | 应用 |
|---|---|---|---|
| 原代细胞[ | 生理相关性 能够保留患者特定的疾病病理特征 | 增殖能力有限 获取较困难 存在个体差异 | 研究肌肉发育、疾病机制和评估治疗干预措施的理想材料 个性化医疗 |
| 细胞系[ | 易于获取 增殖速度快 生理一致性 | 缺乏生理相关性 人源细胞系缺乏 | 初步的机制研究与药物筛选 高通量研究 |
| 干细胞[ | 无限增殖 易于操控 | 分化效率不确定 | 肌肉发育、遗传性肌肉疾病研究 再生医学 |
| 材料 | 优势 | 缺点 |
|---|---|---|
| 甲基丙烯酰化明胶(GelMA)[ | 良好的生物相容性 可调交联密度以控制材料的渗透性和机械性能 可以引入其他丙烯酸系链基团以制成复合系统 | 分子量与成分存在批次间差异 不能与光敏或自由基敏感的酶或因子结合使用 |
| 胶原蛋白[ | 良好的生物相容性 可促进细胞黏附与增殖 通过调整比例可改变机械性能 | 分子量与成分存在批次间差异 成本相对较高 |
| 纤维蛋白(fibrin)[ | 良好的生物相容性 可促进细胞黏附与增殖 易将细胞封装在内部 | 由于在凝血级联反应中的作用,不能与血流结合 复合蛋白,因此不容易修饰 |
| Matrigel[ | 良好的生物相容性 支持细胞迁移和分化 促血管生成 | 分子量与成分存在批次间差异 成本相对较高 |
| 聚己内酯(PCL)[ | 良好的生物相容性 易于加工 无免疫原性 | 细胞黏附性较差 缺乏生物活性,通常与其他生物活性材料结合使用 |
| 聚乳酸-乙醇酸共聚物(PLGA)[ | 易于加工 可降解性 | 生物相容性相对较差,需要额外的涂层或处理来提高细胞黏附性 降解过程中会产生乳酸和乙醇酸,可能影响细胞活性 |
表2 用于骨骼肌芯片的生物材料
Table 2 Biomaterials for skeletal muscle-on-a-chip
| 材料 | 优势 | 缺点 |
|---|---|---|
| 甲基丙烯酰化明胶(GelMA)[ | 良好的生物相容性 可调交联密度以控制材料的渗透性和机械性能 可以引入其他丙烯酸系链基团以制成复合系统 | 分子量与成分存在批次间差异 不能与光敏或自由基敏感的酶或因子结合使用 |
| 胶原蛋白[ | 良好的生物相容性 可促进细胞黏附与增殖 通过调整比例可改变机械性能 | 分子量与成分存在批次间差异 成本相对较高 |
| 纤维蛋白(fibrin)[ | 良好的生物相容性 可促进细胞黏附与增殖 易将细胞封装在内部 | 由于在凝血级联反应中的作用,不能与血流结合 复合蛋白,因此不容易修饰 |
| Matrigel[ | 良好的生物相容性 支持细胞迁移和分化 促血管生成 | 分子量与成分存在批次间差异 成本相对较高 |
| 聚己内酯(PCL)[ | 良好的生物相容性 易于加工 无免疫原性 | 细胞黏附性较差 缺乏生物活性,通常与其他生物活性材料结合使用 |
| 聚乳酸-乙醇酸共聚物(PLGA)[ | 易于加工 可降解性 | 生物相容性相对较差,需要额外的涂层或处理来提高细胞黏附性 降解过程中会产生乳酸和乙醇酸,可能影响细胞活性 |
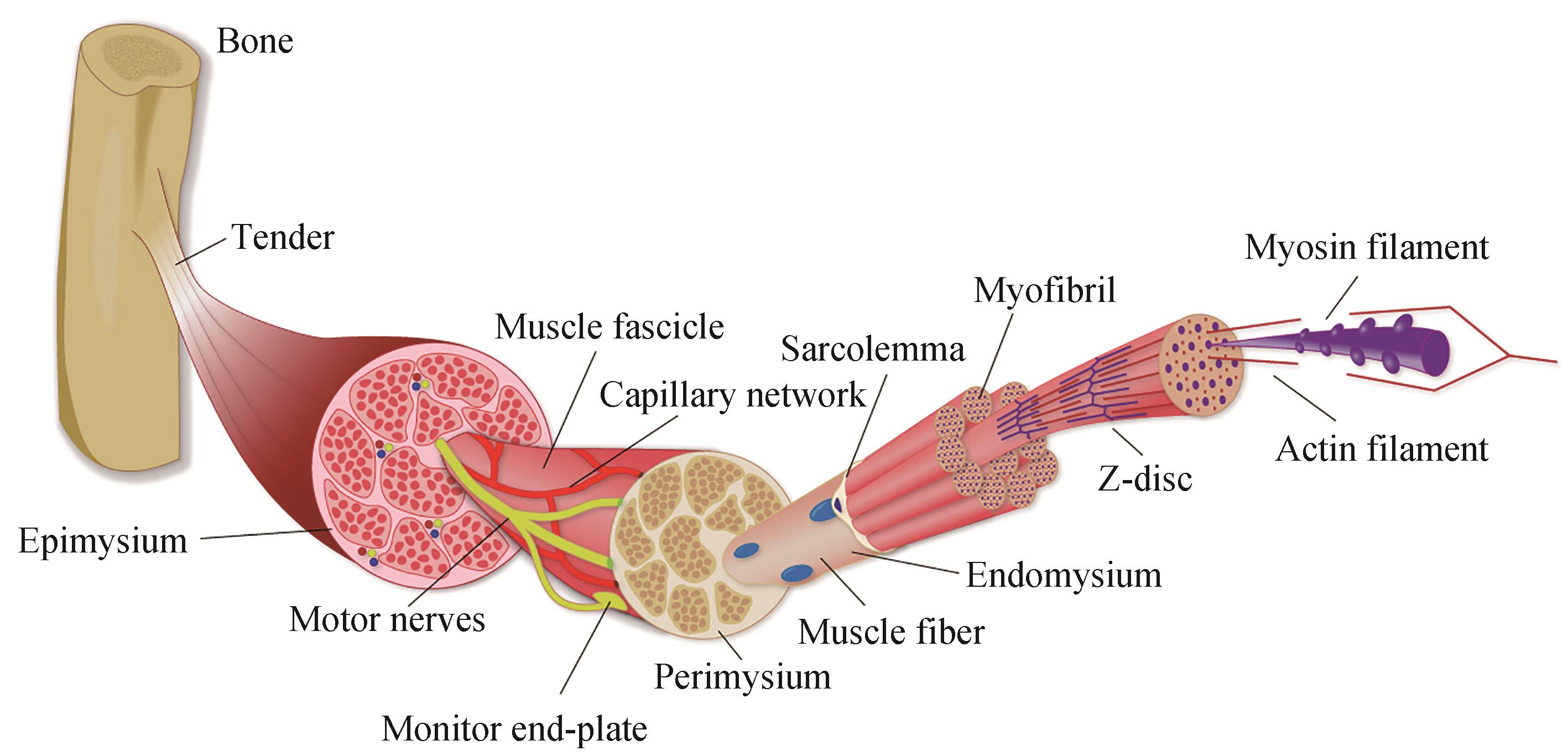
| 1 | FRONTERA W R, OCHALA J. Skeletal muscle: a brief review of structure and function[J]. Calcified Tissue International, 2015, 96(3): 183-195. |
| 2 | HAY M, THOMAS D W, CRAIGHEAD J L, et al. Clinical development success rates for investigational drugs[J]. Nature Biotechnology, 2014, 32(1): 40-51. |
| 3 | WANG J, KHODABUKUS A, RAO L J, et al. Engineered skeletal muscles for disease modeling and drug discovery[J]. Biomaterials, 2019, 221: 119416. |
| 4 | ORTEGA M A, FERNÁNDEZ-GARIBAY X, CASTAÑO A G, et al. Muscle-on-a-chip with an on-site multiplexed biosensing system for in situ monitoring of secreted IL-6 and TNF-α[J]. Lab on a Chip, 2019, 19(15): 2568-2580. |
| 5 | BAKER M. Tissue models: a living system on a chip[J]. Nature, 2011, 471(7340): 661-665. |
| 6 | UZEL S G M, PAVESI A, KAMM R D. Microfabrication and microfluidics for muscle tissue models[J]. Progress in Biophysics and Molecular Biology, 2014, 115(2-3): 279-293. |
| 7 | SMOAK M M, PEARCE H A, MIKOS A G. Microfluidic devices for disease modeling in muscle tissue[J]. Biomaterials, 2019, 198: 250-258. |
| 8 | HUH D, HAMILTON G A, INGBER D E. From 3D cell culture to organs-on-chips[J]. Trends in Cell Biology, 2011, 21(12): 745-754. |
| 9 | ZHAO M Q, LIU H T, ZHANG X, et al. Advances in skeletal muscle engineering in biomedical research[J]. Advanced Materials Technologies, 2023, 8(22): 2300595. |
| 10 | MA C, PENG Y S, LI H T, et al. Organ-on-a-chip: a new paradigm for drug development[J]. Trends in Pharmacological Sciences, 2021, 42(2): 119-133. |
| 11 | SACKMANN E K, FULTON A L, BEEBE D J. The present and future role of microfluidics in biomedical research[J]. Nature, 2014, 507(7491): 181-189. |
| 12 | MEHLINGM, TAY S. Microfluidic cell culture[J]. Current Opinion in Biotechnology, 2014, 25: 95-102. |
| 13 | ZHANG X, HABIBALLA L, AVERSA Z, et al. Characterization of cellular senescence in aging skeletal muscle[J]. Nature Aging, 2022, 2(7): 601-615. |
| 14 | SCHIAFFINO S, REGGIANI C. Fiber types in mammalian skeletal muscles[J]. Physiological Reviews, 2011, 91(4): 1447-1531. |
| 15 | LUO W B, LIU H, WANG C Y, et al. Bioprinting of human musculoskeletal interface[J]. Advanced Engineering Materials, 2019, 21(7): 1900019. |
| 16 | SEFTON E M, KARDON G. Connecting muscle development, birth defects, and evolution: an essential role for muscle connective tissue[J]. Current Topics in Developmental Biology, 2019, 132: 137-176. |
| 17 | ROSSI D, BARONE V, GIACOMELLO E, et al. The sarcoplasmic reticulum: an organized patchwork of specialized domains[J]. Traffic, 2008, 9(7): 1044-1049. |
| 18 | HANSON J, HUXLEY H E. Structural basis of the cross-striations in muscle[J]. Nature, 1953, 172(4377): 530-532. |
| 19 | HUXLEY H E. Electron microscope studies of the organisation of the filaments in striated muscle[J]. Biochimica et Biophysica Acta, 1953, 12(1/2): 387-394. |
| 20 | OSTROVIDOV S, SALEHI S, COSTANTINI M, et al. 3D bioprinting in skeletal muscle tissue engineering[J]. Small, 2019, 15(24): e1805530. |
| 21 | OLIVER C E, PATEL H, HONG J, et al. Tissue engineered skeletal muscle model of rheumatoid arthritis using human primary skeletal muscle cells[J]. Journal of Tissue Engineering and Regenerative Medicine, 2022, 16(2): 128-139. |
| 22 | MONTARRAS D, MORGAN J, COLLINS C, et al. Direct isolation of satellite cells for skeletal muscle regeneration[J]. Science, 2005, 309(5743): 2064-2067. |
| 23 | CHOI K H, YOON J W, KIM M S, et al. Muscle stem cell isolation and in vitro culture for meat production: a methodological review[J]. Comprehensive Reviews in Food Science and Food Safety, 2021, 20(1): 429-457. |
| 24 | RAJABIAN N, SHAHINI A, ASMANI M, et al. Bioengineered skeletal muscle as a model of muscle aging and regeneration[J]. Tissue Engineering Part A, 2021, 27(1-2): 74-86. |
| 25 | ZHAO M Q, LIU H T, ZHANG X, et al. A flexible microfluidic strategy to generate grooved microfibers for guiding cell alignment[J]. Biomaterials Science, 2021, 9(14): 4880-4890. |
| 26 | EBRAHIMI M, LAD H, FUSTO A, et al. De novo revertant fiber formation and therapy testing in a 3D culture model of Duchenne muscular dystrophy skeletal muscle[J]. Acta Biomaterialia, 2021, 132: 227-244. |
| 27 | FERREIRA M J S, MANCINI F E, HUMPHREYS P A, et al. Pluripotent stem cells for skeletal tissue engineering[J]. Critical Reviews in Biotechnology, 2022, 42(5): 774-793. |
| 28 | RAO L J, QIAN Y, KHODABUKUS A, et al. Engineering human pluripotent stem cells into a functional skeletal muscle tissue[J]. Nature Communications, 2018, 9(1): 126. |
| 29 | ABUJAROUR R, VALAMEHR B. Generation of skeletal muscle cells from pluripotent stem cells: advances and challenges[J]. Frontiers in Cell and Developmental Biology, 2015, 3: 29. |
| 30 | URCIUOLO A, SERENA E, GHUA R, et al. Engineering a 3D in vitro model of human skeletal muscle at the single fiber scale[J]. PLoS One, 2020, 15(5): e0232081. |
| 31 | CHEESBROUGH A, SCISCIONE F, RICCIO F, et al. Biobased elastomer nanofibers guide light-controlled human-iPSC-derived skeletal myofibers[J]. Advanced Materials, 2022, 34(18): e2110441. |
| 32 | SHIMA A, MORIMOTO Y, SWEENEY H L, et al. Three-dimensional contractile muscle tissue consisting of human skeletal myocyte cell line[J]. Experimental Cell Research, 2018, 370(1): 168-173. |
| 33 | VAN DER WAL E, IULIANO A, 'T GROEN S L M IN, et al. Highly contractile 3D tissue engineered skeletal muscles from human iPSCs reveal similarities with primary myoblast-derived tissues[J]. Stem Cell Reports, 2023, 18(10): 1954-1971. |
| 34 | WANG Z H, LIU S T, HAN M Y, et al. 3D printing-electrospinning hybrid nanofibrous scaffold as LEGO-like bricks for modular assembling skeletal muscle-on-a-chip functional platform[J/OL]. Advanced Fiber Materials. (2024-06-04)[2024-08-01]. . |
| 35 | NISHIYORI M, UEDA H. Prolonged gabapentin analgesia in an experimental mouse model of fibromyalgia[J]. Molecular Pain, 2008, 4: 52. |
| 36 | CHOI J S, LEE S J, CHRIST G J, et al. The influence of electrospun aligned poly(Epsilon-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes[J]. Biomaterials, 2008, 29(19): 2899-2906. |
| 37 | PARAFATI M, GIZA S, SHENOY T S, et al. Human skeletal muscle tissue chip autonomous payload reveals changes in fiber type and metabolic gene expression due to spaceflight[J]. NPJ Microgravity, 2023, 9(1): 77. |
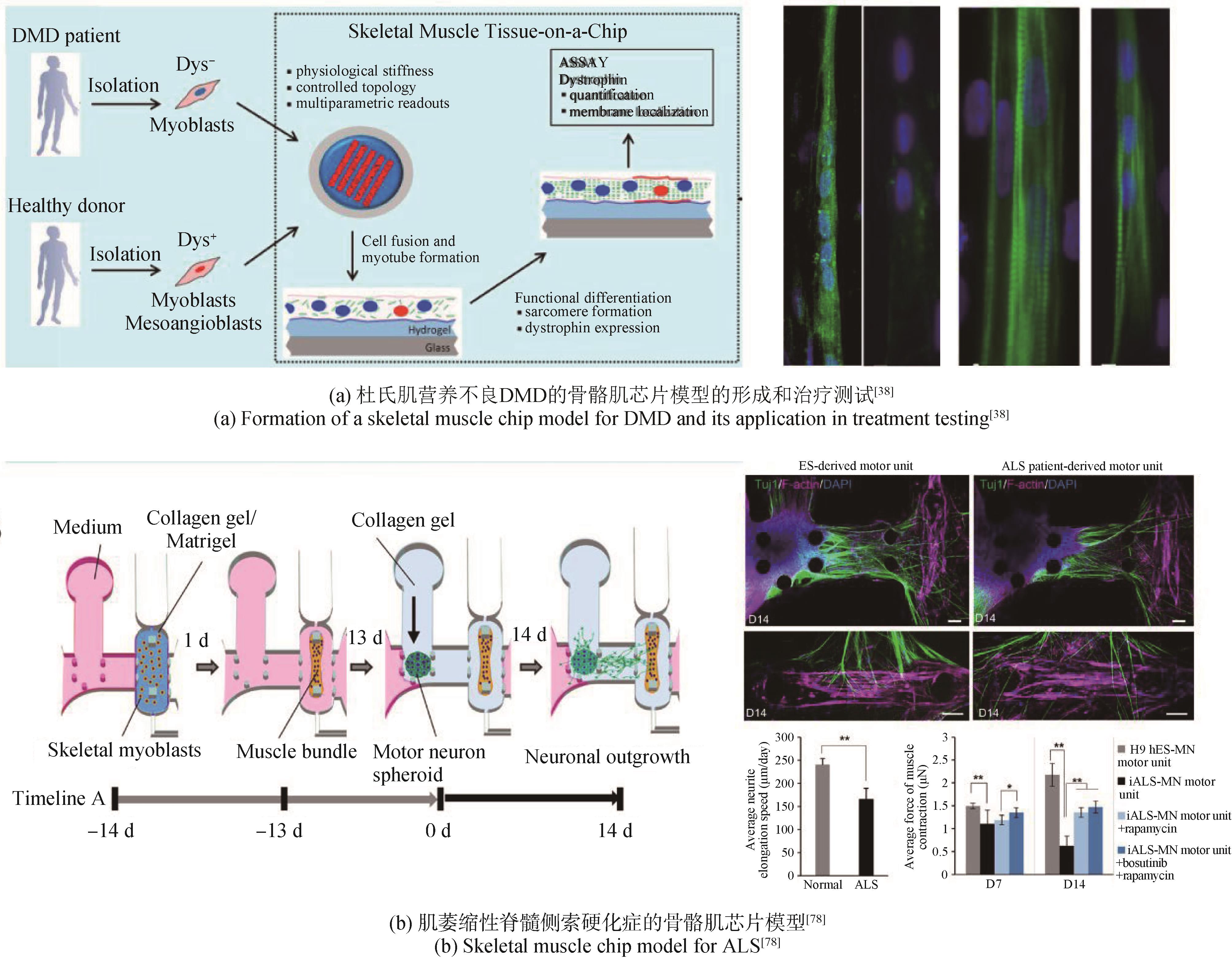
| 38 | SERENA E, ZATTI S, ZOSO A, et al. Skeletal muscle differentiation on a chip shows human donor mesoangioblasts' efficiency in restoring dystrophin in a Duchenne muscular dystrophy model[J]. Stem Cells Translational Medicine, 2016, 5(12): 1676-1683. |
| 39 | FUJIE T, SHI X T, OSTROVIDOV S, et al. Spatial coordination of cell orientation directed by nanoribbon sheets[J]. Biomaterials, 2015, 53: 86-94. |
| 40 | JACOT J G, MCCULLOCH A D, OMENS J H. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes[J]. Biophysical Journal, 2008, 95(7): 3479-3487. |
| 41 | ENGLER A J, GRIFFIN M A, SEN S, et al. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments[J]. Journal of Cell Biology, 2004, 166(6): 877-887. |
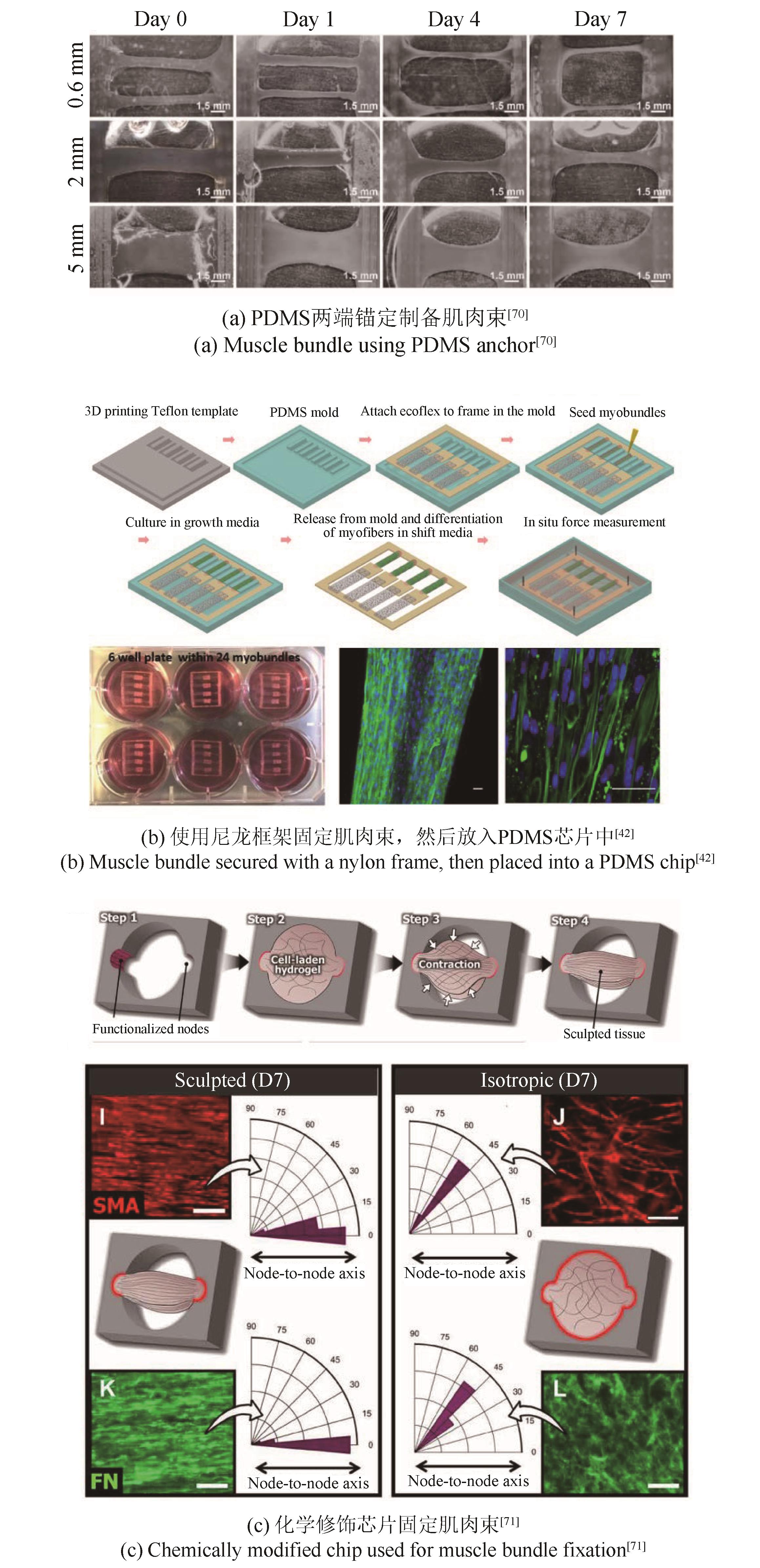
| 42 | ZHANG X, HONG S, YEN R, et al. A system to monitor statin-induced myopathy in individual engineered skeletal muscle myobundles[J]. Lab on a Chip, 2018, 18(18): 2787-2796. |
| 43 | RANGARAJAN S, MADDEN L, BURSAC N. Use of flow, electrical, and mechanical stimulation to promote engineering of striated muscles[J]. Annals of Biomedical Engineering, 2014, 42(7): 1391-1405. |
| 44 | FERNÁNDEZ-COSTA J M, TEJEDERA-VILAFRANCA A, FERNÁNDEZ-GARIBAY X, et al. Muscle-on-a-chip devices: a new era for in vitro modelling of muscular dystrophies[J]. Disease Models & Mechanisms, 2023, 16(6): dmm050107. |
| 45 | OKANO T, MATSUDA T. Hybrid muscular tissues: preparation of skeletal muscle cell-incorporated collagen gels[J]. Cell Transplantation, 1997, 6(2): 109-118. |
| 46 | JACKMAN C P, CARLSON A L, BURSAC N. Dynamic culture yields engineered myocardium with near-adult functional output[J]. Biomaterials, 2016, 111: 66-79. |
| 47 | SHADRIN I Y, ALLEN B W, QIAN Y, et al. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues[J]. Nature Communications, 2017, 8(1): 1825. |
| 48 | JUHAS M, ENGELMAYR G C JR, FONTANELLA A N, et al. Biomimetic engineered muscle with capacity for vascular integration and functional maturation in vivo [J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(15): 5508-5513. |
| 49 | CHEEMA U, YANG S Y, MUDERA V, et al. 3-D in vitro model of early skeletal muscle development[J]. Cell Motility and the Cytoskeleton, 2003, 54(3): 226-236. |
| 50 | CHEEMA U, BROWN R, MUDERA V, et al. Mechanical signals and IGF-Ⅰ gene splicing in vitro in relation to development of skeletal muscle[J]. Journal of Cellular Physiology, 2005, 202(1): 67-75. |
| 51 | HANDSCHIN C, MORTEZAVI A, PLOCK J, et al. External physical and biochemical stimulation to enhance skeletal muscle bioengineering[J]. Advanced Drug Delivery Reviews, 2015, 82-83: 168-175. |
| 52 | DUGAN J M, CARTMELL S H, GOUGH J E. Uniaxial cyclic strain of human adipose-derived mesenchymal stem cells and C2C12 myoblasts in coculture[J]. Journal of Tissue Engineering, 2014, 5: 2041731414530138. |
| 53 | BOONEN K J, LANGELAAN M L, POLAK R B, et al. Effects of a combined mechanical stimulation protocol: value for skeletal muscle tissue engineering[J]. Journal of Biomechanics, 2010, 43(8): 1514-1521. |
| 54 | HEHER P, MALEINER B, PRÜLLER J, et al. A novel bioreactor for the generation of highly aligned 3D skeletal muscle-like constructs through orientation of fibrin via application of static strain[J]. Acta Biomaterialia, 2015, 24: 251-265. |
| 55 | CHEN Z W, LI B J, ZHAN R Z, et al. Exercise mimetics and JAK inhibition attenuate IFN-γ-induced wasting in engineered human skeletal muscle[J]. Science Advances, 2021, 7(4): eabd9502. |
| 56 | KHODABUKUS A, BAAR K. Defined electrical stimulation emphasizing excitability for the development and testing of engineered skeletal muscle[J]. Tissue Engineering Part C, Methods, 2012, 18(5): 349-357. |
| 57 | KHODABUKUS A, BAEHR L M, BODINE S C, et al. Role of contraction duration in inducing fast-to-slow contractile and metabolic protein and functional changes in engineered muscle[J]. Journal of Cellular Physiology, 2015, 230(10): 2489-2497. |
| 58 | HUANG Y C, DENNIS R G, BAAR K. Cultured slow vs. fast skeletal muscle cells differ in physiology and responsiveness to stimulation[J]. American Journal of Physiology Cell Physiology, 2006, 291(1): C11-C17. |
| 59 | EKEN T, ELDER G C B, LØMO T. Development of tonic firing behavior in rat soleus muscle[J]. Journal of Neurophysiology, 2008, 99(4): 1899-1905. |
| 60 | BANAN SADEGHIAN R, EBRAHIMI M, SALEHI S. Electrical stimulation of microengineered skeletal muscle tissue: effect of stimulus parameters on myotube contractility and maturation[J]. Journal of Tissue Engineering and Regenerative Medicine, 2018, 12(4): 912-922. |
| 61 | BIAN W N, BURSAC N. Soluble miniagrin enhances contractile function of engineered skeletal muscle[J]. FASEB Journal, 2012, 26(2): 955-965. |
| 62 | HARDMAN D, NGUYEN M L, DESCROIX S, et al. Mathematical modelling of oxygen transport in a muscle-on-chip device[J]. Interface Focus, 2022, 12(5): 20220020. |
| 63 | WAN L, FLEGLE J, OZDOGANLAR B, et al. Toward vasculature in skeletal muscle-on-a-chip through thermo-responsive sacrificial templates[J]. Micromachines, 2020, 11(10): 907. |
| 64 | ARRIGONI C, PETTA D, BERSINI S, et al. Engineering complex muscle-tissue interfaces through microfabrication[J]. Biofabrication, 2019, 11(3): 032004. |
| 65 | SU W W, YANG Q, LI T, et al. Electrospun aligned nanofiber yarns constructed biomimetic M-type interface integrated into precise co-culture system as muscle-tendon junction-on-a-chip for drug development[J]. Small Methods, 2024: e2301754. |
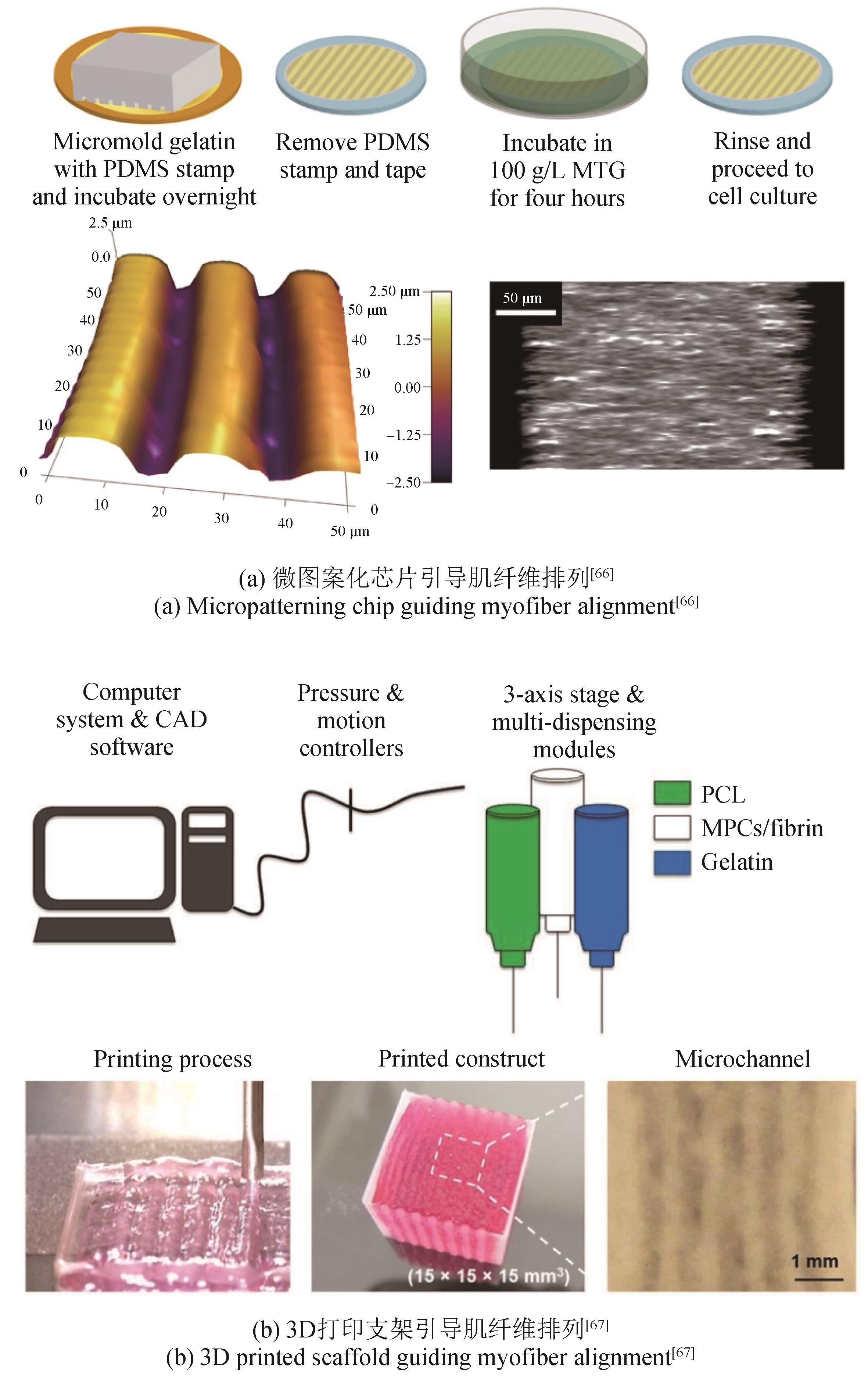
| 66 | BETTADAPUR A, SUH G C, GEISSE N A, et al. Prolonged culture of aligned skeletal myotubes on micromolded gelatin hydrogels [J]. Scientific Reports, 2016, 6(1): 28855. |
| 67 | KIM J H, SEOL Y J, KO I K, et al. 3D bioprinted human skeletal muscle constructs for muscle function restoration [J]. Scientific Reports, 2018, 8(1): 12307. |
| 68 | YEO M J, KIM G H. Micro/nano-hierarchical scaffold fabricated using a cell electrospinning/3D printing process for co-culturing myoblasts and HUVECs to induce myoblast alignment and differentiation[J]. Acta Biomaterialia, 2020, 107: 102-114. |
| 69 | NGUYEN M L, DEMRI N, LAPIN B, et al. Studying the impact of geometrical and cellular cues on myogenesis with a skeletal muscle-on-chip[J]. Lab on a Chip, 2024, 24(17): 4147-4160. |
| 70 | FAN T, WANG S, JIANG Z, et al. Controllable assembly of skeletal muscle-like bundles through 3D bioprinting[J]. Biofabrication, 2021, 14(1): 015009. |
| 71 | MONDRINOS M J, ALISAFAEI F, YI A Y, et al. Surface-directed engineering of tissue anisotropy in microphysiological models of musculoskeletal tissue[J]. Science Advances, 2021, 7(11): eabe9446. |
| 72 | MILLS R J, PARKER B L, MONNOT P, et al. Development of a human skeletal micro muscle platform with pacing capabilities[J]. Biomaterials, 2019, 198: 217-227. |
| 73 | AFSHAR BAKOOSHLI M, LIPPMANN E S, MULCAHY B, et al. A 3D culture model of innervated human skeletal muscle enables studies of the adult neuromuscular junction[J]. eLife, 2019, 8: e44530. |
| 74 | AFSHAR M E, ABRAHA H Y, BAKOOSHLI M A, et al. A 96-well culture platform enables longitudinal analyses of engineered human skeletal muscle microtissue strength[J]. Scientific Reports, 2020, 10(1): 6918. |
| 75 | KHODABUKUS A, MADDEN L, PRABHU N K, et al. Electrical stimulation increases hypertrophy and metabolic flux in tissue-engineered human skeletal muscle[J]. Biomaterials, 2019, 198: 259-269. |
| 76 | KHODABUKUS A, KAZA A, WANG J, et al. Tissue-engineered human myobundle system as a platform for evaluation of skeletal muscle injury biomarkers[J]. Toxicological Sciences, 2020, 176(1): 124-136. |
| 77 | AGRAWAL G, AUNG A, VARGHESE S. Skeletal muscle-on-a-chip: an in vitro model to evaluate tissue formation and injury[J]. Lab on a Chip, 2017, 17(20): 3447-3461. |
| 78 | OSAKI T, UZEL S G M, KAMM R D. Microphysiological 3D model of amyotrophic lateral sclerosis (ALS) from human iPS-derived muscle cells and optogenetic motor neurons[J]. Science Advances, 2018, 4(10): eaat5847. |
| 79 | VANDENBURGH H, SHANSKY J, BENESCH-LEE F, et al. Automated drug screening with contractile muscle tissue engineered from dystrophic myoblasts[J]. FASEB Journal, 2009, 23(10): 3325-3334. |
| 80 | GILBERT-HONICK J, IYER S R, SOMERS S M, et al. Engineering functional and histological regeneration of vascularized skeletal muscle[J]. Biomaterials, 2018, 164: 70-79. |
| 81 | LI J, ZHANG W H, LIU A Q, et al. An engineered anisotropic skeletal muscle organoid-on-a-chip for deciphering muscle response under intermittent hypoxia[J]. Advanced Functional Materials, 2024: 2401564. |
| 82 | O J 4th JUHL, BUETTMANN E G, FRIEDMAN M A, et al. Update on the effects of microgravity on the musculoskeletal system[J]. NPJ Microgravity, 2021, 7(1): 28. |
| 83 | REN Z P, AHN E H, DO M, et al. Simulated microgravity attenuates myogenesis and contractile function of 3D engineered skeletal muscle tissues[J]. NPJ Microgravity, 2024, 10(1): 18. |
| 84 | KIM S C, AYAN B, SHAYAN M, et al. Skeletal muscle-on-a-chip in microgravity as a platform for regeneration modeling and drug screening[J]. Stem Cell Reports, 2024, 19(8): 1061-1073. |
| [1] | 陈汐玥, 王亚清, 包芳, 秦建华. 肝器官芯片在生物医学研究中的应用进展[J]. 合成生物学, 2024, 5(4): 813-830. |
| [2] | 曹荣凯, 秦建华, 王亚清. 胎盘芯片及其在生殖医学领域的研究进展[J]. 合成生物学, 2024, 5(4): 831-850. |
| [3] | 洪源, 刘妍. 脑类器官在再生医学中的研究进展[J]. 合成生物学, 2024, 5(4): 754-769. |
| [4] | 胡可儿, 王汉奇, 黄儒麒, 张灿阳, 邢新会, 马少华. 整合设计策略下的工程化类器官与类器官芯片技术[J]. 合成生物学, 2024, 5(4): 883-897. |
| [5] | 陈子苓, 向阳飞. 类器官技术与合成生物学协同研究进展[J]. 合成生物学, 2024, 5(4): 795-812. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||