| 1 |
SANGER F, NICKLEN S, COULSON A R. DNA sequencing with chain-terminating inhibitors[J]. Proceedings of the National Academy of Sciences of the United States of America, 1977, 74(12): 5463-5467.
|
| 2 |
STEUERNAGEL B, TAUDIEN S, GUNDLACH H, et al. De novo 454 sequencing of barcoded BAC pools for comprehensive gene survey and genome analysis in the complex genome of barley[J]. BMC Genomics, 2009, 10(1): 547.
|
| 3 |
ROTHBERG J M, HINZ W, REARICK T M, et al. An integrated semiconductor device enabling non-optical genome sequencing[J]. Nature, 2011, 475(7356): 348-352.
|
| 4 |
CELLO J, PAUL A V, WIMMER E. Chemical synthesis of poliovirus cDNA: generation of infectious virus in the absence of natural template[J]. Science, 2002, 297(5583): 1016-1018.
|
| 5 |
SMITH H O, HUTCHISON C A, PFANNKOCH C, et al. Generating a synthetic genome by whole genome assembly: φX174 bacteriophage from synthetic oligonucleotides[J]. Proceedings of the National Academy of Sciences of the United States of America, 2003, 100(26): 15440-15445.
|
| 6 |
THAO T T N, LABROUSSAA F, EBERT N, et al. Rapid reconstruction of SARS-CoV-2 using a synthetic genomics platform[J]. Nature, 2020.
|
| 7 |
GIBSON D G, BENDERS G A, ANDREWS-PFANNKOCH C, et al. Complete chemical synthesis, assembly, and cloning of a Mycoplasma genitalium genome[J]. Science, 2008, 319(5867): 1215-1220.
|
| 8 |
GIBSON D G, GLASS J I, LARTIGUE C, et al. Creation of a bacterial cell controlled by a chemically synthesized genome[J]. Science, 2010, 329(5987): 52-56.
|
| 9 |
ZIMMER R, GIBBINS A M. Construction and characterization of a large-fragment chicken bacterial artificial chromosome library[J]. Genomics, 1997, 42(2): 217-226.
|
| 10 |
DE LISE A M, TUAN R S. Electroporation-mediated DNA transfection of embryonic chick limb mesenchymal cells[J]. Methods in Molecular Biology, 2000: 377-382.
|
| 11 |
CO D O, BOROWSKI A H, LEUNG J D, et al. Generation of transgenic mice and germline transmission of a mammalian artificial chromosome introduced into embryos by pronuclear microinjection[J]. Chromosome Research, 2000, 8(3):183-191.
|
| 12 |
PAULIS M. Chromosome transfer via cell fusion[J]. Methods of Molecular Biology, 2011,738: 57-67.
|
| 13 |
BROWN D M, CHAN Y A, DESAI P J, et al. Efficient size-independent chromosome delivery from yeast to cultured cell lines[J]. Nucleic Acids Research, 2016(7):e50.
|
| 14 |
SUZUKI T, KAZUKI Y, HARA T, et al. Current advances in microcell-mediated chromosome transfer technology and its applications[J]. Experimental Cell Research, 2020, 390(1):111915.
|
| 15 |
LAJOIE M J, ROVNER A J, GOODMAN D B, et al. Genomically recoded organisms expand biological functions[J]. Science, 2013, 342(6156): 357-360.
|
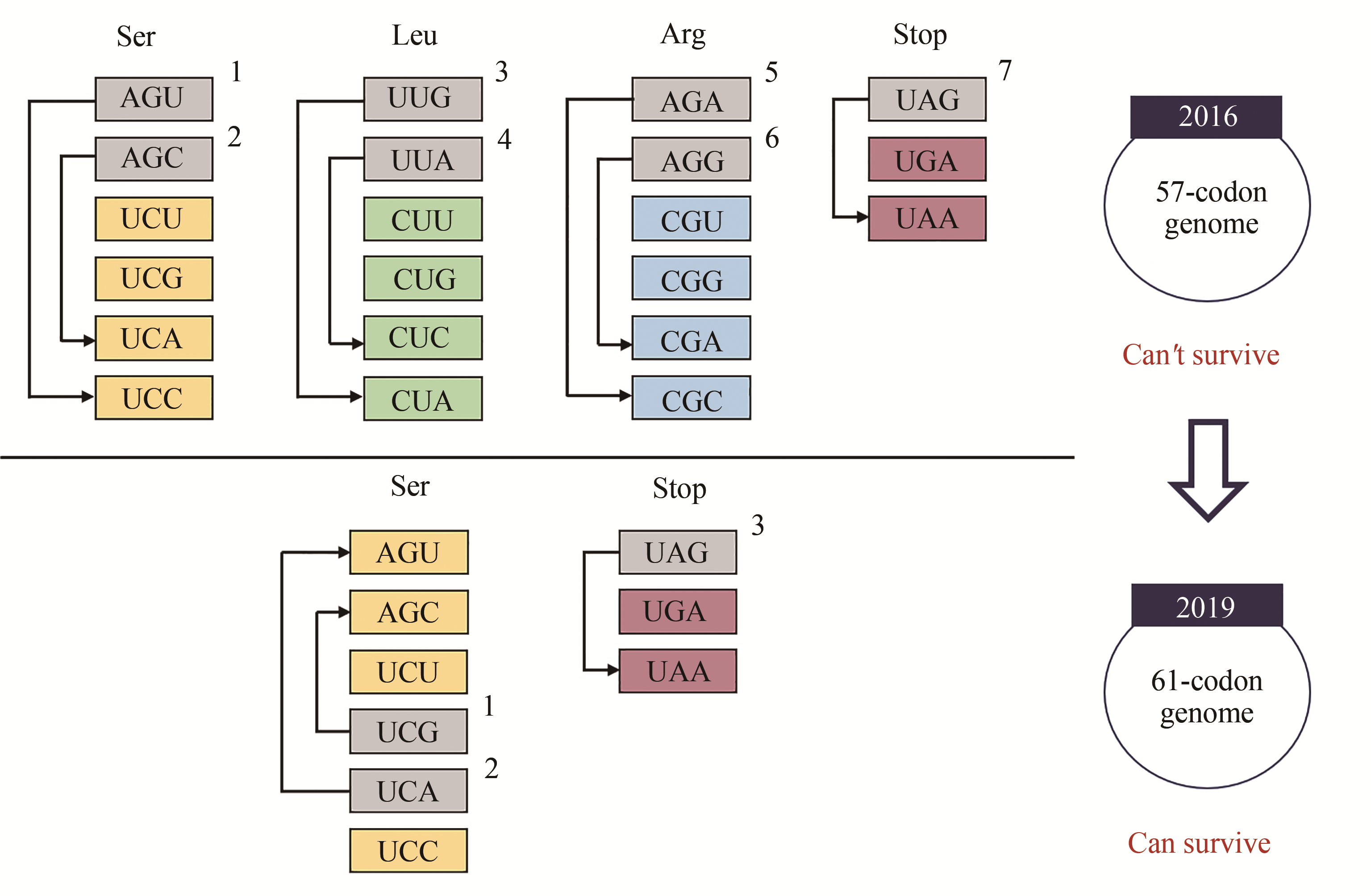
| 16 |
OSTROV N, LANDON M, GUELL M, et al. Design, synthesis, and testing toward a 57-codon genome[J]. Science, 2016, 353(6301): 819-822.
|
| 17 |
WANG K, FREDENS J, BRUNNER S F, et al. Defining synonymous codon compression schemes by genome recoding[J]. Nature, 2016, 539(7627): 59-64.
|
| 18 |
FREDENS J, WANG K, DE LA TORRE D, et al. Total synthesis of Escherichia coli with a recoded genome[J]. Nature, 2019, 569(7757): 514-518.
|
| 19 |
HUTCHISON C A, CHUANG R Y, NOSKOV V N, et al. Design and synthesis of a minimal bacterial genome[J]. Science, 2016, 351(6280).
|
| 20 |
DYMOND J S, RICHARDSON S M, COOMBES C E, et al. Synthetic chromosome arms function in yeast and generate phenotypic diversity by design[J]. Nature, 2011, 477(7365): 471-476.
|
| 21 |
ANNALURU N, MULLER H, MITCHELL L A, et al. Total synthesis of a functional d esigner eukaryotic chromosome[J]. Science, 2014, 344(6179): 55-58.
|
| 22 |
SHEN Y, WANG Y, CHEN T, et al. Deep functional analysis of synII, a 770-kilobase synthetic yeast chromosome[J]. Science, 2017, 355(6329): eaaf4791.
|
| 23 |
XIE Z X, LI B Z, MITCHELL L A, et al. "Perfect" designer chromosome V and behavior of a ring derivative[J]. Science, 2017, 355(6329): eaaf4704.
|
| 24 |
MITCHELL L A, WANG A, STRACQUADANIO G, et al. Synthesis, debugging, and effects of synthetic chromosome consolidation: synVI and beyond[J]. Science, 2017, 355(6329): eaaf4831.
|
| 25 |
WU Y, LI B Z, ZHAO M, et al. Bug mapping and fitness testing of chemically synthesized chromosome X[J]. Science, 2017, 355(6329): eaaf4706.
|
| 26 |
ZHANG W, ZHAO G, LUO Z, et al. Engineering the ribosomal DNA in a megabase synthetic chromosome[J]. Science, 2017, 355(6329): eaaf3981.
|
| 27 |
SHAO Y, LU N, WU Z, et al. Creating a functional single-chromosome yeast[J]. Nature, 2018, 560(7718): 331-335.
|
| 28 |
SHAO Y, LU N, CAI C, et al. A single circular chromosome yeast[J]. Cell Research, 2019, 29(1): 87-89.
|
| 29 |
OSTROV N, BEAL J, ELLIS T, et al. Technological challenges and milestones for writing genomes[J]. Science, 2019, 366(6463): 310-312.
|
| 30 |
柴梦哲, 贾斌, 李炳志,等. 人工基因组合成与重排研究进展[J]. 生命科学, 2019, 31(4):46-53.
|
|
CHAI M Z, JIA B, LI B Z, et al. Progress in research on artificial gene assembly and rearrangement [J]. Chinese Bulletin Life Science, 2019, 31(4): 46-53.
|
| 31 |
罗周卿, 戴俊彪. 合成基因组学:设计与合成的艺术[J]. 生物工程学报, 2017, 33(3): 331-342.
|
|
LUO Z Q, DAI J B. Synthetic genomics: the art of design and synthesis [J]. Chinese Journal of Biotechnology, 2017, 33(3): 331-342.
|
| 32 |
GUSTAFSSON C, GOVINDARAJAN S, MINSHULL J. Codon bias and heterologous protein expression[J]. Trends in Biotechnology, 2004, 22(7): 346-353.
|
| 33 |
CHIN J X, CHUNG B K S, Lee D Y. Codon Optimization OnLine (COOL): a web-based multi-objective optimization platform for synthetic gene design[J]. Bioinformatics, 2014, 30(15): 2210-2212.
|
| 34 |
CANNAROZZI G, SCHRAUDOLPH N N, FATY M, et al. A role for codon order in translation dynamics[J]. Cell, 2010, 141(2): 355-367.
|
| 35 |
RICHARDSON S M, MITCHELL L A, STRACQUADANIO G, et al. Design of a synthetic yeast genome[J]. Science, 2017, 355(6329): 1040-1044.
|
| 36 |
LUO Z, WANG L, WANG Y, et al. Identifying and characterizing SCRaMbLEd synthetic yeast using ReSCuES[J]. Nature Communications, 2018, 9(1): 1-10.
|
| 37 |
MA L, LI Y, CHEN X, et al. SCRaMbLE generates evolved yeasts with increased alkali tolerance[J]. Microbial Cell Factories, 2019, 18(1): 52.
|
| 38 |
HOESS R H, WIERZBICKI A, ABREMSKI K. The role of the loxP spacer region in PI site-specific recombination[J]. Nucleic Acids Research, 1986, 14(5): 2287-2300.
|
| 39 |
SHEN Y, STRACQUADANIO G, WANG Y, et al. SCRaMbLE generates designed combinatorial stochastic diversity in synthetic chromosomes[J]. Genome Research, 2016, 26(1): 36-49.
|
| 40 |
JIA B, WU Y, LI B Z, et al. Precise control of SCRaMbLE in synthetic haploid and diploid yeast[J]. Nature Communications, 2018, 9(1): 1-13.
|
| 41 |
LIN Q, QI H, WU Y, et al. Robust orthogonal recombination system for versatile genomic elements rearrangement in yeast Saccharomyces cerevisiae [J]. Scientific Reports, 2015, 5: 15249.
|
| 42 |
HOCHREIN L, MITCHELL L A, SCHULZ K, et al. L-SCRaMbLE as a tool for light-controlled Cre-mediated recombination in yeast[J]. Nature Communications, 2018, 9(1): 1-10.
|
| 43 |
SHEN M J, WU Y, YANG K, et al. Heterozygous diploid and interspecies SCRaMbLEing[J]. Nature Communications, 2018, 9(1): 1-8.
|
| 44 |
WU Y, ZHU R Y, Mitchell L A, et al. In vitro DNA SCRaMbLE[J]. Nature Communications, 2018, 9(1): 1-9.
|
| 45 |
LIU W, LUO Z, WANG Y, et al. Rapid pathway prototyping and engineering using in vitro and in vivo synthetic genome SCRaMbLE-in methods[J]. Nature Communications, 2018, 9(1): 1-12.
|
| 46 |
BLOUNT B A, GOWERS G O F, HO J C H, et al. Rapid host strain improvement by in vivo rearrangement of a synthetic yeast chromosome[J]. Nature Communications, 2018, 9(1): 1-10.
|
| 47 |
WANG J, JIA B, XIE Z, et al. Improving prodeoxyviolacein production via multiplex SCRaMbLE iterative cycles[J]. Frontiers of Chemical Science and Engineering, 2018, 12(4): 806-814.
|
| 48 |
WANG P X, XU H, LI H, et al. SCRaMbLEing of a synthetic yeast chromosome with clustered essential genes reveals synthetic lethal interactions[J].ACS Synthetic Biology,2020,9(5):1181-1189.
|
| 49 |
LUO Z Q, YU K, XIE S Q, et al. Compacting a synthetic yeast chromosome arm[J]. Genome Biology, 2021, 22. DOI: 10.1186/s 13059-020-02232-8 .
|
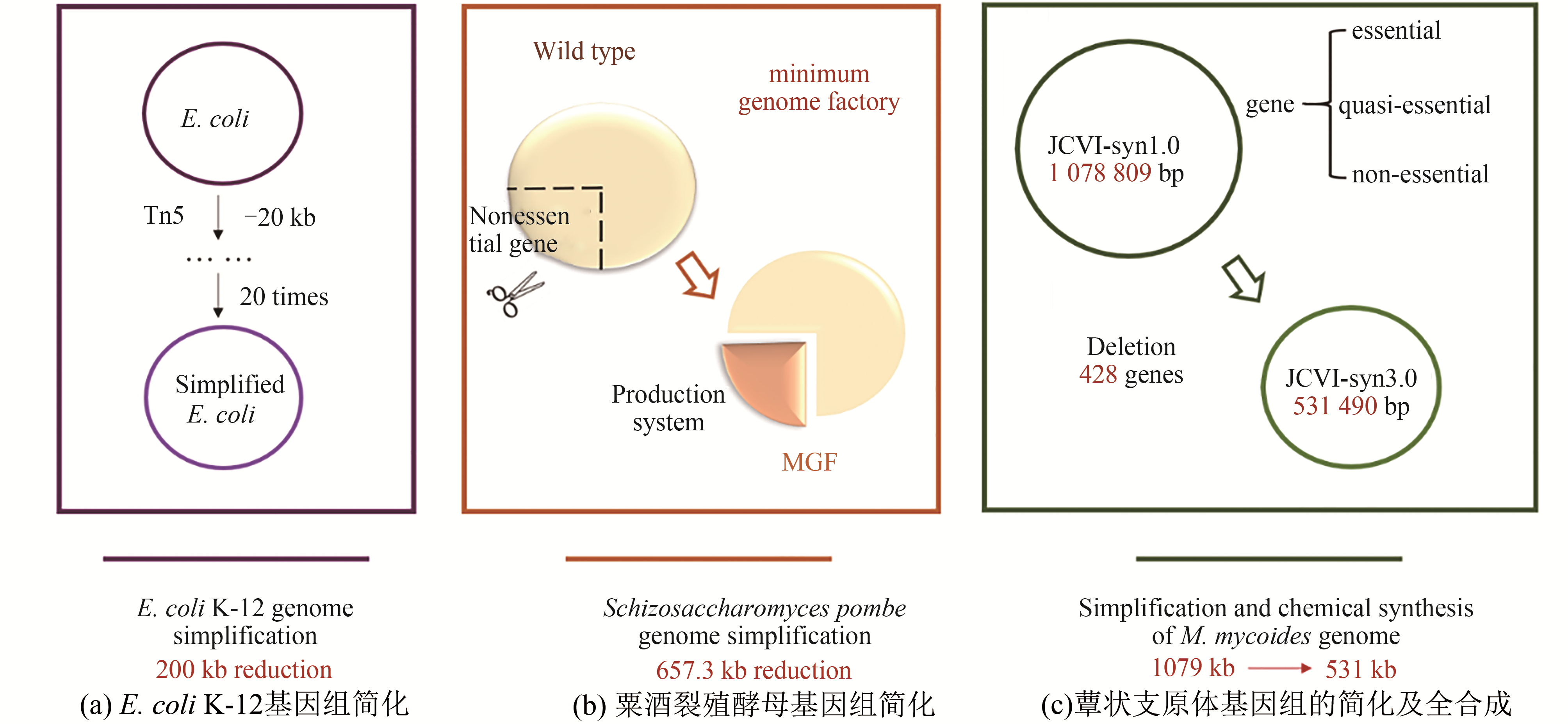
| 50 |
GORYSHIN I Y, NAUMANN T A, APODACA J, et al. Chromosomal deletion formation system based on Tn5 double transposition: use for making minimal genomes and essential gene analysis[J]. Genome Research, 2003, 13(4): 644-653.
|
| 51 |
KUMAGAI H, SASAKI M, IDIRIS A, et al. Minimum genome factories in Schizosaccharomyces pombe [M]//Microbial production, Tokyo: Springer, 2014: 17-24.
|
| 52 |
BOEKE J D, CHURCH G, HESSEL A, et al. The genome project-write[J]. Science, 2016, 353(6295): 126-127.
|
| 53 |
MEINKE G, BOHM A, HAUBER J, et al. Cre recombinase and other tyrosine recombinases[J]. Chemical Reviews, 2016, 116(20): 12785-12820.
|