合成生物学 ›› 2020, Vol. 1 ›› Issue (5): 503-515.DOI: 10.12211/2096-8280.2020-013
基因组的“读-改-写”技术
- 1.深圳大学生命与海洋科学学院,广东 深圳 518055
2.中国科学院深圳先进技术研究院,深圳合成生物学创新研究院,合成基因组学研究中心,广东省合成基因组学重点实验室,深圳合成基因组学重点实验室,广东 深圳 518055
-
收稿日期:2020-02-29修回日期:2020-04-19出版日期:2020-10-31发布日期:2020-12-03 -
通讯作者:戴俊彪,罗周卿 -
作者简介:作者简介:王会(1993—),女,硕士研究生,主要研究方向为合成生物学。E-mail:15225377578@163.com
戴俊彪(1974—),男,博士,研究员,主要研究方向为合成生物学。E-mail:junbiao.dai@siat.ac.cn
罗周卿(1990—),男,博士,副研究员,主要研究方向为合成生物学。E-mail:zq.luo@siat.ac.cn -
基金资助:国家重点研发计划“合成生物学”重点专项(2018YFA0900100);国家自然科学基金(31725002);深圳合成基因组学重点实验室项目(ZDSYS201802061806209);广东省合成基因组学重点实验室(2019B030301006);深圳市科技计划(KQTD20180413181837372)
Reading, editing, and writing techniques for genome research
WANG Hui1,2( ), DAI Junbiao1,2, LUO Zhouqing2
), DAI Junbiao1,2, LUO Zhouqing2
- 1.College of Life Sciences and Oceanography,Shenzhen University,Shenzhen 518055,Guangdong,China
2.Guangdong Provincial Key Laboratory of Synthetic Genomics,Shenzhen Key Laboratory of Synthetic Genomics,Center for Synthetic Genomics,Shenzhen Institute of Synthetic Biology,Shenzhen Institutes of Advanced Technology,Chinese Academy of Sciences,Shenzhen 518055,Guangdong,China
-
Received:2020-02-29Revised:2020-04-19Online:2020-10-31Published:2020-12-03 -
Contact:DAI Junbiao, LUO Zhouqing
摘要:
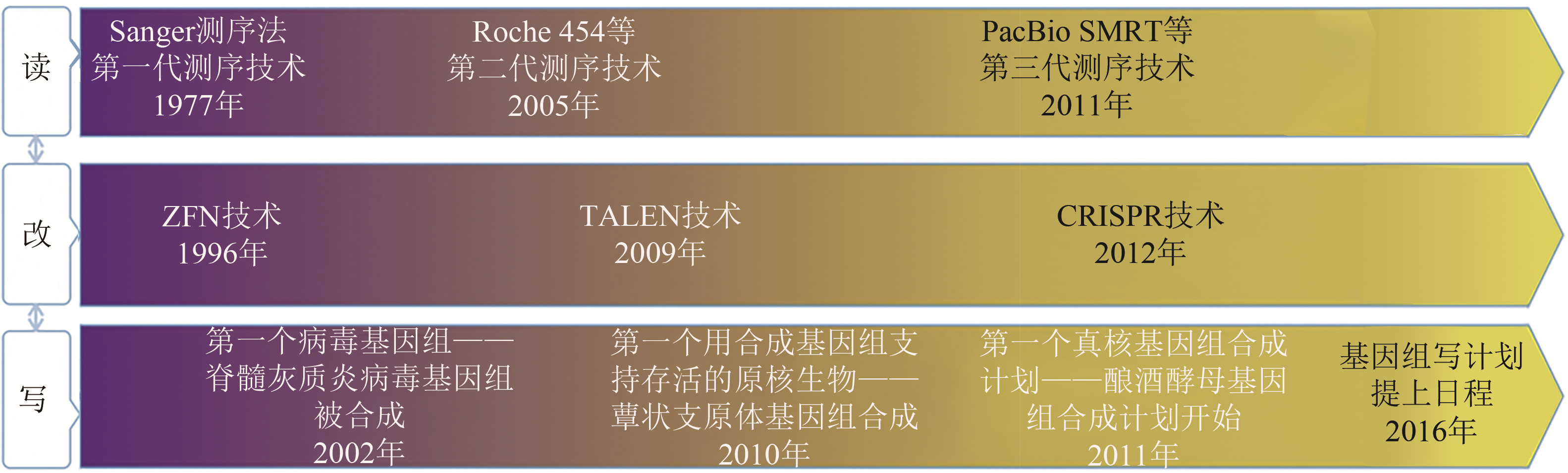
基因组是生命系统的指令中枢,对基因组的研究是生命科学的核心内容,基因组研究相关技术的开发是深化对基因组序列和功能认识的重要推动力量。通过基因组测序获取基因组全序列,通过人工诱变、定点编辑研究基因组局部序列的功能与调控,通过对基因组的从头设计与化学再造实现对生命性状的定制,是基因组研究的三个不同层面。从一代测序到三代测序,基因组“读”技术极大地降低了成本和难度,提升了速度和精准度,引领着复杂基因组、大型基因组从草图走向完成图时代。通过人工诱变、定点编辑等技术可以改变野生型基因组的局部序列,研究基因组序列的功能与调控。从人工诱变到定点编辑,从ZFN到CRISPR,基因组“改”技术在效率、适用对象和简便性上有了显著的提高,为“基因型-表型”研究提供了有力工具,精准编辑、高通量编辑逐步走向应用。通过对基因组的从头设计与化学再造,书写人工基因组,可以获得对基因组全局的系统认识,实现对生命性状的定制。从病毒基因组合成、细菌基因组合成到酵母基因组合成,再到国际基因组写计划,基因组“写”技术在适用对象上不断拓展,人工设计、化学再造正成为复杂生物学问题研究和已有性状优化、新性状引入的一把利器。本文主要综述了基因组测序(读)、基因组编辑(改)和基因组合成(写)技术的发展历程、各自的特征、目前的研究进展及在基因组研究方面的一些应用,并对近期相关技术的可能突破点进行了总结和展望。“读-改-写”技术互为支撑,推动基因组研究在致知和致用领域两面开花。
中图分类号:
引用本文
王会, 戴俊彪, 罗周卿. 基因组的“读-改-写”技术[J]. 合成生物学, 2020, 1(5): 503-515.
WANG Hui, DAI Junbiao, LUO Zhouqing. Reading, editing, and writing techniques for genome research[J]. Synthetic Biology Journal, 2020, 1(5): 503-515.
| 物种 | 基因组 大小/Mb | 编码区占比/% | 非编码区占比/% | 参考数据库 |
|---|---|---|---|---|
| Homo sapiens | 3107 | 2.8 | 97.2 | UCSC Genome Browser (hg18) |
| Drosophila melanogaster | 168.7 | 18.3 | 81.7 | FlyBase |
| Saccharomyces cerevisiae | 12.2 | 72.9 | 27.1 | Saccharomyces Genome Database |
| Escherichia coli K12 | 4.6 | 88 | 12 | EcoCyc |
表1 不同生物基因组中含有的编码序列与非编码序列的比较
Tab. 1 Comparison of the the coding sequence and non-coding sequence contents of several genomes
| 物种 | 基因组 大小/Mb | 编码区占比/% | 非编码区占比/% | 参考数据库 |
|---|---|---|---|---|
| Homo sapiens | 3107 | 2.8 | 97.2 | UCSC Genome Browser (hg18) |
| Drosophila melanogaster | 168.7 | 18.3 | 81.7 | FlyBase |
| Saccharomyces cerevisiae | 12.2 | 72.9 | 27.1 | Saccharomyces Genome Database |
| Escherichia coli K12 | 4.6 | 88 | 12 | EcoCyc |
| 测序技术 | 应用 | 读长 | 优点 | 缺点 |
|---|---|---|---|---|
| 一代测序 | 早期简单基因组测序;日常PCR产物、质粒等的测序 | 约1000 bp | 读长较长,准确度高(99.999%) | 成本高,通量低 |
| 二代测序 | 目前大部分基因组、转录组的测序 | 200~500 bp | 成本低,通量高,准确度大于99.94% | 读长短 |
| 三代测序 (SMRT sequencing) | 复杂基因组、全长转录组等的测序 | 10~100 kb | 读长长,准确度高,可直接检测DNA或者RNA上的修饰 | 测序成本高,每个SMRT cell的数据产出有限(约10 Gb),文库准备需要大量的起始材料,目前读长还比较有限(约80 kb) |
| 三代测序 (nanopore sequencing) | 复杂基因组的测序,结构变异的鉴定等 | kb~Mb | 超长读长,经济高效,可直接检测DNA或者RNA上的修饰 | 错误率高,对于多碱基重复存在系统误差,文库准备需要大量的起始材料 |
表2 不同测序技术比较
Tab. 2 Comparison of different sequencing technologies
| 测序技术 | 应用 | 读长 | 优点 | 缺点 |
|---|---|---|---|---|
| 一代测序 | 早期简单基因组测序;日常PCR产物、质粒等的测序 | 约1000 bp | 读长较长,准确度高(99.999%) | 成本高,通量低 |
| 二代测序 | 目前大部分基因组、转录组的测序 | 200~500 bp | 成本低,通量高,准确度大于99.94% | 读长短 |
| 三代测序 (SMRT sequencing) | 复杂基因组、全长转录组等的测序 | 10~100 kb | 读长长,准确度高,可直接检测DNA或者RNA上的修饰 | 测序成本高,每个SMRT cell的数据产出有限(约10 Gb),文库准备需要大量的起始材料,目前读长还比较有限(约80 kb) |
| 三代测序 (nanopore sequencing) | 复杂基因组的测序,结构变异的鉴定等 | kb~Mb | 超长读长,经济高效,可直接检测DNA或者RNA上的修饰 | 错误率高,对于多碱基重复存在系统误差,文库准备需要大量的起始材料 |
| 种类 | 大小(氨基酸数) | PAM | 来源及特色 | 参考文献 |
|---|---|---|---|---|
| SpCas9 | 1368 | NGG | Streptococcus pyogenes,应用广泛 | [ |
| SaCas9 | 1053 | NNGRRT | Staphylococcus aureus 体积小,单个AAV载体即可承载SaCas9和对应的guide RNA | [ |
| NmeCas9 | 1082 | NNNNGATT | Neisseria meningitidis 识别的PAM位点较长,切割活性较SpCas9低,但特异性较好 | [ |
| CjCas9 | 984 | NNNNACAC 和 NNNNRYAC | Campylobacter jejuni 迄今为止发现的最小的Cas9蛋白,单个AAV载体即可承载CjCas9和对应的guide RNA | [ |
| SpCas9-NG | 1368 | NGH | 理性设计的SpCas9突变体,具有与SpCas9类似的特异性 | [ |
| xCas9 | 1368 | NG,GAA和GTA | 用噬菌体辅助的连续进化技术对SpCas9改造而来,相比SpCas9有更好的特异性 | [ |
表3 Cas9蛋白的不同来源及相关改造
Tab. 3 Different sources of Cas9 and related engineerings
| 种类 | 大小(氨基酸数) | PAM | 来源及特色 | 参考文献 |
|---|---|---|---|---|
| SpCas9 | 1368 | NGG | Streptococcus pyogenes,应用广泛 | [ |
| SaCas9 | 1053 | NNGRRT | Staphylococcus aureus 体积小,单个AAV载体即可承载SaCas9和对应的guide RNA | [ |
| NmeCas9 | 1082 | NNNNGATT | Neisseria meningitidis 识别的PAM位点较长,切割活性较SpCas9低,但特异性较好 | [ |
| CjCas9 | 984 | NNNNACAC 和 NNNNRYAC | Campylobacter jejuni 迄今为止发现的最小的Cas9蛋白,单个AAV载体即可承载CjCas9和对应的guide RNA | [ |
| SpCas9-NG | 1368 | NGH | 理性设计的SpCas9突变体,具有与SpCas9类似的特异性 | [ |
| xCas9 | 1368 | NG,GAA和GTA | 用噬菌体辅助的连续进化技术对SpCas9改造而来,相比SpCas9有更好的特异性 | [ |
| Cas蛋白 | 蛋白大小 (氨基酸数) | 导向分子大小 (核苷酸数) | 靶标核酸的 主要类型 | 靶标序列限制 | 活性控制 | 精确性 | 应用领域 |
|---|---|---|---|---|---|---|---|
| Cas9 | 约1000~1600 | 约105 (sgRNA) | dsDNA | 含有PAM序列(对SpCas9而言, 为NGG),PAM位点近端产生平末端 | 由RuvC和HNH结构域负责靶DNA的切割,结合并切割靶向DNA | 可容忍DNA链上的单个错配 | 基因组编辑、基因表达调控、成像等 |
| Cas12 | 约1300 | 约42~44 (crRNA) | dsDNA | 含有PAM序列(对Cas12a而言, 为TTTV),在PAM位点远端产生5' 突出末端 | 由RuvC和Nuc结构域负责靶DNA的切割,结合并切割靶向DNA | 可有效区分双链DNA上一个碱基的差异 | 基因组编辑和基于双链DNA的分子诊断等 |
| Cas13 | 约1400 | 约64 (crRNA) | ssRNA | RNA结构影响其活性,不同来源的Cas13蛋白对PFS序列的要求不同,但都相对较弱 | 由两个HEPN结构域负责靶RNA的切割;在体外及细菌体内,Cas13a识别并切割靶向序列后即转入酶促“激活”状态,结合并切割其他的ssRNA | 很难区分一个碱基的差异 | RNA敲低和基于RNA的分子诊断等 |
| Cas14 | 约400~700 | 约140 (sgRNA) | ssDNA | 对单链DNA没有限制,双链DNA必须含有PAM序列(对Cas14a而言,为TTTR) | 由RuvC结构域负责靶DNA的切割,识别并切割靶向序列后即转入酶促“激活”状态,这时它将结合并切割其他的 ssDNA | 可有效区分单链DNA上一个碱基的差异 | 基于单链DNA的分子诊断等 |
表4 不同CRISPR/Cas系统的比较
Tab. 4 Comparison of different CRISPR/Cas systems
| Cas蛋白 | 蛋白大小 (氨基酸数) | 导向分子大小 (核苷酸数) | 靶标核酸的 主要类型 | 靶标序列限制 | 活性控制 | 精确性 | 应用领域 |
|---|---|---|---|---|---|---|---|
| Cas9 | 约1000~1600 | 约105 (sgRNA) | dsDNA | 含有PAM序列(对SpCas9而言, 为NGG),PAM位点近端产生平末端 | 由RuvC和HNH结构域负责靶DNA的切割,结合并切割靶向DNA | 可容忍DNA链上的单个错配 | 基因组编辑、基因表达调控、成像等 |
| Cas12 | 约1300 | 约42~44 (crRNA) | dsDNA | 含有PAM序列(对Cas12a而言, 为TTTV),在PAM位点远端产生5' 突出末端 | 由RuvC和Nuc结构域负责靶DNA的切割,结合并切割靶向DNA | 可有效区分双链DNA上一个碱基的差异 | 基因组编辑和基于双链DNA的分子诊断等 |
| Cas13 | 约1400 | 约64 (crRNA) | ssRNA | RNA结构影响其活性,不同来源的Cas13蛋白对PFS序列的要求不同,但都相对较弱 | 由两个HEPN结构域负责靶RNA的切割;在体外及细菌体内,Cas13a识别并切割靶向序列后即转入酶促“激活”状态,结合并切割其他的ssRNA | 很难区分一个碱基的差异 | RNA敲低和基于RNA的分子诊断等 |
| Cas14 | 约400~700 | 约140 (sgRNA) | ssDNA | 对单链DNA没有限制,双链DNA必须含有PAM序列(对Cas14a而言,为TTTR) | 由RuvC结构域负责靶DNA的切割,识别并切割靶向序列后即转入酶促“激活”状态,这时它将结合并切割其他的 ssDNA | 可有效区分单链DNA上一个碱基的差异 | 基于单链DNA的分子诊断等 |
| 物种 | 基因组大小 | 基因数目 | 合成时间 |
|---|---|---|---|
| Poliovirus | 7.7 kb | 10 | 2002年 |
| PhiX174 | 5.4 kb | 11 | 2003年 |
| Mycoplasma genitalium | 580 kb | 525 | 2008年 |
| Mycoplasma mycoides | 1.2 Mb | 985 | 2010年 |
| Escherichia coli | 3.97 Mb | 3730 | 2019年 |
| Saccharomyces cerevisiae | 1.25 Mb | 5770 | 2011年 |
| Homo sapiens | 3.3 Gb | 约21 000 | 2016年 |
表5 基因组合成对象复杂程度对比
Tab. 5 Comparison of the complexity of synthetic genomes
| 物种 | 基因组大小 | 基因数目 | 合成时间 |
|---|---|---|---|
| Poliovirus | 7.7 kb | 10 | 2002年 |
| PhiX174 | 5.4 kb | 11 | 2003年 |
| Mycoplasma genitalium | 580 kb | 525 | 2008年 |
| Mycoplasma mycoides | 1.2 Mb | 985 | 2010年 |
| Escherichia coli | 3.97 Mb | 3730 | 2019年 |
| Saccharomyces cerevisiae | 1.25 Mb | 5770 | 2011年 |
| Homo sapiens | 3.3 Gb | 约21 000 | 2016年 |
| SCRaMbLE对象 | 对后续合成菌株的应用所具有的价值 | 参考文献 |
|---|---|---|
| synⅨR(环形) | 对SCRaMbLE系统的首次实验验证,证实了SCRaMbLE系统可以高效地产生删除、 倒换和重复等基因组结构变异 | [ |
| synⅤ | 利用纳米孔测序技术解析重排基因组,证实了SCRaMbLE所造成的基因组重排可用于外源代谢途径特异的底盘细胞改良 | [ |
| 含有loxPsym 位点的质粒 | 建立光控的Cre重组酶活性控制系统(L-SCRaMbLE),实现对其活性更加精准的控制 | [ |
| synⅤ | 利用与门电路精准控制Cre重组酶的活性以及利用多次SCRaMbLE实现带有合成染色体的异源二倍体菌株中的外源代谢途径的产量提升 | [ |
| synⅡ | 通过对loxPsym等位点的设计改造建立SCRaMbLE-in技术,实现外源代谢途径的优化和底盘细胞的适配 | [ |
| synⅫ,synⅢ等 | 基于loxP与loxPsym位点的正交性建立ReSCuES基因组重排报告系统,实现重排菌株的高效筛选、耐受性进化和机制解析 | [ |
| synⅤ,synⅩ | 表征了带有合成染色体的种内杂交菌株或种间杂交菌株的SCRaMbLE行为,为利用合成染色体优化工业菌株性状奠定基础 | [ |
| synⅤ(环形) | 环形染色体SCRaMbLE可以产生非合成染色体的数目变化,非整倍体菌株可用于提高prodeoxyviolacein的产量 | [ |
| β-胡萝卜素途径 | 利用SCRaMbLE原理设计了基于结构变异的DNA文库体外构建方法,可用于外源代谢途径的体外优化 | [ |
| synⅤ | 将自动化样品制备、超快速LC-MS方法和条形码纳米孔测序相结合,解决了重排菌株代谢性能快速表征的技术障碍 | [ |
表6 合成酵母SCRaMbLE系统的相关研究
Tab. 6 Studies on the synthetic yeast SCRaMbLE system
| SCRaMbLE对象 | 对后续合成菌株的应用所具有的价值 | 参考文献 |
|---|---|---|
| synⅨR(环形) | 对SCRaMbLE系统的首次实验验证,证实了SCRaMbLE系统可以高效地产生删除、 倒换和重复等基因组结构变异 | [ |
| synⅤ | 利用纳米孔测序技术解析重排基因组,证实了SCRaMbLE所造成的基因组重排可用于外源代谢途径特异的底盘细胞改良 | [ |
| 含有loxPsym 位点的质粒 | 建立光控的Cre重组酶活性控制系统(L-SCRaMbLE),实现对其活性更加精准的控制 | [ |
| synⅤ | 利用与门电路精准控制Cre重组酶的活性以及利用多次SCRaMbLE实现带有合成染色体的异源二倍体菌株中的外源代谢途径的产量提升 | [ |
| synⅡ | 通过对loxPsym等位点的设计改造建立SCRaMbLE-in技术,实现外源代谢途径的优化和底盘细胞的适配 | [ |
| synⅫ,synⅢ等 | 基于loxP与loxPsym位点的正交性建立ReSCuES基因组重排报告系统,实现重排菌株的高效筛选、耐受性进化和机制解析 | [ |
| synⅤ,synⅩ | 表征了带有合成染色体的种内杂交菌株或种间杂交菌株的SCRaMbLE行为,为利用合成染色体优化工业菌株性状奠定基础 | [ |
| synⅤ(环形) | 环形染色体SCRaMbLE可以产生非合成染色体的数目变化,非整倍体菌株可用于提高prodeoxyviolacein的产量 | [ |
| β-胡萝卜素途径 | 利用SCRaMbLE原理设计了基于结构变异的DNA文库体外构建方法,可用于外源代谢途径的体外优化 | [ |
| synⅤ | 将自动化样品制备、超快速LC-MS方法和条形码纳米孔测序相结合,解决了重排菌株代谢性能快速表征的技术障碍 | [ |
| 1 | MCKUSICK V A. Genomics: structural and functional studies of genomes[J]. Genomics, 1997, 45(2):244-249. |
| 2 | ALEXANDER R P, FANG G, ROZOWSKY J, et al. Annotating non-coding regions of the genome[J]. Nature Reviews Genetics, 2010, 11(8):559-571. |
| 3 | CRICK F. The double helix: a personal view[J]. Nature, 1974, 248(5451):766-769. |
| 4 | SANGER F, NICKLEN S, COULSON A R. DNA sequencing with chain-terminating inhibitors[J]. Proceedings of the National Academy of Sciences of the United States of America, 1977, 74(12):5463-5467. |
| 5 | JACKSON D A, SYMONS R H, BERG P. Biochemical method for inserting new genetic information into DNA of Simian Virus 40: circular SV40 DNA molecules containing lambda phage genes and the galactose operon of Escherichia coli [J]. Proceedings of the National Academy of Sciences of the United States of America,1972, 69(10):2904-2909. |
| 6 | CHANG A C, COHEN S N. Genome construction between bacterial species in vitro: replication and expression of Staphylococcus plasmid genes in Escherichia coli [J].Proceedings of the National Academy of Sciences of the United States of America, 1974, 71(4):1030-1034. |
| 7 | VENTER J C, ADAMS M D, MYERS E W, et al. The sequence of the human genome[J]. Science, 2001, 291(5507):1304-1351. |
| 8 | International human genome sequencing C: finishing the euchromatic sequence of the human genome[J]. Nature, 2004, 431(7011):931-945. |
| 9 | CELLO J, PAUL A V, WIMMER E. Chemical synthesis of Poliovirus cDNA: generation of infectious virus in the absence of natural template[J]. Science, 2002, 297(5583):1016-1018. |
| 10 | JINEK M, CHYLINSKI K, FONFARA I, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity[J]. Science, 2012, 337(6096):816-821. |
| 11 | CONG L, RAN F A, COX D, et al. Multiplex genome engineering using CRISPR/Cas systems[J]. Science, 2013, 339(6121):819-823. |
| 12 | MARGULIES M, EGHOLM M, ALTMAN W E, et al. Genome sequencing in microfabricated high-density picolitre reactors[J]. Nature, 2005, 437(7057):376-380. |
| 13 | LEVY S E, MYERS R M. Advancements in next-generation sequencing[J]. Annual Review of Genomics and Human Genetics, 2016, 17:95-115. |
| 14 | VALOUEV A, ICHIKAWA J, TONTHAT T, et al. A high-resolution, nucleosome position map of C. elegans reveals a lack of universal sequence-dictated positioning[J]. Genome Research, 2008, 18(7):1051-1063. |
| 15 | GOODWIN S, MCPHERSON J D, MCCOMBIE W R. Coming of age: ten years of next-generation sequencing technologies[J].Nature Reviews Genetics, 2016, 17(6):333-351. |
| 16 | EID J, FEHR A, GRAY J, et al. Real-time DNA sequencing from single polymerase molecules[J]. Science, 2009, 323(5910):133-138. |
| 17 | ASHTON P M, NAIR S, DALLMAN T, et al. MinION nanopore sequencing identifies the position and structure of a bacterial antibiotic resistance island[J]. Nature Biotechnology, 2015, 33(3):296-300. |
| 18 | FLUSBERG B A, WEBSTER D R, LEE J H, et al. Direct detection of DNA methylation during single-molecule, real-time sequencing[J]. Nature Methods, 2010, 7(6):461-465. |
| 19 | TRAVERS K J, CHIN C S, RANK D R, et al. A flexible and efficient template format for circular consensus sequencing and SNP detection[J]. Nucleic Acids Research, 2010, 38(15):e159. |
| 20 | DIJK E L VAN, JASZCZYZYN Y, NAQUIN D, et al. The third revolution in sequencing technology[J]. Trends in Genetics, 2018, 34(9):666-681. |
| 21 | MANRAO E A, DERRINGTON I M, LASZLO A H, et al. Reading DNA at single-nucleotide resolution with a mutant MspA nanopore and phi29 DNA polymerase[J]. Nature Biotechnology, 2012, 30(4):349-353. |
| 22 | JAIN M, KOREN S, MIGA K H, et al. Nanopore sequencing and assembly of a human genome with ultra-long reads[J]. Nature Biotechnology, 2018, 36(4):338-345. |
| 23 | JAIN M, OLSEN H E, TURNER D J, et al. Linear assembly of a human centromere on the Y chromosome[J]. Nature Biotechnology, 2018, 36(4):321-323. |
| 24 | JAIN M, TYSON J R, LOOSE M, et al. MinION analysis and reference consortium: phase 2 data release and analysis of R9.0 chemistry[J]. F1000Research, 2017, 6:760. |
| 25 | FRIEDBERG E C. DNA damage and repair[J]. Nature, 2003, 421(6921):436-440. |
| 26 | ABREMSKI K, HOESS R. Bacteriophage P1 site-specific recombination. Purification and properties of the Cre recombinase protein[J]. Journal of Biological Chemistry, 1984, 259(3):1509-1514. |
| 27 | SENECOFF J F, BRUCKNER R C, COX M M. The FLP recombinase of the yeast 2-micron plasmid: characterization of its recombination site[J]. Proceedings of the National Academy of Sciences of the United States of America, 1985, 82(21):7270-7274. |
| 28 | GAJ T, GERSBACH C A, C F 3rd BARBAS. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering[J]. Trends in Biotechnology, 2013, 31(7):397-405. |
| 29 | KIM Y G, CHA J, CHANDRASEGARAN S. Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain[J]. Proceedings of the National Academy of Sciences of the United States of America, 1996, 93(3):1156-1160. |
| 30 | URNOV F D, REBAR E J, HOLMES M C, et al. Genome editing with engineered zinc finger nucleases[J]. Nature Reviews Genetics, 2010, 11(9):636-646. |
| 31 | GUILLINGER J P, PATTANAYAK V, REYONN D, et al. Broad specificity profiling of TALENs results in engineered nucleases with improved DNA-cleavage specificity[J]. Nature Methods, 2014, 11(4):429-435. |
| 32 | CHANDRASEGARAN S, CARROLL D. Origins of programmable nucleases for genome engineering[J]. Journal of Molecular Biology, 2016, 428(5PtB):963-989. |
| 33 | BARRANGOU R, FREMAUX C, DEVEAU H, et al. CRISPR provides acquired resistance against viruses in prokaryotes[J]. Science, 2007, 315(5819):1709-1712. |
| 34 | RAN F A, CONG L, YAN W X, et al. In vivo genome editing using Staphylococcus aureus Cas9[J]. Nature, 2015, 520(7546):186-191. |
| 35 | LEE C M, CRADICK T J, BAO G. The Neisseria meningitidis CRISPR-Cas9 system enables specific genome editing in mammalian cells[J]. Molecular Therapy : the Journal of the American Society of Gene Therapy, 2016, 24(3):645-654. |
| 36 | KIM E, KOO T, PARK S W, et al. In vivo genome editing with a small Cas9 orthologue derived from Campylobacter jejuni [J]. Nature Communications, 2017, 8:14500. |
| 37 | HU J H, MILLER S M, GEURTSS M H, et al. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity[J]. Nature, 2018, 556(7699):57-63. |
| 38 | NISHIMASU H, SHI X, ISHIGURO S, et al. Engineered CRISPR-Cas9 nuclease with expanded targeting space[J]. Science, 2018, 361(6408):1259-1262. |
| 39 | ZETSCHE B, GOOTENBERG J S, ABUDAYYEH O O, et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system[J]. Cell, 2015, 163(3):759-771. |
| 40 | TENG F, CUI T, FENG G, et al. Repurposing CRISPR-Cas12b for mammalian genome engineering[J]. Cell Discovery, 2018, 4:1-15. |
| 41 | STRECKER J, JONES S, KOOPAL B, et al. Engineering of CRISPR-Cas12b for human genome editing[J]. Nature Communications, 2019, 10(1):212. |
| 42 | ADUDAYYEH O O, GOOTENBERGG J S, KONERMAN S, et al. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector[J]. Science, 2016, 353(2699):aaf5573. |
| 43 | BARRANGOU R, GERSBACH C A. Expanding the CRISPR toolbox: targeting RNA with Cas13b[J]. Molecular Cell, 2017, 65:582-584. |
| 44 | HARRINGTON L B, BURSTEIN D, CHEN J S, et al. Programmed DNA destruction by miniature CRISPR-Cas14 enzymes[J]. Science, 2018, 362(6416):839-842. |
| 45 | QI L S, LARSON M H, GILBERT L A, et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression[J]. Cell, 2013, 152(5):1173-1183. |
| 46 | KOMER A C, KIM Y B, PACKER M S, et al. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage[J]. Nature, 2016, 533(7603):420-424. |
| 47 | GAUDELLI N M, KOMER A C, REES H A, et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage[J]. Nature, 2017, 551(7681):464-471. |
| 48 | GRUNEWALD J, ZHOU R, GARCIA S P, et al. Transcriptome-wide off-target RNA editing induced by CRISPR-guided DNA base editors[J]. Nature, 2019, 569(7756):433-437. |
| 49 | JIN S, ZONG Y, GAO Q, et al. Cytosine, but not adenine, base editors induce genome-wide off-target mutations in rice[J]. Science, 2019, 364(6437):292-295. |
| 50 | ZHOU C, SUN Y, YAN R, et al. Off-target RNA mutation induced by DNA base editing and its elimination by mutagenesis[J]. Nature, 2019, 571(7764):275-278. |
| 51 | ZUO E, SUN Y, WEI W, et al. Cytosine base editor generates substantial off-target single-nucleotide variants in mouse embryos[J]. Science, 2019, 364(6437):289-292. |
| 52 | ANZALONE A V, RANDOLPH P B, DAVIS J R, et al. Search-and-replace genome editing without double-strand breaks or donor DNA[J]. Nature, 2019, 576(7758):149-157. |
| 53 | WALTON R T, CHRISTIE K A, WHITTAKER M N, et al. Unconstrained genome targeting with near-PAMless engineered CRISPR-Cas9 variants[J]. Science, 2020, 368(6488): 290-296. |
| 54 | BEAUCAGE S, CARUTHERS M. Deoxynucleoside phosphoramidites—a new class of key intermediates for deoxypolynucleotide synthesis[J]. Tetrahedron Letters, 1981, 22(20):1859-1862. |
| 55 | CARUTHERS M, BARONE A, BEAUCAGE S, et al. Chemical synthesis of deoxyoligonucleotides by the phosphoramidite method[J]. Method Enzymol, 1987, 154: 287-313. |
| 56 | LEPROUST E M, PECK B J, SPIRIN K, et al. Synthesis of high-quality libraries of long (150mer) oligonucleotides by a novel depurination controlled process[J]. Nucleic Acids Research, 2010, 38(8):2522-2540. |
| 57 | KOSURI S, CHURCH G M. Large-scale de novo DNA synthesis: technologies and applications[J]. Nature Methods, 2014, 11(5):499-507. |
| 58 | BOLLUM F J. Oligodeoxyribonucleotide-primed reactions catalyzed by calf thymus polymerase[J]. Journal of Biological Chemistry, 1962, 237(6):1945-1949. |
| 59 | JENSEN M A, DAVIS R W. Template-independent Enzymatic Oligonucleotide Synthesis (TiEOS): its history, prospects, and challenges[J]. Biochemistry, 2018, 57(12):1821-1832. |
| 60 | PALLUK S, ARLOW D H, DE ROND T, et al. De novo DNA synthesis using polymerase-nucleotide conjugates[J]. Nature Biotechnology, 2018, 36(7):645. |
| 61 | SMITH H O, C A 3rd HUTCHISON, PFANNKOCH C, et al. Generating a synthetic genome by whole genome assembly: phiX174 bacteriophage from synthetic oligonucleotides[J]. Proceedings of the National Academy of Sciences of the United States of America, 2003, 100(26):15440-15445. |
| 62 | ENGLER C, KANDZIA R, MARILLONNET S. A one pot, one step, precision cloning method with high throughput capability[J]. PLoS One, 2008, 3(11):e3647. |
| 63 | GIBSON D G, YOUNG L, CHUANG R Y, et al. Enzymatic assembly of DNA molecules up to several hundred kilobases[J]. Nature Methods, 2009, 6(5):343-345. |
| 64 | GIDSON D G, BENDERS G A, ANDERWS-PFANNKOCH C, et al. Complete chemical synthesis, assembly, and cloning of a Mycoplasma genitalium genome[J]. Science, 2008, 319(5867):1215-1220. |
| 65 | GIBSON D G, GLASS J I, LARTIGUE C, et al. Creation of a bacterial cell controlled by a chemically synthesized genome[J]. Science, 2010, 329(5987):52-56. |
| 66 | C A 3rd HUTCHISON, CHUANG R Y, NOSKOV V N, et al. Design and synthesis of a minimal bacterial genome[J]. Science, 2016, 351(6280):aad6253. |
| 67 | FRASER C M, GOCAYNE J D, WHITE O, et al. The minimal gene complement of Mycoplasma genitalium [J]. Science, 1995, 270(5235):397-403. |
| 68 | HUTCHISON C A, PETERSON S N, GILL S R, et al. Global transposon mutagenesis and a minimal Mycoplasma genome[J]. Science, 1999, 286(5447):2165-2169. |
| 69 | GIBSON D G, BENDERS G A, AXELROD K C, et al. One-step assembly in yeast of 25 overlapping DNA fragments to form a complete synthetic Mycoplasma genitalium genome[J]. Proceedings of the National Academy of Sciences, 2008, 105(51):20404-20409. |
| 70 | LARTIGUE C, VASHEE S, ALGIRE M A, et al. Creating bacterial strains from genomes that have been cloned and engineered in yeast[J]. Science, 2009, 325(5948):1693-1696. |
| 71 | FREDENS J, WANG K, DE LA TORRE D, et al. Total synthesis of Escherichia coli with a recoded genome[J]. Nature, 2019, 569(7757):514-518. |
| 72 | DYMOND J S, RICHARDSON S M, COOMDES C E, et al. Synthetic chromosome arms function in yeast and generate phenotypic diversity by design[J]. Nature, 2011, 477(7365):471-476. |
| 73 | ANNALURU N, MULLER H, MITCHELL LA, et al. Total synthesis of a functional designer eukaryotic chromosome[J]. Science, 2014, 344(6179):55-58. |
| 74 | MITCHELL L A, WANG A, STRACQUADANIO G, et al. Synthesis, debugging, and effects of synthetic chromosome consolidation: synVI and beyond[J]. Science, 2017, 355(6322):aaf4831. |
| 75 | RICHARDSON S M, MITCHELL L A, STRACQUADANIO G, et al. Design of a synthetic yeast genome[J]. Science, 2017, 355(6329):1040-1044. |
| 76 | SHEN Y, WANG Y, CHEN T, et al. Deep functional analysis of synII, a 770-kilobase synthetic yeast chromosome[J]. Science, 2017, 355(6322):aaf4791. |
| 77 | WU Y, LI B Z, ZHAO M, et al. Bug mapping and fitness testing of chemically synthesized chromosome X[J]. Science, 2017, 355(6322):aaf4706. |
| 78 | XIE Z X, LI B Z, MITCHELL L A, et al. "Perfect" designer chromosome V and behavior of a ring derivative[J]. Science, 2017, 355(6322):aaf4704. |
| 79 | ZHANG W, ZHAO G, LUO Z, et al. Engineering the ribosomal DNA in a megabase synthetic chromosome[J]. Science, 2017, 355(6322):aaf3981. |
| 80 | LUO Z, YANG Q, GENG B, et al. Whole genome engineering by synthesis[J]. Science China Life Sciences, 2018, 61(12):1515-1527. |
| 81 | BOEKE J D, CHURCH G, HESSEL A, et al. Genome Engineering. The Genome Project-Write[J]. Science, 2016, 353(6295):126-127. |
| 82 | MERCY G, MOZZICONACCI J, SCOLARI V F, et al. 3D organization of synthetic and scrambled chromosomes[J]. Science, 2017, 355(6322):aaf4597. |
| 83 | SHEN Y, STRACQUADANIO G, WANG Y, et al. SCRaMbLE generates designed combinatorial stochastic diversity in synthetic chromosomes[J]. Genome Research, 2016, 26(1):36-49. |
| 84 | BLOUNT B A, GOWERS G F, HO J C H, et al. Rapid host strain improvement by in vivo rearrangement of a synthetic yeast chromosome[J]. Nature Communications, 2018, 9(1):1932. |
| 85 | HOCHREIN L, MITCHELL L A, SCHULZ K, et al. L-SCRaMbLE as a tool for light-controlled Cre-mediated recombination in yeast[J]. Nature Communications, 2018, 9(1):1931. |
| 86 | JIA B, WU Y, LI B Z, et al. Precise control of SCRaMbLE in synthetic haploid and diploid yeast[J]. Nature Communications, 2018, 9(1):1933. |
| 87 | LIU W, LUO Z, WANG Y, et al. Rapid pathway prototyping and engineering using in vitro and in vivo synthetic genome SCRaMbLE-in methods[J]. Nature Communications, 2018, 9(1):1936. |
| 88 | LUO Z, WANG L, WANG Y, et al. Identifying and characterizing SCRaMbLEd synthetic yeast using ReSCuES[J]. Nature Communications, 2018, 9(1):1930. |
| 89 | SHEN M J, WU Y, YANG K, et al. Heterozygous diploid and interspecies SCRaMbLEing[J]. Nature Communications, 2018, 9(1):1934. |
| 90 | WANG J, XIE Z X, MA Y, et al. Ring synthetic chromosome V SCRaMbLE[J]. Nature Communications, 2018, 9(1): 3783. |
| 91 | WU Y, ZHU R Y, MITCHELL L A, et al. In vitro DNA SCRaMbLE[J]. Nature Communications, 2018, 9(1):1935. |
| 92 | GOWERS G F, CHEE S M, BELL D, et al. Improved betulinic acid biosynthesis using synthetic yeast chromosome recombination and semi-automated rapid LC-MS screening[J]. Nature Communications, 2020, 11(1):868. |
| 93 | GARAJ S, HUBBARD W, REINA A, et al. Graphene as a subnanometre trans-electrode membrane[J]. Nature, 2010, 467:190-193. |
| 94 | GOTO Y, AKAHORI R, YANAGI I, et al. Solid-state nanopores towards single-molecule DNA sequencing[J]. Journal of Human Genetics, 2020, 65(1):69-77. |
| 95 | KOLMOGOROV M, KENNEDY E, DONG Z X, et al. Single-molecule protein identification by sub-nanopore sensors[J]. PLoS Computational Biology, 2017, 13(5):e1005356. |
| 96 | MASCHER M, GUNDLACH H, HIMMELBACH A, et al. A chromosome conformation capture ordered sequence of the barley genome[J]. Nature, 2017, 544(7651):427-433. |
| 97 | SEO J S, RHIE A, KIM J, et al. De novo assembly and phasing of a Korean human genome[J]. Nature, 2016, 538(7624):243-247. |
| 98 | CAMPA C C, WEISBACH N R, SANTINHA A J, et al. Multiplexed genome engineering by Cas12a and CRISPR arrays encoded on single transcripts[J]. Nature Methods, 2019, 16(9):887-893. |
| 99 | ISAACS F J, CARR P A, WANG H H, et al. Precise manipulation of chromosomes in vivo enables genome-wide codon replacement[J]. Science, 2011, 333(6040):348-353. |
| 100 | LAJOIE M J, ROVNER A J, GOODMAN D B, et al. Genomically recoded organisms expand biological functions[J]. Science, 2013, 342(6156):357-360. |
| 101 | PERKEL J M. The race for enzymatic DNA synthesis heats up[J]. Nature, 2019, 566(7745):565. |
| 102 | JUHAS M, AJIOKA J W. High molecular weight DNA assembly in vivo for synthetic biology applications[J]. Critical Reviews in Biotechnology, 2017, 37(3):277-286. |
| 103 | OSTROV N, BEAL J, ELLIS T, et al. Technological challenges and milestones for writing genomes[J]. Science, 2019, 366(6463):310-312. |
| 104 | BROWN D M, CHAN Y A, DESAI P J, et al. Efficient size-independent chromosome delivery from yeast to cultured cell lines[J]. Nucleic Acids Research, 2017, 45(7):e50. |
| [1] | 高歌, 边旗, 王宝俊. 合成基因线路的工程化设计研究进展与展望[J]. 合成生物学, 2025, 6(1): 45-64. |
| [2] | 李冀渊, 吴国盛. 合成生物学视域下有机体的两种隐喻[J]. 合成生物学, 2025, 6(1): 190-202. |
| [3] | 焦洪涛, 齐蒙, 邵滨, 蒋劲松. DNA数据存储技术的法律治理议题[J]. 合成生物学, 2025, 6(1): 177-189. |
| [4] | 唐兴华, 陆钱能, 胡翌霖. 人类世中对合成生物学的哲学反思[J]. 合成生物学, 2025, 6(1): 203-212. |
| [5] | 徐怀胜, 石晓龙, 刘晓光, 徐苗苗. DNA存储的关键技术:编码、纠错、随机访问与安全性[J]. 合成生物学, 2025, 6(1): 157-176. |
| [6] | 石婷, 宋展, 宋世怡, 张以恒. 体外生物转化(ivBT):生物制造的新前沿[J]. 合成生物学, 2024, 5(6): 1437-1460. |
| [7] | 柴猛, 王风清, 魏东芝. 综合利用木质纤维素生物转化合成有机酸[J]. 合成生物学, 2024, 5(6): 1242-1263. |
| [8] | 邵明威, 孙思勉, 杨时茂, 陈国强. 基于极端微生物的生物制造[J]. 合成生物学, 2024, 5(6): 1419-1436. |
| [9] | 陈雨, 张康, 邱以婧, 程彩云, 殷晶晶, 宋天顺, 谢婧婧. 微生物电合成技术转化二氧化碳研究进展[J]. 合成生物学, 2024, 5(5): 1142-1168. |
| [10] | 郑皓天, 李朝风, 刘良叙, 王嘉伟, 李恒润, 倪俊. 负碳人工光合群落的设计、优化与应用[J]. 合成生物学, 2024, 5(5): 1189-1210. |
| [11] | 夏孔晨, 徐维华, 吴起. 光酶催化混乱性反应的研究进展[J]. 合成生物学, 2024, 5(5): 997-1020. |
| [12] | 陈子苓, 向阳飞. 类器官技术与合成生物学协同研究进展[J]. 合成生物学, 2024, 5(4): 795-812. |
| [13] | 蔡冰玉, 谭象天, 李伟. 合成生物学在干细胞工程化改造中的研究进展[J]. 合成生物学, 2024, 5(4): 782-794. |
| [14] | 谢皇, 郑义蕾, 苏依婷, 阮静怡, 李永泉. 放线菌聚酮类化合物生物合成体系重构研究进展[J]. 合成生物学, 2024, 5(3): 612-630. |
| [15] | 查文龙, 卜兰, 訾佳辰. 中药药效成分群的合成生物学研究进展[J]. 合成生物学, 2024, 5(3): 631-657. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||