合成生物学 ›› 2024, Vol. 5 ›› Issue (6): 1437-1460.DOI: 10.12211/2096-8280.2024-004
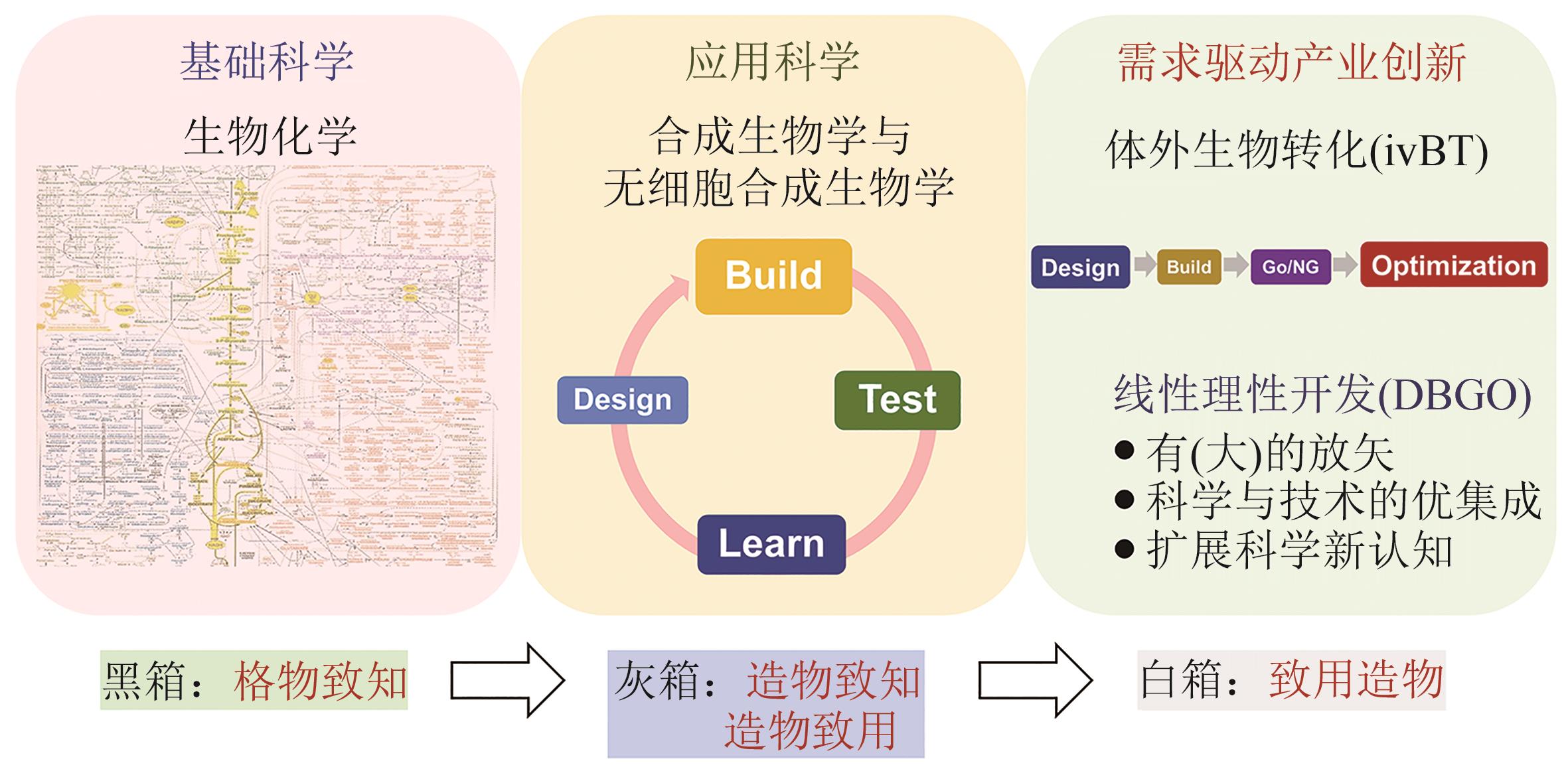
体外生物转化(ivBT):生物制造的新前沿
石婷1,2,3, 宋展1,2,3,4, 宋世怡1,2,5, 张以恒1,2,3
- 1.中国科学院天津工业生物技术研究所低碳合成工程生物学(全国)重点实验室,天津 300308
2.中国科学院天津工业生物技术研究所体外合成生物学中心,天津 300308
3.合成生物学海河实验室,天津 300308
4.上海交通大学生命科学技术学院,微生物代谢国家重点实验室,上海 200240
5.华东理工大学生物反应器工程国家重点实验室,上海 200237
-
收稿日期:2024-01-07修回日期:2024-03-11出版日期:2024-12-31发布日期:2025-01-10 -
通讯作者:张以恒 -
作者简介:石婷 (1984—),女,博士,副研究员。研究方向为体外合成生物学、酶工程与微生物代谢工程。 E-mail:shi_ting@tib.cas.cn宋展 (1996—),女,博士研究生。研究方向为体外合成生物学、酶工程和代谢工程。 E-mail:song_zhan@sjtu.edu.cn张以恒 (1971—),男,博士,研究员,中国科学院天津工业生物技术研究所低碳合成工程生物学(全国)重点实验室主任,曾任美国弗吉尼亚理工大学终身正教授。研究方向为体外合成生物学、新质生物制造、生物炼制和淀粉储能。E-mail:zhang_xw@tib.cas.cn -
基金资助:国家重点研发计划(2022YFA0912300);国家自然科学基金面上项目(NSFC32271544);合成生物学海河实验室颠覆性创新项目(22HHSWSS000155);天津市合成生物技术创新能力提升行动项目(TSBICIP-CXRC-067)
In vitro BioTransformation (ivBT): a new frontier of industrial biomanufacturing
SHI Ting1,2,3, SONG Zhan1,2,3,4, SONG Shiyi1,2,5, ZHANG Yi-Heng P. Job1,2,3
- 1.Key Laboratory of Engineering Biology for Low-Carbon Manufacturing,Tianjin Institute of Industrial Biotechnology,Chinese Academy of Sciences,Tianjin 300308,China
2.In Vitro Synthetic Biology Center,Tianjin Institute of Industrial Biotechnology,Chinese Academy of Sciences,Tianjin 300308,China
3.Haihe Laboratory of Synthetic Biology,Tianjin 300308,China
4.State Key Laboratory of Microbial Metabolism,School of Life Sciences and Biotechnology,Shanghai JiaoTong University,Shanghai 200240,China
5.State Key Laboratory of Bioreactor Engineering,East China University of Science and Technology,Shanghai 200237,China
-
Received:2024-01-07Revised:2024-03-11Online:2024-12-31Published:2025-01-10 -
Contact:ZHANG Yi-Heng P. Job
摘要:
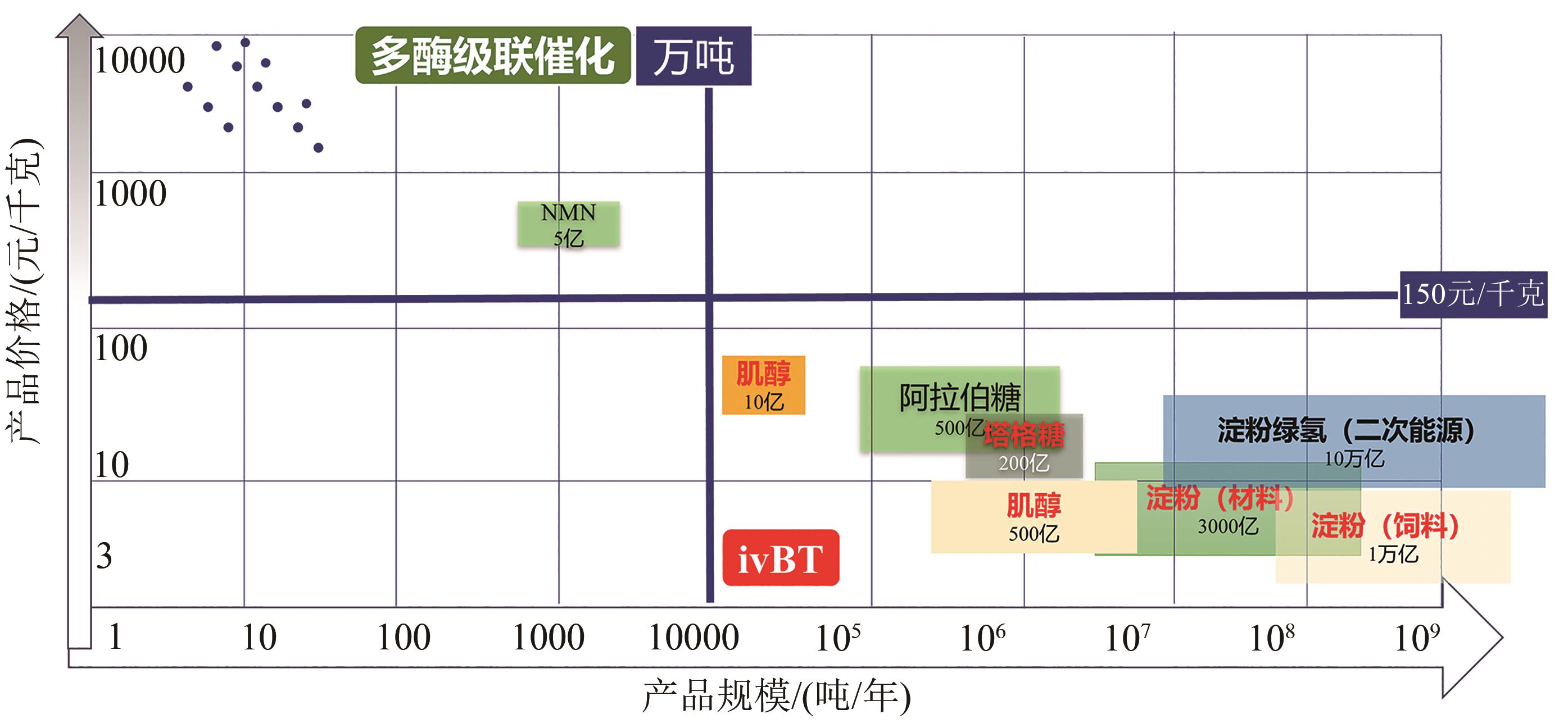
人类社会的重大挑战(如粮食安全、能源安全、气候变化与双碳目标等)驱动全社会寻求创新型技术解决方案。体外生物转化(in vitro biotransformation,ivBT)是介于微生物发酵与酶催化之间的新质生物制造平台,多酶分子机器是其超限生物催化剂。它基于大道至简原则,利用多个天然酶、人工酶以及(仿生/天然)辅酶等重构生化途径,摆脱生物体生存局限(如细胞复制、基础代谢、复杂调控和能量供给等),超越细胞合成极限,实现重要生物转化与超限能量转换,尤其是生产低值大宗产品与新能源产品等。工业生物制造的三个平台技术分别是基于细胞工厂的发酵、基于酶分子的生物催化与基于多酶分子机器的ivBT。本综述对ivBT给出明确定义,阐明其多酶途径设计原则与产业化技术研发路径,比较该平台与现有生物制造平台相似性与不同点,介绍多个代表性案例,以及讨论其未来的机会与挑战。ivBT技术发展采用设计-构建-判决-优化的线性策略,开发能够满足国家需求的超高效多酶分子机器。利用ivBT有望形成超过30万亿元生物产品的工业生物制造,助力实现人类社会的多项重要需求,如粮食安全、新型能源体系等。人造淀粉不仅可以帮助中国端牢粮食饭碗,而且将是一个全新且安全的高密度储氢载体(比压缩氢气高2.5倍)与高能储电介质(比锂电池高10倍)。
中图分类号:
引用本文
石婷, 宋展, 宋世怡, 张以恒. 体外生物转化(ivBT):生物制造的新前沿[J]. 合成生物学, 2024, 5(6): 1437-1460.
SHI Ting, SONG Zhan, SONG Shiyi, ZHANG Yi-Heng P. Job. In vitro BioTransformation (ivBT): a new frontier of industrial biomanufacturing[J]. Synthetic Biology Journal, 2024, 5(6): 1437-1460.
| 生物制造平台 | 催化剂 | 标志性产品 | 产品种类 | 产品规模 | 浓度(Titer) | 得率(Yield) | 速率(Rate) | 生物安全 | 技术壁垒 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 微生物发酵(Fermentation) | 细胞 工厂 | 初级代谢产物 | 次级代谢产物 | 生物大分子 | 微生物蛋白 | 极多 | 小、中、大 | 低 | 较低 | 低 | 有挑战、强监管 | 低 |
| 酶催化(Biocatalysis) | 酶分子、级联多酶 | 果葡 糖浆 | 生物 质糖 | 医药 原料 | NMN | 较多: 优选水解、异构、手性合成等方式合成产品 | 小 | 高 | 高 | 最高 | 好 | 高 |
| 体外生物转化 | 多酶分子机器 | 肌醇、塔格糖 | 合成 淀粉 | 糖水制绿氢 | 糖酶燃料电池 | 较少:优选异构、糖苷键重排、分解与合成代谢等方式合成产品 | 超大(如能源、粮食) | 高 | 高 | 高 | 最好 | 最高 |
表1 生物制造平台的比较
Table 1 Comparison of biomanufacturing platforms
| 生物制造平台 | 催化剂 | 标志性产品 | 产品种类 | 产品规模 | 浓度(Titer) | 得率(Yield) | 速率(Rate) | 生物安全 | 技术壁垒 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 微生物发酵(Fermentation) | 细胞 工厂 | 初级代谢产物 | 次级代谢产物 | 生物大分子 | 微生物蛋白 | 极多 | 小、中、大 | 低 | 较低 | 低 | 有挑战、强监管 | 低 |
| 酶催化(Biocatalysis) | 酶分子、级联多酶 | 果葡 糖浆 | 生物 质糖 | 医药 原料 | NMN | 较多: 优选水解、异构、手性合成等方式合成产品 | 小 | 高 | 高 | 最高 | 好 | 高 |
| 体外生物转化 | 多酶分子机器 | 肌醇、塔格糖 | 合成 淀粉 | 糖水制绿氢 | 糖酶燃料电池 | 较少:优选异构、糖苷键重排、分解与合成代谢等方式合成产品 | 超大(如能源、粮食) | 高 | 高 | 高 | 最好 | 最高 |
| 区分项目 | 体外生物转化 (ivBT) | 多酶级联催化 (CEB) | 无细胞蛋白质合成 (CFPS) |
|---|---|---|---|
| 目标 | 大规模生物制造 产品规模:>1万吨,甚至10亿吨级 | 精细生物制造 精细产品:约1000千克级,<100吨 | 研究工具 特殊制造(快速生产克级蛋白质) |
| 代表性产品 | 粮食、能源、材料 (如淀粉、绿氢、肌醇、塔格糖) | 药物中间体(NMN) | 疫苗合成 |
| 产品市场规模 (每个产品) | 5亿(最小市场) →100亿(塔格糖) →10万亿(绿氢) | 千万(最大市场低于5亿) | NA |
| 目标产品数目 | 约100(粮食、能源等大宗产品) | 约10 000(精细化学品) | NA |
| 原料成本/ 产品价格 | >50%→90%(最大) | 5%→20% | NA |
| 合成途径设计 | 非天然途径与人造电子传递链 | 主反应与辅酶再生,利用部分天然途径 | 利用天然合成途径 |
| 催化元件 | 天然(超稳)酶、人工酶、固定化多酶、(仿生)辅酶再生 | 天然(常温)酶、改造酶、固定化酶、辅酶再生 | 细胞裂解液或纯化元件、外加氨基酸、ATP 供体、DNA 模板 |
| 元件需求 | 超低成本酶、超稳定固定化酶、价廉且稳定(仿生)辅酶 | 酶成本不敏感,利用天然辅酶 | 利用细胞裂解液中的有效成分 |
| 生产周期 | 天、周、月(多次,连续) | 时、天(一次,极少多次) | 时(一次) |
表2 ivBT 与相似技术的区分
Table 2 Comparison of ivBT with similar biotechnologies
| 区分项目 | 体外生物转化 (ivBT) | 多酶级联催化 (CEB) | 无细胞蛋白质合成 (CFPS) |
|---|---|---|---|
| 目标 | 大规模生物制造 产品规模:>1万吨,甚至10亿吨级 | 精细生物制造 精细产品:约1000千克级,<100吨 | 研究工具 特殊制造(快速生产克级蛋白质) |
| 代表性产品 | 粮食、能源、材料 (如淀粉、绿氢、肌醇、塔格糖) | 药物中间体(NMN) | 疫苗合成 |
| 产品市场规模 (每个产品) | 5亿(最小市场) →100亿(塔格糖) →10万亿(绿氢) | 千万(最大市场低于5亿) | NA |
| 目标产品数目 | 约100(粮食、能源等大宗产品) | 约10 000(精细化学品) | NA |
| 原料成本/ 产品价格 | >50%→90%(最大) | 5%→20% | NA |
| 合成途径设计 | 非天然途径与人造电子传递链 | 主反应与辅酶再生,利用部分天然途径 | 利用天然合成途径 |
| 催化元件 | 天然(超稳)酶、人工酶、固定化多酶、(仿生)辅酶再生 | 天然(常温)酶、改造酶、固定化酶、辅酶再生 | 细胞裂解液或纯化元件、外加氨基酸、ATP 供体、DNA 模板 |
| 元件需求 | 超低成本酶、超稳定固定化酶、价廉且稳定(仿生)辅酶 | 酶成本不敏感,利用天然辅酶 | 利用细胞裂解液中的有效成分 |
| 生产周期 | 天、周、月(多次,连续) | 时、天(一次,极少多次) | 时(一次) |
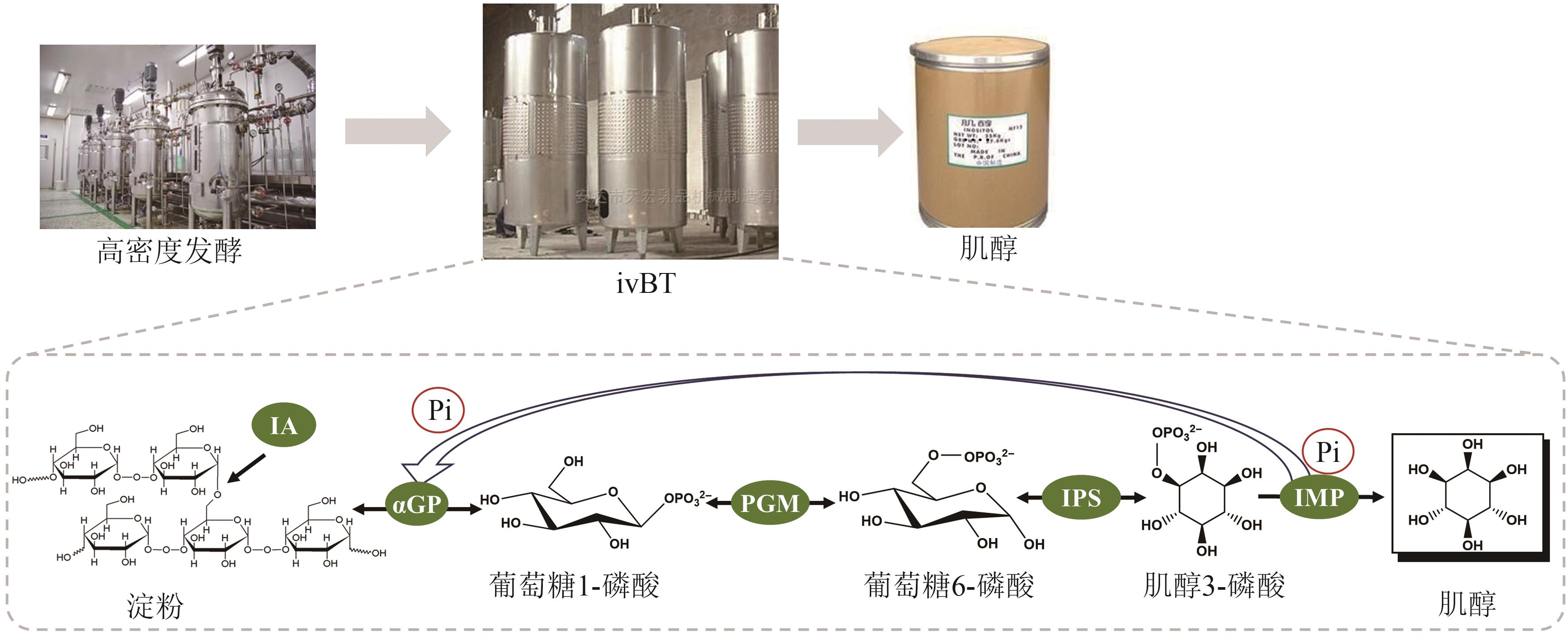
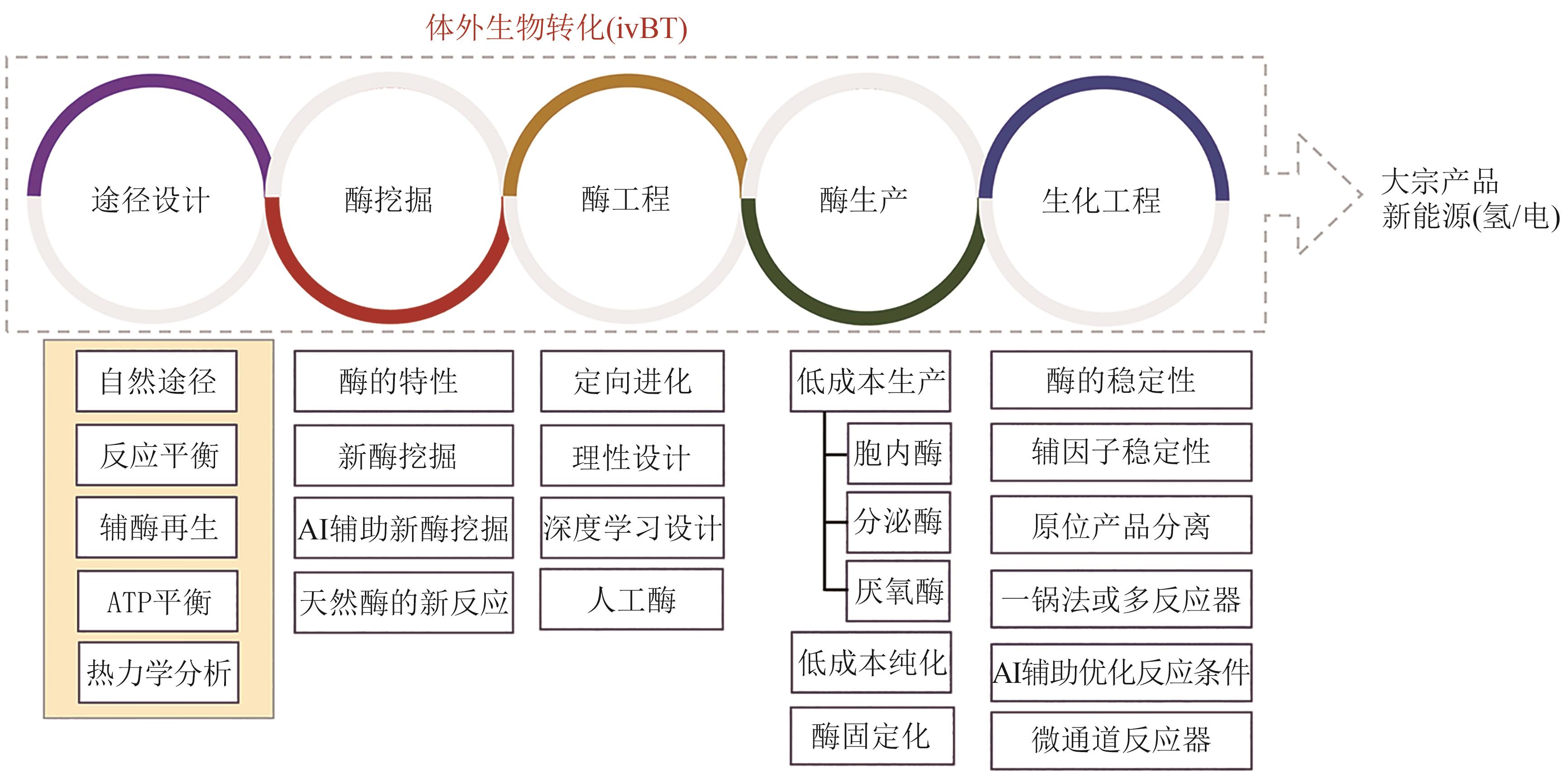
图4 ivBT 的体外肌醇合成途径αGP—α-葡聚糖磷酸化酶;PGM—葡萄糖 6-磷酸异构酶;IPS—肌醇 3-磷酸合成酶;IMP—肌醇单磷酸磷酸酶;IA—异淀粉酶
Fig. 4 Inositol synthesis pathway of ivBTαGP—α-glucan phosphorylase; PGM—phosphoglucomutase; IPS—inositol 3-phosphate synthase; IMP—inositol monophosphatase; IA—isoamylase
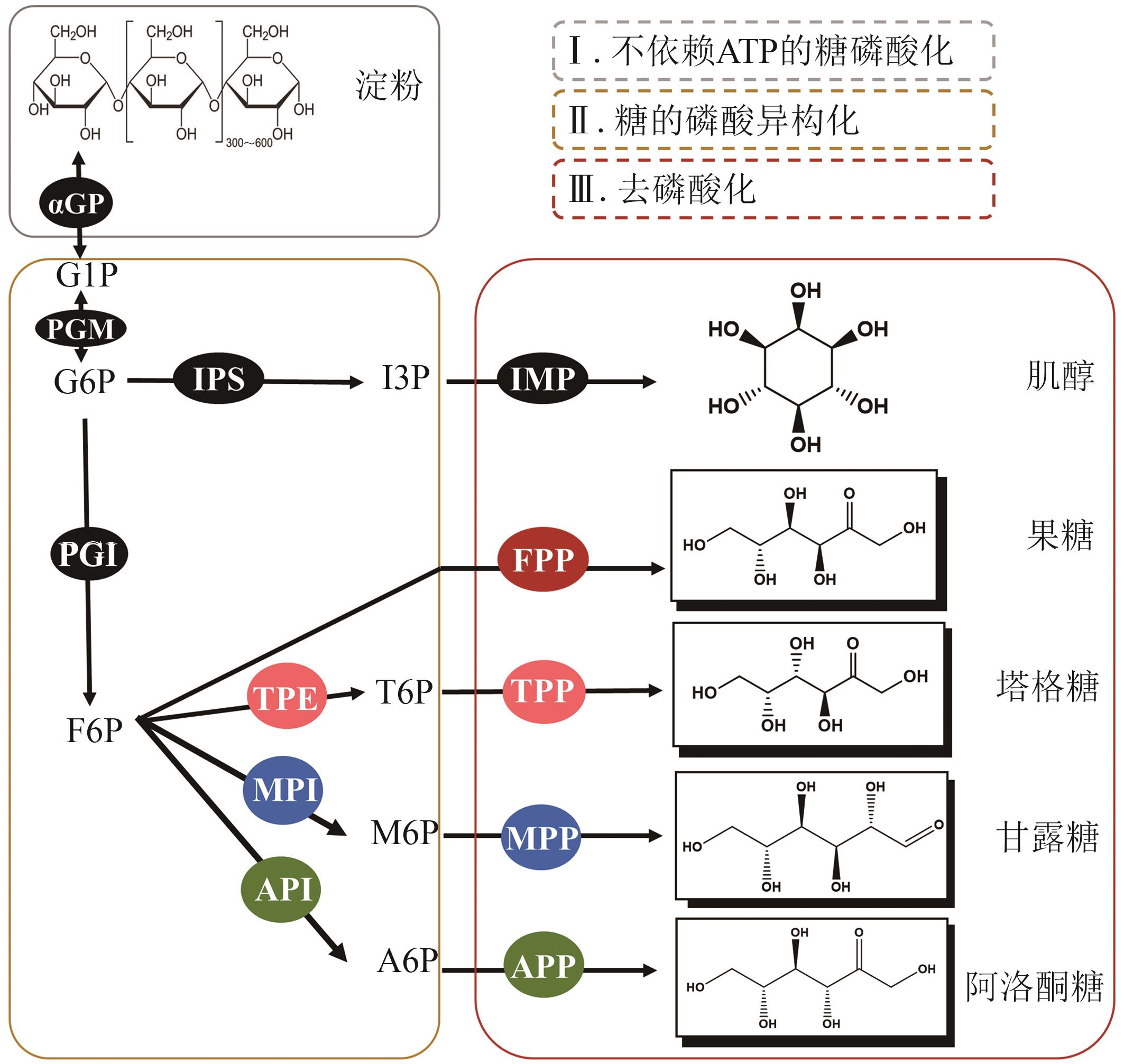
图6 ivBT 利用淀粉合成健康糖的人工合成途径(αGP、PGM、IPS、IMP 同肌醇合成途径)PGI—葡萄糖 6-磷酸异构酶;FPP—果糖 6-磷酸磷酸酶;TPE—塔格糖 6-磷酸 4-差向异构酶;TPP—塔格糖 6-磷酸磷酸酶;MPI—甘露糖 6-磷酸异构酶;MPP—甘露糖 6-磷酸磷酸酶;API—阿洛酮糖 6-磷酸异构酶;APP—阿洛酮糖 6-磷酸磷酸酶
Fig. 6 Rare sugars artificial synthesis pathway of ivBT(Enzymes of αGP, PGM, IPS, IMP are the same as inositol synthesis pathway.)PGI—phosphoglucose isomerase; FPP—fructose 6-phosphatase; TPE—tagatose 6-phosphate 4-epimerase; TPP—tagatose 6-phosphatase; MPI—mannose 6-phosphate isomerase; MPP—mannose 6-phosphatase; API—allulose 6-phosphate isomerase; APP—allulose 6-phosphatase

图7 ivBT 的体外纤维素合成淀粉途径(EG—内切葡聚糖酶;CBH—纤维二糖水解酶;CBP—纤维二糖磷酸化酶;PGP—马铃薯 α-葡聚糖磷酸化酶)
Fig. 7 Cellulose-amylose synthesis pathway of ivBT(EG—endoglucanase; CBH—cellobiohydrolase; CBP—cellobiose phosphorylase; PGP—potato α-glucan phosphorylase)
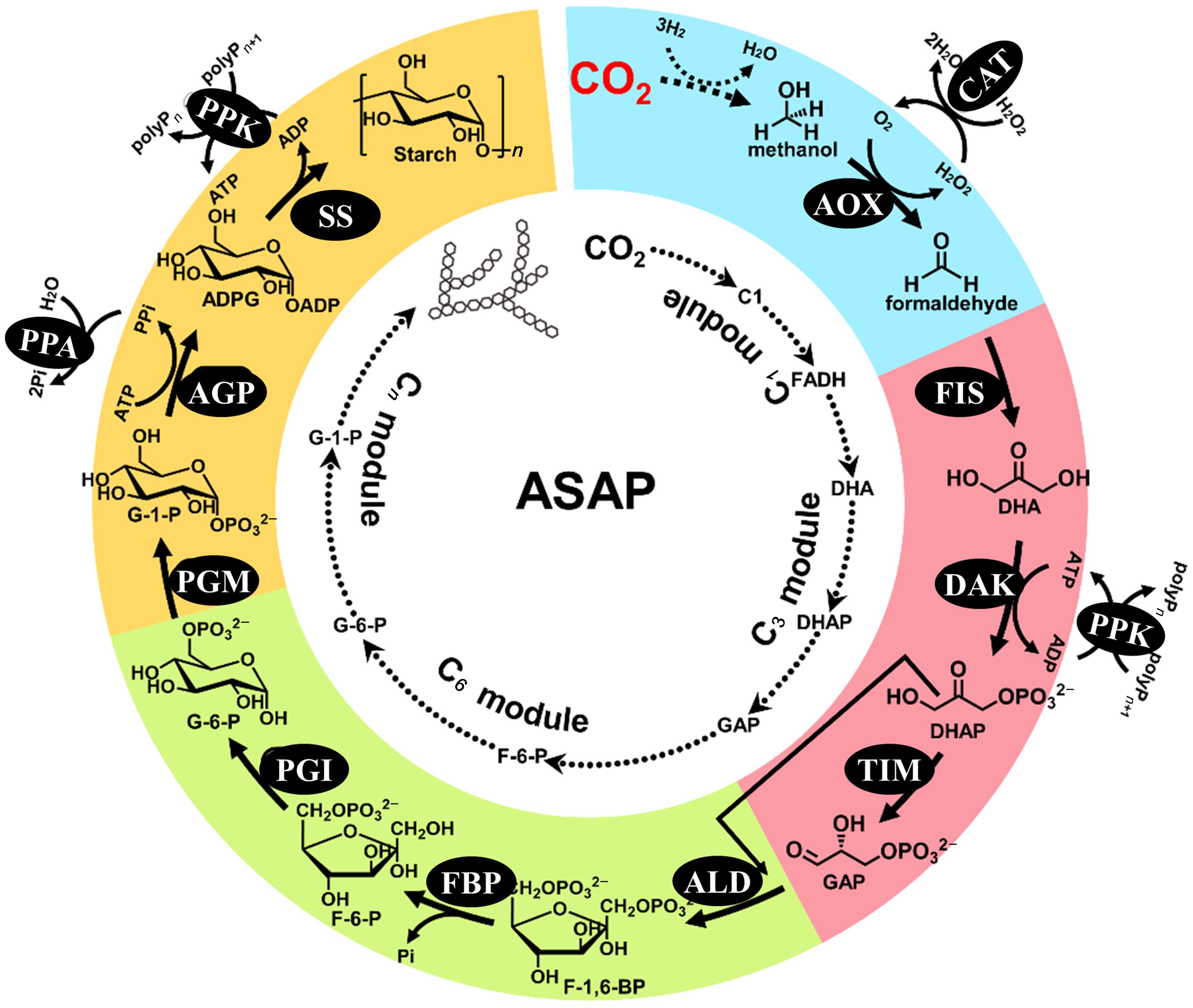
图8 ivBT 的体外 CO2 合成淀粉途径[96]AOX—醇氧化酶;FIS—甲醛酶;DAK—二羟基丙酮激酶;TIM—磷酸甘油醛异构酶;ALD—果糖 1,6-二磷酸醛缩酶;FBP—果糖 1,6-二磷酸酶;PGI—葡萄糖 6-磷酸异构酶;PGM—葡萄糖6-磷酸变位酶;AGP—ADP-葡萄糖焦磷酸化酶;SS—淀粉合成酶;CAT—过氧化氢酶;PPK—多聚磷酸激酶;PPA—焦磷酸酶
Fig. 8 CO2-Starch synthesis pathway of ivBT[96]AOX—alcohol oxidase; FIS—formolase; DAK—dihydroxyacetone kinase; TIM—triose phosphate isomerase; ALD—fructose-bisphosphate aldolase; FBP—fructose bisphosphatase; PGI—phosphoglucose isomerase; PGM—phosphoglucomutase; AGP—ADP-glucose pyrophosphorylase; SS—starch synthase; CAT—catalase; PPK—polyphosphate kinase; PPA—pyrophosphatase
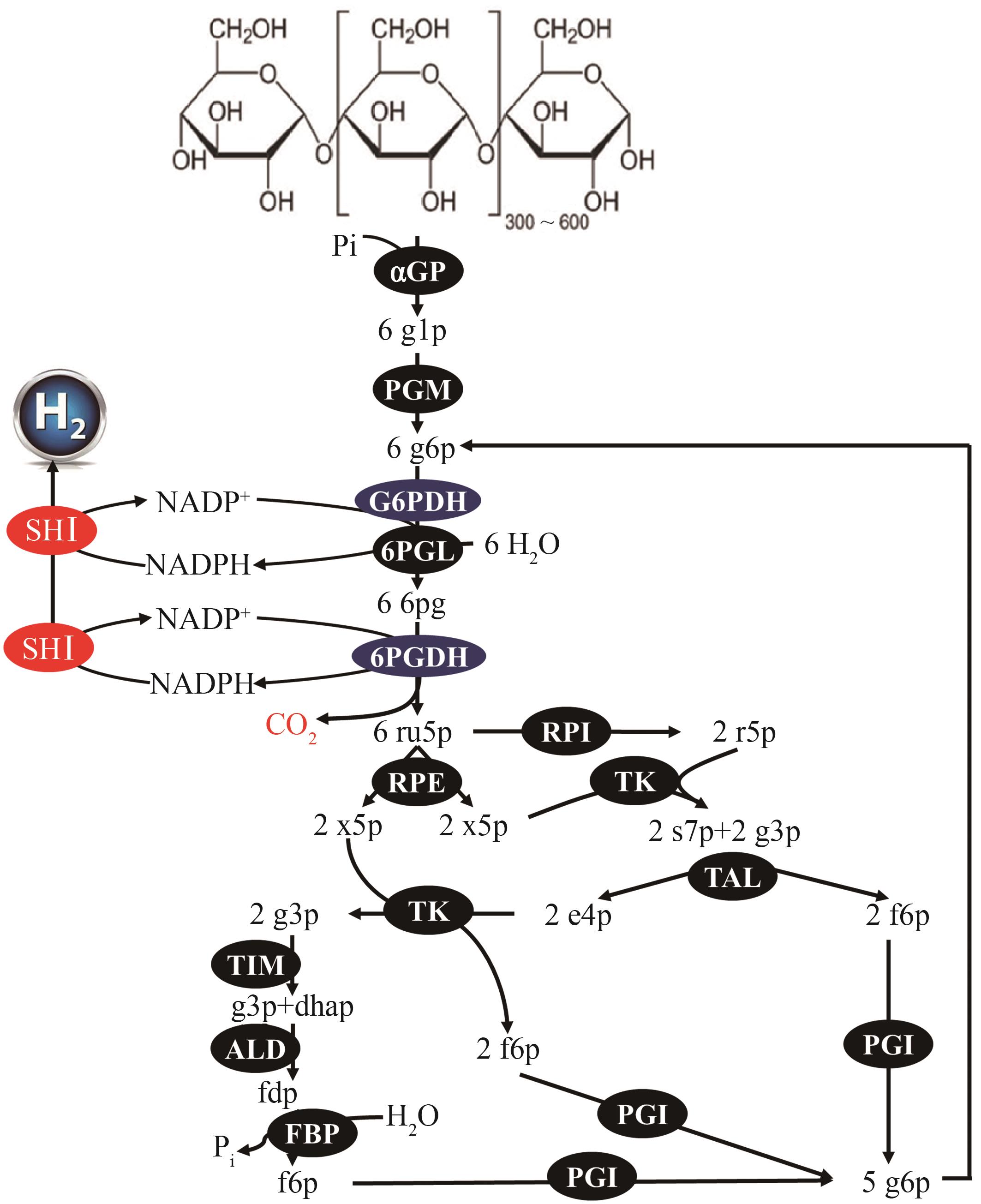
图9 淀粉体外合成氢气途径αGP 和 PGM 同肌醇合成途径。G6PDH—葡萄糖 6-磷酸脱氢酶;6PGL—6-磷酸葡萄糖内酯酶;6PGDH—6-磷酸葡萄糖酸脱氢酶;RPI—核糖 5-磷酸异构酶;RPE—核酮糖 5-磷酸 3-差向异构酶;TK—转酮酶;TAL—转醛酶;TIM—磷酸甘油醛异构酶;ALD—果糖1,6-二磷酸醛缩酶;FBP—果糖1,6-二磷酸酶;PGI—葡萄糖6-磷酸异构酶;SHⅠ—氢酶。代谢物:g1p—葡萄糖 1-磷酸;g6p—葡萄糖 6-磷酸;6pg—6-磷酸葡萄糖酸酯;ru5p—核酮糖 5-磷酸;x5p—木糖 5-磷酸;r5p—核糖 5-磷酸;s7p—景天庚酮糖 7-磷酸;g3p—甘油醛 3-磷酸;e4p—赤藓糖 4-磷酸;dhap—磷酸二羟丙酮;fdp—果糖1,6-二磷酸;f6p—果糖 6-磷酸
Fig. 9 Hydrogen synthesis pathway of ivBTEnzymes of αGP and PGM are the same as inositol synthesis pathway. G6PDH—glucose 6-phosphate dehydrogenase; 6PGL—6-phosphogluconolactonase; 6PGDH—6-phosphogluconate dehydrogenase; RPI—ribose 5-phosphate isomerase; RPE—ribulose 5-phosphate 3-epimerase; TK—transketolase; TAL—transaldolase; TIM—triose phosphate isomerase; ALD—fructose-bisphosphate aldolase; FBP—fructosebisphosphatase; PGI—phosphoglucose isomerase; SHⅠ—soluble hydrogenaseⅠ. The metabolites are: g1p—glucose 1-phosphate; g6p—glucose 6-phosphate; 6pg—6-phospho-D-gluconolactone; ru5p—ribulose 5-phosphate; x5p—xylulose 5-phosphate; r5p—ribose 5-phosphate; s7p—sedoheptulose 7-phosphate; g3p—glyceraldehyde 3-phosphate; e4p—erythrose 4-phosphate; dhap—dihydroxacetone phosphate; fdp—fructose 1,6-diphosphate; f6p—fructose 6-phosphate
| 比较内容 | 细胞工厂的局限 | 体外生物转化的优势 |
|---|---|---|
| 生长偶联 | 细胞生长繁殖与产品制造的耦合,存在有限资源竞争、分配与调控等难题 | 催化剂合成与产品制造时空分离,产品制造是唯一目标 |
| 产品得率 | 产品得率低(由于细胞繁殖、副产物生成等问题) | 近理论得率 |
| 能量限制 | 生物能量学限制,需ATP与还原力的净合成 | ATP 与还原力的平衡,不浪费生物能量 |
| 反应速率 | 反应速度(Rxn)低 | Rxn提升10倍以上 |
| 生物大分子合成 | 生物大分子不能通过细胞 | 没有细胞膜,大分子降解与合成耦合 |
| 特殊极性分子合成 | 特殊极性分子合成如磷酸糖不能过细胞膜 | 没有细胞膜,自由扩散 |
| 三传限制 | 细胞体内三传“动量传递、热量传递、质量传递”限制 | 超限制造(微通道反应器)超越传统“三传”限制 |
表3 ivBT 作为工业生物制造平台的技术优势
Table 3 Advantages of ivBT for biomanufacturing
| 比较内容 | 细胞工厂的局限 | 体外生物转化的优势 |
|---|---|---|
| 生长偶联 | 细胞生长繁殖与产品制造的耦合,存在有限资源竞争、分配与调控等难题 | 催化剂合成与产品制造时空分离,产品制造是唯一目标 |
| 产品得率 | 产品得率低(由于细胞繁殖、副产物生成等问题) | 近理论得率 |
| 能量限制 | 生物能量学限制,需ATP与还原力的净合成 | ATP 与还原力的平衡,不浪费生物能量 |
| 反应速率 | 反应速度(Rxn)低 | Rxn提升10倍以上 |
| 生物大分子合成 | 生物大分子不能通过细胞 | 没有细胞膜,大分子降解与合成耦合 |
| 特殊极性分子合成 | 特殊极性分子合成如磷酸糖不能过细胞膜 | 没有细胞膜,自由扩散 |
| 三传限制 | 细胞体内三传“动量传递、热量传递、质量传递”限制 | 超限制造(微通道反应器)超越传统“三传”限制 |
| 16 | HAN P P, WANG X Y, LI Y J, et al. Synthesis of a healthy sweetener D-tagatose from starch catalyzed by semiartificial cell factories[J]. Journal of Agricultural and Food Chemistry, 2023, 71(8): 3813-3820. |
| 17 | LI Y J, SHI T, HAN P P, et al. Thermodynamics-driven production of value-added D-allulose from inexpensive starch by an in vitro enzymatic synthetic biosystem[J]. ACS Catalysis, 2021, 11(9): 5088-5099. |
| 18 | YOU C, SHI T, LI Y J, et al. An in vitro synthetic biology platform for the industrial biomanufacturing of myo-inositol from starch[J]. Biotechnology and Bioengineering, 2017, 114(8): 1855-1864. |
| 19 | CHEN H G, ZHANG Y H P J. Enzymatic regeneration and conservation of ATP: challenges and opportunities[J]. Critical Reviews in Biotechnology, 2021, 41(1): 16-33. |
| 20 | SHI T, HAN P P, YOU C, et al. An in vitro synthetic biology platform for emerging industrial biomanufacturing: bottom-up pathway design[J]. Synthetic and Systems Biotechnology, 2018, 3(3): 186-195. |
| 21 | WICHMANN R, VASIC-RACKI D. Cofactor regeneration at the lab scale[J]. Advances in Biochemical Engineering/Biotechnology, 2005, 92: 225-260. |
| 22 | ZHU Z G, WANG Y R, MINTEER S D, et al. Maltodextrin-powered enzymatic fuel cell through a non-natural enzymatic pathway[J]. Journal of Power Sources, 2011, 196(18): 7505-7509. |
| 23 | NOWAK C, PICK A, LOMMES P, et al. Enzymatic reduction of nicotinamide biomimetic cofactors using an engineered glucose dehydrogenase: providing a regeneration system for artificial cofactors[J]. ACS Catalysis, 2017, 7(8): 5202-5208. |
| 24 | SONG Y H, LIU M X, XIE L P, et al. A recombinant 12-His tagged Pyrococcus furiosus soluble [NiFe]-hydrogenaseⅠoverexpressed in Thermococcus kodakarensis KOD1 facilitates hydrogen-powered in vitro NADH regeneration[J]. Biotechnology Journal, 2019, 14(4): e1800301. |
| 25 | ANNE A, BOURDILLON C, DANINOS S, et al. Can the combination of electrochemical regeneration of NAD+, selectivity of L-alpha-amino-acid dehydrogenase, and reductive amination of alpha-keto-acid be applied to the inversion of configuration of a L-alpha-amino-acid?[J]. Biotechnology and Bioengineering, 1999, 64(1): 101-107. |
| 26 | TISHKOV V I, POPOV V O. Protein engineering of formate dehydrogenase[J]. Biomolecular Engineering, 2006, 23(2/3): 89-110. |
| 27 | WANDREY C. Biochemical reaction engineering for redox reactions[J]. Chemical Record, 2004, 4(4): 254-265. |
| 28 | INOUE K, MAKINO Y, ITOH N. Purification and characterization of a novel alcohol dehydrogenase from Leifsonia sp. strain S749: a promising biocatalyst for an asymmetric hydrogen transfer bioreduction[J]. Applied and Environmental Microbiology, 2005, 71(7): 3633-3641. |
| 29 | JOHANNES T W, WOODYER R D, ZHAO H M. Directed evolution of a thermostable phosphite dehydrogenase for NAD(P)H regeneration[J]. Applied and Environmental Microbiology, 2005, 71(10): 5728-5734. |
| 30 | WANG Y R, ZHANG Y H P. Overexpression and simple purification of the Thermotoga maritima 6-phosphogluconate dehydrogenase in Escherichia coli and its application for NADPH regeneration[J]. Microbial Cell Factories, 2009, 8(1): 30. |
| 31 | WANG Y R, HUANG W D, SATHITSUKSANOH N, et al. Biohydrogenation from biomass sugar mediated by in vitro synthetic enzymatic pathways[J]. Chemistry & Biology, 2011, 18(3): 372-380. |
| 32 | HUANG H, PANDYA C, LIU C L, et al. Panoramic view of a superfamily of phosphatases through substrate profiling[J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(16): E1974-E1983. |
| 33 | VERHEES C H, AKERBOOM J, SCHILTZ E, et al. Molecular and biochemical characterization of a distinct type of fructose-1,6-bisphosphatase from Pyrococcus furiosus [J]. Journal of Bacteriology, 2002, 184(12): 3401-3405. |
| 34 | TIAN C Y, YANG J G, LIU C, et al. Engineering substrate specificity of HAD phosphatases and multienzyme systems development for the thermodynamic-driven manufacturing sugars[J]. Nature Communications, 2022, 13(1): 3582. |
| 35 | WANG W, LIU M X, YOU C, et al. ATP-free biosynthesis of a high-energy phosphate metabolite fructose 1,6-diphosphate by in vitro metabolic engineering[J]. Metabolic Engineering, 2017, 42: 168-174. |
| 36 | ZHOU W, YOU C, MA H W, et al. One-pot biosynthesis of high-concentration α-glucose 1-phosphate from starch by sequential addition of three hyperthermophilic enzymes[J]. Journal of Agricultural and Food Chemistry, 2016, 64(8): 1777-1783. |
| 37 | SRIVASTAVA D K, BERNHARD S A. Metabolite transfer via enzyme-enzyme complexes[J]. Science, 1986, 234(4780): 1081-1086. |
| 38 | YOU C, MYUNG S, ZHANG Y H P. Facilitated substrate channeling in a self-assembled trifunctional enzyme complex[J]. Angewandte Chemie International Edition, 2012, 51(35): 8787-8790. |
| 39 | ZHU Z G, SONG H Y, WANG Y M, et al. Protein engineering for electrochemical biosensors[J]. Current Opinion in Biotechnology, 2022, 76: 102751. |
| 40 | ZHOU W, HUANG R, ZHU Z G, et al. Coevolution of both thermostability and activity of polyphosphate glucokinase from Thermobifida fusca YX[J]. Applied and Environmental Microbiology, 2018, 84(16): e01224-18. |
| 41 | LIU W J, HONG J, BEVAN D R, et al. Fast identification of thermostable beta-glucosidase mutants on cellobiose by a novel combinatorial selection/screening approach[J]. Biotechnology and Bioengineering, 2009, 103(6): 1087-1094. |
| 42 | MYUNG S, WANG Y R, ZHANG Y H P. Fructose-1,6-bisphosphatase from a hyper-thermophilic bacterium Thermotoga maritima: characterization, metabolite stability, and its implications[J]. Process Biochemistry, 2010, 45(12): 1882-1887. |
| 43 | ROLLIN J A, TAM T K, ZHANG Y H P. New biotechnology paradigm: cell-free biosystems for biomanufacturing[J]. Green Chemistry, 2013, 15(7): 1708-1719. |
| 44 | HUANG R, CHEN H, ZHONG C, et al. High-throughput screening of coenzyme preference change of thermophilic 6-phosphogluconate dehydrogenase from NADP+ to NAD+ [J]. Scientific Reports, 2016, 6: 32644. |
| 45 | HUANG R, CHEN H, UPP D M, et al. A high-throughput method for directed evolution of NAD(P)+-dependent dehydrogenases for the reduction of biomimetic nicotinamide analogues[J]. ACS Catalysis, 2019, 9(12): 11709-11719. |
| 46 | MENG D D, LIU M X, SU H, et al. Coenzyme engineering of glucose-6-phosphate dehydrogenase on a nicotinamide-based biomimic and its application as a glucose biosensor[J]. ACS Catalysis, 2023, 13(3): 1983-1998. |
| 47 | MA C L, WU R R, HUANG R, et al. Directed evolution of a 6-phosphogluconate dehydrogenase for operating an enzymatic fuel cell at lowered anodic pHs[J]. Journal of Electroanalytical Chemistry, 2019, 851: 113444. |
| 48 | MA C L, LIU M X, YOU C, et al. Engineering a diaphorase via directed evolution for enzymatic biofuel cell application[J]. Bioresources and Bioprocessing, 2020, 7: 23. |
| 49 | JUMPER J, EVANS R, PRITZEL A, et al. Highly accurate protein structure prediction with AlphaFold[J]. Nature, 2021, 596(7873): 583-589. |
| 50 | LIU J, GUO Z Y, WU T Q, et al. Enhancing alphafold-multimer-based protein complex structure prediction with MULTICOM in CASP15[J]. Communications Biology, 2023, 6: 1140. |
| 51 | STAHL K, GRAZIADEI A, DAU T, et al. Protein structure prediction with in-cell photo-crosslinking mass spectrometry and deep learning[J]. Nature Biotechnology, 2023, 41(12): 1810-1819. |
| 52 | MYUNG S, ZHANG Y H. Non-complexed four cascade enzyme mixture: simple purification and synergetic co-stabilization[J]. PLoS One, 2013, 8(4): e61500. |
| 1 | ZHANG Y H P, SUN J B, MA Y H. Biomanufacturing: history and perspective[J]. Journal of Industrial Microbiology & Biotechnology, 2017, 44(4-5): 773-784. |
| 2 | CLOMBURG J M, CRUMBLEY A M, GONZALEZ R. Industrial biomanufacturing: the future of chemical production[J]. Science, 2017, 355(6320): aag0804. |
| 3 | SCOWN C D. Prospects for carbon-negative biomanufacturing[J]. Trends in Biotechnology, 2022, 40(12): 1415-1424. |
| 4 | ROLLIN J A, YE X H, MARTIN DEL CAMPO J, et al. Novel hydrogen bioreactor and detection apparatus[M/OL]//BAO J, YE Q, ZHONG J J. Bioreactor engineering research and industrial applications Ⅱ. Advances in biochemical engineering/biotechnology. Berlin, Heidelberg: Springer, 2014 [2023-12-01]. . |
| 5 | ZHANG Y H P. Production of biocommodities and bioelectricity by cell-free synthetic enzymatic pathway biotransformations: challenges and opportunities[J]. Biotechnology and Bioengineering, 2010, 105(4): 663-677. |
| 6 | ZHANG Y H P. Substrate channeling and enzyme complexes for biotechnological applications[J]. Biotechnology Advances, 2011, 29(6): 715-725. |
| 7 | ZHANG Y H P. What is vital (and not vital) to advance economically-competitive biofuels production[J]. Process Biochemistry, 2011, 46(11): 2091-2110. |
| 8 | ZHANG Y H P. Production of biofuels and biochemicals by in vitro synthetic biosystems: opportunities and challenges[J]. Biotechnology Advances, 2015, 33(7): 1467-1483. |
| 9 | ZHANG Y H P, ZHU Z G, YOU C, et al. In vitro BioTransformation (ivBT): definitions, opportunities, and challenges[J]. Synthetic Biology and Engineering, 2023, 1(2): 10013. |
| 10 | THAUER R K, JUNGERMANN K, DECKER K. Energy conservation in chemotrophic anaerobic bacteria[J]. Bacteriological Reviews, 1977, 41(1): 100-180. |
| 11 | ZHANG Y H, EVANS B R, MIELENZ J R, et al. High-yield hydrogen production from starch and water by a synthetic enzymatic pathway[J]. PLoS One, 2007, 2(5): e456. |
| 12 | KIM J E, KIM E J, CHEN H, et al. Advanced water splitting for green hydrogen gas production through complete oxidation of starch by in vitro metabolic engineering[J]. Metabolic Engineering, 2017, 44: 246-252. |
| 13 | YOU C, CHEN H G, MYUNG S, et al. Enzymatic transformation of nonfood biomass to starch[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(18): 7182-7187. |
| 14 | XU X X, ZHANG W, YOU C, et al. Biosynthesis of artificial starch and microbial protein from agricultural residue[J]. Science Bulletin, 2023, 68(2): 214-223. |
| 15 | ZHANG Y H P. Simpler is better: high-yield and potential low-cost biofuels production through cell-free synthetic pathway biotransformation (SyPaB)[J]. ACS Catalysis, 2011, 1(9): 998-1009. |
| 53 | FREEMAN A, WOODLEY J M, LILLY M D. In situ product removal as a tool for bioprocessing[J]. Nature Biotechnology, 1993, 11(9): 1007-1012. |
| 54 | LI H P, YOU Z N, LIU Y Y, et al. Continuous-flow microreactor-enhanced clean NAD+ regeneration for biosynthesis of 7-oxo-lithocholic acid[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(1): 456-463. |
| 55 | VASIC-RACKI D. History of industrial biotransformations-dreams and realities[M/OL]//LIESE A, SEEBALD S, WANDREY C. 2nd Edition. Industrial biotransformations. Weinheim: Wiley-VCH, KGaA, 2006[2023-12-01]. . |
| 56 | MICHELS P, ROSAZZA J. The evolution of microbial transformations for industrial applications[J]. SIM News, 2009, 2009: 36-52. |
| 57 | FU J L, YANG Y R, JOHNSON-BUCK A, et al. Multi-enzyme complexes on DNA scaffolds capable of substrate channelling with an artificial swinging arm[J]. Nature Nanotechnology, 2014, 9(7): 531-536. |
| 58 | LIN J L, PALOMEC L, WHEELDON I. Design and analysis of enhanced catalysis in scaffolded multienzyme cascade reactions[J]. ACS Catalysis, 2014, 4(2): 505-511. |
| 59 | FRANCE S P, HEPWORTH L J, TURNER N J, et al. Constructing biocatalytic cascades: in vitro and in vivo approaches to de novo multi-enzyme pathways[J]. ACS Catalysis, 2017, 7(1): 710-724. |
| 60 | WOODLEY J M. Accelerating the implementation of biocatalysis in industry[J]. Applied Microbiology and Biotechnology, 2019, 103(12): 4733-4739. |
| 61 | DE WILDEMAN S M, SONKE T, SCHOEMAKER H E, et al. Biocatalytic reductions: from lab curiosity to “first choice”[J]. Accounts of Chemical Research, 2007, 40(12): 1260-1266. |
| 62 | BOZIC M, PRICELIUS S, GUEBITZ G M, et al. Enzymatic reduction of complex redox dyes using NADH-dependent reductase from Bacillus subtilis coupled with cofactor regeneration[J]. Applied Microbiology and Biotechnology, 2010, 85(3): 563-571. |
| 63 | XU Z N, JING K J, LIU Y, et al. High-level expression of recombinant glucose dehydrogenase and its application in NADPH regeneration[J]. Journal of Industrial Microbiology & Biotechnology, 2007, 34(1): 83-90. |
| 64 | MERTENS R, LIESE A. Biotechnological applications of hydrogenases[J]. Current Opinion in Biotechnology, 2004, 15(4): 343-348. |
| 65 | JOHANNES T W, WOODYER R D, ZHAO H M. Efficient regeneration of NADPH using an engineered phosphite dehydrogenase[J]. Biotechnology and Bioengineering, 2007, 96(1): 18-26. |
| 66 | NAM K Y, STRUCK D K, HOLTZAPPLE M T. ATP regeneration by thermostable ATP synthase[J]. Biotechnology and Bioengineering, 1996, 51(3): 305-316. |
| 67 | RESNICK S M, ZEHNDER A J. In vitro ATP regeneration from polyphosphate and AMP by polyphosphate: AMP phosphotransferase and adenylate kinase from Acinetobacter johnsonii 210A[J]. Applied and Environmental Microbiology, 2000, 66(5): 2045-2051. |
| 68 | FRANKE D, MACHAJEWSKI T, HSU C C, et al. One-pot synthesis of L-fructose using coupled multienzyme systems based on rhamnulose-1-phosphate aldolase[J]. The Journal of Organic Chemistry, 2003, 68(17): 6828-6831. |
| 69 | SCHOEVAART R, VAN RANTWIJK F, SHELDON R A. A four-step enzymatic cascade for the one-pot synthesis of non-natural carbohydrates from glycerol[J]. The Journal of Organic Chemistry, 2000,65(21): 6940-6943. |
| 70 | ZHANG J B, SHAO J, KOWAL P, et al., Enzymatic synthesis of oligosaccharides[M/OL]//WONG C H. Carbohydrate-based drug discovery. Weinheim: Wiley-VCH Verlag GmbH & Co, KGaA, 2005, 137-167 [2023-12-01]. . |
| 71 | FESSNER W D, HELAINE V. Biocatalytic synthesis of hydroxylated natural products using aldolases and related enzymes[J]. Current Opinion in Biotechnology, 2001, 12(6): 574-586. |
| 72 | FESSNER W D. Enzyme mediated C—C bond formation[J]. Current Opinion in Chemical Biology, 1998, 2(1): 85-97. |
| 73 | ENDO T, KOIZUMI S. Large-scale production of oligosaccharides using engineered bacteria[J]. Current Opinion in Structural Biology, 2000, 10(5): 536-541. |
| 74 | FESSNER W D. Systems Biocatalysis: development and engineering of cell-free “artificial metabolisms” for preparative multi-enzymatic synthesis[J]. New Biotechnology, 2015, 32(6): 658-664. |
| 75 | HUANG K T, WU B C, LIN C C, et al. Multi-enzyme one-pot strategy for the synthesis of sialyl Lewis X-containing PSGL-1 glycopeptide[J]. Carbohydrate Research, 2006, 341(12): 2151-2155. |
| 76 | SWARTZ J R. Cell-free bioprocessing[J]. Chemical Engineering Progress, 2013, 2013(11): 40-45. |
| 77 | CARLSON E D, GAN R, HODGMAN C E, et al. Cell-free protein synthesis: applications come of age[J]. Biotechnology Advances, 2012, 30(5): 1185-1194. |
| 78 | SHIMIZU Y, INOUE A, TOMARI Y, et al. Cell-free translation reconstituted with purified components[J]. Nature Biotechnology, 2001, 19(8): 751-755. |
| 79 | KARIM A S, JEWETT M C. A cell-free framework for rapid biosynthetic pathway prototyping and enzyme discovery[J]. Metabolic Engineering, 2016, 36: 116-126. |
| 80 | HARRIS D C, JEWETT M C. Cell-free biology: exploiting the interface between synthetic biology and synthetic chemistry[J]. Current Opinion in Biotechnology, 2012, 23(5): 672-678. |
| 81 | CHIBA C H, KNIRSCH M C, AZZONI A R, et al. Cell-free protein synthesis: advances on production process for biopharmaceuticals and immunobiological products[J]. BioTechniques, 2021, 70(2): 126-133. |
| 82 | PARDEE K, SLOMOVIC S, NGUYEN P Q, et al. Portable, on-demand biomolecular manufacturing[J]. Cell, 2016, 167(1): 248-259.e12. |
| 83 | STAMATIS C, FARID S S. Process economics evaluation of cell-free synthesis for the commercial manufacture of antibody drug conjugates[J]. Biotechnology Journal, 2021, 16(4): e2000238. |
| 84 | STECH M, RAKOTOARINORO N, TEICHMANN T, et al. Synthesis of fluorescently labeled antibodies using non-canonical amino acids in eukaryotic cell-free systems[J]. Methods in Molecular Biology, 2021, 2305: 175-190. |
| 85 | LÜDDECKE T, PAAS A, TALMANN L, et al. A spider toxin exemplifies the promises and pitfalls of cell-free protein production for venom biodiscovery[J]. Toxins, 2021, 13(8): 575. |
| 86 | RAMM F, JACK L, KASER D, et al. Cell-free systems enable the production of AB5 toxins for diagnostic applications[J]. Toxins, 2022, 14(4): 233. |
| 87 | PE’ERY T, MATHEWS M B. Synthesis and purification of single-stranded RNA for use in experiments with PKR and in cell-free translation systems[J]. Methods, 1997, 11(4): 371-381. |
| 88 | LYND L R, WYMAN C E, GERNGROSS T U. Biocommodity engineering[J]. Biotechnology Progress, 1999, 15(5): 777-793. |
| 89 | ROLLIN J A, MARTIN DEL CAMPO J, MYUNG S, et al. High-yield hydrogen production from biomass by in vitro metabolic engineering: mixed sugars coutilization and kinetic modeling[J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(16): 4964-4969. |
| 90 | OPGENORTH P H, KORMAN T P, BOWIE J U. A synthetic biochemistry module for production of bio-based chemicals from glucose[J]. Nature Chemical Biology, 2016, 12(6): 393-395. |
| 91 | ZHU Z G, KIN TAM T, SUN F F, et al. A high-energy-density sugar biobattery based on a synthetic enzymatic pathway[J]. Nature Communications, 2014, 5: 3026. |
| 92 | CHENG K, ZHENG W M, CHEN H G, et al. Upgrade of wood sugar D-xylose to a value-added nutraceutical by in vitro metabolic engineering[J]. Metabolic Engineering, 2019, 52: 1-8. |
| 93 | SHI T, LIU S, ZHANG Y P J. CO2 fixation for malate synthesis energized by starch via in vitro metabolic engineering[J]. Metabolic Engineering, 2019, 55: 152-160. |
| 94 | KIM E J, WU C H, ADAMS M W, et al. Exceptionally high rates of biological hydrogen production by biomimetic in vitro synthetic enzymatic pathways[J]. Chemistry, 2016, 22(45): 16047-16051. |
| 95 | ZHONG C, WEI P, ZHANG Y H P. Enhancing functional expression of codon-optimized heterologous enzymes in Escherichia coli BL21(DE3) by selective introduction of synonymous rare codons[J]. Biotechnology and Bioengineering, 2017, 114(5): 1054-1064. |
| 96 | CAI T, SUN H B, QIAO J, et al. Cell-free chemoenzymatic starch synthesis from carbon dioxide[J]. Science, 2021, 373(6562): 1523-1527. |
| 97 | DENG X L, FAN M, WU M, et al. Continuous-flow enzymatic synthesis of chiral lactones in a three-dimensional microfluidic reactor[J]. Chinese Chemical Letters, 2024, 35(3): 108684. |
| 98 | DUDLEY Q M, KARIM A S, JEWETT M C. Cell-free metabolic engineering: biomanufacturing beyond the cell[J]. Biotechnology Journal, 2015, 10(1): 69-82. |
| 99 | OPGENORTH P H, KORMAN T P, IANCU L, et al. A molecular rheostat maintains ATP levels to drive a synthetic biochemistry system[J]. Nature Chemical Biology, 2017, 13(9): 938-942. |
| 100 | HOLD C, BILLERBECK S, PANKE S. Forward design of a complex enzyme cascade reaction[J]. Nature Communications, 2016, 7: 12971. |
| 101 | KORMAN T P, OPGENORTH P H, BOWIE J U. A synthetic biochemistry platform for cell free production of monoterpenes from glucose[J]. Nature Communications, 2017, 8: 15526. |
| 102 | GUTERL J K, GARBE D, CARSTEN J, et al. Cell-free metabolic engineering: production of chemicals by minimized reaction cascades[J]. ChemSusChem, 2012, 5(11): 2165-2172. |
| 103 | CHEN K, ARNOLD F H. Tuning the activity of an enzyme for unusual environments: sequential random mutagenesis of subtilisin E for catalysis in dimethylformamide[J]. Proceedings of the National Academy of Sciences of the United States of America, 1993, 90(12): 5618-5622. |
| 104 | KIM E J, KIM J E, ZHANG Y H P J. Ultra-rapid rates of water splitting for biohydrogen gas production through in vitro artificial enzymatic pathways[J]. Energy & Environmental Science, 2018, 11(8): 2064-2072. |
| 105 | ZHANG Y H, ZHOU W. D-xylose 4-epimerase, mutant thereof and use thereof: CN113122528A[P]. 2021-07-16. |
| 106 | SONG Z, LI Y, LI Y J, et al., Aminomutation catalyzed by CO 2 self-sufficient cascade amino acid decarboxylases[EB/OL]. bioRxiv, 2023.08.12.552924. (2023-08-12)[2023-12-01]. . |
| 107 | ZHONG C, YOU C, WEI P, et al. Thermal cycling cascade biocatalysis of myo-inositol synthesis from sucrose[J]. ACS Catalysis, 2017, 7(9): 5992-5999. |
| 108 | COLODNY L, HOFFMAN R L. Inositol: clinical applications for exogenous use[J]. Alternative Medicine Review, 1998, 3(6): 432-447. |
| 109 | CHENG K, ZHANG F, SUN F F, et al. Doubling power output of starch biobattery treated by the most thermostable isoamylase from an archaeon Sulfolobus tokodaii [J]. Scientific Reports, 2015, 5: 13184. |
| 110 | JEON B S, TAGUCHI H, SAKAI H, et al. 4-alpha-glucanotransferase from the hyperthermophilic archaeon Thermococcus litoralis: enzyme purification and characterization, and gene cloning, sequencing and expression in Escherichia coli [J]. European Journal of Biochemistry, 1997, 248(1): 171-178. |
| 111 | LIAO H H, MYUNG S, ZHANG Y H P. One-step purification and immobilization of thermophilic polyphosphate glucokinase from Thermobifida fusca YX: glucose-6-phosphate generation without ATP[J]. Applied Microbiology and Biotechnology, 2012, 93(3): 1109-1117. |
| 112 | FLAMHOLZ A, NOOR E, BAR-EVEN A, et al. eQuilibrator: the biochemical thermodynamics calculator[J]. Nucleic Acids Research, 2012, 40(D1): D770-D775. |
| 113 | HAN P P, ZHOU X G, YOU C. Efficient multi-enzymes immobilized on porous microspheres for producing inositol from starch[J]. Frontiers in Bioengineering and Biotechnology, 2020, 8: 380. |
| 114 | HAN P P, YOU C, LI Y J, et al. High-titer production of myo-inositol by a co-immobilized four-enzyme cocktail in biomimetic mineralized microcapsules[J]. Chemical Engineering Journal, 2023, 461: 141946. |
| 115 | TANG E J, SHEN X L, WANG J, et al. Synergetic utilization of glucose and glycerol for efficient myo-inositol biosynthesis[J]. Biotechnology and Bioengineering, 2020, 117(4): 1247-1252. |
| 116 | YOU R, WANG L, SHI C R, et al. Efficient production of myo-inositol in Escherichia coli through metabolic engineering[J]. Microbial Cell Factories, 2020, 19(1): 109. |
| 117 | 欧阳平凯. 我国工业生物技术发展回顾及展望[J]. 生物工程学报, 2022, 38(11): 3991-4000. |
| OUYANG P K. The industrial biotechnology in China: development and outlook[J]. Chinese Journal of Biotechnology, 2022, 38(11): 3991-4000. | |
| 118 | FUJISAWA T, FUJINAGA S, ATOMI H. An in vitro enzyme system for the production of myo-inositol from starch[J]. Applied and Environmental Microbiology, 2017, 83(16): e00550-17. |
| 119 | LU Y P, WANG L, TENG F, et al. Production of myo-inositol from glucose by a novel trienzymatic cascade of polyphosphate glucokinase, inositol 1-phosphate synthase and inositol monophosphatase[J]. Enzyme and Microbial Technology, 2018, 112: 1-5. |
| 120 | MENG D D, WEI X L, ZHANG Y H P J, et al. Stoichiometric conversion of cellulosic biomass by in vitro synthetic enzymatic biosystems for biomanufacturing[J]. ACS Catalysis, 2018, 8(10): 9550-9559. |
| 121 | GRANSTRÖM T B, TAKATA G, TOKUDA M, et al. Izumoring: a novel and complete strategy for bioproduction of rare sugars[J]. Journal of Bioscience and Bioengineering, 2004, 97(2): 89-94. |
| 122 | ZHANG W L, ZHANG T, JIANG B, et al. Enzymatic approaches to rare sugar production[J]. Biotechnology Advances, 2017, 35(2): 267-274. |
| 123 | IZUMORI K. Izumoring: a strategy for bioproduction of all hexoses[J]. Journal of Biotechnology, 2006, 124(4): 717-722. |
| 124 | LEVIN G V. Tagatose, the new GRAS sweetener and health product[J]. Journal of Medicinal Food, 2002, 5(1): 23-36. |
| 125 | CHEETHAM P S J, WOOTTON A N. Bioconversion of D-galactose into D-tagatose[J]. Enzyme and Microbial Technology, 1993, 15(2): 105-108. |
| 126 | RHIMI M, AGHAJARI N, JUY M, et al. Rational design of Bacillus stearothermophilus US100 L-arabinose isomerase: potential applications for D-tagatose production[J]. Biochimie, 2009, 91(5): 650-653. |
| 127 | BOSSHART A, HEE C S, BECHTOLD M, et al. Directed divergent evolution of a thermostable D-tagatose epimerase towards improved activity for two hexose substrates[J]. ChemBioChem, 2015, 16(4): 592-601. |
| 128 | OH H J, KIM H J, OH D K. Increase in D-tagatose production rate by site-directed mutagenesis of L-arabinose isomerase from Geobacillus thermodenitrificans [J]. Biotechnology Letters, 2006, 28(3): 145-149. |
| 129 | WICHELECKI D J, VETTING M W, CHOU L, et al. ATP-binding cassette (ABC) transport system solute-binding protein-guided identification of novel D-altritol and galactitol catabolic pathways in Agrobacterium tumefaciens C58[J]. Journal of Biological Chemistry, 2015, 290(48): 28963-28976. |
| 130 | MORADIAN A, BENNER S A. A biomimetic biotechnological process for converting starch to fructose: thermodynamic and evolutionary considerations in applied enzymology[J]. Journal of the American Chemical Society, 1992, 114(18): 6980-6987. |
| 131 | WICHELECKI D J, ZHANG Y H P. Enzymatic synthesis of D-tagatose: US62/236226[P]. 2015-10-02. |
| 132 | MA Y H, SUN Y X. Tagatose preparation method: CN106399427A[P]. 2016-11-01. |
| 133 | ZHANG Y H, YOU C. Inositol preparation method: CN106148425B[P]. 2015-04-17. |
| 134 | OH D K, HONG S H, LEE S H. Aldolase, aldolase mutant, and method and composition for producing tagatose by using same: WO2015016544 A1[P]. 2014-07-25. |
| 135 | MA Y H, SUN Y X, YANG J A, et al. Method for preparing tagatose through whole-cell catalysis: CN107988286B[P]. 2017-11-02. |
| 136 | MA Y H, SHI T, LI Y J, et al. Bacillus subtilis gene engineering bacteria for producing tagatose and method for preparing tagatose: CN112342179B[P]. 2021-01-05. |
| 137 | MA Y H, SUN Y X, YANG J G, et al. Engineering strain for producing tagatose, and construction method and application thereof: CN109666620A[P]. 2019-02-20. |
| 138 | DAI Y W, ZHANG T, JIANG B, et al. Dictyoglomus turgidum DSM 6724 α-glucan phosphorylase: characterization and its application in multi-enzyme cascade reaction for D-tagatose production[J]. Applied Biochemistry and Biotechnology, 2021, 193(11): 3719-3731. |
| 139 | DAI Y W, ZHANG J X, ZHANG T, et al. Characteristics of a fructose 6-phosphate 4-epimerase from Caldilinea aerophila DSM 14535 and its application for biosynthesis of tagatose[J]. Enzyme and Microbial Technology, 2020, 139: 109594. |
| 140 | DAI Y W, LI C C, ZHENG L H, et al. Enhanced biosynthesis of D-tagatose from maltodextrin through modular pathway engineering of recombinant Escherichia coli [J]. Biochemical Engineering Journal, 2022, 178: 108303. |
| 141 | ZHANG W L, YU S H, ZHANG T, et al. Recent advances in D-allulose: physiological functionalities, applications, and biological production[J]. Trends in Food Science & Technology, 2016, 54: 127-137. |
| 142 | JIANG S W, XIAO W, ZHU X X, et al. Review on D-allulose: in vivo metabolism, catalytic mechanism, engineering strain construction, bio-production technology[J]. Frontiers in Bioengineering and Biotechnology, 2020, 8: 26. |
| 143 | MATSUO T, SUZUKI H, HASHIGUCHI M, et al. D-psicose is a rare sugar that provides no energy to growing rats[J]. Journal of Nutritional Science and Vitaminology, 2002, 48(1): 77-80. |
| 144 | ZENG Y, ZHANG X X, GUAN Y P, et al. Characteristics and antioxidant activity of Maillard reaction products from psicose-lysine and fructose-lysine model systems[J]. Journal of Food Science, 2011, 76(3): C398-C403. |
| 145 | HAYASHI N, IIDA T, YAMADA T, et al. Study on the postprandial blood glucose suppression effect of D-psicose in borderline diabetes and the safety of long-term ingestion by normal human subjects[J]. Bioscience, Biotechnology, and Biochemistry, 2010, 74(3): 510-519. |
| 146 | CHUNG M Y, OH D K, LEE K W. Hypoglycemic health benefits of D-psicose[J]. Journal of Agricultural and Food Chemistry, 2012, 60(4): 863-869. |
| 147 | MOLLER D E, BERGER J P. Role of PPARs in the regulation of obesity-related insulin sensitivity and inflammation[J]. International Journal of Obesity, 2003, 27(S3): S17-S21. |
| 148 | YANG S J, CHO H K, LEE Y M, et al. Thermostable fructose 6-phosphate-3-epimerase and a method for producing allulose using the same: KR102063908B1[P]. 2017-12-27. |
| 149 | WICHELECKI D J, ROGERS E. Enzymatic production of hexoses: WO2018169957A1[P]. 2018-03-13. |
| 150 | MACEACHRAN D, CUNNINGHAM D S, BLAKE W J, et al. Cell-free production of sugars: US20180320210A1[P]. 2018-07-12. |
| 151 | TORRETTA S, SCAGLIOLA A, RICCI L, et al. D-mannose suppresses macrophage IL-1β production[J]. Nature Communications, 2020, 11(1): 6343. |
| 152 | GONZALEZ P S, O’PREY J, CARDACI S, et al. Mannose impairs tumour growth and enhances chemotherapy[J]. Nature, 2018, 563(7733): 719-723. |
| 153 | ZHANG D F, CHIA C, JIAO X, et al. D-mannose induces regulatory T cells and suppresses immunopathology[J]. Nature Medicine, 2017, 23(9): 1036-1045. |
| 154 | TIAN C Y, YANG J G, LI Y J, et al. Artificially designed routes for the conversion of starch to value-added mannosyl compounds through coupling in vitro and in vivo metabolic engineering strategies[J]. Metabolic Engineering, 2020, 61: 215-224. |
| 155 | ZHANG Y H P. Next generation biorefineries will solve the food, biofuels, and environmental trilemma in the energy-food-water nexus[J]. Energy Science & Engineering, 2013, 1(1): 27-41. |
| 156 | CHEN H G, ZHANG Y H P. New biorefineries and sustainable agriculture: increased food, biofuels, and ecosystem security[J]. Renewable & Sustainable Energy Reviews, 2015, 47: 117-132. |
| 157 | CASILLAS C E, KAMMEN D M. The energy-poverty-climate nexus[J]. Science, 2010, 330(6008): 1181-1182. |
| 158 | SHEPPARD A W, GILLESPIE I, HIRSCH M, et al. Biosecurity and sustainability within the growing global bioeconomy[J]. Current Opinion in Environmental Sustainability, 2011, 3(1-2): 4-10. |
| 159 | ZHANG Y H P, HUANG W D. Constructing the electricity-carbohydrate-hydrogen cycle for a sustainability revolution[J]. Trends in Biotechnology, 2012, 30(6): 301-306. |
| 160 | ZHANG Y H P. A sweet out-of-the-box solution to the hydrogen economy: is the sugar-powered car science fiction?[J]. Energy & Environmental Science, 2009, 2(3): 272-282. |
| 161 | HARNISCH F, MOREJÓN M C. Hydrogen from water is more than a fuel: hydrogenations and hydrodeoxygenations for a biobased economy[J]. Chemical Record, 2021, 21(9): 2277-2289. |
| 162 | THAUER R K, KASTER A K, SEEDORF H, et al. Methanogenic Archaea: ecologically relevant differences in energy conservation[J]. Nature Reviews Microbiology, 2008, 6(8): 579-591. |
| 163 | CHHEDA J N, HUBER G W, DUMESIC J A. Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals[J]. Angewandte Chemie International Edition, 2007, 46(38): 7164-7183. |
| 164 | HUBER G W, SHABAKER J W, DUMESIC J A. Raney Ni-Sn catalyst for H2 production from biomass-derived hydrocarbons[J]. Science, 2003, 300(5628): 2075-2077. |
| 165 | MAEDA T, SANCHEZ-TORRES V, WOOD T K. Metabolic engineering to enhance bacterial hydrogen production[J]. Microbial Biotechnology, 2008, 1(1): 30-39. |
| 166 | MAEDA T, SANCHEZ-TORRES V, WOOD T K. Hydrogen production by recombinant Escherichia coli strains[J]. Microbial Biotechnology, 2012, 5(2): 214-225. |
| 167 | YE X H, WANG Y R, HOPKINS R C, et al. Spontaneous high-yield production of hydrogen from cellulosic materials and water catalyzed by enzyme cocktails[J]. ChemSusChem, 2009, 2(2): 149-152. |
| 168 | MYUNG S, ROLLIN J, YOU C, et al. In vitro metabolic engineering of hydrogen production at theoretical yield from sucrose[J]. Metabolic Engineering, 2014, 24: 70-77. |
| 169 | MARTÍN DEL CAMPO J S, ROLLIN J, MYUNG S, et al. High-yield production of dihydrogen from xylose by using a synthetic enzyme cascade in a cell-free system[J]. Angewandte Chemie International Edition, 2013, 52(17): 4587-4590. |
| 170 | BEREZINA O V, ZVERLOV V V, LUNINA N A, et al. Gene and properties of thermostable 4-alpha-glucanotransferase of Thermotoga neapolitana[J]. Journal of Molecular Biology, 1999, 33: 801-806. |
| 171 | CHEN H, HUANG R, KIM E J, et al. Building a thermostable metabolon for facilitating coenzyme transport and in vitro hydrogen production at elevated temperature[J]. ChemSusChem, 2018, 11(18): 3120-3130. |
| 172 | HUANG R, CHEN H, ZHOU W, et al. Engineering a thermostable highly active glucose 6-phosphate dehydrogenase and its application to hydrogen production in vitro [J]. Applied Microbiology and Biotechnology, 2018, 102(7): 3203-3215. |
| 173 | 张以恒. 中国哲学思想“道法术器”对生物制造的启示[J]. 合成生物学, 2024, 5(6):1231-1241. |
| ZHANG Y-H P J. The enlightenment of the Chinese philosophy “Tao-Fa-Shu-Qi” to industrial biomanufacturing[J]. Synthetic Biology Journal, 2024, 5(6):1231-1241. | |
| 174 | WANG X D, SABA T, YIU H H P, et al. Cofactor NAD(P)H regeneration inspired by heterogeneous pathways[J]. Chem, 2017, 2(5): 621-654. |
| 175 | ALI I, KHAN T, OMANOVIC S. Direct electrochemical regeneration of the cofactor NADH on bare Ti, Ni, Co and Cd electrodes: the influence of electrode potential and electrode material[J]. Journal of Molecular Catalysis A: Chemical, 2014, 387: 86-91. |
| 176 | MORELLO G, MEGARITY C F, ARMSTRONG F A. The power of electrified nanoconfinement for energising, controlling and observing long enzyme cascades[J]. Nature Communications, 2021, 12: 340. |
| 177 | CASTAÑEDA-LOSADA L, ADAM D, PACZIA N, et al. Bioelectrocatalytic cofactor regeneration coupled to CO2 fixation in a redox-active hydrogel for stereoselective C—C bond formation[J]. Angewandte Chemie International Edition, 2021, 60(38): 21056-21061. |
| [1] | 李怡霏, 陈艾, 孙俊松, 张以恒. 体外多酶分子机器产氢应用中的氢酶研究[J]. 合成生物学, 2024, 5(6): 1461-1484. |
| [2] | 张以恒. 中国哲学思想“道法术器”对生物制造的启示[J]. 合成生物学, 2024, 5(6): 1231-1241. |
| [3] | 刘建明, 曾安平. 无细胞多酶分子机器赋能二氧化碳的高值利用及其挑战[J]. 合成生物学, 2022, 3(5): 825-832. |
| [4] | 张以恒. 忆王义翘教授对生物炼制的贡献和我对此领域未来发展的观点[J]. 合成生物学, 2021, 2(4): 497-508. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||