合成生物学 ›› 2024, Vol. 5 ›› Issue (2): 221-238.DOI: 10.12211/2096-8280.2023-079
合成生物学在基于微生物载体肿瘤疫苗设计中的应用
谭子斌, 梁康, 陈有海
- 深圳理工大学药学院,中国科学院深圳先进技术研究院癌症免疫中心,广东 深圳 518055
-
收稿日期:2023-11-20修回日期:2024-02-05出版日期:2024-04-30发布日期:2024-04-28 -
通讯作者:陈有海 -
作者简介:谭子斌 (1990—),男,助理研究员。研究方向为肿瘤疫苗与免疫治疗。E-mail:tanzibin108@gmail.com陈有海 (1963—),博士生导师,欧洲科学院(Academia Europaea)院士,美国医学与生物工程院(AIMBE)Fellow,国家特聘教授,教育部长江学者,深圳理工大学药学院讲席教授、院长。研究方向为肿瘤免疫治疗。E-mail:yh.chen@siat.ac.cn -
基金资助:国家重点研发计划(2022YFA0912400);深圳市科技计划(JCYJ20220818100806015);国家自然科学基金(32130040);深圳市医学科研基金(B2301006)
Applications of synthetic biology in developing microbial-vectored cancer vaccines
TAN Zibin, LIANG Kang, CHEN Youhai
- Center for Cancer Immunology,Faculty of Pharmaceutical Sciences,Shenzhen Institute of Advanced Technology,Shenzhen University of Advanced Technology,Chinese Academy of Sciences (CAS),Shenzhen 518055,Guangdong,China
-
Received:2023-11-20Revised:2024-02-05Online:2024-04-30Published:2024-04-28 -
Contact:CHEN Youhai
摘要:
合成生物学有望创造具备独特优势的抗肿瘤微生物疫苗,合成生物学改造的微生物更能适应肿瘤微环境并在其中富集与增殖,削弱或者逆转免疫抑制细胞的功能,并增强肿瘤抗原的呈递,诱发多种先天与适应性抗肿瘤免疫反应,所以合成生物学已成为肿瘤疫苗研究的重要工具。本文总结了合成生物学在细菌和病毒载体肿瘤疫苗开发中的几个关键应用,其中包括减弱微生物载体毒性的方法,例如去除、失活或修改其致病基因等。讨论了增强它们在肿瘤组织中的趋向性和适应性的策略,如改变它们的细胞入侵分子或引入环境控制的基因表达系统等;也讨论了降低全身毒性的方法。为了充分利用微生物复制引起的肿瘤微环境改变的潜力,多种合成生物学手段被用于改造微生物载体,这些方法包括将外源基因引入微生物基因组,使其生产诸如细胞因子、趋化因子或单克隆抗体等分子,这些分子可以增强先天和适应性免疫细胞的招募和激活,促进肿瘤细胞免疫原性死亡,并增强肿瘤相关抗原的呈递。此外,还探讨了将肿瘤抗原引入载体中的方法,例如不同的装载方式、位置和释放机制。开发微生物载体肿瘤疫苗存在重大挑战,包括安全性问题、抗载体免疫与抗肿瘤免疫的复杂关系和肿瘤生物学的复杂性,克服这些困难将成为未来研究的重要方向。
中图分类号:
引用本文
谭子斌, 梁康, 陈有海. 合成生物学在基于微生物载体肿瘤疫苗设计中的应用[J]. 合成生物学, 2024, 5(2): 221-238.
TAN Zibin, LIANG Kang, CHEN Youhai. Applications of synthetic biology in developing microbial-vectored cancer vaccines[J]. Synthetic Biology Journal, 2024, 5(2): 221-238.
| 疫苗名称 | 载体 类型 | 来源 | 临床状态 | 肿瘤类型 | 临床试验编号 | 方法 免疫 | 肿瘤特异性抗原 | 结合疗法 | 参考 文献 |
|---|---|---|---|---|---|---|---|---|---|
| BCG | 减毒 活细菌 | 牛结核菌 | 临床使用 | 膀胱癌 | — | 瘤内 | 无 | 手术 | [ |
| T-VEC | 工程化病毒 | 单纯疱疹 病毒1型 | 临床使用 | 无法切除的转移性ⅢB/C-ⅣM1a期黑色素瘤 | — | 瘤内 | 无 | 无 | [ |
| 工程化病毒 | 单纯疱疹 病毒1型 | 临床使用 | 复发性神经胶质瘤 | UMIN000002661 UMIN000015995 | 瘤内 | 无 | 无 | [ | |
| REOLYSIN | 工程化病毒 | 呼肠孤病毒Dearing type 3 | Ⅰb | 高级神经胶质瘤、脑转移 | EudraCT 2011-005635-10 | 静脉 | 无 | 手术 | [ |
| Delta-24-RGD | 工程化病毒 | 腺病毒Ad5 | Ⅰ | 儿童弥散内生型脑桥胶质瘤(DIPG) | 瘤内 | 无 | 标准放疗+/化疗 | [ | |
| T-VEC | 工程化病毒 | 单纯疱疹 病毒1型 | Ⅱ | 可手术的ⅢB/C-ⅣM1a期黑色素瘤 | NCT02211131 | 瘤内 | 无 | 手术 | [ |
| NOUS-209 | 工程化病毒 | GAd、MVA | Ⅰ/Ⅱ | 一/二线转移性dMMR/MSI-H结直肠癌、胃癌、胃食管交界腺癌 | NCT04041310 | 瘤内 | 209个dMMR 移码肽 | PD-1单抗帕博利珠 | [ |
| GRANITE | 工程化病毒 | 猩猩腺病毒ChAd68、 委内瑞拉 马脑炎病毒 | Ⅰ/Ⅱ | 多种转移性实体瘤,包括非小细胞肺癌、结直肠癌、胃食管交界腺癌、泌尿上皮癌 | 肌肉 | 个性化 新生抗原 | 标准化疗,PD-1单抗纳武利尤,CTLA-4单抗易普利姆玛 | [ | |
| 工程化病毒 | 腺病毒 | Ⅰ | 多种晚期上皮瘤,包括肺癌、乳癌、卵巢癌、前列腺癌、肠癌 | 皮下 | 分泌型MUC-1-CD40L 融合蛋白 | 标准化疗 | [ | ||
| CAN-3110 | 工程化病毒 | 单纯疱疹 病毒1型 | Ⅰ | 恶性胶质母细胞瘤,恶性星形细胞瘤,少突胶质细胞瘤 | 瘤内 | 无 | 化疗 | [ | |
| Delta-24-RGD | 工程化病毒 | 腺病毒Ad5 | Ⅰ/Ⅱ | 神经胶质瘤,神经内分泌瘤 | 瘤内 | 无 | PD-1单抗帕博利珠 | [ | |
| T-VEC | 工程化病毒 | 单纯疱疹 病毒1型 | Ⅱ | 二-三期三阴性乳腺癌 | NCT02779855 | 瘤内 | 无 | 新辅助化疗,手术 | [ |
表1 目前临床中使用的或近期发表临床试验结果的基于微生物载体的肿瘤疫苗
Table 1 A summary of current clinical microbial-vectored cancer vaccines and recently reported studies on clinical trials
| 疫苗名称 | 载体 类型 | 来源 | 临床状态 | 肿瘤类型 | 临床试验编号 | 方法 免疫 | 肿瘤特异性抗原 | 结合疗法 | 参考 文献 |
|---|---|---|---|---|---|---|---|---|---|
| BCG | 减毒 活细菌 | 牛结核菌 | 临床使用 | 膀胱癌 | — | 瘤内 | 无 | 手术 | [ |
| T-VEC | 工程化病毒 | 单纯疱疹 病毒1型 | 临床使用 | 无法切除的转移性ⅢB/C-ⅣM1a期黑色素瘤 | — | 瘤内 | 无 | 无 | [ |
| 工程化病毒 | 单纯疱疹 病毒1型 | 临床使用 | 复发性神经胶质瘤 | UMIN000002661 UMIN000015995 | 瘤内 | 无 | 无 | [ | |
| REOLYSIN | 工程化病毒 | 呼肠孤病毒Dearing type 3 | Ⅰb | 高级神经胶质瘤、脑转移 | EudraCT 2011-005635-10 | 静脉 | 无 | 手术 | [ |
| Delta-24-RGD | 工程化病毒 | 腺病毒Ad5 | Ⅰ | 儿童弥散内生型脑桥胶质瘤(DIPG) | 瘤内 | 无 | 标准放疗+/化疗 | [ | |
| T-VEC | 工程化病毒 | 单纯疱疹 病毒1型 | Ⅱ | 可手术的ⅢB/C-ⅣM1a期黑色素瘤 | NCT02211131 | 瘤内 | 无 | 手术 | [ |
| NOUS-209 | 工程化病毒 | GAd、MVA | Ⅰ/Ⅱ | 一/二线转移性dMMR/MSI-H结直肠癌、胃癌、胃食管交界腺癌 | NCT04041310 | 瘤内 | 209个dMMR 移码肽 | PD-1单抗帕博利珠 | [ |
| GRANITE | 工程化病毒 | 猩猩腺病毒ChAd68、 委内瑞拉 马脑炎病毒 | Ⅰ/Ⅱ | 多种转移性实体瘤,包括非小细胞肺癌、结直肠癌、胃食管交界腺癌、泌尿上皮癌 | 肌肉 | 个性化 新生抗原 | 标准化疗,PD-1单抗纳武利尤,CTLA-4单抗易普利姆玛 | [ | |
| 工程化病毒 | 腺病毒 | Ⅰ | 多种晚期上皮瘤,包括肺癌、乳癌、卵巢癌、前列腺癌、肠癌 | 皮下 | 分泌型MUC-1-CD40L 融合蛋白 | 标准化疗 | [ | ||
| CAN-3110 | 工程化病毒 | 单纯疱疹 病毒1型 | Ⅰ | 恶性胶质母细胞瘤,恶性星形细胞瘤,少突胶质细胞瘤 | 瘤内 | 无 | 化疗 | [ | |
| Delta-24-RGD | 工程化病毒 | 腺病毒Ad5 | Ⅰ/Ⅱ | 神经胶质瘤,神经内分泌瘤 | 瘤内 | 无 | PD-1单抗帕博利珠 | [ | |
| T-VEC | 工程化病毒 | 单纯疱疹 病毒1型 | Ⅱ | 二-三期三阴性乳腺癌 | NCT02779855 | 瘤内 | 无 | 新辅助化疗,手术 | [ |
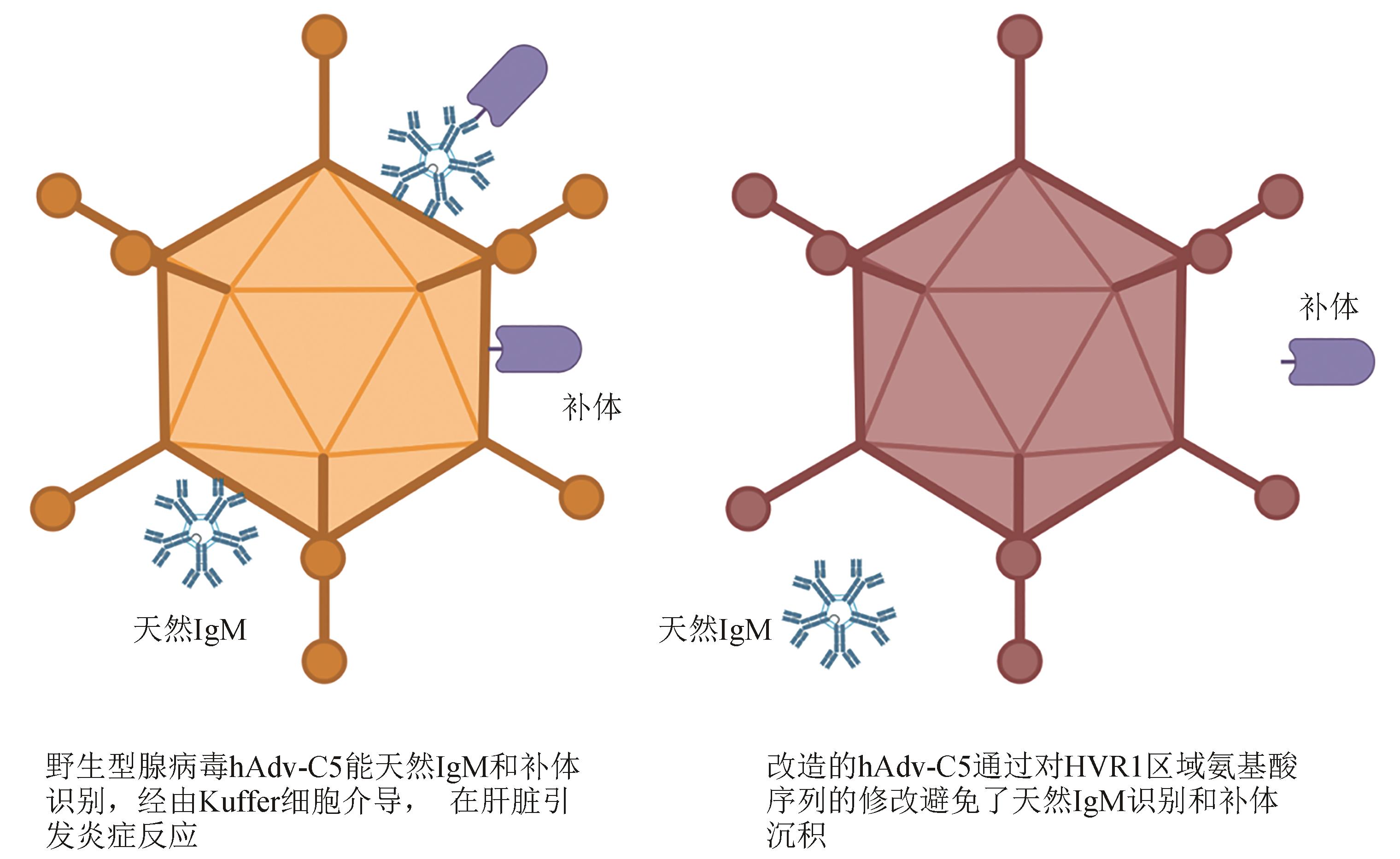
图3 通过编辑腺病毒表面抗原可以限制系统给药相关的炎症反应
Fig. 3 Surface antigen site-specific mutagenesis for reducing the risk of systemic inflammation caused by the intravenous administration of adenoviral vectored vaccines
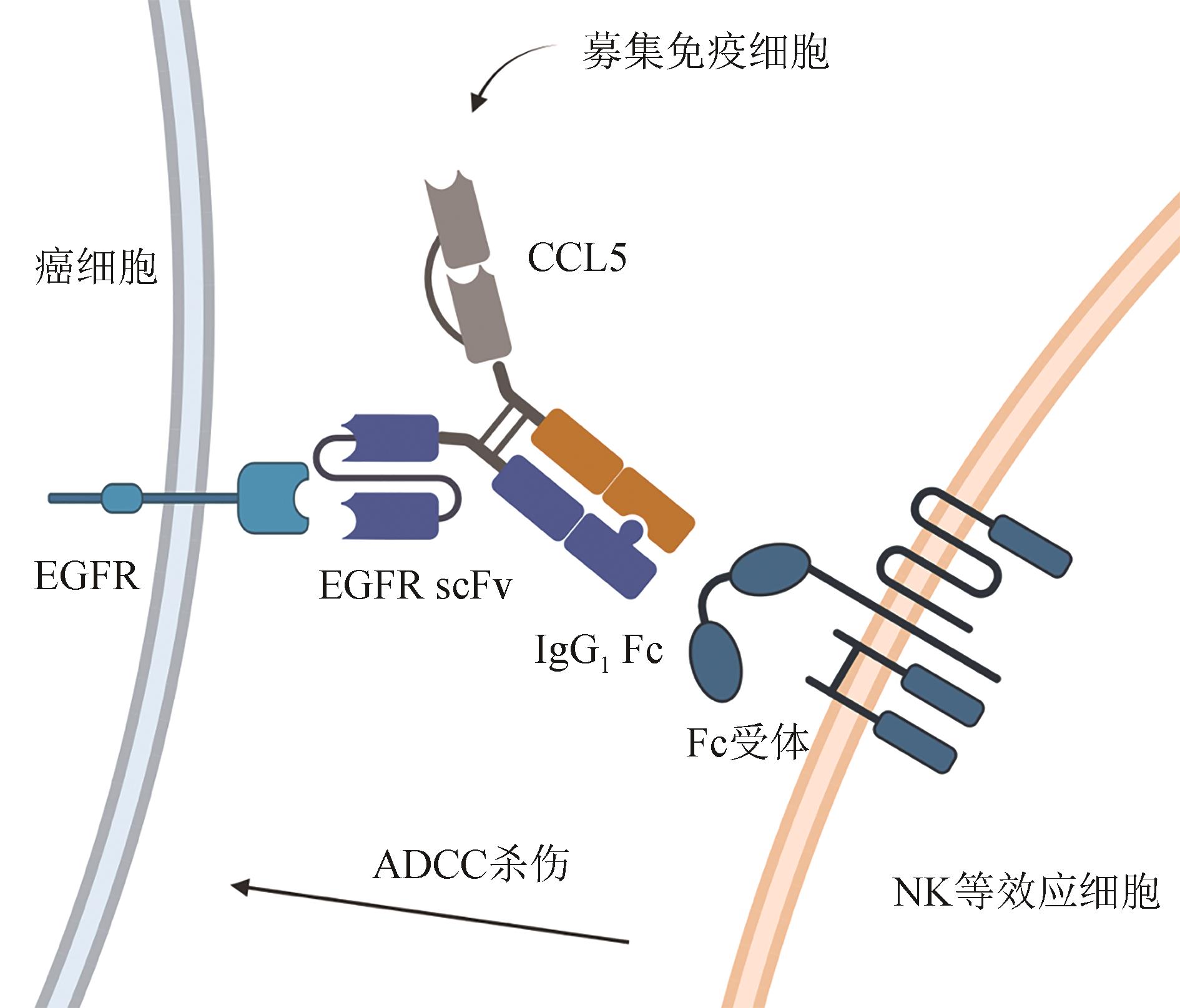
图4 双特异性抗体样蛋白能将趋化因子锚定到肿瘤细胞,招募免疫细胞,并同时通过Fc端介导ADCC
Fig. 4 A bispecific antibody-like protein with multiple function domains for multi-purpose TME modifications to bind EGFR on cancer cell surface, recruiting immune cells to mediate ADCC killing against cancer cells
| 1 | JU W, ZHENG R S, ZHANG S W, et al. Cancer statistics in Chinese older people, 2022: current burden, time trends, and comparisons with the US, Japan, and the Republic of Korea[J]. Science China Life Sciences, 2023, 66(5): 1079-1091. |
| 2 | ROJAS L A, SETHNA Z, SOARES K C, et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer[J]. Nature, 2023, 618(7963): 144-150. |
| 3 | Vaccins anti-hépatite B: note de synthèse de l’OMS-juillet 2017[J/OL]. (2017-07-07)[2023-08-01]. Relevé épidémiologique hebdomadaire, 2017, 92(27): 369-392. [J/OL]. (2017-07-07)[2023-08-01]. Weekly epidemiological record, 2017, 92(27): 369-392. . |
| 4 | RODEN R, WU T C. How will HPV vaccines affect cervical cancer?[J]. Nature Reviews Cancer, 2006, 6(10): 753-763. |
| 5 | PLOTKIN S. History of vaccination[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(34): 12283-12287. |
| 6 | MCCANN N, O’CONNOR D, LAMBE T, et al. Viral vector vaccines[J]. Current Opinion in Immunology, 2022, 77: 102210. |
| 7 | LIN I, VAN T, SMOOKER P. Live-attenuated bacterial vectors: tools for vaccine and therapeutic agent delivery[J]. Vaccines, 2015, 3(4): 940-972. |
| 8 | TOUSSAINT B, CHAUCHET X, WANG Y, et al. Live-attenuated bacteria as a cancer vaccine vector[J]. Expert Review of Vaccines, 2013, 12(10): 1139-1154. |
| 9 | SASSO E, D’ALISE A M, ZAMBRANO N, et al. New viral vectors for infectious diseases and cancer[J]. Seminars in Immunology, 2020, 50: 101430. |
| 10 | D’ALISE A M, BRASU N, INTINIS C D, et al. Adenoviral-based vaccine promotes neoantigen-specific CD8+ T cell stemness and tumor rejection[J]. Science Translational Medicine, 2022, 14(657): eabo7604. |
| 11 | DUMMER R, GYORKI D E, HYNGSTROM J, et al. Neoadjuvant talimogene laherparepvec plus surgery versus surgery alone for resectable stage ⅢB-ⅣM1a melanoma: a randomized, open-label, phase 2 trial[J]. Nature Medicine, 2021, 27(10): 1789-1796. |
| 12 | FERRUCCI P F, PALA L, CONFORTI F, et al. Talimogene laherparepvec (T-VEC): an intralesional cancer immunotherapy for advanced melanoma[J]. Cancers, 2021, 13(6): 1383. |
| 13 | LING A L, SOLOMON I H, LANDIVAR A M, et al. Clinical trial links oncolytic immunoactivation to survival in glioblastoma[J]. Nature, 2023, 623(7985): 157-166. |
| 14 | MARTÍNEZ-VÉLEZ N, GARCIA-MOURE M, MARIGIL M, et al. The oncolytic virus Delta-24-RGD elicits an antitumor effect in pediatric glioma and DIPG mouse models[J]. Nature Communications, 2019, 10(1): 2235. |
| 15 | NASSIRI F, PATIL V, YEFET L S, et al. Oncolytic DNX-2401 virotherapy plus pembrolizumab in recurrent glioblastoma: a phase 1/2 trial[J]. Nature Medicine, 2023, 29(6): 1370-1378. |
| 16 | OVERMAN M, FAKIH M, LE D, et al. 410 Phase Ⅰ interim study results of Nous-209, an off-the-shelf immunotherapy, with pembrolizumab, for the treatment of tumors with a deficiency in mismatch repair/microsatellite instability (dMMR/MSI)[J]. Journal for ImmunoTherapy of Cancer, 2021, 9(): A441. |
| 17 | OVERMAN M J, MAUREL J, OBERSTEIN P E, et al. Results of phase Ⅰ-Ⅱ bridging study for Nous-209, a neoantigen cancer immunotherapy, in combination with pembrolizumab as first line treatment in patients with advanced dMMR/MSI-h colorectal cancer[J]. Journal of Clinical Oncology, 2023, 41(): e14665. |
| 18 | PALMER C D, RAPPAPORT A R, DAVIS M J, et al. Individualized, heterologous chimpanzee adenovirus and self-amplifying mRNA neoantigen vaccine for advanced metastatic solid tumors: phase 1 trial interim results[J]. Nature Medicine, 2022, 28(8): 1619-1629. |
| 19 | SAMSON A, SCOTT K J, TAGGART D, et al. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade[J]. Science Translational Medicine, 2018, 10(422): eaam7577. |
| 20 | SOLIMAN H, HOGUE D, HAN H, et al. Oncolytic T-VEC virotherapy plus neoadjuvant chemotherapy in nonmetastatic triple-negative breast cancer: a phase 2 trial[J]. Nature Medicine, 2023, 29(2): 450-457. |
| 21 | TAN T J, GLADYS ANG W X G, WANG W W, et al. A phase Ⅰ study of an adenoviral vector delivering a MUC1/CD40-ligand fusion protein in patients with advanced adenocarcinoma[J]. Nature Communications, 2022, 13(1): 6453. |
| 22 | TODO T, INO Y, OHTSU H, et al. A phase Ⅰ/Ⅱ study of triple-mutated oncolytic herpes virus G47∆ in patients with progressive glioblastoma[J]. Nature Communications, 2022, 13(1): 4119. |
| 23 | TODO T, ITO H, INO Y, et al. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: a phase 2 trial[J]. Nature Medicine, 2022, 28(8): 1630-1639. |
| 24 | REDELMAN-SIDI G, GLICKMAN M S, BOCHNER B H. The mechanism of action of BCG therapy for bladder cancer: a current perspective[J]. Nature Reviews Urology, 2014, 11(3): 153-162. |
| 25 | MCCARTHY E F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas[J]. The Iowa Orthopaedic Journal, 2006, 26: 154-158. |
| 26 | NAGHAVIAN R, FAIGLE W, OLDRATI P, et al. Microbial peptides activate tumour-infiltrating lymphocytes in glioblastoma[J]. Nature, 2023, 617(7962): 807-817. |
| 27 | LUO X, LI Z, LIN S, et al. Antitumor effect of VNP20009, an attenuated Salmonella, in murine tumor models[J]. Oncology Research, 2001, 12(11-12): 501-508. |
| 28 | ZHENG J H, NGUYEN V H, JIANG S N, et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin[J]. Science Translational Medicine, 2017, 9(376): eaak9537. |
| 29 | SU L, ZHANG Y W, ZHANG X, et al. Combination immunotherapy with two attenuated Listeria strains carrying shuffled HPV-16 E6E7 protein causes tumor regression in a mouse tumor model[J]. Scientific Reports, 2021, 11(1): 13404. |
| 30 | SELVANESAN B C, CHANDRA D, QUISPE-TINTAYA W, et al. Listeria delivers tetanus toxoid protein to pancreatic tumors and induces cancer cell death in mice[J]. Science Translational Medicine, 2022, 14(637): eabc1600. |
| 31 | JAWALAGATTI V, KIRTHIKA P, LEE J H. Targeting primary and metastatic tumor growth in an aggressive breast cancer by engineered tryptophan auxotrophic Salmonella typhimurium [J]. Molecular Therapy Oncolytics, 2022, 25: 350-363. |
| 32 | YUE Y L, XU J Q, LI Y, et al. Antigen-bearing outer membrane vesicles as tumour vaccines produced in situ by ingested genetically engineered bacteria[J]. Nature Biomedical Engineering, 2022, 6(7): 898-909. |
| 33 | SAVAGE T M, VINCENT R L, RAE S S, et al. Chemokines expressed by engineered bacteria recruit and orchestrate antitumor immunity[J]. Science Advances, 2023, 9(10): eadc9436. |
| 34 | CHENG K M, ZHAO R F, LI Y, et al. Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via plug-and-display technology[J]. Nature Communications, 2021, 12(1): 2041. |
| 35 | ZHU J M, KE Y H, LIU Q, et al. Engineered Lactococcus lactis secreting Flt3L and OX40 ligand for in situ vaccination-based cancer immunotherapy[J]. Nature Communications, 2022, 13(1): 7466. |
| 36 | CHEN Y E, BOUSBAINE D, VEINBACHS A, et al. Engineered skin bacteria induce antitumor T cell responses against melanoma[J]. Science, 2023, 380(6641): 203-210. |
| 37 | KITAGAWA K, GONOI R, TATSUMI M, et al. Preclinical development of a WT1 oral cancer vaccine using a bacterial vector to treat castration-resistant prostate cancer[J]. Molecular Cancer Therapeutics, 2019, 18(5): 980-990. |
| 38 | KITAGAWA K, TATSUMI M, KATO M, et al. An oral cancer vaccine using a Bifidobacterium vector suppresses tumor growth in a syngeneic mouse bladder cancer model[J]. Molecular Therapy Oncolytics, 2021, 22: 592-603. |
| 39 | UEKI H, KITAGAWA K, KATO M, et al. An oral cancer vaccine using Bifidobacterium vector augments combination of anti-PD-1 and anti-CTLA-4 antibodies in mouse renal cell carcinoma model[J]. Scientific Reports, 2023, 13(1): 9994. |
| 40 | WEYANT K B, OLOYEDE A, PAL S, et al. A modular vaccine platform enabled by decoration of bacterial outer membrane vesicles with biotinylated antigens[J]. Nature Communications, 2023, 14(1): 464. |
| 41 | KROEMER G, GALASSI C, ZITVOGEL L, et al. Immunogenic cell stress and death[J]. Nature Immunology, 2022, 23(4): 487-500. |
| 42 | WANG W G, XU H H, YE Q S, et al. Systemic immune responses to irradiated tumours via the transport of antigens to the tumour periphery by injected flagellate bacteria[J]. Nature Biomedical Engineering, 2022, 6(1): 44-53. |
| 43 | WIELAND A, PATEL M R, CARDENAS M A, et al. Defining HPV-specific B cell responses in patients with head and neck cancer[J]. Nature, 2021, 597(7875): 274-278. |
| 44 | FERREIRO-IGLESIAS A, MCKAY J D, BRENNER N, et al. Germline determinants of humoral immune response to HPV-16 protect against oropharyngeal cancer[J]. Nature Communications, 2021, 12(1): 5945. |
| 45 | EBERHARDT C S, KISSICK H T, PATEL M R, et al. Functional HPV-specific PD-1+ stem-like CD8 T cells in head and neck cancer[J]. Nature, 2021, 597(7875): 279-284. |
| 46 | ROSATO P C, WIJEYESINGHE S, STOLLEY J M, et al. Virus-specific memory T cells populate tumors and can be repurposed for tumor immunotherapy[J]. Nature Communications, 2019, 10(1): 567. |
| 47 | STRICKLEY J D, MESSERSCHMIDT J L, AWAD M E, et al. Immunity to commensal papillomaviruses protects against skin cancer[J]. Nature, 2019, 575(7783): 519-522. |
| 48 | RESTREPO J, HERRERA T, SAMAKOSES R, et al. Ten-year follow-up of 9-valent human papillomavirus vaccine: immunogenicity, effectiveness, and safety[J]. Pediatrics, 2023, 152(4): e2022060993. |
| 49 | CLARK K T, TRIMBLE C L. Current status of therapeutic HPV vaccines[J]. Gynecologic Oncology, 2020, 156(2): 503-510. |
| 50 | BANASZYNSKI L A, LIU C W, WANDLESS T J. Characterization of the FKBP.rapamycin.FRB ternary complex[J]. Journal of the American Chemical Society, 2005, 127(13): 4715-4721. |
| 51 | AZAD T, REZAEI R, SINGARAVELU R, et al. Synthetic virology approaches to improve the safety and efficacy of oncolytic virus therapies[J]. Nature Communications, 2023, 14(1): 3035. |
| 52 | HEILMANN E, KIMPEL J, HOFER B, et al. Chemogenetic ON and OFF switches for RNA virus replication[J]. Nature Communications, 2021, 12(1): 1362. |
| 53 | KELLY E J, HADAC E M, GREINER S, et al. Engineering microRNA responsiveness to decrease virus pathogenicity[J]. Nature Medicine, 2008, 14(11): 1278-1283. |
| 54 | HUANG H Y, LIU Y Q, LIAO W X, et al. Oncolytic adenovirus programmed by synthetic gene circuit for cancer immunotherapy[J]. Nature Communications, 2019, 10(1): 4801. |
| 55 | GUO L, HU C, LIU Y, et al. Directed natural evolution generates a next-generation oncolytic virus with a high potency and safety profile[J]. Nature Communications, 2023, 14(1): 3410. |
| 56 | DAS K, BELNOUE E, ROSSI M, et al. A modular self-adjuvanting cancer vaccine combined with an oncolytic vaccine induces potent antitumor immunity[J]. Nature Communications, 2021, 12(1): 5195. |
| 57 | MEDINA-ECHEVERZ J, HINTERBERGER M, TESTORI M, et al. Synergistic cancer immunotherapy combines MVA-CD40L induced innate and adaptive immunity with tumor targeting antibodies[J]. Nature Communications, 2019, 10(1): 5041. |
| 58 | ATASHEVA S, EMERSON C C, YAO J, et al. Systemic cancer therapy with engineered adenovirus that evades innate immunity[J]. Science Translational Medicine, 2020, 12(571): eabc6659. |
| 59 | EVGIN L, KOTTKE T, TONNE J, et al. Oncolytic virus-mediated expansion of dual-specific CAR T cells improves efficacy against solid tumors in mice[J]. Science Translational Medicine, 2022, 14(640): eabn2231. |
| 60 | KENNEDY E M, DENSLOW A, HEWETT J, et al. Development of intravenously administered synthetic RNA virus immunotherapy for the treatment of cancer[J]. Nature Communications, 2022, 13(1): 5907. |
| 61 | NIEMANN J, WOLLER N, BROOKS J, et al. Molecular retargeting of antibodies converts immune defense against oncolytic viruses into cancer immunotherapy[J]. Nature Communications, 2019, 10(1): 3236. |
| 62 | SVENSSON-ARVELUND J, CUADRADO-CASTANO S, PANTSULAIA G, et al. Expanding cross-presenting dendritic cells enhances oncolytic virotherapy and is critical for long-term anti-tumor immunity[J]. Nature Communications, 2022, 13(1): 7149. |
| 63 | XU B, TIAN L, CHEN J, et al. An oncolytic virus expressing a full-length antibody enhances antitumor innate immune response to glioblastoma[J]. Nature Communications, 2021, 12(1): 5908. |
| 64 | WANG S Q, YAN W, KONG L K, et al. Oncolytic viruses engineered to enforce cholesterol efflux restore tumor-associated macrophage phagocytosis and anti-tumor immunity in glioblastoma[J]. Nature Communications, 2023, 14(1): 4367. |
| 65 | NAKAO S, ARAI Y, TASAKI M, et al. Intratumoral expression of IL-7 and IL-12 using an oncolytic virus increases systemic sensitivity to immune checkpoint blockade[J]. Science Translational Medicine, 2020, 12(526): eaax7992. |
| 66 | LIU Z Q, GE Y, WANG H Y, et al. Modifying the cancer-immune set point using vaccinia virus expressing re-designed interleukin-2[J]. Nature Communications, 2018, 9(1): 4682. |
| 67 | WANG G, KANG X, CHEN K S, et al. An engineered oncolytic virus expressing PD-L1 inhibitors activates tumor neoantigen-specific T cell responses[J]. Nature Communications, 2020, 11: 1395. |
| 68 | SHEKARIAN T, SIVADO E, JALLAS A C, et al. Repurposing rotavirus vaccines for intratumoral immunotherapy can overcome resistance to immune checkpoint blockade[J]. Science Translational Medicine, 2019, 11(515): eaat5025. |
| 69 | RIVADENEIRA D B, DEPEAUX K, WANG Y Y, et al. Oncolytic viruses engineered to enforce leptin expression reprogram tumor-infiltrating T cell metabolism and promote tumor clearance[J]. Immunity, 2019, 51(3): 548-560.e4. |
| 70 | RUSSELL L, SWANNER J, JAIME-RAMIREZ A C, et al. PTEN expression by an oncolytic herpesvirus directs T-cell mediated tumor clearance[J]. Nature Communications, 2018, 9(1): 5006. |
| 71 | LU Y, HE W B, HUANG X, et al. Strategies to package recombinant Adeno-Associated Virus expressing the N-terminal gasdermin domain for tumor treatment[J]. Nature Communications, 2021, 12: 7155. |
| 72 | LIN J, SUN S H, ZHAO K, et al. Oncolytic Parapoxvirus induces Gasdermin E-mediated pyroptosis and activates antitumor immunity[J]. Nature Communications, 2023, 14(1): 224. |
| 73 | WU A L, LI Z Y, WANG Y L, et al. Recombinant measles virus vaccine rMV-Hu191 exerts an oncolytic effect on esophageal squamous cell carcinoma via caspase-3/GSDME-mediated pyroptosis[J]. Cell Death Discovery, 2023, 9(1): 171. |
| 74 | TIAN L, XU B, CHEN Y Q, et al. Specific targeting of glioblastoma with an oncolytic virus expressing a cetuximab-CCL5 fusion protein via innate and adaptive immunity[J]. Nature Cancer, 2022, 3(11): 1318-1335. |
| 75 | JI D Z, ZHANG Y J, SUN J Q, et al. An engineered influenza virus to deliver antigens for lung cancer vaccination[J/OL]. Nature Biotechnology, 2023[2023-08-01]. . |
| 76 | FUSCIELLO M, FONTANA F, TÄHTINEN S, et al. Artificially cloaked viral nanovaccine for cancer immunotherapy[J]. Nature Communications, 2019, 10(1): 5747. |
| 77 | ROY D G, GEOFFROY K, MARGUERIE M, et al. Adjuvant oncolytic virotherapy for personalized anti-cancer vaccination[J]. Nature Communications, 2021, 12(1): 2626. |
| 78 | D’ALISE A M, LEONI G, COTUGNO G, et al. Adenoviral vaccine targeting multiple neoantigens as strategy to eradicate large tumors combined with checkpoint blockade[J]. Nature Communications, 2019, 10(1): 2688. |
| 79 | RING S S, CUPOVIC J, ONDER L, et al. Viral vector-mediated reprogramming of the fibroblastic tumor stroma sustains curative melanoma treatment[J]. Nature Communications, 2021, 12(1): 4734. |
| 80 | SMITH R, WAFA E I, GEARY S M, et al. Cationic nanoparticles enhance T cell tumor infiltration and antitumor immune responses to a melanoma vaccine[J]. Science Advances, 2022, 8(29): eabk3150. |
| 81 | MOSAHEB M M, DOBRIKOVA E Y, BROWN M C, et al. Genetically stable poliovirus vectors activate dendritic cells and prime antitumor CD8 T cell immunity[J]. Nature Communications, 2020, 11(1): 524. |
| 82 | NATH S, MUKHERJEE P. MUC1: a multifaceted oncoprotein with a key role in cancer progression[J]. Trends in Molecular Medicine, 2014, 20(6): 332-342. |
| 83 | GRECO B, MALACARNE V, GIRARDI F D, et al. Disrupting N-glycan expression on tumor cells boosts chimeric antigen receptor T cell efficacy against solid malignancies[J]. Science Translational Medicine, 2022, 14(628): eabg3072. |
| 84 | RASKA M, CZERNEKOVA L, MOLDOVEANU Z, et al. Differential glycosylation of envelope gp120 is associated with differential recognition of HIV-1 by virus-specific antibodies and cell infection[J]. AIDS Research and Therapy, 2014, 11: 23. |
| 85 | DOORES K J. The HIV glycan shield as a target for broadly neutralizing antibodies[J]. The FEBS Journal, 2015, 282(24): 4679-4691. |
| 86 | LEK A, WONG B, KEELER A, et al. Death after high-dose rAAV9 gene therapy in a patient with duchenne’s muscular dystrophy[J]. New England Journal of Medicine, 2023, 389(13): 1203-1210. |
| 87 | GANESHAN K, NIKKANEN J, MAN K, et al. Energetic trade-offs and hypometabolic states promote disease tolerance[J]. Cell, 2019, 177(2): 399-413.e12. |
| 88 | SAXENA M, VAN T T H, BAIRD F J, et al. Pre-existing immunity against vaccine vectors-friend or foe?[J]. Microbiology, 2013, 159(Pt_1): 1-11. |
| 89 | WANG W C, SAYEDAHMED E E, MITTAL S K. Significance of preexisting vector immunity and activation of innate responses for adenoviral vector-based therapy[J]. Viruses, 2022, 14(12): 2727. |
| 90 | GLORIOSO J C, COHEN J B, GOINS W F, et al. Oncolytic HSV vectors and anti-tumor immunity[J]. Current Issues in Molecular Biology, 2021, 41: 381-468. |
| 91 | SHAW A R, SUZUKI M. Immunology of adenoviral vectors in cancer therapy[J]. Molecular Therapy Methods & Clinical Development, 2019, 15: 418-429. |
| 92 | AUGUSTO D G, MURDOLO L D, CHATZILEONTIADOU D S M, et al. A common allele of HLA is associated with asymptomatic SARS-CoV-2 infection[J]. Nature, 2023, 620(7972): 128-136. |
| [1] | 王步森, 徐婧含, 高智强, 侯利华. 病毒载体疫苗研究进展[J]. 合成生物学, 2024, 5(2): 281-293. |
| [2] | 方超, 黄卫人. 合成生物学在肿瘤疫苗设计中的应用进展[J]. 合成生物学, 2024, 5(2): 239-253. |
| [3] | 涂辉阳, 韩为东, 张斌. 肿瘤新抗原疫苗的设计与优化策略[J]. 合成生物学, 2024, 5(2): 254-266. |
| [4] | 谢君鸿, 何晶晶, 周鹏辉. 合成生物学与工程化T细胞治疗[J]. 合成生物学, 2023, 4(2): 373-393. |
| [5] | 林思思, 潘超, 张一帆, 刘尽尧. 基于表面涂层益生菌的肿瘤抗原口服递送系统[J]. 合成生物学, 2022, 3(4): 810-820. |
| [6] | 许仕琳, 许海燕. 双特异性抗体及纳米技术在肿瘤免疫治疗中的应用进展[J]. 合成生物学, 2022, 3(2): 352-368. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||