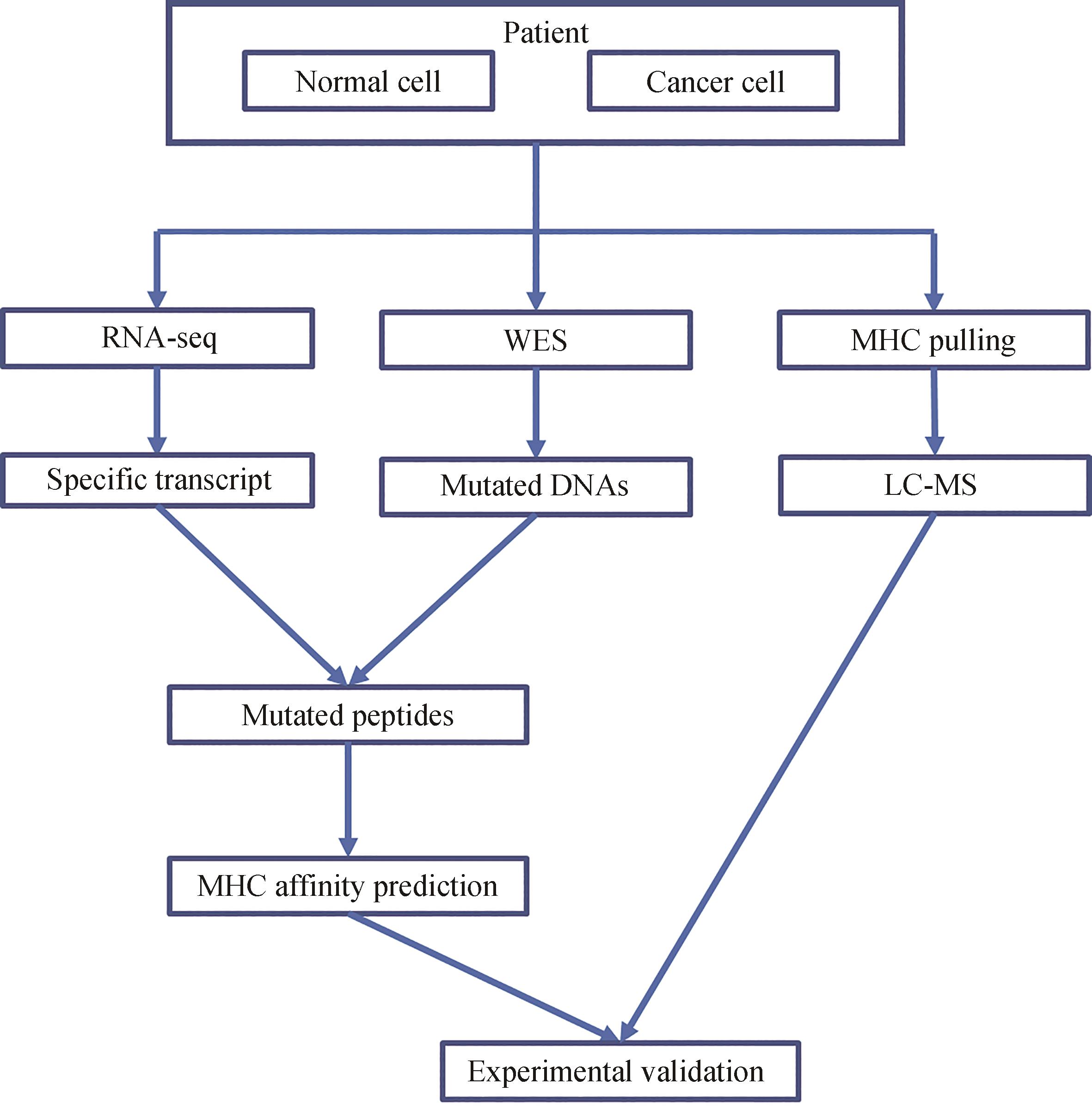
合成生物学 ›› 2024, Vol. 5 ›› Issue (2): 239-253.DOI: 10.12211/2096-8280.2023-061
合成生物学在肿瘤疫苗设计中的应用进展
方超1, 黄卫人1,2,3
- 1.中国科学院深圳先进技术研究院,合成生物学研究所,广东 深圳 518035
2.深圳大学第一附属医院,深圳市第二人民医院,泌尿外科,深圳转化医学研究院,广东 深圳 518035
3.广东省泌尿生殖肿瘤系统生物学与合成生物学重点实验室,广东 深圳 518035
-
收稿日期:2023-08-25修回日期:2024-02-29出版日期:2024-04-30发布日期:2024-04-28 -
通讯作者:黄卫人 -
作者简介:方超 (1990—),男,博士后。研究方向:(1)肿瘤合成生物学;(2)肿瘤表观遗传学。E-mail:c.fang@siat.ac.cn黄卫人 (1980—),男,研究员,博士生导师,深圳市转化医学研究院副院长。研究方向:(1)肿瘤基因组学,应用多组学手段鉴定肿瘤及微环境诊疗标志物,开发相关临床应用;(2)肿瘤类器官,利用体外培养系统还原肿瘤体内生长,药物筛选及耐药机制研究;(3)医学合成生物学,创新肿瘤治疗新方法。E-mail:pony8980@163.com -
基金资助:国家重点研发计划(2019YFA0906003);国家自然科学基金(81972368);广东省培养高层次人才特殊支持计划(2021JC06Y578)
Progress with the application of synthetic biology in designing of cancer vaccines
FANG Chao1, HUANG Weiren1,2,3
- 1.Institute of Synthetic Biology,Shenzhen Institute of Advanced Technology,Chinese Academy of Sciences,Shenzhen 518035,Guangdong,China
2.Department of Urology,The First Affiliated Hospital of Shenzhen University,Shenzhen Second People’s Hospital,Shenzhen Institute of Translational Medicine,Shenzhen 518035,Guangdong,China
3.Guangdong Provincial Key Laboratory of Systems Biology and Synthetic Biology for Urogenital Tumors,Shenzhen 518035,Guangdong,China
-
Received:2023-08-25Revised:2024-02-29Online:2024-04-30Published:2024-04-28 -
Contact:HUANG Weiren
摘要:
根据中心法则和细胞免疫学原则,利用合成生物学设计和生产新型肿瘤疫苗代表了癌症免疫治疗中的一个重要途径。本文概述了利用合成生物学针对两个主要方面(抗原选择和疫苗设计)的创新治疗性肿瘤疫苗的最新研究进展。针对肿瘤相关或特定抗原,开发更精确和有效的肿瘤疫苗引起了广泛关注。传统方法在抗原选择中主要针对肿瘤中的特定基因,而以高通量测序及质谱为基础筛选新抗原的方法明显改善了疫苗的靶向性及免疫原性。在疫苗类别方面,与传统多肽疫苗相比,通过对DNA、mRNA、病毒/细菌、细胞的工程化修饰而成的新型疫苗显著扩大了肿瘤疫苗的范围,从而大幅增强了不同肿瘤疫苗的免疫效果。合成生物学的快速发展将加速对肿瘤疫苗的实验研究进度,最终导致临床治疗效果的持续增强。
中图分类号:
引用本文
方超, 黄卫人. 合成生物学在肿瘤疫苗设计中的应用进展[J]. 合成生物学, 2024, 5(2): 239-253.
FANG Chao, HUANG Weiren. Progress with the application of synthetic biology in designing of cancer vaccines[J]. Synthetic Biology Journal, 2024, 5(2): 239-253.
| 肿瘤抗原 | 抗原类型 | 表达位置 | 高丰度表达位置 |
|---|---|---|---|
| 肿瘤相关性抗原 | 高表达蛋白或者多肽 | 肿瘤或正常细胞 | 肿瘤 |
| 肿瘤种系抗原 | 肿瘤,生殖细胞 | 肿瘤,生殖细胞 | |
| 肿瘤特异性抗原 | 肿瘤病毒 | 病毒性肿瘤 | 病毒性肿瘤 |
| 肿瘤新抗原 | 肿瘤 | 肿瘤 |
表1 肿瘤抗原分类
Table 1 Classification of cancer antigens
| 肿瘤抗原 | 抗原类型 | 表达位置 | 高丰度表达位置 |
|---|---|---|---|
| 肿瘤相关性抗原 | 高表达蛋白或者多肽 | 肿瘤或正常细胞 | 肿瘤 |
| 肿瘤种系抗原 | 肿瘤,生殖细胞 | 肿瘤,生殖细胞 | |
| 肿瘤特异性抗原 | 肿瘤病毒 | 病毒性肿瘤 | 病毒性肿瘤 |
| 肿瘤新抗原 | 肿瘤 | 肿瘤 |
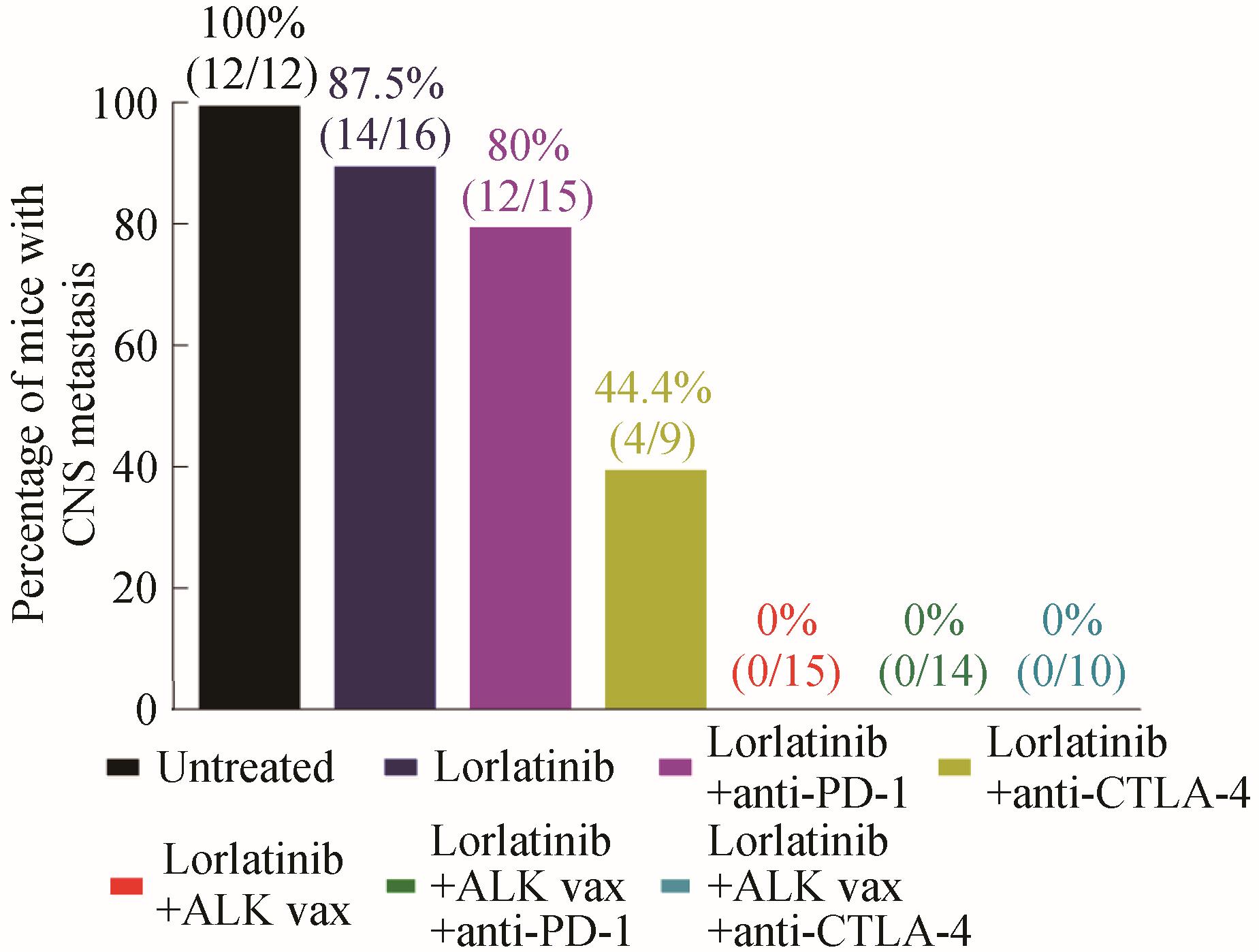
图2 ALK 多肽疫苗抑制肿瘤转移效果[72](中枢神经系统转移肿瘤实验鼠数量的比例)
Fig. 2 Inhibition of tumor metastasis by ALK peptide vaccine characterized by the number of tested mice with metastatic tumors in their central nervous systems[72]
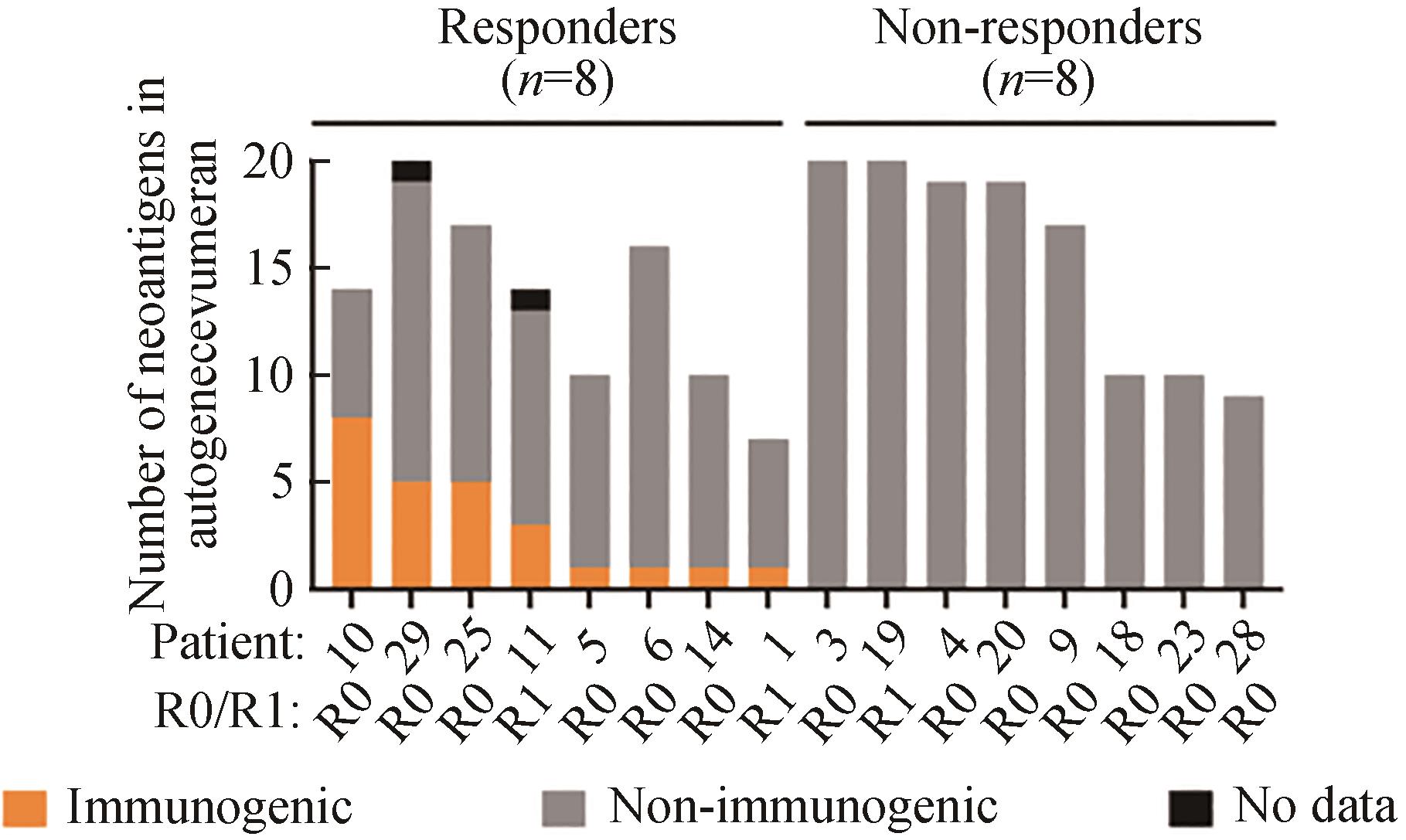
图3 病人对autogene cevumera疫苗的免疫反应[88](接种新疫苗抗原后,在患者体内收集的PBMC中疫苗诱导的IFNγ+ T细胞数量。R0/R1表示手术切除边缘的状态)
Fig. 3 Immune response of the patients to the autogene cevumera vaccine[88](The number of vaccine-induced IFNγ+ T cells in PBMC collected from the patients after vaccination with new vaccine antigens. R0/R1 indicates surgical resection margin status. Adapted with permission from reference.)
| 1 | BOYLSTON A. The origins of inoculation[J]. Journal of the Royal Society of Medicine, 2012, 105(7): 309-313. |
| 2 | DEMARIA P J, BILUSIC M. Cancer vaccines[J]. Hematology/Oncology Clinics of North America, 2019, 33(2): 199-214. |
| 3 | GARY E N, WEINER D B. DNA vaccines: prime time is now[J]. Current Opinion in Immunology, 2020, 65: 21-27. |
| 4 | BECK J D, REIDENBACH D, SALOMON N, et al. mRNA therapeutics in cancer immunotherapy[J]. Molecular Cancer, 2021, 20(1): 69. |
| 5 | GARG A D, COULIE P G, VAN DEN EYNDE B J, et al. Integrating next-generation dendritic cell vaccines into the current cancer immunotherapy landscape[J]. Trends in Immunology, 2017, 38(8): 577-593. |
| 6 | FAN T, ZHANG M N, YANG J X, et al. Therapeutic cancer vaccines: advancements, challenges, and prospects[J]. Signal Transduction and Targeted Therapy, 2023, 8(1): 450. |
| 7 | GEBRE M S, BRITO L A, TOSTANOSKI L H, et al. Novel approaches for vaccine development[J]. Cell, 2021, 184(6): 1589-1603. |
| 8 | MINATI R, PERREAULT C, THIBAULT P. A roadmap toward the definition of actionable tumor-specific antigens[J]. Frontiers in Immunology, 2020, 11: 583287. |
| 9 | CHEN G, HUANG A C, ZHANG W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response[J]. Nature, 2018, 560(7718): 382-386. |
| 10 | KALAORA S, NAGLER A, WARGO J A, et al. Mechanisms of immune activation and regulation: lessons from melanoma[J]. Nature Reviews Cancer, 2022, 22(4): 195-207. |
| 11 | CHIA W K, WANG W W, TEO M, et al. A phase Ⅱ study evaluating the safety and efficacy of an adenovirus-ΔLMP1-LMP2 transduced dendritic cell vaccine in patients with advanced metastatic nasopharyngeal carcinoma[J]. Annals of Oncology, 2012, 23(4): 997-1005. |
| 12 | KENTER G G, WELTERS M J P, VALENTIJN A R P M, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia[J]. The New England Journal of Medicine, 2009, 361(19): 1838-1847. |
| 13 | TRIMBLE C L, MORROW M P, KRAYNYAK K A, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial[J]. The Lancet, 2015, 386(10008): 2078-2088. |
| 14 | HARPER D M, NIEMINEN P, DONDERS G, et al. The efficacy and safety of Tipapkinogen Sovacivec therapeutic HPV vaccine in cervical intraepithelial neoplasia grades 2 and 3: randomized controlled phase Ⅱ trial with 2.5 years of follow-up[J]. Gynecologic Oncology, 2019, 153(3): 521-529. |
| 15 | KIM T J, JIN H T, HUR S Y, et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients[J]. Nature Communications, 2014, 5: 5317. |
| 16 | PIPERNO-NEUMANN S, HASSEL J C, RUTKOWSKI P, et al. Abstract CT002: phase 3 randomized trial comparing tebentafusp with investigator’s choice in first line metastatic uveal melanoma[J]. Cancer Research, 2021, 81(): CT002. |
| 17 | ROSENBERG S A, YANG J C, SCHWARTZENTRUBER D J, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma[J]. Nature Medicine, 1998, 4(3): 321-327. |
| 18 | CUNHA A C, WEIGLE B, KIESSLING A, et al. Tissue-specificity of prostate specific antigens: comparative analysis of transcript levels in prostate and non-prostatic tissues[J]. Cancer Letters, 2006, 236(2): 229-238. |
| 19 | KANTOFF P W, HIGANO C S, SHORE N D, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer[J]. The New England Journal of Medicine, 2010, 363(5): 411-422. |
| 20 | O’ROURKE D M, NASRALLAH M P, DESAI A, et al. A single dose of peripherally infused EGFRvⅢ-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma[J]. Science Translational Medicine, 2017, 9(399): eaaa0984. |
| 21 | ZAHEDIPOUR F, ZAMANI P, MASHREGHI M, et al. Nanoliposomal VEGF-R2 peptide vaccine acts as an effective therapeutic vaccine in a murine B16F10 model of melanoma[J]. Cancer Nanotechnology, 2023, 14(1): 62. |
| 22 | SCHUSTER J, LAI R K, RECHT L D, et al. A phase Ⅱ, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT Ⅲ study[J]. Neuro-oncology, 2015, 17(6): 854-861. |
| 23 | REARDON D A, DESJARDINS A, VREDENBURGH J J, et al. Rindopepimut with bevacizumab for patients with relapsed EGFRvⅢ-expressing glioblastoma (ReACT): results of a double-blind randomized phase Ⅱ trial[J]. Clinical Cancer Research, 2020, 26(7): 1586-1594. |
| 24 | WELLER M, BUTOWSKI N, TRAN D D, et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvⅢ-expressing glioblastoma (ACT Ⅳ): a randomised, double-blind, international phase 3 trial[J]. The Lancet Oncology, 2017, 18(10): 1373-1385. |
| 25 | LIU C, YE D Y, YANG H L, et al. RAS-targeted cancer therapy: advances in drugging specific mutations[J]. MedComm, 2023, 4(3): e285. |
| 26 | BANNOURA S F, UDDIN M H, NAGASAKA M, et al. Targeting KRAS in pancreatic cancer: new drugs on the horizon[J]. Cancer Metastasis Reviews, 2021, 40(3): 819-835. |
| 27 | LIU X Q, HUANG P, YANG R S, et al. mRNA cancer vaccines: construction and boosting strategies[J]. ACS Nano, 2023, 17(20): 19550-19580. |
| 28 | CHEEVER M A, ALLISON J P, FERRIS A S, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research[J]. Clinical Cancer Research, 2009, 15(17): 5323-5337. |
| 29 | QI X W, ZHANG F, WU H, et al. Wilms’ tumor 1 (WT1) expression and prognosis in solid cancer patients: a systematic review and meta-analysis[J]. Scientific Reports, 2015, 5: 8924. |
| 30 | OKA Y, TSUBOI A, TAGUCHI T, et al. Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression[J]. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(38): 13885-13890. |
| 31 | MASLAK P G, TAO D, BERNAL Y, et al. Phase 2 trial of a multivalent WT1 peptide vaccine (galinpepimut-S) in acute myeloid leukemia[J]. Blood Advances, 2018, 2(3): 224-234. |
| 32 | KEILHOLZ U, LETSCH A, BUSSE A, et al. A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS[J]. Blood, 2009, 113(26): 6541-6548. |
| 33 | ANGUILLE S, VAN DE VELDE A L, SMITS E L, et al. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia[J]. Blood, 2017, 130(15): 1713-1721. |
| 34 | VANSTEENKISTE J, ZIELINSKI M, LINDER A, et al. Adjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: phase Ⅱ randomized study results[J]. Journal of Clinical Oncology, 2013, 31(19): 2396-2403. |
| 35 | KRUIT W H J, SUCIU S, DRENO B, et al. Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: results of a randomized phase Ⅱ study of the European Organisation for Research and Treatment of Cancer Melanoma Group in Metastatic Melanoma[J]. Journal of Clinical Oncology, 2013, 31(19): 2413-2420. |
| 36 | SLINGLUFF C L, LEWIS K D, ANDTBACKA R, et al. Multicenter, double-blind, placebo-controlled trial of seviprotimut-L polyvalent melanoma vaccine in patients with post-resection melanoma at high risk of recurrence[J]. Journal for Immunotherapy of Cancer, 2021, 9(10): e003272. |
| 37 | THOMAS R, AL-KHADAIRI G, ROELANDS J, et al. NY-ESO-1 based immunotherapy of cancer: current perspectives[J]. Frontiers in Immunology, 2018, 9: 947. |
| 38 | DHODAPKAR M V, SZNOL M, ZHAO B W, et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205[J]. Science Translational Medicine, 2014, 6(232): 232ra51. |
| 39 | GASSER O, SHARPLES K J, BARROW C, et al. A phase Ⅰ vaccination study with dendritic cells loaded with NY-ESO-1 and α-galactosylceramide: induction of polyfunctional T cells in high-risk melanoma patients[J]. Cancer Immunology, Immunotherapy, 2018, 67(2): 285-298. |
| 40 | ODUNSI K, QIAN F, MATSUZAKI J, et al. Vaccination with an NY-ESO-1 peptide of HLA class Ⅰ/Ⅱ specificities induces integrated humoral and T cell responses in ovarian cancer[J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(31): 12837-12842. |
| 41 | MITTENDORF E A, ARDAVANIS A, SYMANOWSKI J, et al. Primary analysis of a prospective, randomized, single-blinded phase Ⅱ trial evaluating the HER2 peptide AE37 vaccine in breast cancer patients to prevent recurrence[J]. Annals of Oncology, 2016, 27(7): 1241-1248. |
| 42 | MITTENDORF E A, LU B, MELISKO M, et al. Efficacy and safety analysis of nelipepimut-S vaccine to prevent breast cancer recurrence: a randomized, multicenter, phase Ⅲ clinical trial[J]. Clinical Cancer Research, 2019, 25(14): 4248-4254. |
| 43 | LIN M H, SHEN K Y, LIU B S, et al. Immunological evaluation of a novel HLA-A2 restricted phosphopeptide of tumor associated antigen, TRAP1, on cancer therapy[J]. Vaccine: Ⅹ, 2019, 1: 100017. |
| 44 | CLIFTON G T, HALE D, VREELAND T J, et al. Results of a randomized phase Ⅱb trial of nelipepimut-S+trastuzumab versus trastuzumab to prevent recurrences in patients with high-risk HER2 low-expressing breast cancer[J]. Clinical Cancer Research, 2020, 26(11): 2515-2523. |
| 45 | COX K E, LIU S L, LWIN T M, et al. The mucin family of proteins: candidates as potential biomarkers for colon cancer[J]. Cancers, 2023, 15(5): 1491. |
| 46 | PACILIO C, ROSATI G, CRISPO A, et al. An overview of the roles of CDK4/6 inhibitors in metastatic breast cancer elderly patients[J]. In Vivo, 2023, 37(4): 1445-1449. |
| 47 | CHUNG V M, KOS F, HARDWICK N, et al. A phase 1 study of p53MVA vaccine in combination with pembrolizumab[J]. Journal of Clinical Oncology, 2018, 36(): 206. |
| 48 | KIM C, LIU S V, SUBRAMANIAM D S, et al. Phase Ⅰ study of the 177Lu-DOTA0-Tyr3-Octreotate (lutathera) in combination with nivolumab in patients with neuroendocrine tumors of the lung[J]. Journal for Immunotherapy of Cancer, 2020, 8(2): e000980. |
| 49 | ANTONIA S J, MIRZA N, FRICKE I, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer[J]. Clinical Cancer Research, 2006, 12(3 Pt 1): 878-887. |
| 50 | HARDWICK N R, FRANKEL P, RUEL C, et al. p53-Reactive T cells are associated with clinical benefit in patients with platinum-resistant epithelial ovarian cancer after treatment with a p53 vaccine and gemcitabine chemotherapy[J]. Clinical Cancer Research, 2018, 24(6): 1315-1325. |
| 51 | SPEETJENS F M, KUPPEN P J K, WELTERS M J P, et al. Induction of p53-specific immunity by a p53 synthetic long peptide vaccine in patients treated for metastatic colorectal cancer[J]. Clinical Cancer Research, 2009, 15(3): 1086-1095. |
| 52 | CHUNG V, KOS F J, HARDWICK N, et al. Evaluation of safety and efficacy of p53MVA vaccine combined with pembrolizumab in patients with advanced solid cancers[J]. Clinical & Translational Oncology, 2019, 21(3): 363-372. |
| 53 | QUANDT J, SCHLUDE C, BARTOSCHEK M, et al. Long-peptide vaccination with driver gene mutations in p53 and Kras induces cancer mutation-specific effector as well as regulatory T cell responses[J]. Oncoimmunology, 2018, 7(12): e1500671. |
| 54 | KJELDSEN J W, LORENTZEN C L, MARTINENAITE E, et al. A phase 1/2 trial of an immune-modulatory vaccine against IDO/PD-L1 in combination with nivolumab in metastatic melanoma[J]. Nature Medicine, 2021, 27(12): 2212-2223. |
| 55 | SCHUMACHER T N, SCHREIBER R D. Neoantigens in cancer immunotherapy[J]. Science, 2015, 348(6230): 69-74. |
| 56 | XIE N, SHEN G B, GAO W, et al. Neoantigens: promising targets for cancer therapy[J]. Signal Transduction and Targeted Therapy, 2023, 8(1): 9. |
| 57 | WANG L, SHAMARDANI K, BABIKIR H, et al. The evolution of alternative splicing in glioblastoma under therapy[J]. Genome Biology, 2021, 22(1): 48. |
| 58 | JUHARI W K W, AHMAD AMIN NOORDIN K B, ZAKARIA A D, et al. Whole-genome profiles of Malay colorectal cancer patients with intact MMR proteins[J]. Genes, 2021, 12(9): 1448. |
| 59 | HANSEN U K, RAMSKOV S, BJERREGAARD A M, et al. Tumor-infiltrating T cells from clear cell renal cell carcinoma patients recognize neoepitopes derived from point and frameshift mutations[J]. Frontiers in Immunology, 2020, 11: 373. |
| 60 | LU S X, NEEF E D, THOMAS J D, et al. Pharmacologic modulation of RNA splicing enhances anti-tumor immunity[J]. Cell, 2021, 184(15): 4032-4047.e31. |
| 61 | DAO T, MUN S S, MOLVI Z, et al. A TCR mimic monoclonal antibody reactive with the “public” phospho-neoantigen pIRS2/HLA-A*02: 01 complex[J]. JCI Insight, 2022, 7(5): e151624. |
| 62 | KRUMP N A, YOU J X. From merkel cell polyomavirus infection to merkel cell carcinoma oncogenesis[J]. Frontiers in Microbiology, 2021, 12: 739695. |
| 63 | ZHANG W T, ZHU G L, XU W Q, et al. Association of PD-1/PD-L1 expression and Epstein: Barr virus infection in patients with invasive breast cancer[J]. Diagnostic Pathology, 2022, 17(1): 61. |
| 64 | CHAN C K, AIMAGAMBETOVA G, UKYBASSOVA T, et al. Human papillomavirus infection and cervical cancer: epidemiology, screening, and vaccination-review of current perspectives[J]. Journal of Oncology, 2019, 2019: 3257939. |
| 65 | PURCELL A W, RAMARATHINAM S H, TERNETTE N. Mass spectrometry-based identification of MHC-bound peptides for immunopeptidomics[J]. Nature Protocols, 2019, 14: 1687-1707. |
| 66 | KRISTENSEN N P, HEEKE C, TVINGSHOLM S A, et al. Neoantigen-reactive CD8+ T cells affect clinical outcome of adoptive cell therapy with tumor-infiltrating lymphocytes in melanoma[J]. The Journal of Clinical Investigation, 2022, 132(2): e150535. |
| 67 | HOLM J S, FUNT S A, BORCH A, et al. Neoantigen-specific CD8 T cell responses in the peripheral blood following PD-L1 blockade might predict therapy outcome in metastatic urothelial carcinoma[J]. Nature Communications, 2022, 13(1): 1935. |
| 68 | BISWAS N, CHAKRABARTI S, PADUL V, et al. Designing neoantigen cancer vaccines, trials, and outcomes[J]. Frontiers in Immunology, 2023, 14: 1105420. |
| 69 | VITA R, MAHAJAN S, OVERTON J A, et al. The Immune Epitope Database (IEDB): 2018 update[J]. Nucleic Acids Research, 2019, 47(D1): D339-D343. |
| 70 | ZHOU W J, QU Z, SONG C Y, et al. NeoPeptide: an immunoinformatic database of T-cell-defined neoantigens[J]. Database, 2019, 2019: baz128. |
| 71 | CHAROENTONG P, FINOTELLO F, ANGELOVA M, et al. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade[J]. Cell Reports, 2017, 18(1): 248-262. |
| 72 | WU J C, ZHAO W Y, ZHOU B B, et al. TSNAdb: a database for tumor-specific neoantigens from immunogenomics data analysis[J]. Genomics, Proteomics & Bioinformatics, 2018, 16(4): 276-282. |
| 73 | SHAW A T, BAUER T M, MARINIS F D, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer[J]. The New England Journal of Medicine, 2020, 383(21): 2018-2029. |
| 74 | PETERS S, CAMIDGE D R, SHAW A T, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer[J]. The New England Journal of Medicine, 2017, 377(9): 829-838. |
| 75 | CAMIDGE D R, KIM H R, AHN M J, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer[J]. The New England Journal of Medicine, 2018, 379(21): 2027-2039. |
| 76 | KWAK E L, BANG Y J, CAMIDGE D R, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer[J]. The New England Journal of Medicine, 2010, 363(18): 1693-1703. |
| 77 | SHAW A T, KIM T M, CRINÒ L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial[J]. The Lancet Oncology, 2017, 18(7): 874-886. |
| 78 | ZHANG I, ZAORSKY N G, PALMER J D, et al. Targeting brain metastases in ALK-rearranged non-small-cell lung cancer[J]. The Lancet Oncology, 2015, 16(13): e510-e521. |
| 79 | JOHUNG K L, YEH N, DESAI N B, et al. Extended survival and prognostic factors for patients with ALK-rearranged non-small-cell lung cancer and brain metastasis[J]. Journal of Clinical Oncology, 2016, 34(2): 123-129. |
| 80 | MOTA I, PATRUCCO E, MASTINI C, et al. ALK peptide vaccination restores the immunogenicity of ALK-rearranged non-small cell lung cancer[J]. Nature Cancer, 2023, 4(7): 1016-1035. |
| 81 | CHOI Y M, KIM D H, JANG J, et al. A hepatitis B virus-derived peptide combined with HBsAg exerts an anti-HBV effect in an HBV transgenic mouse model as a therapeutic vaccine[J]. Frontiers in Immunology, 2023, 14: 1155637. |
| 82 | SURI S, DAKSHANAMURTHY S. IntegralVac: a machine learning-based comprehensive multivalent epitope vaccine design method[J]. Vaccines, 2022, 10(10): 1678. |
| 83 | HU Z T, LEET D E, ALLESØE R L, et al. Personal neoantigen vaccines induce persistent memory T cell responses and epitope spreading in patients with melanoma[J]. Nature Medicine, 2021, 27(3): 515-525. |
| 84 | WIEDERMANN U, GARNER-SPITZER E, CHAO Y E, et al. Clinical and immunologic responses to a B-cell epitope vaccine in patients with HER2/neu-overexpressing advanced gastric cancer-results from phase ib trial IMU.ACS.001[J]. Clinical Cancer Research, 2021, 27(13): 3649-3660. |
| 85 | PANDYA A, SHAH Y, KOTHARI N, et al. The future of cancer immunotherapy: DNA vaccines leading the way[J]. Medical Oncology, 2023, 40(7): 200. |
| 86 | STRIOGA M M, DARINSKAS A, PASUKONIENE V, et al. Xenogeneic therapeutic cancer vaccines as breakers of immune tolerance for clinical application: to use or not to use?[J]. Vaccine, 2014, 32(32): 4015-4024. |
| 87 | RICCARDO F, BOLLI E, MACAGNO M, et al. Chimeric DNA vaccines: an effective way to overcome immune tolerance[M/OL]//SAVELYEVA N, OTTENSMEIER C. Current topics in microbiology and immunology: cancer vaccines. Cham: Springer International Publishing, 2014: 99-122 [2023-12-01]. . |
| 88 | SAFAVI A, KEFAYAT A, ABIRI A, et al. In silico analysis of transmembrane protein 31 (TMEM31) antigen to design novel multiepitope peptide and DNA cancer vaccines against melanoma[J]. Molecular Immunology, 2019, 112: 93-102. |
| 89 | LI L J, ZHANG X L, WANG X L, et al. Optimized polyepitope neoantigen DNA vaccines elicit neoantigen-specific immune responses in preclinical models and in clinical translation[J]. Genome Medicine, 2021, 13(1): 56. |
| 90 | DURÁNTEZ M, LÓPEZ-VÁZQUEZ A B, DE CERIO A L D, et al. Induction of multiepitopic and long-lasting immune responses against tumour antigens by immunization with peptides, DNA and recombinant adenoviruses expressing minigenes[J]. Scandinavian Journal of Immunology, 2009, 69(2): 80-89. |
| 91 | KESKIN D B, ANANDAPPA A J, SUN J, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial[J]. Nature, 2019, 565(7738): 234-239. |
| 92 | MIAO L, ZHANG Y, HUANG L. mRNA vaccine for cancer immunotherapy[J]. Molecular Cancer, 2021, 20(1): 41. |
| 93 | TEWS B A, MEYERS G. Self-replicating RNA[M/OL]//KRAMPS T, ELBERS K. Methods in molecular Biology: RNA vaccines. New York, NY: Springer New York, 2017, 1499: 15-35 [2023-12-01]. . |
| 94 | SIEGEL R L, MILLER K D, WAGLE N S, et al. Cancer statistics, 2023[J]. CA: A Cancer Journal for Clinicians, 2023, 73(1): 17-48. |
| 95 | BAILEY P, CHANG D K, FORGET M A, et al. Exploiting the neoantigen landscape for immunotherapy of pancreatic ductal adenocarcinoma[J]. Scientific Reports, 2016, 6: 35848. |
| 96 | BALACHANDRAN V P, ŁUKSZA M, ZHAO J N, et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer[J]. Nature, 2017, 551(7681): 512-516. |
| 97 | ŁUKSZA M, SETHNA Z M, ROJAS L A, et al. Neoantigen quality predicts immunoediting in survivors of pancreatic cancer[J]. Nature, 2022, 606(7913): 389-395. |
| 98 | ROJAS L A, SETHNA Z, SOARES K C, et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer[J]. Nature, 2023, 618(7963): 144-150. |
| 99 | KUAI R, OCHYL L J, BAHJAT K S, et al. Designer vaccine nanodiscs for personalized cancer immunotherapy[J]. Nature Materials, 2017, 16(4): 489-496. |
| 100 | SCHLOSSER E, MUELLER M, FISCHER S, et al. TLR ligands and antigen need to be coencapsulated into the same biodegradable microsphere for the generation of potent cytotoxic T lymphocyte responses[J]. Vaccine, 2008, 26(13): 1626-1637. |
| 101 | FISCHER N O, RASLEY A, CORZETT M, et al. Colocalized delivery of adjuvant and antigen using nanolipoprotein particles enhances the immune response to recombinant antigens[J]. Journal of the American Chemical Society, 2013, 135(6): 2044-2047. |
| 102 | KOERNER J, HORVATH D, HERRMANN V L, et al. PLGA-particle vaccine carrying TLR3/RIG-I ligand Riboxxim synergizes with immune checkpoint blockade for effective anti-cancer immunotherapy[J]. Nature Communications, 2021, 12(1): 2935. |
| 103 | HEIDEGGER S, KREPPEL D, BSCHEIDER M, et al. RIG-I activating immunostimulatory RNA boosts the efficacy of anticancer vaccines and synergizes with immune checkpoint blockade[J]. EBioMedicine, 2019, 41: 146-155. |
| 104 | LI W Z, LIU J Q, CHEN M, et al. Circular RNA in cancer development and immune regulation[J]. Journal of Cellular and Molecular Medicine, 2022, 26(6): 1785-1798. |
| 105 | YU L L, XIAO Q, YU B, et al. CircRNAs in tumor immunity and immunotherapy: perspectives from innate and adaptive immunity[J]. Cancer Letters, 2023, 564: 216219. |
| 106 | BALAN S, SAXENA M, BHARDWAJ N. Dendritic cell subsets and locations[M/OL]//International review of cell and molecular biology. Amsterdam: Elsevier, 2019, 348: 1-68 [2023-12-01]. . |
| 107 | SALLUSTO F, LANZAVECCHIA A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha[J]. The Journal of Experimental Medicine, 1994, 179(4): 1109-1118. |
| 108 | LIAU L M, ASHKAN K, BREM S, et al. Association of autologous tumor lysate-loaded dendritic cell vaccination with extension of survival among patients with newly diagnosed and recurrent glioblastoma: a phase 3 prospective externally controlled cohort trial[J]. JAMA Oncology, 2023, 9(1): 112-121. |
| 109 | GABBA A, ATTARIYA R, BEHREN S, et al. MUC1 glycopeptide vaccine modified with a GalNAc glycocluster targets the macrophage galactose C-type lectin on dendritic cells to elicit an improved humoral response[J]. Journal of the American Chemical Society, 2023, 145(24): 13027-13037. |
| 110 | LIU C, LIU X, XIANG X C, et al. A nanovaccine for antigen self-presentation and immunosuppression reversal as a personalized cancer immunotherapy strategy[J]. Nature Nanotechnology, 2022, 17(5): 531-540. |
| 111 | SOLIMAN H, HOGUE D, HAN H, et al. Oncolytic T-VEC virotherapy plus neoadjuvant chemotherapy in nonmetastatic triple-negative breast cancer: a phase 2 trial[J]. Nature Medicine, 2023, 29(2): 450-457. |
| 112 | ZHU J M, KE Y H, LIU Q, et al. Engineered Lactococcus lactis secreting Flt3L and OX40 ligand for in situ vaccination-based cancer immunotherapy[J]. Nature Communications, 2022, 13(1): 7466. |
| 113 | WANG W G, XU H H, YE Q S, et al. Systemic immune responses to irradiated tumours via the transport of antigens to the tumour periphery by injected flagellate bacteria[J]. Nature Biomedical Engineering, 2022, 6(1): 44-53. |
| [1] | 高歌, 边旗, 王宝俊. 合成基因线路的工程化设计研究进展与展望[J]. 合成生物学, 2025, 6(1): 45-64. |
| [2] | 李冀渊, 吴国盛. 合成生物学视域下有机体的两种隐喻[J]. 合成生物学, 2025, 6(1): 190-202. |
| [3] | 焦洪涛, 齐蒙, 邵滨, 蒋劲松. DNA数据存储技术的法律治理议题[J]. 合成生物学, 2025, 6(1): 177-189. |
| [4] | 唐兴华, 陆钱能, 胡翌霖. 人类世中对合成生物学的哲学反思[J]. 合成生物学, 2025, 6(1): 203-212. |
| [5] | 徐怀胜, 石晓龙, 刘晓光, 徐苗苗. DNA存储的关键技术:编码、纠错、随机访问与安全性[J]. 合成生物学, 2025, 6(1): 157-176. |
| [6] | 石婷, 宋展, 宋世怡, 张以恒. 体外生物转化(ivBT):生物制造的新前沿[J]. 合成生物学, 2024, 5(6): 1437-1460. |
| [7] | 柴猛, 王风清, 魏东芝. 综合利用木质纤维素生物转化合成有机酸[J]. 合成生物学, 2024, 5(6): 1242-1263. |
| [8] | 邵明威, 孙思勉, 杨时茂, 陈国强. 基于极端微生物的生物制造[J]. 合成生物学, 2024, 5(6): 1419-1436. |
| [9] | 陈雨, 张康, 邱以婧, 程彩云, 殷晶晶, 宋天顺, 谢婧婧. 微生物电合成技术转化二氧化碳研究进展[J]. 合成生物学, 2024, 5(5): 1142-1168. |
| [10] | 郑皓天, 李朝风, 刘良叙, 王嘉伟, 李恒润, 倪俊. 负碳人工光合群落的设计、优化与应用[J]. 合成生物学, 2024, 5(5): 1189-1210. |
| [11] | 夏孔晨, 徐维华, 吴起. 光酶催化混乱性反应的研究进展[J]. 合成生物学, 2024, 5(5): 997-1020. |
| [12] | 陈子苓, 向阳飞. 类器官技术与合成生物学协同研究进展[J]. 合成生物学, 2024, 5(4): 795-812. |
| [13] | 蔡冰玉, 谭象天, 李伟. 合成生物学在干细胞工程化改造中的研究进展[J]. 合成生物学, 2024, 5(4): 782-794. |
| [14] | 谢皇, 郑义蕾, 苏依婷, 阮静怡, 李永泉. 放线菌聚酮类化合物生物合成体系重构研究进展[J]. 合成生物学, 2024, 5(3): 612-630. |
| [15] | 查文龙, 卜兰, 訾佳辰. 中药药效成分群的合成生物学研究进展[J]. 合成生物学, 2024, 5(3): 631-657. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||