合成生物学 ›› 2024, Vol. 5 ›› Issue (5): 1072-1101.DOI: 10.12211/2096-8280.2024-018
糖蛋白合成的研究进展
杨皓然1, 叶发荣1, 黄平1, 王平1,2
- 1.上海交通大学化学化工学院,张江高等研究院,糖化学生物学中心,变革性分子前沿科学中心,上海市手性药物分子工程重点实验室,上海 200240
2.上海交通大学四川研究院,四川 成都 610218
-
收稿日期:2024-02-04修回日期:2024-03-20出版日期:2024-10-31发布日期:2024-11-20 -
通讯作者:黄平,王平 -
作者简介:杨皓然 (2000—),男,硕士研究生。研究方向为糖蛋白的合成及其生物活性评价。 E-mail:amphibiouskii@sjtu.edu.cn黄平 (1983—),男,助理研究员。研究方向为药物递送与肿瘤治疗。 E-mail:hp158@sjtu.edu.cn王平 (1978—),男,研究员,博士生导师。研究方向为糖蛋白的精确合成及生物学。 E-mail:wangp1@sjtu.edu.cn -
基金资助:国家杰出青年科学基金(22225701);国家自然科学基金(92253302);上海基础研究特区计划(21TQ1400210)
Recent advances in glycoprotein synthesis
YANG Haoran1, YE Farong1, HUANG Ping1, WANG Ping1,2
- 1.Shanghai Jiao Tong University,School of Chemistry and Chemical Engineering,Zhangjiang Institute for Advanced Study,Center for Chemical Glycobiology,Frontiers Science Center for Transformative Molecules,Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs,Shanghai 200240,China
2.Shanghai Jiao Tong University,Sichuan Institute,Chengdu 610218,Sichuan,China
-
Received:2024-02-04Revised:2024-03-20Online:2024-10-31Published:2024-11-20 -
Contact:HUANG Ping, WANG Ping
摘要:
糖基化修饰在各类生理活动中起着非常关键的作用。然而,天然糖蛋白中糖型结构的微观异质性,严重阻碍了糖型结构与糖蛋白功能之间构效关系的研究。因此,从分子层面精确控制糖蛋白结构的化学合成方法是解决这一难题的重要手段。随着蛋白质合成和糖工程技术的不断进步,研究者们发展多种蛋白质连接策略和糖苷键构建策略来精确合成均质糖蛋白。本综述将从化学、酶法构建糖苷键的角度梳理现有的糖蛋白全合成以及结合生物表达方法的半合成策略,分别介绍这些策略在合成带有N-糖基化、O-糖基化修饰等不同糖基化类型的复杂均质糖蛋白时所取得的成果,例如带有多个复杂N-糖基化修饰的HSV gD和含有长疏水片段的IL-2;以及运用这些合成的复杂糖蛋白了解糖基化修饰在各项生理过程中的构效关系所作出的突破,例如抗原糖蛋白中糖链长短与免疫原性的关系、O-GlcNAc调节神经元突触功能的机制等。最后总结了现有合成策略在糖苷键构建、纯化策略、蛋白溶解度等方面取得的进展,并指出选择性和合成产率的进一步优化仍是糖蛋白合成领域亟待解决的问题,而糖蛋白合成技术在开发免疫疗法以及理解各种疾病分子机理等方面的广泛应用,从理解原理到开发产品方面拓展了合成科学在生命健康领域的发展方向。
中图分类号:
引用本文
杨皓然, 叶发荣, 黄平, 王平. 糖蛋白合成的研究进展[J]. 合成生物学, 2024, 5(5): 1072-1101.
YANG Haoran, YE Farong, HUANG Ping, WANG Ping. Recent advances in glycoprotein synthesis[J]. Synthetic Biology Journal, 2024, 5(5): 1072-1101.
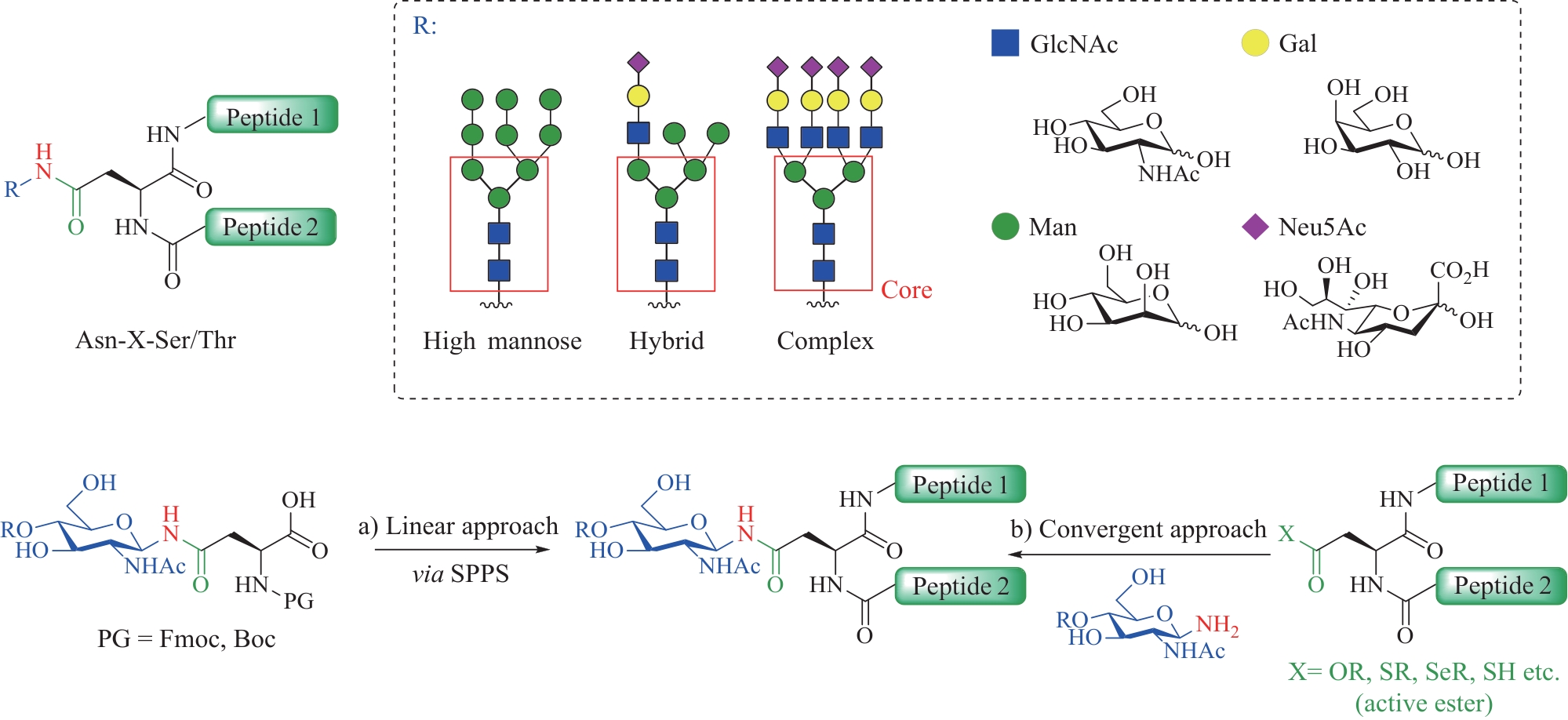
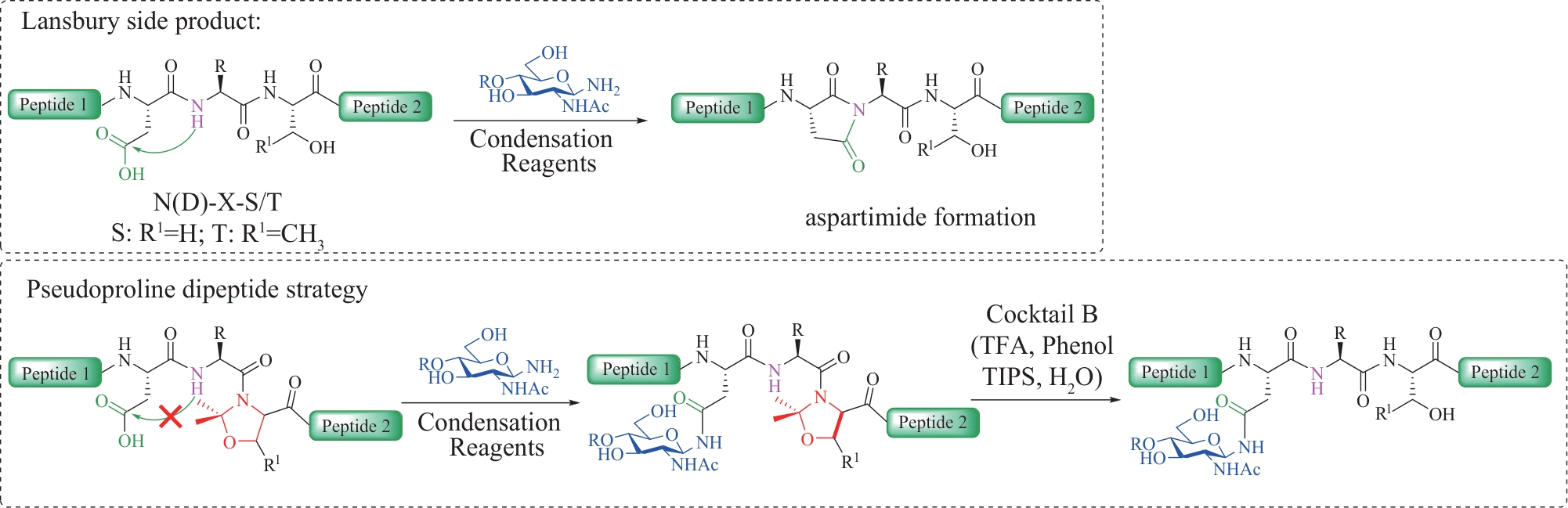
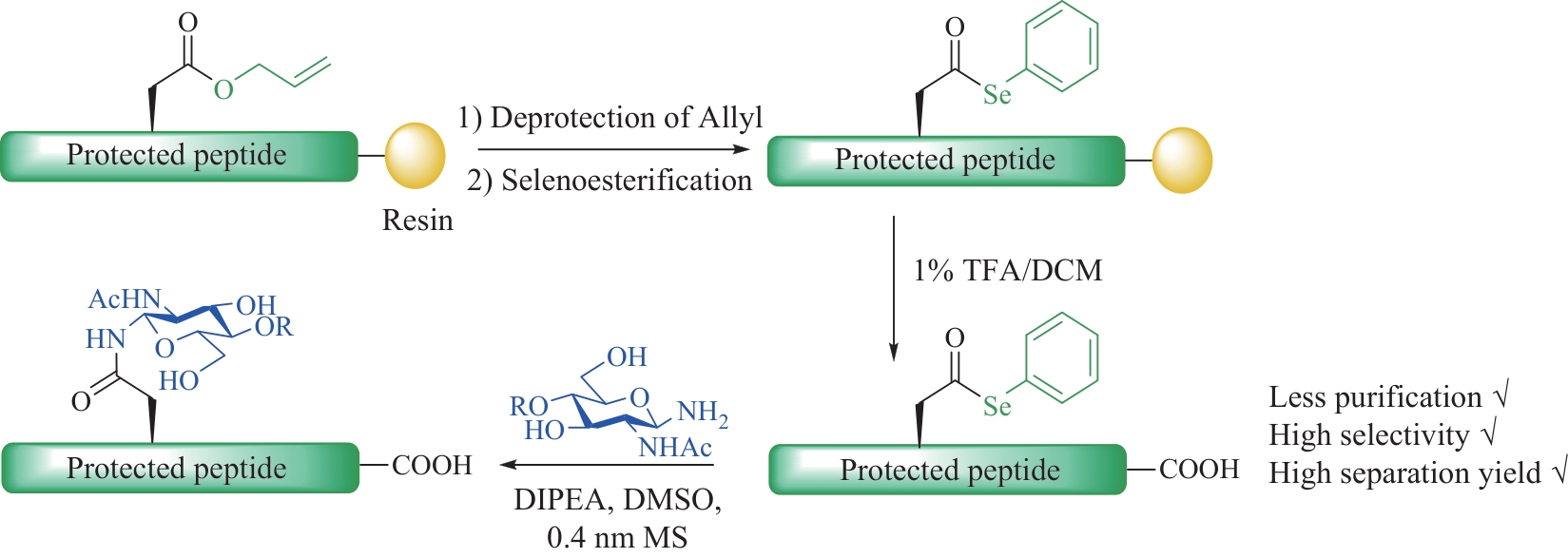
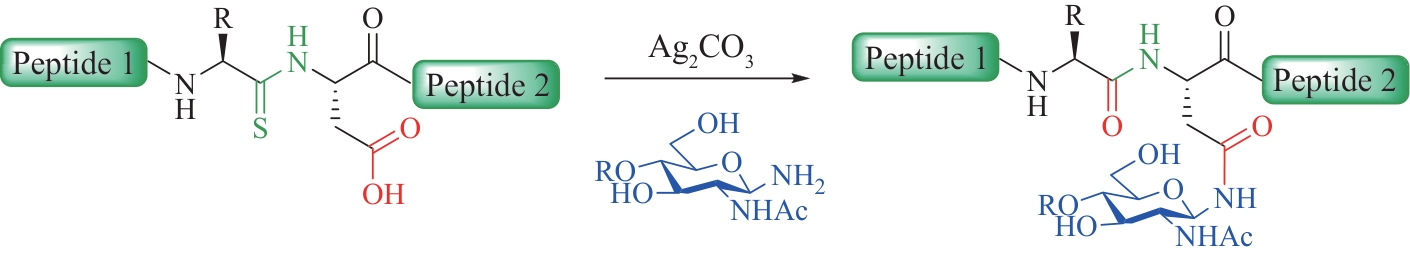
图1 N-连接糖基化修饰的特点与N-连接糖肽主要的化学合成方法
Fig.1 Characteristics of N-linked glycosylation modifications and the main chemical synthesis methods of N-linked glycopeptides
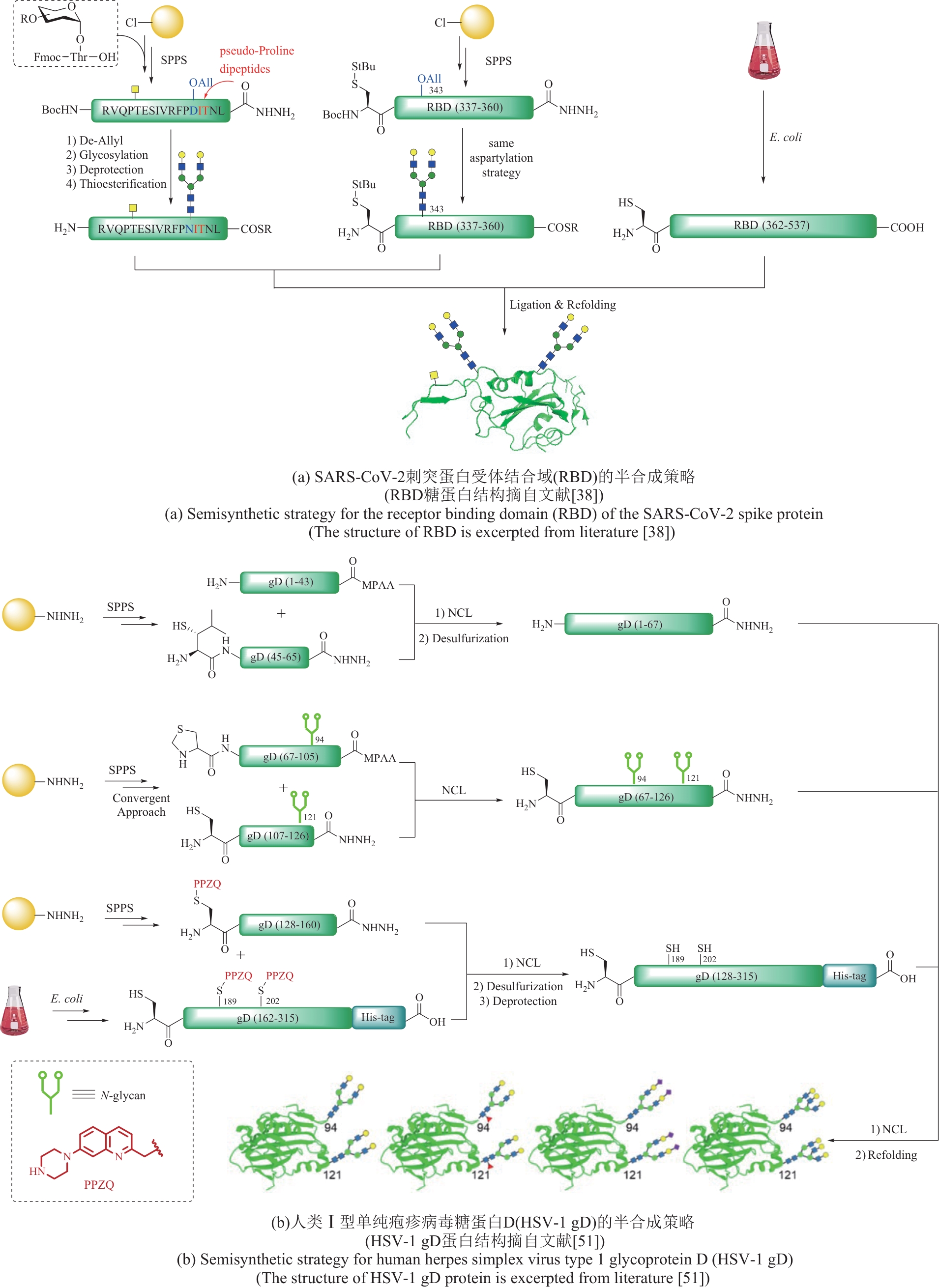
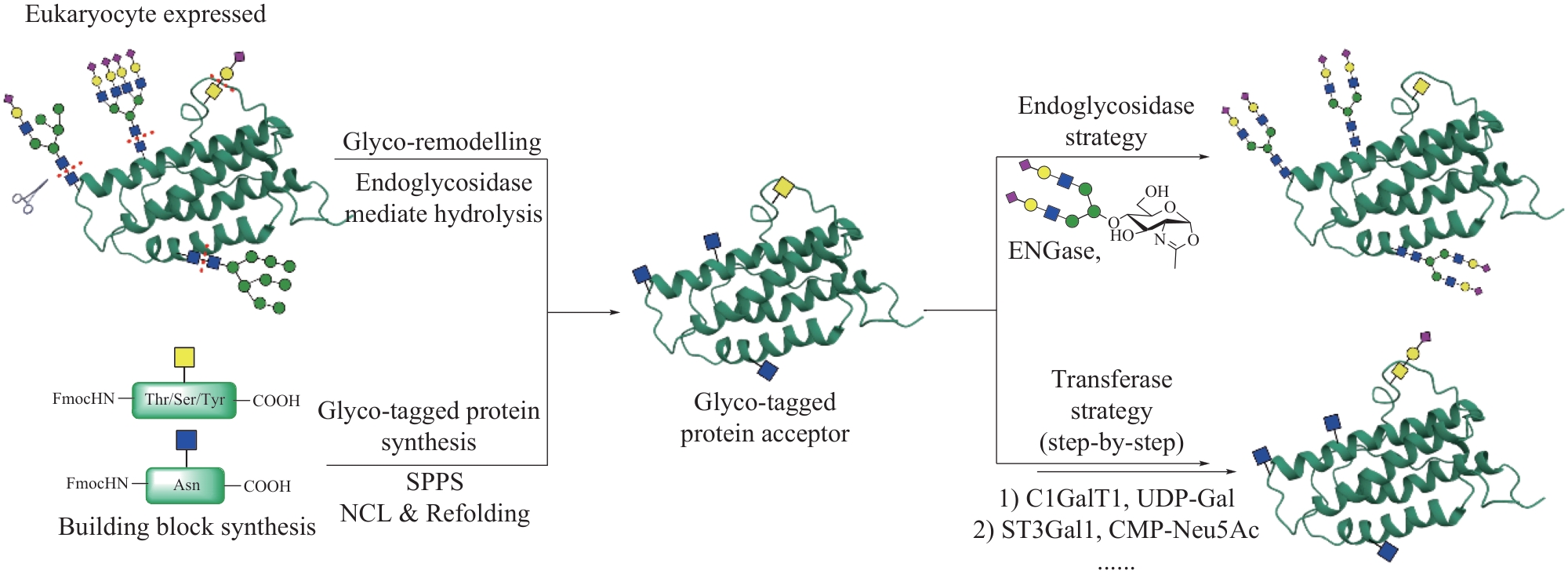
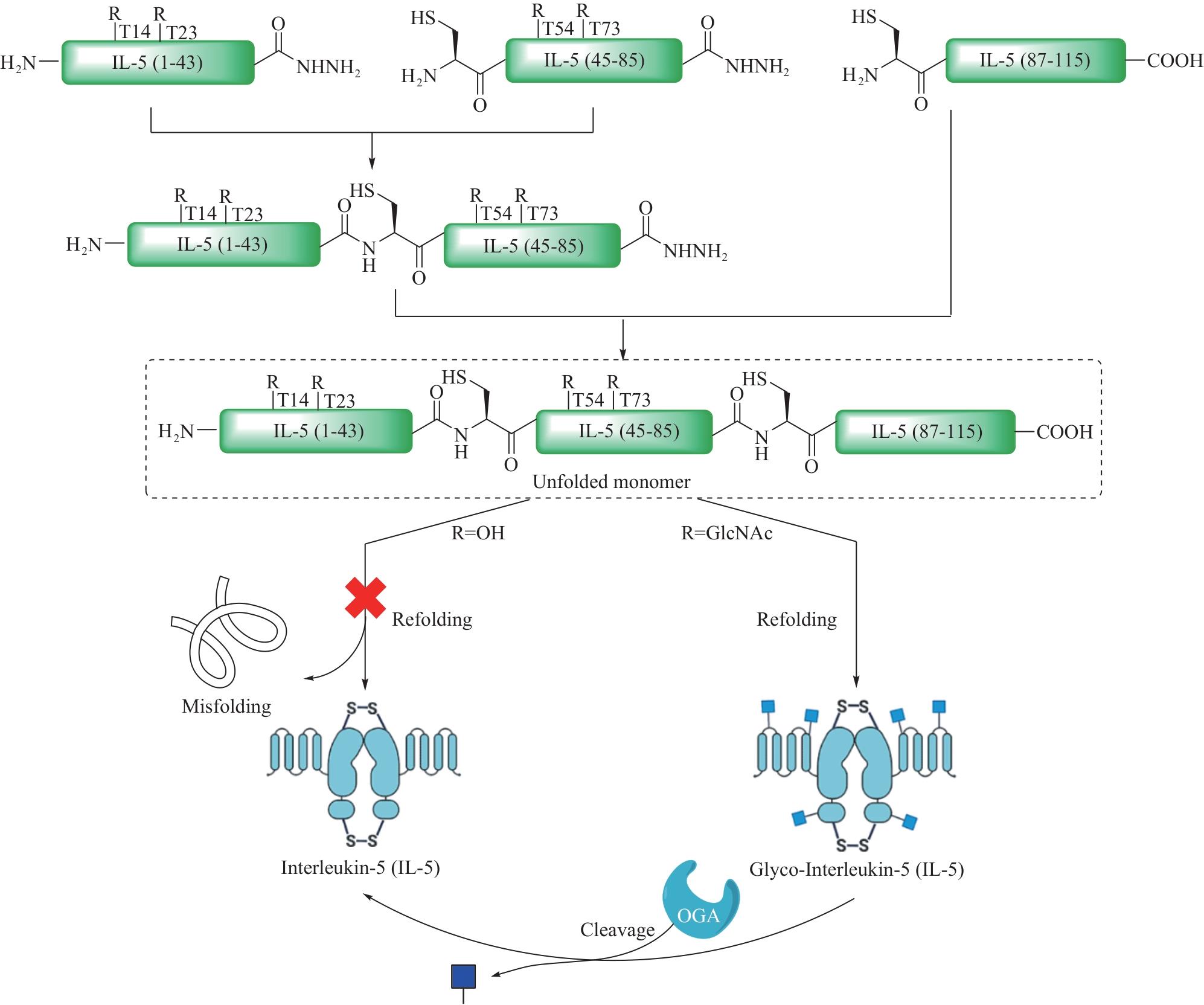
图5 基于表达蛋白连接(EPL)的半合成策略在复杂糖蛋白合成中的应用
Fig. 5 Application of semisynthetic strategy based on expressed protein ligation (EPL) in the synthesis of complex glycoproteins
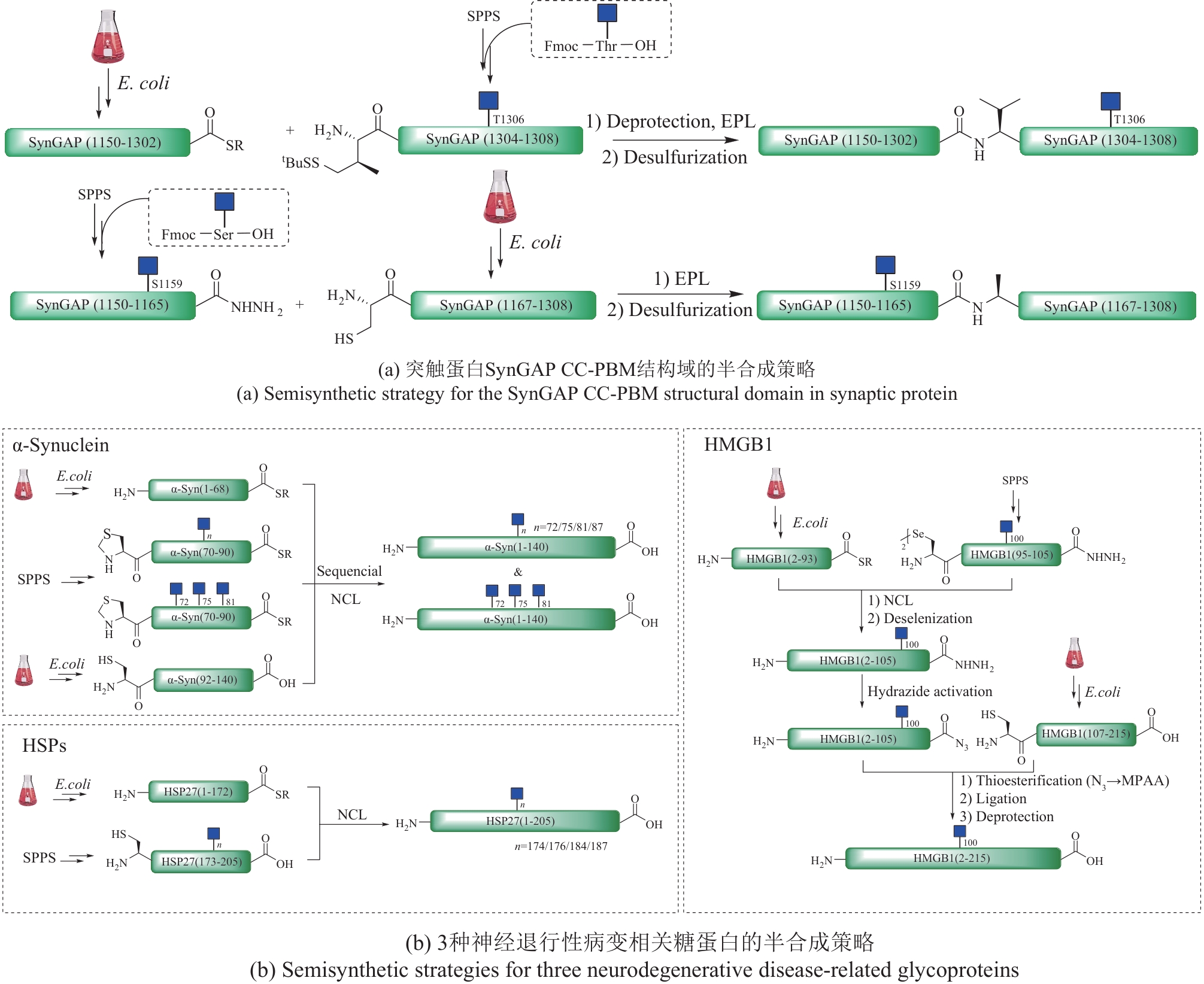
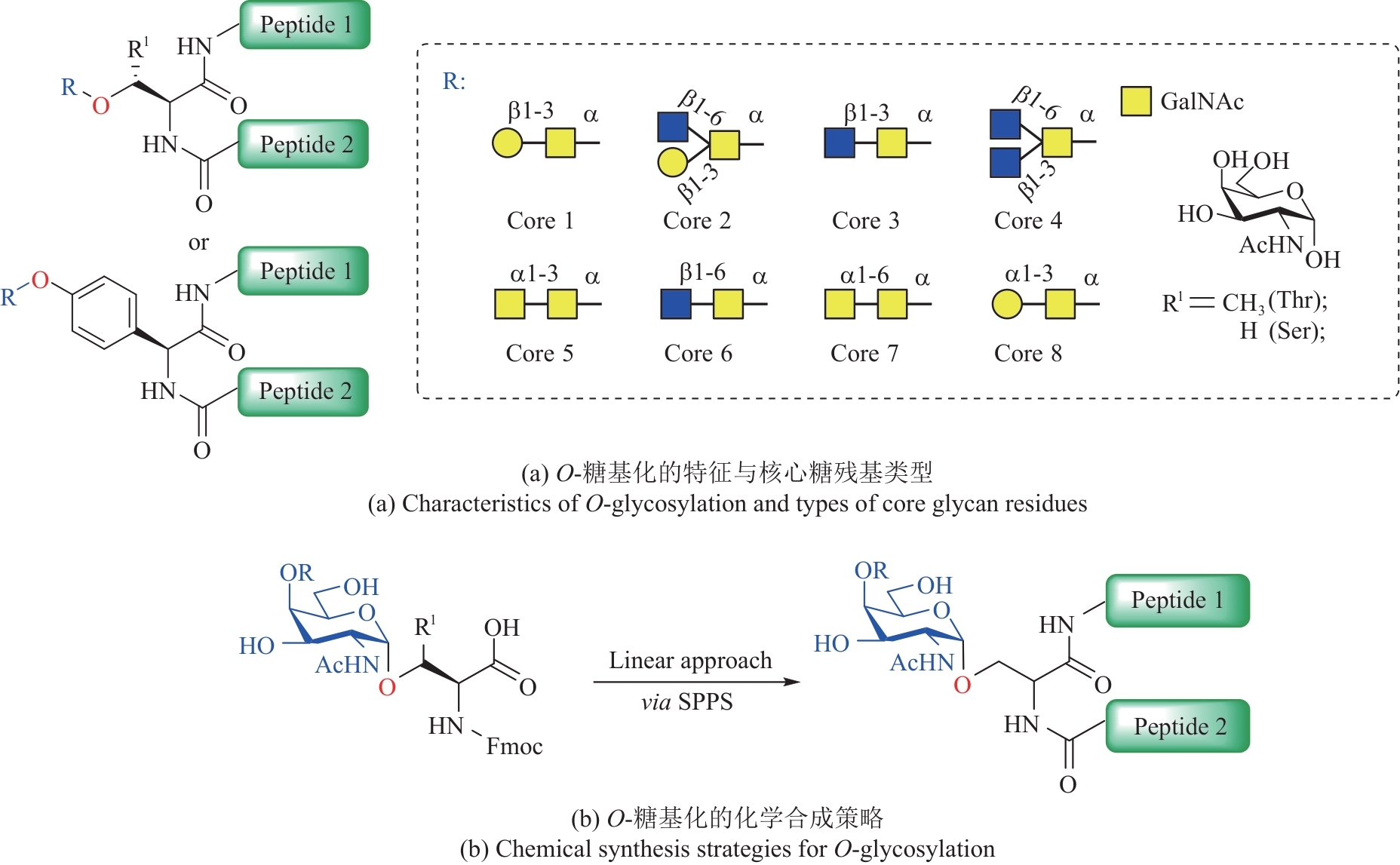
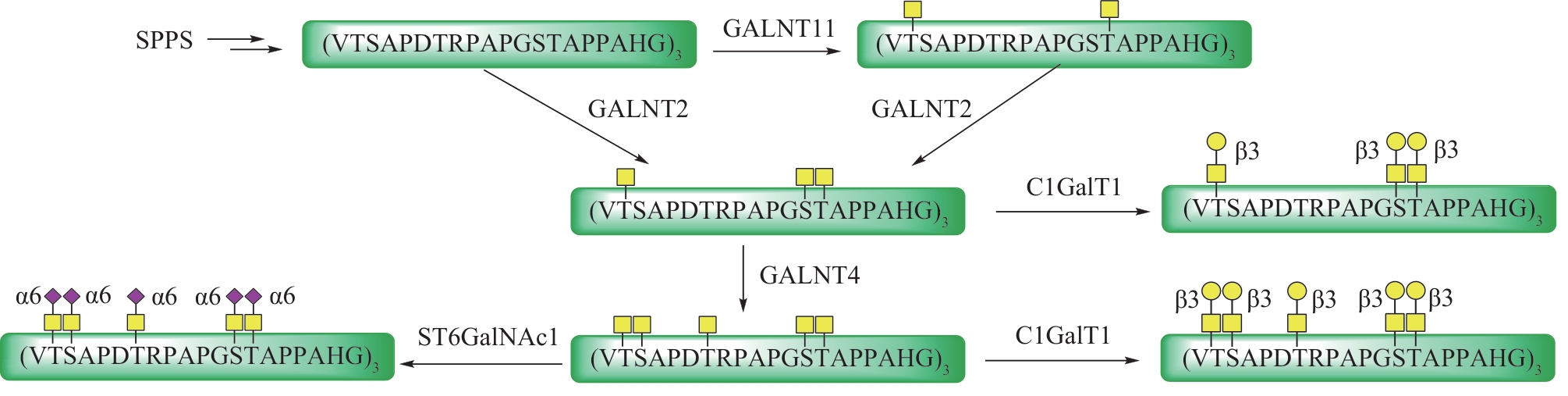
图11 各种合成策略在O-GlcNAc修饰糖蛋白构效关系研究中的应用
Fig.11 Application of various synthesis strategies in study of structure-activity relationships of O-GlcNAc modified glycoproteins
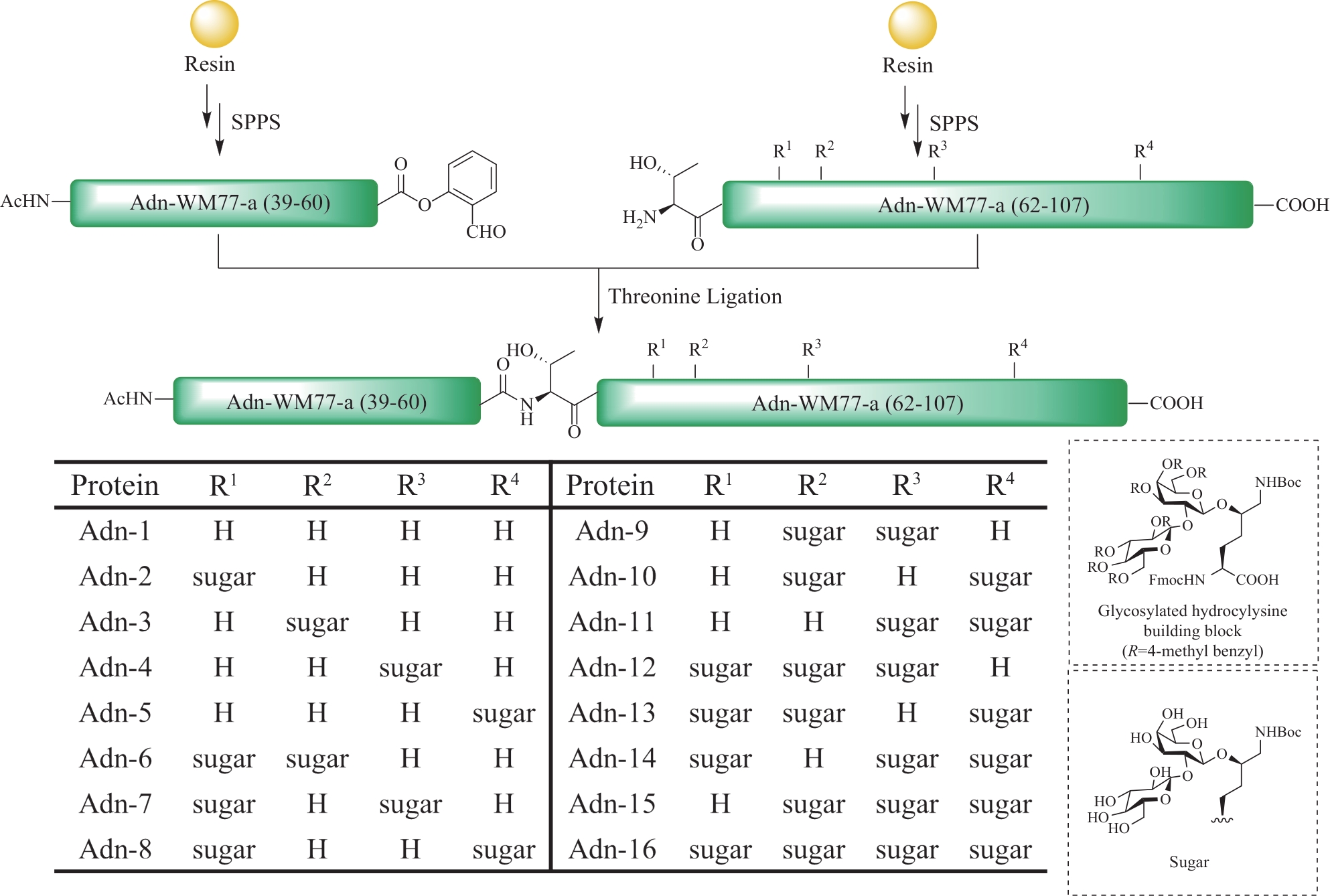
图12 使用丝氨酸/苏氨酸连接策略全合成糖基化脂肪连蛋白胶原结构域(GlyACD)(表格及糖基化羟基赖氨酸结构摘自文献[84])
Fig.12 Total synthesis of glycosylated adiponectin collagen domain (GlyACD) using serine/threonine ligation strategy(Table and structure of glycosylated hydroxylysine excerpted from literature [84])
| 1 | BENKOVIC S J, HAMMES-SCHIFFER S. A perspective on enzyme catalysis[J]. Science, 2003, 301(5637): 1196-1202. |
| 2 | KOSTERLITZ H W. The effects of changes in dietary protein on the composition and structure of the liver cell[J]. The Journal of Physiology, 1947, 106(2): 194-210. |
| 3 | CAHILL D J. Protein and antibody arrays and their medical applications[J]. Journal of Immunological Methods, 2001, 250(1/2): 81-91. |
| 4 | KULKARNI S S, SAYERS J, PREMDJEE B, et al. Rapid and efficient protein synthesis through expansion of the native chemical ligation concept[J]. Nature Reviews Chemistry, 2018, 2: 122. |
| 5 | KHOURY G A, BALIBAN R C, FLOUDAS C A. Proteome-wide post-translational modification statistics: frequency analysis and curation of the Swiss-prot database[J]. Scientific Reports, 2011, 1: 90. |
| 6 | HART G W, KELLY W G, BLOMBERG M A, et al. Glycosylation of nuclear and cytoplasmic proteins is as abundant and as dynamic as phosphorylation[C/OL]// WIELAND F, REUTTER W. Glyco-and cellbiology. Berlin, Heidelberg: Springer, 1994: 91-103 [2023-12-01]. . |
| 7 | SCHJOLDAGER K T, NARIMATSU Y, JOSHI H J, et al. Global view of human protein glycosylation pathways and functions[J]. Nature Reviews Molecular Cell Biology, 2020, 21(12): 729-749. |
| 8 | APWEILER R, HERMJAKOB H, SHARON N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database[J]. Biochimica et Biophysica Acta - General Subjects, 1999, 1473(1): 4-8. |
| 9 | RAMAZI S, ZAHIRI J. Post-translational modifications in proteins: resources, tools and prediction methods[J]. Database, 2021, 2021: baab012. |
| 10 | REILY C, STEWART T J, RENFROW M B, et al. Glycosylation in health and disease[J]. Nature Reviews Nephrology, 2019, 15(6): 346-366. |
| 11 | CHIANG W F, CHENG T M, CHANG C C, et al. Carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM6) promotes EGF receptor signaling of oral squamous cell carcinoma metastasis via the complex N-glycosylation[J]. Oncogene, 2018, 37(1): 116-127. |
| 12 | WOLF B, PIKSA M, BELEY I, et al. Therapeutic antibody glycosylation impacts antigen recognition and immunogenicity[J]. Immunology, 2022, 166(3): 380-407. |
| 13 | BROCKHAUSEN I, SCHUTZBACH J, KUHNS W. Glycoproteins and their relationship to human disease[J]. Acta Anatomica, 1998, 161(1/2/3/4): 36-78. |
| 14 | SCHIEL J E. Glycoprotein analysis using mass spectrometry: unraveling the layers of complexity[J]. Analytical and Bioanalytical Chemistry, 2012, 404(4): 1141-1149. |
| 15 | FERNÁNDEZ-TEJADA A, BRAILSFORD J, ZHANG Q, et al. Total synthesis of glycosylated proteins[M]// LIU L. Protein ligation and total synthesisⅠ. Cham: Springer International Publishing, 2014: 1-26 [2023-12-01]. . |
| 16 | GROGAN M J, PRATT M R, MARCAURELLE L A, et al. Homogeneous glycopeptides and glycoproteins for biological investigation[J]. Annual Review of Biochemistry, 2002, 71: 593-634. |
| 17 | DAWSON P E, MUIR T W, CLARK-LEWIS I, et al. Synthesis of proteins by native chemical ligation[J]. Science, 1994, 266(5186): 776-779. |
| 18 | YAN L Z, DAWSON P E. Synthesis of peptides and proteins without cysteine residues by native chemical ligation combined with desulfurization[J]. Journal of the American Chemical Society, 2001, 123(4): 526-533. |
| 19 | WANG S Y, THOPATE Y A, ZHOU Q Q, et al. Chemical protein synthesis by native chemical ligation and variations thereof[J]. Chinese Journal of Chemistry, 2019, 37(11): 1181-1193. |
| 20 | FANG G M, LI Y M, SHEN F, et al. Protein chemical synthesis by ligation of peptide hydrazides[J]. Angewandte Chemie International Edition, 2011, 50(33): 7645-7649. |
| 21 | ZHENG J S, TANG S, QI Y K, et al. Chemical synthesis of proteins using peptide hydrazides as thioester surrogates[J]. Nature Protocols, 2013, 8(12): 2483-2495. |
| 22 | ZHANG Y F, XU C, LAM H Y, et al. Protein chemical synthesis by serine and threonine ligation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(17): 6657-6662. |
| 23 | MUIR T W, SONDHI D, COLE P A. Expressed protein ligation: a general method for protein engineering[J]. Proceedings of the National Academy of Sciences of the United States of America, 1998, 95(12): 6705-6710. |
| 24 | BODE J W, FOX R M, BAUCOM K D. Chemoselective amide ligations by decarboxylative condensations of N-alkylhydroxylamines and α-ketoacids[J]. Angewandte Chemie International Edition, 2006, 45(8): 1248-1252. |
| 25 | MITCHELL N J, MALINS L R, LIU X Y, et al. Rapid additive-free selenocystine-selenoester peptide ligation[J]. Journal of the American Chemical Society, 2015, 137(44): 14011-14014. |
| 26 | ZHANG B C, ZHENG Y P, CHU G C, et al. Backbone-installed split intein-assisted ligation for the chemical synthesis of mirror-image proteins[J]. Angewandte Chemie International Edition, 2023, 62(33): e202306270. |
| 27 | MERRIFIELD R B. Solid phase peptide synthesis.Ⅰ. The synthesis of a tetrapeptide[J]. Journal of the American Chemical Society, 1963, 85: 2149-2154. |
| 28 | BEHRENDT R, WHITE P, OFFER J. Advances in Fmoc solid-phase peptide synthesis[J]. Journal of Peptide Science, 2016, 22(1): 4-27. |
| 29 | WANG P, DONG S W, SHIEH J H, et al. Erythropoietin derived by chemical synthesis[J]. Science, 2013, 342(6164): 1357-1360. |
| 30 | MAKI Y, OKAMOTO R, IZUMI M, et al. Chemical synthesis of an erythropoietin glycoform having a triantennary N-glycan: significant change of biological activity of glycoprotein by addition of a small molecular weight trisaccharide[J]. Journal of the American Chemical Society, 2020, 142(49): 20671-20679. |
| 31 | PIONTEK C, RING P, HARJES O, et al. Semisynthesis of a homogeneous glycoprotein enzyme: ribonuclease C: part 1[J]. Angewandte Chemie International Edition, 2009, 48(11): 1936-1940. |
| 32 | PIONTEK C, VARÓN SILVA D, HEINLEIN C, et al. Semisynthesis of a homogeneous glycoprotein enzyme: ribonuclease C: part 2[J]. Angewandte Chemie International Edition, 2009, 48(11): 1941-1945. |
| 33 | ZHAO J, LIU J Z, LIU X N, et al. Revealing functional significance of interleukin-2 glycoproteoforms enabled by expressed serine ligation[J]. Chinese Journal of Chemistry, 2022, 40(7): 787-793. |
| 34 | MAMAHIT Y P, MAKI Y, OKAMOTO R, et al. Semisynthesis of homogeneous misfolded glycoprotein interleukin-8[J]. Carbohydrate Research, 2023, 531: 108847. |
| 35 | LI H X, ZHANG J, AN C J, et al. Probing N-glycan functions in human interleukin-17A based on chemically synthesized homogeneous glycoforms[J]. Journal of the American Chemical Society, 2021, 143(7): 2846-2856. |
| 36 | SHI W W, SHI C W, WANG T Y, et al. Total chemical synthesis of correctly folded disulfide-rich proteins using a removable O-linked β-N-acetylglucosamine strategy[J]. Journal of the American Chemical Society, 2022, 144(1): 349-357. |
| 37 | ZHAO J, LIU J Z, LIU X L, et al. Semi-synthesis of interleukin-1α via expressed threonine ligation and native chemical ligation-desulfurization[J]. Tetrahedron Letters, 2022, 104: 154024. |
| 38 | YE F R, ZHAO J, XU P, et al. Synthetic homogeneous glycoforms of the SARS-CoV-2 spike receptor-binding domain reveals different binding profiles of monoclonal antibodies[J]. Angewandte Chemie International Edition, 2021, 60(23): 12904-12910. |
| 39 | YE F R, LI C, LIU F L, et al. Semisynthesis of homogeneous spike RBD glycoforms from SARS-CoV-2 for profiling the correlations between glycan composition and function[J]. National Science Review, 2024, 11(2): nwae030. |
| 40 | CHONG Y K, CHANDRASHEKAR C, ZHAO D L, et al. Optimizing the semisynthesis towards glycosylated interferon-β-polypeptide by utilizing bacterial protein expression and chemical modification[J]. Organic & Biomolecular Chemistry, 2022, 20(9): 1907-1915. |
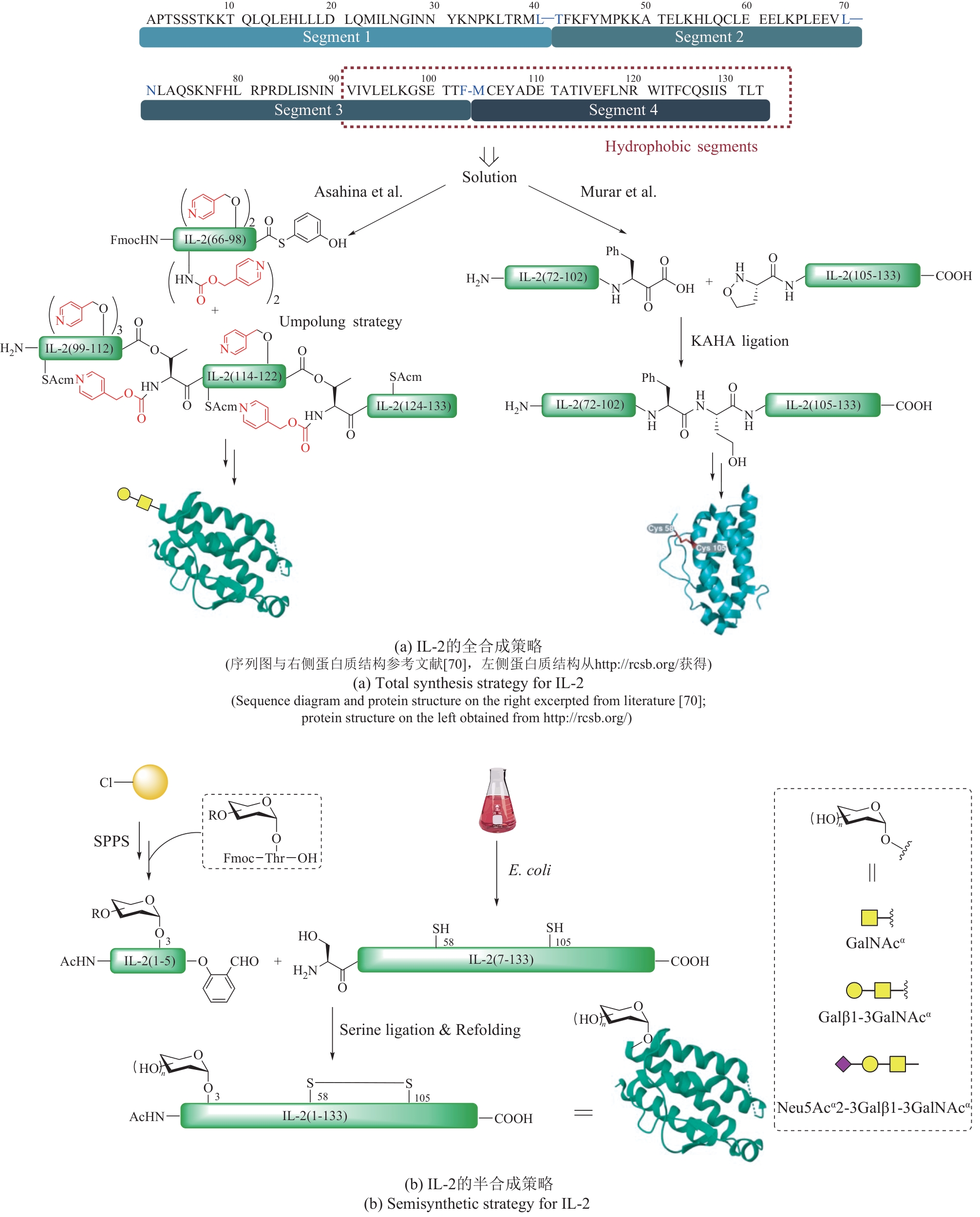
| 41 | WANG X Y, ASHHURST A S, DOWMAN L J, et al. Total synthesis of glycosylated human interferon-Γ[J]. Organic Letters, 2020, 22(17): 6863-6867. |
| 42 | BRIK A, YANG Y Y, FICHT S, et al. Sugar-assisted glycopeptide ligation[J]. Journal of the American Chemical Society, 2006, 128(17): 5626-5627. |
| 43 | ANISFELD S T, LANSBURY P T. A convergent approach to the chemical synthesis of asparagine-linked glycopeptides[J]. The Journal of Organic Chemistry, 1990, 55(21): 5560-5562. |
| 44 | AGOURIDAS V, MAHDI O EL, DIEMER V, et al. Native chemical ligation and extended methods: mechanisms, catalysis, scope, and limitations[J]. Chemical Reviews, 2019, 119(12): 7328-7443. |
| 45 | MCGRATH N A, RAINES R T. Chemoselectivity in chemical biology: acyl transfer reactions with sulfur and selenium[J]. Accounts of Chemical Research, 2011, 44(9): 752-761. |
| 46 | YAMAMOTO N, TAKAYANAGI A, YOSHINO A, et al. An approach for a synthesis of asparagine-linked sialylglycopeptides having intact and homogeneous complex-type undecadisialyloligosaccharides[J]. Chemistry, 2007, 13(2): 613-625. |
| 47 | EVERS D L, HUNG R L, THOMAS V H, et al. Preparative purification of a high-mannose type N-glycan from soy bean agglutinin by hydrazinolysis and tyrosinamide derivatization[J]. Analytical Biochemistry, 1998, 265(2): 313-316. |
| 48 | ULLMANN V, RÄDISCH M, BOOS I, et al. Convergent solid-phase synthesis of N-glycopeptides facilitated by pseudoprolines at consensus-sequence Ser/Thr residues[J]. Angewandte Chemie International Edition, 2012, 51(46): 11566-11570. |
| 49 | WANG P, AUSSEDAT B, VOHRA Y, et al. An advance in the chemical synthesis of homogeneous N-linked glycopolypeptides by convergent aspartylation[J]. Angewandte Chemie International Edition, 2012, 51(46): 11571-11575. |
| 50 | ZENG C, SUN B, CAO X F, et al. Chemical synthesis of homogeneous human E-cadherin N-linked glycopeptides: stereoselective convergent glycosylation and chemoselective solid-phase aspartylation[J]. Organic Letters, 2020, 22(21): 8349-8353. |
| 51 | ZHAO J, LIU X L, LIU J L, et al. Chemical synthesis creates single glycoforms of the ectodomain of herpes simplex virus-1 glycoprotein D[J]. Journal of the American Chemical Society, 2024, 146(4): 2615-2623. |
| 52 | DU J J, GAO X F, XIN L M, et al. Convergent synthesis of N-linked glycopeptides via aminolysis of ω-asp p-nitrophenyl thioesters in solution[J]. Organic Letters, 2016, 18(19): 4828-4831. |
| 53 | DU J J, ZHANG L, GAO X F, et al. Peptidyl ω-asp selenoesters enable efficient synthesis of N-linked glycopeptides[J]. Frontiers in Chemistry, 2020, 8: 396. |
| 54 | LI Y X, LIU J Z, ZHOU Q Q, et al. Preparation of peptide selenoesters from their corresponding acyl hydrazides[J]. Chinese Journal of Chemistry, 2021, 39(7): 1861-1866. |
| 55 | LU D, YIN H L, WANG S Y, et al. Chemical synthesis of the homogeneous granulocyte-macrophage colony-stimulating factor through Se-auxiliary-mediated ligation[J]. The Journal of Organic Chemistry, 2020, 85(3): 1652-1660. |
| 56 | TARESH A B, HUTTON C A. Site specific preparation of N-glycosylated peptides: thioamide-directed activation of aspartate[J]. Angewandte Chemie International Edition, 2022, 61(43): e202210367. |
| 57 | LIU X L, GAO Z J, ZHAO J, et al. Encouraging solution to the problem of synthesizing protein α-thioester[J]. Chinese Journal of Chemistry, 2024, 42(10): 1114-1120. |
| 58 | BONDALAPATI S, JBARA M, BRIK A. Expanding the chemical toolbox for the synthesis of large and uniquely modified proteins[J]. Nature Chemistry, 2016, 8(5): 407-418. |
| 59 | BURKE H M, MCSWEENEY L, SCANLAN E M. Exploring chemoselective S-to-N acyl transfer reactions in synthesis and chemical biology[J]. Nature Communications, 2017, 8: 15655. |
| 60 | MOYAL T, HEMANTHA H P, SIMAN P, et al. Highly efficient one-pot ligation and desulfurization[J]. Chemical Science, 2013, 4(6): 2496. |
| 61 | TANG S, LIANG L J, SI Y Y, et al. Practical chemical synthesis of atypical ubiquitin chains by using an isopeptide-linked ub isomer[J]. Angewandte Chemie International Edition, 2017, 56(43): 13333-13337. |
| 62 | REIMANN O, SMET-NOCCA C, HACKENBERGER C P R. Traceless purification and desulfurization of tau protein ligation products[J]. Angewandte Chemie International Edition, 2015, 54(1): 306-310. |
| 63 | YIN H L, ZHENG M J, CHEN H, et al. Stereoselective and divergent construction of β-thiolated/selenolated amino acids via photoredox-catalyzed asymmetric giese reaction[J]. Journal of the American Chemical Society, 2020, 142(33): 14201-14209. |
| 64 | SHANGGUAN H R, HUANG P, DAI Z Y, et al. Synthesis and application of β-thiolated amino acids[J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3089. |
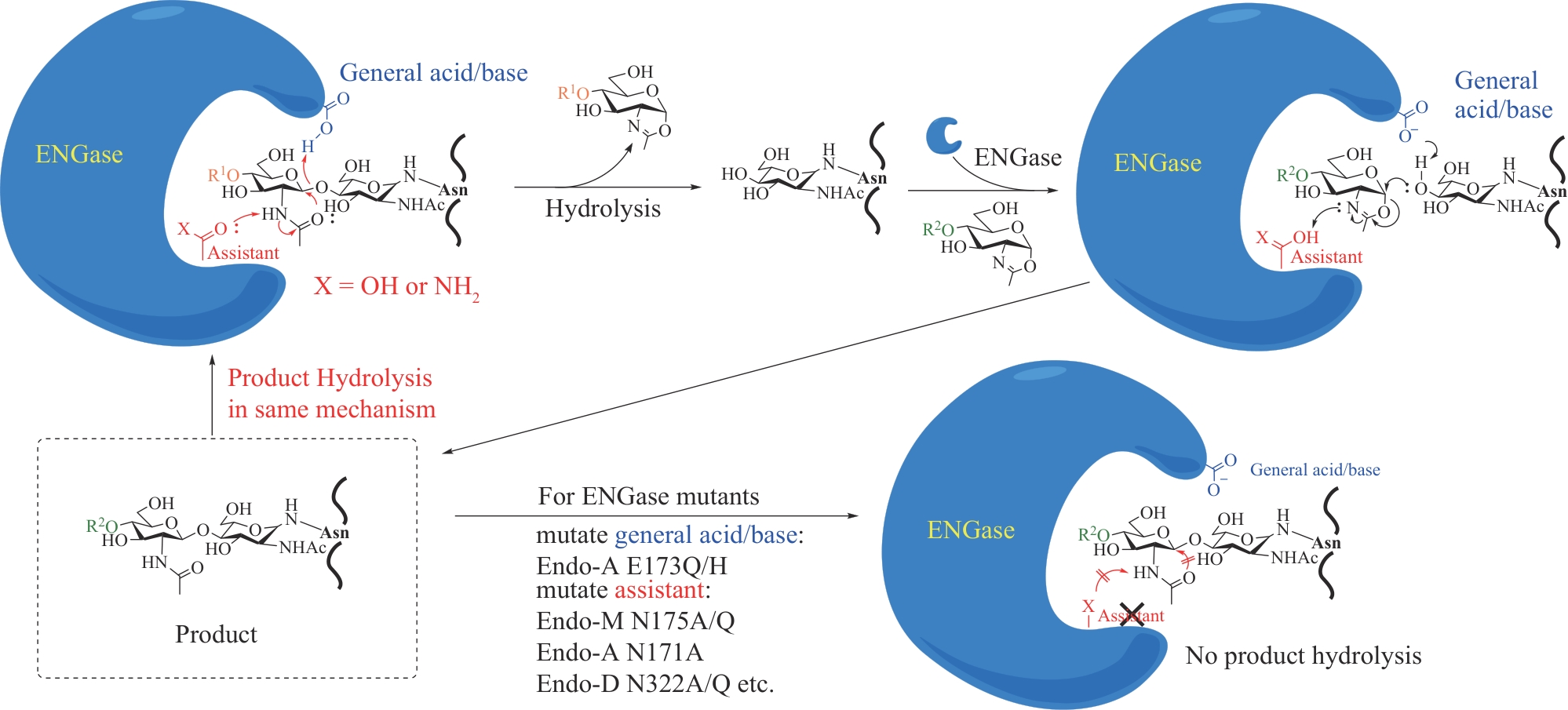
| 65 | ZHENG M J, YIN H, WANG S Y, et al. Stereoselective synthesis of β-thiolated aryl amino acids[J]. Synthesis, 2022, 54(20): e5. |
| 66 | WANG S Y, ZHOU Q Q, LI Y X, et al. Quinoline-based photolabile protection strategy facilitates efficient protein assembly[J]. Journal of the American Chemical Society, 2022, 144(3): 1232-1242. |
| 67 | WANG P, DANISHEFSKY S J. Promising general solution to the problem of ligating peptides and glycopeptides[J]. Journal of the American Chemical Society, 2010, 132(47): 17045-17051. |
| 68 | WANG P, LI X C, ZHU J L, et al. Encouraging progress in the ω-aspartylation of complex oligosaccharides as a general route to β-N-linked glycopolypeptides[J]. Journal of the American Chemical Society, 2011, 133(5): 1597-1602. |
| 69 | NOMURA K, MAKI Y, OKAMOTO R, et al. Glycoprotein semisynthesis by chemical insertion of glycosyl asparagine using a bifunctional thioacid-mediated strategy[J]. Journal of the American Chemical Society, 2021, 143(27): 10157-10167. |
| 70 | ZHAO J, YE F R, HUANG P, et al. Recent advances in chemical synthesis of O-linked glycopeptides and glycoproteins: an advanced synthetic tool for exploring the biological realm[J]. Current Opinion in Chemical Biology, 2023, 77: 102405. |
| 71 | MURAR C E, NINOMIYA M, SHIMURA S, et al. Chemical synthesis of interleukin-2 and disulfide stabilizing analogues[J]. Angewandte Chemie International Edition, 2020, 59(22): 8425-8429. |
| 72 | ASAHINA Y, KOMIYA S, OHAGI A, et al. Chemical synthesis of O-glycosylated human interleukin-2 by the reverse polarity protection strategy[J]. Angewandte Chemie International Edition, 2015, 54(28): 8226-8230. |
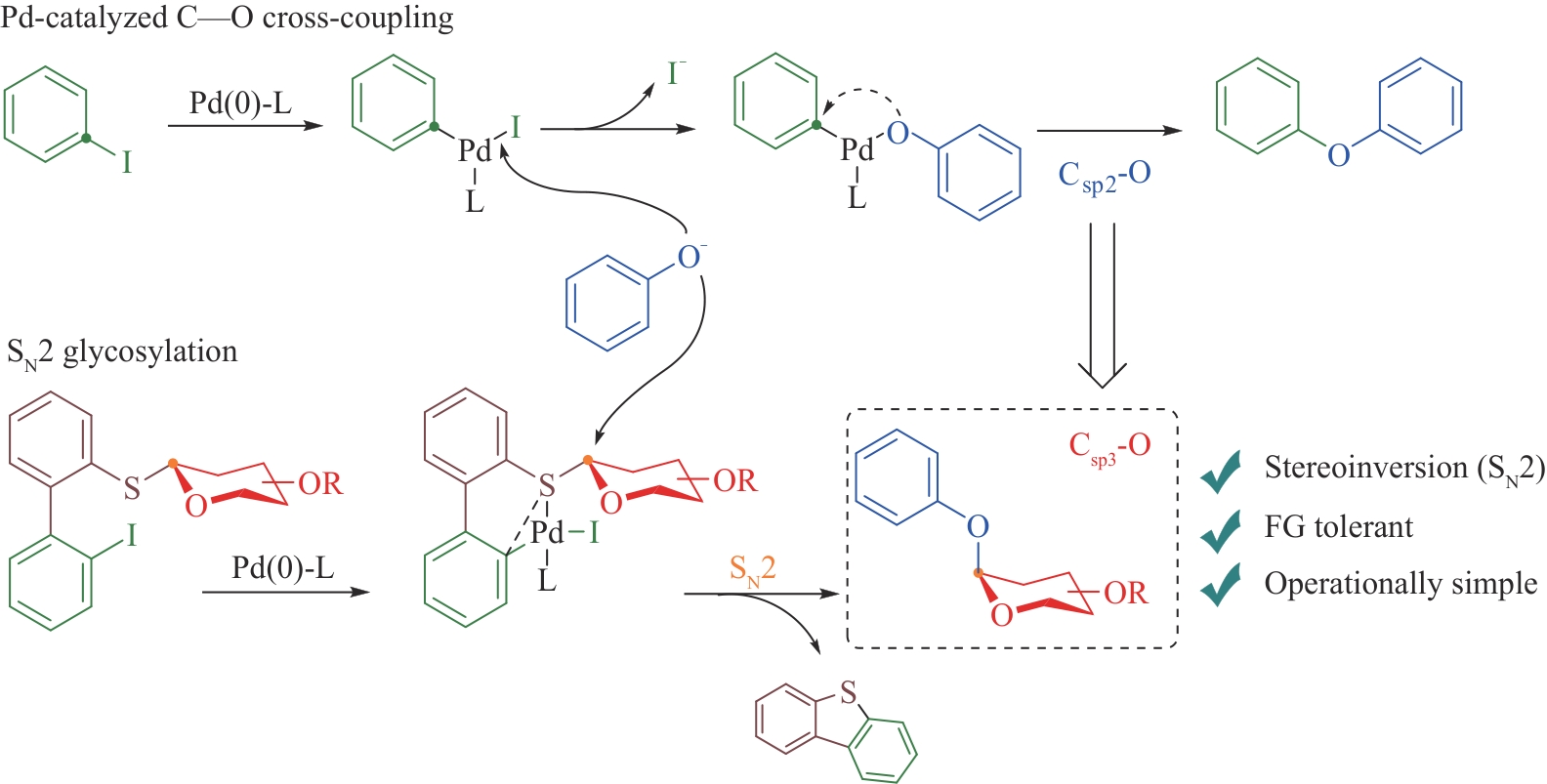
| 73 | DENG L F, WANG Y W, XU S Y, et al. Palladium catalysis enables cross-coupling-like SN2-glycosylation of phenols[J]. Science, 2023, 382(6673): 928-935. |
| 74 | MA W J, DENG Y Q, XU Z J, et al. Integrated chemoenzymatic approach to streamline the assembly of complex glycopeptides in the liquid phase[J]. Journal of the American Chemical Society, 2022, 144(20): 9057-9065. |
| 75 | LIU X Y, LIU J Z, WU Z C, et al. Photo-cleavable purification/protection handle assisted synthesis of giant modified proteins with tandem repeats[J]. Chemical Science, 2019, 10(37): 8694-8700. |
| 76 | LIU D L, WEI Q J, XIA W C, et al. O-glycosylation induces amyloid-β to form new fibril polymorphs vulnerable for degradation[J]. Journal of the American Chemical Society, 2021, 143(48): 20216-20223. |
| 77 | YANG X Y, QIAN K. Protein O-GlcNAcylation: emerging mechanisms and functions[J]. Nature Reviews Molecular Cell Biology, 2017, 18(7): 452-465. |
| 78 | LV P O, DU Y F, HE C D, et al. O-GlcNAcylation modulates liquid-liquid phase separation of SynGAP/PSD-95[J]. Nature Chemistry, 2022, 14(7): 831-840. |
| 79 | WAN Q, DANISHEFSKY S J. Free-radical-based, specific desulfurization of cysteine: a powerful advance in the synthesis of polypeptides and glycopolypeptides[J]. Angewandte Chemie International Edition, 2007, 46(48): 9248-9252. |
| 80 | LEVINE P M, GALESIC A, BALANA A T, et al. α-Synuclein O-GlcNAcylation alters aggregation and toxicity, revealing certain residues as potential inhibitors of Parkinson’s disease[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(5): 1511-1519. |
| 81 | BALANA A T, MUKHERJEE A, NAGPAL H, et al. O-GlcNAcylation of high mobility group box 1 (HMGB1) alters its DNA binding and DNA damage processing activities[J]. Journal of the American Chemical Society, 2021, 143(39): 16030-16040. |
| 82 | BALANA A T, LEVINE P M, CRAVEN T W, et al. O-GlcNAc modification of small heat shock proteins enhances their anti-amyloid chaperone activity[J]. Nature Chemistry, 2021, 13(5): 441-450. |
| 83 | WANG Y, XU A M, KNIGHT C, et al. Hydroxylation and glycosylation of the four conserved lysine residues in the collagenous domain of adiponectin. Potential role in the modulation of its insulin-sensitizing activity[J]. Journal of Biological Chemistry, 2002, 277(22): 19521-19529. |
| 84 | WU H X, ZHANG Y W, LI Y X, et al. Chemical synthesis and biological evaluations of adiponectin collagenous domain glycoforms[J]. Journal of the American Chemical Society, 2021, 143(20): 7808-7818. |
| 85 | LAFITE P, DANIELLOU R. Rare and unusual glycosylation of peptides and proteins[J]. Natural Product Reports, 2012, 29(7): 729-738. |
| 86 | HOFSTEENGE J, MÜLLER D R, DE BEER T, et al. New type of linkage between a carbohydrate and a protein: C-glycosylation of a specific tryptophan residue in human RNase Us[J]. Biochemistry, 1994, 33(46): 13524-13530. |
| 87 | MINAKATA S, MANABE S, INAI Y, et al. Protein C-mannosylation and C-mannosyl tryptophan in chemical biology and medicine[J]. Molecules, 2021, 26(17): 5258. |
| 88 | WANG Q Q, FU Y, ZHU W J, et al. Total synthesis of C-α-mannosyl tryptophan via palladium-catalyzed C-H glycosylation[J]. CCS Chemistry, 2021, 3(6): 1729-1736. |
| 89 | MAO R Y, XI S Y, SHAH S, et al. Synthesis of C-mannosylated glycopeptides enabled by Ni-catalyzed photoreductive cross-coupling reactions[J]. Journal of the American Chemical Society, 2021, 143(32): 12699-12707. |
| 90 | LIU Y H, XIA Y N, GULZAR T, et al. Facile access to C-glycosyl amino acids and peptides via Ni-catalyzed reductive hydroglycosylation of alkynes[J]. Nature Communications, 2021, 12(1): 4924. |
| 91 | OMAN T J, BOETTCHER J M, WANG H, et al. Sublancin is not a lantibiotic but an S-linked glycopeptide[J]. Nature Chemical Biology, 2011, 7(2): 78-80. |
| 92 | WANG H, OMAN T J, ZHANG R, et al. The glycosyltransferase involved in thurandacin biosynthesis catalyzes both O- and S-glycosylation[J]. Journal of the American Chemical Society, 2014, 136(1): 84-87. |
| 93 | WAN L Q, ZHANG X, ZOU Y K, et al. Nonenzymatic stereoselective S-glycosylation of polypeptides and proteins[J]. Journal of the American Chemical Society, 2021, 143(31): 11919-11926. |
| 94 | LI G F, DAO Y K, MO J, et al. Protection-free site-directed peptide or protein S-glycosylation and its application in the glycosylation of glucagon-like peptide 1[J]. CCS Chemistry, 2022, 4(6): 1930-1937. |
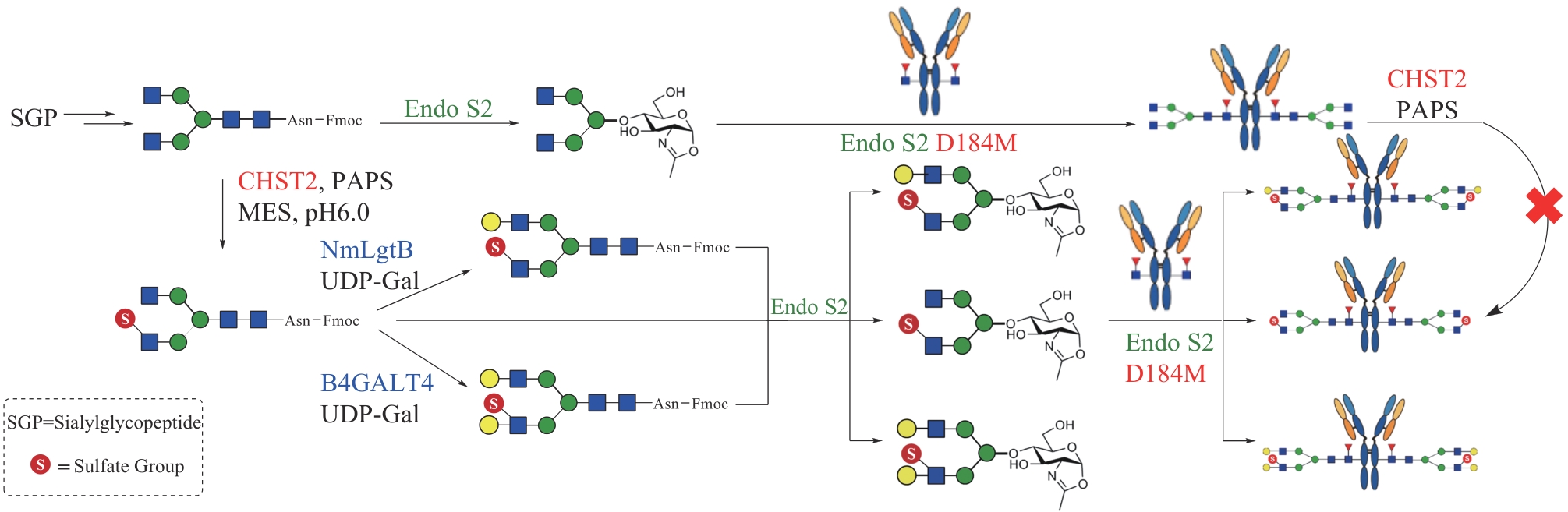
| 95 | TRIMBLE R B, ATKINSON P H, TARENTINO A L, et al. Transfer of glycerol by endo-β-N-acetylglucosaminidase F to oligosaccharides during chitobiose core cleavage[J]. Journal of Biological Chemistry, 1986, 261(26): 12000-12005. |
| 96 | YAMAMOTO K. Endo-enzymes[M/OL]// TANIGUCHI N, ENDO T, HART G, et al. Glycoscience: biology and medicine. Tokyo: Springer, 2015: 391-399 [2023-12-01]. . |
| 97 | TAKEGAWA K, TABUCHI M, YAMAGUCHI S, et al. Synthesis of neoglycoproteins using oligosaccharide-transfer activity with endo-β-N-acetylglucosaminidase[J]. Journal of Biological Chemistry, 1995, 270(7): 3094-3099. |
| 98 | MIZUNO M, HANEDA K, IGUCHI R, et al. Synthesis of a glycopeptide containing oligosaccharides: chemoenzymatic synthesis of eel calcitonin analogues having natural N-linked oligosaccharides[J]. Journal of the American Chemical Society, 1999, 121(2): 284-290. |
| 99 | SINGH S, NI J H, WANG L X. Chemoenzymatic synthesis of high-mannose type HIV-1 gp120 glycopeptides[J]. Bioorganic & Medicinal Chemistry Letters, 2003, 13(3): 327-330. |
| 100 | LI H G, SINGH S, ZENG Y, et al. Chemoenzymatic synthesis of CD52 glycoproteins carrying native N-glycans[J]. Bioorganic & Medicinal Chemistry Letters, 2005, 15(4): 895-898. |
| 101 | MACKENZIE L F, WANG Q P, WARREN R A J, et al. Glycosynthases: mutant glycosidases for oligosaccharide synthesis[J]. Journal of the American Chemical Society, 1998, 120(22): 5583-5584. |
| 102 | HUANG W, LI C S, LI B, et al. Glycosynthases enable a highly efficient chemoenzymatic synthesis of N-glycoproteins carrying intact natural N-glycans[J]. Journal of the American Chemical Society, 2009, 131(6): 2214-2223. |
| 103 | FUJITA M, SHODA S, HANEDA K, et al. A novel disaccharide substrate having 1,2-oxazoline moiety for detection of transglycosylating activity of endoglycosidases[J]. Biochimica et Biophysica Acta - General Subjects, 2001, 1528(1): 9-14. |
| 104 | PRIYANKA P, PARSONS T B, MILLER A, et al. Chemoenzymatic synthesis of a phosphorylated glycoprotein[J]. Angewandte Chemie International Edition, 2016, 55(16): 5058-5061. |
| 105 | SCHMALTZ R M, HANSON S R, WONG C H. Enzymes in the synthesis of glycoconjugates[J]. Chemical Reviews, 2011, 111(7): 4259-4307. |
| 106 | ARMSTRONG Z, WITHERS S G. Synthesis of glycans and glycopolymers through engineered enzymes[J]. Biopolymers, 2013, 99(10): 666-674. |
| 107 | LI C, WANG L X. Chemoenzymatic methods for the synthesis of glycoproteins[J]. Chemical Reviews, 2018, 118(17): 8359-8413. |
| 108 | AMIN M N, MCLELLAN J S, HUANG W, et al. Synthetic glycopeptides reveal the glycan specificity of HIV-neutralizing antibodies[J]. Nature Chemical Biology, 2013, 9(8): 521-526. |
| 109 | ORWENYO J, CAI H, GIDDENS J, et al. Systematic synthesis and binding study of HIV V3 glycopeptides reveal the fine epitopes of several broadly neutralizing antibodies[J]. ACS Chemical Biology, 2017, 12(6): 1566-1575. |
| 110 | YANG Q, WANG L X. Mammalian α-1,6-fucosyltransferase (FUT8) is the sole enzyme responsible for the N-acetylglucosaminyltransferase I-independent core fucosylation of high-mannose N-glycans[J]. Journal of Biological Chemistry, 2016, 291(21): 11064-11071. |
| 111 | YANG Q, AN Y M, ZHU S L, et al. Glycan remodeling of human erythropoietin (EPO) through combined mammalian cell engineering and chemoenzymatic transglycosylation[J]. ACS Chemical Biology, 2017, 12(6): 1665-1673. |
| 112 | SMITH E L, GIDDENS J P, IAVARONE A T, et al. Chemoenzymatic Fc glycosylation via engineered aldehyde tags[J]. Bioconjugate Chemistry, 2014, 25(4): 788-795. |
| 113 | PARSONS T B, STRUWE W B, GAULT J, et al. Optimal synthetic glycosylation of a therapeutic antibody[J]. Angewandte Chemie International Edition, 2016, 55(7): 2361-2367. |
| 114 | HUANG W, GIDDENS J, FAN S Q, et al. Chemoenzymatic glycoengineering of intact IgG antibodies for gain of functions[J]. Journal of the American Chemical Society, 2012, 134(29): 12308-12318. |
| 115 | KUROGOCHI M, MORI M, OSUMI K, et al. Glycoengineered monoclonal antibodies with homogeneous glycan (M3, G0, G2, and A2) using a chemoenzymatic approach have different affinities for FcγRIIIa and variable antibody-dependent cellular cytotoxicity activities[J]. PLoS One, 2015, 10(7): e0132848. |
| 116 | ZHANG X, LIU H Y, HE J, et al. Site-specific chemoenzymatic conjugation of high-affinity M6P glycan ligands to antibodies for targeted protein degradation[J]. ACS Chemical Biology, 2022, 17(11): 3013-3023. |
| 117 | BANIK S M, PEDRAM K, WISNOVSKY S, et al. Lysosome-targeting chimaeras for degradation of extracellular proteins[J]. Nature, 2020, 584(7820): 291-297. |
| 118 | TANG F, YANG Y, TANG Y B, et al. One-pot N-glycosylation remodeling of IgG with non-natural sialylglycopeptides enables glycosite-specific and dual-payload antibody-drug conjugates[J]. Organic & Biomolecular Chemistry, 2016, 14(40): 9501-9518. |
| 119 | SHI W, LI W Z, ZHANG J X, et al. One-step synthesis of site-specific antibody-drug conjugates by reprograming IgG glycoengineering with LacNAc-based substrates[J]. Acta Pharmaceutica Sinica B, 2022, 12(5): 2417-2428. |
| 120 | TANG C H, ZENG Y, ZHANG J X, et al. One-pot assembly of dual-site-specific antibody-drug conjugates via glycan remodeling and affinity-directed traceless conjugation[J]. Bioconjugate Chemistry, 2023, 34(4): 748-755. |
| 121 | ZOU X M, LIU Z, LIU L Y, et al. Enhanced transglycosylation activity of an Endo-F3 mutant by ligand-directed localization[J]. Organic & Biomolecular Chemistry, 2022, 20(15): 3086-3095. |
| 122 | TAYLOR M E, DRICKAMER K. Concepts of glycobiology[M/OL]// Introduction to Glycobiology. Oxford: Oxford University Press, 2011[2023-12-01]. . |
| 123 | LAIRSON L L, HENRISSAT B, DAVIES G J, et al. Glycosyltransferases: structures, functions, and mechanisms[J]. Annual Review of Biochemistry, 2008, 77: 521-555. |
| 124 | UNVERZAGT C, KUNZ H, PAULSON J C. High-efficiency synthesis of sialyloligosaccharides and sialoglycopeptides[J]. Journal of the American Chemical Society, 1990, 112(25): 9308-9309. |
| 125 | HUANG K, LI C, ZONG G H, et al. Site-selective sulfation of N-glycans by human GlcNAc-6-O-sulfotransferase 1 (CHST2) and chemoenzymatic synthesis of sulfated antibody glycoforms[J]. Bioorganic Chemistry, 2022, 128: 106070. |
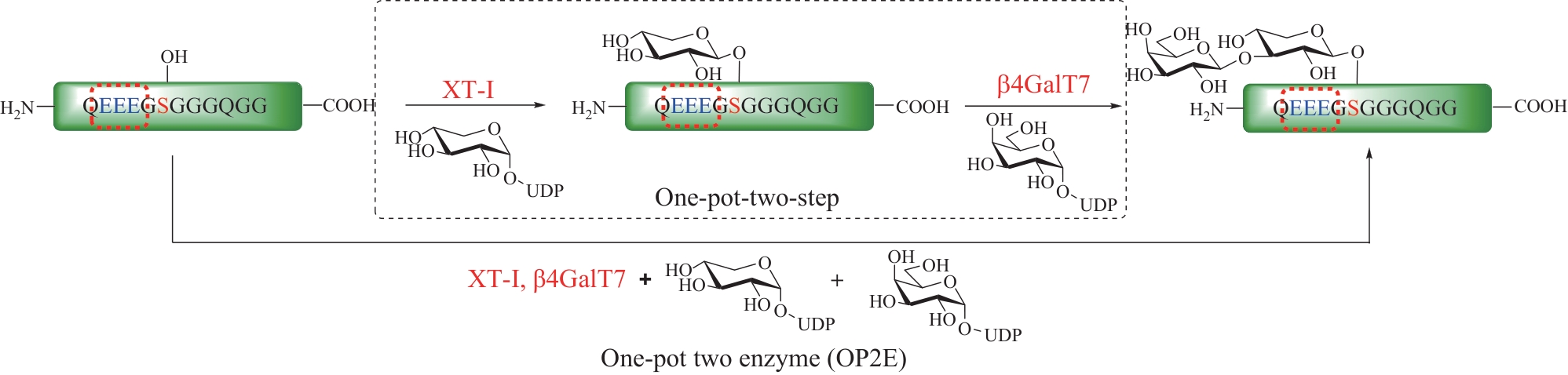
| 126 | GAO J, LIN P H, NICK S T, et al. Exploration of human xylosyltransferase for chemoenzymatic synthesis of proteoglycan linkage region[J]. Organic & Biomolecular Chemistry, 2021, 19(15): 3374-3378. |
| 127 | CHAO Q, LI T L, JIA J X, et al. Spore-encapsulating glycosyltransferase catalysis tandem reactions: facile chemoenzymatic synthesis of complex human glycans[J]. ACS Catalysis, 2022, 12(5): 3181-3188. |
| 128 | RUIZ-CANADA C, KELLEHER D J, GILMORE R. Cotranslational and posttranslational N-glycosylation of polypeptides by distinct mammalian OST isoforms[J]. Cell, 2009, 136(2): 272-283. |
| 129 | LUKOSE V, WHITWORTH G, GUAN Z Q, et al. Chemoenzymatic assembly of bacterial glycoconjugates for site-specific orthogonal labeling[J]. Journal of the American Chemical Society, 2015, 137(39): 12446-12449. |
| 130 | GLOVER K J, WEERAPANA E, NUMAO S, et al. Chemoenzymatic synthesis of glycopeptides with PglB, a bacterial oligosaccharyl transferase from Campylobacter jejuni [J]. Chemistry & Biology, 2005, 12(12): 1311-1315. |
| 131 | VALDERRAMA-RINCON J D, FISHER A C, MERRITT J H, et al. An engineered eukaryotic protein glycosylation pathway in Escherichia coli [J]. Nature Chemical Biology, 2012, 8(5): 434-436. |
| 132 | SCHWARZ F, HUANG W, LI C S, et al. A combined method for producing homogeneous glycoproteins with eukaryotic N-glycosylation[J]. Nature Chemical Biology, 2010, 6(4): 264-266. |
| 133 | KOWARIK M, NUMAO S, FELDMAN M F, et al. N-linked glycosylation of folded proteins by the bacterial oligosaccharyltransferase[J]. Science, 2006, 314(5802): 1148-1150. |
| 134 | KOWARIK M, YOUNG N M, NUMAO S, et al. Definition of the bacterial N-glycosylation site consensus sequence[J]. The EMBO Journal, 2006, 25(9): 1957-1966. |
| 135 | NITA-LAZAR M, WACKER M, SCHEGG B, et al. The N-X-S/T consensus sequence is required but not sufficient for bacterial N-linked protein glycosylation[J]. Glycobiology, 2005, 15(4): 361-367. |
| 136 | OHUCHI T, IKEDA-ARAKI A, WATANABE-SAKAMOTO A, et al. Cloning and expression of a gene encoding N-glycosyltransferase (ngt) from Saccarothrix aerocolonigenes ATCC39243[J]. The Journal of Antibiotics, 2000, 53(4): 393-403. |
| 137 | GRASS S, BUSCHER A Z, SWORDS W E, et al. The Haemophilus influenzae HMW1 adhesin is glycosylated in a process that requires HMW1C and phosphoglucomutase, an enzyme involved in lipooligosaccharide biosynthesis[J]. Molecular Microbiology, 2003, 48(3): 737-751. |
| 138 | GROSS J, GRASS S, DAVIS A E, et al. The Haemophilus influenzae HMW1 adhesin is a glycoprotein with an unusual N-linked carbohydrate modification[J]. Journal of Biological Chemistry, 2008, 283(38): 26010-26015. |
| 139 | GRASS S, LICHTI C F, TOWNSEND R R, et al. The Haemophilus influenzae HMW1C protein is a glycosyltransferase that transfers hexose residues to asparagine sites in the HMW1 adhesin[J]. PLoS Pathogens, 2010, 6(5): e1000919. |
| 140 | CHOI K J, GRASS S, PAEK S, et al. The Actinobacillus pleuropneumoniae HMW1C-like glycosyltransferase mediates N-linked glycosylation of the Haemophilus influenzae HMW1 adhesin[J]. PLoS One, 2010, 5(12): e15888. |
| 141 | SCHWARZ F, FAN Y Y, SCHUBERT M, et al. Cytoplasmic N-glycosyltransferase of Actinobacillus pleuropneumoniae is an inverting enzyme and recognizes the NX(S/T) consensus sequence[J]. Journal of Biological Chemistry, 2011, 286(40): 35267-35274. |
| 142 | LOMINO J V, NAEGELI A, ORWENYO J, et al. A two-step enzymatic glycosylation of polypeptides with complex N-glycans[J]. Bioorganic & Medicinal Chemistry, 2013, 21(8): 2262-2270. |
| 143 | SØRENSEN A L, REIS C A, TARP M A, et al. Chemoenzymatically synthesized multimeric Tn/STn MUC1 glycopeptides elicit cancer-specific anti-MUC1 antibody responses and override tolerance[J]. Glycobiology, 2006, 16(2): 96-107. |
| 144 | WU Z G, JIANG K, ZHU H L, et al. Site-directed glycosylation of peptide/protein with homogeneous O-linked eukaryotic N-glycans[J]. Bioconjugate Chemistry, 2016, 27(9): 1972-1975. |
| 145 | PARODI A J. Protein glucosylation and its role in protein folding[J]. Annual Review of Biochemistry, 2000, 69: 69-93. |
| 146 | CHAFFEY P K, GUAN X Y, WANG X F, et al. Quantitative effects of O-linked glycans on protein folding[J]. Biochemistry, 2017, 56(34): 4539-4548. |
| 147 | KIUCHI T, IZUMI M, MUKOGAWA Y, et al. Monitoring of glycoprotein quality control system with a series of chemically synthesized homogeneous native and misfolded glycoproteins[J]. Journal of the American Chemical Society, 2018, 140(50): 17499-17507. |
| 148 | SHI W W, WANG T Y, YANG Z Y, et al. L-glycosidase-cleavable natural glycans facilitate the chemical synthesis of correctly folded disulfide-bonded D-proteins[J]. Angewandte Chemie International Edition, 2024, 63(9): e202313640. |
| 149 | CHENG C W, WU C Y, WANG S W, et al. Low-sugar universal mRNA vaccine against coronavirus variants with deletion of glycosites in the S2 or stem of SARS-CoV-2 spike messenger RNA (mRNA)[J]. Proceedings of the National Academy of Sciences of the United States of America, 2023, 120(49): e2314392120. |
| 150 | ZHANG Y N, PAYNTER J, ANTANASIJEVIC A, et al. Single-component multilayered self-assembling protein nanoparticles presenting glycan-trimmed uncleaved prefusion optimized envelope trimers as HIV-1 vaccine candidates[J]. Nature Communications, 2023, 14(1): 1985. |
| 151 | LIU C P, TSAI T I, CHENG T, et al. Glycoengineering of antibody (herceptin) through yeast expression and in vitro enzymatic glycosylation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(4): 720-725. |
| 152 | WANG L X, TONG X, LI C, et al. Glycoengineering of antibodies for modulating functions[J]. Annual Review of Biochemistry, 2019, 88: 433-459. |
| 153 | JACKSON H J, RAFIQ S, BRENTJENS R J. Driving CAR T-cells forward[J]. Nature Reviews Clinical Oncology, 2016, 13(6): 370-383. |
| 154 | BECKWITH D M, CUDIC M. Tumor-associated O-glycans of MUC1: carriers of the glyco-code and targets for cancer vaccine design[J]. Seminars in Immunology, 2020, 47: 101389. |
| [1] | 谢向前, 郭雯, 王欢, 李进. 含氨基乙烯半胱氨酸核糖体肽的生物合成与化学合成[J]. 合成生物学, 2024, 5(5): 981-996. |
| [2] | 汤志军, 胡友财, 刘文. 酶促4+2和2+2环加成反应:区域与立体选择性的理解与应用[J]. 合成生物学, 2024, 5(3): 401-407. |
| [3] | 袁燕燕, 陈慧芳, 杨思慧, 王洪辉, 聂舟. 人工调控受体聚集的化学合成生物学策略及应用[J]. 合成生物学, 2024, 5(1): 53-76. |
| [4] | 闫汉, 肖鹏峰, 刘全俊, 陆祖宏. DNA微阵列原位化学合成[J]. 合成生物学, 2021, 2(3): 354-370. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||