合成生物学 ›› 2024, Vol. 5 ›› Issue (5): 1102-1124.DOI: 10.12211/2096-8280.2024-008
与核酸兼容的化学反应开发进展
刘子健1,2, 穆柏杨3, 段志强1, 王璇1, 陆晓杰1,2
- 1.中国科学院上海药物研究所,原创新药研究全国重点实验室,上海 201203
2.中国科学院大学,北京 100049
3.山东第二医科大学,山东 潍坊 261053
-
收稿日期:2024-01-17修回日期:2024-05-30出版日期:2024-10-31发布日期:2024-11-20 -
通讯作者:陆晓杰 -
作者简介:刘子健 (2000—),男,硕士研究生。研究方向为on-DNA化学反应开发和DNA编码化合物库的构建和筛选。 E-mail:liuzijian@simm.ac.cn穆柏杨 (2000—),男,硕士研究生。研究方向为on-DNA化学反应开发和苗头化合物发现和优化。 E-mail:mubaiyang@simm.ac.cn陆晓杰 (1983—),男,博士,研究员,博士生导师。研究方向为DNA编码化合库技术的开发和应用。 E-mail:xjlu@simm.ac.cn -
基金资助:国家自然科学基金(22377139);国家重点研发计划(91953203)
Advances in the development of DNA-compatible chemistries
LIU Zijian1,2, MU Baiyang3, DUAN Zhiqiang1, WANG Xuan1, LU Xiaojie1,2
- 1.State Key Laboratory of Drug Research,Shanghai Institute of Materia Medica,Chinese Academic of Sciences,Shanghai 201203,China
2.University of Chinese Academy of Sciences,Beijing 100049,China
3.Shandong Second Medical University,Weifang 261053,Shandong,China
-
Received:2024-01-17Revised:2024-05-30Online:2024-10-31Published:2024-11-20 -
Contact:LU Xiaojie
摘要:
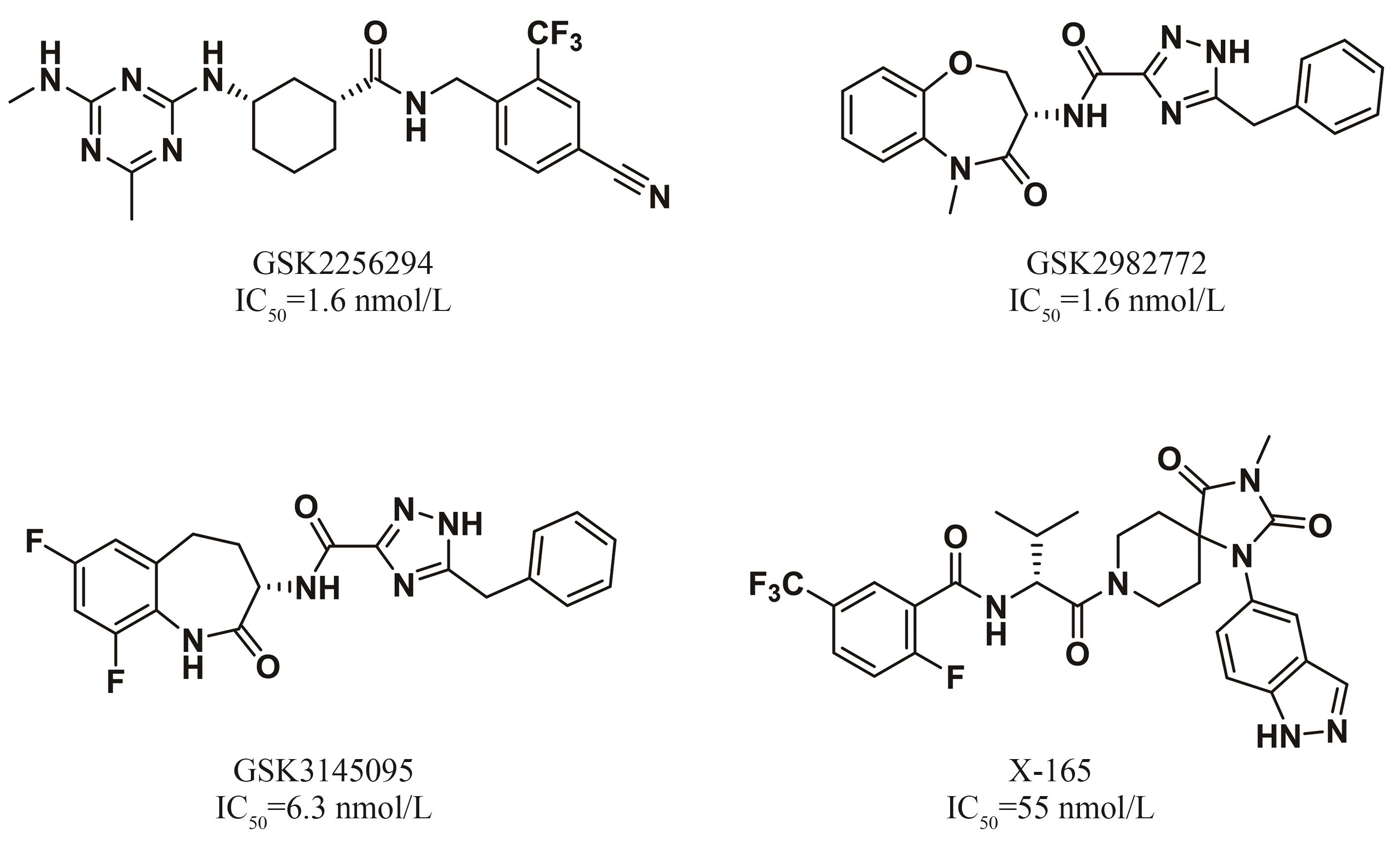
DNA编码化合物库(DNA-Encoded Library,DEL)技术作为一种新兴的小分子药物筛选手段已经成为新药研发中不可或缺的重要技术平台。与核酸兼容(on-DNA)的化学反应对于构建具有丰富化学空间和结构多样性的DEL具有重要意义。近年来,on-DNA化学反应的数量不断增加,极大地拓宽了可用于DEL构建的化学反应范畴。同时,一系列创新性的反应方法,诸如光催化、固相合成以及生物合成等,亦在on-DNA化学反应领域不断涌现,进一步推动了该领域的发展。本文系统综述了近年来金属催化的on-DNA化学反应,包括:C(sp2)—C(sp2)键生成反应、C(sp3)—C(sp3)键生成反应、C(sp2)—C(sp3)键生成反应以及C(sp2)—X键生成反应;采用目标导向合成策略和多样性导向合成策略合成具有单环、稠环、螺环等on-DNA优势骨架;光催化和酶催化on-DNA化学反应等的研究进展。然而,目前开发的on-DNA化学反应仍然存在诸如与核酸的兼容性、底物适用性等问题,开发更高效、更稳定且能在温和条件下进行的on-DNA化学反应,发展新型的on-DNA化学反应类型,以及结合高通量筛选和计算机辅助的on-DNA反应开发仍然具有重要意义。
中图分类号:
引用本文
刘子健, 穆柏杨, 段志强, 王璇, 陆晓杰. 与核酸兼容的化学反应开发进展[J]. 合成生物学, 2024, 5(5): 1102-1124.
LIU Zijian, MU Baiyang, DUAN Zhiqiang, WANG Xuan, LU Xiaojie. Advances in the development of DNA-compatible chemistries[J]. Synthetic Biology Journal, 2024, 5(5): 1102-1124.
| 条目 | 反应式 | 反应条件 | 参考文献 |
|---|---|---|---|
| 1 | 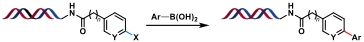 | Pd (PPh3)4, Na2CO3, H2O/DMA/CH3CN, 80 ℃ | [ |
| 2 |  | POPd/sSPhos, KOH, H2O/DMA, 80 ℃ | [ |
| 3 | 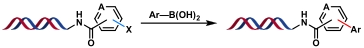 | Pd(OAc)2, Et3N H2O/DMA, rt, 2 h | [ |
| 4 | 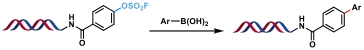 | Pd(OAc)2, Et3N H2O/DMA, rt, 2 h | [ |
| 5 |  | Pd(OAc)2, (rac)-BIDIME, K2CO3, DMA/H2O, 95℃, 2 h | [ |
| 6 | 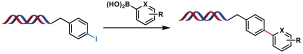 | Pd(OAc)2/TPPTS, K2CO3, H2O/DMA, 70℃, 2 h | [ |
| 7 | 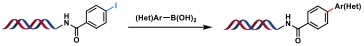 | Na2PdCl4/sSPhos, K2CO3, H2O/ACN, 37℃, 28 h | [ |
| 8 | 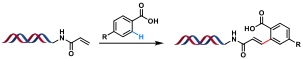 | [Ru], KOAc, DMF/H2O, 60℃, 10 h | [ |
| 9 |  | 1: PdCl2(dppf)DCM, K2CO3, DMSO/H2O, 80 ℃ 2: PdCl2(COD), NaOAc, DMA/H2O, 80 ℃ | [ |
表1 金属催化的C(sp2)—C(sp2)键生成反应
Table 1 Metal-catalyzed C(sp2)—C(sp2) bond formation reactions
| 条目 | 反应式 | 反应条件 | 参考文献 |
|---|---|---|---|
| 1 |  | Pd (PPh3)4, Na2CO3, H2O/DMA/CH3CN, 80 ℃ | [ |
| 2 |  | POPd/sSPhos, KOH, H2O/DMA, 80 ℃ | [ |
| 3 | 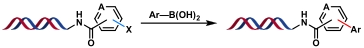 | Pd(OAc)2, Et3N H2O/DMA, rt, 2 h | [ |
| 4 |  | Pd(OAc)2, Et3N H2O/DMA, rt, 2 h | [ |
| 5 |  | Pd(OAc)2, (rac)-BIDIME, K2CO3, DMA/H2O, 95℃, 2 h | [ |
| 6 |  | Pd(OAc)2/TPPTS, K2CO3, H2O/DMA, 70℃, 2 h | [ |
| 7 |  | Na2PdCl4/sSPhos, K2CO3, H2O/ACN, 37℃, 28 h | [ |
| 8 |  | [Ru], KOAc, DMF/H2O, 60℃, 10 h | [ |
| 9 |  | 1: PdCl2(dppf)DCM, K2CO3, DMSO/H2O, 80 ℃ 2: PdCl2(COD), NaOAc, DMA/H2O, 80 ℃ | [ |
| 条目 | DNA兼容反应 | 反应条件 | 参考文献 |
|---|---|---|---|
| 1 | 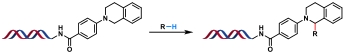 | CuOTf, TBHP ACN/H2O, 50/70 ℃, 10 h | [ |
| 2 | 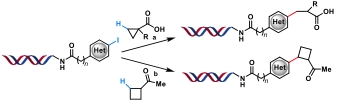 | a: Pd(OAc)2, AgOAc, Li2CO3, H2O/DMA, 80℃, 20 h b: Pd(OAc)2/ligand AgTFA, NaOAc H2O/DMA, 80℃, 20 h | [ |
表2 金属催化的C(sp2)—C(sp3)和C(sp2)—C(sp)键生成反应
Table 2 Metal-catalyzed C(sp2)—C(sp3) and C(sp2)—C(sp) bond formation reactions
| 条目 | DNA兼容反应 | 反应条件 | 参考文献 |
|---|---|---|---|
| 1 |  | CuOTf, TBHP ACN/H2O, 50/70 ℃, 10 h | [ |
| 2 |  | a: Pd(OAc)2, AgOAc, Li2CO3, H2O/DMA, 80℃, 20 h b: Pd(OAc)2/ligand AgTFA, NaOAc H2O/DMA, 80℃, 20 h | [ |
| 条目 | DNA兼容反应 | 反应条件 | 参考文献 |
|---|---|---|---|
| 1 | 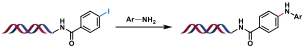 | t-BuXPhos Pd G1, CsOH, H2O/DMA, 100 ℃, 3 h | [ |
| 2 | 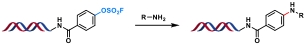 | t-Brettphos Pd G3, Et3N, H2O/DMA, 60 ℃, 2 h | [ |
| 3 | 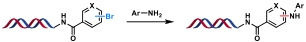 | t-BuXPhos-Pd-G3, NaOH, H2O/DMA, 60 ℃, 2 h | [ |
| 4 | 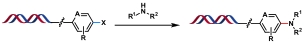 | Pd-PEPPSI-iPentCl-pyr, Na ascorbate, CsOH, DMA/H2O, 95 ℃, 15 min | [ |
| 5 |  | Pd(OAc)2/BippyPhos, Na ascorbate, K3PO4, DMA/H2O, 95 ℃, 15 min | [ |
| 6 |  | t-BuXPhos-Pd-G1, NaOH, H2O/DMA, 80 ℃, 3 h | [ |
| 7 | 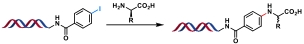 | 1: CuSO4·5H2O, Na ascorbate, H2O/DMA, 100 ℃, 2 h 2: CuSO4·5H2O, Proline, KOH, Na ascorbate, H2O/DMA, 100 ℃, 2 h | [ |
| 8 | 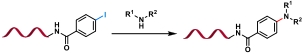 | Cu(OAc)2 /ligand, Na ascorbate, K3PO4, DMSO/H2O, 40℃, 3 h | [ |
| 9 | 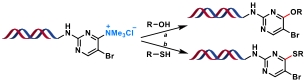 | a: K2CO3 or KOH, DMA/H2O, rt, or 60 ℃, 10 h b: K2CO3, DMA/H2O, rt, or 80 ℃, 10 h | [ |
| 10 | 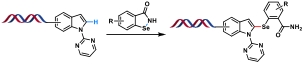 | [Rh], PBS (pH 4.2)-DMA (7∶1) 80 ℃, 6 h | [ |
| 11 | 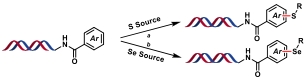 | a: I2, BSA, MeOH/H2O, 40, ℃, 150 min b: (1) BME, RT, 10 min (2) I2, BSA, MeOH/H2O, 40, ℃, 150 min | [ |
表3 金属催化的C(sp2)—X偶联反应
Table 3 Metal-catalyzed C—X coupling reactions
| 条目 | DNA兼容反应 | 反应条件 | 参考文献 |
|---|---|---|---|
| 1 |  | t-BuXPhos Pd G1, CsOH, H2O/DMA, 100 ℃, 3 h | [ |
| 2 |  | t-Brettphos Pd G3, Et3N, H2O/DMA, 60 ℃, 2 h | [ |
| 3 |  | t-BuXPhos-Pd-G3, NaOH, H2O/DMA, 60 ℃, 2 h | [ |
| 4 |  | Pd-PEPPSI-iPentCl-pyr, Na ascorbate, CsOH, DMA/H2O, 95 ℃, 15 min | [ |
| 5 |  | Pd(OAc)2/BippyPhos, Na ascorbate, K3PO4, DMA/H2O, 95 ℃, 15 min | [ |
| 6 |  | t-BuXPhos-Pd-G1, NaOH, H2O/DMA, 80 ℃, 3 h | [ |
| 7 |  | 1: CuSO4·5H2O, Na ascorbate, H2O/DMA, 100 ℃, 2 h 2: CuSO4·5H2O, Proline, KOH, Na ascorbate, H2O/DMA, 100 ℃, 2 h | [ |
| 8 |  | Cu(OAc)2 /ligand, Na ascorbate, K3PO4, DMSO/H2O, 40℃, 3 h | [ |
| 9 |  | a: K2CO3 or KOH, DMA/H2O, rt, or 60 ℃, 10 h b: K2CO3, DMA/H2O, rt, or 80 ℃, 10 h | [ |
| 10 |  | [Rh], PBS (pH 4.2)-DMA (7∶1) 80 ℃, 6 h | [ |
| 11 |  | a: I2, BSA, MeOH/H2O, 40, ℃, 150 min b: (1) BME, RT, 10 min (2) I2, BSA, MeOH/H2O, 40, ℃, 150 min | [ |
| 条目 | DNA兼容反应 | 反应条件 | 参考文献 |
|---|---|---|---|
| 1 | 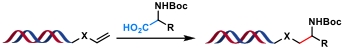 | [Ir], blue light, K2HPO4, DMSO/H2O, N2, rt, 6 h | [ |
| 2 | 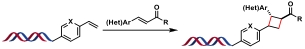 | [Ir], blue light, DMSO/H2O, glycerol, rt, 2 h | [ |
| 3 | 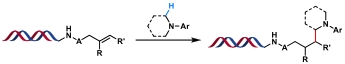 | [Ir], blue light, K2HPO4, DMSO/H2O, N2, rt, 2 h | [ |
| 4 | 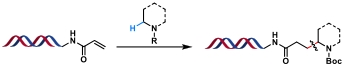 | [Ir], blue light, quinuclidine, DMSO/H2O, N2, rt, 45 min | [ |
| 5 | 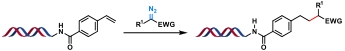 | [Ru], blue light, Hantzsch ester, 4-methylbenzenethiol DMA/H2O, rt, 3 h | [ |
| 6 | 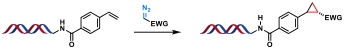 | [Ru], blue light, I2, DMA/H2O, rt, 3 h | [ |
| 7 | 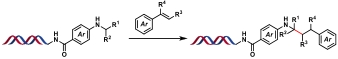 | [Ir], blue light, quinuclidine, DMF/H2O, N2, rt, 1.5 h | [ |
| 8 | 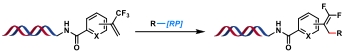 | [Ir], blue light, 2,6-lutidine, DMSO/H2O, rt, 10 min | [ |
| 9 | 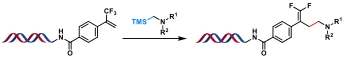 | [Ir], Kessil lamp, DMSO/H2O, rt, 5 min | [ |
| 10 | 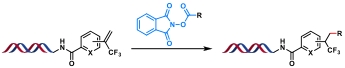 | Hantzsch ester, blue Kessil, DMSO/H2O, rt, 5 min | [ |
表4 光催化的C(sp3)—C(sp3)键生成反应
Table 4 Photocatalytic C(sp3)—C(sp3) bond formation reactions
| 条目 | DNA兼容反应 | 反应条件 | 参考文献 |
|---|---|---|---|
| 1 |  | [Ir], blue light, K2HPO4, DMSO/H2O, N2, rt, 6 h | [ |
| 2 |  | [Ir], blue light, DMSO/H2O, glycerol, rt, 2 h | [ |
| 3 |  | [Ir], blue light, K2HPO4, DMSO/H2O, N2, rt, 2 h | [ |
| 4 |  | [Ir], blue light, quinuclidine, DMSO/H2O, N2, rt, 45 min | [ |
| 5 |  | [Ru], blue light, Hantzsch ester, 4-methylbenzenethiol DMA/H2O, rt, 3 h | [ |
| 6 |  | [Ru], blue light, I2, DMA/H2O, rt, 3 h | [ |
| 7 |  | [Ir], blue light, quinuclidine, DMF/H2O, N2, rt, 1.5 h | [ |
| 8 |  | [Ir], blue light, 2,6-lutidine, DMSO/H2O, rt, 10 min | [ |
| 9 |  | [Ir], Kessil lamp, DMSO/H2O, rt, 5 min | [ |
| 10 |  | Hantzsch ester, blue Kessil, DMSO/H2O, rt, 5 min | [ |
| 条目 | DNA兼容反应 | 反应条件 | 参考文献 |
|---|---|---|---|
| 1 | 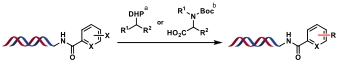 | a: 4CzIPN, Ni(TMHD)2, blue LED, DMSO/H2O, 45 min b: [Ir], Ni(TMHD)2, blue LED, TMG, MOPS pH 8 buffer, DMSO/H2O, 10 min | [ |
| 2 | 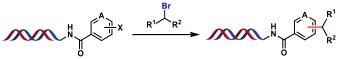 | [Ir], [Ni], blue Kessil, MgCl2, Et3N, DMSO/H2O, rt, 45 min | [ |
| 3 | 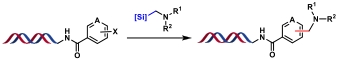 | [Ir], [Ni], blue Kessil, DMSO/H2O, rt, 15 min | [ |
| 4 | 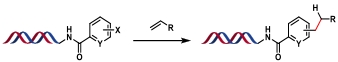 | [Ir], Hantzsch ester, blue Kessil, DMSO/H2O, rt, 5 min | [ |
| 5 | 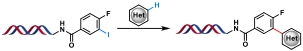 | [Ir], DIPEA, blue Kessil, DMSO/H2O, rt, 5 min | [ |
表5 光催化的C(sp2)—C键生成反应
Table 5 Photocatalytic C(sp2)—C bond formation reactions
| 条目 | DNA兼容反应 | 反应条件 | 参考文献 |
|---|---|---|---|
| 1 |  | a: 4CzIPN, Ni(TMHD)2, blue LED, DMSO/H2O, 45 min b: [Ir], Ni(TMHD)2, blue LED, TMG, MOPS pH 8 buffer, DMSO/H2O, 10 min | [ |
| 2 |  | [Ir], [Ni], blue Kessil, MgCl2, Et3N, DMSO/H2O, rt, 45 min | [ |
| 3 |  | [Ir], [Ni], blue Kessil, DMSO/H2O, rt, 15 min | [ |
| 4 |  | [Ir], Hantzsch ester, blue Kessil, DMSO/H2O, rt, 5 min | [ |
| 5 |  | [Ir], DIPEA, blue Kessil, DMSO/H2O, rt, 5 min | [ |
| 条目 | 杂环结构 | 合成策略 | 参考文献 |
|---|---|---|---|
| 1 | 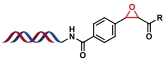 | 醛和α-氯代酮缩合环化 | [ |
| 2 | 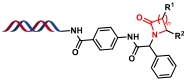 | 醛、氨基酸和异腈之间多组分缩合环化 | [ |
| 3 |  | Clauson-Kaas反应合成吡咯核心;再通过碘代和交叉偶联实现功能化 | [ |
| 4 | 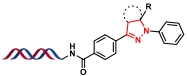 | 醛、苯磺酰肼和重氮盐环化生成二取代四唑;再与末端烯烃进行环加成反应 | [ |
| 5 | 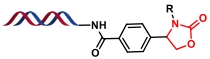 | 环氧化物开环生成β-氨基醇,再与氯甲酸酯环化 | [ |
| 6 | 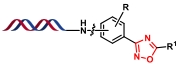 | 羟胺和腈生成偕胺肟,再由羧酸酰化后发生脱水环化 | [ |
| 7 | 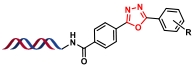 | 苯甲酰肼与醛缩合环化 | [ |
| 8 | 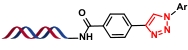 | 芳基硼酸先转化芳基叠氮化物,再与炔进行环加成 | [ |
| 9 |  | 腈与叠氮化物之间的环加成反应 | [ |
| 10 | 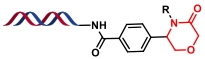 | 环氧化物开环生成β-氨基醇,再与氯乙酰氯环化 | [ |
| 11 | 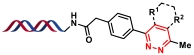 | 四嗪与烯基/羰基化合物进行IEDDA反应(烯基产物需要额外氧化) | [ |
| 12 | 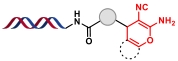 | 烯醇前体与羰基化合物和丙二腈之间的多组分缩合环化反应 | [ |
表6 单环合成反应
Table 6 Single-ring synthesis reactions
| 条目 | 杂环结构 | 合成策略 | 参考文献 |
|---|---|---|---|
| 1 |  | 醛和α-氯代酮缩合环化 | [ |
| 2 |  | 醛、氨基酸和异腈之间多组分缩合环化 | [ |
| 3 |  | Clauson-Kaas反应合成吡咯核心;再通过碘代和交叉偶联实现功能化 | [ |
| 4 |  | 醛、苯磺酰肼和重氮盐环化生成二取代四唑;再与末端烯烃进行环加成反应 | [ |
| 5 |  | 环氧化物开环生成β-氨基醇,再与氯甲酸酯环化 | [ |
| 6 |  | 羟胺和腈生成偕胺肟,再由羧酸酰化后发生脱水环化 | [ |
| 7 |  | 苯甲酰肼与醛缩合环化 | [ |
| 8 |  | 芳基硼酸先转化芳基叠氮化物,再与炔进行环加成 | [ |
| 9 |  | 腈与叠氮化物之间的环加成反应 | [ |
| 10 |  | 环氧化物开环生成β-氨基醇,再与氯乙酰氯环化 | [ |
| 11 |  | 四嗪与烯基/羰基化合物进行IEDDA反应(烯基产物需要额外氧化) | [ |
| 12 |  | 烯醇前体与羰基化合物和丙二腈之间的多组分缩合环化反应 | [ |
| 条目 | 杂环结构 | 合成策略 | 参考文献 |
|---|---|---|---|
| 1 | 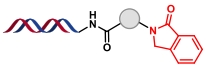 | 伯胺与邻苯二甲醛缩合环化反应 | [ |
| 2 | 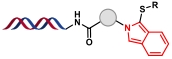 | 伯胺、邻苯二甲醛和4-叔丁基苯硫醇之间的多组分缩合环化反应 | |
| 3 | 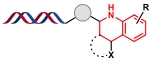 | 苯胺与醛缩合生成的亚胺,由布朗斯特酸活化后进行烯烃亲电加成、苯环亲电取代环化和消除反应 | [ |
| 4 | 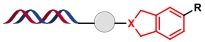 | 苯乙炔和1,6-庚二炔之间的环加成反应 | [ |
| 5 | 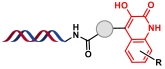 | 靛红与醛在苯甲酰肼促进下的环加成反应 | [ |
| 6 | 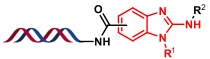 | 芳基伯胺与TCDI反应生成异硫氰酸酯,再与邻位仲胺生成硫脲;最后脱硫环化 | [ |
| 7 | 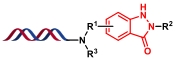 | 硝基芳烃经硼酸还原为亚硝基中间体,再与邻位酰胺氮原子亲核加成 | [ |
| 8 | 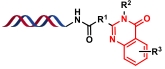 | 醛与邻氨基苯甲酰胺或醛与靛红酸酐和伯胺进行环化反应,再对环化产物进行氧化 | [ |
| 9 | 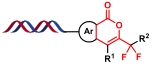 | 苯甲酸C—H活化后与炔发生环加成反应 | [ |
| 10 | 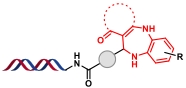 | 醛、邻苯二胺和1,3-二羰基化合物之间的多组分缩合环化反应 | [ |
表7 稠环合成反应
Table 7 Fused-ring synthesis reactions
| 条目 | 杂环结构 | 合成策略 | 参考文献 |
|---|---|---|---|
| 1 |  | 伯胺与邻苯二甲醛缩合环化反应 | [ |
| 2 |  | 伯胺、邻苯二甲醛和4-叔丁基苯硫醇之间的多组分缩合环化反应 | |
| 3 |  | 苯胺与醛缩合生成的亚胺,由布朗斯特酸活化后进行烯烃亲电加成、苯环亲电取代环化和消除反应 | [ |
| 4 |  | 苯乙炔和1,6-庚二炔之间的环加成反应 | [ |
| 5 |  | 靛红与醛在苯甲酰肼促进下的环加成反应 | [ |
| 6 |  | 芳基伯胺与TCDI反应生成异硫氰酸酯,再与邻位仲胺生成硫脲;最后脱硫环化 | [ |
| 7 |  | 硝基芳烃经硼酸还原为亚硝基中间体,再与邻位酰胺氮原子亲核加成 | [ |
| 8 |  | 醛与邻氨基苯甲酰胺或醛与靛红酸酐和伯胺进行环化反应,再对环化产物进行氧化 | [ |
| 9 |  | 苯甲酸C—H活化后与炔发生环加成反应 | [ |
| 10 |  | 醛、邻苯二胺和1,3-二羰基化合物之间的多组分缩合环化反应 | [ |
| 条目 | 杂环结构 | 合成策略 | 参考文献 |
|---|---|---|---|
| 1 | 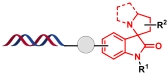 | 丙烯酰胺、靛红和脯氨酸之间的三组分环化反应 | [ |
| 2 | 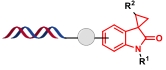 | 3-重氮吲哚酮和缺电子烯烃之间的环加成反应 | [ |
| 3 | 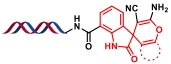 | 靛红、烯醇化物和丙二腈之间的环化反应 | [ |
| 4 | 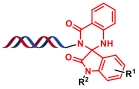 | 靛红和邻氨基苯甲酰胺之间的缩合环化反应 | [ |
| 5 | 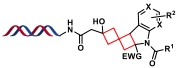 | 亚甲基环丁烷与不饱和杂环分子间的光环加成反应 | [ |
表8 螺环合成反应
Table 8 Spiro-ring synthesis reactions
| 条目 | 杂环结构 | 合成策略 | 参考文献 |
|---|---|---|---|
| 1 |  | 丙烯酰胺、靛红和脯氨酸之间的三组分环化反应 | [ |
| 2 |  | 3-重氮吲哚酮和缺电子烯烃之间的环加成反应 | [ |
| 3 |  | 靛红、烯醇化物和丙二腈之间的环化反应 | [ |
| 4 |  | 靛红和邻氨基苯甲酰胺之间的缩合环化反应 | [ |
| 5 |  | 亚甲基环丁烷与不饱和杂环分子间的光环加成反应 | [ |
| 条目 | 多功能核心 | 衍生杂环 | 参考文献 |
|---|---|---|---|
| 1 | 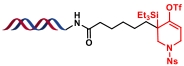 | 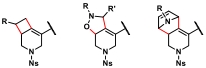 | [ |
| 2 | 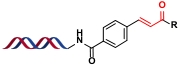 |  | [ |
| 3 |  |  | [ |
| 4 |  | 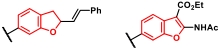 | [ |
表9 DOS导向的优势骨架合成反应
Table 9 DOS-directed privileged heterocycles synthesis reactions
| 条目 | 多功能核心 | 衍生杂环 | 参考文献 |
|---|---|---|---|
| 1 |  |  | [ |
| 2 |  |  | [ |
| 3 |  |  | [ |
| 4 |  | 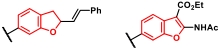 | [ |
| 1 | FOLMER R H A. Integrating biophysics with HTS-driven drug discovery projects[J]. Drug Discovery Today, 2016, 21(3): 491-498. |
| 2 | STARK J L, POWERS R. Application of NMR and molecular docking in structure-based drug discovery[J]. Topics in Current Chemistry, 2012, 326: 1-34. |
| 3 | ERLANSON D A, FESIK S W, HUBBARD R E, et al. Twenty years on: the impact of fragments on drug discovery[J]. Nature Reviews Drug Discovery, 2016, 15(9): 605-619. |
| 4 | BRENNER S, LERNER R A. Encoded combinatorial chemistry[J]. Proceedings of the National Academy of Sciences of the United States of America, 1992, 89(12): 5381-5383. |
| 5 | CASTAÑÓN J, ROMÁN J P, JESSOP T C, et al. Design and development of a technology platform for DNA-encoded library production and affinity selection[J]. SLAS Discovery, 2018, 23(5): 387-396. |
| 6 | DECURTINS W, WICHERT M, FRANZINI R M, et al. Automated screening for small organic ligands using DNA-encoded chemical libraries[J]. Nature Protocols, 2016, 11(4): 764-780. |
| 7 | LITOVCHICK A, DUMELIN C E, HABESHIAN S, et al. Encoded library synthesis using chemical ligation and the discovery of sEH inhibitors from a 334-million member library[J]. Scientific Reports, 2015, 5: 10916. |
| 8 | WANG J, LUNDBERG H, ASAI S, et al. Kinetically guided radical-based synthesis of C(sp3)—C(sp3) linkages on DNA[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(28): E6404-E6410. |
| 9 | BURROWS C J, MULLER J G. Oxidative nucleobase modifications leading to strand scission[J]. Chemical Reviews, 1998, 98(3): 1109-1152. |
| 10 | MA P X, ZHANG S N, HUANG Q P, et al. Evolution of chemistry and selection technology for DNA-encoded library[J]. Acta Pharmaceutica Sinica B, 2024, 14(2): 492-516. |
| 11 | KUNIG V, POTOWSKI M, GOHLA A, et al. DNA-encoded libraries—an efficient small molecule discovery technology for the biomedical sciences[J]. Biological Chemistry, 2018, 399(7): 691-710. |
| 12 | SHI Y, WU Y R, YU J Q, et al. DNA-encoded libraries (DELs): a review of on-DNA chemistries and their output[J]. RSC Advances, 2021, 11(4): 2359-2376. |
| 13 | FAIR R J, WALSH R T, HUPP C D. The expanding reaction toolkit for DNA-encoded libraries[J]. Bioorganic & Medicinal Chemistry Letters, 2021, 51: 128339. |
| 14 | SAHU R, YADAV S, NATH S, et al. DNA-encoded libraries via late-stage functionalization strategies: a review[J]. Chemical Communications, 2023, 59(41): 6128-6147. |
| 15 | FRANZINI R M, RANDOLPH C. Chemical space of DNA-encoded libraries[J]. Journal of Medicinal Chemistry, 2016, 59(14): 6629-6644. |
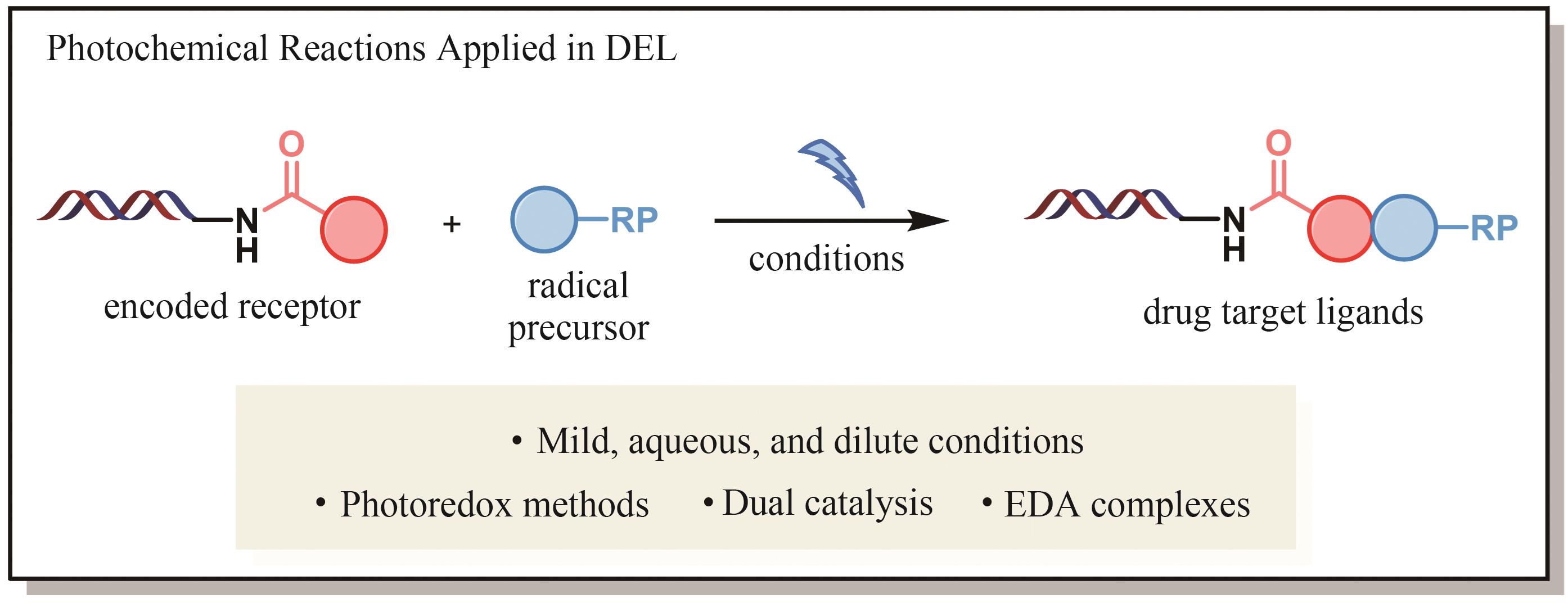
| 16 | MATSUO B, GRANADOS A, LEVITRE G, et al. Photochemical methods applied to DNA encoded library (DEL) synthesis[J]. Accounts of Chemical Research, 2023, 56(3): 385-401. |
| 17 | ADAMIK R, BUCHHOLCZ B, DARVAS F, et al. The potential of micellar media in the synthesis of DNA-encoded libraries[J]. Chemistry, 2022, 28(20): e202103967. |
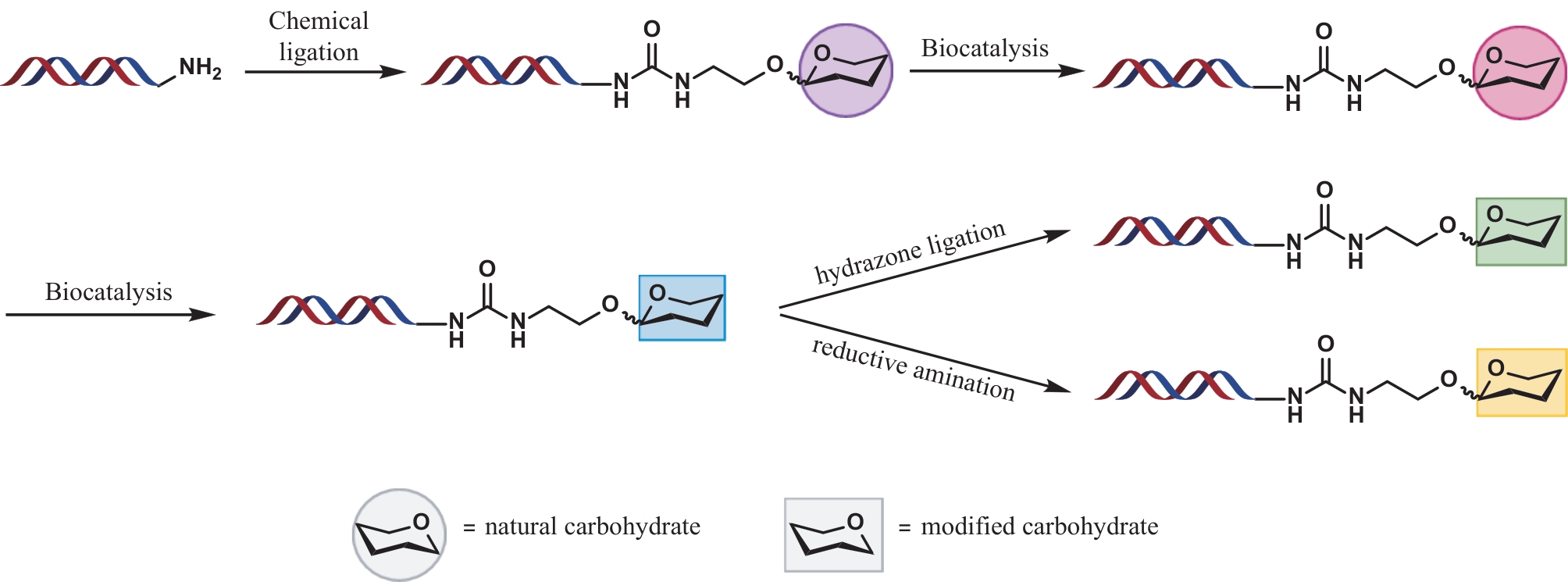
| 18 | THOMAS B, LU X J, BIRMINGHAM W R, et al. Application of biocatalysis to on-DNA carbohydrate library synthesis[J]. ChemBioChem, 2017, 18(9): 858-863. |
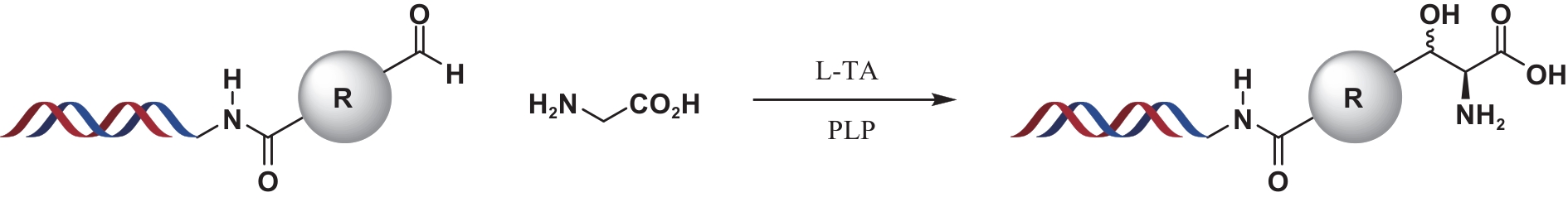
| 19 | CHAI J, LU X J, ARICO-MUENDEL C C, et al. Application of l-threonine aldolase to on-DNA reactions[J]. Bioconjugate Chemistry, 2021, 32(9): 1973-1978. |
| 20 | KADU B S. Suzuki-Miyaura cross coupling reaction: recent advancements in catalysis and organic synthesis[J]. Catalysis Science & Technology, 2021, 11(4): 1186-1221. |
| 21 | OMUMI A, BEACH D G, BAKER M, et al. Postsynthetic guanine arylation of DNA by Suzuki-Miyaura cross-coupling[J]. Journal of the American Chemical Society, 2011, 133(1): 42-50. |
| 22 | DING Y, CLARK M A. Robust Suzuki-Miyaura cross-coupling on DNA-linked substrates[J]. ACS Combinatorial Science, 2015, 17(1): 1-4. |
| 23 | DING Y, DELOREY J L, CLARK M A. Novel catalyst system for Suzuki-Miyaura coupling of challenging DNA-linked aryl chlorides[J]. Bioconjugate Chemistry, 2016, 27(11): 2597-2600. |
| 24 | LI J Y, HUANG H B. Development of DNA-compatible Suzuki-Miyaura reaction in aqueous media[J]. Bioconjugate Chemistry, 2018, 29(11): 3841-3846. |
| 25 | XU H T, MA F, WANG N, et al. DNA-encoded libraries: aryl fluorosulfonates as versatile electrophiles enabling facile on-DNA Suzuki, Sonogashira, and Buchwald reactions[J]. Advanced Science, 2019, 6(23): 1901551. |
| 26 | QU Y, LIU S X, WEN H N, et al. Palladium-mediated Suzuki-Miyaura cross-coupling reaction of potassium Boc-protected aminomethyltrifluoroborate with DNA-conjugated aryl bromides for DNA-encoded chemical library synthesis[J]. Biochemical and Biophysical Research Communications, 2020, 533(2): 209-214. |
| 27 | FAVALLI N, BASSI G, BIANCHI D, et al. Large screening of DNA-compatible reaction conditions for Suzuki and Sonogashira cross-coupling reactions and for reverse amide bond formation[J]. Bioorganic & Medicinal Chemistry, 2021, 41: 116206. |
| 28 | SIRIPURAM V K, SUNKARI Y K, NGUYEN T L, et al. DNA-compatible Suzuki-Miyaura cross-coupling reaction of aryl iodides with (hetero)aryl boronic acids for DNA-encoded libraries[J]. Frontiers in Chemistry, 2022, 10: 894603. |
| 29 | WANG X, SUN H, LIU J X, et al. Ruthenium-promoted C—H activation reactions between DNA-conjugated acrylamide and aromatic acids[J]. Organic Letters, 2018, 20(16): 4764-4768. |
| 30 | WANG X, SUN H, LIU J X, et al. Palladium-promoted DNA-compatible Heck reaction[J]. Organic Letters, 2019, 21(3): 719-723. |
| 31 | KRANTHIKUMAR R. Recent advances in C(sp3)—C(sp3) cross-coupling chemistry: a dominant performance of nickel catalysts[J]. Organometallics, 2022, 41(6): 667-679. |
| 32 | WEN X, DUAN Z Q, LIU J X, et al. On-DNA cross-dehydrogenative coupling reaction toward the synthesis of focused DNA-encoded tetrahydroisoquinoline libraries[J]. Organic Letters, 2020, 22(15): 5721-5725. |
| 33 | FAN Z L, ZHAO S, LIU T, et al. Merging C(sp3)—H activation with DNA-encoding[J]. Chemical Science, 2020, 11(45): 12282-12288. |
| 34 | LU X J, ROBERTS S E, FRANKLIN G J, et al. On-DNA Pd and Cu promoted C—N cross-coupling reactions[J]. MedChemComm, 2017, 8(8): 1614-1617. |
| 35 | FORERO-CORTÉS P A, HAYDL A M. The 25th anniversary of the Buchwald-Hartwig amination: development, applications, and outlook[J]. Organic Process Research & Development, 2019, 23(8): 1478-1483. |
| 36 | DE PEDRO BEATO E, PRIEGO J, GIRONDA-MARTÍNEZ A, et al. Mild and efficient palladium-mediated C—N cross-coupling reaction between DNA-conjugated aryl bromides and aromatic amines[J]. ACS Combinatorial Science, 2019, 21(2): 69-74. |
| 37 | CHEN Y C, FAVER J C, KU A F, et al. C—N coupling of DNA-conjugated (hetero)aryl bromides and chlorides for DNA-encoded chemical library synthesis[J]. Bioconjugate Chemistry, 2020, 31(3): 770-780. |
| 38 | CHHEDA P R, SIMMONS N, SCHUMAN D P, et al. Palladium-mediated C—N coupling of DNA-conjugated (hetero)aryl halides with aliphatic and (hetero)aromatic amines[J]. Organic Letters, 2022, 24(18): 3401-3406. |
| 39 | YANG J, XIA S D, LIU J X, et al. DNA-encoded focused indazole library synthesis by a palladium-mediated CN(sp2) cross-coupling reaction between DNA-linked (hetero)aryl halides and aromatic nitrogen heterocycles[J]. Tetrahedron Letters, 2022, 96: 153732. |
| 40 | YANG Q, ZHAO Y S, MA D W. Cu-mediated ullmann-type cross-coupling and industrial applications in route design, process development, and scale-up of pharmaceutical and agrochemical processes[J]. Organic Process Research & Development, 2022, 26(6): 1690-1750. |
| 41 | RUFF Y, BERST F. Efficient copper-catalyzed amination of DNA-conjugated aryl iodides under mild aqueous conditions[J]. MedChemComm, 2018, 9(7): 1188-1193. |
| 42 | WANG D Y, WEN X, XIONG C D, et al. Non-transition metal-mediated diverse aryl-heteroatom bond formation of arylammonium salts[J]. iScience, 2019, 15: 307-315. |
| 43 | XU H T, GU Y A, ZHANG S N, et al. A chemistry for incorporation of selenium into DNA-encoded libraries[J]. Angewandte Chemie International Edition, 2020, 59(32): 13273-13280. |
| 44 | YANG S L, ZHAO G X, GAO Y T, et al. In-solution direct oxidative coupling for the integration of sulfur/selenium into DNA-encoded chemical libraries[J]. Chemical Science, 2022, 13(9): 2604-2613. |
| 45 | TELLIS J C, PRIMER D N, MOLANDER G A. Dual catalysis. Single-electron transmetalation in organoboron cross-coupling by photoredox/nickel dual catalysis[J]. Science, 2014, 345(6195): 433-436. |
| 46 | PATEL S, BADIR S O, MOLANDER G A. Developments in photoredox-mediated alkylation for DNA-encoded libraries[J]. Trends in Chemistry, 2021, 3(3): 161-175. |
| 47 | KÖLMEL D K, LOACH R P, KNAUBER T, et al. Employing photoredox catalysis for DNA-encoded chemistry: decarboxylative alkylation of α-amino acids[J]. ChemMedChem, 2018, 13(20): 2159-2165. |
| 48 | KÖLMEL D K, RATNAYAKE A S, FLANAGAN M E, et al. Photocatalytic [2 + 2] cycloaddition in DNA-encoded chemistry[J]. Organic Letters, 2020, 22(8): 2908-2913. |
| 49 | WU R F, DU T, SUN W B, et al. Functionalization of DNA-tagged alkenes enabled by visible-light-induced C—H activation of N-aryl tertiary amines[J]. Organic Letters, 2021, 23(9): 3486-3490. |
| 50 | SHAN J M, LING X, LIU J X, et al. DNA-encoded CH functionality via photoredox-mediated hydrogen atom transformation catalysis[J]. Bioorganic & Medicinal Chemistry, 2021, 42: 116234. |
| 51 | FU X, TANG J, HUA R Y, et al. Functionalization of DNA-tagged alkenes with diazo compounds via photocatalysis[J]. Organic Letters, 2022, 24(11): 2208-2213. |
| 52 | MAHDAVI-AMIRI Y, HU M S J, FRIAS N, et al. Photoredox-catalysed hydroaminoalkylation of on-DNA N-arylamines[J]. Organic & Biomolecular Chemistry, 2023, 21(7): 1463-1467. |
| 53 | MÜLLER K, FAEH C, DIEDERICH F. Fluorine in pharmaceuticals: looking beyond intuition[J]. Science, 2007, 317(5846): 1881-1886. |
| 54 | PURSER S, MOORE P R, SWALLOW S, et al. Fluorine in medicinal chemistry[J]. Chemical Society Reviews, 2008, 37(2): 320-330. |
| 55 | PHELAN J P, LANG S B, SIM J H, et al. Open-air alkylation reactions in photoredox-catalyzed DNA-encoded library synthesis[J]. Journal of the American Chemical Society, 2019, 141(8): 3723-3732. |
| 56 | BADIR S O, SIM J H, BILLINGS K, et al. Multifunctional building blocks compatible with photoredox-mediated alkylation for DNA-encoded library synthesis[J]. Organic Letters, 2020, 22(3): 1046-1051. |
| 57 | BADIR S O, LIPP A, KRUMB M, et al. Photoredox-mediated hydroalkylation and hydroarylation of functionalized olefins for DNA-encoded library synthesis[J]. Chemical Science, 2021, 12(36): 12036-12045. |
| 58 | CHENG J P, LU Y, ZHU X Q, et al. Heterolytic and homolytic N—H bond dissociation energies of 4-substituted Hantzsch 2,6-dimethyl-1,4-dihydropyridines and the effect of one-electron transfer on the N—H bond activation[J]. The Journal of Organic Chemistry, 2000, 65(12): 3853-3857. |
| 59 | DING H, GREENBERG M M. DNA damage and interstrand cross-link formation upon irradiation of aryl iodide C-nucleotide analogues[J]. The Journal of Organic Chemistry, 2010, 75(3): 535-544. |
| 60 | KRUMB M, KAMMER L M, BADIR S O, et al. Photochemical C—H arylation of heteroarenes for DNA-encoded library synthesis[J]. Chemical Science, 2022, 13(4): 1023-1029. |
| 61 | JAMPILEK J. Heterocycles in medicinal chemistry[J]. Molecules, 2019, 24(21): 3839. |
| 62 | LIPINSKI C A, LOMBARDO F, DOMINY B W, et al. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings[J]. Advanced Drug Delivery Reviews, 2001, 46(1-3): 3-26. |
| 63 | WEN X, WU X Y, JIN R, et al. Privileged heterocycles for DNA-encoded library design and hit-to-lead optimization[J]. European Journal of Medicinal Chemistry, 2023, 248: 115079. |
| 64 | GAO Y T, ZHAO G X, HE P Y, et al. DNA-compatible synthesis of α,β-epoxyketones for DNA-encoded chemical libraries[J]. Bioconjugate Chemistry, 2022, 33(1): 105-110. |
| 65 | MANTELL M A, MARCAURELLE L, DING Y. One reaction served three ways: the on-DNA ugi 4C-3C reaction for the formation of lactams[J]. Organic Letters, 2023, 25(8): 1241-1245. |
| 66 | QI J J, LIU S X, SEYDIMEMET M, et al. A general set of DNA-compatible reactions for preparing DNA-tagged multisubstituted pyrroles[J]. Bioconjugate Chemistry, 2021, 32(11): 2290-2294. |
| 67 | ZHANG J, LI X F, WEI H M, et al. Sequential DNA-encoded building block fusion for the construction of polysubstituted pyrazoline core libraries[J]. Organic Letters, 2021, 23(21): 8429-8433. |
| 68 | FAN L J, DAVIE C P. Zirconium(Ⅳ)-catalyzed ring opening of on-DNA epoxides in water[J]. Chembiochem, 2017, 18(9): 843-847. |
| 69 | DU H C, BANGS M C, SIMMONS N, et al. Multistep synthesis of 1,2,4-oxadiazoles via DNA-conjugated aryl nitrile substrates[J]. Bioconjugate Chemistry, 2019, 30(5): 1304-1308. |
| 70 | MA F, LI J, ZHANG S N, et al. DNA-encoded libraries: hydrazide as a pluripotent precursor for on-DNA synthesis of various azole derivatives[J]. Chemistry, 2021, 27(31): 8214-8220. |
| 71 | COSTA M S, BOECHAT N, RANGEL E A, et al. Synthesis, tuberculosis inhibitory activity, and SAR study of N-substituted-phenyl-1,2,3-triazole derivatives[J]. Bioorganic & Medicinal Chemistry, 2006, 14(24): 8644-8653. |
| 72 | GIFFIN M J, HEASLET H, BRIK A, et al. A copper(Ⅰ)- catalyzed 1,2,3-triazole azide-alkyne click compound is a potent inhibitor of a multidrug-resistant HIV-1 protease variant[J]. Journal of Medicinal Chemistry, 2008, 51(20): 6263-6270. |
| 73 | MELDAL M, TORNØE C W. Cu-catalyzed azide-alkyne cycloaddition[J]. Chemical Reviews, 2008, 108(8): 2952-3015. |
| 74 | HEIN J E, TRIPP J C, KRASNOVA L B, et al. Copper (Ⅰ)-catalyzed cycloaddition of organic azides and 1-iodoalkynes[J]. Angewandte Chemie International Edition, 2009, 48(43): 8018-8021. |
| 75 | GIRONDA-MARTÍNEZ A, NERI D, SAMAIN F, et al. DNA-compatible diazo-transfer reaction in aqueous media suitable for DNA-encoded chemical library synthesis[J]. Organic Letters, 2019, 21(23): 9555-9558. |
| 76 | KABOUDIN B, ABEDI Y, YOKOMATSU T. One-pot synthesis of 1,2,3-triazoles from boronic acids in water using Cu(Ⅱ)-β-cyclodextrin complex as a nanocatalyst[J]. Organic & Biomolecular Chemistry, 2012, 10(23): 4543-4548. |
| 77 | QU Y, WEN H N, GE R, et al. Copper-mediated DNA-compatible one-pot click reactions of alkynes with aryl borates and TMS-N3 [J]. Organic Letters, 2020, 22(11): 4146-4150. |
| 78 | SINGH H, CHAWLA A S, KAPOOR V K, et al. Medicinal chemistry of tetrazoles[J]. Progress in Medicinal Chemistry, 1980, 17: 151-183. |
| 79 | DU H C, MATZUK M M, CHEN Y C. Synthesis of 5-substituted tetrazoles via DNA-conjugated nitrile[J]. Organic & Biomolecular Chemistry, 2020, 18(45): 9221-9226. |
| 80 | LI H L, SUN Z, WU W T, et al. Inverse-electron-demand Diels-Alder reactions for the synthesis of pyridazines on DNA[J]. Organic Letters, 2018, 20(22): 7186-7191. |
| 81 | GAO Y T, SUN Y, ZHAO G X, et al. On-DNA synthesis of functionalized 4H-pyran scaffolds for focused DNA-encoded chemical libraries[J]. Organic Letters, 2022, 24(36): 6664-6669. |
| 82 | NIE Q G, FANG X F, LIU C Y, et al. DNA-compatible ortho-phthalaldehyde (OPA)-mediated 2-substituted isoindole core formation and applications[J]. The Journal of Organic Chemistry, 2022, 87(5): 2551-2558. |
| 83 | ŠKOPIĆ M K, GÖTTE K, GRAMSE C, et al. Micellar Brønsted acid mediated synthesis of DNA-tagged heterocycles[J]. Journal of the American Chemical Society, 2019, 141(26): 10546-10555. |
| 84 | SUO Y R, XU M, SUN M M, et al. Ruthenium-mediated [2+2+2]cyclization: a route to forge indane and isoindoline core and its application in DNA-encoded library technology[J]. Organic Letters, 2022, 24(49): 9092-9096. |
| 85 | FANG X F, LIAO H L, FAN X H, et al. Incorporation of viridicatin alkaloid-like scaffolds into DNA-encoded chemical libraries[J]. Organic & Biomolecular Chemistry, 2023, 21(10): 2162-2166. |
| 86 | SU L Q, FENG J, PENG T, et al. Synthesis of multifunctional 2-aminobenzimidazoles on DNA via iodine-promoted cyclization[J]. Organic Letters, 2020, 22(4): 1290-1294. |
| 87 | BAO Y P, DENG Z F, FENG J, et al. A B2(OH)4-mediated synthesis of 2-substituted indazolone and its application in a DNA-encoded library[J]. Organic Letters, 2020, 22(16): 6277-6282. |
| 88 | WEN X, ZHANG M M, DUAN Z Q, et al. Discovery, SAR study of GST inhibitors from a novel quinazolin-4(1H)-one focused DNA-encoded library[J]. Journal of Medicinal Chemistry, 2023, 66(16): 11118-11132. |
| 89 | GAO H, LIN S, ZHANG S N, et al. Gem-difluoromethylene alkyne-enabled diverse C—H functionalization and application to the on-DNA synthesis of difluorinated isocoumarins[J]. Angewandte Chemie International Edition, 2021, 60(4): 1959-1966. |
| 90 | ZHAO G X, WANG H H, LUO J, et al. Multicomponent DNA-compatible synthesis of an annelated benzodiazepine scaffold for focused chemical libraries[J]. Organic Letters, 2023, 25(4): 665-670. |
| 91 | HIESINGER K, DAR’IN D, PROSCHAK E, et al. Spirocyclic scaffolds in medicinal chemistry[J]. Journal of Medicinal Chemistry, 2021, 64(1): 150-183. |
| 92 | WANG X, LIU J X, YAN Z Q, et al. Diversified strategy for the synthesis of DNA-encoded oxindole libraries[J]. Chemical Science, 2021, 12(8): 2841-2847. |
| 93 | NIE Q G, SUN J, FANG X F, et al. Antimony salt-promoted cyclization facilitating on-DNA syntheses of dihydroquinazolinone derivatives and its applications[J]. Chinese Chemical Letters, 2023, 34(8): 108132. |
| 94 | LI L B, MATSUO B, LEVITRE G, et al. Dearomative intermolecular [2+2] photocycloaddition for construction of C(sp3)-rich heterospirocycles on-DNA[J]. Chemical Science, 2023, 14(10): 2713-2720. |
| 95 | GALLOWAY W R J D, ISIDRO-LLOBET A, SPRING D R. Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules[J]. Nature Communications, 2010, 1: 80. |
| 96 | SPRING D R. Diversity-oriented synthesis; a challenge for synthetic chemists[J]. Organic & Biomolecular Chemistry, 2003, 1(22): 3867-3870. |
| 97 | WESTPHAL M V, HUDSON L, MASON J W, et al. Water-compatible cycloadditions of oligonucleotide-conjugated strained allenes for DNA-encoded library synthesis[J]. Journal of the American Chemical Society, 2020, 142(17): 7776-7782. |
| 98 | LIU S X, QI J J, LU W W, et al. Synthetic studies toward DNA-encoded heterocycles based on the on-DNA formation of α,β-unsaturated ketones[J]. Organic Letters, 2021, 23(3): 908-913. |
| 99 | FANG X F, WANG Y T, HE P Y, et al. Visible light-promoted divergent benzoheterocyclization from aldehydes for DNA-encoded chemical libraries[J]. Organic Letters, 2022, 24(17): 3291-3296. |
| 100 | ZHANG S L, ZHANG H M, LIU X W, et al. Mask and release strategy-enabled diversity-oriented synthesis for DNA-encoded library[J]. Advanced Science, 2024, 11(6): e2307049. |
| 101 | TRUPPO M D. Biocatalysis in the pharmaceutical industry: the need for speed[J]. ACS Medicinal Chemistry Letters, 2017, 8(5): 476-480. |
| [1] | 仲泉周, 单依怡, 裴清云, 金艳芸, 王艺涵, 孟璐远, 王歆韵, 张雨鑫, 刘坤媛, 王慧中, 冯尚国. 生物合成法生产α-熊果苷的研究进展[J]. 合成生物学, 2025, 6(1): 118-135. |
| [2] | 竺方欢, 岑雪聪, 陈振. 微生物合成二元醇研究进展[J]. 合成生物学, 2024, 5(6): 1367-1385. |
| [3] | 刘益宁, 蒲伟, 杨金星, 王钰. ω-氨基酸与内酰胺的生物合成研究进展[J]. 合成生物学, 2024, 5(6): 1350-1366. |
| [4] | 李庚, 申晓林, 孙新晓, 王佳, 袁其朋. 过氧化物酶的重组表达和应用研究进展[J]. 合成生物学, 2024, 5(6): 1498-1517. |
| [5] | 郑皓天, 李朝风, 刘良叙, 王嘉伟, 李恒润, 倪俊. 负碳人工光合群落的设计、优化与应用[J]. 合成生物学, 2024, 5(5): 1189-1210. |
| [6] | 程晓雷, 刘天罡, 陶慧. 萜类化合物的非常规生物合成研究进展[J]. 合成生物学, 2024, 5(5): 1050-1071. |
| [7] | 张守祺, 王涛, 孔尧, 邹家胜, 刘元宁, 徐正仁. 天然产物的化学-酶法合成:方法与策略的演进[J]. 合成生物学, 2024, 5(5): 913-940. |
| [8] | 谢向前, 郭雯, 王欢, 李进. 含氨基乙烯半胱氨酸核糖体肽的生物合成与化学合成[J]. 合成生物学, 2024, 5(5): 981-996. |
| [9] | 汤志军, 胡友财, 刘文. 酶促4+2和2+2环加成反应:区域与立体选择性的理解与应用[J]. 合成生物学, 2024, 5(3): 401-407. |
| [10] | 张俊, 金诗雪, 云倩, 瞿旭东. 聚酮化合物非天然延伸单元的生物合成与结构改造应用[J]. 合成生物学, 2024, 5(3): 561-570. |
| [11] | 陈锡玮, 张华然, 邹懿. 真菌源非核糖体肽类药物生物合成及代谢工程[J]. 合成生物学, 2024, 5(3): 571-592. |
| [12] | 冯金, 潘海学, 唐功利. 近十年天然产物药物的生物合成研究进展[J]. 合成生物学, 2024, 5(3): 408-446. |
| [13] | 奚萌宇, 胡逸灵, 顾玉诚, 戈惠明. 基因组挖掘指导天然药物分子的发现[J]. 合成生物学, 2024, 5(3): 447-473. |
| [14] | 施鑫杰, 杜艺岭. 双嵌入家族抗肿瘤非核糖体肽的生物合成研究进展[J]. 合成生物学, 2024, 5(3): 593-611. |
| [15] | 宋永相, 张秀凤, 李艳芹, 肖华, 闫岩. 自抗性基因导向的活性天然产物挖掘[J]. 合成生物学, 2024, 5(3): 474-491. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||