合成生物学 ›› 2024, Vol. 5 ›› Issue (5): 981-996.DOI: 10.12211/2096-8280.2024-037
含氨基乙烯半胱氨酸核糖体肽的生物合成与化学合成
谢向前1, 郭雯1, 王欢1, 李进1,2
- 1.南京大学化学与生物医药创新研究院,配位化学国家重点实验室,南京大学化学化工学院,江苏省先进有机材料重点实验室,江苏 南京 210093
2.南京药石科技股份有限公司,江苏 南京 210032
-
收稿日期:2024-04-17修回日期:2024-07-26出版日期:2024-10-31发布日期:2024-11-20 -
通讯作者:李进 -
作者简介:谢向前 (1997—),男,博士研究生。研究方向为天然产物的生物合成等。E-mail:dg21240123@smail.nju.edu.cn李进 (1987—),男,研究员,执行总监。研究方向为非天然氨基酸分子砌块的合成与应用。E-mail:li_jin@PharmaBlock.com -
基金资助:国家自然科学基金(22325702)
Biosynthesis and chemical synthesis of ribosomally synthesized and post-translationally modified peptides containing aminovinyl cysteine
XIE Xiangqian1, GUO Wen1, WANG Huan1, LI Jin1,2
- 1.State Key Laboratory of Coordination Chemistry,Chemistry and Biomedicine Innovation Center of Nanjing University,Jiangsu Key Laboratory of Advanced Organic Materials,School of Chemistry and Chemical Engineering,Nanjing University,Nanjing 210093,Jiangsu,China
2.PharmaBlock Sciences (Nanjing),INC. ,Nanjing 210032,Jiangsu,China
-
Received:2024-04-17Revised:2024-07-26Online:2024-10-31Published:2024-11-20 -
Contact:LI Jin
摘要:
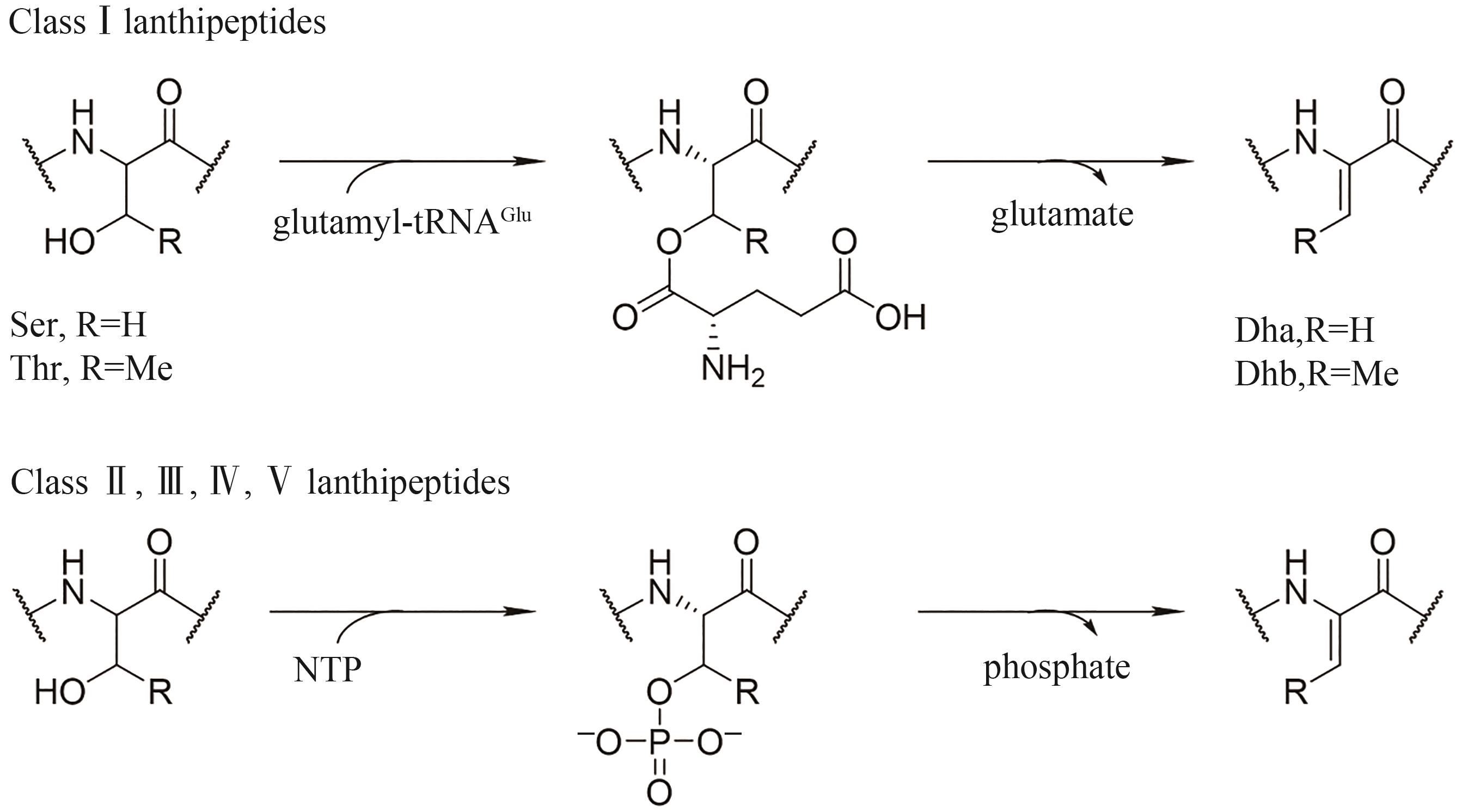
核糖体肽是一类拥有化学结构和生物活性多样性的多肽天然产物家族,在药物开发方面具有巨大的发展潜力。氨基乙烯半胱氨酸(AviCys)是部分核糖体肽类天然产物中存在的一种特殊C末端交联结构单元。含有AviCys单元的核糖体肽类天然环肽往往具有优良的抗菌或抗肿瘤活性,且AviCys大环结构对其生物活性至关重要。本文围绕此类天然环肽的生物合成和化学合成进行了总结:①羊毛硫肽、lipolanthines、linaridins和thioamitides四类核糖体肽天然产物中AviCys结构单元的生物合成研究进展,主要包括C末端半胱氨酸的氧化脱羧反应,丝氨酸/苏氨酸或半胱氨酸脱水或脱硫反应,以及AviCys环化反应;②针对含AviCys结构单元环肽的化学合成方法,包括自由基硫醇-炔偶联、氧化脱羧/脱羰、酰胺与缩醛缩合等。本综述同时对相关研究中存在的若干挑战和尚待解决的问题进行了梳理和总结,包括生物合成过程中尚未得到深入解析的环化步骤、化学合成中尚未解决的立体选择性和化学兼容性等。综上,对含AviCys结构单元天然环肽的生物合成途径全面解析及化学合成方法的开发, 有望为此类生物功能环肽及其衍生物的制备与生物工程改造奠定基础,推动该类功能环肽在生命科学和药物科学领域的应用。
中图分类号:
引用本文
谢向前, 郭雯, 王欢, 李进. 含氨基乙烯半胱氨酸核糖体肽的生物合成与化学合成[J]. 合成生物学, 2024, 5(5): 981-996.
XIE Xiangqian, GUO Wen, WANG Huan, LI Jin. Biosynthesis and chemical synthesis of ribosomally synthesized and post-translationally modified peptides containing aminovinyl cysteine[J]. Synthetic Biology Journal, 2024, 5(5): 981-996.
| 亚家族 | 天然产物 | 产生菌株 | 生物活性 |
|---|---|---|---|
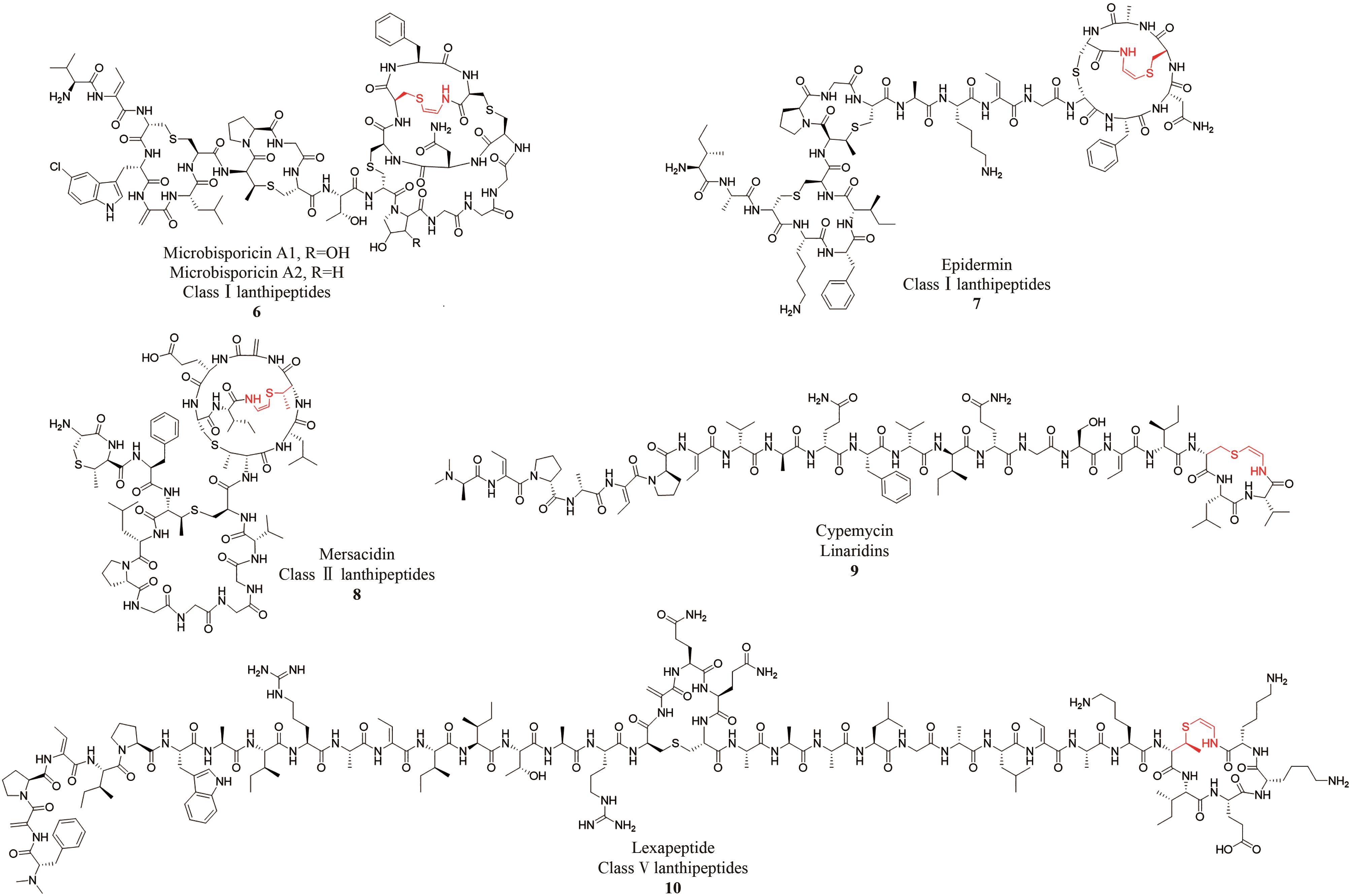
| 羊毛硫肽 | Microbisporicins[ | Microbispora ATCC PTA-5024 | 对MRSA、Streptococcus pneumoniae等有抗菌活性 |
| Epidermin[ | Staphylococcus epidermidis Tü 3298 | 对Mariniluteicoccus flavus、Staphylococcus simulans等有抗菌活性 | |
| Mersacidin[ | Bacillus amyloliquefaciens | 对Staphylococcus aureus、MRSA等有抗菌活性 | |
| Lexapeptide[ | Streptomyces rochei Sal35 | 对MRSA、MRSE等有抗菌活性 | |
| Lipolanthines | Microvionin[ | Microbacterium arborescens | 对MRSA、Streptococcus pneumoniae等有抗菌活性 |
| Nocavionin[ | Nocardia terpenica | 尚未报道 | |
| Goadvionins[ | Streptomyces sp. TP-A0584 | 对Staphylococcus aureus、Bacillus subtilis等有抗菌活性 | |
| Lipoavitides[ | Streptomyces sp. NRRL S-1521 | 溶血活性 | |
| Linaridins | Cypemycin[ | Streptomyces sp. OH-4156 | 对P388白血病细胞有细胞毒性,对Micrococcus luteus等有抗菌活性 |
| Salinipeptins[ | Streptomyces sp. strain GSL-6C | 对Streptococcus pyogenes M1T1等有抗菌作用 | |
| Thioamitides | Thioviridamide[ | Streptomyces olivoviridis NA005001 | 诱导细胞凋亡 |
| Thioholgamides[ | Streptomyces malayseiense | 抗增殖活性、细胞毒性 |
表1 含有Avi(Me)Cys结构单元天然产物的产生菌株及其生物活性
Table 1 Bacterial producers and bioactivity of Avi(Me)Cys-containing peptides
| 亚家族 | 天然产物 | 产生菌株 | 生物活性 |
|---|---|---|---|
| 羊毛硫肽 | Microbisporicins[ | Microbispora ATCC PTA-5024 | 对MRSA、Streptococcus pneumoniae等有抗菌活性 |
| Epidermin[ | Staphylococcus epidermidis Tü 3298 | 对Mariniluteicoccus flavus、Staphylococcus simulans等有抗菌活性 | |
| Mersacidin[ | Bacillus amyloliquefaciens | 对Staphylococcus aureus、MRSA等有抗菌活性 | |
| Lexapeptide[ | Streptomyces rochei Sal35 | 对MRSA、MRSE等有抗菌活性 | |
| Lipolanthines | Microvionin[ | Microbacterium arborescens | 对MRSA、Streptococcus pneumoniae等有抗菌活性 |
| Nocavionin[ | Nocardia terpenica | 尚未报道 | |
| Goadvionins[ | Streptomyces sp. TP-A0584 | 对Staphylococcus aureus、Bacillus subtilis等有抗菌活性 | |
| Lipoavitides[ | Streptomyces sp. NRRL S-1521 | 溶血活性 | |
| Linaridins | Cypemycin[ | Streptomyces sp. OH-4156 | 对P388白血病细胞有细胞毒性,对Micrococcus luteus等有抗菌活性 |
| Salinipeptins[ | Streptomyces sp. strain GSL-6C | 对Streptococcus pyogenes M1T1等有抗菌作用 | |
| Thioamitides | Thioviridamide[ | Streptomyces olivoviridis NA005001 | 诱导细胞凋亡 |
| Thioholgamides[ | Streptomyces malayseiense | 抗增殖活性、细胞毒性 |

图7 自由基硫醇-炔偶联的可能机制(a)和半胱氨酸衍生物与炔酰胺的自由基硫醇-炔偶联反应(b)AIBN—2,2′-偶氮二(2-甲基丙腈);Cbz—羧基苄基;PMB—对甲氧基苄基
Fig. 7 Postulated mechanism for radical thiol-yne reaction for the synthesis of an AviCys derivative by Castle et al. (a) and Attempted radical thiol-yne coupling of cysteine derivative with ynamides by Castle et al. (b)AIBN—2,2′-Azobis(2-methylpropionitrile); Cbz—Carboxybenzyl; PMB—para-Methoxybenzyl

图8 硫酯的脱羰基反应(a),氧化脱羧/脱羰反应构建mersacidin的C末端环(b)和氧化脱羧/脱羰反应的可能机制(c)Ni(COD)2—双(1,5-环辛二烯)镍(0);CuTC—硫代苯-2-甲酸铜(Ⅰ);Cbz—羧基苄基
Fig. 8 Decarbonylation of thioesters to give AviMeCys derivatives and building blocks (a). Oxidative decarboxylation/decarbonylation of the C-terminal ring of mersacidin by VanNieuwenhze et al (b). Postulated mechanism of oxidative decarboxylation/decarbonylation (c)Ni(COD)2—Bis(1,5-cyclooctadiene)nickel(0); CuTC—Copper(Ⅰ) Thiophene-2-carboxylate; Cbz—Carboxybenzyl

图9 通过乙酰胺与缩醛 22 缩合合成 AviCys 衍生物(a)和通过醛25与酰胺(Tcp)Val-NH2缩合及肽链延伸、内酰胺化合成cypemycin中的AviCys结构(b)Pht—邻苯二甲酰亚胺;Tcp—3,4,5,6-四氯邻苯二甲酰亚胺
Fig. 9 Synthesis of AviCys derivativesa via condensation of acetamide upon acetal 22 in the presence of a mild lewis acid (a) and Synthesis of the AviCys-containing ring of cypemycin via condensation of aldehyde 25 with amide (Tcp) Val-NH2, followed by elongation of the peptide chain and lactamization to give 29 in 4.6% yield from 25 (b)Pht—Phthalimide; Tcp—3,4,5,6-Tetrachlorophthalimide
| 1 | ARNISON P G, BIBB M J, BIERBAUM G, et al. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature[J]. Natural Product Reports, 2013, 30(1): 108-160. |
| 2 | MONTALBÁN-LÓPEZ M, SCOTT T A, RAMESH S, et al. New developments in RiPP discovery, enzymology and engineering[J]. Natural Product Reports, 2021, 38(1): 130-239. |
| 3 | SCHEIDLER C M, KICK L M, SCHNEIDER S. Ribosomal peptides and small proteins on the rise[J]. Chembiochem, 2019, 20(12): 1479-1486. |
| 4 | CHENG B T, XUE Y Q, DUAN Y T, et al. Enzymatic formation of an aminovinyl cysteine residue in ribosomal peptide natural products[J]. ChemPlusChem, 2024, 89(6): e202400047. |
| 5 | GRANT-MACKIE E S, WILLIAMS E T, HARRIS P W R, et al. Aminovinyl cysteine containing peptides: a unique motif that imparts key biological activity[J]. JACS Au, 2021, 1(10): 1527-1540. |
| 6 | DISCHINGER J, BASI CHIPALU S, BIERBAUM G. Lantibiotics: promising candidates for future applications in health care[J]. International Journal of Medical Microbiology, 2014, 304(1): 51-62. |
| 7 | HAYAKAWA Y, SASAKI K, ADACHI H, et al. Thioviridamide, a novel apoptosis inducer in transformed cells from Streptomyces olivoviridis [J]. Journal of Antibiotics, 2006, 59(1): 1-5. |
| 8 | CASTIGLIONE F, LAZZARINI A, CARRANO L, et al. Determining the structure and mode of action of microbisporicin, a potent lantibiotic active against multiresistant pathogens[J]. Chemistry & Biology, 2008, 15(1): 22-31. |
| 9 | ALLGAIER H, JUNG G, WERNER R G, et al. Epidermin: sequencing of a heterodetic tetracyclic 21-peptide amide antibiotic[J]. European Journal of Biochemistry, 1986, 160(1): 9-22. |
| 10 | CHATTERJEE S, CHATTERJEE S, LAD S J, et al. Mersacidin, a new antibiotic from Bacillus. Fermentation, isolation, purification and chemical characterization[J]. Journal of Antibiotics, 1992, 45(6): 832-838. |
| 11 | XU M, ZHANG F, CHENG Z, et al. Functional genome mining reveals a classⅤlanthipeptide containing a D-amino acid introduced by an F420H2-dependent reductase[J]. Angewandte Chemie International Edition, 2020, 59(41): 18029-18035. |
| 12 | WIEBACH V, MAINZ A, SIEGERT M A J, et al. The anti-staphylococcal lipolanthines are ribosomally synthesized lipopeptides[J]. Nature Chemical Biology, 2018, 14(7): 652-654. |
| 13 | KOZAKAI R, ONO T, HOSHINO S, et al. Acyltransferase that catalyses the condensation of polyketide and peptide moieties of goadvionin hybrid lipopeptides[J]. Nature Chemistry, 2020, 12(9): 869-877. |
| 14 | REN H Q, HUANG C S, PAN Y W, et al. Non-modular fatty acid synthases yield distinct N-terminal acylation in ribosomal peptides[J/OL]. Nature Chemistry, 2024. (2024-03-25)[2024-04-01]. . |
| 15 | KOMIYAMA K, OTOGURO K, SEGAWA T, et al. A new antibiotic, cypemycin. Taxonomy, fermentation, isolation and biological characteristics[J]. Journal of Antibiotics, 1993, 46(11): 1666-1671. |
| 16 | SHANG Z, WINTER J M, KAUFFMAN C A, et al. Salinipeptins: integrated genomic and chemical approaches reveal unusual D-amino acid-containing ribosomally synthesized and post-translationally modified peptides (RiPPs) from a great salt lake Streptomyces sp[J]. ACS Chemical Biology, 2019, 14(3): 415-425. |
| 17 | DAHLEM C, SIOW W X, LOPATNIUK M, et al. Thioholgamide A, a new anti-proliferative anti-tumor agent, modulates macrophage polarization and metabolism[J]. Cancers, 2020, 12(5): 1288. |
| 18 | ONGEY E L, NEUBAUER P. Lanthipeptides: chemical synthesis versus in vivo biosynthesis as tools for pharmaceutical production[J]. Microbial Cell Factories, 2016, 15: 97. |
| 19 | ONGEY E L, YASSI H, PFLUGMACHER S, et al. Pharmacological and pharmacokinetic properties of lanthipeptides undergoing clinical studies[J]. Biotechnology Letters, 2017, 39(4): 473-482. |
| 20 | BANERJEE B, LITVINOV D N, KANG J, et al. Stereoselective additions of thiyl radicals to terminal ynamides[J]. Organic Letters, 2010, 12(11): 2650-2652. |
| 21 | GARCÍA-REYNAGA P, CARRILLO A K, VANNIEUWENHZE M S. Decarbonylative approach to the synthesis of enamides from amino acids: stereoselective synthesis of the (Z)- aminovinyl-D-cysteine unit of mersacidin[J]. Organic Letters, 2012, 14(4): 1030-1033. |
| 22 | CARRILLO A K, VANNIEUWENHZE M S. Synthesis of the AviMeCys-containing D-ring of mersacidin[J]. Organic Letters, 2012, 14(4): 1034-1037. |
| 23 | LUTZ J A, SUBASINGHEGE DON V, KUMAR R, et al. Influence of sulfur on acid-mediated enamide formation[J]. Organic Letters, 2017, 19(19): 5146-5149. |
| 24 | LUTZ J A, TAYLOR C M. Synthesis of the aminovinylcysteine-containing C-terminal macrocycle of the linaridins[J]. Organic Letters, 2020, 22(5): 1874-1877. |
| 25 | KUMASHIRO M, OHSAWA K, DOI T. Photocatalyzed oxidative decarboxylation forming aminovinylcysteine containing peptides[J]. Catalysts, 2022, 12(12): 1615. |
| 26 | ROGERS L A, WHITTIER E O. Limiting factors in the lactic fermentation[J]. Journal of Bacteriology, 1928, 16(4): 211-229. |
| 27 | REPKA L M, CHEKAN J R, NAIR S K, et al. Mechanistic understanding of lanthipeptide biosynthetic enzymes[J]. Chemical Reviews, 2017, 117(8): 5457-5520. |
| 28 | GENG M X, SMITH L. Improving the attrition rate of Lanthipeptide discovery for commercial applications[J]. Expert Opinion on Drug Discovery, 2018, 13(2): 155-167. |
| 29 | BAKHTIARY A, COCHRANE S A, MERCIER P, et al. Insights into the mechanism of action of the two-peptide lantibiotic lacticin 3147[J]. Journal of the American Chemical Society, 2017, 139(49): 17803-17810. |
| 30 | BREUKINK E, DE KRUIJFF B. Lipid Ⅱ as a target for antibiotics[J]. Nature Reviews Drug Discovery, 2006, 5(4): 321-323. |
| 31 | BRÖTZ H, JOSTEN M, WIEDEMANN I, et al. Role of lipid-bound peptidoglycan precursors in the formation of pores by nisin, epidermin and other lantibiotics[J]. Molecular Microbiology, 1998, 30(2): 317-327. |
| 32 | HSU S T D, BREUKINK E, TISCHENKO E, et al. The nisin-lipid Ⅱ complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics[J]. Nature Structural & Molecular Biology, 2004, 11(10): 963-967. |
| 33 | DICKMAN R, MITCHELL S A, FIGUEIREDO A M, et al. Molecular recognition of lipid Ⅱ by lantibiotics: synthesis and conformational studies of analogues of nisin and mutacin rings A and B[J]. Journal of Organic Chemistry, 2019, 84(18): 11493-11512. |
| 34 | POKHREL R, BHATTARAI N, BARAL P, et al. Molecular mechanisms of pore formation and membrane disruption by the antimicrobial lantibiotic peptide Mutacin 1140[J]. Physical Chemistry Chemical Physics, 2019, 21(23): 12530-12539. |
| 35 | HSU S T D, BREUKINK E, BIERBAUM G, et al. NMR study of mersacidin and lipid Ⅱ interaction in dodecylphosphocholine micelles. Conformational changes are a key to antimicrobial activity[J]. Journal of Biological Chemistry, 2003, 278(15): 13110-13117. |
| 36 | KRUSZEWSKA D, SAHL H G, BIERBAUM G, et al. Mersacidin eradicates methicillin-resistant Staphylococcus aureus (MRSA) in a mouse rhinitis model[J]. Journal of Antimicrobial Chemotherapy, 2004, 54(3): 648-653. |
| 37 | BLAESSE M, KUPKE T, HUBER R, et al. Crystal structure of the peptidyl-cysteine decarboxylase EpiD complexed with a pentapeptide substrate[J]. EMBO Journal, 2000, 19(23): 6299-6310. |
| 38 | BLAESSE M, KUPKE T, HUBER R, et al. Structure of MrsD, an FAD-binding protein of the HFCD family[J]. Acta Crystallographica Section D, Biological Crystallography, 2003, 59(Pt 8): 1414-1421. |
| 39 | MO T L, YUAN H, WANG F T, et al. Convergent evolution of the Cys decarboxylases involved in aminovinyl-cysteine (AviCys) biosynthesis[J]. FEBS Letters, 2019, 593(6): 573-580. |
| 40 | SIT C S, YOGANATHAN S, VEDERAS J C. Biosynthesis of aminovinyl-cysteine-containing peptides and its application in the production of potential drug candidates[J]. Accounts of Chemical Research, 2011, 44(4): 261-268. |
| 41 | LU J X, LI J, WU Y, et al. Characterization of the FMN-dependent cysteine decarboxylase from thioviridamide biosynthesis[J]. Organic Letters, 2019, 21(12): 4676-4679. |
| 42 | KUPKE T, KEMPTER C, JUNG G, et al. Oxidative decarboxylation of peptides catalyzed by flavoprotein EpiD. Determination of substrate specificity using peptide libraries and neutral loss mass spectrometry[J]. Journal of Biological Chemistry, 1995, 270(19): 11282-11289. |
| 43 | XIA Y Z, YI Y C, SHI Y, et al. Enzymatic generation of thioaldehyde motifs by flavin-dependent cysteine decarboxylases for peptide bioconjugation and macrocyclization[J]. Organic Letters, 2023, 25(32): 6035-6039. |
| 44 | WANG S, WU K W, TANG Y J, et al. Dehydroamino acid residues in bioactive natural products[J]. Natural Product Reports, 2024, 41(2): 273-297. |
| 45 | BOTHWELL I R, COGAN D P, KIM T, et al. Characterization of glutamyl-tRNA-dependent dehydratases using nonreactive substrate mimics[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(35): 17245-17250. |
| 46 | CHATTERJEE C, MILLER L M, LEUNG Y L, et al. Lacticin 481 synthetase phosphorylates its substrate during lantibiotic production[J]. Journal of the American Chemical Society, 2005, 127(44): 15332-15333. |
| 47 | DONG S H, TANG W X, LUKK T, et al. The enterococcal cytolysin synthetase has an unanticipated lipid kinase fold[J]. eLife, 2015, 4: e07607. |
| 48 | HUANG S Q, WANG Y, CAI C X, et al. Discovery of a unique structural motif in lanthipeptide synthetases for substrate binding and interdomain interactions[J]. Angewandte Chemie International Edition, 2022, 61(45): e202211382. |
| 49 | HERNANDEZ GARCIA A, NAIR S K. Structure and function of a class Ⅲ metal-independent lanthipeptide synthetase[J]. ACS Central Science, 2023, 9(10): 1944-1956. |
| 50 | SIGURDSSON A, MARTINS B M, DÜTTMANN S A, et al. Discovery of the lanthipeptide curvocidin and structural insights into its trifunctional synthetase CuvL[J]. Angewandte Chemie International Edition, 2023, 62(23): e202302490. |
| 51 | LIANG H Q, LOPEZ I J, SÁNCHEZ-HIDALGO M, et al. Mechanistic studies on dehydration in class Ⅴ lanthipeptides[J]. ACS Chemical Biology, 2022, 17(9): 2519-2527. |
| 52 | XUE Y Q, LI M, HU L, et al. Mechanistic investigations into the catalytic mode of a dehydratase complex involved in the biosynthesis of lantibiotic cacaoidin[J]. Chinese Journal of Chemistry, 2023, 41(24): 3579-3586. |
| 53 | LI B, YU J P, BRUNZELLE J S, et al. Structure and mechanism of the lantibiotic cyclase involved in nisin biosynthesis[J]. Science, 2006, 311(5766): 1464-1467. |
| 54 | MUKHERJEE S, VAN DER DONK W A. Mechanistic studies on the substrate-tolerant lanthipeptide synthetase ProcM[J]. Journal of the American Chemical Society, 2014, 136(29): 10450-10459. |
| 55 | THIBODEAUX C J, HA T, VAN DER DONK W A. A price to pay for relaxed substrate specificity: a comparative kinetic analysis of the class Ⅱ lanthipeptide synthetases ProcM and HalM2[J]. Journal of the American Chemical Society, 2014, 136(50): 17513-17529. |
| 56 | YANG X, VAN DER DONK W A. Michael-type cyclizations in lantibiotic biosynthesis are reversible[J]. ACS Chemical Biology, 2015, 10(5): 1234-1238. |
| 57 | YU Y, MUKHERJEE S, VAN DER DONK W A. Product formation by the promiscuous lanthipeptide synthetase ProcM is under kinetic control[J]. Journal of the American Chemical Society, 2015, 137(15): 5140-5148. |
| 58 | LU J X, LI Y Q, BAI Z B, et al. Enzymatic macrocyclization of ribosomally synthesized and posttranslational modified peptides via C—S and C—C bond formation[J]. Natural Product Reports, 2021, 38(5): 981-992. |
| 59 | WIEBACH V, MAINZ A, SCHNEGOTZKI R, et al. An amphipathic alpha-helix guides maturation of the ribosomally-synthesized lipolanthines[J]. Angewandte Chemie International Edition, 2020, 59(38): 16777-16785. |
| 60 | CHU L X, CHENG J D, ZHOU C Z, et al. Hijacking a linaridin biosynthetic intermediate for lanthipeptide production[J]. ACS Chemical Biology, 2022, 17(11): 3198-3206. |
| 61 | XUE Y Q, WANG X F, LIU W. Reconstitution of the linaridin pathway provides access to the family-determining activity of two membrane-associated proteins in the formation of structurally underestimated cypemycin[J]. Journal of the American Chemical Society, 2023, 145(12): 7040-7047. |
| 62 | CLAESEN J, BIBB M. Genome mining and genetic analysis of cypemycin biosynthesis reveal an unusual class of posttranslationally modified peptides[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(37): 16297-16302. |
| 63 | CLAESEN J, BIBB M J. Biosynthesis and regulation of grisemycin, a new member of the linaridin family of ribosomally synthesized peptides produced by Streptomyces griseus IFO 13350[J]. Journal of Bacteriology, 2011, 193(10): 2510-2516. |
| 64 | WANG F T, WEI W Q, ZHAO J F, et al. Genome mining and biosynthesis study of a type B linaridin reveals a highly versatile α-N-methyltransferase[J]. CCS Chemistry, 2020, 3(3): 1049-1057. |
| 65 | GEORGIOU M A, DOMMARAJU S R, GUO X R, et al. Bioinformatic and reactivity-based discovery of linaridins[J]. ACS Chemical Biology, 2020, 15(11): 2976-2985. |
| 66 | LU J X, WU Y, LI Y Q, et al. The utilization of lanthipeptide synthetases is a general strategy for the biosynthesis of 2-aminovinyl-cysteine motifs in thioamitides[J]. Angewandte Chemie International Edition, 2021, 60(4): 1951-1958. |
| 67 | DENOËL T, LEMAIRE C, LUXEN A. Progress in lanthionine and protected lanthionine synthesis[J]. Chemistry, 2018, 24(58): 15421-15441. |
| 68 | JIMÉNEZ J C, BAYÓ N, CHAVARRÍA B, et al. Synthesis of peptides containing α,β-didehydroamino acids. Scope and limitations[J]. Letters in Peptide Science, 2002, 9(2): 135-141. |
| [1] | 仲泉周, 单依怡, 裴清云, 金艳芸, 王艺涵, 孟璐远, 王歆韵, 张雨鑫, 刘坤媛, 王慧中, 冯尚国. 生物合成法生产α-熊果苷的研究进展[J]. 合成生物学, 2025, 6(1): 118-135. |
| [2] | 竺方欢, 岑雪聪, 陈振. 微生物合成二元醇研究进展[J]. 合成生物学, 2024, 5(6): 1367-1385. |
| [3] | 刘益宁, 蒲伟, 杨金星, 王钰. ω-氨基酸与内酰胺的生物合成研究进展[J]. 合成生物学, 2024, 5(6): 1350-1366. |
| [4] | 李庚, 申晓林, 孙新晓, 王佳, 袁其朋. 过氧化物酶的重组表达和应用研究进展[J]. 合成生物学, 2024, 5(6): 1498-1517. |
| [5] | 郑皓天, 李朝风, 刘良叙, 王嘉伟, 李恒润, 倪俊. 负碳人工光合群落的设计、优化与应用[J]. 合成生物学, 2024, 5(5): 1189-1210. |
| [6] | 程晓雷, 刘天罡, 陶慧. 萜类化合物的非常规生物合成研究进展[J]. 合成生物学, 2024, 5(5): 1050-1071. |
| [7] | 杨皓然, 叶发荣, 黄平, 王平. 糖蛋白合成的研究进展[J]. 合成生物学, 2024, 5(5): 1072-1101. |
| [8] | 程中玉, 李付琸. 基于P450选择性氧化的天然产物化学-酶法合成进展[J]. 合成生物学, 2024, 5(5): 960-980. |
| [9] | 刘子健, 穆柏杨, 段志强, 王璇, 陆晓杰. 与核酸兼容的化学反应开发进展[J]. 合成生物学, 2024, 5(5): 1102-1124. |
| [10] | 张守祺, 王涛, 孔尧, 邹家胜, 刘元宁, 徐正仁. 天然产物的化学-酶法合成:方法与策略的演进[J]. 合成生物学, 2024, 5(5): 913-940. |
| [11] | 汤志军, 胡友财, 刘文. 酶促4+2和2+2环加成反应:区域与立体选择性的理解与应用[J]. 合成生物学, 2024, 5(3): 401-407. |
| [12] | 张俊, 金诗雪, 云倩, 瞿旭东. 聚酮化合物非天然延伸单元的生物合成与结构改造应用[J]. 合成生物学, 2024, 5(3): 561-570. |
| [13] | 陈锡玮, 张华然, 邹懿. 真菌源非核糖体肽类药物生物合成及代谢工程[J]. 合成生物学, 2024, 5(3): 571-592. |
| [14] | 虞旭昶, 吴辉, 李雷. 文库构建与基因簇靶向筛选驱动的微生物天然产物高效发现[J]. 合成生物学, 2024, 5(3): 492-506. |
| [15] | 冯金, 潘海学, 唐功利. 近十年天然产物药物的生物合成研究进展[J]. 合成生物学, 2024, 5(3): 408-446. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||