合成生物学 ›› 2024, Vol. 5 ›› Issue (5): 913-940.DOI: 10.12211/2096-8280.2024-028
天然产物的化学-酶法合成:方法与策略的演进
张守祺, 王涛, 孔尧, 邹家胜, 刘元宁, 徐正仁
- 北京大学药学院,天然药物及仿生药物全国重点实验室,北京 100191
-
收稿日期:2024-03-25修回日期:2024-05-28出版日期:2024-10-31发布日期:2024-11-20 -
通讯作者:徐正仁 -
作者简介:张守祺 (1998—),男,博士研究生。研究方向为活性二萜的化学-酶法合成。 E-mail:shouqizhang_pku@pku.edu.cn王涛 (1991—),男,博士研究生。研究方向为活性二萜的化学-酶法合成。 E-mail:1005704167@qq.com徐正仁 (1983—),男,研究员,博士生导师。研究方向为活性天然产物的化学-酶法合成、新颖生物催化反应的发现与应用、天然药物化学等。 E-mail:zhengrenxu@bjmu.edu.cn -
基金资助:国家自然科学基金(81991525)
Chemoenzymatic synthesis of natural products: evolution of synthetic methodology and strategy
ZHANG Shouqi, WANG Tao, KONG Yao, ZOU Jiasheng, LIU Yuanning, XU Zhengren
- State Key Laboratory of Natural and Biomimetic Drugs,School of Pharmaceutical Sciences,Peking University,Beijing 100191,China
-
Received:2024-03-25Revised:2024-05-28Online:2024-10-31Published:2024-11-20 -
Contact:XU Zhengren
摘要:
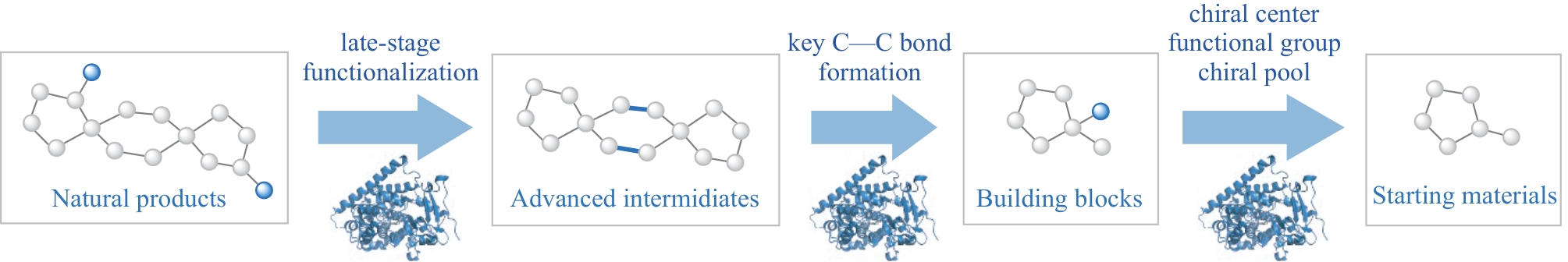
天然产物是小分子药物和探针的重要来源,其合成研究一直以来是有机合成中一个备受关注而又极具挑战性的领域。随着色谱分离技术和结构分析技术的不断发展,微量活性天然产物的发现速度不断加快,其结构的多样性和复杂性也不断增加,而对其构效关系、靶标鉴定、体内活性等方面的研究则需要供应足够量的天然产物,因而对天然产物的合成在效率、经济性和规模等方面都提出了更高的要求。化学-酶法的方式为天然产物的合成研究提供了多维的视角,一方面提供了高效高选择性的酶催化合成方法,另一方面,酶催化反应的引入可以给原先合成策略的设计模式带来突破,并快速、高效地实现天然产物的多样化合成,从而成为近期研究的热点。其中酶催化反应如何有机地整合到天然产物的合成中便成为目前化学-酶法合成成功的关键,本文从当前天然产物化学-酶法的合成实践中总结了酶催化反应所发挥的三方面作用:①对合成起点的改变,即酶催化反应可以在合成原料中引入关键的手性中心或官能团,以体外酶促或体内发酵的方式提供复杂的合成前体,如多取代芳(杂)环、手性池等;②合成后期通过酶催化方式对多官能团底物或复杂骨架的惰性位置进行化学、区域和立体选择性的官能团化;③酶催化反应作为关键步骤在母核骨架构建中关键碳碳键形成方面的策略性应用。最后,本文从合成策略的设计、合成方法的开发以及研究人员思维等三个方面讨论了化学-酶法策略在当下所面临的挑战和未来的发展趋势。在此背景下,化学合成与生物催化等多学科手段的深度交叉融合将为天然产物的合成科学带来新的活力。
中图分类号:
引用本文
张守祺, 王涛, 孔尧, 邹家胜, 刘元宁, 徐正仁. 天然产物的化学-酶法合成:方法与策略的演进[J]. 合成生物学, 2024, 5(5): 913-940.
ZHANG Shouqi, WANG Tao, KONG Yao, ZOU Jiasheng, LIU Yuanning, XU Zhengren. Chemoenzymatic synthesis of natural products: evolution of synthetic methodology and strategy[J]. Synthetic Biology Journal, 2024, 5(5): 913-940.
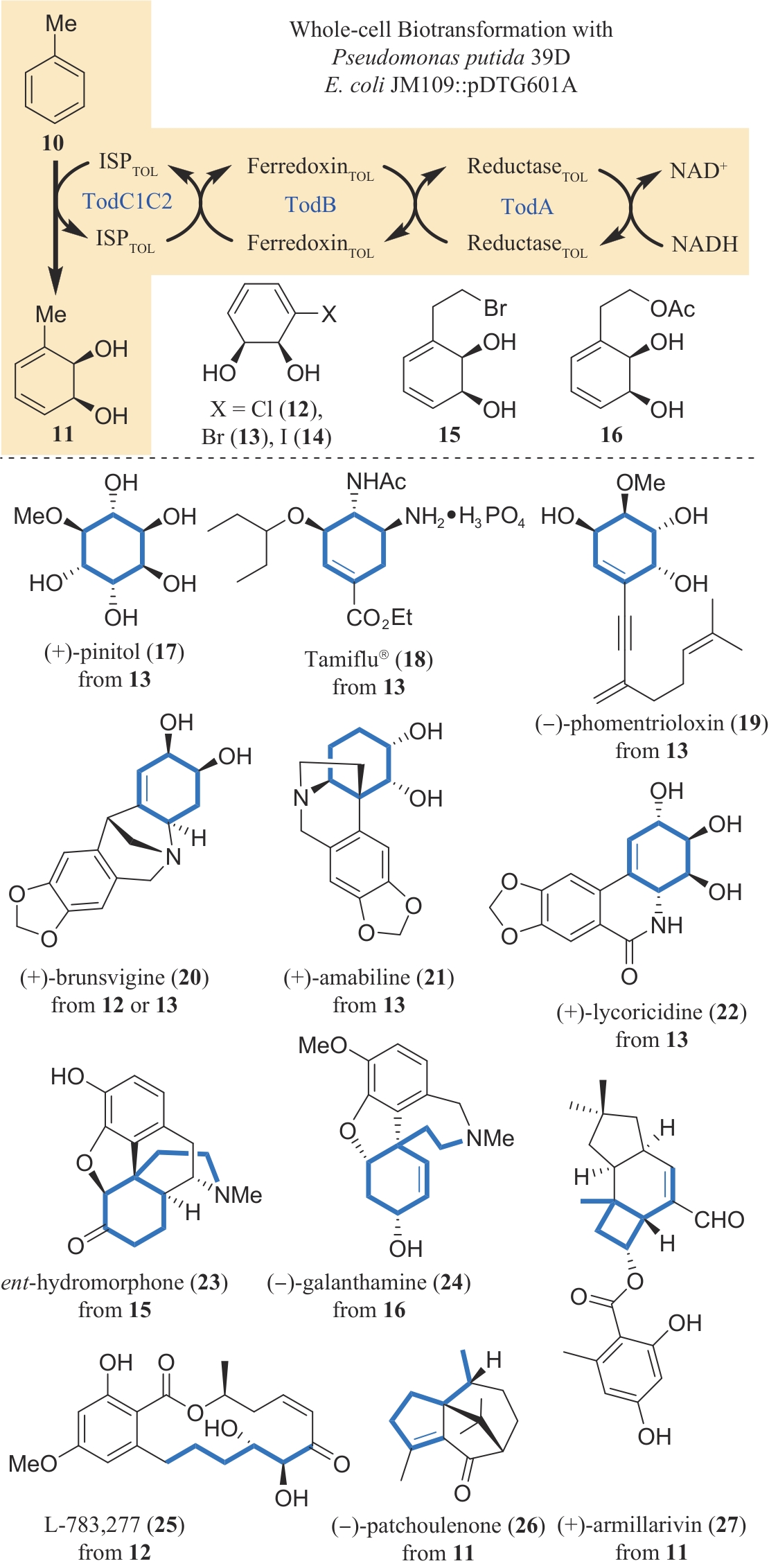
图3 甲苯双加氧酶催化的底物关键手性中心引入及其在多种天然产物合成中的应用
Fig. 3 Introduction of the key chiral center(s) to a simple substrate by the action of toluene dioxygenase and its application in the synthesis of various natural products

图5 α-酮戊二酸依赖的非血红素铁双加氧酶催化的底物关键官能团引入
Fig. 5 Introduction of the key functional group(s) to the substrate via α-ketoglutarate-dependent non-heme iron dioxygenase
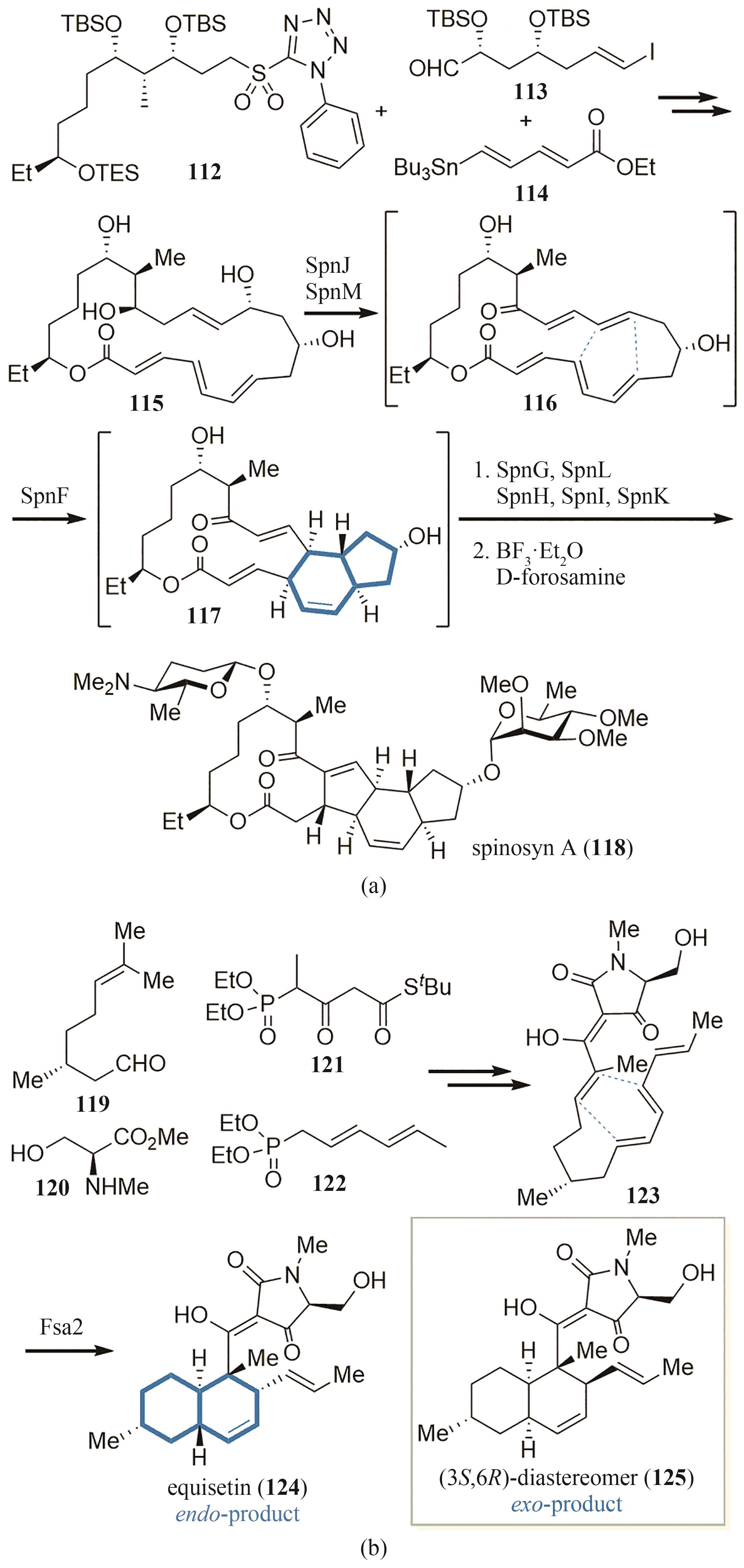
图11 环化酶在天然产物spinosyn A(a)和equisetin(b)骨架形成中的应用
Fig. 11 Application of cyclases to the skeleton-forming step in the synthesis of spinosyn A (a) and equisetin (b)
| 1 | NEWMAN D J, CRAGG G M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019[J]. Journal of Natural Products, 2020, 83(3): 770-803. |
| 2 | CRAGG G M, NEWMAN D J. Natural products: a continuing source of novel drug leads[J]. Biochimica et Biophysica Acta (BBA) - General Subjects, 2013, 1830(6): 3670-3695. |
| 3 | CARLSON E E. Natural products as chemical probes[J]. ACS Chemical Biology, 2010, 5(7): 639-653. |
| 4 | PYE C R, BERTIN M J, LOKEY R S, et al. Retrospective analysis of natural products provides insights for future discovery trends[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(22): 5601-5606. |
| 5 | BUTLER M S. The role of natural product chemistry in drug discovery[J]. Journal of Natural Products, 2004, 67(12): 2141-2153. |
| 6 | KOEHN F E, CARTER G T. The evolving role of natural products in drug discovery[J]. Nature Reviews Drug Discovery, 2005, 4(3): 206-220. |
| 7 | ATANASOV A G, ZOTCHEV S B, DIRSCH V M, et al. Natural products in drug discovery: advances and opportunities[J]. Nature Reviews Drug Discovery, 2021, 20(3): 200-216. |
| 8 | WILSON B A P, THORNBURG C C, HENRICH C J, et al. Creating and screening natural product libraries[J]. Natural Product Reports, 2020, 37(7): 893-918. |
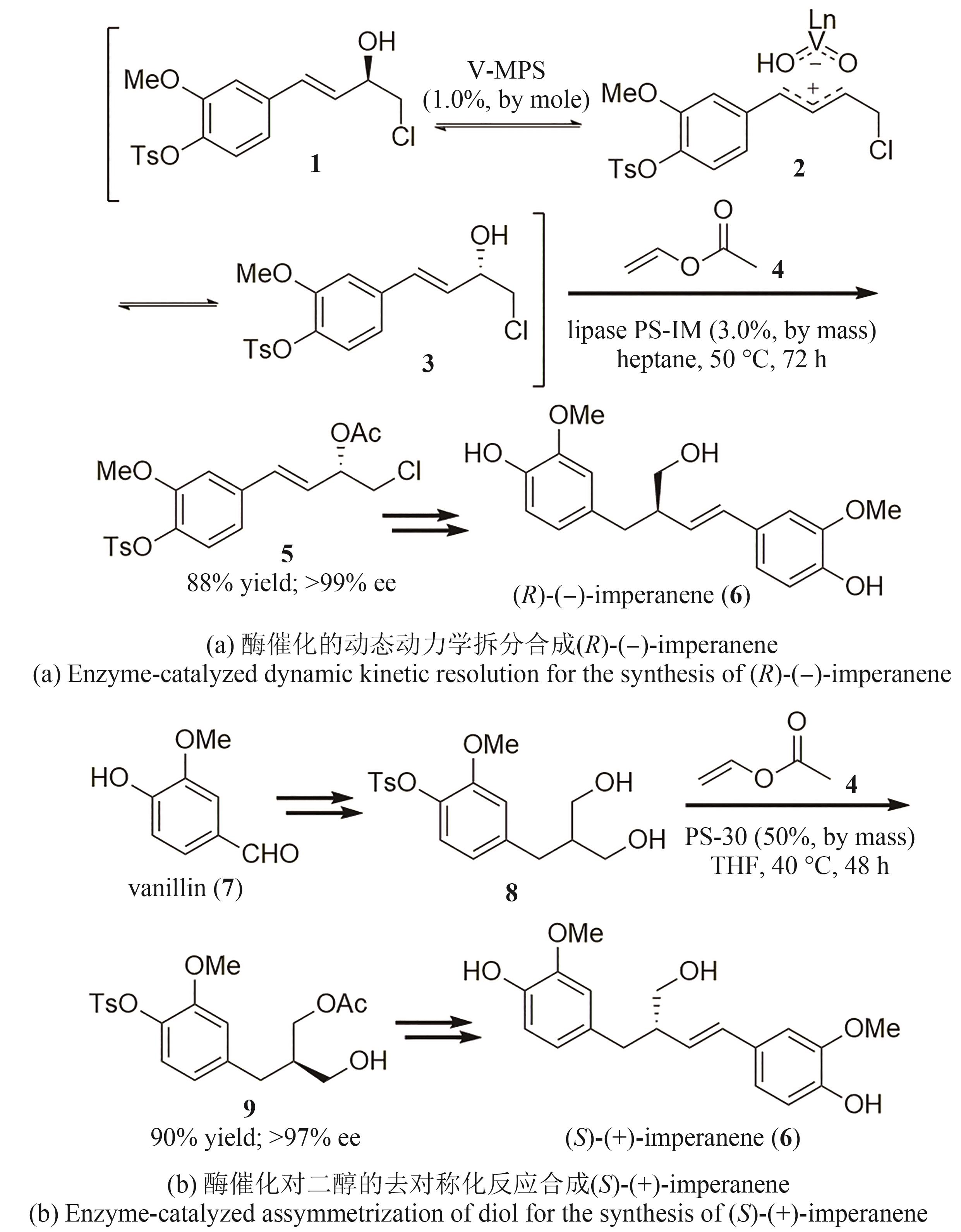
| 9 | NEWMAN D J. Natural products as leads to potential drugs: an old process or the new hope for drug discovery?[J]. Journal of Medicinal Chemistry, 2008, 51(9): 2589-2599. |
| 10 | LI L, CHEN Z, ZHANG X W, et al. Divergent strategy in natural product total synthesis[J]. Chemical Reviews, 2018, 118(7): 3752-3832. |
| 11 | MULZER J. Trying to rationalize total synthesis[J]. Natural Product Reports, 2014, 31(4): 595-603. |
| 12 | WENDER P A. Toward the ideal synthesis and molecular function through synthesis-informed design[J]. Natural Product Reports, 2014, 31(4): 433-440. |
| 13 | DRAUZ K . GRÖGER H . MAY O. Enzyme catalysis in organic synthesis[M/OL]. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA, 2012. (2012-02-22)[2024-03-01]. . |
| 14 | RODRÍGUEZ BENÍTEZ A, NARAYAN A R H. Frontiers in biocatalysis: profiling function across sequence space[J]. ACS Central Science, 2019, 5(11): 1747-1749. |
| 119 | NAKAMURA H, SCHULTZ E E, BALSKUS E P. A new strategy for aromatic ring alkylation in cylindrocyclophane biosynthesis[J]. Nature Chemical Biology, 2017, 13(8): 916-921. |
| 120 | WANG H Q, MOU S B, XIAO W, et al. Structural basis for the Friedel-Crafts alkylation in cyclindrocyclophane biosynthesis [J]. ACS Catalysis, 2022, 12(3): 2108-2117. |
| 121 | CHEN K Y, WANG H Q, YUAN Y, et al. Chemoenzymatic synthesis of cylindrocyclophanes A and F and merocyclophanes A and D[J]. Angewandte Chemie International Edition, 2023, 62(46): e202307602. |
| 122 | GAO J M, LIU S N, ZHOU C, et al. A pyridoxal 5'-phosphate-dependent Mannich cyclase[J]. Nature Catalysis, 2023, 6: 476-486. |
| 123 | LIU S N, HAI Y. Chemoenzymatic approaches to izidine alkaloids: an efficient total synthesis of (+)-absouline and laburnamine[J]. ACS Catalysis, 2023, 13(24): 15725-15729. |
| 124 | WU X M, GUAN Q Y, HAN Y B, et al. Regeneration of phytochemicals by structure-driven organization of microbial biosynthetic steps[J]. Angewandte Chemie International Edition, 2022, 61(8): e202114919. |
| 125 | KIEFER A F, LIU Y C, GUMMERER R, et al. An artificial in vitro metabolism to angiopterlactone B inspired by traditional retrosynthesis[J]. Angewandte Chemie International Edition, 2023, 62(23): e202301178. |
| 126 | YI D, BAYER T, BADENHORST C P S, et al. Recent trends in biocatalysis[J]. Chemical Society Reviews, 2021, 50(14): 8003-8049. |
| 127 | YANG Y, ARNOLD F H. Navigating the unnatural reaction space: directed evolution of heme proteins for selective carbene and nitrene transfer[J]. Accounts of Chemical Research, 2021, 54(5): 1209-1225. |
| 128 | PAN Y J, LI G B, LIU R X, et al. Unnatural activities and mechanistic insights of cytochrome P450 PikC gained from site-specific mutagenesis by non-canonical amino acids[J]. Nature Communications, 2023, 14(1): 1669. |
| 129 | ROMERO E O, SAUCEDO A T, HERNÁNDEZ-MELÉNDEZ J R, et al. Enabling broader adoption of biocatalysis in organic chemistry[J]. Journal of the American Chemical Society Au, 2023, 3(8): 2073-2085. |
| 15 | LEWIS R D, FRANCE S P, MARTINEZ C A. Emerging technologies for biocatalysis in the pharmaceutical industry[J]. ACS Catalysis, 2023, 13(8): 5571-5577. |
| 16 | TRUPPO M D. Biocatalysis in the pharmaceutical industry: the need for speed[J]. ACS Medicinal Chemistry Letters, 2017, 8(5): 476-480. |
| 17 | REED J H, SEEBECK F P. Reagent engineering for group transfer biocatalysis[J]. Angewandte Chemie International Edition, 2024, 63(7): e202311159. |
| 18 | WU S K, SNAJDROVA R, MOORE J C, et al. Biocatalysis: enzymatic synthesis for industrial applications[J]. Angewandte Chemie International Edition, 2021, 60(1): 88-119. |
| 19 | TURNER N J, O’REILLY E. Biocatalytic retrosynthesis[J]. Nature Chemical Biology, 2013, 9(5): 285-288. |
| 20 | KIRSCHNING A, HAHN F. Merging chemical synthesis and biosynthesis: a new chapter in the total synthesis of natural products and natural product libraries[J]. Angewandte Chemie International Edition, 2012, 51(17): 4012-4022. |
| 21 | FRIEDRICH S, HAHN F. Opportunities for enzyme catalysis in natural product chemistry[J]. Tetrahedron, 2015, 71(10): 1473-1508. |
| 22 | MURRAY L A M, MCKINNIE S M K, MOORE B S, et al. Meroterpenoid natural products from Streptomyces bacteria- the evolution of chemoenzymatic syntheses[J]. Natural Product Reports, 2020, 37(10): 1334-1366. |
| 23 | CHAKRABARTY S, ROMERO E O, PYSER J B, et al. Chemoenzymatic total synthesis of natural products[J]. Accounts of Chemical Research, 2021, 54(6): 1374-1384. |
| 24 | ZHANG H L, TANG X Y. Combining microbial and chemical syntheses for the production of complex natural products[J]. Chinese Journal of Natural Medicines, 2022, 20(10): 729-736. |
| 25 | RODDAN R, CARTER E M, THAIR B, et al. Chemoenzymatic approaches to plant natural product inspired compounds[J]. Natural Product Reports, 2022, 39(7): 1375-1382. |
| 26 | STOUT C N, WASFY N M, CHEN F, et al. Charting the evolution of chemoenzymatic strategies in the syntheses of complex natural products[J]. Journal of the American Chemical Society, 2023, 145(33): 18161-18181. |
| 27 | BRILL Z G, CONDAKES M L, TING C P, et al. Navigating the chiral pool in the total synthesis of complex terpene natural products[J]. Chemical Reviews, 2017, 117(18): 11753-11795. |
| 28 | BREUER M, DITRICH K, HABICHER T, et al. Industrial methods for the production of optically active intermediates[J]. Angewandte Chemie International Edition, 2004, 43(7): 788-824. |
| 29 | FACIN B R, MELCHIORS M S, VALÉRIO A, et al. Driving immobilized lipases as biocatalysts: 10 years state of the art and future prospects[J]. Industrial & Engineering Chemistry Research, 2019, 58(14): 5358-5378. |
| 30 | WANG H H, ZHANG Q, YU X, et al. Application of lipase B from Candida antarctica in the pharmaceutical industry [J]. Industrial & Engineering Chemistry Research, 2023, 62(39): 15733-15751. |
| 31 | SLABU I, GALMAN J L, LLOYD R C, et al. Discovery, engineering, and synthetic application of transaminase biocatalysts[J]. ACS Catalysis, 2017, 7(12): 8263-8284. |
| 32 | TOOGOOD H S, SCRUTTON N S. Discovery, characterization, engineering and applications of ene-reductases for industrial biocatalysis[J]. ACS Catalysis, 2018, 8(4): 3532-3549. |
| 33 | YANG L C, DENG H P, RENATA H. Recent progress and developments in chemoenzymatic and biocatalytic dynamic kinetic resolution[J]. Organic Process Research & Development, 2022, 26(7): 1925-1943. |
| 34 | EGI M, SUGIYAMA K, SANETO M, et al. A mesoporous-silica-immobilized oxovanadium cocatalyst for the lipase-catalyzed dynamic kinetic resolution of racemic alcohols[J]. Angewandte Chemie International Edition, 2013, 52(13): 3654-3658. |
| 35 | MATSUNAGA K, SHIBUYA M, Imperanene OHIZUMI Y., a novel phenolic compound with platelet aggregation inhibitory activity from Imperata cylindrica [J]. Journal of Natural Products, 1995, 58(1): 138-139. |
| 36 | CARR J A, BISHT K S. Enantioselective synthesis of imperanene via enzymatic asymmetrization of an intermediary 1,3-diol[J]. Organic Letters, 2004, 6(19): 3297-3300. |
| 37 | GIBSON D T, KOCH J R, KALLIO R E. Oxidative degradation of aromatic hydrocarbons by microorganisms.Ⅰ. Enzymatic formation of catechol from benzene[J]. Biochemistry, 1968, 7(7): 2653-2662. |
| 38 | GIBSON D T, KOCH J R, SCHULD C L, et al. Oxidative degradation of aromatic hydrocarbons by microorganisms.Ⅱ. Metabolism of halogenated aromatic hydrocarbons[J]. Biochemistry, 1968, 7(11): 3795-3802. |
| 39 | GIBSON D T, HENSLEY M, YOSHIOKA H, et al. Formation of (+)-cis-2,3-dihydroxy-1-methylcyclohexa-4,6-diene from toluene by Pseudomonas putida [J]. Biochemistry, 1970, 9(7): 1626-1630. |
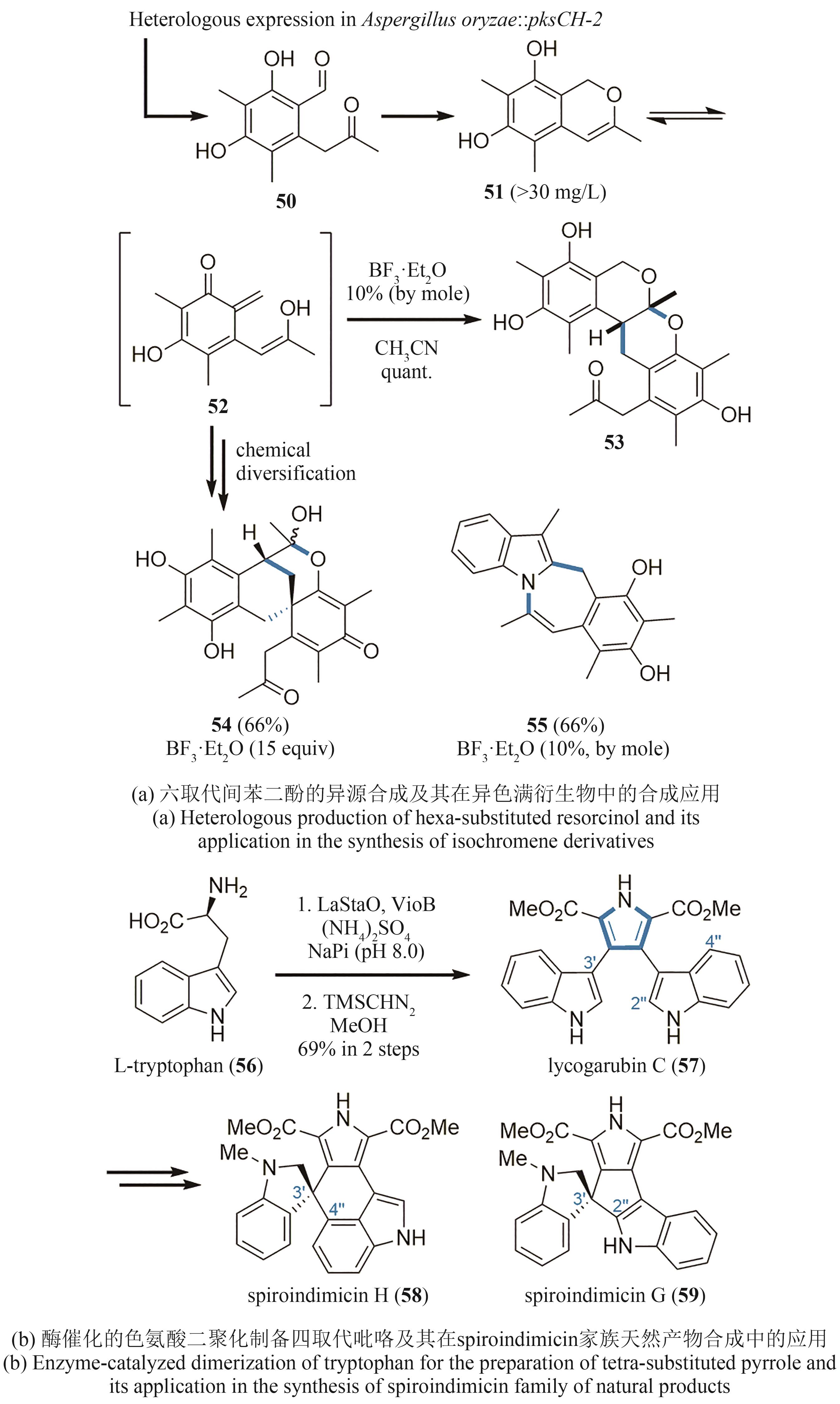
| 40 | ZYLSTRA G J, GIBSON D T. Toluene degradation by Pseudomonas putida F1. Nucleotide sequence of the todC1C2BADE genes and their expression in Escherichia coli [J]. The Journal of Biological Chemistry, 1989, 264(25): 14940-14946. |
| 41 | HUDLICKY T, GONZALEZ D, GIBSON D T. Enzymatic dihydroxylation of aromatics in enantioselective synthesis: expanding asymmetric methodology [J]. Aldrichimica Acta, 1999, 32(2): 35-62. |
| 42 | HUDLICKY T, REED J. Celebrating 20 years of SYNLETT- special account on the merits of biocatalysis and the impact of arene cis-dihydrodiolson enantioselective synthesis[J]. Synlett, 2009(5): 685-703. |
| 43 | LEWIS S E. Applications of biocatalytic arene ipso, ortho cis-dihydroxylation in synthesis[J]. Chemical Communications, 2014, 50(22): 2821-2830. |
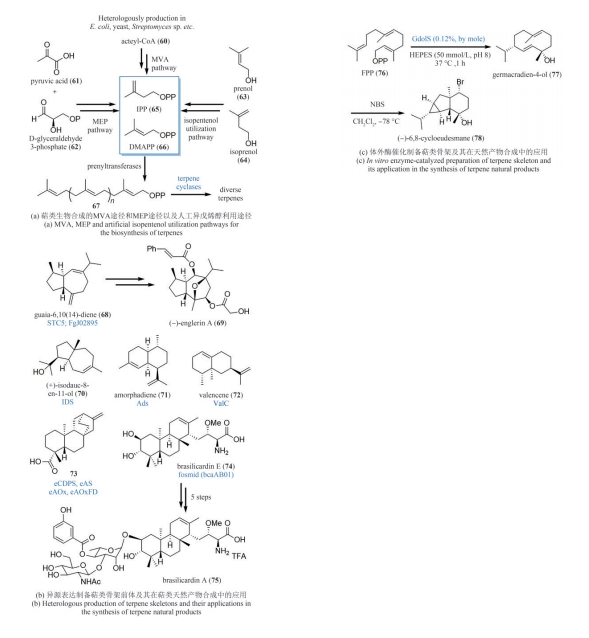
| 44 | TAHER E S, BANWELL M G, BUCKLER J N, et al. The exploitation of enzymatically-derived cis-1,2-dihydrocatechols and related compounds in the synthesis of biologically active natural products[J]. Chemical Record, 2018, 18(2): 239-264. |
| 45 | LAN P, YE S, BANWELL M G. The application of dioxygenase-based chemoenzymatic processes to the total synthesis of natural products[J]. Chemistry, an Asian Journal, 2019, 14(22): 4001-4012. |
| 46 | HUDLICKY T, PRICE J D, FAN R L, et al. Efficient and enantiodivergent synthesis of (+)- and (-)-pinitol[J]. Journal of the American Chemical Society, 1990, 112(25): 9439-9440. |
| 47 | MATVEENKO M, WILLIS A C, BANWELL M G. A chemoenzymatic synthesis of the anti-influenza agent Tamiflu®[J]. Tetrahedron Letters, 2008, 49(49): 7018-7020. |
| 48 | MA X H, BANWELL M G, WILLIS A C. Chemoenzymatic total synthesis of the phytotoxic geranylcyclohexentriol (-)-phomentrioloxin[J]. Journal of Natural Products, 2013, 76(8): 1514-1518. |
| 49 | BANWELL M G, KOKAS O J, WILLIS A C. Chemoenzymatic approaches to the montanine alkaloids: a total synthesis of (+)-brunsvigine[J]. Organic Letters, 2007, 9(18): 3503-3506. |
| 50 | FINDLAY A D, BANWELL M G. A chemoenzymatic total synthesis of (+)-amabiline[J]. Organic Letters, 2009, 11(14): 3160-3162. |
| 51 | HUDLICKY T, OLIVO H F. A short synthesis of (+)- lycoricidine[J]. Journal of the American Chemical Society, 1992, 114(24): 9694-9696. |
| 52 | ENDOMA-ARIAS M A A, HUDLICKY T. Chemoenzymatic total synthesis of (+)-galanthamine and (+)-narwedine from phenethyl acetate[J]. Chemistry, 2016, 22(41): 14540-14543. |
| 53 | VARGHESE V, HUDLICKY T. Short chemoenzymatic total synthesis of ent-hydromorphone: an oxidative dearomatization/intramolecular [4+2] cycloaddition/amination sequence[J]. Angewandte Chemie International Edition, 2014, 53(17): 4355-4358. |
| 54 | LIN A, WILLIS A C, BANWELL M G. A chemoenzymatic and enantioselective total synthesis of the resorcylic acid lactone L-783, 290, the trans-isomer of L-783, 277[J]. Tetrahedron Letters, 2010, 51(7): 1044-1047. |
| 55 | BANWELL M G, HOCKLESS D C R, MCLEOD M D. Chemoenzymatic total syntheses of the sesquiterpene (-)-patchoulenone[J]. New Journal of Chemistry, 2003, 27(1): 50-59. |
| 56 | SCHWARTZ B D, MATOUŠOVÁ E, WHITE R, et al. A chemoenzymatic total synthesis of the protoilludane aryl ester (+)-armillarivin[J]. Organic Letters, 2013, 15(8): 1934-1937. |
| 57 | BAKER DOCKREY S A, NARAYAN A R H. Flavin-dependent biocatalysts in synthesis[J]. Tetrahedron, 2019, 75(9): 1115-1121. |
| 58 | FAHAD A A, ABOOD A, FISCH K M, et al. Oxidative dearomatisation: the key step of sorbicillinoid biosynthesis[J]. Chemical Science, 2014, 5(2): 523-527. |
| 59 | SIB A, GULDER T A M. Stereoselective total synthesis of bisorbicillinoid natural products by enzymatic oxidative dearomatization/dimerization[J]. Angewandte Chemie International Edition, 2017, 56(42): 12888-12891. |
| 60 | BAKER DOCKREY S A, LUKOWSKI A L, BECKER M R, et al. Biocatalytic site- and enantioselective oxidative dearomatization of phenols[J]. Nature Chemistry, 2018, 10(2): 119-125. |
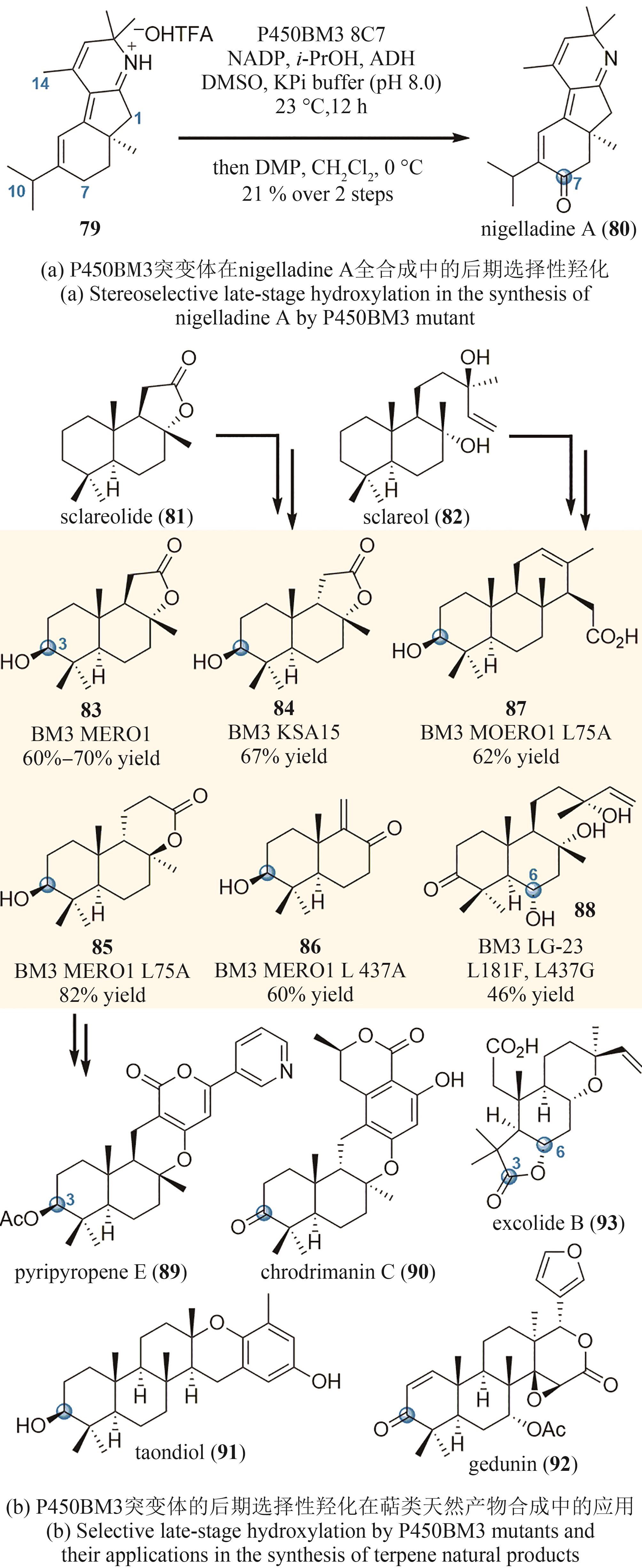
| 61 | DAVISON J, FAHAD A A, CAI M H, et al. Genetic, molecular, and biochemical basis of fungal tropolone biosynthesis[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(20): 7642-7647. |
| 62 | ZABALA A O, XU W, CHOOI Y H, et al. Characterization of a silent azaphilone gene cluster from Aspergillus niger ATCC 1015 reveals a hydroxylation-mediated pyran-ring formation[J]. Chemistry & Biology, 2012, 19(8): 1049-1059. |
| 63 | SOMOZA A D, LEE K H, CHIANG Y M, et al. Reengineering an azaphilone biosynthesis pathway in Aspergillus nidulans to create lipoxygenase inhibitors[J]. Organic Letters, 2012, 14(4): 972-975. |
| 64 | PYSER J B, BAKER DOCKREY S A, BENÍTEZ A R, et al. Stereodivergent, chemoenzymatic synthesis of azaphilone natural products[J]. Journal of the American Chemical Society, 2019, 141(46): 18551-18559. |
| 65 | HERR C Q, HAUSINGER R P. Amazing diversity in biochemical roles of Fe(Ⅱ)/2-oxoglutarate oxygenases[J]. Trends in Biochemical Sciences, 2018, 43(7): 517-532. |
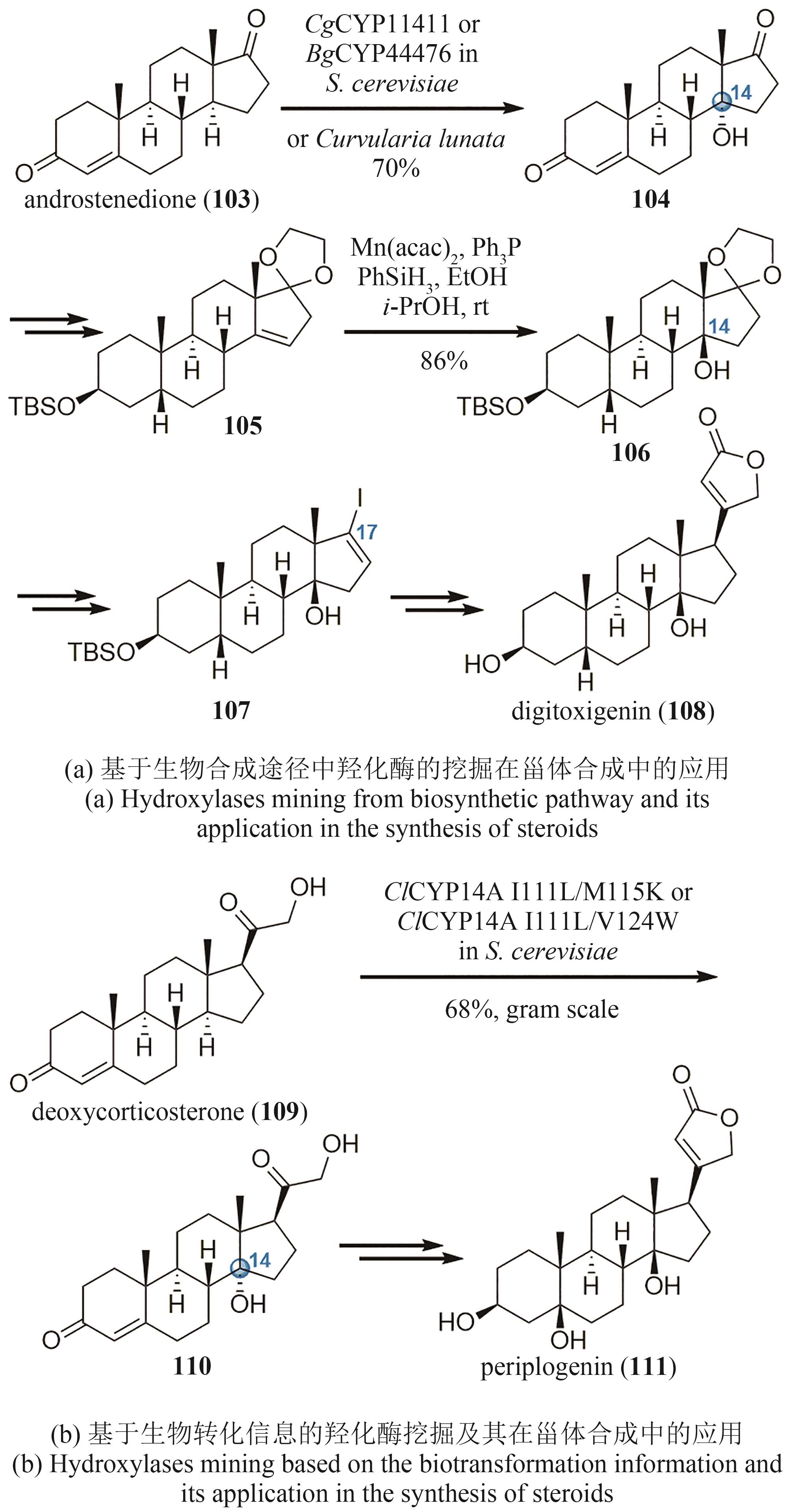
| 66 | ITOH H, INOUE M. Comprehensive structure-activity relationship studies of macrocyclic natural products enabled by their total syntheses[J]. Chemical Reviews, 2019, 119(17): 10002-10031. |
| 67 | BAUD D, SAAIDI P L, MONFLEUR A, et al. Synthesis of mono- and dihydroxylated amino acids with new α-ketoglutarate-dependent dioxygenases: biocatalytic oxidation of C—H bonds[J]. ChemCatChem, 2014, 6(10): 3012-3017. |
| 68 | ZHANG X, KING-SMITH E, RENATA H. Total synthesis of tambromycin by combining chemocatalytic and biocatalytic C—H functionalization[J]. Angewandte Chemie International Edition, 2018, 57(18): 5037-5041. |
| 69 | MATTAY J, HÜTTEL W. Pipecolic acid hydroxylases: a monophyletic clade among cis-selective bacterial proline hydroxylases that discriminates L-proline[J]. ChemBioChem, 2017, 18(15): 1523-1528. |
| 70 | C R Ⅲ ZWICK, SOSA M B, RENATA H. Characterization of a citrulline 4-hydroxylase from nonribosomal peptide GE81112 biosynthesis and engineering of its substrate specificity for the chemoenzymatic synthesis of enduracididine[J]. Angewandte Chemie International Edition, 2019, 58(52): 18854-18858. |
| 71 | C R Ⅲ ZWICK, SOSA M B, RENATA H. Modular chemoenzymatic synthesis of GE81112 B1 and related analogues enables elucidation of its key pharmacophores[J]. Journal of the American Chemical Society, 2021, 143(3): 1673-1679. |
| 72 | FAN J, LIAO G, KINDINGER F, et al. Peniphenone and penilactone formation in Penicillium crustosum via 1,4-Michael additions of ortho-quinone methide from hydroxyclavatol to γ-butyrolactones from crustosic acid[J]. Journal of the American Chemical Society, 2019, 141(10): 4225-4229. |
| 73 | DOYON T J, PERKINS J C, BAKER DOCKREY S A, et al. Chemoenzymatic o-quinone methide formation[J]. Journal of the American Chemical Society, 2019, 141(51): 20269-20277. |
| 74 | ROMERO E O, PERKINS J C, BURCH J E, et al. Chemoenzymatic synthesis of (+)-xyloketal B[J]. Organic Letters, 2023, 25(9): 1547-1552. |
| 75 | ASAI T, YAMAMOTO T, SHIRATA N, et al. Structurally diverse chaetophenol productions induced by chemically mediated epigenetic manipulation of fungal gene expression[J]. Organic Letters, 2013, 15(13): 3346-3349. |
| 76 | ASAI T, TSUKADA K, ISE S, et al. Use of a biosynthetic intermediate to explore the chemical diversity of pseudo-natural fungal polyketides[J]. Nature Chemistry, 2015, 7(9): 737-743. |
| 77 | SÁNCHEZ C, MÉNDEZ C, SALAS J A. Indolocarbazole natural products: occurrence, biosynthesis, and biological activity[J]. Natural Product Reports, 2006, 23(6): 1007-1045. |
| 78 | BLAIR L M, SPERRY J. Total syntheses of (±)- spiroindimicins B and C enabled by a late-stage Schöllkopf-Magnus-Barton-Zard (SMBZ) reaction[J]. Chemical Communications, 2016, 52(4): 800-802. |
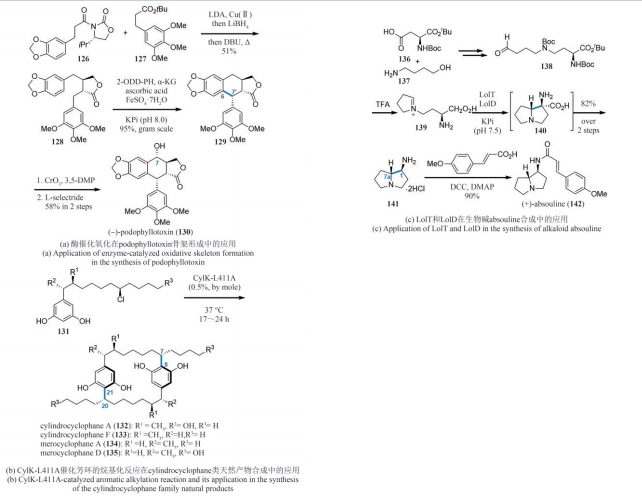
| 79 | ZHANG Z, RAY S, IMLAY L, et al. Total synthesis of (+)-spiroindimicin A and congeners unveils their antiparasitic activity[J]. Chemical Science, 2021, 12(30): 10388-10394. |
| 80 | ZHENG X K, LI Y, GUAN M T, et al. Biomimetic total synthesis of the spiroindimicin family of natural products[J]. Angewandte Chemie International Edition, 2022, 61(38): e202208802. |
| 81 | BUREAU J A, OLIVA M E, DONG Y M, et al. Engineering yeast for the production of plant terpenoids using synthetic biology approaches[J]. Natural Product Reports, 2023, 40(12): 1822-1848. |
| 82 | SIEMON T, WANG Z Q, BIAN G K, et al. Semisynthesis of plant-derived englerin A enabled by microbe engineering of guaia-6,10(14)-diene as building block[J]. Journal of the American Chemical Society, 2020, 142(6): 2760-2765. |
| 83 | MOU S B, XIAO W, WANG H Q, et al. Syntheses of epoxyguaiane sesquiterpenes (-)-englerin A, (-)-oxyphyllol, (+)-orientalol E, and (+)-orientalol F: a synthetic biology approach[J]. Organic Letters, 2020, 22(5): 1976-1979. |
| 84 | MOU S B, XIAO W, WANG H Q, et al. Syntheses of the carotane-type terpenoids (+)-schisanwilsonene A and (+)-tormesol via a two-stage approach[J]. Organic Letters, 2021, 23(2): 400-404. |
| 85 | ZHOU Q H, CHEN X F, MA D W. Asymmetric, protecting-group-free total synthesis of (-)-englerin A[J]. Angewandte Chemie International Edition, 2010, 49(20): 3513-3516. |
| 86 | MOLAWI K, DELPONT N, ECHAVARREN A M. Enantioselective synthesis of (-)-englerins A and B[J]. Angewandte Chemie International Edition, 2010, 49(20): 3517-3519. |
| 87 | GAYDOU M, MILLER R E, DELPONT N, et al. Synthesis of (+)-schisanwilsonene A by tandem gold-catalyzed cyclization/1,5-migration/cyclopropanation[J]. Angewandte Chemie International Edition, 2013, 52(25): 6396-6399. |
| 88 | LIU C G, CUI X Y, CHEN W, et al. Synthesis of oxygenated sesquiterpenoids enabled by combining metabolic engineering and visible-light photocatalysis[J]. Chemistry, 2022, 28(46): e202201230. |
| 89 | HSU S Y, PERUSSE D, HOUGARD T, et al. Semisynthesis of the neuroprotective metabolite, serofendic acid[J]. ACS Synthetic Biology, 2019, 8(10): 2397-2403. |
| 90 | BOTAS A, EITEL M, SCHWARZ P N, et al. Genetic engineering in combination with semi-synthesis leads to a new route for gram-scale production of the immunosuppressive natural product brasilicardin A[J]. Angewandte Chemie International Edition, 2021, 60(24): 13536-13541. |
| 91 | NAKANO C, KUDO F, EGUCHI T, et al. Genome mining reveals two novel bacterial sesquiterpene cyclases: (-)-germacradien-4-ol and (-)-epi-α-bisabolol synthases from Streptomyces citricolor [J]. ChemBioChem, 2011, 12(15): 2271-2275. |
| 92 | GRANT P S, MEYRELLES R, GAJSEK O, et al. Biomimetic cationic cyclopropanation enables an efficient chemoenzymatic synthesis of 6,8-cycloeudesmanes[J]. Journal of the American Chemical Society, 2023, 145(10): 5855-5863. |
| 93 | HONG B K, LUO T P, LEI X G. Late-stage diversification of natural products[J]. ACS Central Science, 2020, 6(5): 622-635. |
| 94 | DECORTE B L. Underexplored opportunities for natural products in drug discovery[J]. Journal of Medicinal Chemistry, 2016, 59(20): 9295-9304. |
| 95 | FASAN R D. Tuning P450 enzymes as oxidation catalysts[J]. ACS Catalysis, 2012, 2(4): 647-666. |
| 96 | MÜNCH J, PÜLLMANN P, ZHANG W Y, et al. Enzymatic hydroxylations of sp3-carbons[J]. ACS Catalysis, 2021, 11(15): 9168-9203. |
| 97 | WHITEHOUSE C J C, BELL S G, WONG L L. P450BM3(CYP102A1): connecting the dots[J]. Chemical Society Reviews, 2012, 41(3): 1218-1260. |
| 98 | LOSKOT S A, ROMNEY D K, ARNOLD F H, et al. Enantioselective total synthesis of nigelladine A via late-stage C—H oxidation enabled by an engineered P450 enzyme[J]. Journal of the American Chemical Society, 2017, 139(30): 10196-10199. |
| 99 | LI J, LI F Z, KING-SMITH E, et al. Merging chemoenzymatic and radical-based retrosynthetic logic for rapid and modular synthesis of oxidized meroterpenoids[J]. Nature Chemistry, 2020, 12(2): 173-179. |
| 100 | LI F Z, RENATA H. A Chiral-Pool-Based strategy to access trans-syn-fused drimane meroterpenoids: chemoenzymatic total syntheses of polysin, N-acetyl-polyveoline and the chrodrimanins[J]. Journal of the American Chemical Society, 2021, 143(43): 18280-18286. |
| 101 | LI J, CHEN F, RENATA H. Concise chemoenzymatic synthesis of gedunin[J]. Journal of the American Chemical Society, 2022, 144(42): 19238-19242. |
| 102 | LI F Z, DENG H P, RENATA H. Remote B-ring oxidation of sclareol with an engineered P450 facilitates divergent access to complex terpenoids[J]. Journal of the American Chemical Society, 2022, 144(17): 7616-7621. |
| 103 | DONG L B, ZHANG X, RUDOLF J D, et al. Cryptic and stereospecific hydroxylation, oxidation, and reduction in platensimycin and platencin biosynthesis[J]. Journal of the American Chemical Society, 2019, 141(9): 4043-4050. |
| 104 | RUDOLF J D, DONG L B, MANOOGIAN K, et al. Biosynthetic origin of the ether ring in platensimycin[J]. Journal of the American Chemical Society, 2016, 138(51): 16711-16721. |
| 105 | ZHANG X, KING-SMITH E, DONG L B, et al. Divergent synthesis of complex diterpenes through a hybrid oxidative approach[J]. Science, 2020, 369(6505): 799-806. |
| 106 | ZHAO Y, ZHANG B, SUN Z Q, et al. Biocatalytic C14-hydroxylation on androstenedione enabled modular synthesis of cardiotonic steroids[J]. ACS Catalysis, 2022, 12(16): 9839-9845. |
| 107 | SONG F Z, ZHENG M M, WANG J L, et al. Chemoenzymatic synthesis of C14-functionalized steroids[J]. Nature Synthesis, 2023, 2(8): 729-739. |
| 108 | JAMIESON C S, OHASHI M, LIU F, et al. The expanding world of biosynthetic pericyclases: cooperation of experiment and theory for discovery[J]. Natural Product Reports, 2019, 36(5): 698-713. |
| 109 | KIM H J, RUSZCZYCKY M W, CHOI S H, et al. Enzyme-catalysed [4+2] cycloaddition is a key step in the biosynthesis of spinosyn A[J]. Nature, 2011, 473(7345): 109-112. |
| 110 | KIM H J, CHOI S H, JEON B S, et al. Chemoenzymatic synthesis of spinosyn A[J]. Angewandte Chemie International Edition, 2014, 53(49): 13553-13557. |
| 111 | LI X J, ZHENG Q F, YIN J, et al. Chemo-enzymatic synthesis of equisetin[J]. Chemical Communications, 2017, 53(34): 4695-4697. |
| 112 | KATO N, NOGAWA T, HIROTA H, et al. A new enzyme involved in the control of the stereochemistry in the decalin formation during equisetin biosynthesis[J]. Biochemical and Biophysical Research Communications, 2015, 460(2): 210-215. |
| 113 | GAO L, SU C, DU X X, et al. FAD-dependent enzyme-catalysed intermolecular [4+2] cycloaddition in natural product biosynthesis[J]. Nature Chemistry, 2020, 12(7): 620-628. |
| 114 | LIU X J, YANG J, GAO L, et al. Chemoenzymatic total syntheses of artoninⅠwith an intermolecular Diels-Alderase[J]. Biotechnology Journal, 2020, 15(11): e2000119. |
| 115 | GAO L, ZOU Y K, LIU X J, et al. Enzymatic control of endo- and exo-stereoselective Diels-Alder reactions with broad substrate scope[J]. Nature Catalysis, 2021, 4(12): 1059-1069. |
| 116 | LAU W, SATTELY E S. Six enzymes from mayapple that complete the biosynthetic pathway to the etoposide aglycone[J]. Science, 2015, 349(6253): 1224-1228. |
| 117 | LI J, ZHANG X, RENATA H. Asymmetric chemoenzymatic synthesis of (-)-podophyllotoxin and related aryltetralin lignans[J]. Angewandte Chemie International Edition, 2019, 58(34): 11657-11660. |
| 118 | LAZZAROTTO M, HAMMERER L, HETMANN M, et al. Chemoenzymatic total synthesis of deoxy-, epi-, and podophyllotoxin and a biocatalytic kinetic resolution of dibenzylbutyrolactones[J]. Angewandte Chemie International Edition, 2019, 58(24): 8226-8230. |
| [1] | 温艳华, 刘合栋, 曹春来, 巫瑞波. 蛋白质工程在医药产业中的应用[J]. 合成生物学, 2025, 6(1): 65-86. |
| [2] | 仲泉周, 单依怡, 裴清云, 金艳芸, 王艺涵, 孟璐远, 王歆韵, 张雨鑫, 刘坤媛, 王慧中, 冯尚国. 生物合成法生产α-熊果苷的研究进展[J]. 合成生物学, 2025, 6(1): 118-135. |
| [3] | 竺方欢, 岑雪聪, 陈振. 微生物合成二元醇研究进展[J]. 合成生物学, 2024, 5(6): 1367-1385. |
| [4] | 刘益宁, 蒲伟, 杨金星, 王钰. ω-氨基酸与内酰胺的生物合成研究进展[J]. 合成生物学, 2024, 5(6): 1350-1366. |
| [5] | 李庚, 申晓林, 孙新晓, 王佳, 袁其朋. 过氧化物酶的重组表达和应用研究进展[J]. 合成生物学, 2024, 5(6): 1498-1517. |
| [6] | 程峰, 邹树平, 徐建妙, 汤恒, 薛亚平, 郑裕国. 生物高纯精草:高光学纯L-草铵膦生物制造的创新与发展[J]. 合成生物学, 2024, 5(6): 1404-1418. |
| [7] | 付雨, 钟芳锐. 化学原理驱动的光生物不对称催化研究进展[J]. 合成生物学, 2024, 5(5): 1021-1049. |
| [8] | 郑皓天, 李朝风, 刘良叙, 王嘉伟, 李恒润, 倪俊. 负碳人工光合群落的设计、优化与应用[J]. 合成生物学, 2024, 5(5): 1189-1210. |
| [9] | 程晓雷, 刘天罡, 陶慧. 萜类化合物的非常规生物合成研究进展[J]. 合成生物学, 2024, 5(5): 1050-1071. |
| [10] | 程中玉, 李付琸. 基于P450选择性氧化的天然产物化学-酶法合成进展[J]. 合成生物学, 2024, 5(5): 960-980. |
| [11] | 刘子健, 穆柏杨, 段志强, 王璇, 陆晓杰. 与核酸兼容的化学反应开发进展[J]. 合成生物学, 2024, 5(5): 1102-1124. |
| [12] | 谢向前, 郭雯, 王欢, 李进. 含氨基乙烯半胱氨酸核糖体肽的生物合成与化学合成[J]. 合成生物学, 2024, 5(5): 981-996. |
| [13] | 汤志军, 胡友财, 刘文. 酶促4+2和2+2环加成反应:区域与立体选择性的理解与应用[J]. 合成生物学, 2024, 5(3): 401-407. |
| [14] | 张俊, 金诗雪, 云倩, 瞿旭东. 聚酮化合物非天然延伸单元的生物合成与结构改造应用[J]. 合成生物学, 2024, 5(3): 561-570. |
| [15] | 陈锡玮, 张华然, 邹懿. 真菌源非核糖体肽类药物生物合成及代谢工程[J]. 合成生物学, 2024, 5(3): 571-592. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||