合成生物学 ›› 2024, Vol. 5 ›› Issue (5): 1021-1049.DOI: 10.12211/2096-8280.2024-005
化学原理驱动的光生物不对称催化研究进展
付雨1, 钟芳锐2
- 1.深圳技术大学药学院,广东 深圳 518118
2.华中科技大学化学与化工学院,生物医用与防护材料湖北省工程研究中心,生物无机化学与药物湖北省重点实验室,湖北 武汉 430074
-
收稿日期:2024-01-08修回日期:2024-03-14出版日期:2024-10-31发布日期:2024-11-20 -
通讯作者:钟芳锐 -
作者简介:付雨 (1992—),男,助理教授。研究方向为化学原理驱动的人工酶设计及其催化应用研究。 E-mail:fuyu@sztu.edu.cn钟芳锐 (1986—),男,教授,博士生导师。研究方向为化学原理驱动的人工酶设计与光生物催化及仿生催化绿色合成。 E-mail:chemzfr@hust.edu.cn -
基金资助:国家重点研发计划(2018YFA0903500)
Recent advances in chemically driven enantioselective photobiocatalysis
FU Yu1, ZHONG Fangrui2
- 1.College of Pharmacy,Shenzhen Technology University,Shenzhen 518118,Guangdong,China
2.Hubei Key Laboratory of Bioinorganic Chemistry & Materia Medica,Hubei Engineering Research Center for Biomaterials and Medical Protective Materials,School of Chemistry and Chemical Engineering,Huazhong University of Science and Technology (HUST),Wuhan 430074,Hubei,China
-
Received:2024-01-08Revised:2024-03-14Online:2024-10-31Published:2024-11-20 -
Contact:ZHONG Fangrui
摘要:
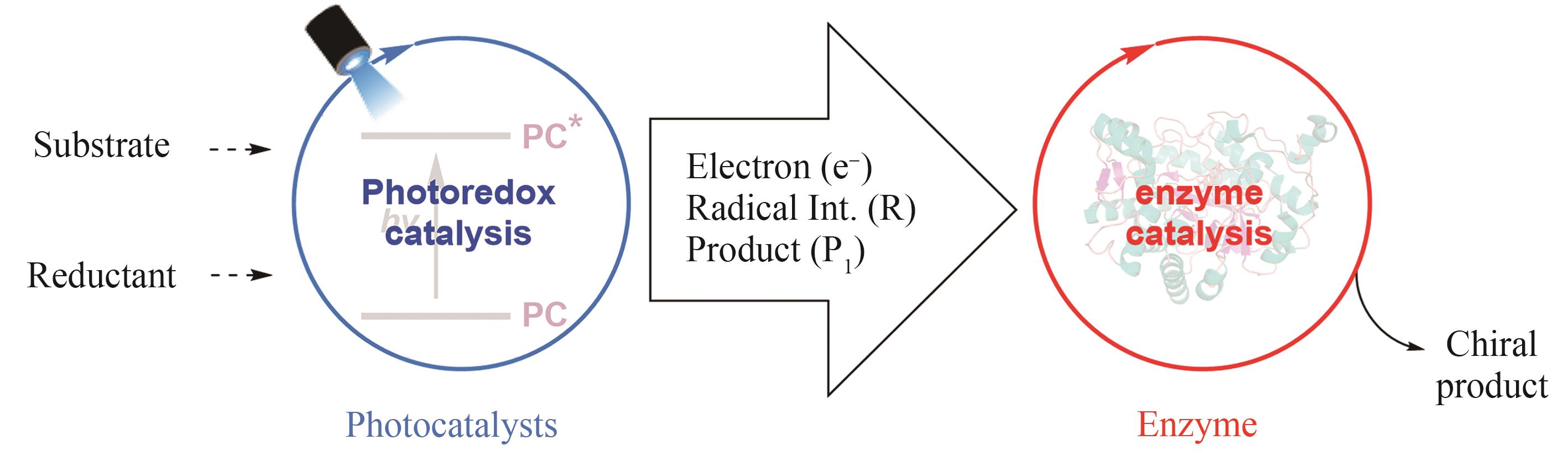
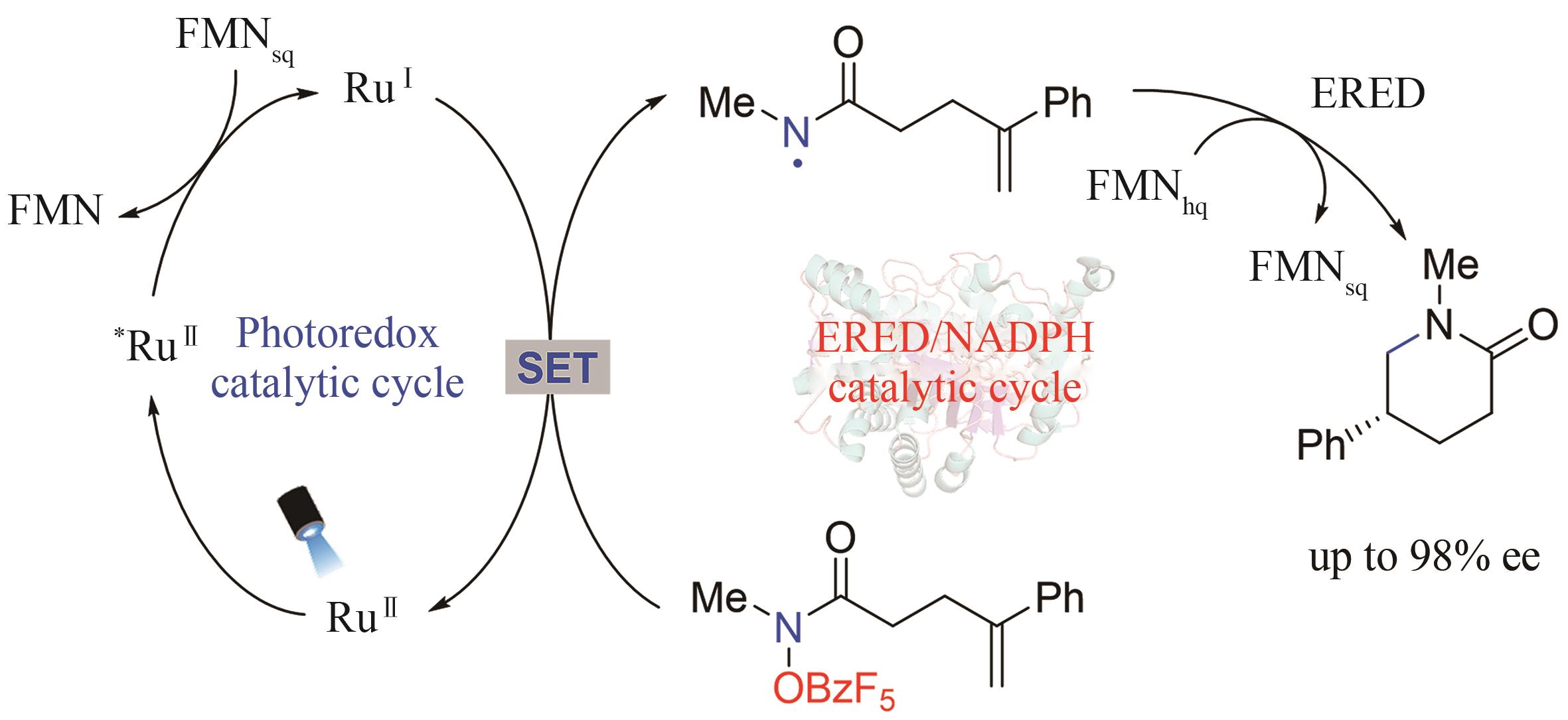
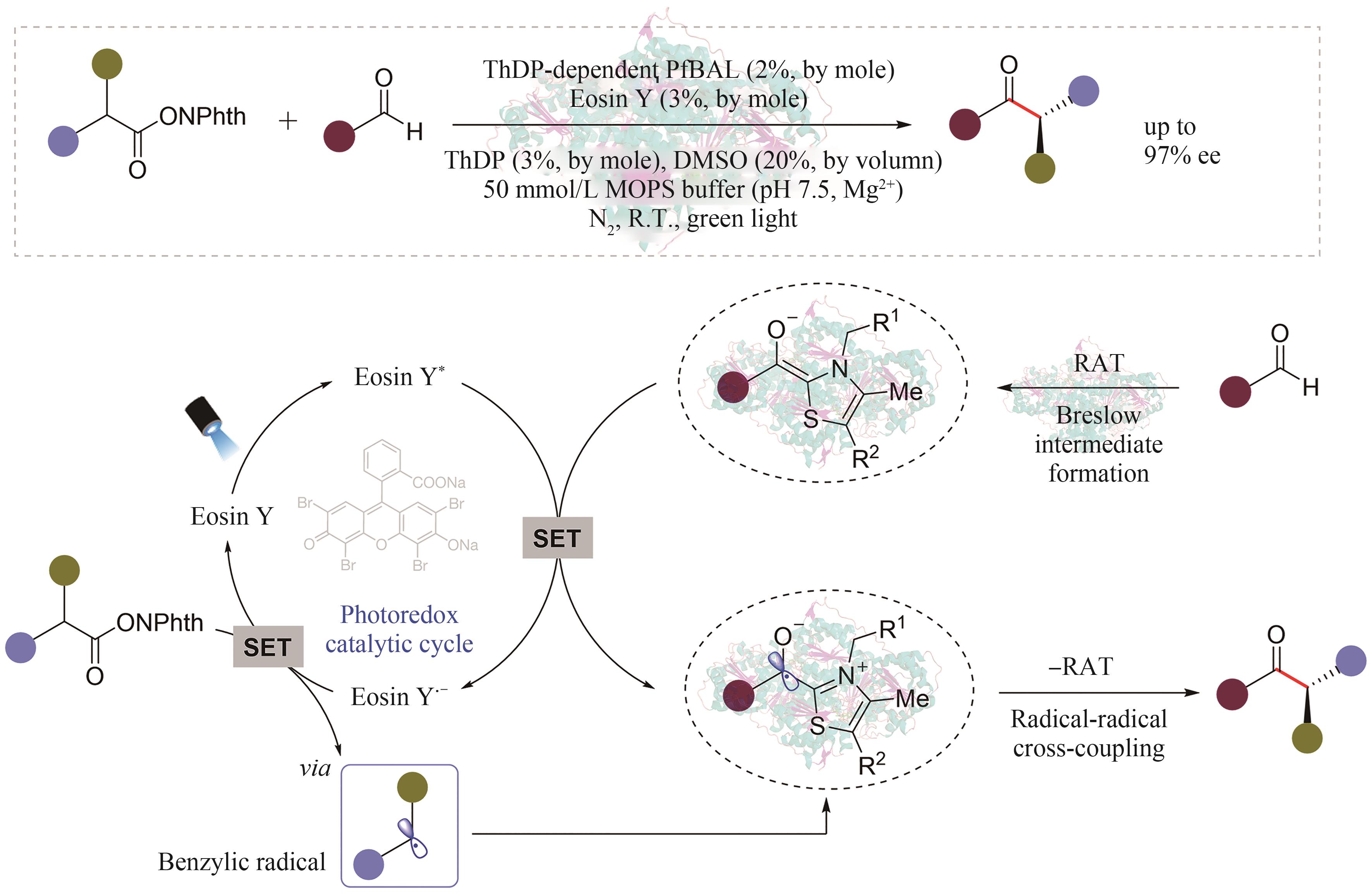
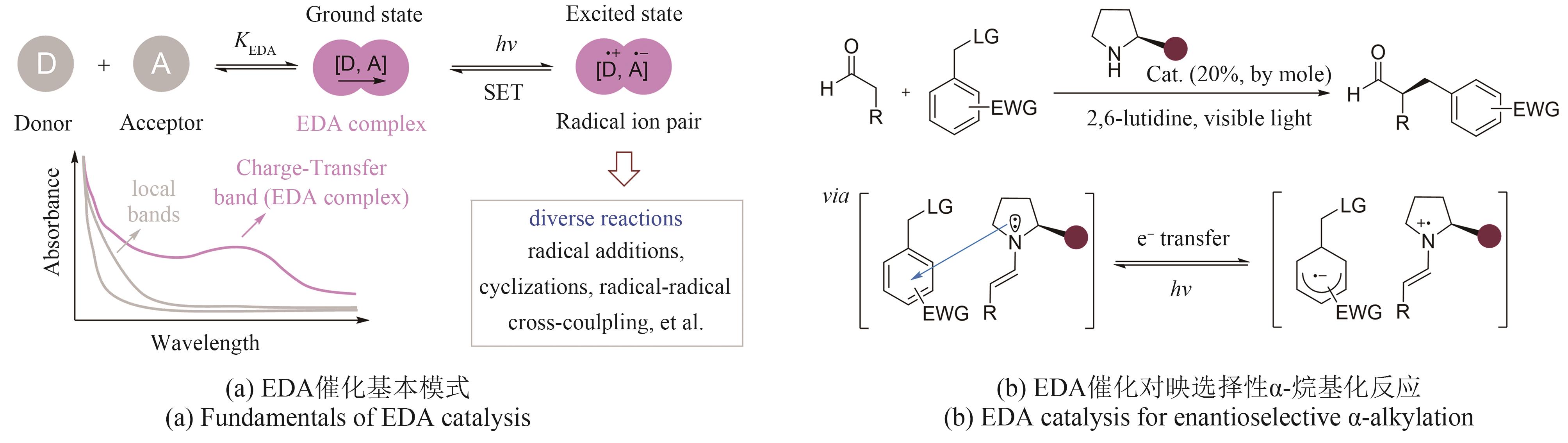
手性选择性的调控一直是合成化学的重要研究主题。酶是绿色、高选择性的天然手性催化剂,但在绿色生物合成中的应用受限于进化形成的分子结构和催化机制,光催化通过捕获光子能量实现底物分子的化学键活化,是引发自由基反应的重要策略,融合光化学和酶的光生物催化正成为不对称催化合成的新兴合成工具。利用蛋白质改造、定向进化等先进分子生物学技术,基于光化学的化学原理挖掘辅酶的非天然催化功能,探索光催化剂与酶的协同耦合作用新模式,理性设计人为定义功能的人工光酶,能突破天然酶催化的底物谱和反应类型,有效弥补天然光酶的稀缺性,拓展生物催化的化学边界和合成空间。本文综述了化学原理驱动光生物催化不对称反应的最新研究进展,依据光和酶之间的耦合模式和催化机制,将文献分为外源光催化剂与天然酶耦合、电子供体-受体复合物激发驱动的光酶催化、辅酶直接光氧化还原和光酶能量转移催化四种不同类型,分别详细探讨其分子活化机制和立体化学调控的化学原理。此外,本文也对光生物不对称催化存在的辅酶类型单调、催化效率低等挑战进行了总结,并从天然酶库的深度挖掘、人工光酶类型的拓展、酶的从头设计和全细胞催化等角度展望了未来的发展方向,通过化学和生物融合实现高价值功能分子的绿色生物制造,推动合成化学的可持续发展。
中图分类号:
引用本文
付雨, 钟芳锐. 化学原理驱动的光生物不对称催化研究进展[J]. 合成生物学, 2024, 5(5): 1021-1049.
FU Yu, ZHONG Fangrui. Recent advances in chemically driven enantioselective photobiocatalysis[J]. Synthetic Biology Journal, 2024, 5(5): 1021-1049.
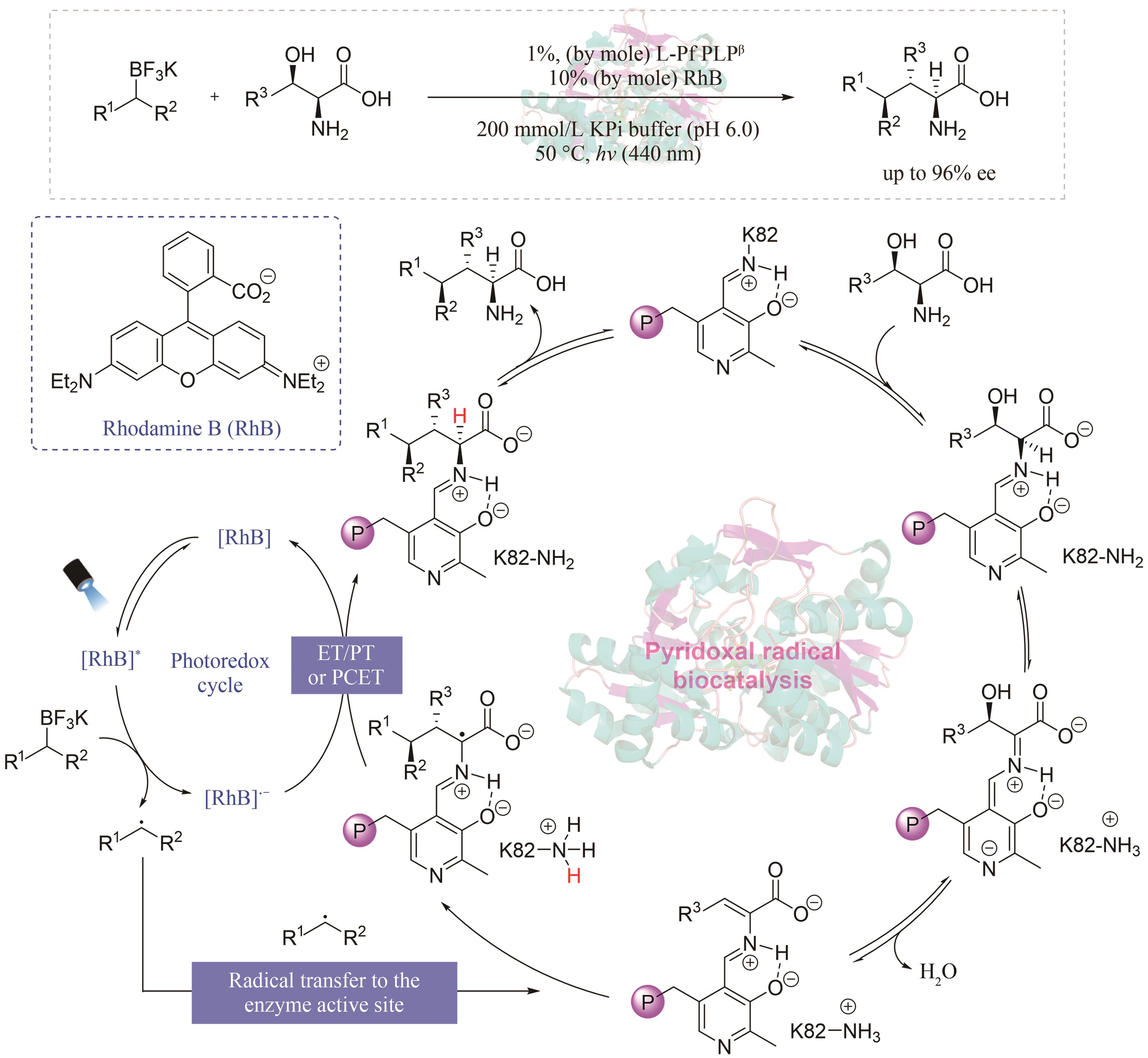
图5 光催化剂-色氨酸合成酶协同催化构建非天然氨基酸
Fig. 5 Stereoselective unnatural amino acid synthesis by synergistic catalysis with photocatalyst and tryptophan synthetase
| 反应类型 | 作者/年份 | 酶的类型 | 参考文献 |
|---|---|---|---|
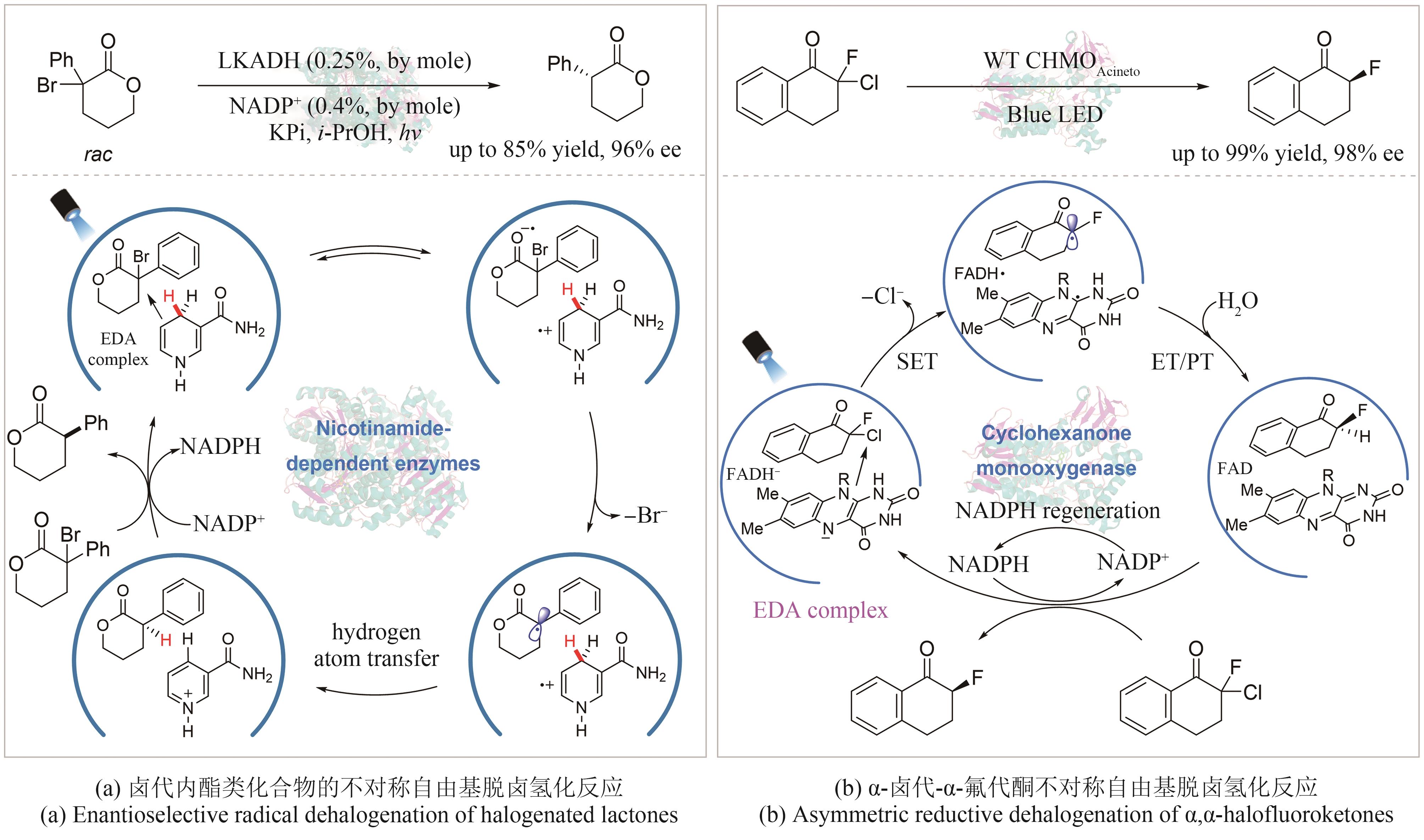
| α-卤代羰基化合物的不对称脱卤还原 | Emmanuel/2016 | 烟酰胺依赖型短链脱氢酶LKADH | [ |
| Peng/2022 | 黄素依赖型环己酮单加氧酶CHMO | [ | |
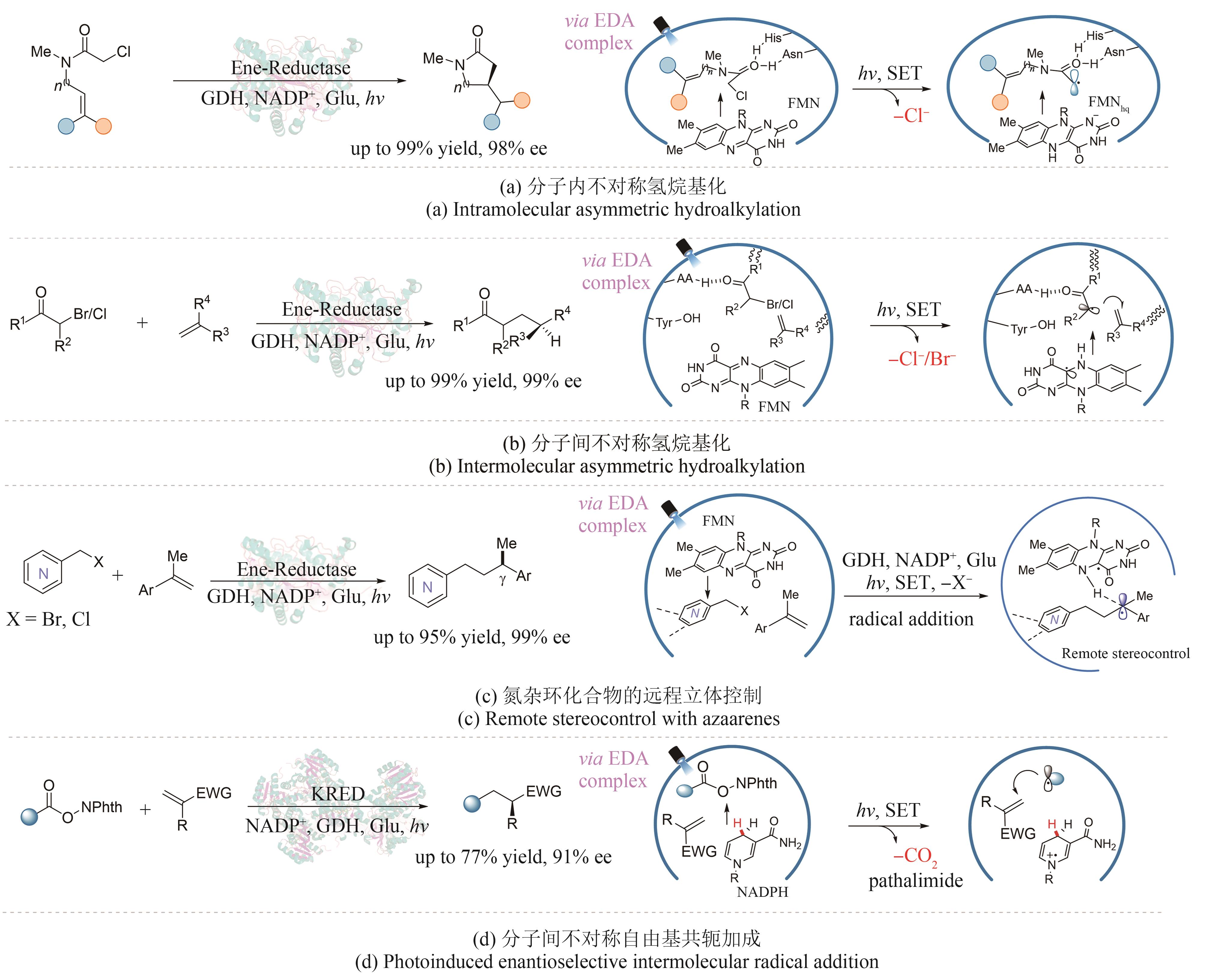
| 烯烃的不对称氢烷基化 | Biegasiewicz/2019 | 黄素依赖型烯烃还原酶GluER | [ |
| Huang/2020 | 黄素依赖型烯烃还原酶OYE1 | [ | |
| Li/2023 | 黄素依赖型烯烃还原酶OYE1 | [ | |
| Huang/2022 | 烟酰胺依赖型酮还原酶KRED | [ | |
| Zhu/2023 | 黄素依赖型烯烃还原酶GluER | [ | |
| Duan/2023 | 黄素依赖型烯烃还原酶OYE1 | [ | |
| Chen/2023 | 黄素依赖型烯烃还原酶OYE1 | [ | |
| 烯烃的不对称烃基羟基化 | Ouyang/2023 | 黄素依赖型烯烃还原酶MorB | [ |
| 不对称C(sp3)-C(sp3)亲电试剂偶联 | Fu/2022 | 黄素依赖型烯烃还原酶CsER | [ |
| Fu/2023 | 黄素依赖型烯烃还原酶GkOYE | [ |
表1 EDA复合物激发驱动的光酶催化不对称反应总结
Table 1 Summary of photoenzymatic asymmetric reactions driven by EDA complex excitation
| 反应类型 | 作者/年份 | 酶的类型 | 参考文献 |
|---|---|---|---|
| α-卤代羰基化合物的不对称脱卤还原 | Emmanuel/2016 | 烟酰胺依赖型短链脱氢酶LKADH | [ |
| Peng/2022 | 黄素依赖型环己酮单加氧酶CHMO | [ | |
| 烯烃的不对称氢烷基化 | Biegasiewicz/2019 | 黄素依赖型烯烃还原酶GluER | [ |
| Huang/2020 | 黄素依赖型烯烃还原酶OYE1 | [ | |
| Li/2023 | 黄素依赖型烯烃还原酶OYE1 | [ | |
| Huang/2022 | 烟酰胺依赖型酮还原酶KRED | [ | |
| Zhu/2023 | 黄素依赖型烯烃还原酶GluER | [ | |
| Duan/2023 | 黄素依赖型烯烃还原酶OYE1 | [ | |
| Chen/2023 | 黄素依赖型烯烃还原酶OYE1 | [ | |
| 烯烃的不对称烃基羟基化 | Ouyang/2023 | 黄素依赖型烯烃还原酶MorB | [ |
| 不对称C(sp3)-C(sp3)亲电试剂偶联 | Fu/2022 | 黄素依赖型烯烃还原酶CsER | [ |
| Fu/2023 | 黄素依赖型烯烃还原酶GkOYE | [ |
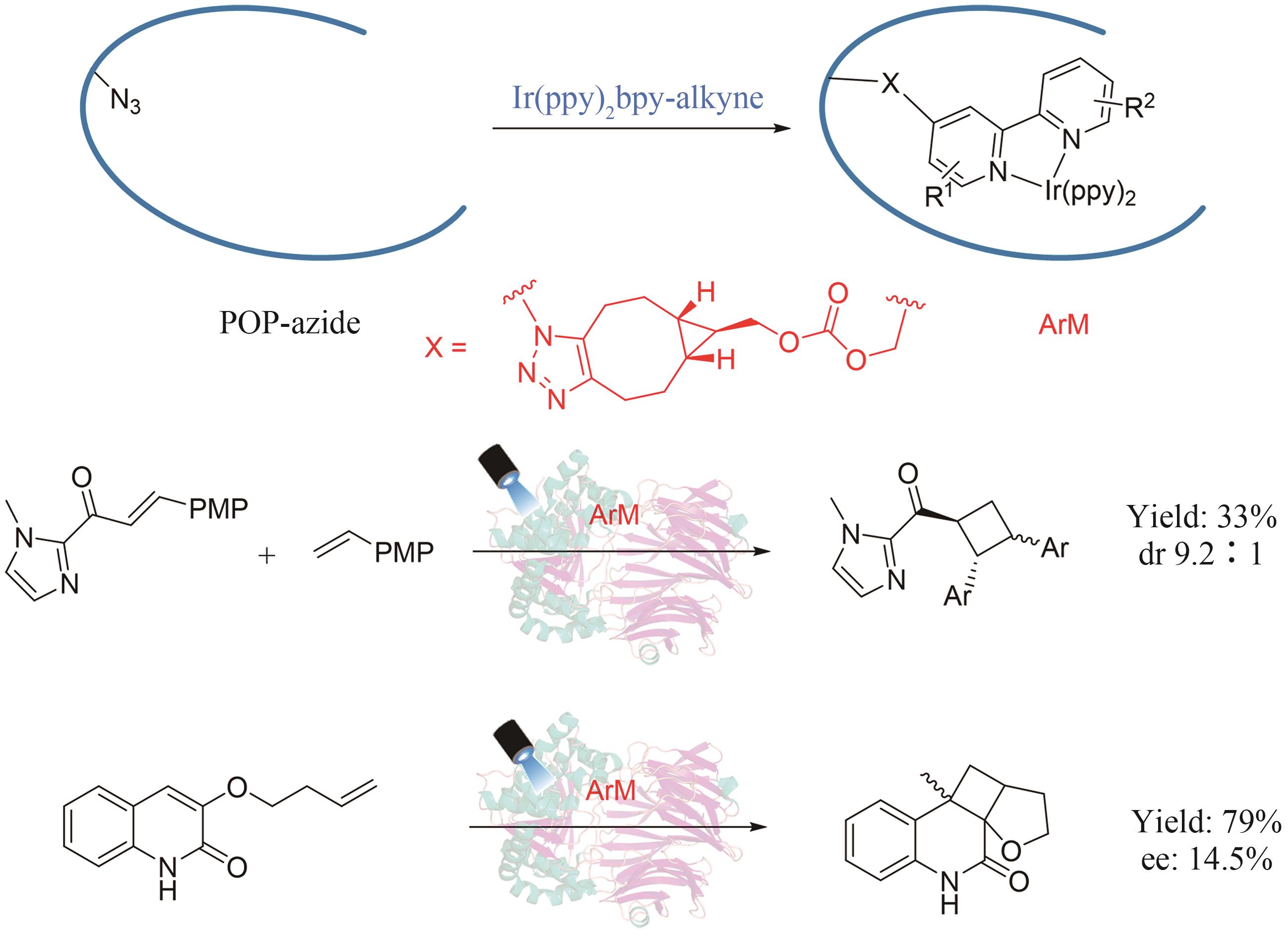
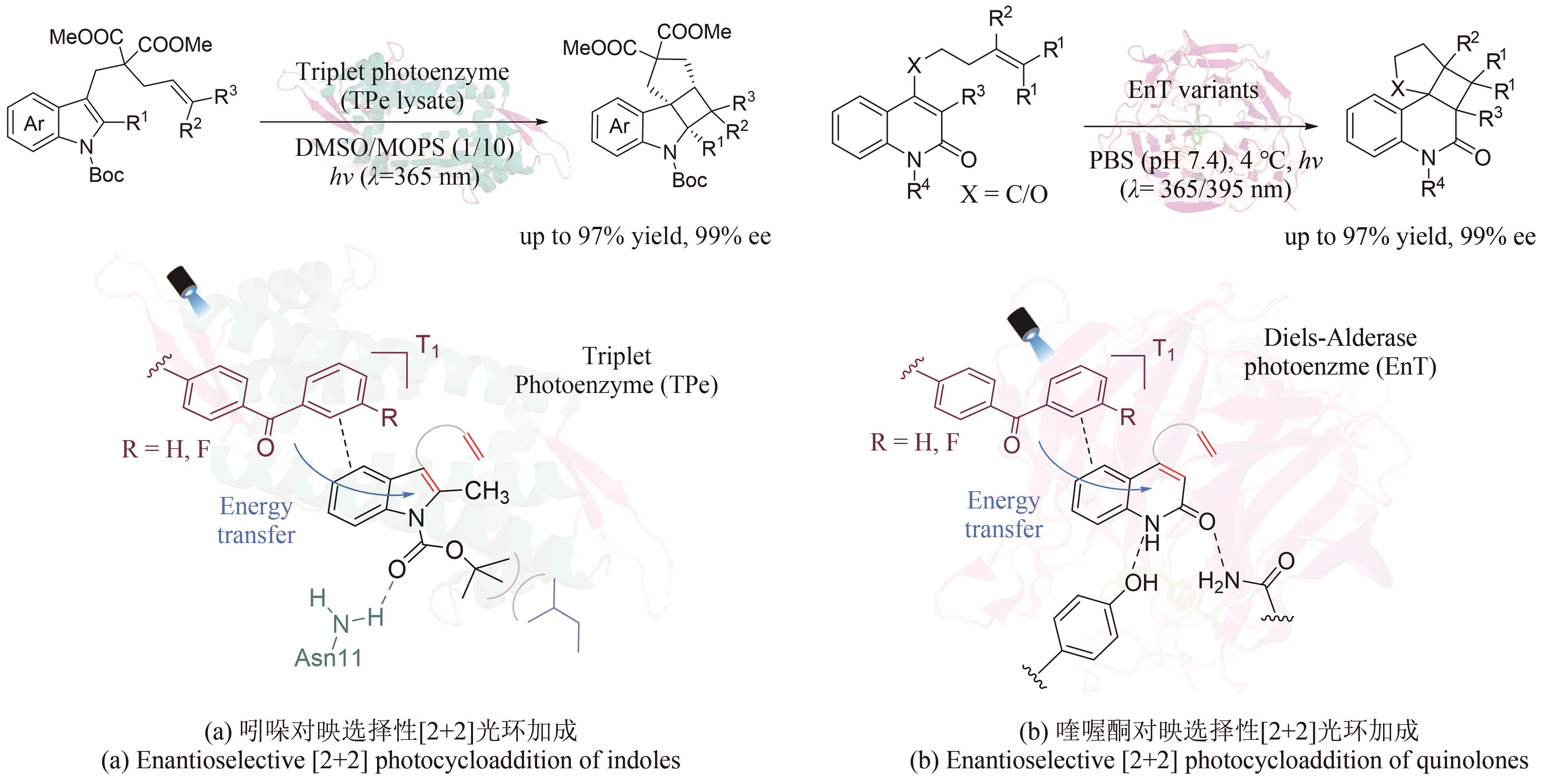
图22 人工光酶催化分子间/分子内不对称[2+2]光环加成(PMP:对甲氧基苯基)
Fig. 22 Artificial photoenzyme catalyzed asymmetric inter/intra-molecular [2+2] photocycloaddition (PMP: p-methoxyphenyl)
| 16 | PACKER M S, LIU D R. Methods for the directed evolution of proteins[J]. Nature Reviews Genetics, 2015, 16(7): 379-394. |
| 17 | ZHANG R K, CHEN K, HUANG X Y, et al. Enzymatic assembly of carbon-carbon bonds via iron-catalysed sp3 C-H functionalization[J]. Nature, 2019, 565(7737): 67-72. |
| 18 | PRIER C K, ZHANG R K, BULLER A R, et al. Enantioselective, intermolecular benzylic C-H amination catalysed by an engineered iron-haem enzyme[J]. Nature Chemistry, 2017, 9(7): 629-634. |
| 19 | YI D, BAYER T, BADENHORST C P S, et al. Recent trends in biocatalysis[J]. Chemical Society Reviews, 2021, 50(14): 8003-8049. |
| 20 | CIAMICIAN G. The photochemistry of the future[J]. Science, 1912, 36(926): 385-394. |
| 21 | STEPHENSON C R J, YOON T P, MACMILLAN D W C. Visible light photocatalysis in organic chemistry[M]. John Wiley & Sons, 2018. |
| 22 | UOYAMA H, GOUSHI K, SHIZU K, et al. Highly efficient organic light-emitting diodes from delayed fluorescence[J]. Nature, 2012, 492(7428): 234-238. |
| 23 | LUO J, ZHANG J. Donor-acceptor fluorophores for visible-light-promoted organic synthesis: photoredox/Ni dual catalytic C(sp3)-C(sp2) cross-coupling[J]. ACS Catalysis, 2016, 6(2): 873-877. |
| 24 | YIN Y L, DAI Y T, JIA H S, et al. Conjugate addition-enantioselective protonation of N-aryl glycines to α-branched 2-vinylazaarenes via cooperative photoredox and asymmetric catalysis[J]. Journal of the American Chemical Society, 2018, 140(19): 6083-6087. |
| 25 | MACKENZIE I A, WANG L F, ONUSKA N P R, et al. Discovery and characterization of an acridine radical photoreductant[J]. Nature, 2020, 580(7801): 76-80. |
| 26 | NIKIAS N F, GKIZIS P L, KOKOTOS C G. Thioxanthone: a powerful photocatalyst for organic reactions[J]. Organic & Biomolecular Chemistry, 2021, 19(24): 5237-5253. |
| 27 | GROBKOPF J, KRATZ T, RIGOTTI T, et al. Enantioselective photochemical reactions enabled by triplet energy transfer[J]. Chemical Reviews, 2021, 122(2): 1626-1653. |
| 28 | BRETTEL K, BYRDIN M. Reaction mechanisms of DNA photolyase[J]. Current Opinion in Structural Biology, 2010, 20(6): 693-701. |
| 29 | SANCAR A. Mechanisms of DNA repair by photolyase and excision nuclease (Nobel Lecture)[J]. Angewandte Chemie International Edition, 2016, 55(30): 8502-8527. |
| 30 | GABRUK M, MYSLIWA-KURDZIEL B. Light-dependent protochlorophyllide oxidoreductase: phylogeny, regulation, and catalytic properties[J]. Biochemistry, 2015, 54(34): 5255-5262. |
| 31 | SORIGUE D, LEGERET B, CUINE S, et al. An algal photoenzyme converts fatty acids to hydrocarbons[J]. Science, 2017, 357(6354): 903-907. |
| 32 | PENG Y Z, CHEN Z C, XU J, et al. Recent advances in photobiocatalysis for selective organic synthesis[J]. Organic Process Research & Development, 2022, 26(7): 1900-1913. |
| 33 | EMMANUEL M A, BENDER S G, BILODEAU C. Photobiocatalytic strategies for organic synthesis[J]. Chemical Reviews, 2023, 123(9), 5459–5520. |
| 34 | 明阳, 陈彬, 黄小强. 光酶催化合成进展[J]. 合成生物学, 2023, 4(4): 651-675. |
| MING Y, CHEN B, HUANG X Q. Recent advances in photoenzymatic synthesis[J]. Synthetic Biology Journal, 2023, 4(4): 651-675. | |
| 35 | ROMERO N A, NICEWICZ D A. Organic photoredox catalysis[J]. Chemical Reviews, 2016, 116(17): 10075-10166. |
| 36 | HUTTON G A M, MARTINDALE B C M, REISNER E. Carbon dots as photosensitisers for solar-driven catalysis[J]. Chemical Society Reviews, 2017, 46(20): 6111-6123. |
| 37 | PAL A K, HANAN G S. Design, synthesis and excited-state properties of mononuclear Ru(Ⅱ) complexes of tridentate heterocyclic ligands[J]. Chemical Society Reviews, 2014, 43(17): 6184-6197. |
| 38 | LI L L, DIAU E W G. Porphyrin-sensitized solar cells[J]. Chemical Society Reviews, 2013, 42(1): 291-304. |
| 39 | ZHANG S H, LIU S S, SUN Y Y, et al. Enzyme-photo-coupled catalytic systems[J]. Chemical Society Reviews, 2021, 50(24): 13449-13466. |
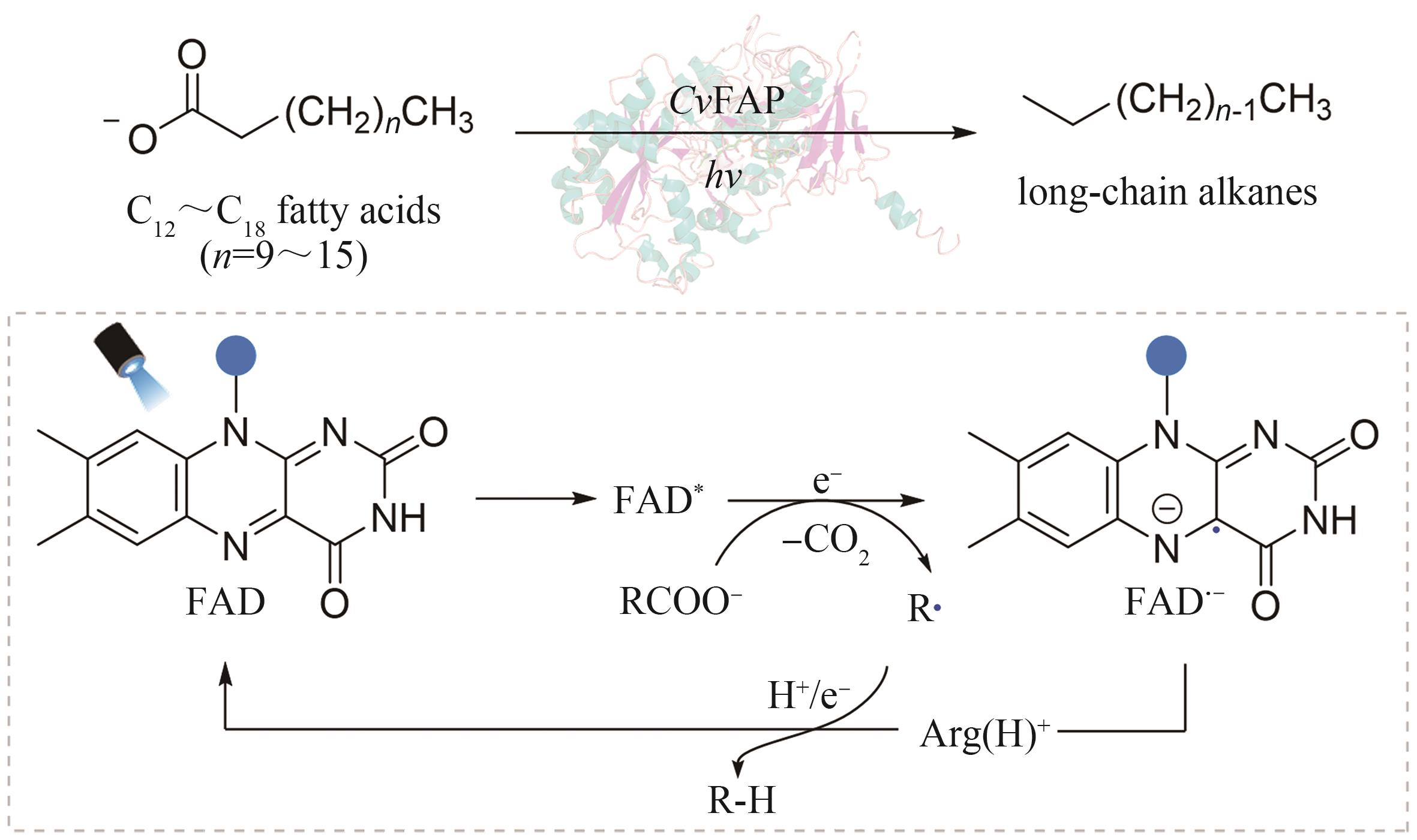
| 40 | TOOGOOD H S, SCRUTTON N S. Discovery, characterization, engineering, and applications of ene-reductases for industrial biocatalysis[J]. ACS catalysis, 2018, 8(4): 3532-3549. |
| 41 | KIM J, LEE S H, TIEVES F, et al. Biocatalytic C=C bond reduction through carbon nanodot‐sensitized regeneration of NADH analogues[J]. Angewandte Chemie, 2018, 130(42): 14021-14024. |
| 42 | BIEGASIEWICZ K F, COOPER S J, EMMANUEL M A, et al. Catalytic promiscuity enabled by photoredox catalysis in nicotinamide-dependent oxidoreductases[J]. Nature Chemistry, 2018, 10(7): 770-775. |
| 43 | NAKANO Y, BLACK M J, MEICHAN A J, et al. Photoenzymatic hydrogenation of heteroaromatic olefins using ene‐reductases with photoredox catalysts[J]. Angewandte Chemie International Edition, 2020, 59(26): 10484-10488. |
| 44 | SANDOVAL B A, KURTOIC S I, CHUNG M M, et al. Photoenzymatic catalysis enables radical‐mediated ketone reduction in ene‐reductases[J]. Angewandte Chemie, 2019, 131(26): 8806-8810. |
| 45 | SUN S Z, NICHOLLS B T, BAIN D, et al. Enantioselective decarboxylative alkylation using synergistic photoenzymatic catalysis[J]. Nature Catalysis, 2023: 1-8. |
| 46 | KWON K, SIMONS R T, NANDAKUMAR M, et al. Strategies to generate nitrogen-centered radicals that may rely on photoredox catalysis: development in reaction methodology and applications in organic synthesis[J]. Chemical Reviews, 2021, 122(2): 2353-2428. |
| 47 | PRATLEY C, FENNER S, MURPHY J A. Nitrogen-centered radicals in functionalization of sp2 systems: generation, reactivity, and applications in synthesis[J]. Chemical Reviews, 2022, 122(9): 8181-8260. |
| 48 | YE Y X, CAO J Z, OBLINSKY D G, et al. Using enzymes to tame nitrogen-centred radicals for enantioselective hydroamination[J]. Nature Chemistry, 2023, 15(2): 206-212. |
| 49 | CHENG L, LI D, MAI B K, et al. Stereoselective amino acid synthesis by synergistic photoredox-pyridoxal radical biocatalysis[J]. Science, 2023, 381(6656): 444-451. |
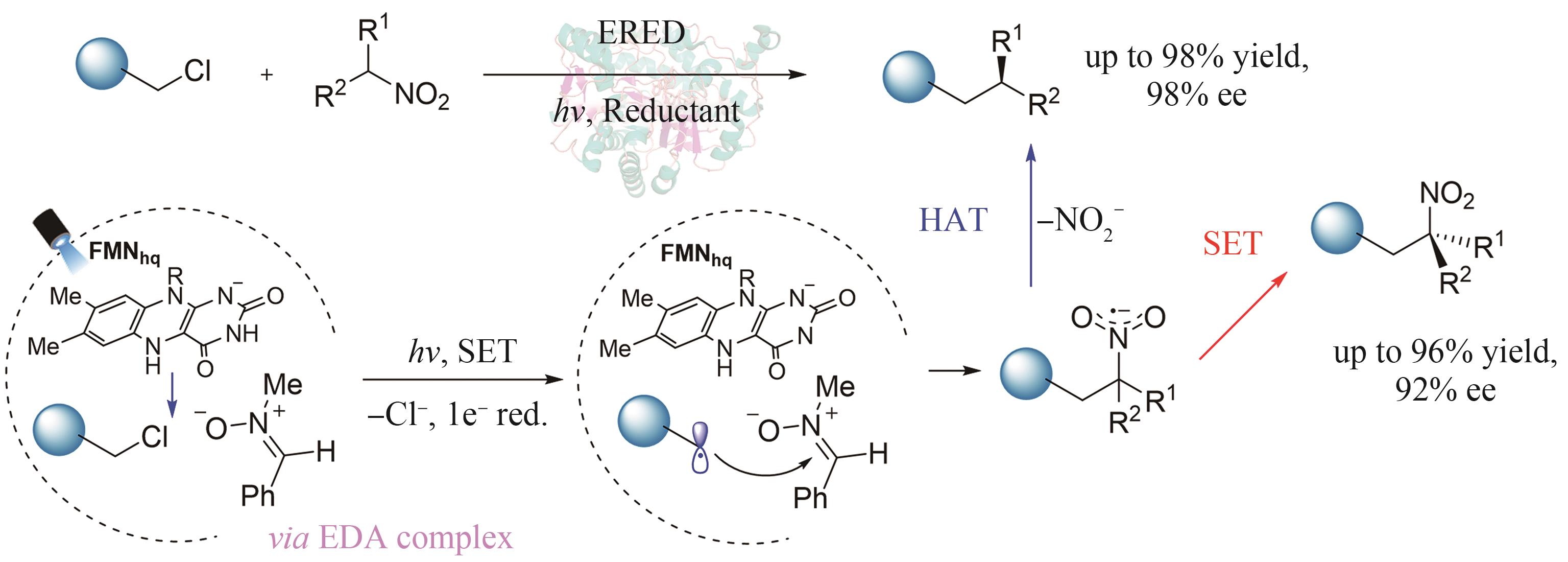
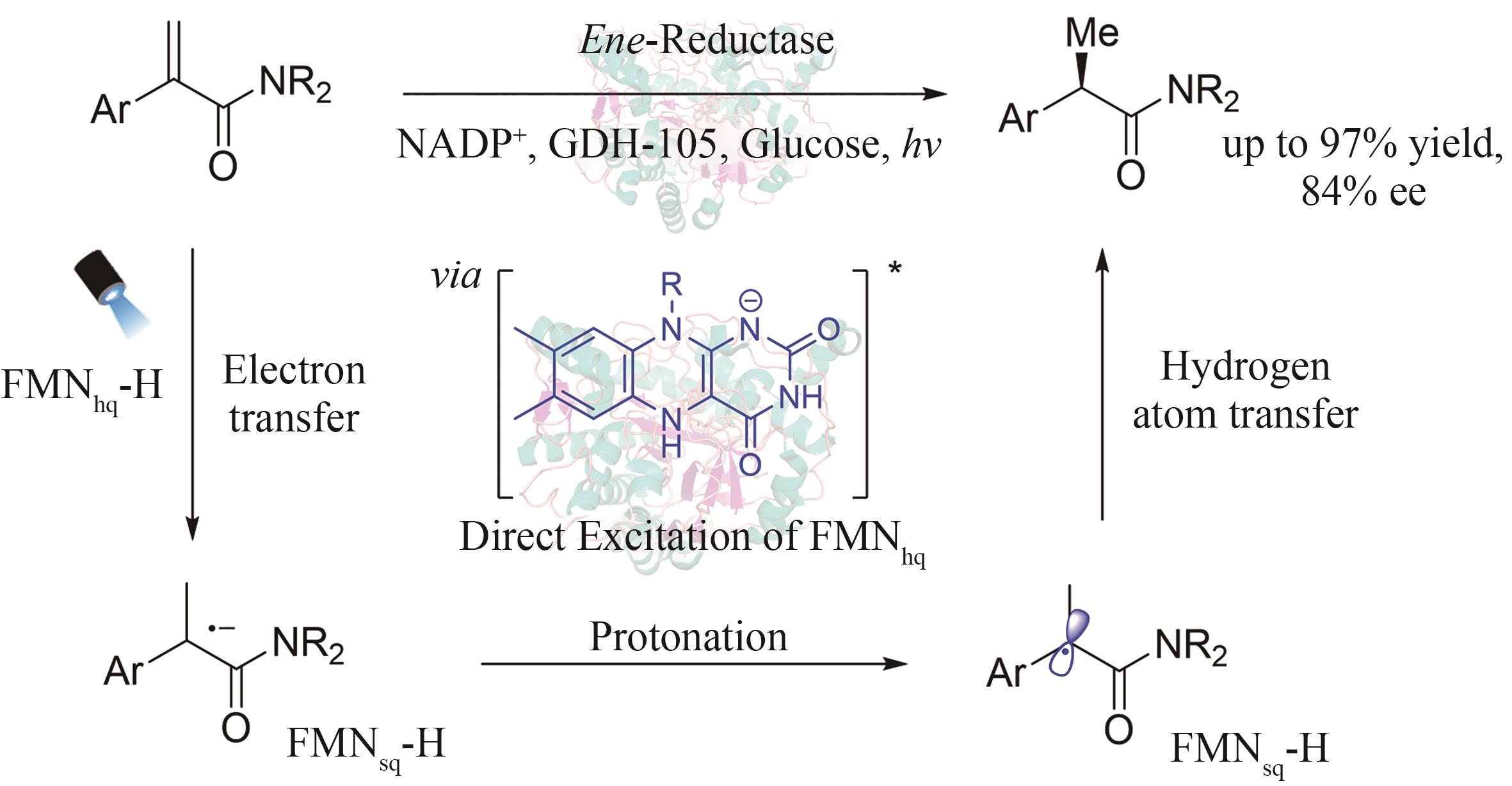
| 50 | XU Y Y, CHEN H W, YU L, et al. A light-driven enzymatic enantioselective radical acylation[J]. Nature, 2024, 625: 74-78. |
| 1 | NOYORI R. Asymmetric catalysis: science and opportunities (Nobel Lecture)[J]. Angewandte Chemie International Edition, 2002, 41(12): 2008-2022. |
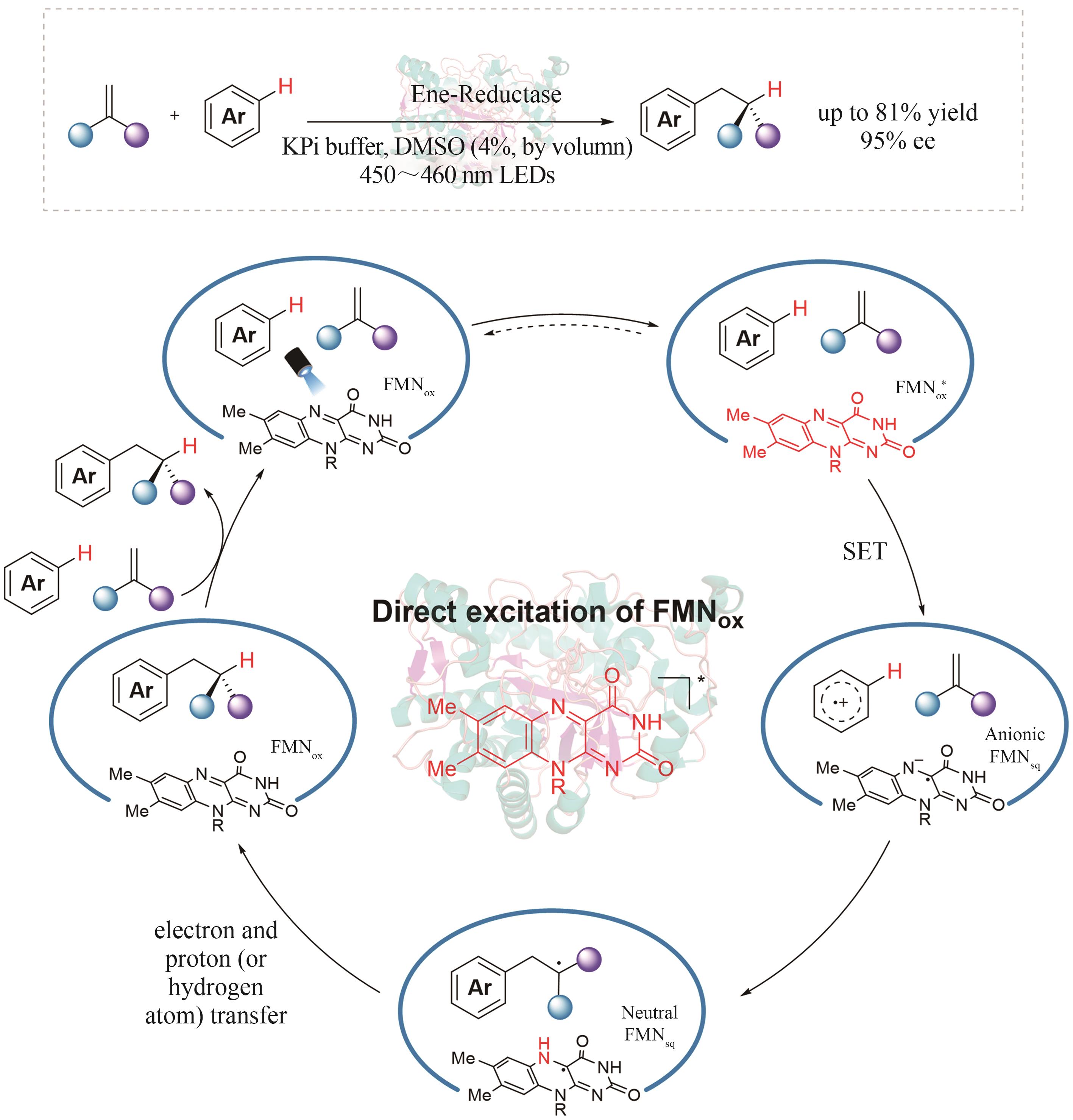
| 2 | YU X H, WANG W. Hydrogen‐bond‐mediated asymmetric catalysis[J]. Chemistry-An Asian Journal, 2008, 3(3): 516-532. |
| 3 | NGUYEN L A, HE H, PHAM-HUY C. Chiral drugs: an overview[J]. International Journal of Biomedical Science: IJBS, 2006, 2(2): 85-100. |
| 4 | DE ALBUQUERQUE N C P, CARRAO D B, HABENSCHUS M D, et al. Metabolism studies of chiral pesticides: a critical review[J]. Journal of Pharmaceutical and Biomedical Analysis, 2018, 147: 89-109. |
| 5 | GONG W, CHEN Z J, DONG J Q, et al. Chiral metal-organic frameworks[J]. Chemical Reviews, 2022, 122(9): 9078-9144. |
| 6 | FARINA V, REEVES J T, SENANAYAKE C H, et al. Asymmetric synthesis of active pharmaceutical ingredients[J]. Chemical Reviews, 2006, 106(7): 2734-2793. |
| 7 | KATSUKI T, SHARPLESS K B. The first practical method for asymmetric epoxidation[J]. Journal of the American Chemical Society, 1980, 102(18): 5974-5976. |
| 8 | ARNOLD F H. Innovation by evolution: bringing new chemistry to life (Nobel Lecture)[J]. Angewandte Chemie International Edition, 2019, 58(41): 14420-14426. |
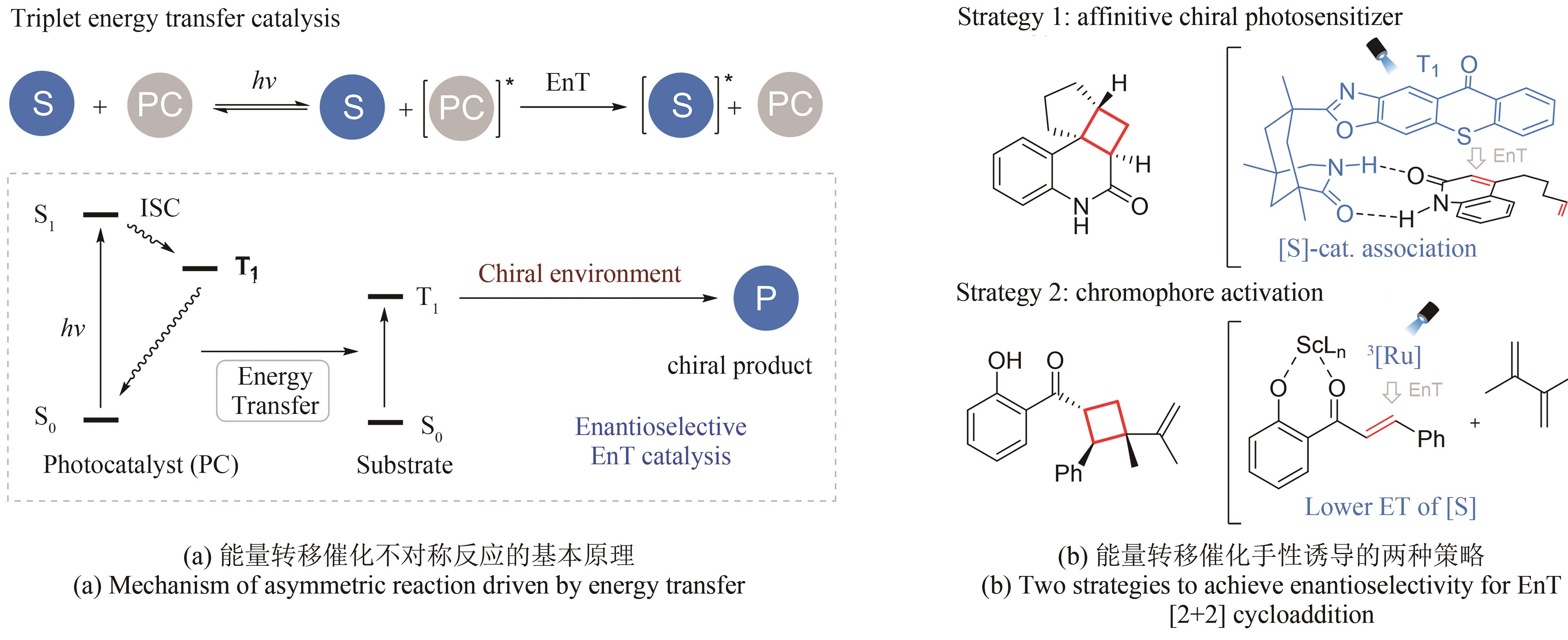
| 9 | SEAYAD J, LIST B. Asymmetric organocatalysis[J]. Organic & Biomolecular Chemistry, 2005, 3(5): 719-724. |
| 10 | MUKHERJEE S, YANG J W, HOFFMANN S, et al. Asymmetric enamine catalysis[J]. Chemical Reviews, 2007, 107(12): 5471-5569. |
| 11 | MACMILLAN D W C. The advent and development of organocatalysis[J]. Nature, 2008, 455(7211): 304-308. |
| 12 | PRIER C K, RANKIC D A, MACMILLAN D W C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis[J]. Chemical Reviews, 2013, 113(7): 5322-5363. |
| 13 | LI C J, TROST B M. Green chemistry for chemical synthesis[J]. Proceedings of the National Academy of Sciences, 2008, 105(36): 13197-13202. |
| 14 | ROSENTHALER L. Durch enzyme bewirkte asymmetrische synthesen[J]. Biochem Z, 1908, 14(1): 238-253. |
| 15 | ARNOLD F H. Directed evolution: creating biocatalysts for the future[J]. Chemical Engineering Science, 1996, 51(23): 5091-5102. |
| 51 | KLUGER R, TITTMANN K. Thiamin diphosphate catalysis: enzymic and nonenzymic covalent intermediates[J]. Chemical Reviews, 2008, 108(6): 1797-1833. |
| 52 | GIOVANNINI P P, BORTOLINI O, MASSI A. Thiamine-diphosphate-dependent enzymes as catalytic tools for the asymmetric benzoin-type reaction[J]. European Journal of Organic Chemistry, 2016, 2016(26): 4441-4459. |
| 53 | YANG Q, ZHAO F Q, ZHANG N, et al. Mild dynamic kinetic resolution of amines by coupled visible-light photoredox and enzyme catalysis[J]. Chemical Communications, 2018, 54(100): 14065-14068. |
| 54 | LITMAN Z C, WANG Y J, ZHAO H M, et al. Cooperative asymmetric reactions combining photocatalysis and enzymatic catalysis[J]. Nature, 2018, 560(7718): 355-359. |
| 55 | WANG Y J, HUANG X Q, HUI J S, et al. Stereoconvergent reduction of activated alkenes by a nicotinamide free synergistic photobiocatalytic system[J]. ACS Catalysis, 2020, 10(16): 9431-9437. |
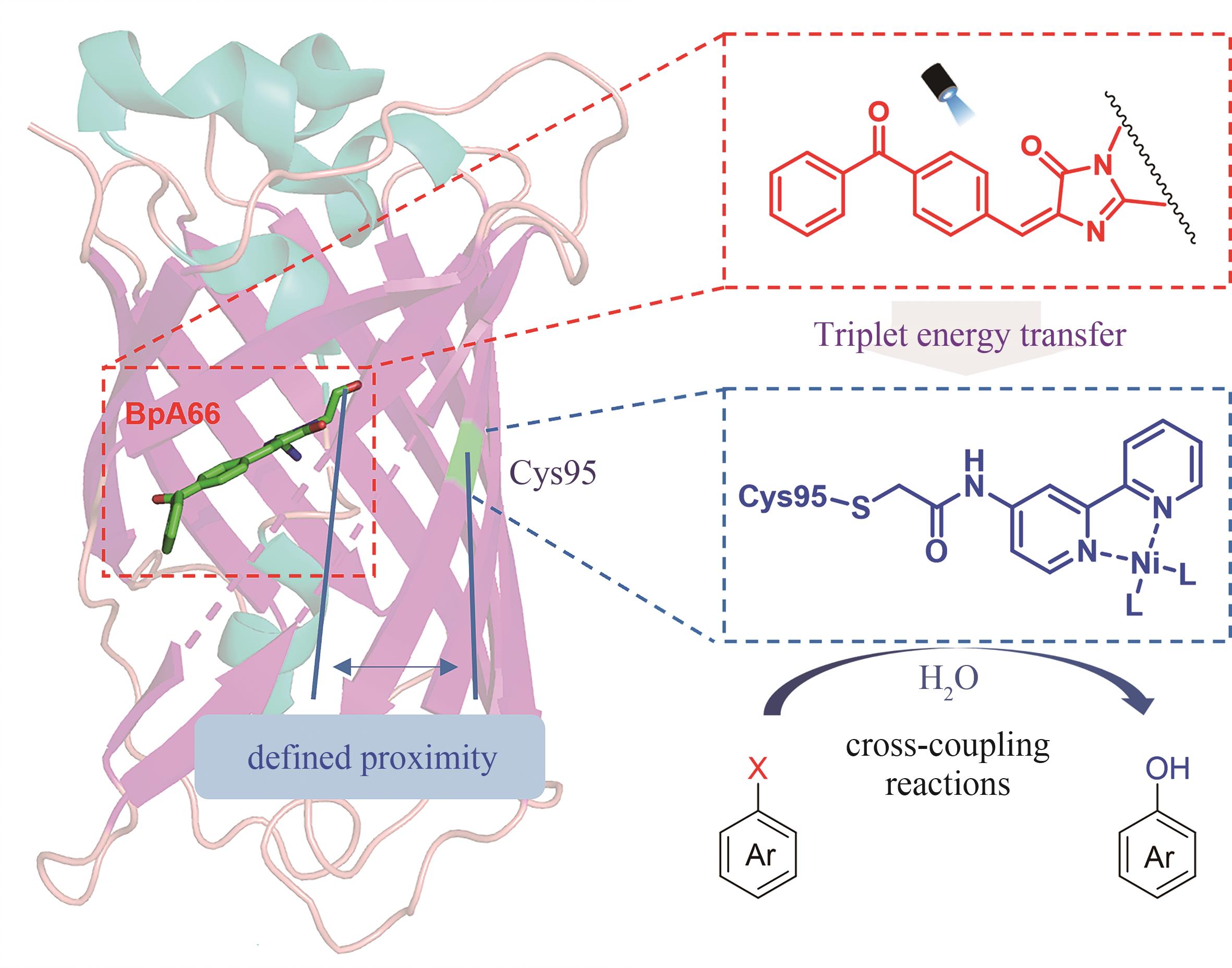
| 56 | DING X, DONG C L, GUAN Z, et al. Concurrent asymmetric reactions combining photocatalysis and enzyme catalysis: direct enantioselective synthesis of 2,2-disubstituted indol‐3-ones from 2-arylindoles[J]. Angewandte Chemie International Edition, 2019, 58 (1): 118-124. |
| 57 | DEHOVITZ J S, LOH Y Y, KAUTZKY J A, et al. Static to inducibly dynamic stereocontrol: the convergent use of racemic β-substituted ketones[J]. Science, 2020, 369(6507): 1113-1118. |
| 58 | LIU Y Y, ZHU L Y, LI X M, et al. Photoredox/enzymatic catalysis enabling redox-neutral decarboxylative asymmetric C-C coupling for asymmetric synthesis of chiral 1,2-amino alcohols[J]. JACS Au, 2023, 3(11): 3005-3013. |
| 59 | RUDZKA A, ANTOS N, REITER T, et al. One-pot sequential two-step photo-biocatalytic deracemization of sec-alcohols combining photocatalytic oxidation and bioreduction[J]. ACS Catalysis, 2024, 14(3): 1808-1823. |
| 60 | ZHANG W Y, FERNANDEZ-FUEYO E, NI Y, et al. Selective aerobic oxidation reactions using a combination of photocatalytic water oxidation and enzymatic oxyfunctionalizations[J]. Nature Catalysis, 2018, 1(1): 55-62. |
| 61 | WORTMAN A K, STEPHENSON C R J. EDA photochemistry: mechanistic investigations and future opportunities[J]. Chem, 2023, 9(9): 2390-2415. |
| 62 | ARCEO E, JURBERG I D, ÁLVAREZ-FERNANDEZ A, et al. Photochemical activity of a key donor-acceptor complex can drive stereoselective catalytic α-alkylation of aldehydes[J]. Nature Chemistry, 2013, 5(9): 750-756. |
| 63 | YUAN Y Q, MAJUMDER S, YANG M H, et al. Recent advances in catalyst-free photochemical reactions via electron-donor-acceptor (EDA) complex process[J]. Tetrahedron Letters, 2020, 61(8): 151506. |
| 64 | HARRISON W, HUANG X Q, ZHAO H M. Photobiocatalysis for abiological transformations[J]. Accounts of Chemical Research, 2022, 55(8): 1087-1096. |
| 65 | EMMANUEL M A, GREENBERG N R, OBLINSKY D G, et al. Accessing non-natural reactivity by irradiating nicotinamide-dependent enzymes with light[J]. Nature, 2016, 540(7633): 414-417. |
| 66 | PENG Y Z, WANG Z G, CHEN Y, et al. Photoinduced promiscuity of cyclohexanone monooxygenase for the enantioselective synthesis of α‐fluoroketones[J]. Angewandte Chemie International Edition, 2022, 61(50): e202211199. |
| 67 | BIEGASIEWICZ K F, COOPER S J, GAO X, et al. Photoexcitation of flavoenzymes enables a stereoselective radical cyclization[J]. Science, 2019, 364(6446): 1166-1169. |
| 68 | HUANG X Q, WANG B J, WANG Y J, et al. Photoenzymatic enantioselective intermolecular radical hydroalkylation[J]. Nature, 2020, 584(7819): 69-74. |
| 69 | LI M L, HARRISON W, ZHANG Z Y, et al. Remote stereocontrol with azaarenes via enzymatic hydrogen atom transfer[J]. Nature Chemistry, 2023: 1-8. |
| 70 | HUANG X Q, FENG J Q, CUI J W, et al. Photoinduced chemomimetic biocatalysis for enantioselective intermolecular radical conjugate addition[J]. Nature Catalysis, 2022, 5(7): 586-593. |
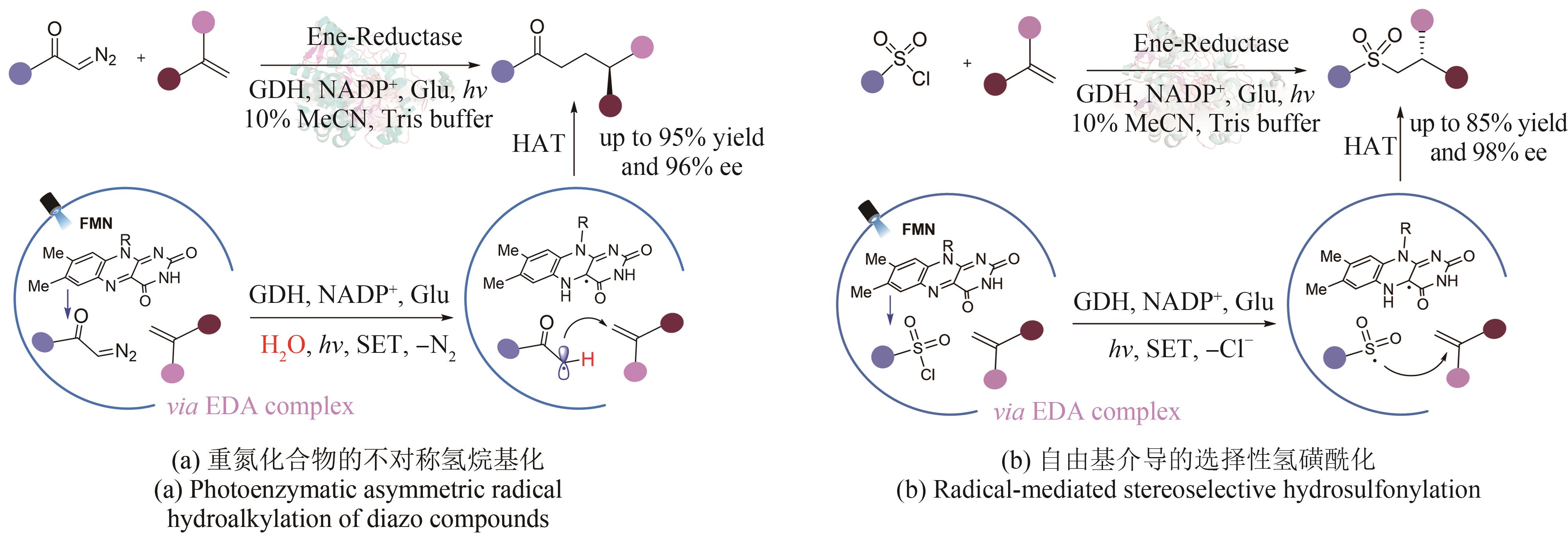
| 71 | ZHU C T, YUAN Z B, DENG Z W, et al. Photoenzymatic enantioselective synthesis of oxygen‐containing benzo‐fused heterocycles[J]. Angewandte Chemie, 2023: e202311762. |
| 72 | DUAN X Y, CUI D, WANG Z G, et al. A photoenzymatic strategy for radical‐mediated stereoselective hydroalkylation with diazo compounds[J]. Angewandte Chemie International Edition, 2023, 62(5): e202214135. |
| 73 | CHEN X Y, ZHENG D N, JIANG L Y, et al. Photoenzymatic hydrosulfonylation for the stereoselective synthesis of chiral sulfones[J]. Angewandte Chemie International Edition, 2023: e202218140. |
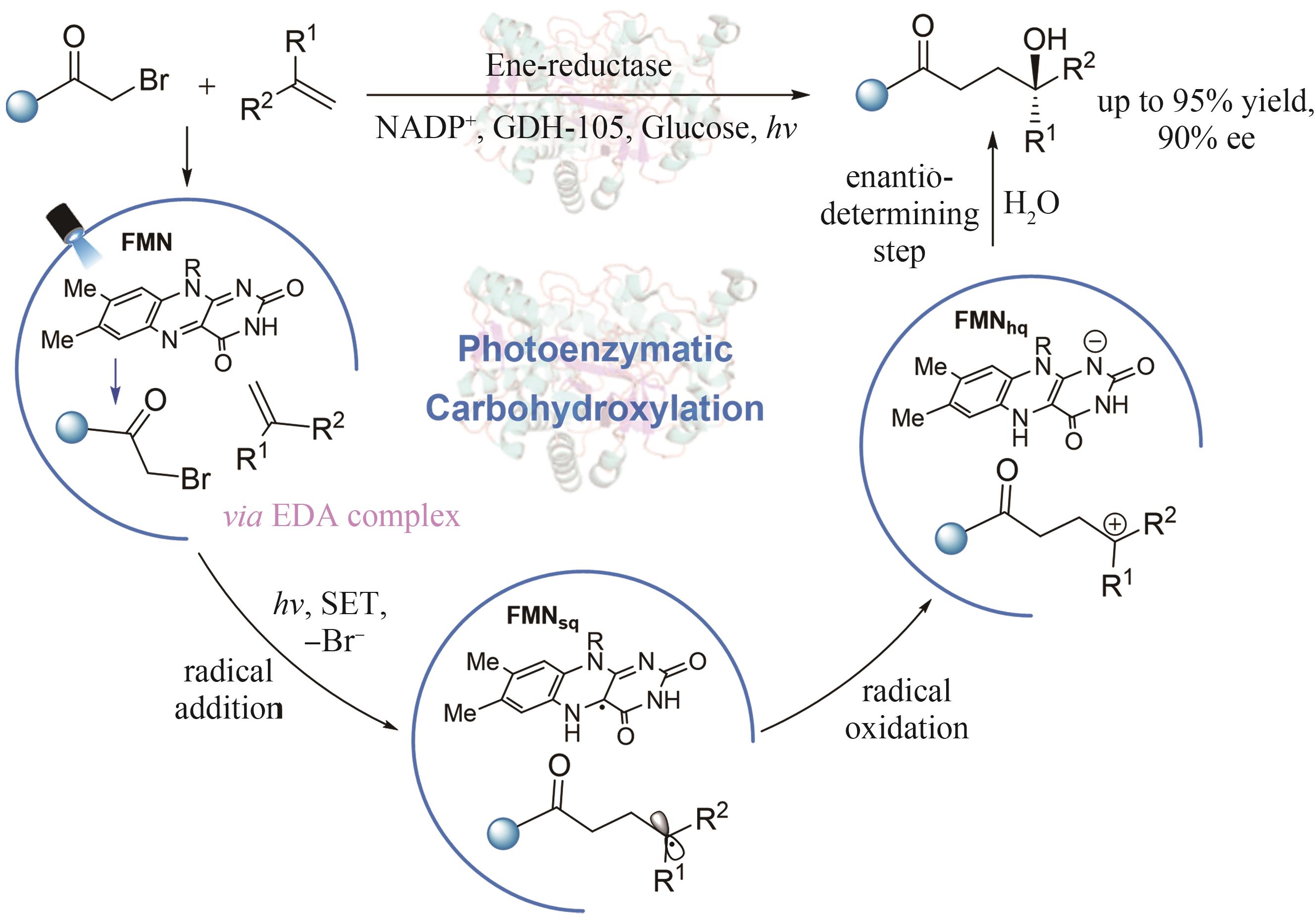
| 74 | OUYANG Y, TUREK-HERMAN J, QIAO T Z, et al. Asymmetric carbohydroxylation of alkenes using photoenzymatic catalysis[J]. Journal of the American Chemical Society, 2023, 145(31): 17018-17022. |
| 75 | FU H G, CAO J Z, QIAO T Z, et al. An asymmetric sp3-sp3 cross-electrophile coupling using ene-reductases[J]. Nature, 2022, 610(7931): 302-307. |
| 76 | FU H G, QIAO T Z, CARCELLER J M, et al. Asymmetric C-alkylation of nitroalkanes via enzymatic photoredox catalysis[J]. Journal of the American Chemical Society, 2023, 145(2): 787-793. |
| 77 | SIEGEL L M. Quantitative determination of noncovalently bound flavins: types and methods of analysis[M]. Methods in Enzymology. Academic Press, 1978, 53: 419-429. |
| 78 | GROSHEVA D, HYSTER T K. Light‐driven flavin‐based biocatalysis[J]. Flavin‐Based Catalysis: Principles and Applications, 2021: 291-313. |
| 79 | SANCAR A. Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors[J]. Chemical Reviews, 2003, 103(6): 2203-2238. |
| 80 | ZHANG M, WANG L J, ZHONG D P. Photolyase: dynamics and electron-transfer mechanisms of DNA repair[J]. Archives of Biochemistry and Biophysics, 2017, 632: 158-174. |
| 81 | CONRAD K S, MANAHAN C C, CRANE B R. Photochemistry of flavoprotein light sensors[J]. Nature Chemical Biology, 2014, 10(10): 801-809. |
| 82 | SORIGUE D, HADJIDEMETRIOU K, BLANGY S, et al. Mechanism and dynamics of fatty acid photodecarboxylase[J]. Science, 2021, 372(6538): eabd5687. |
| 83 | ZHANG W Y, MA M, HUIJBERS M M E, et al. Hydrocarbon synthesis via photoenzymatic decarboxylation of carboxylic acids[J]. Journal of the American Chemical Society, 2019, 141(7): 3116-3120. |
| 84 | XU J, FAN J J, LOU Y J, et al. Light-driven decarboxylative deuteration enabled by a divergently engineered photodecarboxylase[J]. Nature Communications, 2021, 12(1): 3983. |
| 85 | LI D Y, HAN T, XUE J D, et al. Engineering fatty acid photodecarboxylase to enable highly selective decarboxylation of trans fatty acids[J]. Angewandte Chemie, 2021, 133(38): 20863-20867. |
| 86 | AMER M, WOJCIK E Z, SUN C H, et al. Low carbon strategies for sustainable bio-alkane gas production and renewable energy[J]. Energy & Environmental Science, 2020, 13(6): 1818-1831. |
| 87 | QIN Z Y, ZHOU Y, LI Z, et al. Production of biobased ethylbenzene by cascade biocatalysis with an engineered photodecarboxylase[J]. Angewandte Chemie, e202314566. |
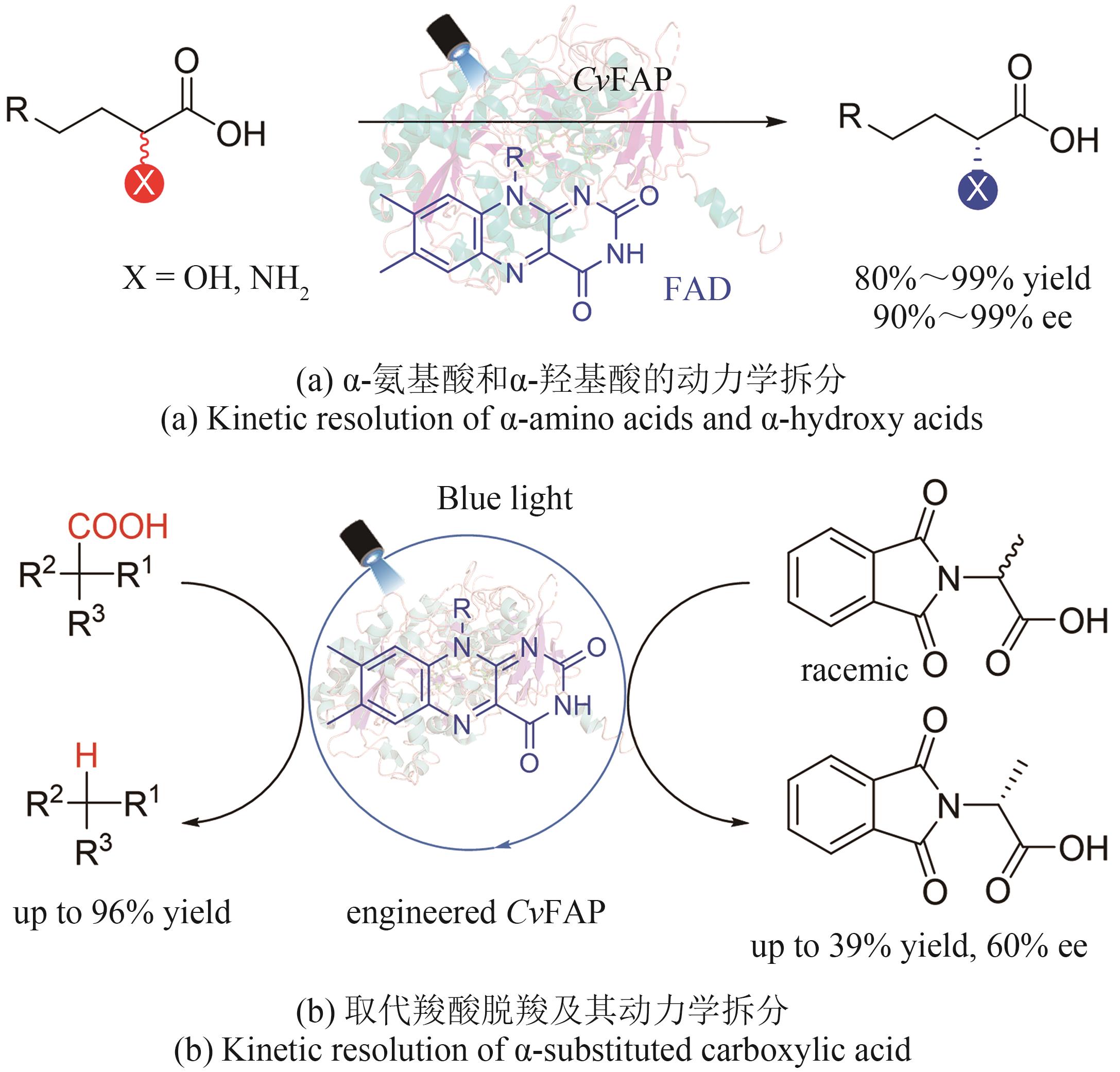
| 88 | XU J, HU Y J, FAN J J, et al. Light‐driven kinetic resolution of α‐functionalized carboxylic acids enabled by an engineered fatty acid photodecarboxylase[J]. Angewandte Chemie International Edition, 2019, 58(25): 8474-8478. |
| 89 | MOU K H, GUO Y, XU W H, et al. Stereodivergent protein engineering of fatty acid photodecarboxylase for light‐driven kinetic resolution of Sec‐alcohol oxalates[J]. Angewandte Chemie International Edition, 2024: e202318374. |
| 90 | ZHENG J, SHEN Z L, GAO J M, et al. Enzymatic photodecarboxylation on secondary and tertiary carboxylic acids[J]. Organic Letters, 2023, 25(48): 8564-8569. |
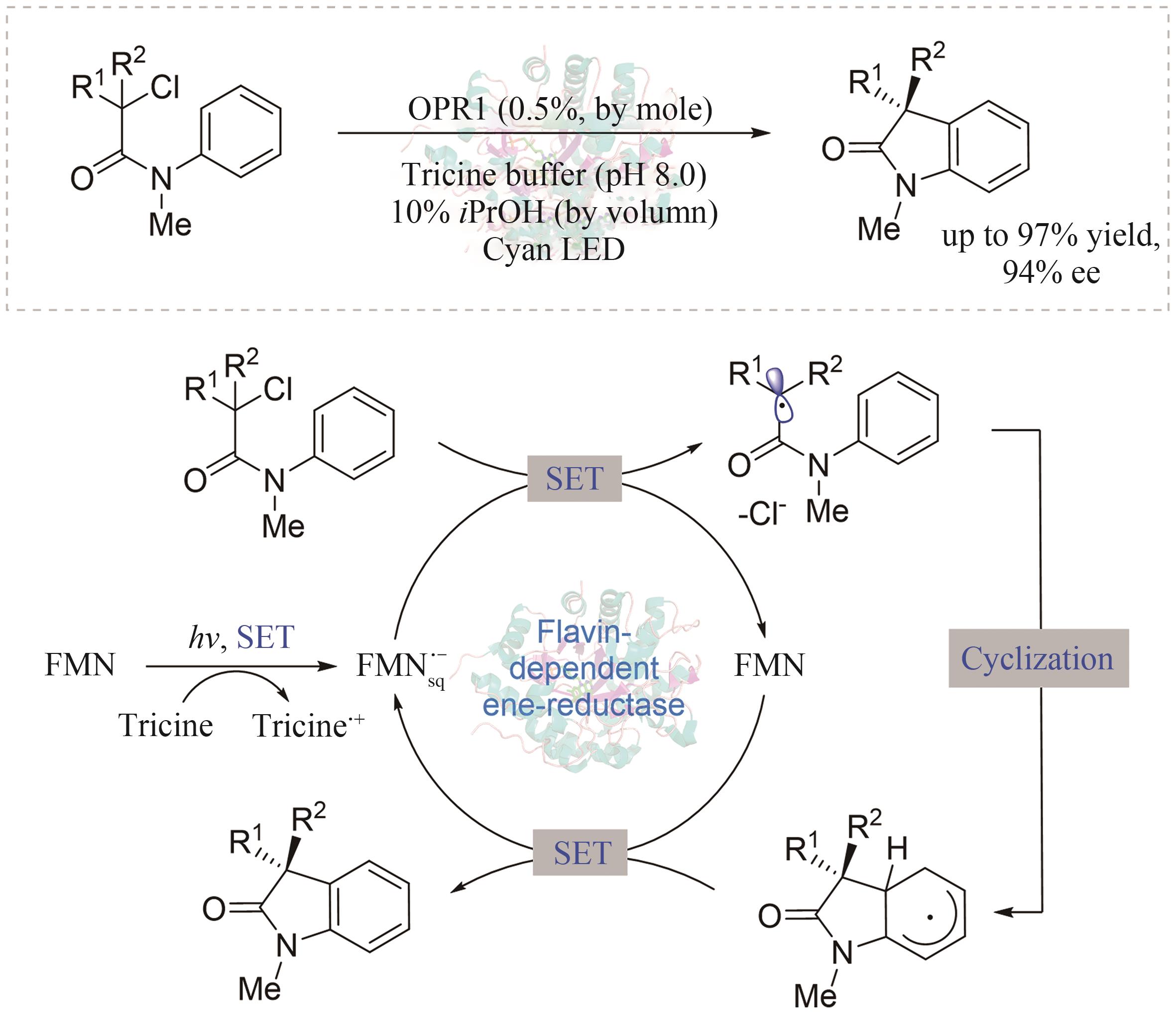
| 91 | BLACK M J, BIEGASIEWICZ K F, MEICHAN A J, et al. Asymmetric redox-neutral radical cyclization catalysed by flavin-dependent ene-reductases[J]. Nature Chemistry, 2020, 12(1): 71-75. |
| 92 | SANDOVAL B A, CLAYMAN P D, OBLINSKY D G, et al. Photoenzymatic reductions enabled by direct excitation of flavin-dependent ene-reductases[J]. Journal of the American Chemical Society, 2020, 143(4): 1735-1739. |
| 93 | ZHANG J W, ZHANG Q Y, CHEN B, et al. Photoenzymatic conversion of enamides to enantioenriched benzylic amines enabled by visible-light-induced single-electron reduction[J]. ACS Catalysis, 2023, 13(24): 15682-15690. |
| 94 | ZHAO B B, FENG J Q, YU L, et al. Direct visible-light-excited flavoproteins for redox-neutral asymmetric radical hydroarylation[J]. Nature Catalysis, 2023, 6(11): 996-1004. |
| 95 | SHI Q L, KANG X W, LIU Z Y, et al. Single-electron oxidation-initiated enantioselective hydrosulfonylation of olefins enabled by photoenzymatic catalysis[J]. Journal of the American Chemical Society, 2024, 146(4): 2748-2756. |
| 96 | DUTTA S, ERCHINGER J E, STRIETH-KALTHOFF F, et al. Energy transfer photocatalysis: exciting modes of reactivity[J]. Chemical Society Reviews, 2024, Advance Article. |
| 97 | POPLATA S, TROSTER A, ZOU Y Q, et al. Recent advances in the synthesis of cyclobutanes by olefin [2+2] photocycloaddition reactions[J]. Chemical Reviews, 2016, 116(17): 9748-9815. |
| 98 | KLEINMANS R, PINKERT T, DUTTA S, et al. Intermolecular [2π+2σ]-photocycloaddition enabled by triplet energy transfer[J]. Nature, 2022, 605(7910): 477-482. |
| 99 | MUNSTER N, PARKER N A, VAN DIJK L, et al. Visible light photocatalysis of 6π heterocyclization[J]. Angewandte Chemie International Edition, 2017, 56(32): 9468-9472. |
| 100 | HUANG M X, ZHANG L, PAN T R, et al. Deracemization through photochemical E/Z isomerization of enamines[J]. Science, 2022, 375(6583): 869-874. |
| 101 | BLUM T R, MILLER Z D, BATES D M, et al. Enantioselective photochemistry through lewis acid-catalyzed triplet energy transfer[J]. Science, 2016, 354(6318): 1391-1395. |
| 102 | ALONSO R, BACH T. A chiral thioxanthone as an organocatalyst for enantioselective [2+2] photocycloaddition reactions induced by visible light[J]. Angewandte Chemie, 2014, 126(17): 4457-4460. |
| 103 | LI X Y, GROBKOPF J, JANDL C, et al. Enantioselective, visible light mediated aza Paternò-Büchi reactions of quinoxalinones[J]. Angewandte Chemie, 2021, 133(5): 2716-2720. |
| 104 | HOLZL-HOBMEIER A, BAUER A, SILVA A V, et al. Catalytic deracemization of chiral allenes by sensitized excitation with visible light[J]. Nature, 2018, 564(7735): 240-243. |
| 105 | NOREN C J, ANTHONY-CAHILL S J, GRIFFITH M C, et al. A general method for site-specific incorporation of unnatural amino acids into proteins[J]. Science, 1989, 244(4901): 182-188. |
| 106 | WANG L, SCHULTZ P G. Expanding the genetic code[J]. Angewandte Chemie International Edition, 2005, 44(1): 34-66. |
| 107 | RYU Y, SCHULTZ P G. Efficient incorporation of unnatural amino acids into proteins in Escherichia coli [J]. Nature Methods, 2006, 3(4): 263-265. |
| 108 | DRIENOVSKA I, ROELFES G. Expanding the enzyme universe with genetically encoded unnatural amino acids[J]. Nature Catalysis, 2020, 3(3): 193-202. |
| 109 | LANG K, CHIN J W. Cellular incorporation of unnatural amino acids and bioorthogonal labeling of proteins[J]. Chemical Reviews, 2014, 114(9): 4764-4806. |
| 110 | YU Y, LIU X H, WANG J Y. Expansion of redox chemistry in designer metalloenzymes[J]. Accounts of Chemical Research, 2019, 52(3): 557-565. |
| 111 | STRIETH-KALTHOFF F, JAMES M J, TEDERS M, et al. Energy transfer catalysis mediated by visible light: principles, applications, directions[J]. Chemical Society Reviews, 2018, 47(19): 7190-7202. |
| 112 | LIU X H, KANG F Y, HU C, et al. A genetically encoded photosensitizer protein facilitates the rational design of a miniature photocatalytic CO2-reducing enzyme[J]. Nature Chemistry, 2018, 10(12): 1201-1206. |
| 113 | FU Y, HUANG J, WU Y Z, et al. Biocatalytic cross-coupling of aryl halides with a genetically engineered photosensitizer artificial dehalogenase[J]. Journal of the American Chemical Society, 2021, 143(2): 617-622. |
| 114 | SUN N N, HUANG J J, QIAN J Y, et al. Enantioselective [2+2]-cycloadditions with triplet photoenzymes[J]. Nature, 2022, 611(7937): 715-720. |
| 115 | TRIMBLE J S, CRAWSHAW R, HARDY F J, et al. A designed photoenzyme for enantioselective [2+2] cycloadditions[J]. Nature, 2022, 611(7937): 709-714. |
| 116 | ROELFES G. LmrR: a privileged scaffold for artificial metalloenzymes[J]. Accounts of Chemical Research, 2019, 52(3): 545-556. |
| 117 | DRIENOVSKA I, MAYER C, DULSON C, et al. A designer enzyme for hydrazone and oxime formation featuring an unnatural catalytic aniline residue[J]. Nature Chemistry, 2018, 10(9): 946-952. |
| 118 | LEWIS J C. Beyond the second coordination sphere: engineering dirhodium artificial metalloenzymes to enable protein control of transition metal catalysis[J]. Accounts of Chemical Research, 2019, 52(3): 576-584. |
| 119 | SANDOVAL B A, HYSTER T K. Emerging strategies for expanding the toolbox of enzymes in biocatalysis[J]. Current Opinion in Chemical Biology, 2020, 55: 45-51. |
| 120 | GU Y F, ELLIS‐GUARDIOLA K, SRIVASTAVA P, et al. Preparation, characterization, and oxygenase activity of a photocatalytic artificial enzyme[J]. ChemBioChem, 2015, 16(13): 1880-1883. |
| 121 | LIU B Q, ZUBI Y S, LEWIS J C. Iridium(Ⅲ) polypyridine artificial metalloenzymes with tunable photophysical properties: a new platform for visible light photocatalysis in aqueous solution[J]. Dalton Transactions, 2023, 52(16): 5034-5038. |
| 122 | FU Y, LIU X H, XIA Y, et al. Whole-cell-catalyzed hydrogenation/deuteration of aryl halides with a genetically repurposed photodehalogenase[J]. Chem, 2023, 9(7): 1897-1909. |
| 123 | WACHTMEISTER J, ROTHER D. Recent advances in whole cell biocatalysis techniques bridging from investigative to industrial scale[J]. Current Opinion in Biotechnology, 2016, 42: 169-177. |
| [1] | 汤志军, 胡友财, 刘文. 酶促4+2和2+2环加成反应:区域与立体选择性的理解与应用[J]. 合成生物学, 2024, 5(3): 401-407. |
| [2] | 孙梦楚, 陆亮宇, 申晓林, 孙新晓, 王佳, 袁其朋. 基于荧光检测的高通量筛选技术和装备助力细胞工厂构建[J]. 合成生物学, 2023, 4(5): 947-965. |
| [3] | 明阳, 陈彬, 黄小强. 光酶催化合成进展[J]. 合成生物学, 2023, 4(4): 651-675. |
| [4] | 康里奇, 谈攀, 洪亮. 人工智能时代下的酶工程[J]. 合成生物学, 2023, 4(3): 524-534. |
| [5] | 阮青云, 黄莘, 孟子钧, 全舒. 蛋白质稳定性计算设计与定向进化前沿工具[J]. 合成生物学, 2023, 4(1): 5-29. |
| [6] | 祁延萍, 朱晋, 张凯, 刘彤, 王雅婕. 定向进化在蛋白质工程中的应用研究进展[J]. 合成生物学, 2022, 3(6): 1081-1108. |
| [7] | 唐宇琦, 叶松涛, 刘嘉, 张鑫. 分子伴侣作用下的蛋白质稳定与进化[J]. 合成生物学, 2022, 3(3): 445-464. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||