• •
蛋白质优化设计与从头合成引领的疫苗研发革命
陈涛1,2, 赖锦涛1, 胡美林1, 马显才1,2
- 1.广州国家实验室,广东 广州 510005
2.中山大学,中山医学院,广东 广州 510080
-
收稿日期:2025-06-30修回日期:2025-08-19出版日期:2025-08-20 -
通讯作者:马显才 -
作者简介:陈涛 (2002—),男,博士研究生。研究方向为抗呼吸道病毒新型纳米颗粒疫苗研发。E-mail:chen_tao02@gzlab.ac.cn赖锦涛 (2001—),男,研究实习员。研究方向为蛋白类仿生纳米材料的人工智能优化与设计。E-mail:lai_jintao@gzlab.ac.cn马显才 (1991—),男,研究员,博士生导师。研究方向为病毒与宿主互作的机制研究及其抗病毒纳米颗粒疫苗研发。E-mail:ma_xiancai@gzlab.ac.cn -
基金资助:广州国家实验室专项项目(GZNL2024A01017);广东省基础与应用基础研究基金项目(2024B1515020068)
Revolution in vaccine development led by protein optimization design and de novo synthesis
CHEN Tao1,2, LAI Jingtao1, HU Meilin1, MA Xiancai1,2
- 1.Guangzhou National Laboratory,Guangzhou 510005,Guangdong,China
2.Zhongshan School of Medicine,Sun Yat-sen University,Guangzhou 510080,Guangdong,China
-
Received:2025-06-30Revised:2025-08-19Online:2025-08-20 -
Contact:MA Xiancai
摘要:
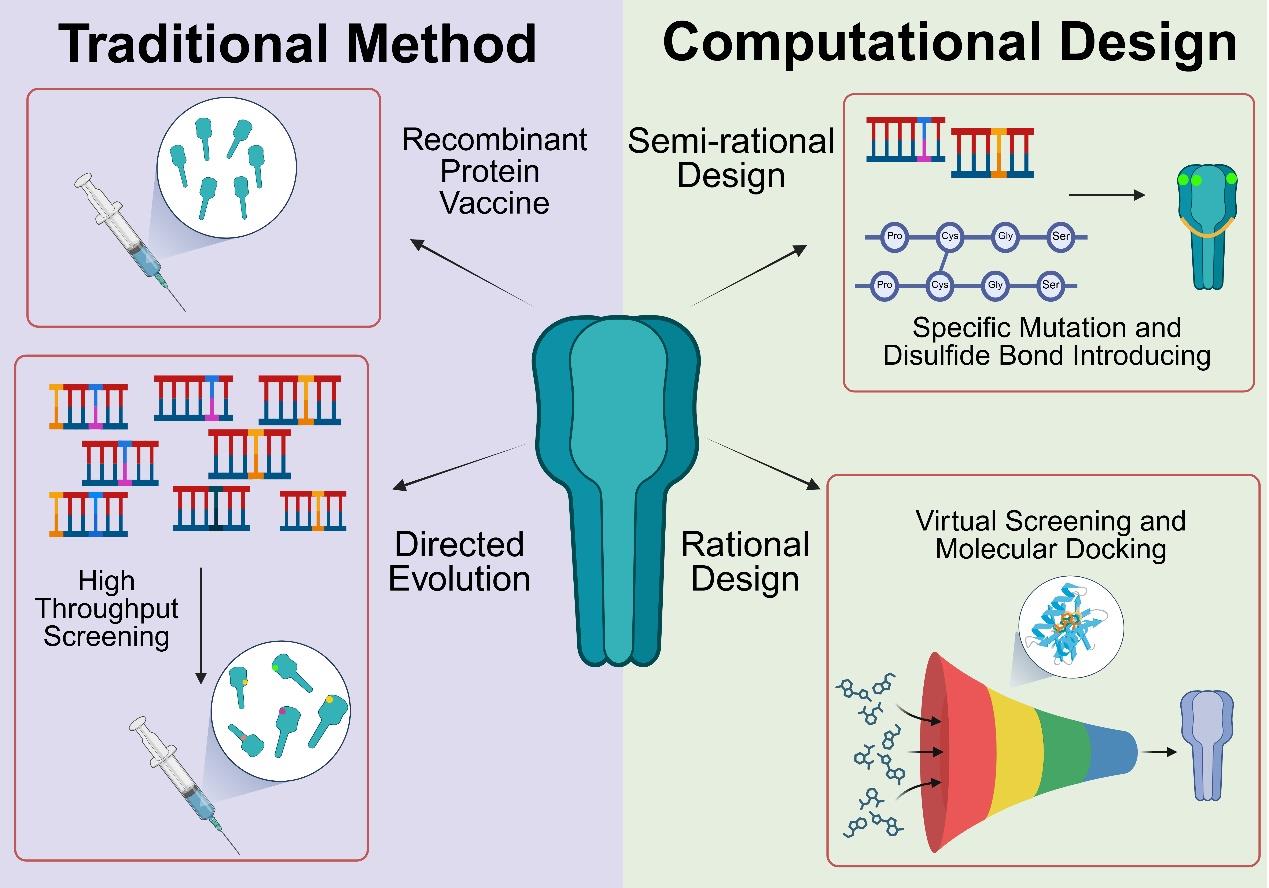
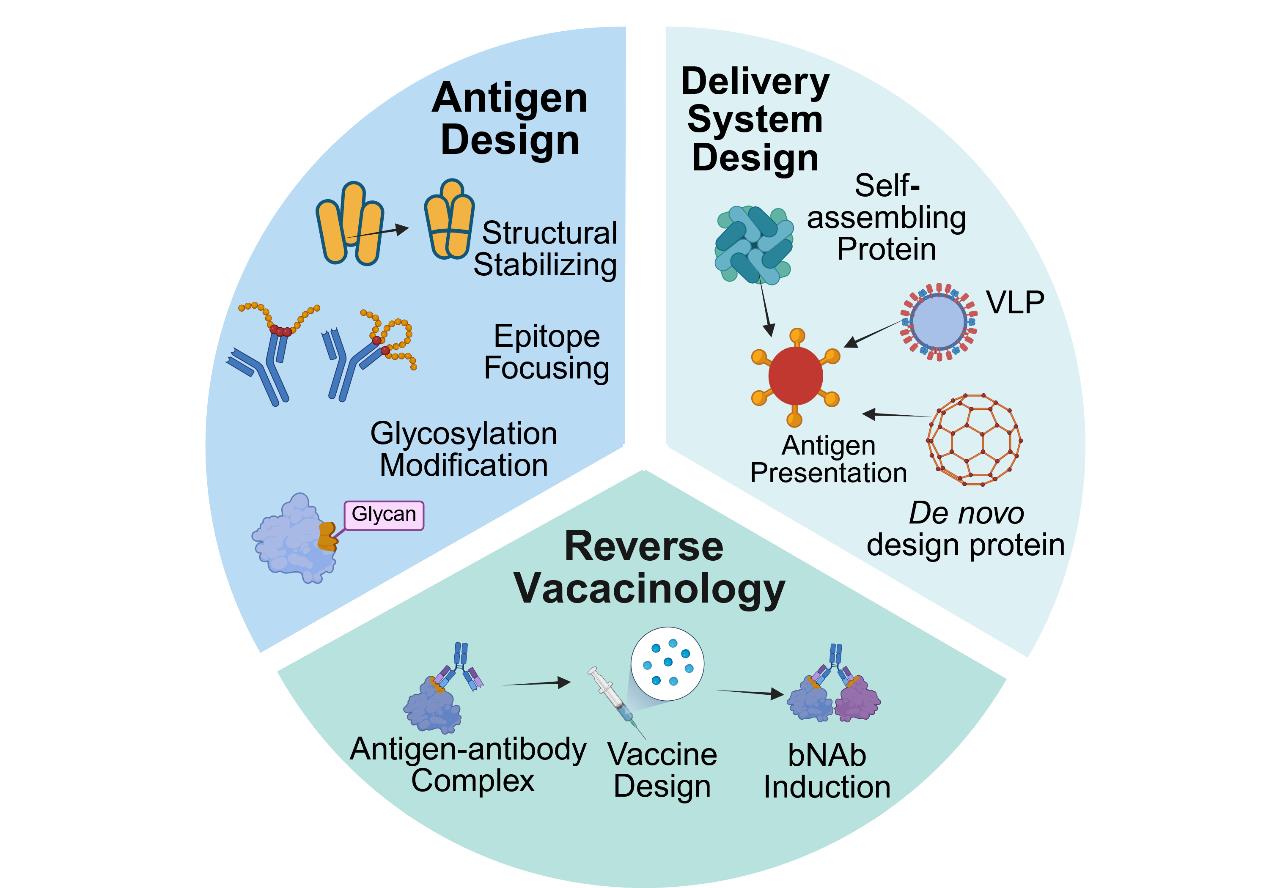
疫苗作为防控传染性疾病的有力手段,在发展应用过程中经历了四次重要革命。近年来计算工具的迅猛发展更是推动疫苗研发走向新的阶段,形成以结构为导向,蛋白质优化与计算设计为核心的合成生物学研究范式。本文系统介绍了蛋白质优化设计中定向进化、半理性设计和理性设计三种策略与从头合成技术在疫苗研发中的应用与价值。在免疫原设计层面,介绍了结构稳定性改造、表位聚焦、糖基化修饰调控等策略对提升抗原免疫原性与广谱性等特性方面的潜力。在递送系统层面,介绍了蛋白纳米颗粒凭借高密度抗原展示与几何构象优势,结合“马赛克”多价展示技术,对诱导交叉中和抗体生成方面的优势。人工智能计算工具的突破性进展实现了从“结构模拟”到“功能定制”的转变,并极大地推动了以抗原-抗体复合物结构为导向的反向疫苗学的发展。整合表位计算筛选与从头蛋白骨架设计,实现了疫苗从天然结构到定制结构的突破。尽管面临高变异病原广谱保护、动态构象模拟等挑战,疫苗设计与计算工具的深度融合加速了新型冠状病毒、呼吸道合胞病毒等疫苗的临床转化,并为未来新发和突发传染病防控提供了通用设计方法。
中图分类号:
引用本文
陈涛, 赖锦涛, 胡美林, 马显才. 蛋白质优化设计与从头合成引领的疫苗研发革命[J]. 合成生物学, DOI: 10.12211/2096-8280.2025-068.
CHEN Tao, LAI Jingtao, HU Meilin, MA Xiancai. Revolution in vaccine development led by protein optimization design and de novo synthesis[J]. Synthetic Biology Journal, DOI: 10.12211/2096-8280.2025-068.
| 计算软件 | 开发时间 | 主要用途 | 核心特点 |
|---|---|---|---|
| MODELLER[ | 1993年 | 基于同源建模的蛋白质三维结构预测,优化侧链构象。 | 依赖已知同源模板,引入空间约束进行优化,适用于结构补全与局部优化。 |
| Rosetta[ | 1998年 | 已知蛋白质稳定性增强、活性位点改造,全新结构蛋白的构建、功能位点设计。 | 基于物理力场与蒙特卡洛搜索,支持大规模构象采样,是蛋白质从头设计的里程碑式工具。 |
| FoldX[ | 2005年 | 快速评估点突变对蛋白质稳定性、结合亲和力的影响。 | 经验力场结合机器学习,适用于高通量虚拟突变筛选。 |
| D-I-TASSER[ | 2008年 | 基于模板的蛋白质结构预测与功能注释,间接支持优化设计。 | 整合多重线程算法和分子动力学优化,提供从结构到功能的综合分析。 |
| AlphaFold[ | 2018年 | 高精度蛋白质结构预测。 | 首次将深度学习大规模应用于结构预测,显著提升精度。 |
| AlphaFold 2[ | 2020年 | 革命性的高精度蛋白质单链及复合物结构预测。 | 基于注意力机制的端到端模型,预测精度接近实验水平,开源后推动结构生物学变革。 |
| RoseTTAFold[ | 2021年 | 结构预测与部分设计功能,支持蛋白质-蛋白质复合物建模。 | 三轨神经网络(序列-结构-进化信息协同处理),计算效率高,可建模蛋白质-蛋白质相互作用。 |
| ProteinMPNN[ | 2022年 | 蛋白质序列设计,根据目标结构生成最优氨基酸序列。 | 基于图神经网络,设计速度更高,支持对称性设计和功能位点约束。 |
| RFdiffusion[ | 2023年 | 从头生成功能性蛋白质结构。 | 基于扩散模型生成三维结构,可直接融入功能约束,开创设计新范式。 |
| AlphaFold3[ | 2024年 | 预测蛋白质与核酸、配体、修饰等的复合结构,支持更广泛的分子相互作用建模。 | 统一框架处理蛋白质+生物分子复合系统,显著提升配体结合位点预测准确性,推动药物设计发展。 |
表1 计算设计的常用软件总结
Table 1 Overview of commonly-used software for computational design
| 计算软件 | 开发时间 | 主要用途 | 核心特点 |
|---|---|---|---|
| MODELLER[ | 1993年 | 基于同源建模的蛋白质三维结构预测,优化侧链构象。 | 依赖已知同源模板,引入空间约束进行优化,适用于结构补全与局部优化。 |
| Rosetta[ | 1998年 | 已知蛋白质稳定性增强、活性位点改造,全新结构蛋白的构建、功能位点设计。 | 基于物理力场与蒙特卡洛搜索,支持大规模构象采样,是蛋白质从头设计的里程碑式工具。 |
| FoldX[ | 2005年 | 快速评估点突变对蛋白质稳定性、结合亲和力的影响。 | 经验力场结合机器学习,适用于高通量虚拟突变筛选。 |
| D-I-TASSER[ | 2008年 | 基于模板的蛋白质结构预测与功能注释,间接支持优化设计。 | 整合多重线程算法和分子动力学优化,提供从结构到功能的综合分析。 |
| AlphaFold[ | 2018年 | 高精度蛋白质结构预测。 | 首次将深度学习大规模应用于结构预测,显著提升精度。 |
| AlphaFold 2[ | 2020年 | 革命性的高精度蛋白质单链及复合物结构预测。 | 基于注意力机制的端到端模型,预测精度接近实验水平,开源后推动结构生物学变革。 |
| RoseTTAFold[ | 2021年 | 结构预测与部分设计功能,支持蛋白质-蛋白质复合物建模。 | 三轨神经网络(序列-结构-进化信息协同处理),计算效率高,可建模蛋白质-蛋白质相互作用。 |
| ProteinMPNN[ | 2022年 | 蛋白质序列设计,根据目标结构生成最优氨基酸序列。 | 基于图神经网络,设计速度更高,支持对称性设计和功能位点约束。 |
| RFdiffusion[ | 2023年 | 从头生成功能性蛋白质结构。 | 基于扩散模型生成三维结构,可直接融入功能约束,开创设计新范式。 |
| AlphaFold3[ | 2024年 | 预测蛋白质与核酸、配体、修饰等的复合结构,支持更广泛的分子相互作用建模。 | 统一框架处理蛋白质+生物分子复合系统,显著提升配体结合位点预测准确性,推动药物设计发展。 |
| [1] | MORENS D M, TAUBENBERGER J K. Influenza Cataclysm, 1918 [J]. New England Journal of Medicine, 2018, 379(24): 2285-2287. |
| [2] | DONNELLY C A, GHANI A C, LEUNG G M, et al. Epidemiological Determinants of Spread of Causal Agent of Severe Acute Respiratory Syndrome in Hong Kong [J]. The Lancet, 2003, 361(9371): 1761-1766. |
| [3] | ARABI Y M, BALKHY H H, HAYDEN F G, et al. Middle East Respiratory Syndrome [J]. New England Journal of Medicine, 2017, 376(6): 584-594. |
| [4] | MSEMBURI W, KARLINSKY A, KNUTSON V, et al. The WHO estimates of excess mortality associated with the COVID-19 pandemic[J]. Nature, 2023, 613(7942): 130-137. |
| [5] | SCHUMACHER A E, KYU H H, AALI A, et al. Global age-sex-specific mortality, life expectancy, and population estimates in 204 countries and territories and 811 subnational locations, 1950–2021, and the impact of the COVID-19 pandemic: a comprehensive demographic analysis for the Global Burden of Disease Study 2021[J]. The Lancet, 2024, 403(10440): 1989-2056. |
| [6] | MURA M, TRIGNOL A, LE DAULT E, et al. Lessons for medical countermeasure development from unforeseen outbreaks[J]. Emerging Microbes & Infections, 2025, 14(1): 2471035. |
| [7] | IWASAKI A, OMER S B. Why and How Vaccines Work [J]. Cell, 2020, 183(2): 290-295. |
| [8] | RAPPUOLI R, ALTER G, PULENDRAN B. Transforming Vaccinology [J]. Cell, 2024, 187(19): 5171-5194. |
| [9] | FINE P E. Variation in Protection by BCG: Implications of and for Heterologous Immunity [J]. The Lancet, 1995, 346(8986): 1339-1345. |
| [10] | FOURNIER J M. [The Current Status of Research on a Cholera Vaccine] [J]. Bulletin de la Société de Pathologie Exotique, 1998, 91(5 Pt 1-2): 412-415. |
| [11] | GERBERDING J L, HAYNES B F. Vaccine Innovations - Past and Future [J]. New England Journal of Medicine, 2021, 384(5): 393-396. |
| [12] | RAPPUOLI R. Reverse Vaccinology [J]. Current Opinion in Microbiology, 2000, 3(5): 445-450. |
| [13] | PULENDRAN B, LI S, NAKAYA H I. Systems Vaccinology [J]. Immunity, 2010, 33(4): 516-529. |
| [14] | WANG Y, XUE P, CAO M, et al. Directed Evolution: Methodologies and Applications [J]. Chemical Reviews, 2021, 121(20): 12384-12444. |
| [15] | PEISAJOVICH S G, TAWFIK D S. Protein Engineers Turned Evolutionists [J]. Nature Methods, 2007, 4(12): 991-994. |
| [16] | PACKER M S, LIU D R. Methods for the Directed Evolution of Proteins [J]. Nature Reviews Genetics, 2015, 16(7): 379-394. |
| [17] | TOKURIKI N, JACKSON C J, AFRIAT-JURNOU L, et al. Diminishing Returns and Tradeoffs Constrain the Laboratory Optimization of an Enzyme [J]. Nature Communications, 2012, 3(1): 1257. |
| [18] | DADONAITE B, AHN J J, ORT J T, et al. Deep Mutational Scanning of H5 Hemagglutinin to Inform Influenza Virus Surveillance [J]. bioRxiv, 2024. |
| [19] | DADONAITE B, BROWN J, MCMAHON T E, et al. Spike Deep Mutational Scanning Helps Predict Success of SARS-CoV-2 Clades [J]. Nature, 2024, 631(8021): 617-626. |
| [20] | CAO Y, JIAN F, WANG J, et al. Imprinted SARS-CoV-2 Humoral Immunity Induces Convergent Omicron RBD Evolution [J]. Nature, 2023, 614(7948): 521-529. |
| [21] | WANG E, COHEN A A, CALDERA L F, et al. Designed Mosaic Nanoparticles Enhance Cross-reactive Immune Responses in Mice [J]. Cell, 2025, 188(4): 1036-1050.e11. |
| [22] | LUTZ S. Beyond Directed Evolution - Semi-rational Protein Engineering and Design [J]. Current Opinion in Biotechnology, 2010, 21(6): 734-743. |
| [23] | GOLDENZWEIG A, FLEISHMAN S J. Principles of Protein Stability and Their Application in Computational Design [J]. Annual Review of Biochemistry, 2018, 87: 105-129. |
| [24] | TOKURIKI N, TAWFIK D S. Stability Effects of Mutations and Protein Evolvability [J]. Current Opinion in Structural Biology, 2009, 19(5): 596-604. |
| [25] | LISTOV D, GOVERDE C A, CORREIA B E, et al. Opportunities and Challenges in Design and Optimization of Protein Function [J]. Nature Reviews Molecular Cell Biology, 2024, 25(8): 639-653. |
| [26] | PING X, HU W, XIONG R, et al. Generation of a Broadly Reactive Influenza H1 Antigen Using a Consensus HA Sequence [J]. Vaccine, 2018, 36(32, Part B): 4837-4845. |
| [27] | ZHAO Y, NI W, LIANG S, et al. Vaccination with S(pan), an Antigen Guided by SARS-CoV-2 S Protein Evolution, Protects Against Challenge with Viral Variants in Mice [J]. Science Translational Medicine, 2023, 15(677): eabo3332. |
| [28] | YANG K K, WU Z, ARNOLD F H. Machine-learning-guided Directed Evolution for Protein Engineering [J]. Nature Methods, 2019, 16(8): 687-694. |
| [29] | CHE Y, GRIBENKO A V, SONG X, et al. Rational Design of a Highly Immunogenic Prefusion-stabilized F Glycoprotein Antigen for a Respiratory Syncytial Virus Vaccine [J]. Science Translational Medicine, 2023, 15(693): eade6422. |
| [30] | LIU G, CARTER B, BRICKEN T, et al. Computationally Optimized SARS-CoV-2 MHC Class I and II Vaccine Formulations Predicted to Target Human Haplotype Distributions [J]. Cell Systems, 2020, 11(2): 131-144.e6. |
| [31] | XIONG W, LIU B, SHEN Y, et al. Protein Engineering Design from Directed Evolution to de Novo Synthesis [J]. Biochemical Engineering Journal, 2021, 174: 108096. |
| [32] | ROMERO-RIVERA A, GARCIA-BORRàS M, OSUNA S. Computational Tools for the Evaluation of Laboratory-engineered Biocatalysts [J]. Chemical Communications, 2016, 53(2): 284-297. |
| [33] | WINNIFRITH A, OUTEIRAL C, HIE B L. Generative Artificial Intelligence for de Novo Protein Design [J]. Current Opinion in Structural Biology, 2024, 86: 102794. |
| [34] | DING W, NAKAI K, GONG H. Protein Design via Deep Learning [J]. Briefings in Bioinformatics, 2022, 23(3) : bbac102. |
| [35] | ANAND N, EGUCHI R, MATHEWS I I, et al. Protein Sequence Design with a Learned Potential [J]. Nature Communications, 2022, 13(1): 746. |
| [36] | WATSON J L, JUERGENS D, BENNETT N R, et al. De Novo Design of Protein Structure and Function with RFdiffusion [J]. Nature, 2023, 620(7976): 1089-1100. |
| [37] | HUANG P-S, BOYKEN S E, BAKER D. The Coming of Age of de Novo Protein Design [J]. Nature, 2016, 537(7620): 320-327. |
| [38] | KORENDOVYCH I V, DEGRADO W F. De Novo Protein Design, a Retrospective [J]. Quarterly Reviews of Biophysics, 2020, 53: e3. |
| [39] | QUINN T P, TWEEDY N B, WILLIAMS R W, et al. Betadoublet: de Novo Design, Synthesis, and Characterization of a Beta-sandwich Protein [J]. Proceedings of the National Academy of Sciences of the United States of America, 1994, 91(19): 8747-8751. |
| [40] | DEGRADO W F, REGAN L, HO S P. The Design of a Four-helix Bundle Protein [J]. Cold Spring Harbor Symposia on Quantitative Biology, 1987, 52: 521-526. |
| [41] | REGAN L, DEGRADO W F. Characterization of a Helical Protein Designed from First Principles [J]. Science, 1988, 241(4868): 976-978. |
| [42] | HANDEL T M, WILLIAMS S A, DEGRADO W F. Metal Ion-dependent Modulation of the Dynamics of a Designed Protein [J]. Science, 1993, 261(5123): 879-885. |
| [43] | KUHLMAN B, DANTAS G, IRETON G C, et al. Design of a Novel Globular Protein Fold with Atomic-level Accuracy [J]. Science, 2003, 302(5649): 1364-1368. |
| [44] | HAYES T, RAO R, AKIN H, et al. Simulating 500 Million Years of Evolution with a Language Model [J]. Science, 2025, 387(6736): 850-858. |
| [45] | CAO L, GORESHNIK I, COVENTRY B, et al. De Novo Design of Picomolar SARS-CoV-2 Miniprotein Inhibitors [J]. Science, 2020, 370(6515): 426-431. |
| [46] | CAO L, COVENTRY B, GORESHNIK I, et al. Design of Protein-binding Proteins from the Target Structure Alone [J]. Nature, 2022, 605(7910): 551-560. |
| [47] | SESTERHENN F, YANG C, BONET J, et al. De Novo Protein Design Enables the Precise Induction of RSV-neutralizing Antibodies [J]. Science, 2020, 368(6492): eaay5051. |
| [48] | AHMAD S, DEMNEH F M, REHMAN B, et al. In Silico Design of a Novel Multi-epitope Vaccine Against HCV Infection through Immunoinformatics Approaches [J]. International Journal of Biological Macromolecules, 2024, 267(Pt 2): 131517. |
| [49] | SABZIAN-MOLAEI F, AHMADI M A, NIKFARJAM Z, et al. Inactivation of Cell-free HIV-1 by Designing Potent Peptides Based on Mutations in the CD4 Binding Site [J]. Medical & Biological Engineering & Computing, 2024, 62(2): 423-436. |
| [50] | KOZAKOV D, HALL D R, XIA B, et al. The ClusPro Web Server for Protein–protein Docking [J]. Nature Protocols, 2017, 12(2): 255-278. |
| [51] | FILIPOVIC J, VAVRA O, PLHAK J, et al. CaverDock: A Novel Method for the Fast Analysis of Ligand Transport [J]. IEEE/ACM Transactions on Computational Biology and Bioinformatics, 2020, 17(5): 1625-1638. |
| [52] | VREVEN T, VANGAVETI S, BORRMAN T M, et al. Performance of ZDOCK and IRAD in CAPRI Rounds 39-45 [J]. Proteins: Structure, Function, and Bioinformatics, 2020, 88(8): 1050-1054. |
| [53] | HONORATO R V, TRELLET M E, JIMéNEZ-GARCíA B, et al. The HADDOCK2.4 Web Server for Integrative Modeling of Biomolecular Complexes [J]. Nature Protocols, 2024, 19(11): 3219-3241. |
| [54] | ŠALI A, BLUNDELL T L. Comparative Protein Modelling by Satisfaction of Spatial Restraints [J]. Journal of Molecular Biology, 1993, 234(3): 779-815. |
| [55] | WEBB B, SALI A. Comparative Protein Structure Modeling Using MODELLER [J]. Current Protocols in Bioinformatics, 2016, 54: 5.6.1-5.6.37. |
| [56] | LEAVER-FAY A, TYKA M, LEWIS S M, et al. ROSETTA3: An Object-oriented Software Suite for the Simulation and Design of Macromolecules [J]. Methods in Enzymology, 2011, 487: 545-574. |
| [57] | GUEROIS R, NIELSEN J E, SERRANO L. Predicting Changes in the Stability of Proteins and Protein Complexes: A Study of More Than 1000 Mutations [J]. Journal of Molecular Biology, 2002, 320(2): 369-387. |
| [58] | ROY A, KUCUKURAL A, ZHANG Y. I-TASSER: A Unified Platform for Automated Protein Structure and Function Prediction [J]. Nature Protocols, 2010, 5(4): 725-738. |
| [59] | ZHENG W, WUYUN Q, LI Y, et al. Deep-learning-based single-domain and multidomain protein structure prediction with D-I-TASSER [J]. Nature Biotechnology, 2025. |
| [60] | JUMPER J, EVANS R, PRITZEL A, et al. Highly Accurate Protein Structure Prediction with AlphaFold [J]. Nature, 2021, 596(7873): 583-589. |
| [61] | BAEK M, DIMAIO F, ANISHCHENKO I, et al. Accurate Prediction of Protein Structures and Interactions Using a Three-track Neural Network [J]. Science, 2021, 373(6557): 871-876. |
| [62] | SUMIDA K H, NúñEZ-FRANCO R, KALVET I, et al. Improving Protein Expression, Stability, and Function with ProteinMPNN [J]. Journal of the American Chemical Society, 2024, 146(3): 2054-2061. |
| [63] | ABRAMSON J, ADLER J, DUNGER J, et al. Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3 [J]. Nature, 2024, 630(8016): 493-500. |
| [64] | SENIOR A W, EVANS R, JUMPER J, et al. Improved Protein Structure Prediction Using Potentials from Deep Learning [J]. Nature, 2020, 577: 706-710. |
| [65] | ALFORD R F, et al. The Rosetta All-atom Energy Function for Macromolecular Modeling and Design [J]. Journal of Chemical Theory and Computation, 2017, 13: 3031-3048. |
| [66] | ADOLF-BRYFOGLE J, KALYUZHNIY O, KUBITZ M, et al. RosettaAntibodyDesign (RAbD): A General Framework for Computational Antibody Design [J]. PLoS Computational Biology, 2018, 14(4): e1006112. |
| [67] | BURTON D R. Antibodies, Viruses and Vaccines [J]. Nature Reviews Immunology, 2002, 2(9): 706-713. |
| [68] | OSCHERWITZ J. The Promise and Challenge of Epitope-focused Vaccines [J]. Human Vaccines & Immunotherapeutics, 2016, 12(8): 2113-2116. |
| [69] | DIAZ D, CARE A, SUNNA A. Bioengineering Strategies for Protein-based Nanoparticles [J]. Genes, 2018, 9(7) : 370. |
| [70] | PATI R, SHEVTSOV M, SONAWANE A. Nanoparticle Vaccines Against Infectious Diseases [J]. Frontiers in Immunology, 2018, 9: 2224. |
| [71] | WALLS A C, TORTORICI M A, SNIJDER J, et al. Tectonic Conformational Changes of a Coronavirus Spike Glycoprotein Promote Membrane Fusion [J]. Proceedings of the National Academy of Sciences of the United States of America. 2017, 114(42):11157-11162. |
| [72] | WRAPP D, WANG N, CORBETT K S, et al. Cryo-EM Structure of the 2019-nCoV Spike in the Prefusion Conformation [J]. Science, 2020, 367(6483): 1260-1263. |
| [73] | C-L HSIEH, GOLDSMITH J A, SCHAUB J M, et al. Structure-based Design of Prefusion-stabilized SARS-CoV-2 Spikes [J]. Science, 2020, 369(6510): 1501-1505. |
| [74] | TAN C W, ZHU F, CHIA W N, et al. Distinctive Serotypes of SARS-related Coronaviruses Defined by Convalescent Sera from Unvaccinated Individuals [J]. Hlife, 2023, 1(1): 26-34. |
| [75] | DU P, WU C, HU S, et al. The Omicron BA.2.86 Subvariant as a New Serotype of SARS-CoV-2 [J]. Lancet Microbe, 2024, 5(6): e516. |
| [76] | DU P, LI J, KONG T, et al. Defining the Serotypes of SARS-CoV-2 Subvariants up to December, 2024 [J]. Lancet Microbe, 2025: 101124. |
| [77] | SUN J, LI M, WANG Y, et al. Elaboration of Tetravalent Antibody Responses Against Dengue Viruses Using a Subunit Vaccine Comprised of a Single Consensus Dengue Envelope Sequence [J]. Vaccine, 2017, 35(46): 6308-6320. |
| [78] | SLIEPEN K, HAN B W, BONTJER I, et al. Structure and Immunogenicity of a Stabilized HIV-1 Envelope Trimer Based on a Group-M Consensus Sequence [J]. Nature Communications, 2019, 10(1): 2355. |
| [79] | REISS E I M M, VAN HAAREN M M, VAN SCHOOTEN J, et al. Fine-mapping the Immunodominant Antibody Epitopes on Consensus Sequence-based HIV-1 Envelope Trimer Vaccine Candidates [J]. npj Vaccines, 2022, 7(1): 152. |
| [80] | RAHMAN M S, HOQUE M N, ISLAM M R, et al. Epitope-based Chimeric Peptide Vaccine Design Against S, M and E Proteins of SARS-CoV-2, the Etiologic Agent of COVID-19 Pandemic: An In Silico Approach [J]. PeerJ, 2020, 8: e9572. |
| [81] | ABDELMAGEED M I, ABDELMONEIM A H, MUSTAFA M I, et al. Design of a Multiepitope-based Peptide Vaccine Against the E Protein of Human COVID-19: An Immunoinformatics Approach [J]. BioMed Research International, 2020, 2020: 2683286. |
| [82] | FEDERICO L, MALONE B, TENNøE S, et al. Experimental Validation of Immunogenic SARS-CoV-2 T Cell Epitopes Identified by Artificial Intelligence [J]. Frontiers in Immunology, 2023, 14: 1265044. |
| [83] | BRAVI B. Development and Use of Machine Learning Algorithms in Vaccine Target Selection [J]. npj Vaccines, 2024, 9(1): 15. |
| [84] | MCLELLAN J S, CHEN M, JOYCE M G, et al. Structure-based Design of a Fusion Glycoprotein Vaccine for Respiratory Syncytial Virus [J]. Science, 2013, 342(6158): 592-598. |
| [85] | GRAHAM B S. Vaccines Against Respiratory Syncytial Virus: The Time Has Finally Come [J]. Vaccine, 2016, 34(30): 3535-3541. |
| [86] | CRANK M C, RUCKWARDT T J, CHEN M, et al. A Proof of Concept for Structure-based Vaccine Design Targeting RSV in Humans [J]. Science, 2019, 365(6452): 505-509. |
| [87] | CHANG L A, PHUNG E, CRANK M C, et al. A Prefusion-stabilized RSV F Subunit Vaccine Elicits B Cell Responses with Greater Breadth and Potency than a Postfusion F Vaccine [J]. Science Translational Medicine, 2022, 14(676): eade0424. |
| [88] | TORRENTS DE LA PEñA A, SANDERS R W. Stabilizing HIV-1 Envelope Glycoprotein Trimers to Induce Neutralizing Antibodies [J]. Retrovirology, 2018, 15(1): 63. |
| [89] | SANDERS R W, DERKING R, CUPO A, et al. A Next-generation Cleaved, Soluble HIV-1 Env Trimer, BG505 SOSIP.664 gp140, Expresses Multiple Epitopes for Broadly Neutralizing but not Non-neutralizing Antibodies [J]. PLoS Pathogens, 2013, 9(9): e1003618. |
| [90] | SHARMA S K, DE VAL N, BALE S, et al. Cleavage-independent HIV-1 Env Trimers Engineered as Soluble Native Spike Mimetics for Vaccine Design [J]. Cell Reports, 2015, 11(4): 539-550. |
| [91] | KONG L, HE L, DE VAL N, et al. Uncleaved Prefusion-optimized gp140 Trimers Derived from Analysis of HIV-1 Envelope Metastability [J]. Nature Communications, 2016, 7: 12040. |
| [92] | HENDERSON R, ANASTI K, MANNE K, et al. Engineering Immunogens that Select for Specific Mutations in HIV Broadly Neutralizing Antibodies [J]. Nature Communications, 2024, 15(1): 9503. |
| [93] | DEJNIRATTISAI W, JUMNAINSONG A, ONSIRISAKUL N, et al. Cross-reacting Antibodies Enhance Dengue Virus Infection in Humans [J]. Science, 2010, 328(5979): 745-748. |
| [94] | STETTLER K, BELTRAMELLO M, ESPINOSA D A, et al. Specificity, Cross-reactivity, and Function of Antibodies Elicited by Zika Virus Infection [J]. Science, 2016, 353(6301): 823-826. |
| [95] | DEJNIRATTISAI W, SUPASA P, WONGWIWAT W, et al. Dengue Virus Sero-cross-reactivity Drives Antibody-dependent Enhancement of Infection with Zika Virus [J]. Nature Immunology, 2016, 17(9): 1102-1108. |
| [96] | HASAN S S, MILLER A, SAPPARAPU G, et al. A Human Antibody against Zika Virus Crosslinks the E Protein to Prevent Infection [J]. Nature Communications, 2017, 8: 14722. |
| [97] | LONG F, DOYLE M, FERNANDEZ E, et al. Structural Basis of a Potent Human Monoclonal Antibody against Zika Virus Targeting a Quaternary Epitope [J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(5): 1591-1596. |
| [98] | SLON-CAMPOS J L, DEJNIRATTISAI W, JAGGER B W, et al. A Protective Zika Virus E-dimer-based Subunit Vaccine Engineered to Abrogate Antibody-dependent Enhancement of Dengue Infection [J]. Nature Immunology, 2019, 20(10): 1291-1298. |
| [99] | DAI L, XU K, LI J, et al. Protective Zika Vaccines Engineered to Eliminate Enhancement of Dengue Infection via Immunodominance Switch [J]. Nature Immunology, 2021, 22(8): 958-968. |
| [100] | SHARMA A, ZHANG X, DEJNIRATTISAI W, et al. The Epitope Arrangement on Flavivirus Particles Contributes to Mab C10's Extraordinary Neutralization Breadth across Zika and Dengue Viruses [J]. Cell, 2021, 184(25): 6052-6066.e18. |
| [101] | CHENG N, LIU M, LI W, et al. Protein Post-translational Modification in SARS-CoV-2 and Host Interaction [J]. Frontiers in Immunology, 2022, 13: 1068449. |
| [102] | SHAJAHAN A, PEPI L E, ROUHANI D S, et al. Glycosylation of SARS-CoV-2: Structural and Functional Insights [J]. Analytical and Bioanalytical Chemistry, 2021, 413(29): 7179-7193. |
| [103] | RINGE R P, OZOROWSKI G, RANTALAINEN K, et al. Reducing V3 Antigenicity and Immunogenicity on Soluble, Native-like HIV-1 Env SOSIP Trimers [J]. Journal of Virology, 2017, 91(15). |
| [104] | DUAN H, CHEN X, BOYINGTON J C, et al. Glycan Masking Focuses Immune Responses to the HIV-1 CD4-binding Site and Enhances Elicitation of VRC01-class Precursor Antibodies [J]. Immunity, 2018, 49(2): 301-311.e5. |
| [105] | WEIDENBACHER P A, KIM P S. Protect, Modify, Deprotect (PMD): A Strategy for Creating Vaccines to Elicit Antibodies Targeting a Specific Epitope [J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(20): 9947-9952. |
| [106] | MUSUNURI S, WEIDENBACHER P A B, KIM P S. Bringing Immunofocusing into Focus [J]. npj Vaccines, 2024, 9(1): 11. |
| [107] | FANG Z, JIANG W, LIU P, et al. Targeting HIV-1 Immune Escape Mechanisms: Key Advances and Challenges in HIV-1 Vaccine Design [J]. Microbiological Research, 2025, 299: 128229. |
| [108] | VAN GILS M J, SANDERS R W. Broadly Neutralizing Antibodies Against HIV-1: Templates for a Vaccine [J]. Virology, 2013, 435(1): 46-56. |
| [109] | WEST A P, SCHARF L, SCHEID J F, et al. Structural Insights on the Role of Antibodies in HIV-1 Vaccine and Therapy [J]. Cell, 2014, 156(4): 633-648. |
| [110] | NG'UNI T, CHASARA C, NDHLOVU Z M. Major Scientific Hurdles in HIV Vaccine Development: Historical Perspective and Future Directions [J]. Frontiers in Immunology, 2020, 11: 590780. |
| [111] | LIU S, HU M, LIU X, CHEN T, ZHU Y, LIANG T, XIAO S, LI P, MA X. Nanoparticles and Antiviral Vaccines [J/OL]. Vaccines, 2023, 12(1): 30. |
| [112] | WUERTZ K M, BARKEI E K, CHEN W-H, et al. A SARS-CoV-2 Spike Ferritin Nanoparticle Vaccine Protects Hamsters Against Alpha and Beta Virus Variant Challenge [J]. npj Vaccines, 2021, 6(1): 129. |
| [113] | JOYCE M G, CHEN W-H, SANKHALA R S, et al. SARS-CoV-2 Ferritin Nanoparticle Vaccines Elicit Broad SARS Coronavirus Immunogenicity [J]. Cell Reports, 2021, 37(12): 110143. |
| [114] | SONG J Y, CHOI W S, HEO J Y, et al. Safety and Immunogenicity of a SARS-CoV-2 Recombinant Protein Nanoparticle Vaccine (GBP510) Adjuvanted with AS03: A Randomised, Placebo-controlled, Observer-blinded Phase 1/2 Trial [J]. EClinicalMedicine, 2022, 51: 101569. |
| [115] | LI Y, ZHANG Y, ZHOU Y, et al. An RBD Virus-like Particle Vaccine for SARS-CoV-2 Induces Cross-variant Antibody Responses in Mice and Macaques [J]. Signal Transduction and Targeted Therapy, 2023, 8(1): 173. |
| [116] | NGUYEN B, TOLIA N H. Protein-based Antigen Presentation Platforms for Nanoparticle Vaccines [J]. npj Vaccines, 2021, 6(1): 70. |
| [117] | LUA L H, CONNORS N K, SAINSBURY F, et al. Bioengineering Virus-like Particles as Vaccines [J]. Biotechnology and Bioengineering, 2014, 111(3): 425-440. |
| [118] | LU Y, CHAN W, KO B Y, et al. Assessing Sequence Plasticity of a Virus-like Nanoparticle by Evolution Toward a Versatile Scaffold for Vaccines and Drug Delivery [J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(40): 12360-12365. |
| [119] | ZHANG X, MEINING W, FISCHER M, et al. X-ray Structure Analysis and Crystallographic Refinement of Lumazine Synthase from the Hyperthermophile Aquifex Aeolicus at 1.6 A Resolution: Determinants of Thermostability Revealed from Structural Comparisons [J]. Journal of Molecular Biology, 2001, 306(5): 1099-1114. |
| [120] | PIOT P, LARSON H J, O'BRIEN K L, et al. Immunization: Vital Progress, Unfinished Agenda [J]. Nature, 2019, 575(7781): 119-129. |
| [121] | BOYOGLU-BARNUM S, ELLIS D, GILLESPIE R A, et al. Quadrivalent Influenza Nanoparticle Vaccines Induce Broad Protection [J]. Nature, 2021, 592(7855): 623-628. |
| [122] | CAO S, MA D, JI S, et al. Self-assembled Ferritin Nanoparticles for Delivery of Antigens and Development of Vaccines: From Structure and Property to Applications [J/OL]. Molecules, 2024, 29(17): 10.3390/molecules29174221. |
| [123] | LADENSTEIN R, MORGUNOVA E. Second Career of a Biosynthetic Enzyme: Lumazine Synthase as a Virus-like Nanoparticle in Vaccine Development [J]. Biotechnology Reports, 2020, 27: e00494. |
| [124] | REUTOVICH A A, SRIVASTAVA A K, AROSIO P, et al. Ferritin Nanocages as Efficient Nanocarriers and Promising Platforms for COVID-19 and Other Vaccines Development [J]. Biochimica et Biophysica Acta (BBA) - General Subjects, 2023, 1867(3): 130288. |
| [125] | KANG Y F, SUN C, ZHUANG Z, et al. Rapid Development of SARS-CoV-2 Spike Protein Receptor-binding Domain Self-assembled Nanoparticle Vaccine Candidates [J]. ACS Nano, 2021, 15(2): 2738-2752. |
| [126] | MARCANDALLI J, FIALA B, OLS S, et al. Induction of Potent Neutralizing Antibody Responses by a Designed Protein Nanoparticle Vaccine for Respiratory Syncytial Virus [J]. Cell, 2019, 176(6): 1420-1431.e17. |
| [127] | TAI W, CHAI B, FENG S, et al. Development of a Ferritin-based Nanoparticle Vaccine Against the SARS-CoV-2 Omicron Variant [J]. Signal Transduction and Targeted Therapy, 2022, 7(1): 173. |
| [128] | KANEKIYO M, JOYCE M G, GILLESPIE R A, et al. Mosaic Nanoparticle Display of Diverse Influenza Virus Hemagglutinins Elicits Broad B Cell Responses [J]. Nature Immunology, 2019, 20(3): 362-372. |
| [129] | KANG Y F, SUN C, SUN J, et al. Quadrivalent Mosaic HexaPro-bearing Nanoparticle Vaccine Protects Against Infection of SARS-CoV-2 Variants [J]. Nature Communications, 2022, 13(1): 2674. |
| [130] | BRINKKEMPER M, SLIEPEN K. Nanoparticle Vaccines for Inducing HIV-1 Neutralizing Antibodies [J]. Vaccines, 2019, 7(3): 105. |
| [131] | STEPHENSON K E, WEGMANN F, TOMAKA F, et al. Comparison of Shortened Mosaic HIV-1 Vaccine Schedules: A Randomised, Double-blind, Placebo-controlled Phase 1 Trial (IPCAVD010/HPX1002) and a Preclinical Study in Rhesus Monkeys (NHP 17–22) [J]. The Lancet HIV, 2020, 7(6): e410-e421. |
| [132] | HILLS R A, TAN T K, COHEN A A, et al. Proactive Vaccination Using Multiviral Quartet Nanocages to Elicit Broad Anti-coronavirus Responses [J]. Nature Nanotechnology, 2024, 19(8): 1216-1223. |
| [133] | LENEIGHAN D B, MIURA K, TAYLOR I J, et al. Nanoassembly Routes Stimulate Conflicting Antibody Quantity and Quality for Transmission-blocking Malaria Vaccines [J]. Scientific Reports, 2017, 7(1): 3811. |
| [134] | ZHANG Y N, PAYNTER J, SOU C, et al. Mechanism of a COVID-19 Nanoparticle Vaccine Candidate that Elicits a Broadly Neutralizing Antibody Response to SARS-CoV-2 Variants [J]. Science Advances, 2021, 7(43): eabj3107. |
| [135] | MA X, ZOU F, YU F, et al. Nanoparticle Vaccines Based on the Receptor Binding Domain (RBD) and Heptad Repeat (HR) of SARS-CoV-2 Elicit Robust Protective Immune Responses [J]. Immunity, 2020, 53(6): 1315-1330.e9. |
| [136] | LI L, FIERER J O, RAPOPORT T A, et al. Structural Analysis and Optimization of the Covalent Association between SpyCatcher and a Peptide Tag [J]. Journal of Molecular Biology, 2014, 426(2): 309-317. |
| [137] | KEEBLE A H, BANERJEE A, FERLA M P, et al. Evolving Accelerated Amidation by SpyTag/SpyCatcher to Analyze Membrane Dynamics [J]. Angewandte Chemie International Edition, 2017, 56(52): 16521-16525. |
| [138] | KEEBLE A H, TURKKI P, STOKES S, et al. Approaching Infinite Affinity through Engineering of Peptide-protein Interaction [J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(52): 26523-26533. |
| [139] | FU D, WANG W, ZHANG Y, et al. Self-assembling Nanoparticle Engineered from the Ferritinophagy Complex as a Rabies Virus Vaccine Candidate [J]. Nature Communications, 2024, 15(1): 8601. |
| [140] | CHOI A, BOUZYA B, CORTéS FRANCO K D, et al. Chimeric Hemagglutinin-based Influenza Virus Vaccines Induce Protective Stalk-specific Humoral Immunity and Cellular Responses in Mice [J]. Immunohorizons, 2019, 3(4): 133-148. |
| [141] | ZHANG J, HAN Z B, LIANG Y, et al. A Mosaic-type Trimeric RBD-based COVID-19 Vaccine Candidate Induces Potent Neutralization Against Omicron and Other SARS-CoV-2 Variants [J]. eLife, 2022, 11: e75643. |
| [142] | OLS S, LENART K, ARCOVERDE CERVEIRA R, et al. Multivalent Antigen Display on Nanoparticle Immunogens Increases B Cell Clonotype Diversity and Neutralization Breadth to Pneumoviruses [J]. Immunity, 2023, 56(10): 2425-2441.e14. |
| [143] | LIU C, XU S, ZHENG Y, et al. Mosaic RBD Nanoparticle Elicits Immunodominant Antibody Responses Across Sarbecoviruses [J]. Cell Reports, 2024, 43(5): 114235. |
| [144] | COHEN A A, YANG Z, GNANAPRAGASAM P N P, et al. Construction, Characterization, and Immunization of Nanoparticles that Display a Diverse Array of Influenza HA Trimers [J]. PLoS One, 2021, 16(3): e0247963. |
| [145] | GEORGIEV I S, JOYCE M G, CHEN R E, et al. Two-component Ferritin Nanoparticles for Multimerization of Diverse Trimeric Antigens [J]. ACS Infectious Diseases, 2018, 4(5): 788-796. |
| [146] | UEDA G, ANTANASIJEVIC A, FALLAS J A, et al. Tailored Design of Protein Nanoparticle Scaffolds for Multivalent Presentation of Viral Glycoprotein Antigens [J]. eLife, 2020, 9: e56789. |
| [147] | BROUWER P J M, ANTANASIJEVIC A, BERNDSEN Z, et al. Enhancing and Shaping the Immunogenicity of Native-like HIV-1 Envelope Trimers with a Two-component Protein Nanoparticle [J]. Nature Communications, 2019, 10(1): 4272. |
| [148] | LEE S, KIBLER R D, AHN G, et al. Four-component Protein Nanocages Designed by Programmed Symmetry Breaking [J]. Nature, 2025, 638(8050): 546-552. |
| [149] | DOWLING Q M, Y-J PARK, FRIES C N, et al. Hierarchical Design of Pseudosymmetric Protein Nanocages [J]. Nature, 2025, 638(8050): 553-561. |
| [150] | ZHAO T, CAI Y, JIANG Y, et al. Vaccine Adjuvants: Mechanisms and Platforms [J]. Signal Transduction and Targeted Therapy, 2023, 8(1): 283. |
| [151] | MONI S S, ABDELWAHAB S I, JABEEN A, et al. Advancements in Vaccine Adjuvants: The Journey from Alum to Nano Formulations [J]. Vaccines, 2023, 11(11): 1323. |
| [152] | STERTMAN L, PALM A E, ZARNEGAR B, et al. The Matrix-M™ Adjuvant: A Critical Component of Vaccines for the 21st Century [J]. Human Vaccines & Immunotherapeutics, 2023, 19(1): 2189885. |
| [153] | SUNG H D, KIM N, LEE Y, et al. Protein-Based Nanoparticle Vaccines for SARS-CoV-2 [J]. International Journal of Molecular Sciences, 2021, 22(24): 13209. |
| [154] | RODRIGUES K A, ZHANG Y J, LAM J, et al. Vaccines Combining Slow Release and Follicle Targeting of Antigens Increase Germinal Center B Cell Diversity and Clonal Expansion [J]. Science Translational Medicine, 2025, 17(803): eadw7499. |
| [155] | CARTER N J. Multicomponent Meningococcal Serogroup B Vaccine (4CMenB; Bexsero®): A Review of Its Use in Primary and Booster Vaccination [J]. BioDrugs, 2013, 27(3): 263-274. |
| [156] | SHIRLEY M, TAHA M K. MenB-FHbp Meningococcal Group B Vaccine (Trumenba®): A Review in Active Immunization in Individuals Aged ≥ 10 Years [J]. Drugs, 2018, 78(2): 257-268. |
| [157] | DORMITZER P R, GRANDI G, RAPPUOLI R. Structural Vaccinology Starts to Deliver [J]. Nature Reviews Microbiology, 2012, 10(12): 807-813. |
| [158] | RAPPUOLI R, BOTTOMLEY M J, D'ORO U, et al. Reverse Vaccinology 2.0: Human Immunology Instructs Vaccine Antigen Design [J]. Journal of Experimental Medicine, 2016, 213(4): 469-481. |
| [159] | DORMITZER P R, ULMER J B, RAPPUOLI R. Structure-based Antigen Design: A Strategy for Next Generation Vaccines [J]. Trends in Biotechnology, 2008, 26(12): 659-667. |
| [160] | STANFIELD R L, J-P JULIEN, PEJCHAL R, et al. Structure-based Design of a Protein Immunogen that Displays an HIV-1 gp41 Neutralizing Epitope [J]. Journal of Molecular Biology, 2011, 414(3): 460-476. |
| [161] | MESHRAM R, KOLTE B, GACCHE R. Reverse Vaccinology Approach for Identification of Epitopes from E1 Protein as Peptide Vaccine Against HCV: A Proof of Concept [J]. Vaccine, 2024, 42(24): 126106. |
| [162] | LAINŠČEK D, FINK T, FORSTNERIČ V, et al. A Nanoscaffolded Spike-RBD Vaccine Provides Protection Against SARS-CoV-2 with Minimal Anti-Scaffold Response [J]. Vaccines, 2021, 9(5): 431. |
| [1] | 袁为锋, 赵永亮, 吴芷萱, 徐可. 合成生物学在新冠病毒广谱疫苗研发中的应用[J]. 合成生物学, 2024, 5(2): 369-384. |
| [2] | 刘泽众, 周洁, 朱赟, 陆路, 姜世勃. 基于重组人Ⅲ型胶原蛋白的三聚体抗原疫苗策略在新冠和流感疫苗中的应用[J]. 合成生物学, 2024, 5(2): 385-395. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||