合成生物学 ›› 2024, Vol. 5 ›› Issue (2): 369-384.DOI: 10.12211/2096-8280.2023-088
合成生物学在新冠病毒广谱疫苗研发中的应用
袁为锋, 赵永亮, 吴芷萱, 徐可
- 武汉大学病毒学国家重点实验室,泰康生命医学中心,生命科学学院,湖北 武汉 430072
-
收稿日期:2023-11-28修回日期:2024-02-18出版日期:2024-04-30发布日期:2024-04-28 -
通讯作者:徐可 -
作者简介:袁为锋 (1995—),男,博士后。研究方向为病毒宿主相互作用与抗病毒药物的靶点发现、新型流感/冠状病毒疫苗研发。E-mail:ywf519@whu.edu.cn徐可 (1982—),女,教授,博士生导师。研究方向为呼吸道RNA病毒分子致病机制、呼吸道病毒共感染、抗呼吸道RNA病毒广谱药物靶标发现、广谱疫苗研发等。E-mail:xuke03@whu.edu.cn -
基金资助:国家重点研发计划(2023YFC2307800);湖北省支持企业技术创新发展项目(2021BAB117);武汉大学示范课堂建设项目(2022ZG090)
Applications of synthetic biology in the development of SARS-CoV-2 broad-spectrum vaccines
YUAN Weifeng, ZHAO Yongliang, WU Zhixuan, XU Ke
- State Key Laboratory of Virology,College of Life Sciences,Taikang Center for Life and Medical Sciences,Wuhan University,Wuhan 430072,Hubei,China
-
Received:2023-11-28Revised:2024-02-18Online:2024-04-30Published:2024-04-28 -
Contact:XU Ke
摘要:
新型冠状病毒(SARS-CoV-2)自2019年底引发疫情至今,已经变异出Alpha、Beta、Delta和Omicron等不同谱系。传统疫苗的抗原序列来源于某一自然分离株的原始序列,疫苗迭代速度跟不上病毒变异的速度,导致突破性感染的发生,研发跨谱系的广谱疫苗是预防这类高变异呼吸道病毒的迫切需求。随着合成生物技术的发展,抗原的多价偶联、核心抗原模块的提取、抗原内部保守表位的工程化设计、抗原表位展示技术、计算指导的抗原重构等抗原“再设计”方案得以实现,提高了抗原的免疫原性和广谱性。合成生物学还体现在疫苗产品的生产工艺环节,基因工程表达的疫苗抗原以纳米颗粒、病毒载体、核酸、亚单位的形式,借助细菌、酵母、植物、昆虫或哺乳动物细胞等表达平台进行规模化生产。本文综述了近年来合成生物技术在广谱疫苗(尤其是广谱新冠病毒疫苗)多种设计策略中的应用情况,总结了合成生物技术如何通过反向疫苗学的设计展示全新的共性抗原表位和交叉抗原位点,达到“以不变应万变”的广谱保护效果。本文还讨论了多种广谱疫苗设计策略的应用场景及面临的挑战。基于合成生物技术的马赛克设计策略、保守表位工程化设计策略、计算共识序列策略和新型佐剂策略,结合不同的疫苗技术路线,可提高疫苗的免疫原性、广谱保护性和安全性。这为高变异病毒的疫苗研发提供了合成生物学的新思路。
中图分类号:
引用本文
袁为锋, 赵永亮, 吴芷萱, 徐可. 合成生物学在新冠病毒广谱疫苗研发中的应用[J]. 合成生物学, 2024, 5(2): 369-384.
YUAN Weifeng, ZHAO Yongliang, WU Zhixuan, XU Ke. Applications of synthetic biology in the development of SARS-CoV-2 broad-spectrum vaccines[J]. Synthetic Biology Journal, 2024, 5(2): 369-384.
| 疫苗类型 | 产品名 | 关键组分或技术 | 保护率或临床阶段 | 研发团队或机构 |
|---|---|---|---|---|
| 灭活疫苗 | CoronaVac | CZ02株 | 50.4%[ | 北京科兴中维生物 |
| BBIBP-CorV | HB02株 | 78.1%[ | 北京生物制品研究所 | |
| WIBP | WIV04株 | 72.8%[ | 武汉生物制品研究所 | |
| BBV152 | NIV-2020-770株 | 77.8%[ | Bharat Biotech | |
| QazVac | Wuhan-Hu-1株 | 90%[ | Kazakhstan RIBSP | |
| 纳米颗粒疫苗 | I53-50 | RBD | Ⅲ期(原始株) | 华盛顿大学[ |
| 铁蛋白 | S | Ⅰ期(原始株) | 斯坦福大学[ | |
| SC003-mi3 | RBD | Ⅱ/Ⅲ期(原始株) | 牛津大学[ | |
| 铁蛋白 | RBD | 临床前(突变株) | 中山大学[ | |
| 亚单位疫苗 | NVXCoV2373 | Matrix-M™佐剂 | 89.7%[ | Novavax |
| COVAX-19 | Advax-CpG55.2™佐剂 | 63.55%[ | Vaxine Pty Ltd | |
| SCB-2019 | CpG 1018、铝佐剂 | 67%[ | 三叶草 | |
| ZF2001 | 氢氧化铝佐剂 | 75.7%[ | 中国科学院微生物研究所 | |
| 威克欣 | MF59样佐剂 | 未公布[ | 威斯克生物 | |
| SCTV01C | 水包油佐剂 | 未公布[ | 神州细胞 | |
| MVC-COV1901 | CpG1018、明矾佐剂 | 未公布[ | Medigen Vaccine Biologics | |
| Soberana | 明矾、B群脑膜炎奈瑟氏菌外膜囊泡 | 未公布[ | 古巴芬利疫苗研究所 | |
| 病毒载体疫苗 | Ad26.COV2.S | Ad26腺病毒载体 | 66.1%[ | Janssen |
| Sputnik V | rAd26和rAd5腺病毒载体 | 91.6%[ | Gamaleya Center | |
| Ad5-nCoV | Ad5腺病毒载体 | 57.5%[ | 康希诺生物、军事医学科学院 | |
| AZD1222 | ChAdOx1腺病毒载体 | 76%[ | 阿斯利康、牛津大学 | |
| dNS1-RBD | dNS1流感病毒载体 | 100%[ | 厦门大学、香港大学、万泰生物 | |
| 核酸疫苗 | BNT162b2 | mRNA疫苗 | 95%[ | Pfizer Inc. |
| mRNA-1273 | mRNA疫苗 | 94.1%[ | Moderna | |
| ARCoV | mRNA疫苗 | 83.75%[ | 艾博生物 | |
| ZyCov-D | DNA疫苗 | 67%[ | Zydus Cadila |
表1 五种技术路线SARS-CoV-2疫苗的研究进展
Table 1 Research progress in SARS-CoV-2 vaccines
| 疫苗类型 | 产品名 | 关键组分或技术 | 保护率或临床阶段 | 研发团队或机构 |
|---|---|---|---|---|
| 灭活疫苗 | CoronaVac | CZ02株 | 50.4%[ | 北京科兴中维生物 |
| BBIBP-CorV | HB02株 | 78.1%[ | 北京生物制品研究所 | |
| WIBP | WIV04株 | 72.8%[ | 武汉生物制品研究所 | |
| BBV152 | NIV-2020-770株 | 77.8%[ | Bharat Biotech | |
| QazVac | Wuhan-Hu-1株 | 90%[ | Kazakhstan RIBSP | |
| 纳米颗粒疫苗 | I53-50 | RBD | Ⅲ期(原始株) | 华盛顿大学[ |
| 铁蛋白 | S | Ⅰ期(原始株) | 斯坦福大学[ | |
| SC003-mi3 | RBD | Ⅱ/Ⅲ期(原始株) | 牛津大学[ | |
| 铁蛋白 | RBD | 临床前(突变株) | 中山大学[ | |
| 亚单位疫苗 | NVXCoV2373 | Matrix-M™佐剂 | 89.7%[ | Novavax |
| COVAX-19 | Advax-CpG55.2™佐剂 | 63.55%[ | Vaxine Pty Ltd | |
| SCB-2019 | CpG 1018、铝佐剂 | 67%[ | 三叶草 | |
| ZF2001 | 氢氧化铝佐剂 | 75.7%[ | 中国科学院微生物研究所 | |
| 威克欣 | MF59样佐剂 | 未公布[ | 威斯克生物 | |
| SCTV01C | 水包油佐剂 | 未公布[ | 神州细胞 | |
| MVC-COV1901 | CpG1018、明矾佐剂 | 未公布[ | Medigen Vaccine Biologics | |
| Soberana | 明矾、B群脑膜炎奈瑟氏菌外膜囊泡 | 未公布[ | 古巴芬利疫苗研究所 | |
| 病毒载体疫苗 | Ad26.COV2.S | Ad26腺病毒载体 | 66.1%[ | Janssen |
| Sputnik V | rAd26和rAd5腺病毒载体 | 91.6%[ | Gamaleya Center | |
| Ad5-nCoV | Ad5腺病毒载体 | 57.5%[ | 康希诺生物、军事医学科学院 | |
| AZD1222 | ChAdOx1腺病毒载体 | 76%[ | 阿斯利康、牛津大学 | |
| dNS1-RBD | dNS1流感病毒载体 | 100%[ | 厦门大学、香港大学、万泰生物 | |
| 核酸疫苗 | BNT162b2 | mRNA疫苗 | 95%[ | Pfizer Inc. |
| mRNA-1273 | mRNA疫苗 | 94.1%[ | Moderna | |
| ARCoV | mRNA疫苗 | 83.75%[ | 艾博生物 | |
| ZyCov-D | DNA疫苗 | 67%[ | Zydus Cadila |
| 疫苗产品 | 疫苗类型 | 抗原 | 临床阶段 | 研发机构 |
|---|---|---|---|---|
| SpFN[ | 亚单位 | 多种突变株的S蛋白 | Ⅰ | 美国沃尔特-里德陆军研究所 |
| RBD-scNP[ | 亚单位 | 多种突变株的RBD蛋白 | 临床前 | 杜克大学 |
| 复必泰[ | mRNA | WT和BA.4/5的S蛋白 | Ⅰ | 复星医药 |
| RBD-sc[ | 亚单位 | 不同突变株的RBD二聚体 | 临床前 | 中国科学院微生物研究所 |
| V-01D-351[ | 亚单位 | Beta和Delta株的RBD二聚体 | Ⅰ | 丽珠集团 |
| SCTV01C[ | 亚单位 | Alpha和Beta株的S蛋白 | Ⅲ | 神州细胞 |
| SCTV01E[ | 亚单位 | Alpha、Beta、Delta和Omicron株的S蛋白 | Ⅲ | 神州细胞 |
表2 马赛克策略的SARS-CoV-2疫苗研究进展
Table 2 Research progress in developing the SARS-CoV-2 vaccine with the Mosaic strategy
| 疫苗产品 | 疫苗类型 | 抗原 | 临床阶段 | 研发机构 |
|---|---|---|---|---|
| SpFN[ | 亚单位 | 多种突变株的S蛋白 | Ⅰ | 美国沃尔特-里德陆军研究所 |
| RBD-scNP[ | 亚单位 | 多种突变株的RBD蛋白 | 临床前 | 杜克大学 |
| 复必泰[ | mRNA | WT和BA.4/5的S蛋白 | Ⅰ | 复星医药 |
| RBD-sc[ | 亚单位 | 不同突变株的RBD二聚体 | 临床前 | 中国科学院微生物研究所 |
| V-01D-351[ | 亚单位 | Beta和Delta株的RBD二聚体 | Ⅰ | 丽珠集团 |
| SCTV01C[ | 亚单位 | Alpha和Beta株的S蛋白 | Ⅲ | 神州细胞 |
| SCTV01E[ | 亚单位 | Alpha、Beta、Delta和Omicron株的S蛋白 | Ⅲ | 神州细胞 |
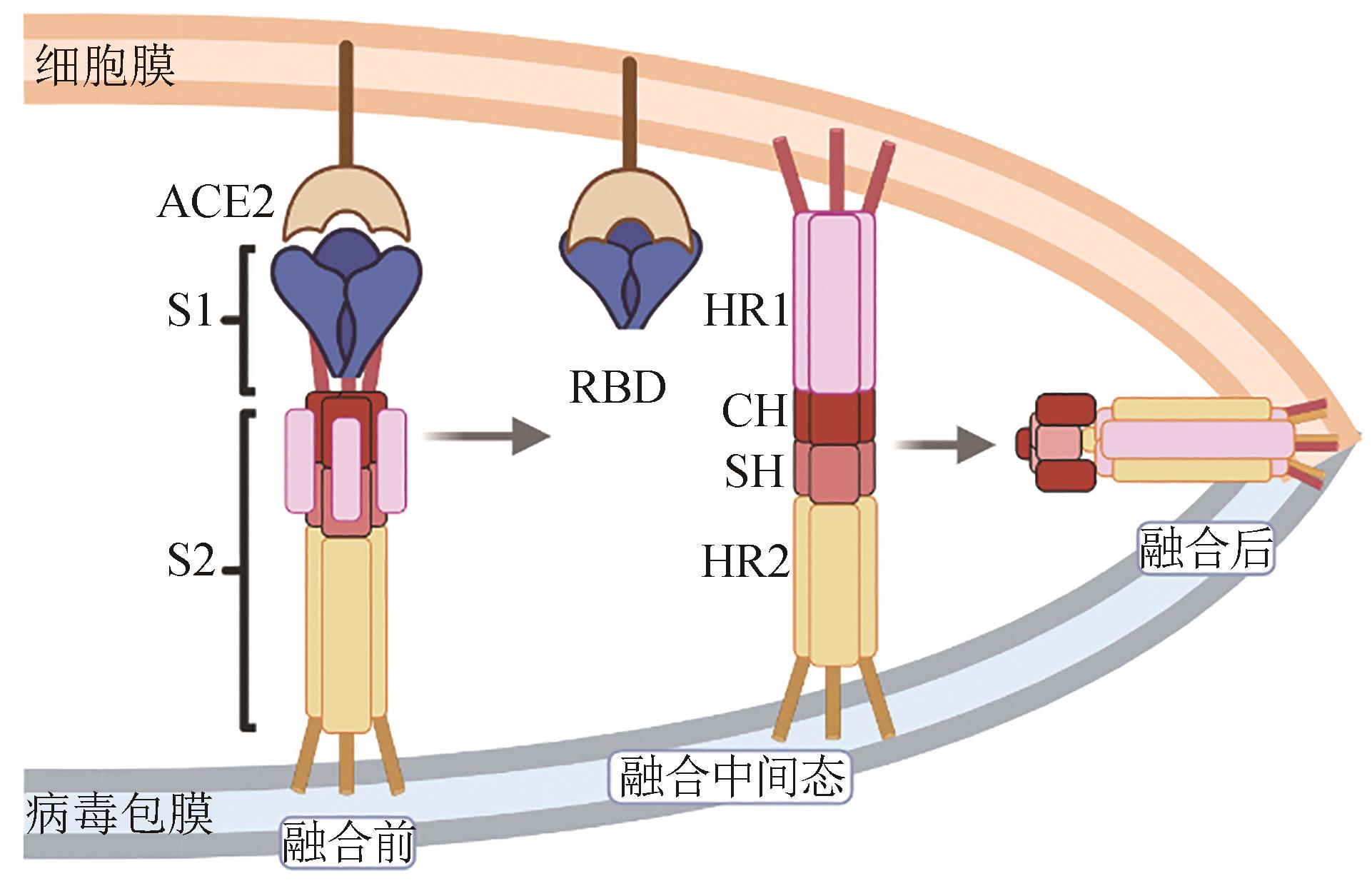
图2 以保守表位HR1、HR2作为广谱疫苗抗原的示意图(新冠病毒S2亚基介导膜融合过程。当新冠病毒结合ACE2受体后,S1与S2解离,S2被宿主蛋白酶切割后发生构象变化,HR1延伸将融合肽FP插入细胞膜,形成融合中间态。随后,HR2折叠结合至HR1三聚体螺旋疏水凹槽中,与HR1形成6螺旋结构,实现病毒包膜与细胞膜融合)
Fig. 2 Schematic diagram for engineering conserved epitopes HR1 and HR2 to develop broad-spectrum vaccine antigens(Membrane fusion mediated by the SARS-CoV-2 S2 subunit. Initially, the standing RBD engages with ACE2, and subsequently, the S1 subunit dissociates from S2, followed by the exposure of the S2 site and the cleavage of S2 by the host proteases. HR1 undergoes a “jack-knife” refolding change to allow the insertion of FP into the host cell membrane. The folding back of the extended SH-HR2 element packs against the long central CH-HR1 coiled-coil, inducing the binding of SH onto the outer region of CH and HR2 to the HR1 groove. Subsequently, membrane fusion occurs between the viral particles and host cells.)

图3 重组蛋白HR121的模式图、3D结构(a)以及重组蛋白HR1LS的模式图、3D结构(b)
Fig. 3 Schematic diagrams of the dimer protein HR121 and predicted structures (a) and the trimeric protein HRILS and predicted structures (b)
| 疫苗产品 | 疫苗类型 | 抗原 | 临床阶段 | 研发机构 |
|---|---|---|---|---|
| HR121[ | 亚单位 | HR1-HR2-HR1串联 | 临床前 | 中国科学院昆明动物研究所 |
| HR1LS[ | 亚单位 | HR1-CH-SH串联 | 临床前 | 复旦大学 |
| MigVax-101[ | 亚单位 | RBD和N蛋白 | 临床前 | MigVax |
| STFK1628x/y[ | 亚单位 | B.1.620-NTD和Gamma-RBD-S2 | 临床前 | 厦门大学 |
| hAd5 S-Fusion+N-ETSD[ | 病毒载体 | S和N蛋白 | Ⅱ | ImmunityBio |
表3 SARS-CoV-2靶向病毒保守表位策略的疫苗研究进展
Table 3 Research progress in vaccines against the conserved epitope of SARS-CoV-2
| 疫苗产品 | 疫苗类型 | 抗原 | 临床阶段 | 研发机构 |
|---|---|---|---|---|
| HR121[ | 亚单位 | HR1-HR2-HR1串联 | 临床前 | 中国科学院昆明动物研究所 |
| HR1LS[ | 亚单位 | HR1-CH-SH串联 | 临床前 | 复旦大学 |
| MigVax-101[ | 亚单位 | RBD和N蛋白 | 临床前 | MigVax |
| STFK1628x/y[ | 亚单位 | B.1.620-NTD和Gamma-RBD-S2 | 临床前 | 厦门大学 |
| hAd5 S-Fusion+N-ETSD[ | 病毒载体 | S和N蛋白 | Ⅱ | ImmunityBio |

图4 共识序列设计策略示意图[62](以新冠病毒共识序列Span为例。①建立序列库;②将序列库中所有毒株序列进行进化计算;③构建毒株进化树,得到每个分支的代表毒株;④使用代表毒株计算得到共识序列,并带回数据库进行优化与修正;⑤得到位于进化中心的共识序列)
Fig. 4 Schematic diagram of strategy for designing consensus sequences[62](Taking the SARS-CoV-2 consensus sequence Span as an example. ① Establishing the whole sequences library; ② Evolutionary calculations of the viruses; ③ Constructing an evolutionary tree for the viral strains to obtain representative ones for each branch; ④ Utilizing representative strains to calculate the consensus sequences, and refining and optimizing them by incorporating back into the database; ⑤ Obtaining the consensus sequences located at the evolutionary center.)
| 疫苗产品 | 疫苗类型 | 抗原 | 佐剂 | 临床阶段 | 研发机构 |
|---|---|---|---|---|---|
| β-CoV-B[ | 亚单位 | WT-RBD | CF501 | 临床前 | 复旦大学 |
| NVX-CoV2373[ | 亚单位 | S | Matrix-M | 已上市 | Novavax |
| VLA2001[ | 灭活疫苗 | WT毒株 | 铝佐剂和CpG 1018 | 已上市 | Valneva SE |
| YS-SC2-010[ | 亚单位 | WT-S | 皮卡佐剂 | Ⅰ | 依生生物 |
表4 SARS-CoV-2新型佐剂策略的疫苗研究进展
Table 4 Research progress of the SARS-CoV-2 vaccines developed based on novel adjuvant strategies
| 疫苗产品 | 疫苗类型 | 抗原 | 佐剂 | 临床阶段 | 研发机构 |
|---|---|---|---|---|---|
| β-CoV-B[ | 亚单位 | WT-RBD | CF501 | 临床前 | 复旦大学 |
| NVX-CoV2373[ | 亚单位 | S | Matrix-M | 已上市 | Novavax |
| VLA2001[ | 灭活疫苗 | WT毒株 | 铝佐剂和CpG 1018 | 已上市 | Valneva SE |
| YS-SC2-010[ | 亚单位 | WT-S | 皮卡佐剂 | Ⅰ | 依生生物 |
| 1 | WILHELM A, WIDERA M, GRIKSCHEIT K, et al. Limited neutralisation of the SARS-CoV-2 Omicron subvariants BA.1 and BA.2 by convalescent and vaccine serum and monoclonal antibodies[J]. EBioMedicine, 2022, 82: 104158. |
| 2 | CAO Y L, YISIMAYI A, JIAN F C, et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection[J]. Nature, 2022, 608(7923): 593-602. |
| 3 | DAI L P, GAO G F. Viral targets for vaccines against COVID-19[J]. Nature Reviews Immunology, 2021, 21(2): 73-82. |
| 4 | COSTA CLEMENS S A, WECKX L, CLEMENS R, et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study[J]. Lancet, 2022, 399(10324): 521-529. |
| 5 | KAABI N A, ZHANG Y T, XIA S L, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial[J]. JAMA, 2021, 326(1): 35-45. |
| 6 | ELLA R, REDDY S, BLACKWELDER W, et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): interim results of a randomised, double-blind, controlled, phase 3 trial[J]. Lancet, 2021, 398(10317): 2173-2184. |
| 7 | KHAIRULLIN B, ZAKARYA K, ORYNBAYEV M, et al. Efficacy and safety of an inactivated whole-virion vaccine against COVID-19, QazCovid-in®, in healthy adults: a multicentre, randomised, single-blind, placebo-controlled phase 3 clinical trial with a 6-month follow-up[J]. EClinicalMedicine, 2022, 50: 101526. |
| 8 | WALLS A C, FIALA B, SCHÄFER A, et al. Elicitation of potent neutralizing antibody responses by designed protein nanoparticle vaccines for SARS-CoV-2[EB/OL]. BioRxiv, 2020: 2020.08.11.247395[2023-08-01]. . |
| 9 | WEIDENBACHER P A, SANYAL M, FRIEDLAND N, et al. A ferritin-based COVID-19 nanoparticle vaccine that elicits robust, durable, broad-spectrum neutralizing antisera in non-human primates[J]. Nature Communications, 2023, 14(1): 2149. |
| 10 | RAHIKAINEN R, RIJAL P, TAN T K, et al. Overcoming symmetry mismatch in vaccine nanoassembly through spontaneous amidation[J]. Angewandte Chemie International Edition, 2021, 60(1): 321-330. |
| 11 | KANG Y F, SUN C, ZHUANG Z, et al. Rapid development of SARS-CoV-2 spike protein receptor-binding domain self-assembled nanoparticle vaccine candidates[J]. ACS Nano, 2021, 15(2): 2738-2752. |
| 12 | ÁÑEZ G, DUNKLE L M, GAY C L, et al. Safety, immunogenicity, and efficacy of the NVX-CoV2373 COVID-19 vaccine in adolescents: a randomized clinical trial[J]. JAMA Network Open, 2023, 6(4): e239135. |
| 13 | LI L, HONDA-OKUBO Y, BALDWIN J, et al. Covax-19/Spikogen® vaccine based on recombinant spike protein extracellular domain with Advax-CpG55.2 adjuvant provides single dose protection against SARS-CoV-2 infection in hamsters[J]. Vaccine, 2022, 40(23): 3182-3192. |
| 14 | BRAVO L, SMOLENOV I, HAN H H, et al. Efficacy of the adjuvanted subunit protein COVID-19 vaccine, SCB-2019: a phase 2 and 3 multicentre, double-blind, randomised, placebo-controlled trial[J]. Lancet, 2022, 399(10323): 461-472. |
| 15 | ZHAO X, ZHANG R, QIAO S T, et al. Omicron SARS-CoV-2 neutralization from inactivated and ZF2001 vaccines[J]. The New England Journal of Medicine, 2022, 387(3): 277-280. |
| 16 | CHAPPELL K J, MORDANT F L, LI Z Y, et al. Safety and immunogenicity of an MF59-adjuvanted spike glycoprotein-clamp vaccine for SARS-CoV-2: a randomised, double-blind, placebo-controlled, phase 1 trial[J]. The Lancet Infectious Diseases, 2021, 21(10): 1383-1394. |
| 17 | WANG G Q, ZHAO K X, HAN J, et al. Safety and immunogenicity of a bivalent SARS-CoV-2 recombinant protein vaccine, SCTV01C in unvaccinated adults: a randomized, double-blinded, placebo-controlled, phase Ⅰ clinical trial[J]. The Journal of Infection, 2023, 86(2): 154-225. |
| 18 | HSIEH S M, LIU M C, CHEN Y H, et al. Safety and immunogenicity of CpG 1018 and aluminium hydroxide-adjuvanted SARS-CoV-2 S-2P protein vaccine MVC-COV1901: interim results of a large-scale, double-blind, randomised, placebo-controlled phase 2 trial in Taiwan[J]. The Lancet Respiratory Medicine, 2021, 9(12): 1396-1406. |
| 19 | EUGENIA-TOLEDO-ROMANÍ M, VERDECIA-SÁNCHEZ L, RODRÍGUEZ-GONZÁLEZ M, et al. Safety and immunogenicity of anti-SARS CoV-2 vaccine SOBERANA 02 in homologous or heterologous scheme: open label phase Ⅰ and phase Ⅱa clinical trials[J]. Vaccine, 2022, 40(31): 4220-4230. |
| 20 | SADOFF J, GRAY G, VANDEBOSCH A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against covid-19[J]. The New England Journal of Medicine, 2021, 384(23): 2187-2201. |
| 21 | SOLTANI S, MATIN B K, GOUYA M M, et al. A prospective cohort study protocol: monitoring and surveillance of adverse events following heterologous booster doses of Oxford AstraZeneca COVID-19 vaccine in previous recipients of two doses of Sinopharm or Sputnik Ⅴ vaccines in Iran[J]. BMC Public Health, 2023, 23(1): 1415. |
| 22 | LI J X, WU S P, GUO X L, et al. Safety and immunogenicity of heterologous boost immunisation with an orally administered aerosolised Ad5-nCoV after two-dose priming with an inactivated SARS-CoV-2 vaccine in Chinese adults: a randomised, open-label, single-centre trial[J]. The Lancet Respiratory Medicine, 2022, 10(8): 739-748. |
| 23 | RAMASAMY M N, KELLY E J, SEEGOBIN S, et al. Immunogenicity and safety of AZD2816, a beta (B.1.351) variant COVID-19 vaccine, and AZD1222 (ChAdOx1 nCoV-19) as third-dose boosters for previously vaccinated adults: a multicentre, randomised, partly double-blinded, phase 2/3 non-inferiority immunobridging study in the UK and Poland[J]. The Lancet Microbe, 2023, 4(11): e863-e874. |
| 24 | ZHU F C, HUANG S J, LIU X H, et al. Safety and efficacy of the intranasal spray SARS-CoV-2 vaccine dNS1-RBD: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial[J]. The Lancet Respiratory Medicine, 2023, 11(12): 1075-1088. |
| 25 | THOMAS S J, MOREIRA E D, KITCHIN N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine through 6 months[J]. The New England Journal of Medicine, 2021, 385(19): 1761-1773. |
| 26 | TSENG H F, ACKERSON B K, SY L S, et al. mRNA-1273 bivalent (original and Omicron) COVID-19 vaccine effectiveness against COVID-19 outcomes in the United States[J]. Nature Communications, 2023, 14(1): 5851. |
| 27 | CHEN G L, LI X F, DAI X H, et al. Safety and immunogenicity of the SARS-CoV-2 ARCoV mRNA vaccine in Chinese adults: a randomised, double-blind, placebo-controlled, phase 1 trial[J]. The Lancet Microbe, 2022, 3(3): e193-e202. |
| 28 | KHOBRAGADE A, BHATE S, RAMAIAH V, et al. Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): the interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India[J]. Lancet, 2022, 399(10332): 1313-1321. |
| 29 | QU D, ZHENG B J, YAO X, et al. Intranasal immunization with inactivated SARS-CoV (SARS-associated coronavirus) induced local and serum antibodies in mice[J]. Vaccine, 2005, 23(7): 924-931. |
| 30 | NEUMANN G, FUJII K, KINO Y, et al. An improved reverse genetics system for influenza A virus generation and its implications for vaccine production[J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(46): 16825-16829. |
| 31 | TLAXCA J L, ELLIS S, REMMELE R L JR. Live attenuated and inactivated viral vaccine formulation and nasal delivery: potential and challenges[J]. Advanced Drug Delivery Reviews, 2015, 93: 56-78. |
| 32 | LIU Y, ZHANG X W, LIU J Y, et al. A live-attenuated SARS-CoV-2 vaccine candidate with accessory protein deletions[J]. Nature Communications, 2022, 13(1): 4337. |
| 33 | YE Z W, ONG C P, TANG K M, et al. Intranasal administration of a single dose of a candidate live attenuated vaccine derived from an NSP16-deficient SARS-CoV-2 strain confers sterilizing immunity in animals[J]. Cellular & Molecular Immunology, 2022, 19(5): 588-601. |
| 34 | JEYANATHANM, AFKHAMIS, SMAILLF, et al. Immunological considerations for COVID-19 vaccine strategies[J]. Nature Reviews Immunology, 2020, 20(10): 615-632. |
| 35 | WARD B J, GOBEIL P, SÉGUIN A, et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19[J]. Nature Medicine, 2021, 27(6): 1071-1078. |
| 36 | HAGER K J, PÉREZ MARC G, GOBEIL P, et al. Efficacy and safety of a recombinant plant-based adjuvanted COVID-19 vaccine[J]. The New England Journal of Medicine, 2022, 386(22): 2084-2096. |
| 37 | LI M C, WANG H, TIAN L L, et al. COVID-19 vaccine development: milestones, lessons and prospects[J]. Signal Transduction and Targeted Therapy, 2022, 7(1): 146. |
| 38 | GEORGIEV I S, JOYCE M G, CHEN R E, et al. Two-component ferritin nanoparticles for multimerization of diverse trimeric antigens[J]. ACS Infectious Diseases, 2018, 4(5): 788-796. |
| 39 | ZHANG X, MEINING W, FISCHER M, et al. X-ray structure analysis and crystallographic refinement of lumazine synthase from the hyperthermophile Aquifex aeolicus at 1.6 A resolution: determinants of thermostability revealed from structural comparisons[J]. Journal of Molecular Biology, 2001, 306(5): 1099-1114. |
| 40 | IZARD T, AEVARSSON A, ALLEN M D, et al. Principles of quasi-equivalence and Euclidean geometry govern the assembly of cubic and dodecahedral cores of pyruvate dehydrogenase complexes[J]. Proceedings of the National Academy of Sciences of the United States of America, 1999, 96(4): 1240-1245. |
| 41 | CARTER DANIEL C, BRENDA W, GRAY J W, et al. A unique protein self-assembling nanoparticle with significant advantages in vaccine development and production[J]. Journal of Nanomaterials, 2020, 2020: 4297937. |
| 42 | KARPIŃSKI T M, OŻAROWSKI M, SEREMAK-MROZIKIEWICZ A, et al. The 2020 race towards SARS-CoV-2 specific vaccines[J]. Theranostics, 2021, 11(4): 1690-1702. |
| 43 | LI Y D, CHI W Y, SU J H, et al. Coronavirus vaccine development: from SARS and MERS to COVID-19[J]. Journal of Biomedical Science, 2020, 27(1): 104. |
| 44 | BHAT E A, KHAN J, SAJJAD N, et al. SARS-CoV-2: insight in genome structure, pathogenesis and viral receptor binding analysis — an updated review[J]. International Immunopharmacology, 2021, 95: 107493. |
| 45 | WU Y, WANG F R, SHEN C G, et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2[J]. Science, 2020, 368(6496): 1274-1278. |
| 46 | HUMPHREYS I R, SEBASTIAN S. Novel viral vectors in infectious diseases[J]. Immunology, 2018, 153(1): 1-9. |
| 47 | LIU X, LIU C, LIU G, et al. COVID-19: progress in diagnostics, therapy and vaccination[J]. Theranostics, 2020, 10(17): 7821-7835. |
| 48 | WANG J L, PENG Y, XU H Y, et al. The COVID-19 vaccine race: challenges and opportunities in vaccine formulation[J]. AAPS PharmSciTech, 2020, 21(6): 225. |
| 49 | MERCADO N B, ZAHN R, WEGMANN F, et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques[J]. Nature, 2020, 586(7830): 583-588. |
| 50 | FOLEGATTI P M, EWER K J, ALEY P K, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial[J]. Lancet, 2020, 396(10249): 467-478. |
| 51 | CHEN J Y, WANG P, YUAN L Z, et al. A live attenuated virus-based intranasal COVID-19 vaccine provides rapid, prolonged, and broad protection against SARS-CoV-2[J]. Science Bulletin, 2022, 67(13): 1372-1387. |
| 52 | KUTZLERM A, WEINERD B. DNA vaccines: ready for prime time?[J]. Nature Reviews Genetics, 2008, 9(10): 776-788. |
| 53 | PARDI N, HOGAN M J, PORTER F W, et al. mRNA vaccines-a new era in vaccinology[J]. Nature Reviews Drug Discovery, 2018, 17(4): 261-279. |
| 54 | WANG Z Y, JACOBUS E J, STIRLING D C, et al. Reducing cell intrinsic immunity to mRNA vaccine alters adaptive immune responses in mice[J]. Molecular Therapy Nucleic Acids, 2023, 34: 102045. |
| 55 | RAUCH S, ROTH N, SCHWENDT K, et al. mRNA-based SARS-CoV-2 vaccine candidate CVnCoV induces high levels of virus-neutralising antibodies and mediates protection in rodents[J]. NPJ Vaccines, 2021, 6(1): 57. |
| 56 | KARIKÓ K, BUCKSTEIN M, NI H P, et al. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA[J]. Immunity, 2005, 23(2): 165-175. |
| 57 | KARIKÓ K, MURAMATSU H, WELSH F A, et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability[J]. Molecular Therapy, 2008, 16(11): 1833-1840. |
| 58 | XIA X H. Detailed dissection and critical evaluation of the pfizer/BioNTech and moderna mRNA vaccines[J]. Vaccines, 2021, 9(7): 734. |
| 59 | QU L, YI Z Y, SHEN Y, et al. Circular RNA vaccines against SARS-CoV-2 and emerging variants[J]. Cell, 2022, 185(10): 1728-1744.e16. |
| 60 | LI D P, MARTINEZ D R, SCHÄFER A, et al. Breadth of SARS-CoV-2 neutralization and protection induced by a nanoparticle vaccine[J]. Nature Communications, 2022, 13(1): 6309. |
| 61 | ZHANG Z R, HE Q R, ZHAO W, et al. A heterologous V-01 or variant-matched bivalent V-01D-351 booster following primary series of inactivated vaccine enhances the neutralizing capacity against SARS-CoV-2 Delta and Omicron strains[J]. Journal of Clinical Medicine, 2022, 11(14): 4164. |
| 62 | ZHAO Y L, NI W J, LIANG S M, et al. Vaccination with Span, an antigen guided by SARS-CoV-2 S protein evolution, protects against challenge with viral variants in mice[J]. Science Translational Medicine, 2023, 15(677): eabo3332. |
| 63 | KANG Y F, SUN C, SUN J, et al. Quadrivalent mosaic HexaPro-bearing nanoparticle vaccine protects against infection of SARS-CoV-2 variants[J]. Nature Communications, 2022, 13(1): 2674. |
| 64 | JOYCE M G, KING H A D, ELAKHAL-NAOUAR I, et al. A SARS-CoV-2 ferritin nanoparticle vaccine elicits protective immune responses in nonhuman primates[J]. Science Translational Medicine, 2022, 14(632): eabi5735. |
| 65 | TAI W B, CHAI B J, FENG S Y, et al. Development of a ferritin-based nanoparticle vaccine against the SARS-CoV-2 Omicron variant[J]. Signal Transduction and Targeted Therapy, 2022, 7(1): 173. |
| 66 | PAN X Y, SHI J, HU X, et al. RBD-homodimer, a COVID-19 subunit vaccine candidate, elicits immunogenicity and protection in rodents and nonhuman primates[J]. Cell Discovery, 2021, 7(1): 82. |
| 67 | DAI L P, DUAN H X, LIU X Y, et al. Omicron neutralisation: RBD-dimer booster versus BF.7 and BA.5.2 breakthrough infection[J]. Lancet, 2023, 402(10403): 687-689. |
| 68 | JOYCE M G, CHEN W H, SANKHALA R S, et al. SARS-CoV-2 ferritin nanoparticle vaccines elicit broad SARS coronavirus immunogenicity[J]. Cell Reports, 2021, 37(12): 110143. |
| 69 | MOREIRA E D Jr, KITCHIN N, XU X, et al. Safety and efficacy of a third dose of BNT162b2 COVID-19 vaccine[J]. The New England Journal of Medicine, 2022, 386(20): 1910-1921. |
| 70 | DAI L P, ZHENG T Y, XU K, et al. A universal design of betacoronavirus vaccines against COVID-19, MERS, and SARS[J]. Cell, 2020, 182(3): 722-733.e11. |
| 71 | WANG R, HUANG X, CAO T S, et al. Development of a thermostable SARS-CoV-2 variant-based bivalent protein vaccine with cross-neutralizing potency against Omicron subvariants[J]. Virology, 2022, 576: 61-68. |
| 72 | WANG R, HUANG H P, YU C L, et al. A spike-trimer protein-based tetravalent COVID-19 vaccine elicits enhanced breadth of neutralization against SARS-CoV-2 Omicron subvariants and other variants[J]. Science China Life Sciences, 2023, 66(8): 1818-1830. |
| 73 | WRAPP D, WANG N S, CORBETT K S, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation[J]. Science, 2020, 367(6483): 1260-1263. |
| 74 | SUN X Y, YI C Y, ZHU Y F, et al. Neutralization mechanism of a human antibody with pan-coronavirus reactivity including SARS-CoV-2[J]. Nature Microbiology, 2022, 7(7): 1063-1074. |
| 75 | MA X C, ZOU F, YU F, et al. Nanoparticle vaccines based on the receptor binding domain (RBD) and heptad repeat (HR) of SARS-CoV-2 elicit robust protective immune responses[J]. Immunity, 2020, 53(6): 1315-1330.e9. |
| 76 | PANG W, LU Y, ZHAO Y B, et al. A variant-proof SARS-CoV-2 vaccine targeting HR1 domain in S2 subunit of spike protein[J]. Cell Research, 2022, 32(12): 1068-1085. |
| 77 | WANG X L, SUN L J, LIU Z Z, et al. An engineered recombinant protein containing three structural domains in SARS-CoV-2 S2 protein has potential to act as a pan-human coronavirus entry inhibitor or vaccine antigen[J]. Emerging Microbes & Infections, 2023, 12(2): 2244084. |
| 78 | YU D W, ZHU Y M, YAN H X, et al. Pan-coronavirus fusion inhibitors possess potent inhibitory activity against HIV-1, HIV-2, and Simian immunodeficiency virus[J]. Emerging Microbes & Infections, 2021, 10(1): 810-821. |
| 79 | BENHAIM M A, MANGALA PRASAD V, GARCIA N K, et al. Structural monitoring of a transient intermediate in the hemagglutinin fusion machinery on influenza virions[J]. Science Advances, 2020, 6(18): eaaz8822. |
| 80 | LADINSKY M S, GNANAPRAGASAM P N, YANG Z, et al. Electron tomography visualization of HIV-1 fusion with target cells using fusion inhibitors to trap the pre-hairpin intermediate[J]. eLife, 2020, 9: e58411. |
| 81 | DOLGIN E. Pan-coronavirus vaccine pipeline takes form[J]. Nature Reviews Drug Discovery, 2022, 21(5): 324-326. |
| 82 | WU Y T, WANG S J, ZHANG Y L, et al. Lineage-mosaic and mutation-patched spike proteins for broad-spectrum COVID-19 vaccine[J]. Cell Host & Microbe, 2022, 30(12): 1732-1744.e7. |
| 83 | RICE A, VERMA M, SHIN A, et al. Intranasal plus subcutaneous prime vaccination with a dual antigen COVID-19 vaccine elicits T-cell and antibody responses in mice[J]. Scientific Reports, 2021, 11(1): 14917. |
| 84 | STERNKE M, TRIPP K W, BARRICK D. Consensus sequence design as a general strategy to create hyperstable, biologically active proteins[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(23): 11275-11284. |
| 85 | TEBAS P, ROBERTS C C, MUTHUMANI K, et al. Safety and immunogenicity of an anti-Zika virus DNA vaccine[J]. The New England Journal of Medicine, 2021, 385(12): e35. |
| 86 | WANG R, ZHENG X Y, SUN J, et al. Vaccination with a single consensus envelope protein ectodomain sequence administered in a heterologous regimen induces tetravalent immune responses and protection against dengue viruses in mice[J]. Frontiers in Microbiology, 2019, 10: 1113. |
| 87 | CHEN M W, CHENG T J, HUANG Y X, et al. A consensus-hemagglutinin-based DNA vaccine that protects mice against divergent H5N1 influenza viruses[J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(36): 13538-13543. |
| 88 | ELLIOTT S T C, KEATON A A, CHU J D, et al. A synthetic micro-consensus DNA vaccine generates comprehensive influenza A H3N2 immunity and protects mice against lethal challenge by multiple H3N2 viruses[J]. Human Gene Therapy, 2018, 29(9): 1044-1055. |
| 89 | PING X Q, HU W B, XIONG R, et al. Generation of a broadly reactive influenza H1 antigen using a consensus HA sequence[J]. Vaccine, 2018, 36(32 Pt B): 4837-4845. |
| 90 | LIU Z Z, XU W, XIA S, et al. RBD-Fc-based COVID-19 vaccine candidate induces highly potent SARS-CoV-2 neutralizing antibody response[J]. Signal Transduction and Targeted Therapy, 2020, 5(1): 282. |
| 91 | SUN S Y, CAI Y Q, SONG T Z, et al. Interferon-armed RBD dimer enhances the immunogenicity of RBD for sterilizing immunity against SARS-CoV-2[J]. Cell Research, 2021, 31(9): 1011-1023. |
| 92 | LIU Z Z, ZHOU J, XU W, et al. A novel STING agonist-adjuvanted pan-sarbecovirus vaccine elicits potent and durable neutralizing antibody and T cell responses in mice, rabbits and NHPs[J]. Cell Research, 2022, 32(3): 269-287. |
| 93 | WANG J, LI P Y, YU Y, et al. Pulmonary surfactant-biomimetic nanoparticles potentiate heterosubtypic influenza immunity[J]. Science, 2020, 367(6480): eaau0810. |
| 94 | LIU Z Z, XU W, CHEN Z G, et al. An ultrapotent pan-β- coronavirus lineage B (β-CoV-B) neutralizing antibody locks the receptor-binding domain in closed conformation by targeting its conserved epitope[J]. Protein & Cell, 2022, 13(9): 655-675. |
| 95 | HEATH P T, GALIZA E P, BAXTER D N, et al. Safety and efficacy of NVX-CoV2373 COVID-19 vaccine[J]. The New England Journal of Medicine, 2021, 385(13): 1172-1183. |
| 96 | LAZARUS R, QUERTON B, CORBIC RAMLJAK I, et al. Immunogenicity and safety of an inactivated whole-virus COVID-19 vaccine (VLA2001) compared with the adenoviral vector vaccine ChAdOx1-S in adults in the UK (COV-COMPARE): interim analysis of a randomised, controlled, phase 3, immunobridging trial[J]. The Lancet Infectious Diseases, 2022, 22(12): 1716-1727. |
| 97 | LIU Y, DAI L P, FENG X L, et al. Fast and long-lasting immune response to S-trimer COVID-19 vaccine adjuvanted by PIKA[J]. Molecular Biomedicine, 2021, 2(1): 29. |
| 98 | XU K J, YU S Y, WANG K, et al. AI and knowledge-based method for rational design of Escherichia coli Sigma70 promoters[J]. ACS Synthetic Biology, 2024, 13(1): 402-407. |
| 99 | LAI C J, KIM D, KANG S, et al. Viral codon optimization on SARS-CoV-2 spike boosts immunity in the development of COVID-19 mRNA vaccines[J]. Journal of Medical Virology, 2023, 95(10): e29183. |
| 100 | BEUKERS M W, KLAASSEN C H, DE GRIP W J, et al. Heterologous expression of rat epitope-tagged histamine H2 receptors in insect Sf9 cells[J]. British Journal of Pharmacology, 1997, 122(5): 867-874. |
| 101 | DENG S F, LIU Y, TAM R C, et al. An intranasal influenza virus-vectored vaccine prevents SARS-CoV-2 replication in respiratory tissues of mice and hamsters[J]. Nature Communications, 2023, 14(1): 2081. |
| 102 | ZHANG H, ZHANG L, LIN A, et al. Algorithm for optimized mRNA design improves stability and immunogenicity[J]. Nature, 2023, 621(7978): 396-403. |
| 103 | SADEGHALVAD M, MANSOURABADI A H, NOORI M, et al. Recent developments in SARS-CoV-2 vaccines: a systematic review of the current studies[J]. Reviews in Medical Virology, 2023, 33(1): e2359. |
| 104 | KORBER B, FISCHER W M, GNANAKARAN S, et al. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus[J]. Cell, 2020, 182(4): 812-827.e19. |
| 105 | GARCIA-BELTRAN W F, LAM E C, DENIS K S, et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity[J]. Cell, 2021, 184(9): 2523. |
| 106 | CELE S, JACKSON L, KHOURY D S, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization[J]. Nature, 2022, 602(7898): 654-656. |
| 107 | GRETEBECK L M, SUBBARAO K. Animal models for SARS and MERS coronaviruses[J]. Current Opinion in Virology, 2015, 13: 123-129. |
| 108 | VAN DOREMALEN N, MUNSTER V J. Animal models of Middle East respiratory syndrome coronavirus infection[J]. Antiviral Research, 2015, 122: 28-38. |
| 109 | HOFFMANN M, KLEINE-WEBER H, SCHROEDER S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor[J]. Cell, 2020, 181(2): 271-280.e8. |
| 110 | BAO L L, DENG W, HUANG B Y, et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice[J]. Nature, 2020, 583(7818): 830-833. |
| 111 | SILVAS J A, VASQUEZ D M, PARK J G, et al. Contribution of SARS-CoV-2 accessory proteins to viral pathogenicity in K18 human ACE2 transgenic mice[J]. Journal of Virology, 2021, 95(17): e0040221. |
| 112 | SUN J, ZHUANG Z, ZHENG J, et al. Generation of a broadly useful model for COVID-19 pathogenesis, vaccination, and treatment[J]. Cell, 2020, 182(3): 734-743.e5. |
| 113 | KIM Y I, KIM S G, KIM S M, et al. Infection and rapid transmission of SARS-CoV-2 in ferrets[J]. Cell Host & Microbe, 2020, 27(5): 704-709.e2. |
| 114 | LI D P, EDWARDS R J, MANNE K, et al. The functions of SARS-CoV-2 neutralizing and infection-enhancing antibodies in vitro and in mice and nonhuman Primates[EB/OL]. bioRxiv, 2021: 2020.12.31.424729[2023-11-01]. . |
| 115 | CHANDRASHEKAR A, LIU J Y, MARTINOT A J, et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques[J]. Science, 2020, 369(6505): 812-817. |
| 116 | BUTT A A, GUERRERO M D, CANLAS E B, et al. Evaluation of mortality attributable to SARS-CoV-2 vaccine administration using national level data from Qatar[J]. Nature Communications, 2023, 14(1): 24. |
| 117 | MULRONEY T E, PÖYRY T, YAM-PUC J C, et al. N1-methylpseudouridylation of mRNA causes +1 ribosomal frameshifting[J]. Nature, 2024, 625(7993): 189-194. |
| 118 | WOO E J, MBA-JONAS A, DIMOVA R B, et al. Association of receipt of the Ad26.COV2.S COVID-19 vaccine with presumptive guillain-barré syndrome, february-july 2021[J]. JAMA, 2021, 326(16): 1606-1613. |
| 119 | GREINACHER A, THIELE T, WARKENTIN T E, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination[J]. The New England Journal of Medicine, 2021, 384(22): 2092-2101. |
| [1] | 高歌, 边旗, 王宝俊. 合成基因线路的工程化设计研究进展与展望[J]. 合成生物学, 2025, 6(1): 45-64. |
| [2] | 李冀渊, 吴国盛. 合成生物学视域下有机体的两种隐喻[J]. 合成生物学, 2025, 6(1): 190-202. |
| [3] | 焦洪涛, 齐蒙, 邵滨, 蒋劲松. DNA数据存储技术的法律治理议题[J]. 合成生物学, 2025, 6(1): 177-189. |
| [4] | 唐兴华, 陆钱能, 胡翌霖. 人类世中对合成生物学的哲学反思[J]. 合成生物学, 2025, 6(1): 203-212. |
| [5] | 徐怀胜, 石晓龙, 刘晓光, 徐苗苗. DNA存储的关键技术:编码、纠错、随机访问与安全性[J]. 合成生物学, 2025, 6(1): 157-176. |
| [6] | 石婷, 宋展, 宋世怡, 张以恒. 体外生物转化(ivBT):生物制造的新前沿[J]. 合成生物学, 2024, 5(6): 1437-1460. |
| [7] | 柴猛, 王风清, 魏东芝. 综合利用木质纤维素生物转化合成有机酸[J]. 合成生物学, 2024, 5(6): 1242-1263. |
| [8] | 邵明威, 孙思勉, 杨时茂, 陈国强. 基于极端微生物的生物制造[J]. 合成生物学, 2024, 5(6): 1419-1436. |
| [9] | 陈雨, 张康, 邱以婧, 程彩云, 殷晶晶, 宋天顺, 谢婧婧. 微生物电合成技术转化二氧化碳研究进展[J]. 合成生物学, 2024, 5(5): 1142-1168. |
| [10] | 郑皓天, 李朝风, 刘良叙, 王嘉伟, 李恒润, 倪俊. 负碳人工光合群落的设计、优化与应用[J]. 合成生物学, 2024, 5(5): 1189-1210. |
| [11] | 夏孔晨, 徐维华, 吴起. 光酶催化混乱性反应的研究进展[J]. 合成生物学, 2024, 5(5): 997-1020. |
| [12] | 陈子苓, 向阳飞. 类器官技术与合成生物学协同研究进展[J]. 合成生物学, 2024, 5(4): 795-812. |
| [13] | 蔡冰玉, 谭象天, 李伟. 合成生物学在干细胞工程化改造中的研究进展[J]. 合成生物学, 2024, 5(4): 782-794. |
| [14] | 谢皇, 郑义蕾, 苏依婷, 阮静怡, 李永泉. 放线菌聚酮类化合物生物合成体系重构研究进展[J]. 合成生物学, 2024, 5(3): 612-630. |
| [15] | 查文龙, 卜兰, 訾佳辰. 中药药效成分群的合成生物学研究进展[J]. 合成生物学, 2024, 5(3): 631-657. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||

