合成生物学 ›› 2021, Vol. 2 ›› Issue (5): 734-750.DOI: 10.12211/2096-8280.2021-056
天然产物中炔基的生物合成机制研究及其应用
吕建明1, 赵欢2, 胡丹1, 高昊1
- 1.暨南大学药学院,中药及天然药物研究所,广东 广州 510632
2.暨南大学中医学院,广东 广州 510632
-
收稿日期:2021-05-07修回日期:2021-07-25出版日期:2021-10-31发布日期:2021-11-19 -
通讯作者:胡丹,高昊 -
作者简介:吕建明 (1985—),男,博士,副研究员。研究方向为真菌天然产物的生物合成。E-mail:ljm21@jnu.edu.cn胡丹 (1979—),男,博士,研究员。研究方向为天然产物的生物合成。E-mail:thudan@jnu.edu.cn高昊 (1979—),男,博士,教授。研究方向为天然药物化学。E-mail:tghao@jnu.edu.cn -
基金资助:国家重点研发计划(2018YFA0903200/2018YFA0903201)
Biosynthesis of alkyne moiety in natural products and application of alkyne biosynthetic machineries
LYU Jianming1, ZHAO Huan2, HU Dan1, GAO Hao1
- 1.Institute of Traditional Chinese Medicine and Natural Products,College of Pharmacy,Jinan University,Guangzhou 510632,Guangdong,China
2.College of Traditional Chinese Medicine,Jinan University,Guangzhou 510632,Guangdong,China
-
Received:2021-05-07Revised:2021-07-25Online:2021-10-31Published:2021-11-19 -
Contact:HU Dan, GAO Hao
摘要:
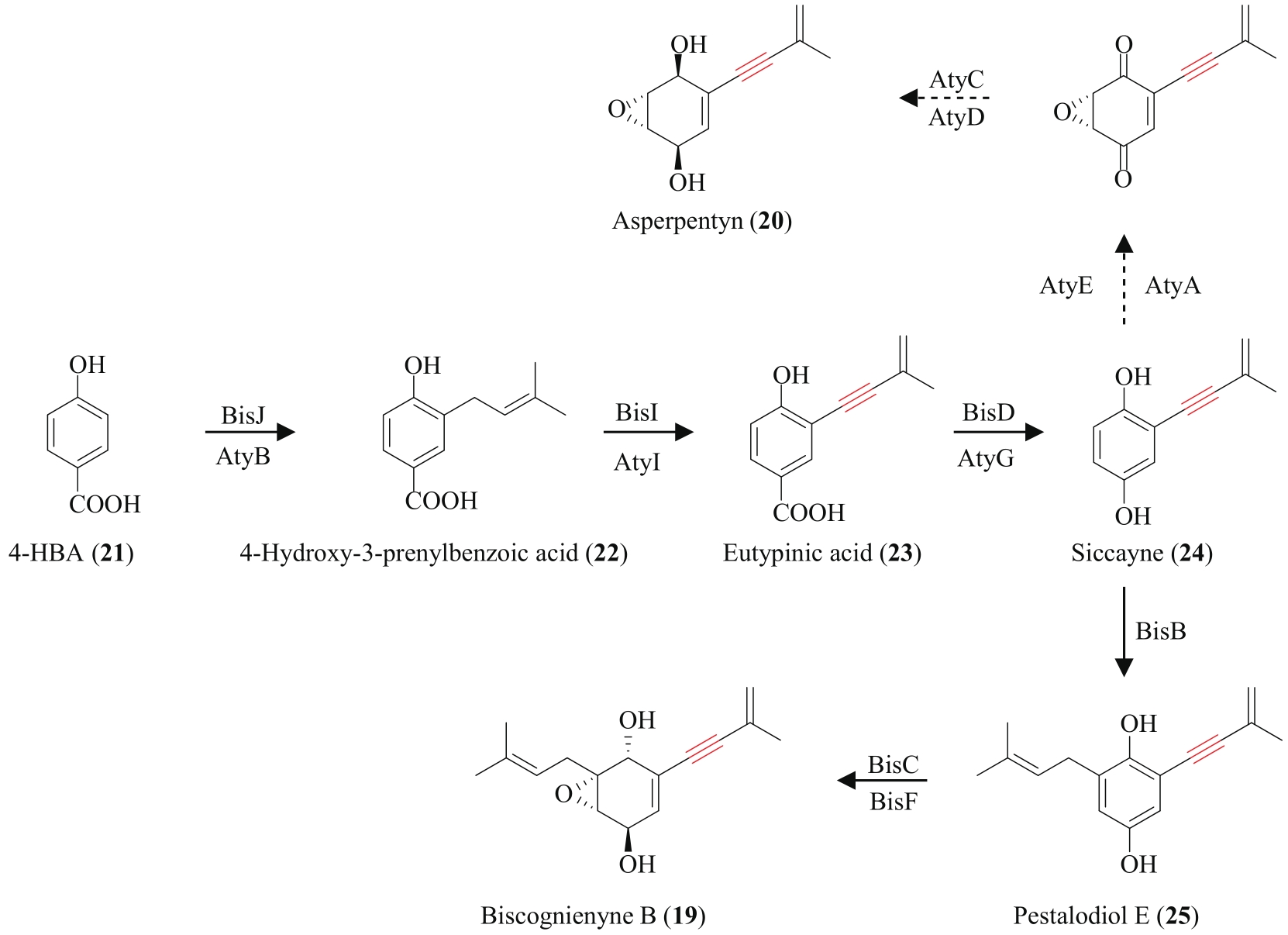
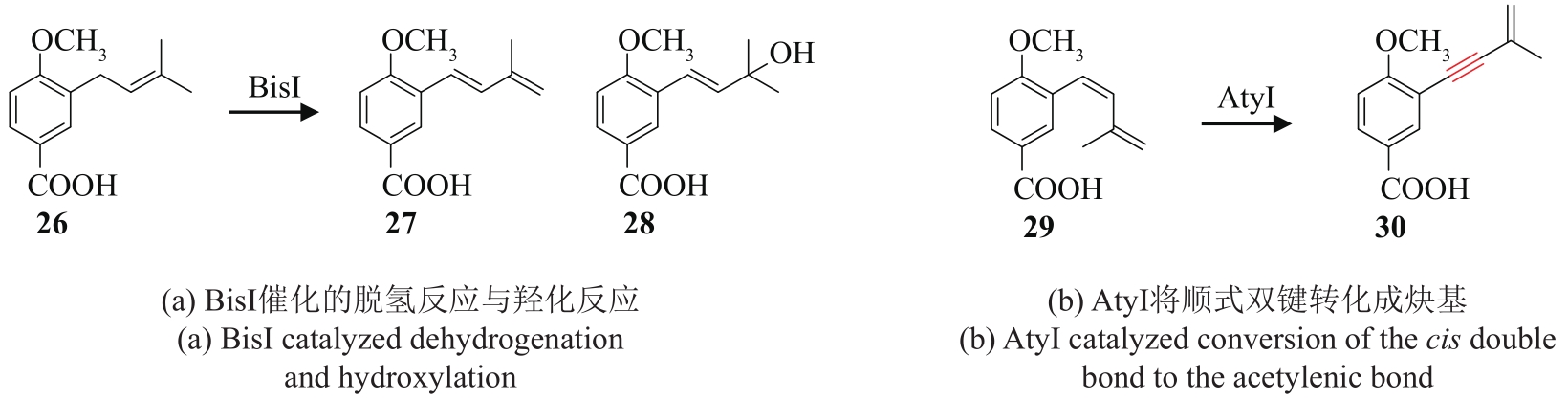
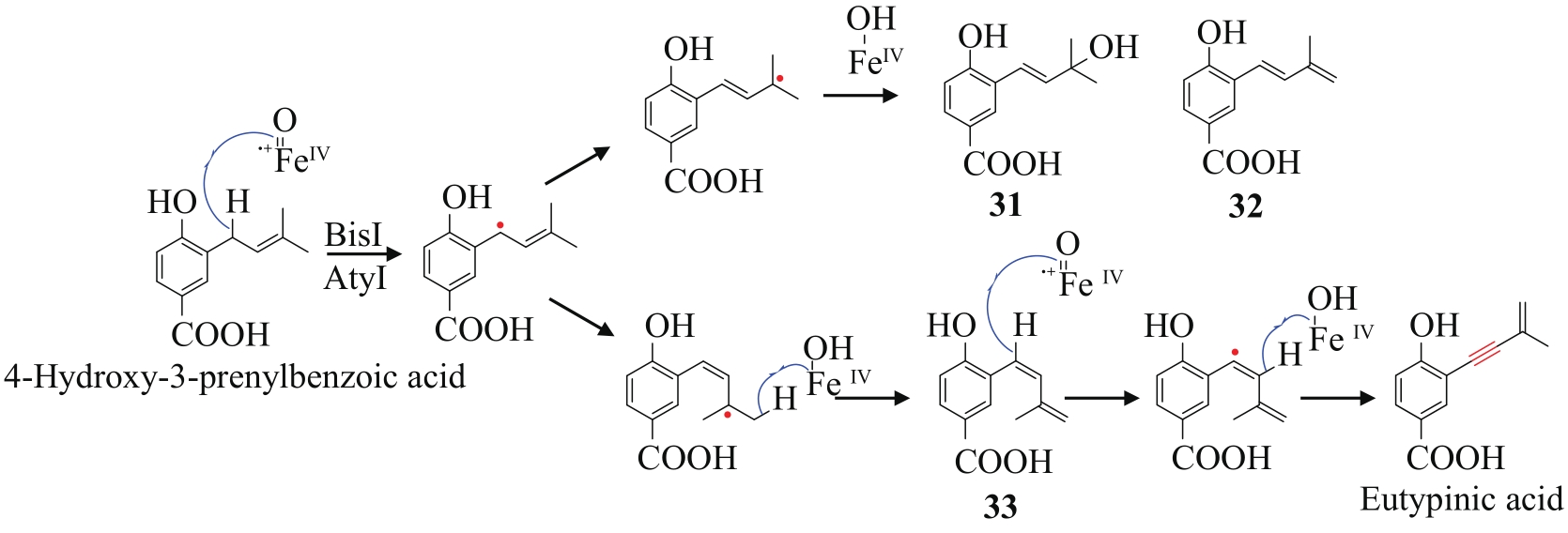
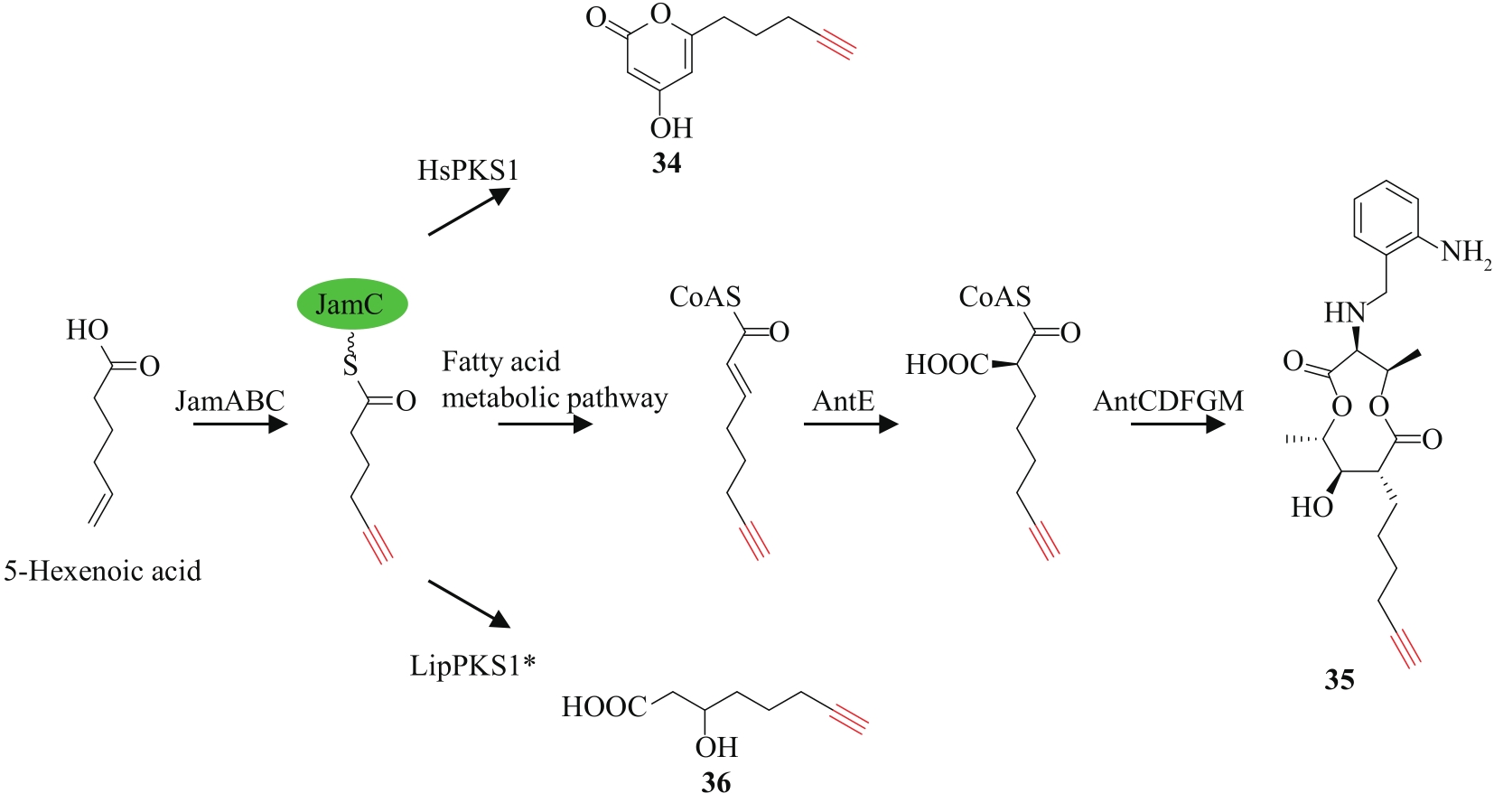
炔基是许多药物、活性天然产物以及功能材料等的重要官能团,因此,开发高效的炔类化合物合成策略具有重要意义。传统的合成策略包括通过化学反应直接制备,或以简单的炔类前体为底物,通过生物转化获得。随着合成生物学技术的飞速发展,从头生物合成有望成为炔类化合物合成的新策略。该策略绿色环保,易于操作,是对传统化学合成以及生物转化策略的有效补充。炔类化合物从头生物合成的关键是阐明天然产物中炔基的生物合成机制,获得炔基合成酶。本文重点总结了不同天然产物中炔基的生物合成研究进展,包括脂肪酸、聚酮、聚酮-非核糖体肽杂合体、氨基酸以及杂萜,并介绍了炔基合成酶在炔类化合物从头生物合成中的应用。尽管炔基的生物合成研究近年来取得了长足发展,但在炔基合成酶的种类挖掘及其底物特异性拓展方面还有待进一步加强,从而为炔类化合物的从头生物合成提供更多可供选择的酶工具。
中图分类号:
引用本文
吕建明, 赵欢, 胡丹, 高昊. 天然产物中炔基的生物合成机制研究及其应用[J]. 合成生物学, 2021, 2(5): 734-750.
LYU Jianming, ZHAO Huan, HU Dan, GAO Hao. Biosynthesis of alkyne moiety in natural products and application of alkyne biosynthetic machineries[J]. Synthetic Biology Journal, 2021, 2(5): 734-750.
| 序号 | 来源 | 乙炔酶名称与NCBI登录号 | 产物 | 文献 | |
|---|---|---|---|---|---|
| 1 | 植物 | Crepis alpina | Crep1 (CAA76158) |  | [ |
| 2 | Petroselinum crispum | PcACET (AAB80697) |  | [ | |
| 3 | Hedera helix | HhACET (AAO38031) |  | [ | |
| 4 | Helianthus annuus | HaACET (AAO38032) |  | [ | |
| 5 | Calendula officinalis | CoACET (CAB64256) |  | [ | |
| 6 | Daucus carota | DCAR_013552 (XP_017247320) |  | [ | |
| 7 | Daucus carota | DCAR_017011 (XP_017253817) |  | [ | |
| 8 | Daucus carota | DCAR_013548 (XP_017246930) |  | [ | |
| 9 | Solanum lycopersicum | ACET1a/b |  | [ | |
| 10 | Ceratodon purpureus | CpAcet6 (Q9LEN0) | 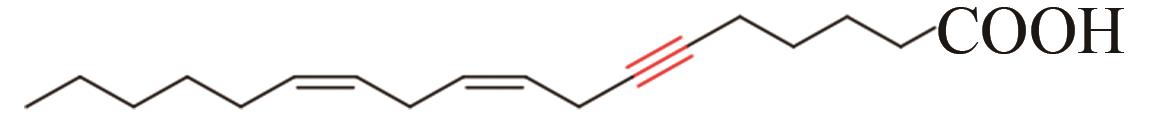 | [ | |
| 11 | Bidens pilosa | BPFAA (MF318525) | 未知 | [ | |
| 12 | 昆虫 | Thaumetopoea pityocampa | Tpi-PGFAD (ABO43722) | 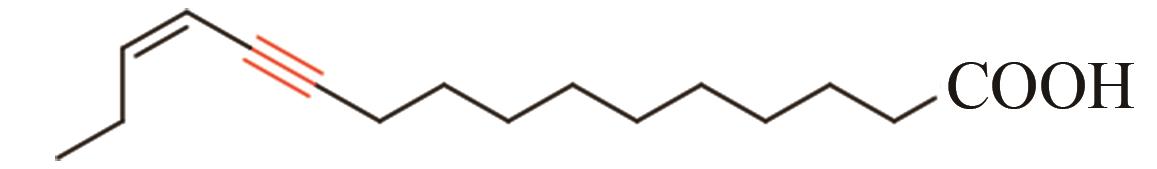 | [ |
| 13 | Chauliognathus lugubris | CL10 (AFJ66832) |  | [ | |
| 14 | Chauliognathus lugubris | CL2-1 (AFJ66828) | 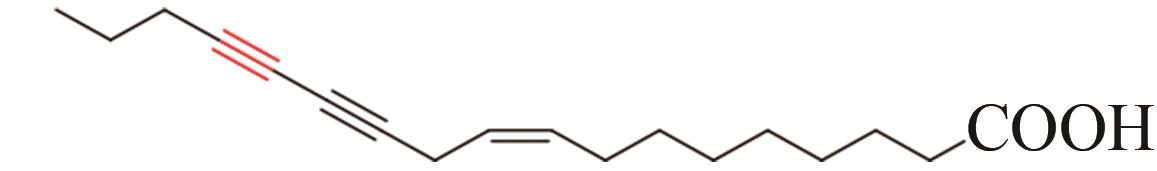 | [ | |
| 15 | Chauliognathus lugubris | CL2-2 (AFJ66829) | 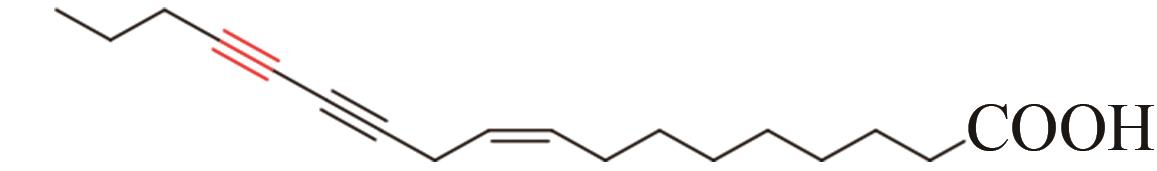 | [ | |
| 16 | 微生物 | Cantharellus formosus | CfACET (ADK24720) |  | [ |
| 17 | Burkholderia caryophylli | CayB (AIG53817) | 未知 | [ | |
| 18 | Burkholderia caryophylli | CayC (AIG53820) | 未知 | [ |
表1 已鉴定的参与炔类脂肪酸生物合成的乙炔酶
Tab. 1 Acetylenases identified for the biosynthesis of acetylenic fatty acids
| 序号 | 来源 | 乙炔酶名称与NCBI登录号 | 产物 | 文献 | |
|---|---|---|---|---|---|
| 1 | 植物 | Crepis alpina | Crep1 (CAA76158) |  | [ |
| 2 | Petroselinum crispum | PcACET (AAB80697) |  | [ | |
| 3 | Hedera helix | HhACET (AAO38031) |  | [ | |
| 4 | Helianthus annuus | HaACET (AAO38032) |  | [ | |
| 5 | Calendula officinalis | CoACET (CAB64256) |  | [ | |
| 6 | Daucus carota | DCAR_013552 (XP_017247320) |  | [ | |
| 7 | Daucus carota | DCAR_017011 (XP_017253817) |  | [ | |
| 8 | Daucus carota | DCAR_013548 (XP_017246930) |  | [ | |
| 9 | Solanum lycopersicum | ACET1a/b |  | [ | |
| 10 | Ceratodon purpureus | CpAcet6 (Q9LEN0) |  | [ | |
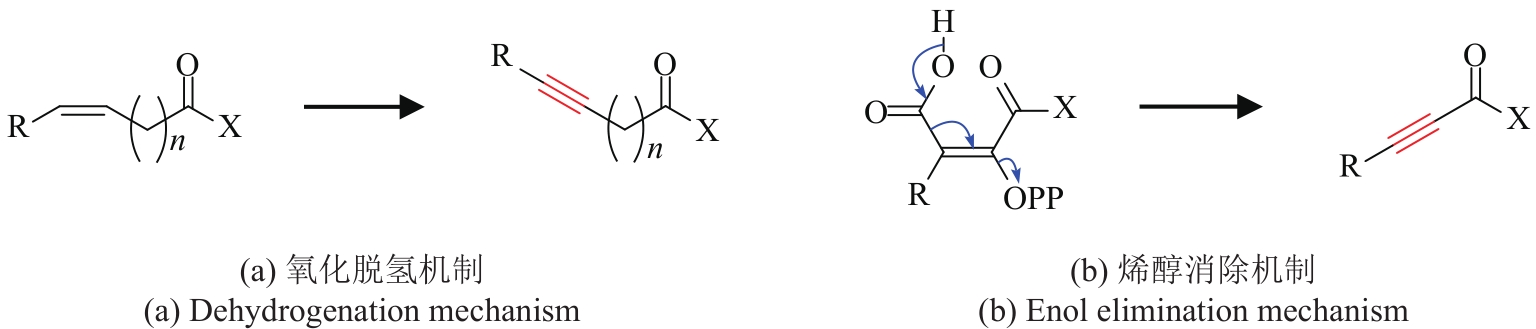
| 11 | Bidens pilosa | BPFAA (MF318525) | 未知 | [ | |
| 12 | 昆虫 | Thaumetopoea pityocampa | Tpi-PGFAD (ABO43722) |  | [ |
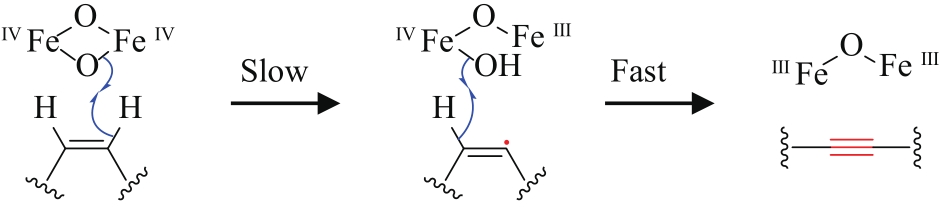
| 13 | Chauliognathus lugubris | CL10 (AFJ66832) |  | [ | |
| 14 | Chauliognathus lugubris | CL2-1 (AFJ66828) |  | [ | |
| 15 | Chauliognathus lugubris | CL2-2 (AFJ66829) |  | [ | |
| 16 | 微生物 | Cantharellus formosus | CfACET (ADK24720) |  | [ |
| 17 | Burkholderia caryophylli | CayB (AIG53817) | 未知 | [ | |
| 18 | Burkholderia caryophylli | CayC (AIG53820) | 未知 | [ |
| 1 | TALELE T T. Acetylene group, friend or foe in medicinal chemistry[J]. Journal of Medicinal Chemistry, 2020, 63(11): 5625-5663. |
| 2 | CHRISTIN-MAITRE S. History of oral contraceptive drugs and their use worldwide[J]. Best Practice & Research Clinical Endocrinology & Metabolism, 2013, 27(1): 3-12. |
| 3 | RAKHMANINA N Y, ANKER J N VAN DEN. Efavirenz in the therapy of HIV infection[J]. Expert Opinion on Drug Metabolism & Toxicology, 2010, 6(1): 95-103. |
| 4 | TAN F H, PUTOCZKI T L, STYLLI S S, et al. Ponatinib: a novel multi-tyrosine kinase inhibitor against human malignancies[J]. OncoTargets and Therapy, 2019, 12: 635-645. |
| 5 | JIRSCHELE K, SAND P K. Oxybutynin: past, present, and future[J]. International Urogynecology Journal, 2013, 24(4): 595-604. |
| 6 | SHEAR N H, VILLARS V V, MARSOLAIS C. Terbinafine: an oral and topical antifungal agent[J]. Clinics in Dermatology, 1991, 9(4): 487-495. |
| 7 | CHANDRARATNA R A S. Tazarotene: the first receptor-selective topical retinoid for the treatment of psoriasis[J]. Journal of the American Academy of Dermatology, 1997, 37(2 pt 3): S12-S17. |
| 8 | KUKLEV D V, DOMB A J, DEMBITSKY V M. Bioactive acetylenic metabolites[J]. Phytomedicine, 2013, 20(13): 1145-1159. |
| 9 | ZHOU Z F, MENNA M, CAI Y S, et al. Polyacetylenes of marine origin: Chemistry and bioactivity[J]. Chemical Reviews, 2015, 115(3): 1543-1596. |
| 10 | NEGRI R. Polyacetylenes from terrestrial plants and fungi: Recent phytochemical and biological advances[J]. Fitoterapia, 2015, 106: 92-109. |
| 11 | CHRISTENSEN L P. Bioactive C17 and C18 acetylenic oxylipins from terrestrial plants as potential lead compounds for anticancer drug development[J]. Molecules, 2020, 25(11): 2568. |
| 12 | DALY J W. Thirty years of discovering arthropod alkaloids in amphibian skin[J]. Journal of Natural Products, 1998, 61(1): 162-172. |
| 13 | CHRISTENSEN L P. Aliphatic C17-polyacetylenes of the falcarinol type as potential health promoting compounds in food plants of the Apiaceae family[J]. Recent Patents on Food, Nutrition & Agriculture, 2011, 3(1): 64-77. |
| 14 | DAWID C, DUNEMANN F, SCHWAB W, et al. Bioactive C17-polyacetylenes in carrots (Daucus carota L.): Current knowledge and future perspectives[J]. Journal of Agricultural and Food Chemistry, 2015, 63(42): 9211-9222. |
| 15 | NICOLAOU K C, CHEN J S, DALBY S M. From nature to the laboratory and into the clinic[J]. Bioorganic & Medicinal Chemistry, 2009, 17(6): 2290-2303. |
| 16 | SHAO R G. Pharmacology and therapeutic applications of enediyne antitumor antibiotics[J]. Current Molecular Pharmacology, 2008, 1(1): 50-60. |
| 17 | TYKWINSKI R R. Evolution in the palladium-catalyzed cross-coupling of sp-and sp2-hybridized carbon atoms[J]. Angewandte Chemie International Edition, 2003, 42(14): 1566-1568. |
| 18 | KOSCHKER P, BREIT B. Branching out: Rhodium-catalyzed allylation with alkynes and allenes[J]. Accounts of Chemical Research, 2016, 49(8): 1524-1536. |
| 19 | GODOI B, SCHUMACHER R F, ZENI G. Synthesis of heterocycles via electrophilic cyclization of alkynes containing heteroatom[J]. Chemical Reviews, 2011, 111(4): 2937-2980. |
| 20 | ZENG X M. Recent advances in catalytic sequential reactions involving hydroelement addition to carbon-carbon multiple bonds[J]. Chemical Reviews, 2013, 113(8): 6864-6900. |
| 21 | PATEL M, SAUNTHWAL R K, VERMA A K. Base-mediated hydroamination of alkynes[J]. Accounts of Chemical Research, 2017, 50(2): 240-254. |
| 22 | TROST B M, MASTERS J T. Transition metal-catalyzed couplings of alkynes to 1, 3-enynes: modern methods and synthetic applications[J]. Chemical Society Reviews, 2016, 45(8): 2212-2238. |
| 23 | TADROSS P M, STOLTZ B M. A comprehensive history of arynes in natural product total synthesis[J]. Chemical Reviews, 2012, 112(6): 3550-3577. |
| 24 | JURÍČEK M, KOUWER P H J, ROWAN A E. Triazole: a unique building block for the construction of functional materials[J]. Chemical Communications, 2011, 47(31): 8740-8749. |
| 25 | THIRUMURUGAN P, MATOSIUK D, JOZWIAK K. Click chemistry for drug development and diverse chemical-biology applications[J]. Chemical Reviews, 2013, 113(7): 4905-4979. |
| 26 | HABRANT D, RAUHALA V, KOSKINEN A M. Conversion of carbonyl compounds to alkynes: general overview and recent developments[J]. Chemical Society Reviews, 2010, 39(6): 2007-2017. |
| 27 | SHAW R, ELAGAMY A, ALTHAGAFI I, et al. Synthesis of alkynes from non-alkyne sources[J]. Organic & Biomolecular Chemistry, 2020, 18(20): 3797-3817. |
| 28 | DUPUIS S N, ROBERTSON A W, VEINOT T, et al. Synthetic diversification of natural products: Semi-synthesis and evaluation of triazole jadomycins[J]. Chemical Science, 2012, 3(5): 1640-1644. |
| 29 | YAN Y, CHEN J, ZHANG L H, et al. Multiplexing of combinatorial chemistry in antimycin biosynthesis: Expansion of molecular diversity and utility[J]. Angewandte Chemie International Edition, 2013, 52(47): 12308-12312. |
| 30 | SANDY M, ZHU X J, RUI Z, et al. Characterization of AntB, a promiscuous acyltransferase involved in antimycin biosynthesis[J]. Organic Letters, 2013, 15(13): 3396-3399. |
| 31 | HARVEY C J, PUGLISI J D, PANDE V S, et al. Precursor directed biosynthesis of an orthogonally functional erythromycin analogue: Selectivity in the ribosome macrolide binding pocket[J]. Journal of the American Chemical Society, 2012, 134(29): 12259-12265. |
| 32 | 林章凛, 张艳, 王胥, 等. 合成生物学研究进展[J]. 化工学报, 2015, 66(8): 2863-2871. |
| LIN Z L, ZHANG Y, WANG X, et al. Recent advances in synthetic biology[J]. CIESC Journal, 2015, 66(8): 2863-2871. | |
| 33 | KEASLING J D. Synthetic biology for synthetic chemistry[J]. ACS Chemical Biology, 2008, 3(1): 64-76. |
| 34 | MINTO R E, BLACKLOCK B J. Biosynthesis and function of polyacetylenes and allied natural products[J]. Progress in Lipid Research, 2008, 47(4): 233-306. |
| 35 | CHAI Q Y, YANG Z, LIN H W, et al. Alkynyl-containing peptides of marine origin: a review[J]. Marine Drugs, 2016, 14(11): 216. |
| 36 | MEINWALD J, MEINWALD Y C, CHALMERS A M, et al. Dihydromatricaria acid: Acetylenic acid secreted by soldier beetle[J]. Science, 1968, 160(3830): 890-892. |
| 37 | BU'LOCK J D, SMITH G N. The origin of naturally-occurring acetylenes[J]. Journal of the Chemical Society C: Organic, 1967, 332-336. |
| 38 | FLEMING I, HARLEY-MASON J. Enol elimination reactions (I): a new synthesis of acetylenic acids[J/OL]. Journal of the Chemical Society, 1963: 4771-4777. |
| CDCAC 75 A932C42E72F36729D947&thid=OIP.5Uql7uEJ1xbUyr_-yZCzyQHaJs&mediaurl=https%3a%2f%2fpubs.rsc.org%2fen%2fImage%2fGet%3fimageInfo.ImageType%3dGA%26imageInfo.ImageIdentifier.ManuscriptID%3dJR9630004771%26imageInfo.ImageIdentifier.Year%3d1963&cdnurl=https%3a%2f%2ftse1-mm.cn.bing.net%2fth%2fid%2fR-C.e54aa5eee109d716d4cabffec990b3c9%3frik%3dR9kpZ%252fNyLsQyqQ%26pid%3dImgRaw%26r%3d0&exph=655&expw=500&q=enol+elimination+reactions+i&simid=607992233410701701&FORM=IRPRST&ck=7D5B10BF8DF7FBE406CF4E280217CA91&selectedIndex=0&qpvt=enol+elimination+reactions+i&ajaxhist=0&ajaxserp=0 | |
| 39 | LEE M, LENMAN M, BANAŚ A, et al. Identification of non-heme diiron proteins that catalyze triple bond and epoxy group formation[J]. Science, 1998, 280(5365): 915-918. |
| 40 | CARLSSON A S, THOMAEUS S, HAMBERG M, et al. Properties of two multifunctional plant fatty acid acetylenase/desaturase enzymes[J]. European Journal of Biochemistry, 2004, 271(14): 2991-2997. |
| 41 | CAHOON E B, SCHNURR J A, HUFFMAN E A, et al. Fungal responsive fatty acid acetylenases occur widely in evolutionarily distant plant families[J]. The Plant Journal, 2003, 34(5): 671-683. |
| 42 | BUSTA L, YIM W C, LABRANT E W, et al. Identification of genes encoding enzymes catalyzing the early steps of carrot polyacetylene biosynthesis[J]. Plant Physiology, 2018, 178(4): 1507-1521. |
| 43 | JEON J E, KIM J G, FISCHER C R, et al. A pathogen-responsive gene cluster for highly modified fatty acids in tomato[J]. Cell, 2020, 180(1): 176-187. |
| 44 | SPERLING P, LEE M, GIRKE T, et al. A bifunctional Δ6-fatty acyl acetylenase/desaturase from the moss Ceratodon purpureus. a new member of the cytochrome b5 superfamily[J]. European Journal of Biochemistry, 2000, 267(12): 3801-3811. |
| 45 | CHEN P Y, HSIEH M J, TSAI Y T, et al. Transformation and characterization of Δ12-fatty acid acetylenase and Δ12-oleate desaturase potentially involved in the polyacetylene biosynthetic pathway from Bidens pilosa [J]. Plants, 2020, 9(11): 1483. |
| 46 | CHUNG H H, TING H M, WANG W H, et al. Elucidation of enzymes involved in the biosynthetic pathway of bioactive polyacetylenes in Bidens pilosa using integrated omics approaches[J]. Journal of Experimental Botany, 2021, 72(2): 525-541. |
| 47 | SERRA M, PIÑA B, ABAD J L, et al. A multifunctional desaturase involved in the biosynthesis of the processionary moth sex pheromone[J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(42): 16444-16449. |
| 48 | HARITOS V S, HORNE I, DAMCEVSKI K, et al. The convergent evolution of defensive polyacetylenic fatty acid biosynthesis genes in soldier beetles[J]. Nature Communications, 2012, 3: 1150. |
| 49 | BLACKLOCK B J, SCHEFFLER B E, SHEPARD M R, et al. Functional diversity in fungal fatty acid synthesis: the first acetylenase from the Pacific golden chanterelle, Cantharellus formosus[J]. The Journal of Biological Chemistry, 2010, 285(37): 28442-28449. |
| 50 | ROSS C, SCHERLACH K, KLOSS F, et al. The molecular basis of conjugated polyyne biosynthesis in phytopathogenic bacteria[J]. Angewandte Chemie International Edition, 2014, 53(30): 7794-7798. |
| 51 | BLOOMFIELD D K, BLOCH K. The formation of Δ9-unsaturated fatty acids[J]. Journal of Biological Chemistry, 1960, 235: 337-345. |
| 52 | SHANKLIN J, CAHOON E B. Desaturation and related modifications of fatty acids[J]. Annual Review of Plant Physiology and Plant Molecular Biology, 1998, 49: 611-641. |
| 53 | BUIST P H. Fatty acid desaturases: Selecting the dehydrogenation channel[J]. Natural Product Reports, 2004, 21(2): 249-262. |
| 54 | SHANKLIN J, GUY J E, MISHRA G, et al. Desaturases: emerging models for understanding functional diversification of diiron-containing enzymes[J]. The Journal of Biological Chemistry, 2009, 284(28): 18559-18563. |
| 55 | BAI Y, MCCOY J G, LEVIN E J, et al. X-ray structure of a mammalian stearoyl-CoA desaturase[J]. Nature, 2015, 524(7564): 252-256. |
| 56 | LINDQVIST Y, HUANG W, SCHNEIDER G, et al. Crystal structure of Δ9 stearoyl-acyl carrier protein desaturase from castor seed and its relationship to other di-iron proteins[J]. The EMBO Journal, 1996, 15(16): 4081-4092. |
| 57 | REED D W, POLICHUK D R, BUIST P H, et al. Mechanistic study of an improbable reaction-alkene dehydrogenation by the Δ12 acetylenase of Crepis alpina [J]. Journal of the American Chemical Society, 2003, 125(35): 10635-10640. |
| 58 | BEHROUZIAN B, FAUCONNOT L, DALIGAULT F, et al. Mechanism of fatty acid desaturation in the green alga Chlorella vulgaris [J]. European Journal of Biochemistry, 2001, 268(12): 3545-3549. |
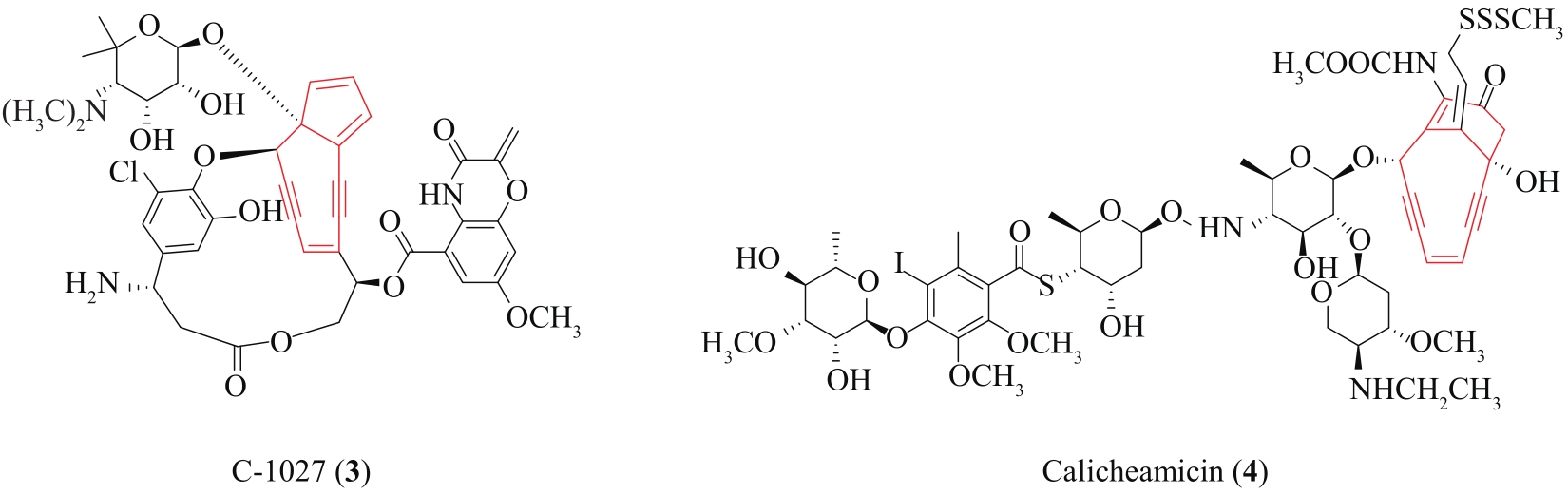
| 59 | LANEN S G VAN, SHEN B. Biosynthesis of enediyne antitumor antibiotics[J]. Current Topics in Medicinal Chemistry, 2008, 8(6): 448-459. |
| 60 | LIANG Z X. Complexity and simplicity in the biosynthesis of enediyne natural products[J]. Natural Product Reports, 2010, 27(4): 499-528. |
| 61 | MAEDA H, SAWA T, KONNO T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS[J]. Journal of Controlled Release, 2001, 74(1-3): 47-61. |
| 62 | DAMLE N K, FROST P. Antibody-targeted chemotherapy with immunoconjugates of calicheamicin[J]. Current Opinion in Pharmacology, 2003, 3(4): 386-390. |
| 63 | HENSENS O D, GINER J L, GOLDBERG I H. Biosynthesis of NCS Chrom A, the chromophore of the antitumor antibiotic neocarzinostatin[J]. Journal of the American Chemical Society, 1989, 111(9): 3295-3299. |
| 64 | LAM K S, VEITCH J A, GOLIK J, et al. Biosynthesis of esperamicin A1, an enediyne antitumor antibiotic[J]. Journal of the American Chemical Society, 1993, 115(26): 12340-12345. |
| 65 | THORSON J S, SHEN B, WHITWAM R E, et al. Enediyne biosynthesis and self-resistance: a progress report[J]. Bioorganic Chemistry, 1999, 27(2): 172-188. |
| 66 | LIU W, CHRISTENSON S D, STANDAGE S, et al. Biosynthesis of the enediyne antitumor antibiotic C-1027[J]. Science, 2002, 297(5584): 1170-1173. |
| 67 | AHLERT J, SHEPARD E, LOMOVSKAYA N, et al. The calicheamicin gene cluster and its iterative type I enediyne PKS[J]. Science, 2002, 297(5584): 1173-1176. |
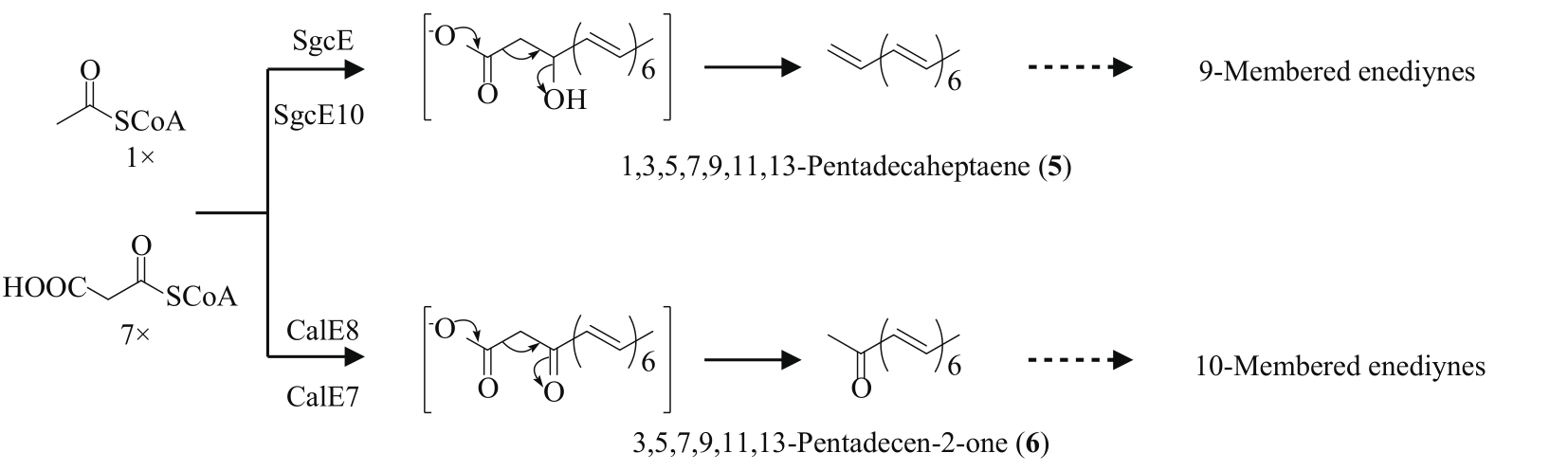
| 68 | ZHANG J, LANEN S G VAN, JU J H, et al. A phosphopantetheinylating polyketide synthase producing a linear polyene to initiate enediyne antitumor antibiotic biosynthesis[J]. Proceeding of the National Academy of Sciences of the United States of America, 2008, 105(5): 1460-1465. |
| 69 | KONG R, GOH L P, LIEW C W, et al. Characterization of a carbonyl-conjugated polyene precursor in 10-membered enediyne biosynthesis[J]. Journal of the American Chemical Society, 2008, 130(26): 8142-8143. |
| 70 | RIVAS L, ROJAS V. Cyanobacterial peptides as a tour de force in the chemical space of antiparasitic agents[J]. Archives of Biochemistry and Biophysics, 2019, 664: 24-39. |
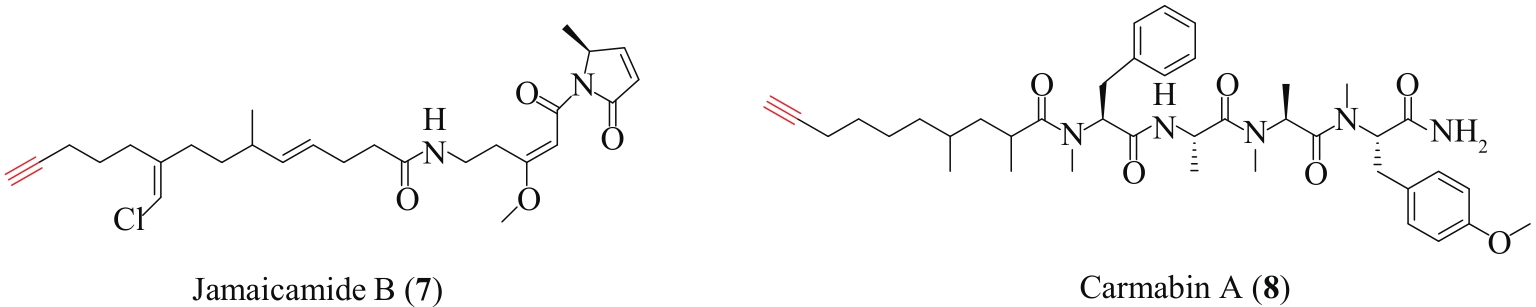
| 71 | EDWARDS D J, MARQUEZ B L, NOGLE L M, et al. Structure and biosynthesis of the jamaicamides, new mixed polyketide-peptide neurotoxins from the marine cyanobacterium Lyngbya majuscula [J]. Chemistry & Biology, 2004, 11(6): 817-833. |
| 72 | JONES A C, MONROE E A, PODELL S, et al. Genomic insights into the physiology and ecology of the marine filamentous cyanobacterium Lyngbya majuscula [J]. Proceeding of the National Academy of Sciences of the United States of America, 2011, 108(21): 8815-8820. |
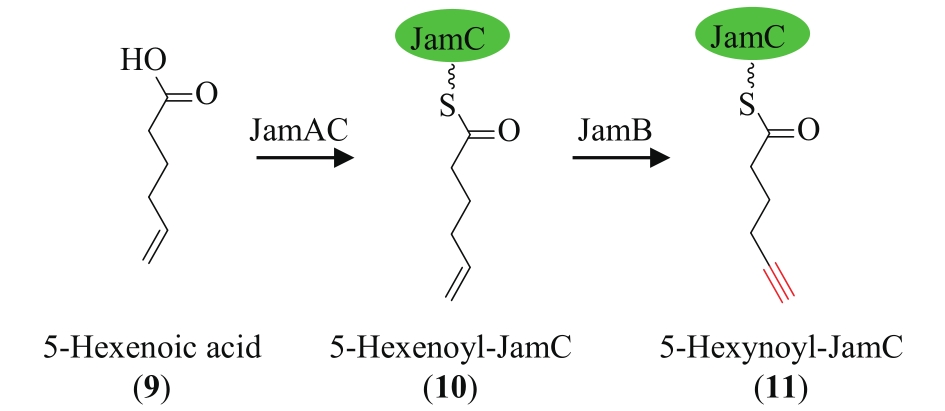
| 73 | ZHU X J, LIU J, ZHANG W J. De novo biosynthesis of terminal alkyne-labeled natural products[J]. Nature Chemical Biology, 2015, 11(2): 115-120. |
| 74 | ZHU X J, SU M, MANICKAM K, et al. Bacterial genome mining of enzymatic tools for alkyne biosynthesis[J]. ACS Chemical Biology, 2015, 10(12): 2785-2793. |
| 75 | POTGIETER H C, VERMEULEN N M J, POTGIETER D J J, et al. A toxic amino acid, 2(S),3(R)-2-amino-3-hydroxypent-4-ynoic acid from the fungus Sclerotium rolfsii [J]. Phytochemistry, 1977, 16(11): 1757-1759. |
| 76 | SANADA M, MIYANO T, IWADARE S. β-Ethynylserine, an antimetabolite of L-threonine, from Streptomyces cattleya [J]. The Journal of Antibiotics, 1986, 39(2): 304-305. |
| 77 | KURODA Y, OKUHARA M, GOTO T, et al. FR-900130, a novel amino acid antibiotic (Ⅱ): Isolation and structure elucidation of the acetyl derivative of FR-900130[J]. The Journal of Antibiotics, 1980, 33(2): 132-136. |
| 78 | HATANAKA S I. Amino acids from mushrooms[J]. Progress in the Chemistry of Organic Natural Products, 1992, 59: 1-140. |
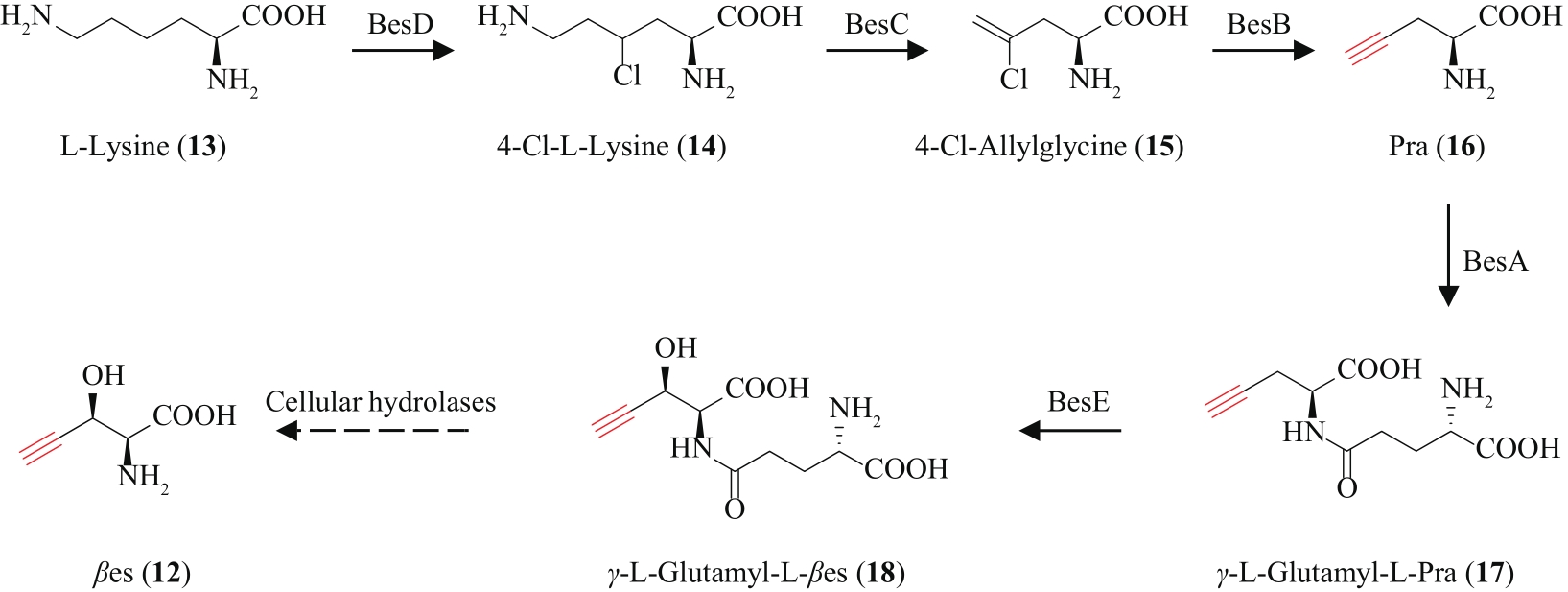
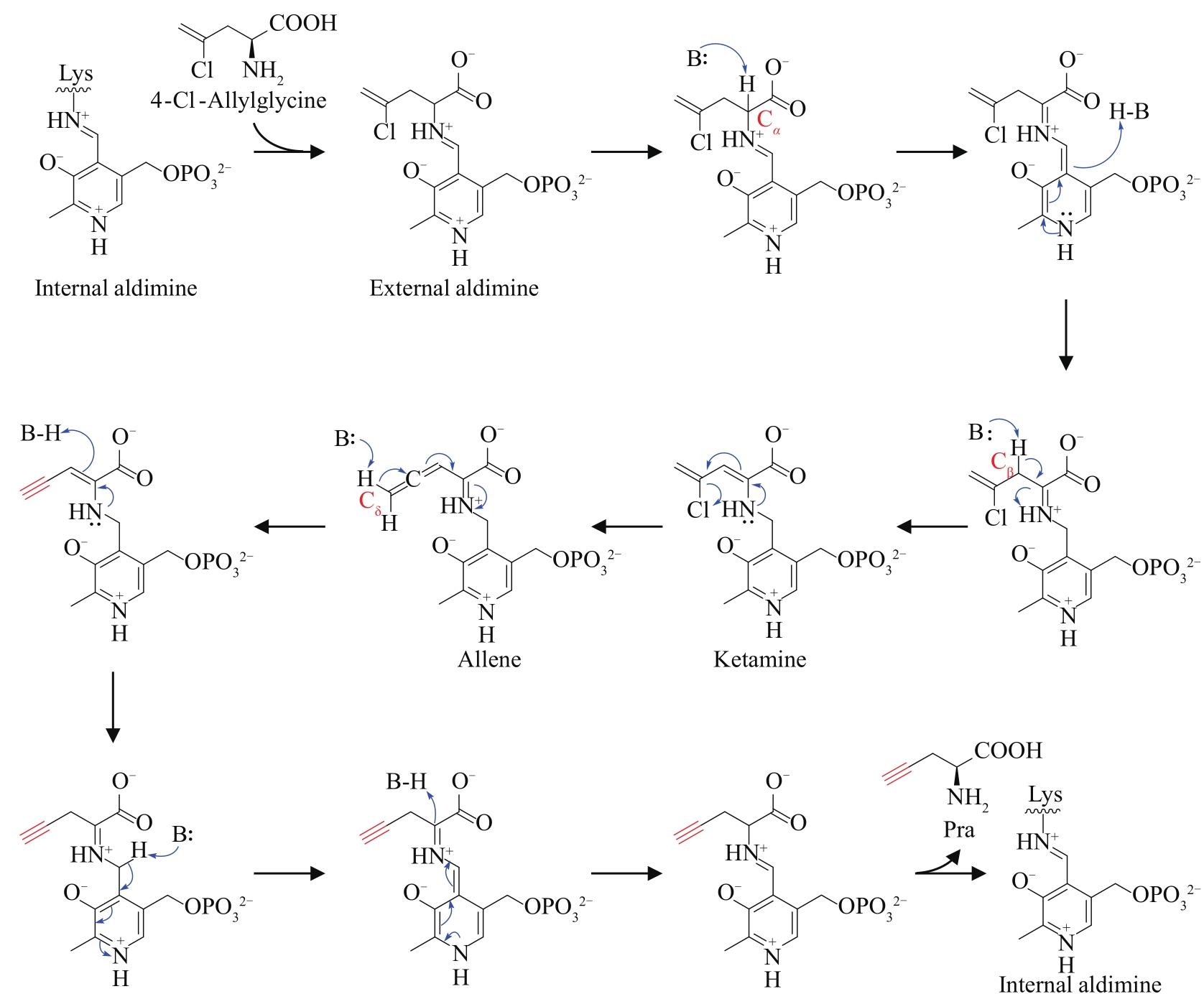
| 79 | MARCHAND J A, NEUGEBAUER M E, ING M C, et al. Discovery of a pathway for terminal-alkyne amino acid biosynthesis[J]. Nature, 2019, 567(7748): 420-424. |
| 80 | SUN Q X, COLLINS R, HUANG S F, et al. Structural basis for the inhibition mechanism of human cystathionine γ-lyase, an enzyme responsible for the production of H2S[J]. Journal of Biological Chemistry, 2009, 284(5): 3076-3085. |
| 81 | ABELES R H, WALSH C T. Acetylenic enzyme inactivators. Inactivation of γ-cystathionase, in vitro and in vivo, by propargylglycine[J]. Journal of the American Chemical Society, 1973, 95(18): 6124-6125. |
| 82 | ZHAO H, CHEN G D, ZOU J, et al. Dimericbiscognienyne A: A meroterpenoid dimer from Biscogniauxia sp. with new skeleton and its activity[J]. Organic Letters, 2017, 19(1): 38-41. |
| 83 | ZHAO H, WANG M Z, CHEN G D, et al. Dimericbiscognienynes B and C: new diisoprenyl-cyclohexene-type meroterpenoid dimers from Biscogniauxia sp.[J]. Chinese Chemical Letters, 2019, 30(1): 51-54. |
| 84 | PAN Y Y, LIU L, GUAN F F, et al. Characterization of a prenyltransferase for iso-A82775C biosynthesis and generation of new congeners of chloropestolides[J]. ACS Chemical Biology, 2018, 13(3): 703-711. |
| 85 | KIM G, KIM M J, CHUNG G, et al. (+)-Dimericbiscognienyne A: total synthesis and mechanistic investigations of the key heterodimerization[J]. Organic Letters, 2018, 20(21): 6886-6890. |
| 86 | SIEBERTZ R, PROKSCH P, WITTE L. Accumulation and biosynthesis of the chromenes precocene I and II in Ageratum houstonianum [J]. Phytochemistry, 1990, 29(7): 2135-2138. |
| 87 | LÜ J M, GAO Y H, ZHAO H, et al. Biosynthesis of biscognienyne B involving a cytochrome P450-dependent alkynylation[J]. Angewandte Chemie International Edition, 2020, 59(32): 13531-13536. |
| 88 | CHEN Y R, NARESH A, LIANG S Y, et al. Discovery of a dual function cytochrome P450 that catalyzes enyne formation in cyclohexanoid terpenoid biosynthesis[J]. Angewandte Chemie International Edition, 2020, 59(32): 13537-13541. |
| 89 | MORITA H, YAMASHITA M, SHI S P, et al. Synthesis of unnatural alkaloid scaffolds by exploiting plant polyketide synthase[J]. Proceeding of the National Academy of Sciences of the United States of America, 2011, 108(33): 13504-13509. |
| 90 | PORTERFIELD W B, POENATEETAI N, ZHANG W J. Engineered biosynthesis of alkyne-tagged polyketides by type I PKSs[J]. iScience, 2020, 23(3): 100938. |
| 91 | HUANG L S, COBESSI D, TUNG E Y, et al. Binding of the respiratory chain inhibitor antimycin to the mitochondrial bc1 complex: A new crystal structure reveals an altered intramolecular hydrogen-bonding pattern[J]. Journal of Molecular Biology, 2005, 351(3): 573-597. |
| 92 | SCHWARTZ P S, MANION M K, EMERSON C B, et al. 2-Methoxy antimycin reveals a unique mechanism for Bcl-xL inhibition[J]. Molecular Cancer Therapeutics, 2007, 6(7): 2073-2080. |
| 93 | BIHLMAIER C, WELLE E, HOFMANN C, et al. Biosynthetic gene cluster for the polyenoyltetramic acid α-lipomycin[J]. Antimicrobial Agents and Chemotherapy, 2006, 50(6): 2113-2121. |
| 94 | YUZAWA S, DENG K, WANG G, et al. Comprehensive in vitro analysis of acyltransferase domain exchanges in modular polyketide synthases and its application for short-chain ketone production[J]. ACS Synthetic Biology, 2017, 6(1): 139-147. |
| 95 | ZHU X J, SHIEH P, SU M, et al. A fluorogenic screening platform enables directed evolution of an alkyne biosynthetic tool[J]. Chemical Communications, 2016, 52(75): 11239-11242. |
| 96 | 谢泽雄, 陈祥荣, 肖文海, 等. 基因组再造与重排构建细胞工厂[J]. 化工学报, 2019, 70(10): 3712-3721. |
| XIE Z X, CHEN X R, XIAO W H, et al. Cell factory construction accelerated by genome synthesis and rearrangement[J]. CIESC Journal, 2019, 70(10): 3712-3721. | |
| 97 | LANG K, CHIN J W. Cellular incorporation of unnatural amino acids and bioorthogonal labeling of proteins[J]. Chemical Reviews, 2014, 114(9): 4764-4806. |
| 98 | BOUTUREIRA O, BERNARDES G J L. Advances in chemical protein modification[J]. Chemical Reviews, 2015, 115(5): 2174-2195. |
| 99 | SAKAMOTO S, HAMACHI I. Recent progress in chemical modification of proteins[J]. Analytical Sciences, 2019, 35(1): 5-27. |
| 100 | 付宪, 林涛, 张帆, 等. 基因密码子拓展技术的方法原理和前沿应用研究进展[J]. 合成生物学, 2020, 1(1): 103-119. |
| FU X, LIN T, ZHANG F, et al. Progress in the study of genetic code expansion related methods, principles and applications[J]. Synthetic Biology Journal, 2020, 1(1): 103-119. | |
| 101 | LIU C C, SCHULTZ P G. Adding new chemistries to the genetic code[J]. Annual Review of Biochemistry, 2010, 79: 413-444. |
| 102 | TRUONG F, YOO T H, LAMPO T J, et al. Two-strain, cell-selective protein labeling in mixed bacterial cultures[J]. Journal of the American Chemical Society, 2012, 134(20): 8551-8556. |
| [1] | 熊亮斌, 宋璐, 赵云秋, 刘坤, 刘勇军, 王风清, 魏东芝. 甾体化合物绿色生物制造:从生物转化到微生物从头合成[J]. 合成生物学, 2021, 2(6): 942-963. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||