合成生物学 ›› 2022, Vol. 3 ›› Issue (1): 98-115.DOI: 10.12211/2096-8280.2021-078
材料-生物杂化体的光驱生物催化
王雪云1,2, 杨文君1,2, 钟超1,2, 高翔1,2
- 1.中国科学院深圳先进技术研究院,深圳合成生物学创新研究院,中国科学院定量工程生物学重点实验室,广东 深圳 518055
2.中国科学院深圳先进技术研究院,深圳合成生物学创新研究院,材料合成生物学中心,广东 深圳 518055
-
收稿日期:2021-07-23修回日期:2021-10-14出版日期:2022-02-28发布日期:2022-03-14 -
通讯作者:高翔 -
作者简介:王雪云 (1993—),女,博士后。研究方向为半导体材料光驱动合成生物学。E-mail:xy.wang5@siat.ac.cn杨文君 (1986—),女,博士后。研究方向为半导体材料光驱动合成生物学。E-mail:wj.yang@siat.ac.cn高翔 (1987—),男,博士,副研究员。研究方向材料-细菌人工杂合系统的设计与应用。E-mail:gaoxiang@siat.ac.cn -
基金资助:国家自然科学基金面上项目(32171426);深圳先进技术研究院优青基金新引进人才项目(E1G022)
Biohybrid materials for light-driven biocatalysis
WANG Xueyun1,2, YANG Wenjun1,2, ZHONG Chao1,2, GAO Xiang1,2
- 1.CAS Key Laboratory of Quantitative Engineering Biology,Shenzhen Institute of Synthetic Biology,Shenzhen Institutes of Advanced Technology,Chinese Academy of Sciences,Shenzhen 518055,Guangdong,China
2.Center for Materials Synthetic Biology,Shenzhen Institute of Synthetic Biology,Shenzhen Institutes of Advanced Technology,Chinese Academy of Sciences,Shenzhen 518055,Guangdong,China
-
Received:2021-07-23Revised:2021-10-14Online:2022-02-28Published:2022-03-14 -
Contact:GAO Xiang
摘要:
材料-生物杂化体的光驱生物催化,又称为半人工光合作用,利用高效捕获光能的材料与高选择性的生物催化相结合,从而实现光能到化学能高效、高特异性的转化。天然光合系统光能到化学能的转换效率低,进而发展了光能捕获和转换效率更高的人工光合作用,然而人工光合系统很难实现特异性合成高能量密度、高附加值的多碳化合物。基于材料-生物杂化体构建的半人工光合作用,同时具备材料和生物系统两者的优势,实现优势互补,为光能到化学能的转化提供新的机遇和应用。本文详细介绍了材料-生物杂化体的构建方式,杂化体通过光吸收剂与催化剂进行复合,其复合方式包括以天然光系统作为光吸收剂与纳米催化剂相结合,和以材料作为光吸收剂与酶或微生物全细胞催化剂相结合;分别总结不同复合方式的研究进展、不同系统之间的优缺点以及不同杂化体的应用方向,并对未来发展方向进行了展望。
中图分类号:
引用本文
王雪云, 杨文君, 钟超, 高翔. 材料-生物杂化体的光驱生物催化[J]. 合成生物学, 2022, 3(1): 98-115.
WANG Xueyun, YANG Wenjun, ZHONG Chao, GAO Xiang. Biohybrid materials for light-driven biocatalysis[J]. Synthetic Biology Journal, 2022, 3(1): 98-115.
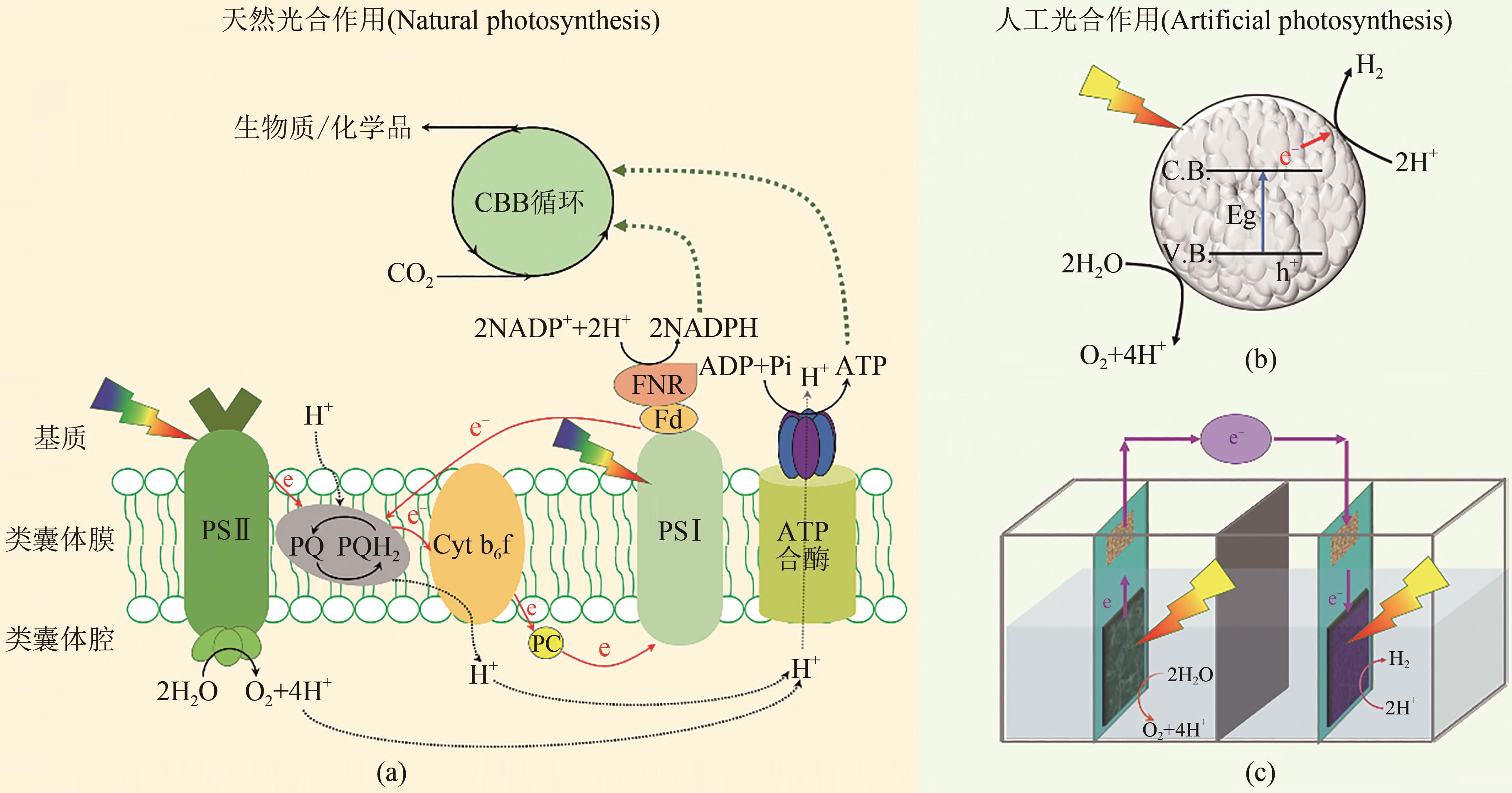
图1 天然光合作用和人工光合作用(a)天然光合作用的电子和能量传递示意图,天然光合作用分为光反应(下)和暗反应(上):光反应通过吸收光能并将能量储存在NADPH和ATP中;暗反应的CBB循环利用NADPH和ATP驱动CO2固定,合成生物质和多碳化合物[3, 10, 15-16]人工光合作用系统包含半导体材料体系和电极体系。(b)利用半导体材料分解水时,材料吸收光能产生电荷分离,e-从价带(V.B.)跃迁到导带(C.B.),在V.B.上留下空穴(h+),水为还原剂消耗h+并释放O2,导带上e-将H+还原成H2[6]。(c)光阳极材料氧化水生成O2并提供e-,并传递到光阴极端,还原H+成H2[3]
Fig. 1 Diagram for natural photosynthesis and artificial photosynthesis(a) Schematic diagram of natural photosynthesis with light reaction (lower) and dark reaction (upper). Photoreaction uses light energy to generate NADPH and ATP, and in the dark reaction, NADPH and ATP are used to drive CO2 fixation through the CBB cycle. (b) The artificial photosynthesis composed of a semiconductor material system and an electrode system [3, 10-12]. The semiconductor material absorbs light and generates electron (e-), e- transitions from the valence band (V.B.) to the conduction band (C.B.) to reduces H+ to H2[6]. The holes (h+) left on V.B. are consumed using water as reducing agent and O2 is released. (c) The photoanode material oxidizes water to generate O2 and provide e-, and the electron is transferred to the photocathode for reducing H+ to H2[3]
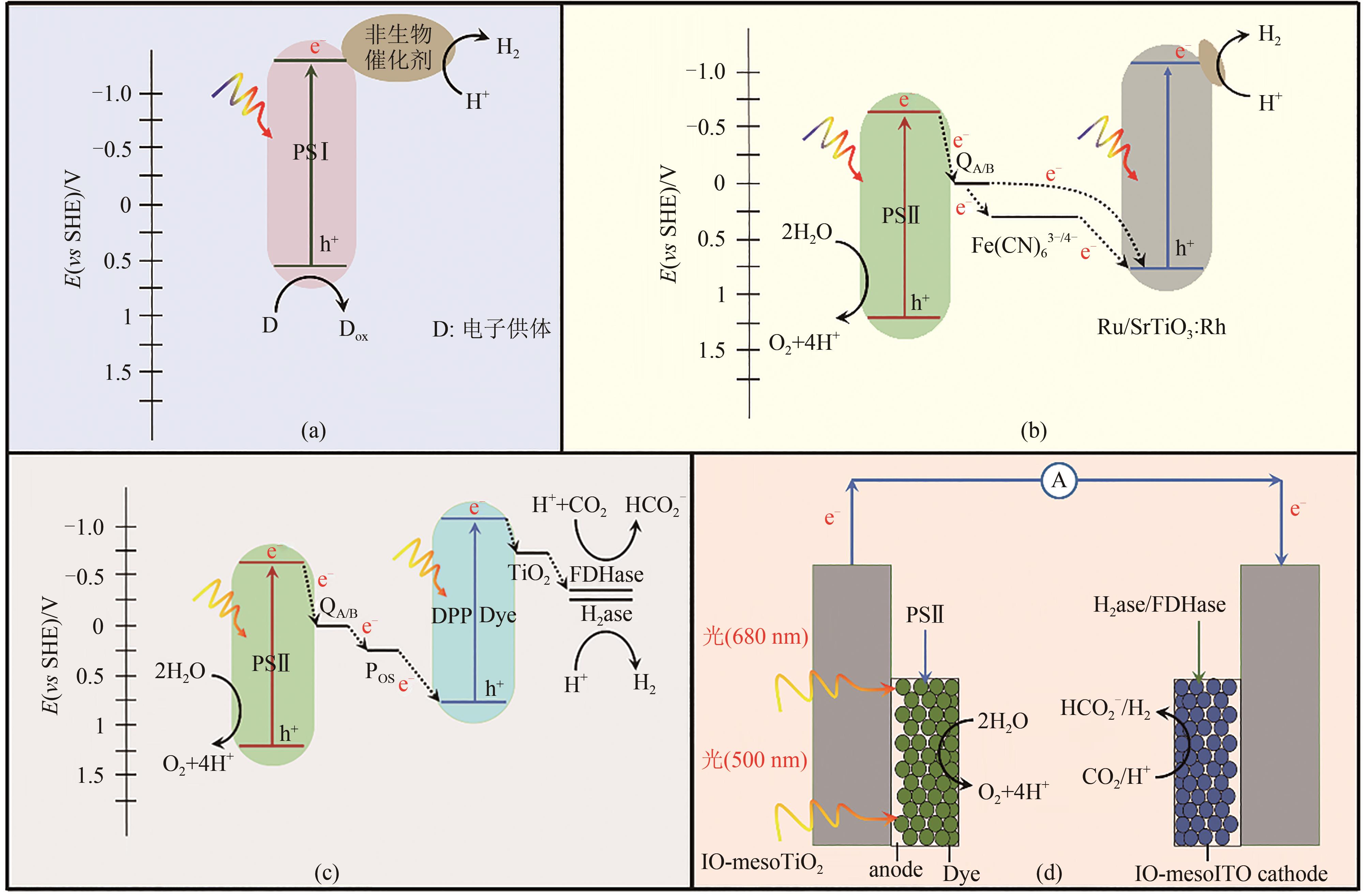
图2 基于生物光吸收剂的杂化体示意图H2ase—氢酶;FDHase—甲酸脱氢酶(a) PSⅠ作为光敏剂,吸收光能后发生电子-空穴分离,导带上e-转移给非生物催化剂,最终还原H+成H2[44];(b) PSⅡ和Ru/SrTiO3:Rh构建Z型方式传递,PSⅡ导带上激发的光电子传递到Ru/SrTiO3:Rh,经过二次激发产生电势较高的电子,用于还原H+成H2[45];(c) PSⅡ和DPP染料构建Z型电子传递链,与H2ase或FDHase构建半导体-酶杂化体[46];DPP染料C.B.的光生电子参与酶催化反应将H+还原为H2或将CO2固定为甲酸盐;(d)在PSⅡ和H2ase/FDHase酶构成的对电极中,PSⅡ作为阳极光解水提供电子,阴极的酶则利用电子进行还原反应[3, 47-48]
Fig. 2 Diagram for biological photosensitizer-material hybrids(a) PSⅠ photosensitizer harvests light and generates e-, which is then transferred to non-biological catalyst for reducing H+ to H2[44]. (b) PSⅡ and Ru/SrTiO3:Rh form a Z-scheme structure. Photoelectrons from PSⅡ neutralize h+ on V.B. of Ru/SrTiO3:Rh, leaving electrons with higher reduction potential on C.B. of Ru/SrTiO3:Rh to reduce H+ to H2[45]. (c) PSⅡ and DPP dye form a Z-scheme electron transfer structure, and together with formate dehydrogenase (H2ase) or formate dehydrogenase (FDHase) to build semiconductor-enzyme hybrid. Electrons on C.B. of DPP dye participate in catalytic reaction of enzyme to reduce H+ to H2 or fix carbon dioxide into formate[46]. (d) PSⅡ acts as photoanode to catalyze water splitting to provide electrons, and enzyme at photocathode uses the electrons to drive reduction reaction[3, 47-48]
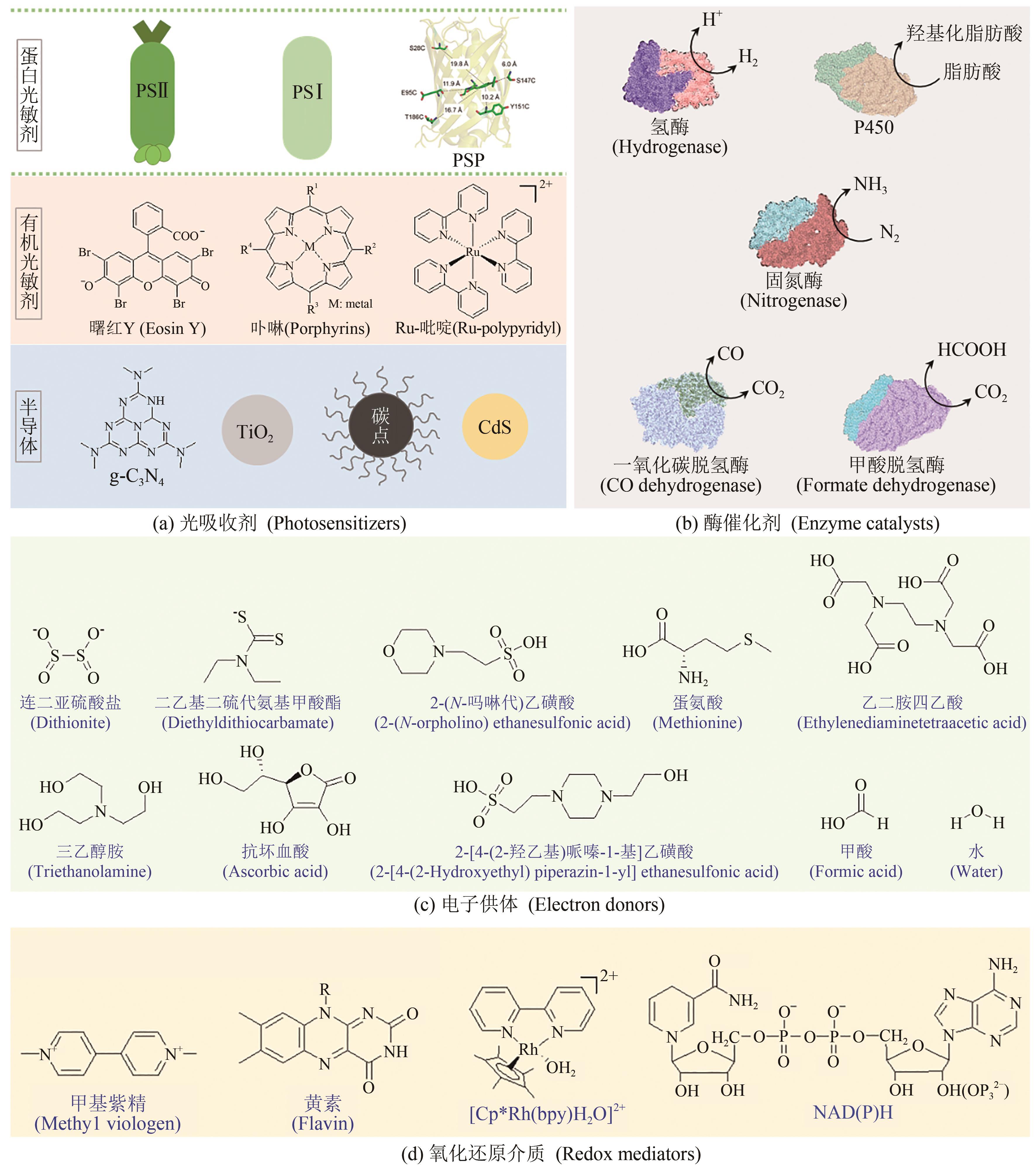
图3 材料-酶杂化体中的主要组成部分(a)常用光吸收剂主要分为蛋白质光敏剂,染料/高分子为主的有机光敏剂和半导体材料[57];(b)用于构建的材料-酶杂化体中常用的酶[3];(c)主要电子供体的结构式,电子供体用于在催化体系中中和空穴,提供电子,使反应顺利连续进行[57];(d)氧化还原介质的结构式,其中[Cp*Rh(bpy)H2O]2+和NAD(P)H是使用最广泛的介质[57];PSP—光敏蛋白质[59]
Fig. 3 Diagram for components in materials-enzymes hybrid systems(a) Major photosensitizers including proteins, organic photosensitizers and semiconductor materials[57]. (b) Representative enzymes used in the material-enzyme hybrids[3]. (c) Major electron donors[57]. (d) Redox mediators. [Cp*Rh(bpy)H2O]2+ and NAD(P)H are the most widely used mediators[57]. PSP—Photo-sensitive protein[59]
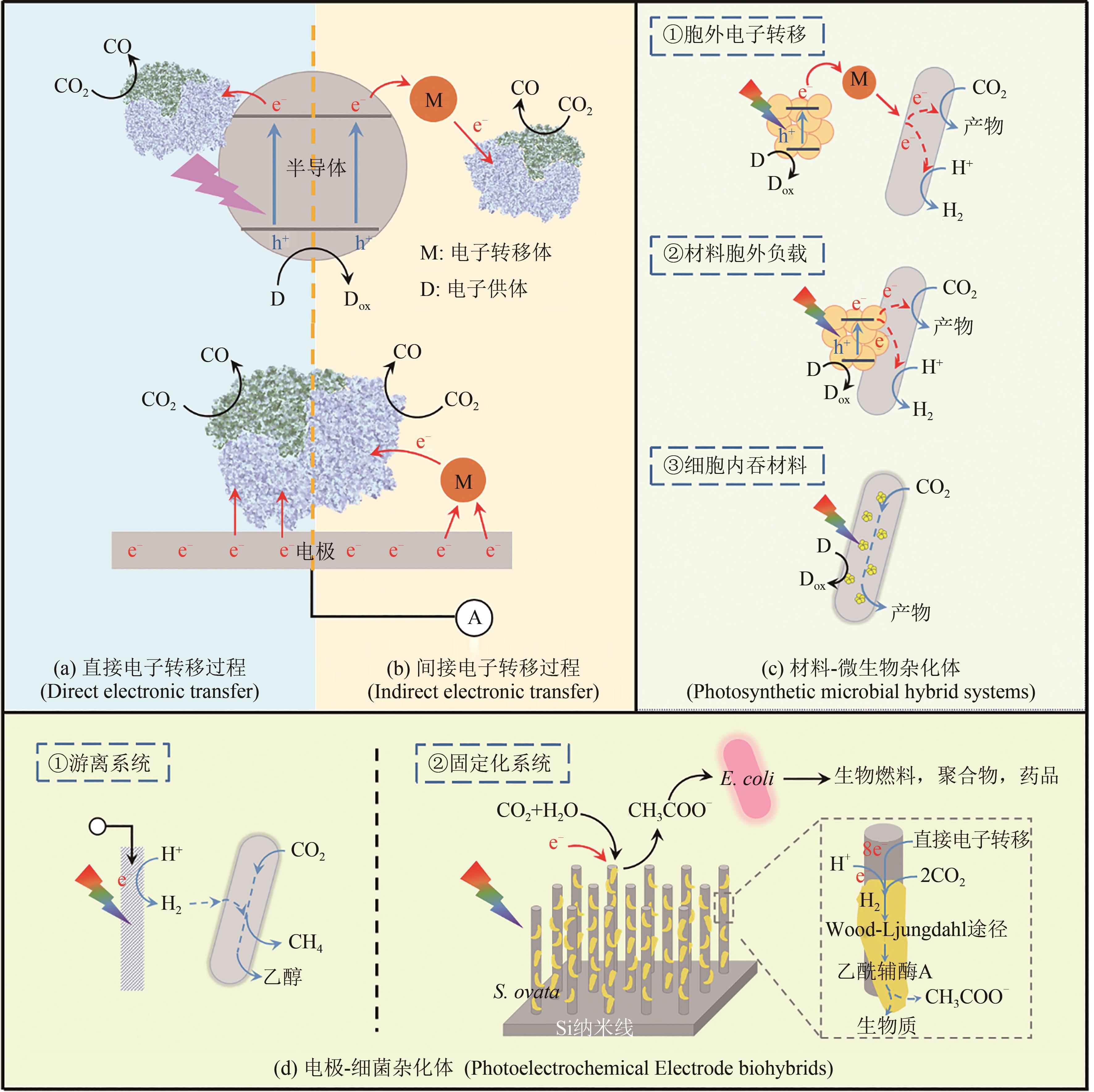
图4 材料-生物杂化体示意图[在基于半导体或电极构筑的材料-酶杂化体系中,电子的转移方式分为直接电子转移(a)和间接电子转移(b)[3, 57];在材料-微生物杂化体(c)中,材料可以分布在细胞外,细胞膜上和细胞内部,材料产生的光电子会进入微生物细胞内,为胞内代谢途径提供能量[3];电极-细菌杂化体(d)分为游离系统和固定化系统[3, 9, 61]]
Fig. 4 Diagram for materials biohybrid systems[In semiconductor/electrode-enzyme hybrid systems, there are two electron transfer routes: direct (a) and indirect electron transfer (b) [3, 57]; In semiconductor-microbial hybrid systems (c), the nanoparticles are distributed at different sites of the cell, including extracellular, surface and intracellular[3]; Electrode-bacteria hybrids (d) include free and immobilized cell systems[3, 9, 61]]
| 1 | ARMAROLI N, BALZANI V. The future of energy supply: challenges and opportunities[J]. Angewandte Chemie International Edition, 2007, 46(1/2): 52-66. |
| 2 | LI X B, TUNG C H, WU L Z. Semiconducting quantum dots for artificial photosynthesis[J]. Nature Reviews Chemistry, 2018, 2(8): 160-173. |
| 3 | FANG X, KALATHIL S, REISNER E. Semi-biological approaches to solar-to-chemical conversion[J]. Chemical Society Reviews, 2020, 49(14): 4926-4952. |
| 4 | NAYAK P K, MAHESH S, SNAITH H J, et al. Photovoltaic solar cell technologies: analysing the state of the art[J]. Nature Reviews Materials, 2019, 4(4): 269-285. |
| 5 | TACHIBANA Y, VAYSSIERES L, DURRANT J R. Artificial photosynthesis for solar water-splitting[J]. Nature Photonics, 2012, 6(8): 511-518. |
| 6 | ALBERO J, PENG Y, GARCÍA H. Photocatalytic CO2 reduction to C2+ products[J]. ACS Catalysis, 2020, 10(10): 5734-5749. |
| 7 | BROWN K A, HARRIS D F, WILKER M B, et al. Light-driven dinitrogen reduction catalyzed by a CdS: nitrogenase MoFe protein biohybrid[J]. Science, 2016, 352(6284): 448-450. |
| 8 | KORNIENKO N, ZHANG J Z, SAKIMOTO K K, et al. Interfacing nature's catalytic machinery with synthetic materials for semi-artificial photosynthesis[J]. Nature Nanotechnology, 2018, 13(10): 890-899. |
| 9 | CESTELLOS-BLANCO S, ZHANG H, KIM J M, et al. Photosynthetic semiconductor biohybrids for solar-driven biocatalysis[J]. Nature Catalysis, 2020, 3(3): 245-255. |
| 10 | BROWN K A, KING P W. Coupling biology to synthetic nanomaterials for semi-artificial photosynthesis[J]. Photosynthesis Research, 2020, 143(2): 193-203. |
| 11 | ZUO W L, ZHANG L, ZHANG Z D, et al. Degradation of organic pollutants by intimately coupling photocatalytic materials with microbes: a review[J]. Critical Reviews in Biotechnology, 2021, 41(2): 273-299. |
| 12 | SAHOO P C, PANT D, KUMAR M, et al. Material-microbe interfaces for solar-driven CO2 bioelectrosynthesis[J]. Trends in Biotechnology, 2020, 38(11): 1245-1261. |
| 13 | 熊威, 冯建勇, 马为民, 等. 基于无机材料-微生物复合的半人工光合作用[J]. 无机化学学报, 2019, 35(9): 1521-1534. |
| XIONG W, FENG J Y, MA W M, et al. Semi-artificial photosynthesis based on inorganic material-microbe hybrids[J]. Chinese Journal of Inorganic Chemistry, 2019, 35(9): 1521-1534. | |
| 14 | FLEMING G R, SCHLAU-COHEN G S, AMARNATH K, et al. Design principles of photosynthetic light-harvesting[J]. Faraday Discussions, 2012, 155: 27-41. |
| 15 | ZHANG J Z, BOMBELLI P, SOKOL K P, et al. Photoelectrochemistry of photosystem Ⅱ in vitro vs in vivo [J]. Journal of the American Chemical Society, 2018, 140(1): 6-9. |
| 16 | SCHMERMUND L, JURKAŠ V, ÖZGEN F F, et al. Photo-biocatalysis: Biotransformations in the presence of light[J]. ACS Catalysis, 2019, 9(5): 4115-4144. |
| 17 | BLANKENSHIP R E, TIEDE D M, BARBER J, et al. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement[J]. Science, 2011, 332(6031): 805-809. |
| 18 | ZHU X G, LONG S P, ORT D R. Improving photosynthetic efficiency for greater yield[J]. Annual Review of Plant Biology, 2010, 61: 235-261. |
| 19 | CHEN M. Chapter Four-Chlorophylls d and f: Synthesis, occurrence, light-harvesting, and pigment organization in chlorophyll-binding protein complexes[M]//GRIMM B. Advances in Botanical Research. Academic Press. 2019: 121-139. |
| 20 | CHEN M, SCHLIEP M, WILLOWS R D, et al. A red-shifted chlorophyll[J]. Science, 2010, 329(5997): 1318-1319. |
| 21 | TROS M, BERSANINI L, SHEN G Z, et al. Harvesting far-red light: Functional integration of chlorophyll f into Photosystem Ⅰ complexes of Synechococcus sp. PCC 7002[J]. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 2020, 1861(8): 148206. |
| 22 | JARCHUM I. Photosynthesis gets a boost[J]. Nature Biotechnology, 2017, 35(1): 30. |
| 23 | KROMDIJK J, GŁOWACKA K, LEONELLI L, et al. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection[J]. Science, 2016, 354(6314): 857-861. |
| 24 | CHEN J H, CHEN S T, HE N Y, et al. Nuclear-encoded synthesis of the D1 subunit of photosystem Ⅱ increases photosynthetic efficiency and crop yield[J]. Nature Plants, 2020, 6(5): 570-580. |
| 25 | MELIS A. Solar energy conversion efficiencies in photosynthesis: minimizing the chlorophyll antennae to maximize efficiency[J]. Plant Science, 2009, 177(4): 272-280. |
| 26 | KRAMER D M, EVANS J R. The importance of energy balance in improving photosynthetic productivity[J]. Plant Physiology, 2010, 155(1): 70-78. |
| 27 | HASUNUMA T, MATSUDA M, SENGA Y H, et al. Overexpression of flv3 improves photosynthesis in the cyanobacterium Synechocystis sp. PCC6803 by enhancement of alternative electron flow[J]. Biotechnology for Biofuels, 2014, 7(1): 493. |
| 28 | CHEN Q, STEEN J B VAN DER, DEKKER H L, et al. Expression of holo-proteorhodopsin in Synechocystis sp. PCC 6803[J]. Metabolic Engineering, 2016, 35: 83-94. |
| 29 | DUCAT D C, SILVER P A. Improving carbon fixation pathways[J]. Current Opinion in Chemical Biology, 2012, 16(3/4): 337-344. |
| 30 | KAMENNAYA N A, AHN S, PARK H, et al. Installing extra bicarbonate transporters in the cyanobacterium Synechocystis sp. PCC6803 enhances biomass production[J]. Metabolic Engineering, 2015, 29: 76-85. |
| 31 | TAMOI M, NAGAOKA M, MIYAGAWA Y, et al. Contribution of fructose-1,6-bisphosphatase and sedoheptulose-1,7-bisphosphatase to the photosynthetic rate and carbon flow in the Calvin cycle in transgenic plants[J]. Plant and Cell Physiology, 2006, 47(3): 380-390. |
| 32 | ZHU X G, DE STURLER E, LONG S P. Optimizing the distribution of resources between enzymes of carbon metabolism can dramatically increase photosynthetic rate: a numerical simulation using an evolutionary algorithm[J]. Plant Physiology, 2007, 145(2): 513-526. |
| 33 | JABLONSKY J, BAUWE H, WOLKENHAUER O. Modeling the Calvin-Benson cycle[J]. BMC Systems Biology, 2011, 5: 185. |
| 34 | ROSENTHAL D M, LOCKE A M, KHOZAEI M, et al. Over-expressing the C3 photosynthesis cycle enzyme sedoheptulose-1-7 bisphosphatase improves photosynthetic carbon gain and yield under fully open air CO2 fumigation (FACE)[J]. BMC Plant Biology, 2011, 11: 123. |
| 35 | KÖHLER I H, RUIZ-VERA U M, VANLOOCKE A, et al. Expression of cyanobacterial FBP/SBPase in soybean prevents yield depression under future climate conditions[J]. Journal of Experimental Botany, 2017, 68(3): 715-726. |
| 36 | JANASCH M, ASPLUND-SAMUELSSON J, STEUER R, et al. Kinetic modeling of the Calvin cycle identifies flux control and stable metabolomes in Synechocystis carbon fixation[J]. Journal of Experimental Botany, 2018, 70(3): 973-983. |
| 37 | HU G P, ZHOU J, CHEN X L, et al. Engineering synergetic CO2-fixing pathways for malate production[J]. Metabolic Engineering, 2018, 47: 496-504. |
| 38 | BAR-EVEN A, NOOR E, LEWIS N E, et al. Design and analysis of synthetic carbon fixation pathways[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(19): 8889-8894. |
| 39 | SCHWANDER T, BORZYSKOWSKI L S VON, BURGENER S, et al. A synthetic pathway for the fixation of carbon dioxide in vitro [J]. Science, 2016, 354(6314): 900-904. |
| 40 | MILLER T E, BENEYTON T, SCHWANDER T, et al. Light-powered CO2 fixation in a chloroplast mimic with natural and synthetic parts[J]. Science, 2020, 368(6491): 649-654. |
| 41 | CARDONA T, SHAO S X, NIXON P J. Enhancing photosynthesis in plants: The light reactions[J]. Essays in Biochemistry, 2018, 62(1): 85-94. |
| 42 | CIAMICIAN G. The Photochemistry of the future[J]. Science, 1912, 36(926): 385-394. |
| 43 | ZHOU G, SHAN Y, HU Y Y, et al. Half-metallic carbon nitride nanosheets with micro grid mode resonance structure for efficient photocatalytic hydrogen evolution[J]. Nature Communications, 2018, 9: 3366. |
| 44 | GRIMME R A, LUBNER C E, BRYANT D A, et al. Photosystem I/molecular wire/metal nanoparticle bioconjugates for the photocatalytic production of H2 [J]. Journal of the American Chemical Society, 2008, 130(20): 6308-6309. |
| 45 | WANG W Y, LI Z, CHEN J, et al. Crucial roles of electron-proton transport relay in the photosystem Ⅱ-photocatalytic hybrid system for overall water splitting[J]. The Journal of Physical Chemistry C, 2017, 121(5): 2605-2612. |
| 46 | MERSCH D, LEE C Y, ZHANG J Z, et al. Wiring of photosystem Ⅱ to hydrogenase for photoelectrochemical water splitting[J]. Journal of the American Chemical Society, 2015, 137(26): 8541-8549. |
| 47 | TIAN Y, ZHOU Y N, ZONG Y C, et al. Construction of functionally compartmental inorganic photocatalyst-enzyme system via imitating chloroplast for efficient photoreduction of CO2 to formic acid[J]. ACS Applied Materials & Interfaces, 2020, 12(31): 34795-34805. |
| 48 | LI Z, WANG W Y, DING C M, et al. Biomimetic electron transport via multiredox shuttles from photosystem Ⅱ to a photoelectrochemical cell for solar water splitting[J]. Energy & Environmental Science, 2017, 10(3): 765-771. |
| 49 | UTSCHIG L M, SOLTAU S R, TIEDE D M. Light-driven hydrogen production from Photosystem I-catalyst hybrids[J]. Current Opinion in Chemical Biology, 2015, 25: 1-8. |
| 50 | UTSCHIG L M, DIMITRIJEVIC N M, POLUEKTOV O G, et al. Photocatalytic hydrogen production from noncovalent biohybrid photosystem I/Pt nanoparticle complexes[J]. The Journal of Physical Chemistry Letters, 2011, 2(3): 236-241. |
| 51 | NOJI T, SUZUKI T, KONDO M, et al. Light-induced hydrogen production by photosystem I-Pt nanoparticle conjugates immobilized in porous glass plate nanopores[J]. Research on Chemical Intermediates, 2016, 42(11): 7731-7742. |
| 52 | ZHAO F Y, CONZUELO F, HARTMANN V, et al. Light induced H2 evolution from a biophotocathode based on photosystem 1-Pt nanoparticles complexes integrated in solvated redox polymers films[J]. The Journal of Physical Chemistry B, 2015, 119(43): 13726-13731. |
| 53 | LUBITZ W, CHRYSINA M, COX N. Water oxidation in photosystem Ⅱ[J]. Photosynthesis Research, 2019, 142(1): 105-125. |
| 54 | UTSCHIG L M, SOLTAU S R, MULFORT K L, et al. Z-scheme solar water splitting via self-assembly of photosystem I-catalyst hybrids in thylakoid membranes[J]. Chemical Science, 2018, 9(45): 8504-8512. |
| 55 | ZHANG J Z, BOMBELLI P, SOKOL K P, et al. Photoelectrochemistry of photosystem Ⅱ in vitro vs in vivo [J]. Journal of the American Chemical Society, 2018, 140(1): 6-9. |
| 56 | MCCORMICK A J, BOMBELLI P, BRADLEY R W, et al. Biophotovoltaics: oxygenic photosynthetic organisms in the world of bioelectrochemical systems[J]. Energy & Environmental Science, 2015, 8(4): 1092-1109. |
| 57 | LEE S H, CHOI D S, KUK S K, et al. Photobiocatalysis: activating redox enzymes by direct or indirect transfer of photoinduced electrons[J]. Angewandte Chemie International Edition, 2018, 57(27): 7958-7985. |
| 58 | CHEN S Y, WANG L W. Thermodynamic oxidation and reduction potentials of photocatalytic semiconductors in aqueous solution[J]. Chemistry of Materials, 2012, 24(18): 3659-3666. |
| 59 | FU Y, HUANG J, WU Y Z, et al. Biocatalytic cross-coupling of aryl halides with a genetically engineered photosensitizer artificial dehalogenase[J]. Journal of the American Chemical Society, 2021, 143(2): 617-622. |
| 60 | PARK J H, LEE S H, CHA G S, et al. Cofactor-free light-driven whole-cell cytochrome P450 catalysis[J]. Angewandte Chemie International Edition, 2015, 54(3): 969-973. |
| 61 | LIU C, GALLAGHER J J, SAKIMOTO K K, et al. Nanowire-bacteria hybrids for unassisted solar carbon dioxide fixation to value-added chemicals[J]. Nano Letters, 2015, 15(5): 3634-3639. |
| 62 | BROWN K A, WILKER M B, BOEHM M, et al. Characterization of photochemical processes for H2 production by CdS nanorod-[FeFe]hydrogenase complexes[J]. Journal of the American Chemical Society, 2012, 134(12): 5627-5636. |
| 63 | BROWN K A, SONG Q, MULDER D W, et al. Diameter dependent electron transfer kinetics in semiconductor-enzyme complexes[J]. ACS Nano, 2014, 8(10): 10790-10798. |
| 64 | HUTTON G A M, REUILLARD B, MARTINDALE B C M, et al. Carbon dots as versatile photosensitizers for solar-driven catalysis with redox enzymes[J]. Journal of the American Chemical Society, 2016, 138(51): 16722-16730. |
| 65 | CAPUTO C A, WANG L D, BERANEK R, et al. Carbon nitride-TiO2 hybrid modified with hydrogenase for visible light driven hydrogen production[J]. Chemical Science, 2015, 6(10): 5690-5694. |
| 66 | REISNER E, ARMSTRONG F A. A TiO2 nanoparticle system for sacrificial solar h2 production prepared by rational combination of a hydrogenase with a ruthenium photosensitizer[M]//WANG P. Nanoscale biocatalysis: methods and protocols. Totowa, NJ; Humana Press, 2011: 107-117. |
| 67 | REISNER E, POWELL D J, CAVAZZA C, et al. Visible light-driven H2 production by hydrogenases attached to dye-sensitized TiO2 nanoparticles[J]. Journal of the American Chemical Society, 2009, 131(51): 18457-18466. |
| 68 | SAKAI T, MERSCH D, REISNER E. Photocatalytic hydrogen evolution with a hydrogenase in a mediator-free system under high levels of oxygen[J]. Angewandte Chemie International Edition, 2013, 52(47): 12313-12316. |
| 69 | WOOLERTON T W, SHEARD S, REISNER E, et al. Efficient and clean photoreduction of CO2 to CO by enzyme-modified TiO2 nanoparticles using visible light[J]. Journal of the American Chemical Society, 2010, 132(7): 2132-2133. |
| 70 | ZHANG Y Y, ZHAO Y J, LI R, et al. Bioinspired NADH regeneration based on conjugated photocatalytic systems[J]. Solar RRL, 2021, 5(2): 2000339. |
| 71 | LIU J, ANTONIETTI M. Bio-inspired NADH regeneration by carbon nitride photocatalysis using diatom templates[J]. Energy & Environmental Science, 2013, 6(5): 1486. |
| 72 | LIU W, HU W, YANG L, et al. Single cobalt atom anchored on carbon nitride with well-defined active sites for photo-enzyme catalysis[J]. Nano Energy, 2020, 73: 104750. |
| 73 | ZHANG S H, ZHANG Y S, CHEN Y, et al. Metal hydride-embedded titania coating to coordinate electron transfer and enzyme protection in photo-enzymatic catalysis[J]. ACS Catalysis, 2021, 11(1): 476-483. |
| 74 | SEELAJAROEN H, BAKANDRITSOS A, OTYEPKA M, et al. Immobilized enzymes on graphene as nanobiocatalyst[J]. ACS Applied Materials & Interfaces, 2020, 12(1): 250-259. |
| 75 | SAKIMOTO K K, WONG A B, YANG P D. Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production[J]. Science, 2016, 351(6268): 74-77. |
| 76 | GURUNATHAN K. Photobiocatalytic production of hydrogen using sensitized TiO2-MV2+ system coupled Rhodopseudomonas capsulata [J]. Journal of Molecular Catalysis A: Chemical, 2000, 156(1/2): 59-67. |
| 77 | WANG B, JIANG Z F, YU J C, et al. Enhanced CO2 reduction and valuable C2 + chemical production by a CdS-photosynthetic hybrid system[J]. Nanoscale, 2019, 11(19): 9296-9301. |
| 78 | WANG B, ZENG C P, CHU K H, et al. Enhanced biological hydrogen production from Escherichia coli with surface precipitated cadmium sulfide nanoparticles[J]. Advanced Energy Materials, 2017, 7(20): 1700611. |
| 79 | WEI W, SUN P Q, LI Z, et al. A surface-display biohybrid approach to light-driven hydrogen production in air[J]. Science Advances, 2018, 4(2): eaap9253. |
| 80 | GUO J L, SUÁSTEGUI M, SAKIMOTO K K, et al. Light-driven fine chemical production in yeast biohybrids[J]. Science, 2018, 362(6416): 813-816. |
| 81 | NICHOLS E M, GALLAGHER J J, LIU C, et al. Hybrid bioinorganic approach to solar-to-chemical conversion[J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(37): 11461-11466. |
| 82 | YE J, YU J, ZHANG Y Y, et al. Light-driven carbon dioxide reduction to methane by Methanosarcina barkeri-CdS biohybrid[J]. Applied Catalysis B: Environmental, 2019, 257: 117916. |
| 83 | TORELLA J P, GAGLIARDI C J, CHEN J S, et al. Efficient solar-to-fuels production from a hybrid microbial-water-splitting catalyst system[J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(8): 2337-2342. |
| 84 | HUANG S F, TANG J H, LIU X, et al. Fast light-driven biodecolorization by a geobacter sulfurreducens-CdS biohybrid[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(18): 15427-15433. |
| 85 | WANG X N, NIU M T, FAN J X, et al. Photoelectric bacteria enhance the in situ production of tetrodotoxin for antitumor therapy[J]. Nano Letters, 2021, 21(10): 4270-4279. |
| 86 | LI X J, SUN H, MAO X M, et al. Enhanced photosynthesis of carotenoids in microalgae driven by light-harvesting gold nanoparticles[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(20): 7600-7608. |
| 87 | ZHANG H, LIU H, TIAN Z Q, et al. Bacteria photosensitized by intracellular gold nanoclusters for solar fuel production[J]. Nature Nanotechnology, 2018, 13(10): 900-905. |
| 88 | DING Y C, BERTRAM J R, ECKERT C, et al. Nanorg microbial factories: Light-driven renewable biochemical synthesis using quantum dot-bacteria nanobiohybrids[J]. Journal of the American Chemical Society, 2019, 141(26): 10272-10282. |
| 89 | LUO B F, WANG Y Z, LI D, et al. A periplasmic photosensitized biohybrid system for solar hydrogen production[J]. Advanced Energy Materials, 2021, 11(19): 2100256. |
| 90 | GUENGERICH F P. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity[J]. Chemical Research in Toxicology, 2001, 14(6): 611-650. |
| 91 | ZENG Y, ZHOU X, QI R L, et al. Photoactive conjugated polymer-based hybrid biosystems for enhancing cyanobacterial photosynthesis and regulating redox state of protein[J]. Advanced Functional Materials, 2021, 31(8): 2007814. |
| 92 | GAI P P, YU W, ZHAO H, et al. Solar-powered organic semiconductor-bacteria biohybrids for CO2 reduction into acetic acid[J]. Angewandte Chemie International Edition, 2020, 59(18): 7224-7229. |
| 93 | DAI N, ZHAO H, YU W, et al. Oligo(p-phenylenevinylene)-rhodium complex as intracellular catalyst for enhancing biosynthesis of polyhydroxybutyrate biomaterials[J]. Science China Chemistry, 2021, 64(1): 143-150. |
| 94 | ZHOU X, ZENG Y, TANG Y Y, et al. Artificial regulation of state transition for augmenting plant photosynthesis using synthetic light-harvesting polymer materials[J]. Science Advances, 2020, 6(35): eabc5237. |
| 95 | QI R L, ZHAO H, ZHOU X, et al. In situ synthesis of photoactive polymers on a living cell surface via bio-palladium catalysis for modulating biological functions[J]. Angewandte Chemie International Edition, 2021, 60(11): 5759-5765. |
| 96 | LIU C, COLÓN B C, ZIESACK M, et al. Water splitting-biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis[J]. Science, 2016, 352(6290): 1210-1213. |
| 97 | LIU C, SAKIMOTO K K, COLÓN B C, et al. Ambient nitrogen reduction cycle using a hybrid inorganic-biological system[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(25): 6450-6455. |
| 98 | RODRIGUES R M, GUAN X, IÑIGUEZ J A, et al. Perfluorocarbon nanoemulsion promotes the delivery of reducing equivalents for electricity-driven microbial CO2 reduction[J]. Nature Catalysis, 2019, 2(5): 407-414. |
| 99 | SU Y D, CESTELLOS-BLANCO S, KIM J M, et al. Close-packed nanowire-bacteria hybrids for efficient solar-driven CO2 fixation[J]. Joule, 2020, 4(4): 800-811. |
| 100 | JI Z, ZHANG H, LIU H, et al. Cytoprotective metal-organic frameworks for anaerobic bacteria[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(42): 10582-10587. |
| 101 | ZHANG R T, HE Y, YI J, et al. Proteomic and metabolic elucidation of solar-powered biomanufacturing by bio-abiotic hybrid system[J]. Chemistry, 2020, 6(1): 234-249. |
| 102 | KORNIENKO N, SAKIMOTO K K, HERLIHY D M, et al. Spectroscopic elucidation of energy transfer in hybrid inorganic-biological organisms for solar-to-chemical production[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(42): 11750-11755. |
| 103 | LI Z D, WU C, GAO X, et al. Exogenous electricity flowing through cyanobacterial photosystem I drives CO2 valorization with high energy efficiency[J]. Energy & Environmental Science, 2021, 14(10): 5480-5490. |
| [1] | 杨健钊, 朱新广. 面向碳达峰与碳中和的植物合成生物学[J]. 合成生物学, 2022, 3(5): 847-869. |
| [2] | 王松, 吴莎, 江亚男, 胡章立. 微藻光合作用的优化升级助力“双碳”目标[J]. 合成生物学, 2022, 3(5): 915-931. |
| [3] | 盛阳阳, 徐秀美, 张巧红, 张立新. 光合作用碳同化的合成生物学研究进展[J]. 合成生物学, 2022, 3(5): 870-883. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
