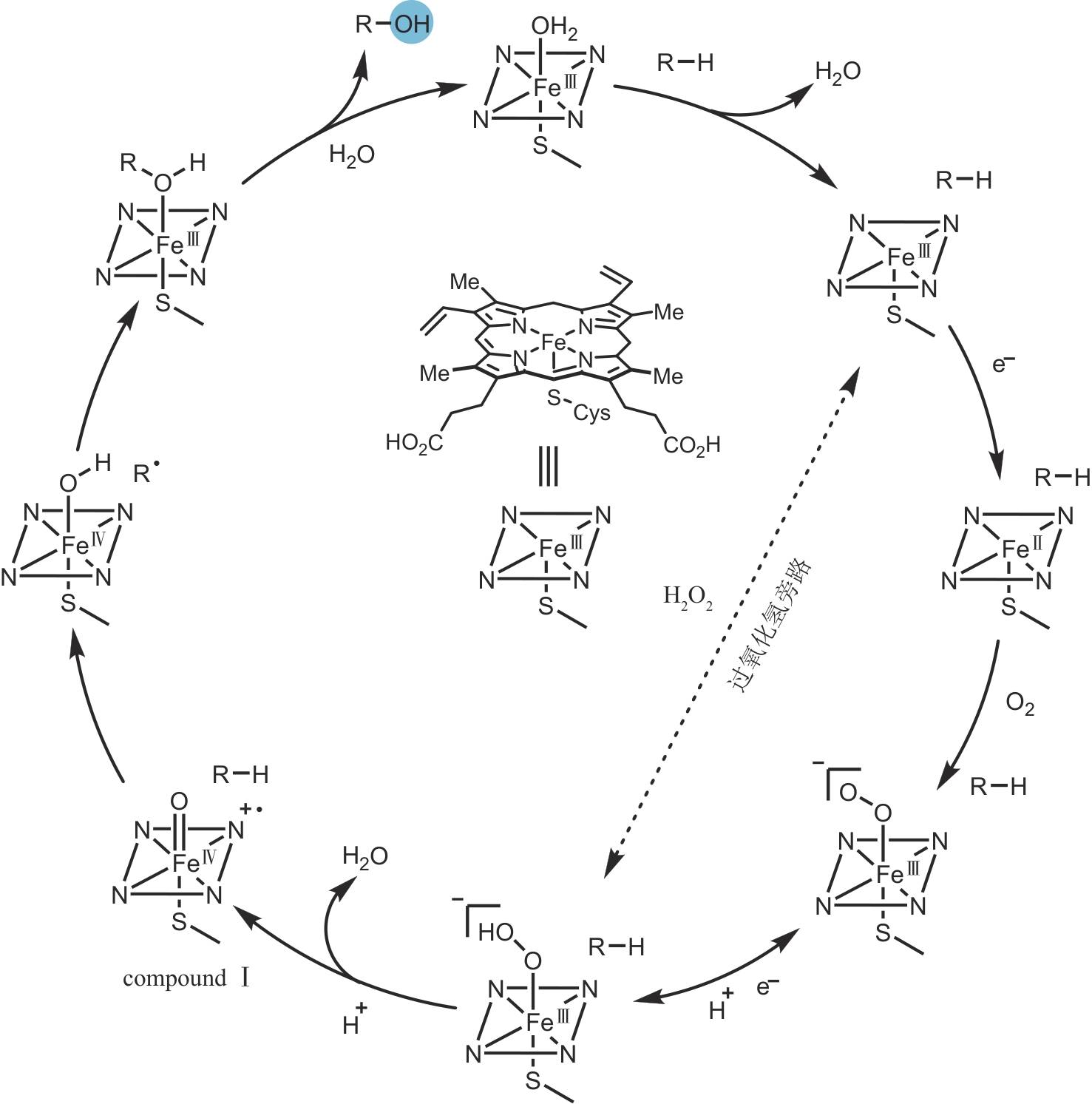
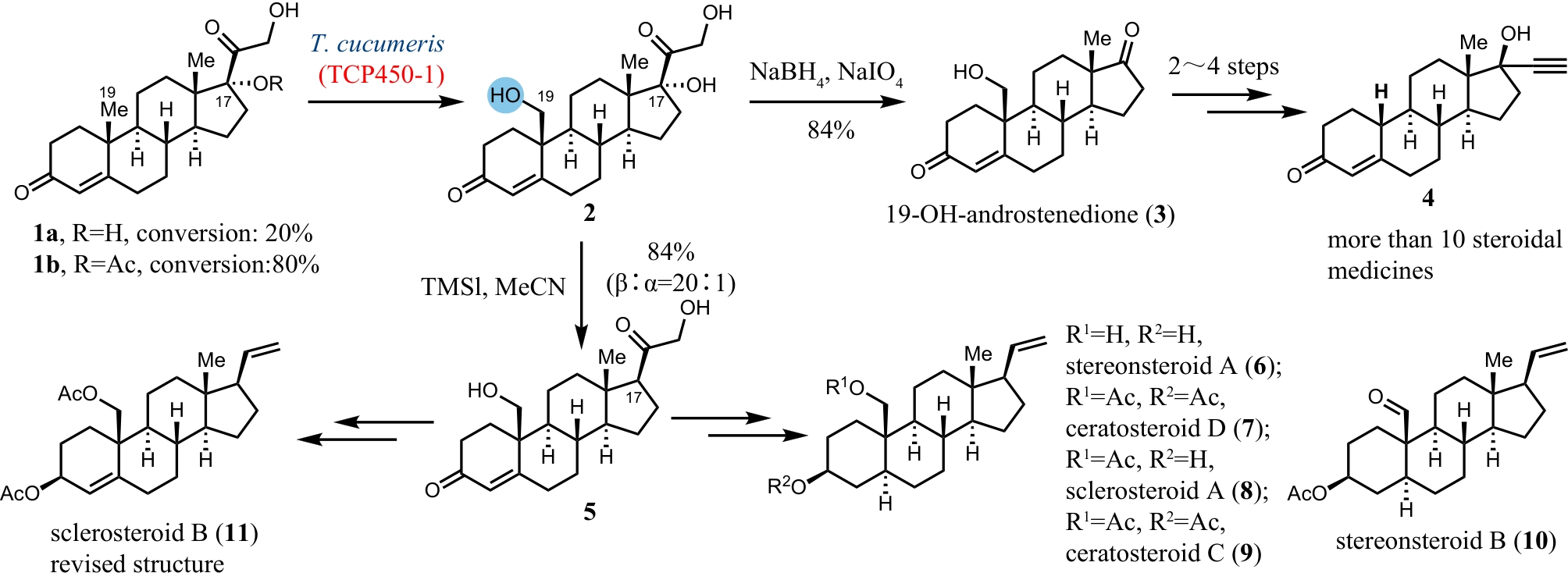
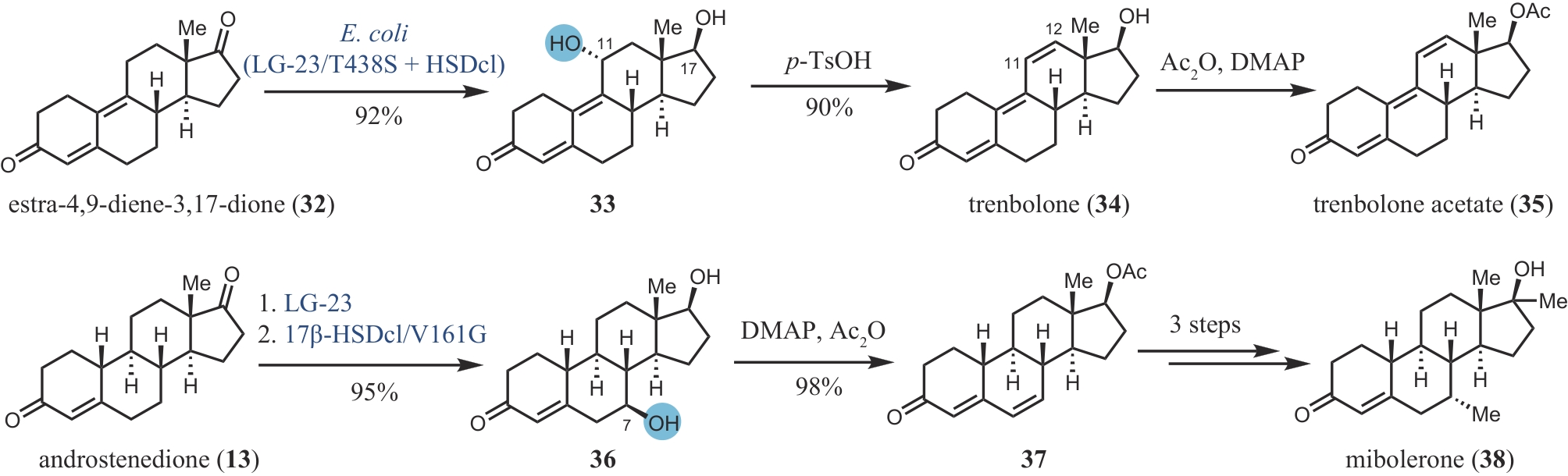
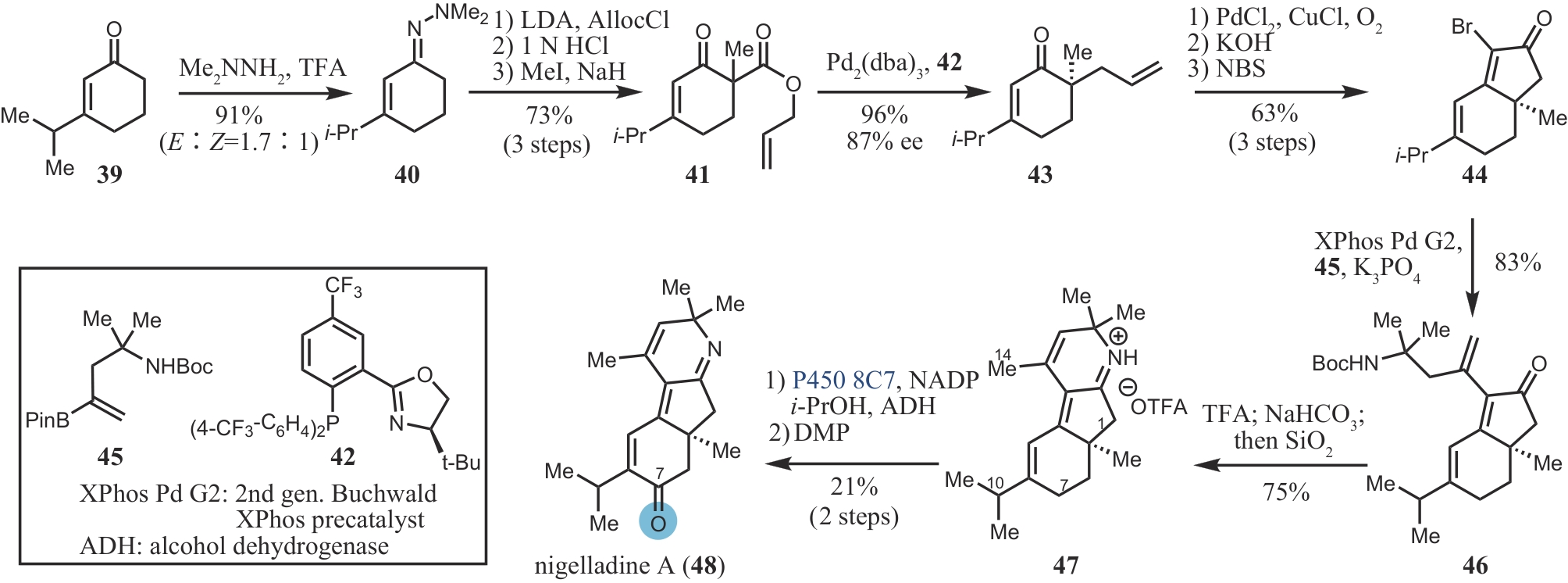
合成生物学 ›› 2024, Vol. 5 ›› Issue (5): 960-980.DOI: 10.12211/2096-8280.2024-017
基于P450选择性氧化的天然产物化学-酶法合成进展
程中玉, 李付琸
- 复旦大学药学院,天然药物学系,上海 201203
-
收稿日期:2024-02-04修回日期:2024-05-21出版日期:2024-10-31发布日期:2024-11-20 -
通讯作者:李付琸 -
作者简介:程中玉 (1998—),男,研究助理。研究方向为活性天然产物化学-酶法合成。 E-mail:zhongyu_cheng@fudan.edu.cn李付琸 (1990—),男,青年研究员,博士生导师。研究方向为酶催化反应开发,活性天然产物化学-酶法合成等。 E-mail:lifuzhuo@fudan.edu.cn -
基金资助:国家自然科学基金(22301041);上海市自然科学基金(23ZR1412900)
Recent advances in chemoenzymatic synthesis of natural products via site- selective P450 oxidation
CHENG Zhongyu, LI Fuzhuo
- Department of Natural Medicine,School of Pharmacy,Fudan University,Shanghai 201203,China
-
Received:2024-02-04Revised:2024-05-21Online:2024-10-31Published:2024-11-20 -
Contact:LI Fuzhuo
摘要:
随着基因挖掘、生物信息学、酶工程等多学科的交叉融合与协同创新,将绿色高效的酶催化反应与现代有机合成方法相结合的化学-酶法合成策略逐渐发展成为合成活性天然产物、药物分子和其他具有重要价值的有机分子的有力工具。其中细胞色素单加氧酶P450能够选择性地实现惰性C—H氧化这一经典的挑战性化学转化,为活性天然产物的高效合成提供了新的思路,成为合成科学领域的研究热点之一。本文以结构类型进行分类,综述了P450选择性氧化在甾体、萜类以及其他类型天然产物化学-酶法合成中的应用进展,并分析了相应的酶催化反应在提高目标产物合成效率方面起到的关键作用。此外,还讨论了该领域目前所面临的挑战,例如主要集中在对酶天然功能的利用,并缺乏对反应位点的准确预测等;同时从酶资源挖掘、酶的改造等多个角度出发展望了未来能够为这些挑战提供解决方案的研究方向和新兴技术,包括高通量筛选技术、AI辅助酶工程等等。
中图分类号:
引用本文
程中玉, 李付琸. 基于P450选择性氧化的天然产物化学-酶法合成进展[J]. 合成生物学, 2024, 5(5): 960-980.
CHENG Zhongyu, LI Fuzhuo. Recent advances in chemoenzymatic synthesis of natural products via site- selective P450 oxidation[J]. Synthetic Biology Journal, 2024, 5(5): 960-980.
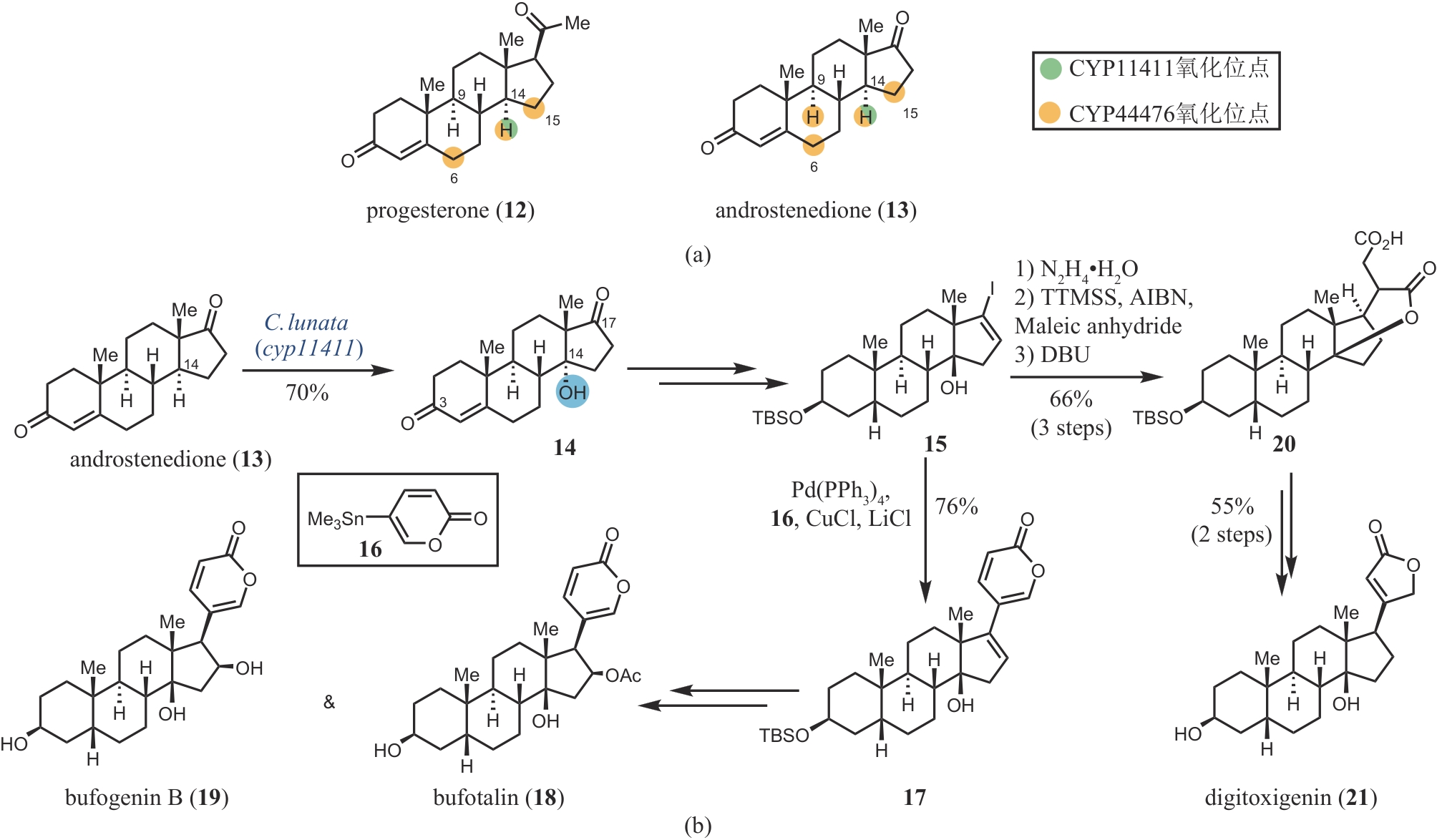
图3 CYP11411和CYP44476的区域选择性(a)和bufotalin、bufogenin和digitoxigenin的化学-酶法合成(b)
Fig. 3 Regioselectivity of CYP11411 and CYP44476 (a) and chemoenzymatic synthesis of bufotalin, bufogenin and digitoxigenin (b)
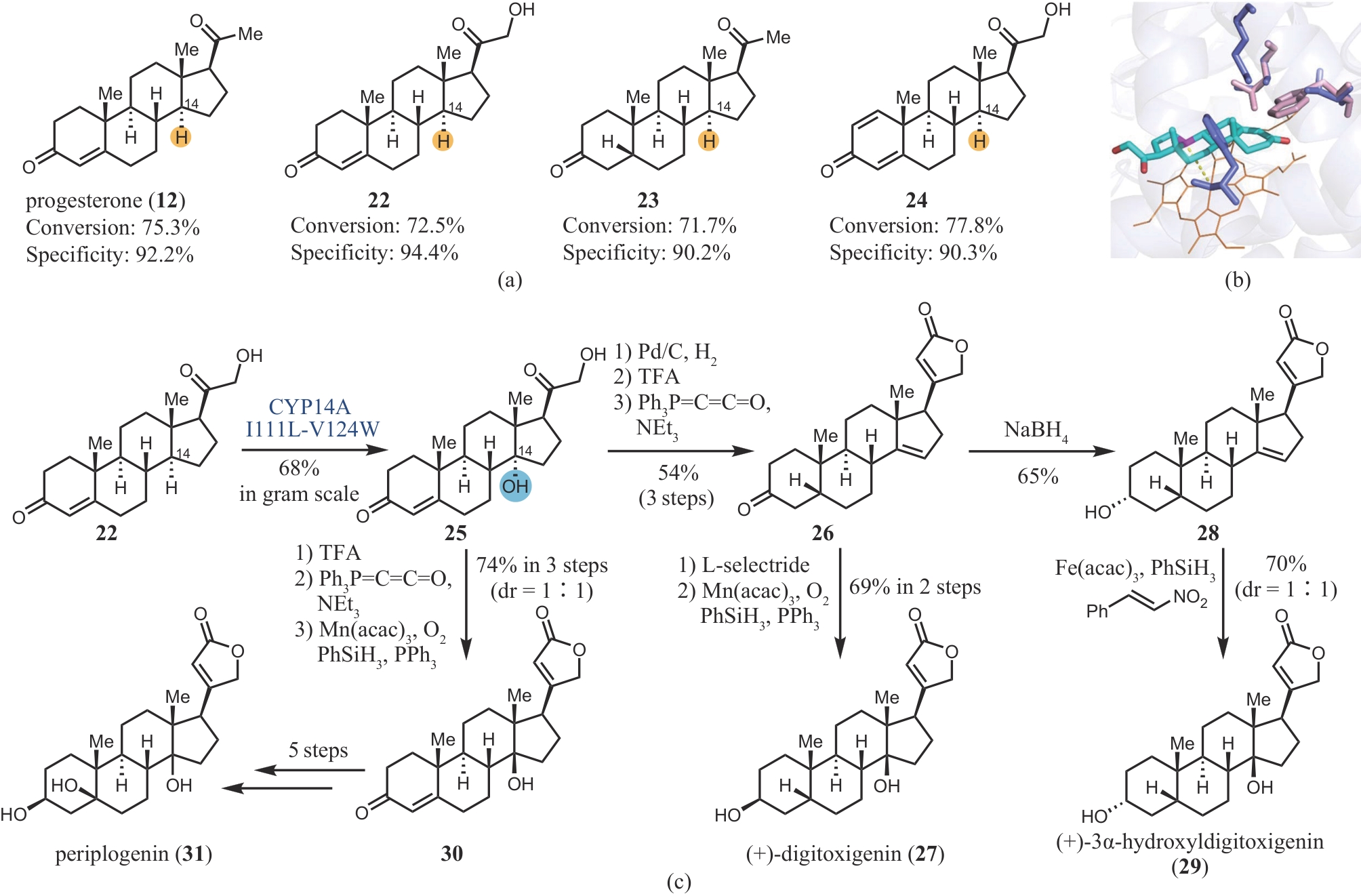
图4 CYP14A I111L-V124W的区域选择性(a),野生型CYP14A和突变体的对比(b)和digitoxigenin、3α-hydroxyldigitoxigenin和periplogenin的化学-酶法合成(c)
Fig. 4 Regioselectivity of CYP14A I111L-V124W(a), comparison of the wide-type CYP14A and mutant(b), and chemoenzymatic synthesis of digitoxigenin, 3α-hydroxyldigitoxigenin and periplogenin(c)
| 1 | NEWMAN D J, CRAGG G M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019[J]. Journal of Natural Products, 2020, 83(3): 770-803. |
| 2 | NEWHOUSE T, BARAN P S, HOFFMANN R W. The economies of synthesis[J]. Chemical Society Reviews, 2009, 38(11): 3010-3021. |
| 3 | KUTTRUFF C A, EASTGATE M D, BARAN P S. Natural product synthesis in the age of scalability[J]. Natural Product Reports, 2014, 31(4): 419-432. |
| 4 | ATANASOV A G, ZOTCHEV S B, DIRSCH V M, et al. Natural products in drug discovery: advances and opportunities[J]. Nature Reviews Drug Discovery, 2021, 20(3): 200-216. |
| 5 | LI J, AMATUNI A, RENATA H. Recent advances in the chemoenzymatic synthesis of bioactive natural products[J]. Current Opinion in Chemical Biology, 2020, 55: 111-118. |
| 6 | SIMIĆ S, ZUKIĆ E, SCHMERMUND L, et al. Shortening synthetic routes to small molecule active pharmaceutical ingredients employing biocatalytic methods[J]. Chemical Reviews, 2022, 122(1): 1052-1126. |
| 7 | CHAKRABARTY S, ROMERO E O, PYSER J B, et al. Chemoenzymatic total synthesis of natural products[J]. Accounts of Chemical Research, 2021, 54(6): 1374-1384. |
| 8 | ABRAMS D J, PROVENCHER P A, SORENSEN E J. Recent applications of C—H functionalization in complex natural product synthesis[J]. Chemical Society Reviews, 2018, 47(23): 8925-8967. |
| 9 | SINHA S K, GHOSH P, JAIN S, et al. Transition-metal catalyzed C—H activation as a means of synthesizing complex natural products[J]. Chemical Society Reviews, 2023, 52(21): 7461-7503. |
| 10 | LI F Z, ZHANG X, RENATA H. Enzymatic C—H functionalizations for natural product synthesis[J]. Current Opinion in Chemical Biology, 2019, 49: 25-32. |
| 11 | TURNER N J. Directed evolution drives the next generation of biocatalysts[J]. Nature Chemical Biology, 2009, 5(8): 567-573. |
| 12 | PACKER M S, LIU D R. Methods for the directed evolution of proteins[J]. Nature Reviews Genetics, 2015, 16(7): 379-394. |
| 13 | DENISOV I G, MAKRIS T M, SLIGAR S G, et al. Structure and chemistry of cytochrome P450[J]. Chemical Reviews, 2005, 105(6): 2253-2278. |
| 14 | GUENGERICH F P. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity[J]. Chemical Research in Toxicology, 2001, 14(6): 611-650. |
| 15 | 蒋媛媛, 李盛英. 细胞色素P450酶在生物合成及有机合成中的催化功能及其应用[J]. 有机化学, 2018, 38(9): 2307-2323. |
| JIANG Y Y, LI S Y. Catalytic function and application of cytochrome P450 enzymes in biosynthesis and organic synthesis[J]. Chinese Journal of Organic Chemistry, 2018, 38(9): 2307-2323. | |
| 16 | FESSNER N D. P450 monooxygenases enable rapid late-stage diversification of natural products via C—H bond activation[J]. ChemCatChem, 2019, 11(9): 2226-2242. |
| 17 | XIONG L B, LIU H H, ZHAO M, et al. Enhancing the bioconversion of phytosterols to steroidal intermediates by the deficiency of kasB in the cell wall synthesis of Mycobacterium neoaurum [J]. Microbial Cell Factories, 2020, 19(1): 80. |
| 18 | ZHENG M M, LIN Z, LIN S J, et al. Chemoenzymatic synthesis of steroidal products: recent advances[J]. European Journal of Organic Chemistry, 2024: e202301066. |
| 19 | ZHANG X D, PENG Y Q, ZHAO J, et al. Bacterial cytochrome P450-catalyzed regio- and stereoselective steroid hydroxylation enabled by directed evolution and rational design[J]. Bioresources and Bioprocessing, 2020, 7(1): 2. |
| 20 | RENATA H, ZHOU Q H, BARAN P S. Strategic redox relay enables a scalable synthesis of ouabagenin, a bioactive cardenolide[J]. Science, 2013, 339(6115): 59-63. |
| 21 | RENATA H, ZHOU Q H, DÜNSTL G, et al. Development of a concise synthesis of ouabagenin and hydroxylated corticosteroid analogues[J]. Journal of the American Chemical Society, 2015, 137(3): 1330-1340. |
| 22 | WOLFF M E, MORIOKA T. C-19 functional steroids. Ⅹ.17β-hydroxy-1β,19-cyclo-5α-androstan-2-one and related compounds[J]. Journal of Organic Chemistry, 1965, 30: 2553-2557. |
| 23 | WANG Y, JU W, TIAN H L, et al. Scalable synthesis of cyclocitrinol[J]. Journal of the American Chemical Society, 2018, 140(30): 9413-9416. |
| 24 | CLARK T A, CHONG R, MADDOX I S. An investigation into the 19-hydroxylation of androstenedione, cortexolone and progesterone by selected fungi[J]. Applied Microbiology and Biotechnology, 1985, 21(1): 132-134. |
| 25 | WANG J L, ZHANG Y N, LIU H H, et al. A biocatalytic hydroxylation-enabled unified approach to C19-hydroxylated steroids[J]. Nature Communications, 2019, 10(1): 3378. |
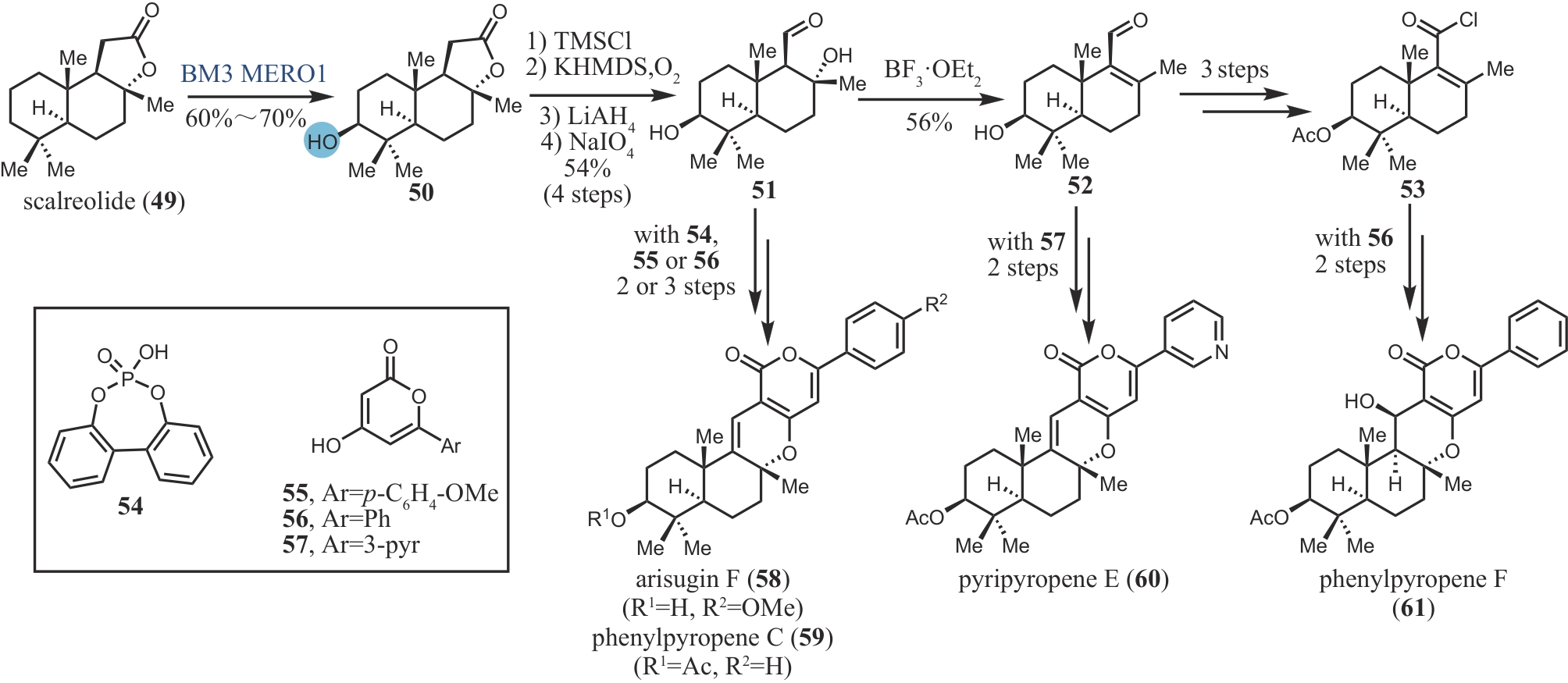
| 26 | PRASSAS I, DIAMANDIS E P. Novel therapeutic applications of cardiac glycosides[J]. Nature Reviews Drug Discovery, 2008, 7(11): 926-935. |
| 27 | DU J, JIANG L J, CHEN F Q, et al. Cardiac glycoside ouabain exerts anticancer activity via downregulation of STAT3[J]. Frontiers in Oncology, 2021, 11: 684316. |
| 28 | ZEITLIN P L, DIENER-WEST M, CALLAHAN K A, et al. Digitoxin for airway inflammation in cystic fibrosis: preliminary assessment of safety, pharmacokinetics, and dose finding[J]. Annals of the American Thoracic Society, 2017, 14(2): 220-229. |
| 29 | RIJSBERGEN M, NIEMEYER-VAN DER KOLK T, HOGENDOORN G, et al. A randomized controlled proof-of-concept trial of digoxin and furosemide in adults with cutaneous warts[J]. British Journal of Dermatology, 2019, 180(5): 1058-1068. |
| 30 | FEJEDELEM Z, CARNEY N, NAGORNY P. Synthesis of cardiotonic steroids oleandrigenin and rhodexin B[J]. The Journal of Organic Chemistry, 2021, 86(15): 10249-10262. |
| 31 | KHATRI H R, BHATTARAI B, KAPLAN W, et al. Modular total synthesis and cell-based anticancer activity evaluation of ouabagenin and other cardiotonic steroids with varying degrees of oxygenation[J]. Journal of the American Chemical Society, 2019, 141(12): 4849-4860. |
| 32 | SHIMIZU S, HAGIWARA K, ITOH H, et al. Unified total synthesis of five bufadienolides[J]. Organic Letters, 2020, 22(21): 8652-8657. |
| 33 | ZHAO Y, ZHANG B, SUN Z, et al. Biocatalytic C14-hydroxylation on androstenedione enabled modular synthesis of cardiotonic steroids[J]. ACS Catalysis, 2022, 12(16): 9839-9845. |
| 34 | SONG F Z, ZHENG M M, WANG J L, et al. Chemoenzymatic synthesis of C14-functionalized steroids[J]. Nature Synthesis, 2023, 2: 729-739. |
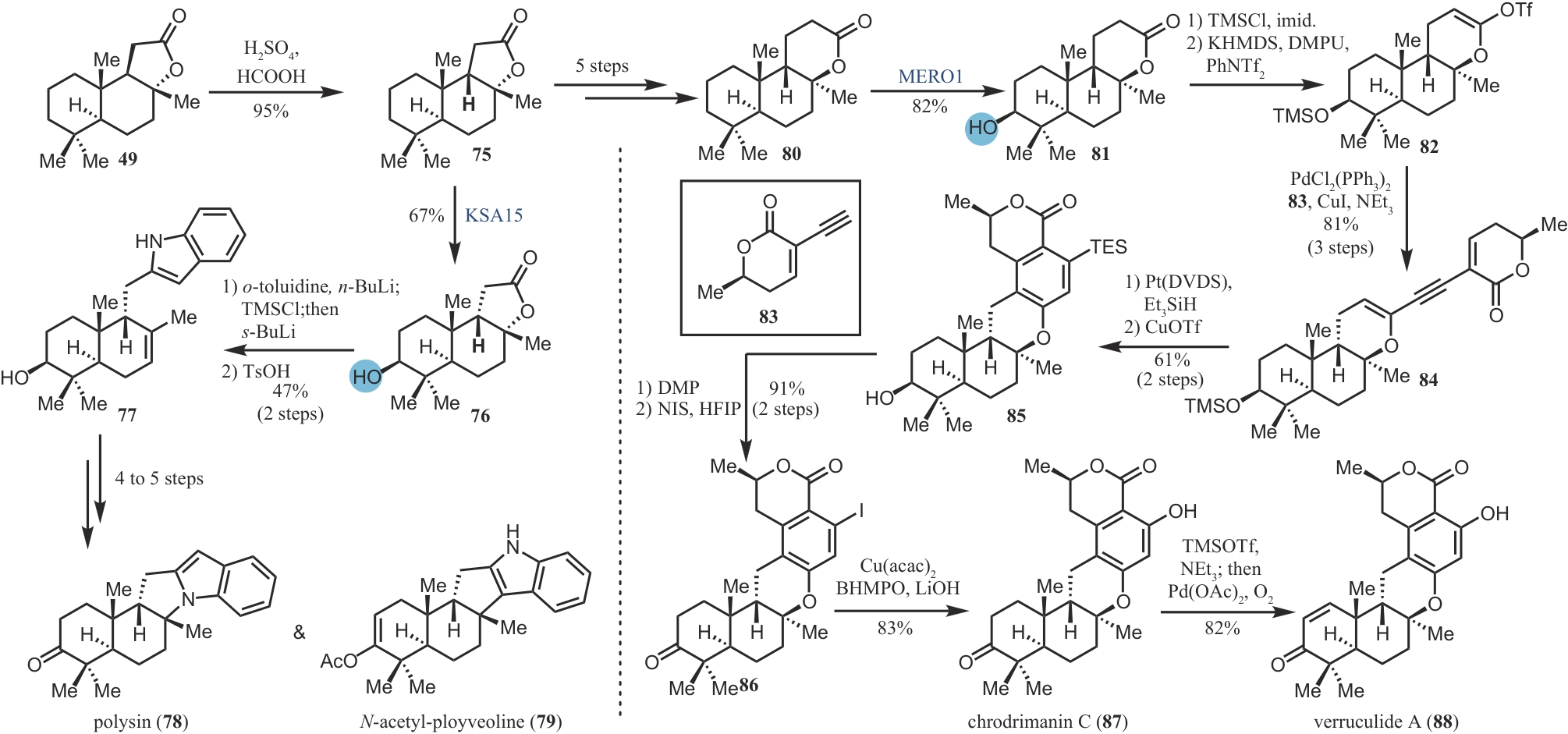
| 35 | YARROW J F, MCCOY S C, BORST S E. Tissue selectivity and potential clinical applications of trenbolone (17β-hydroxyestra-4,9,11-trien-3-one): a potent anabolic steroid with reduced androgenic and estrogenic activity[J]. Steroids, 2010, 75(6): 377-389. |
| 36 | 张沪跃, 陈燕, 施蛟, 等. 高收率合成醋酸群勃龙[J]. 复旦学报(医学版), 2002, 29(3): 211-212. |
| ZHANG H Y, CHEN Y, SHI J, et al. Synthesis of trenbolone acetate in high yield[J]. Fudan University Journal of Medical Science, 2002, 29(3): 211-212. | |
| 37 | LI A T, ACEVEDO-ROCHA C G, D’AMORE L, et al. Regio- and stereoselective steroid hydroxylation at C7 by cytochrome P450 monooxygenase mutants[J]. Angewandte Chemie International Edition, 2020, 59(30): 12499-12505. |
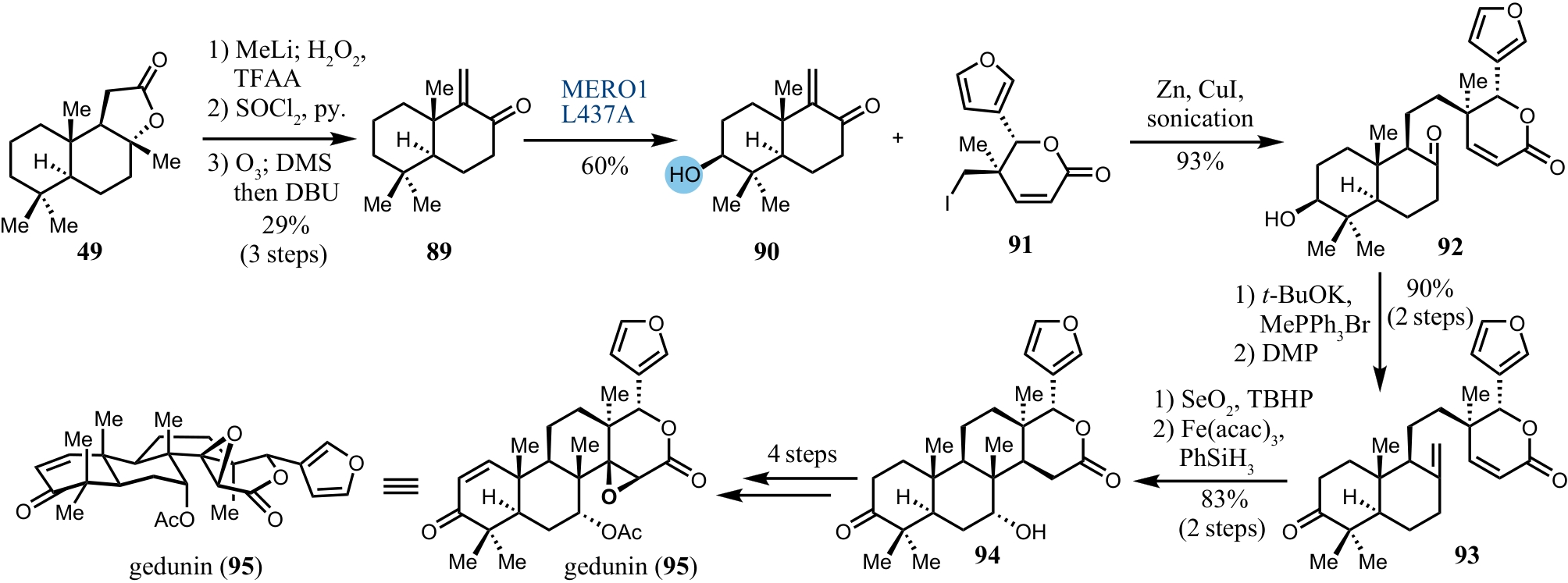
| 38 | PENG Y Q, GAO C H, ZHANG Z L, et al. A chemoenzymatic strategy for the synthesis of steroid drugs enabled by P450 monooxygenase-mediated steroidal core modification[J]. ACS Catalysis, 2022, 12(5): 2907-2914. |
| 39 | LI C, QIU W W, YANG Z F, et al. Stereoselective synthesis of some methyl-substituted steroid hormones and their in vitro cytotoxic activity against human gastric cancer cell line MGC-803[J]. Steroids, 2010, 75(12): 859-869. |
| 40 | ZHANG Z L, GAO C H, ZHAO J, et al. A designed chemoenzymatic route for efficient synthesis of 6-dehydronandrolone acetate: a key precursor in the synthesis of C7-functionalized steroidal drugs[J]. ACS Catalysis, 2023, 13(19): 13111-13116. |
| 41 | CHEN Q B, XIN X L, YANG Y, et al. Highly conjugated norditerpenoid and pyrroloquinoline alkaloids with potent PTP1B inhibitory activity from Nigella glandulifera [J]. Journal of Natural Products, 2014, 77(4): 807-812. |
| 42 | ZHANG S, ZHANG Z Y. PTP1B as a drug target: recent developments in PTP1B inhibitor discovery[J]. Drug Discovery Today, 2007, 12(9-10): 373-381. |
| 43 | LOSKOT S A, ROMNEY D K, ARNOLD F H, et al. Enantioselective total synthesis of nigelladine A via late-stage C—H oxidation enabled by an engineered P450 enzyme[J]. Journal of the American Chemical Society, 2017, 139(30): 10196-10199. |
| 44 | BRUNO N C, TUDGE M T, BUCHWALD S L. Design and preparation of new palladium precatalysts for C—C and C—N cross-coupling reactions[J]. Chemical Science, 2013, 4: 916-920. |
| 45 | LEWIS J C, MANTOVANI S M, FU Y, et al. Combinatorial alanine substitution enables rapid optimization of cytochrome P450BM3 for selective hydroxylation of large substrates[J]. ChemBioChem, 2010, 11(18): 2502-2505. |
| 46 | SUNAZUKA T, ŌMURA S. Total synthesis of α-pyrone meroterpenoids, novel bioactive microbial metabolites[J]. Chemical Reviews, 2005, 105(12): 4559-4580. |
| 47 | COREY E J, NOE M C, LIN S Z. A mechanistically designed bis-cinchona alkaloid ligand allows position- and enantioselective dihydroxylation of farnesol and other oligoprenyl derivatives at the terminal isopropylidene unit[J]. Tetrahedron Letters, 1995, 36(48): 8741-8744. |
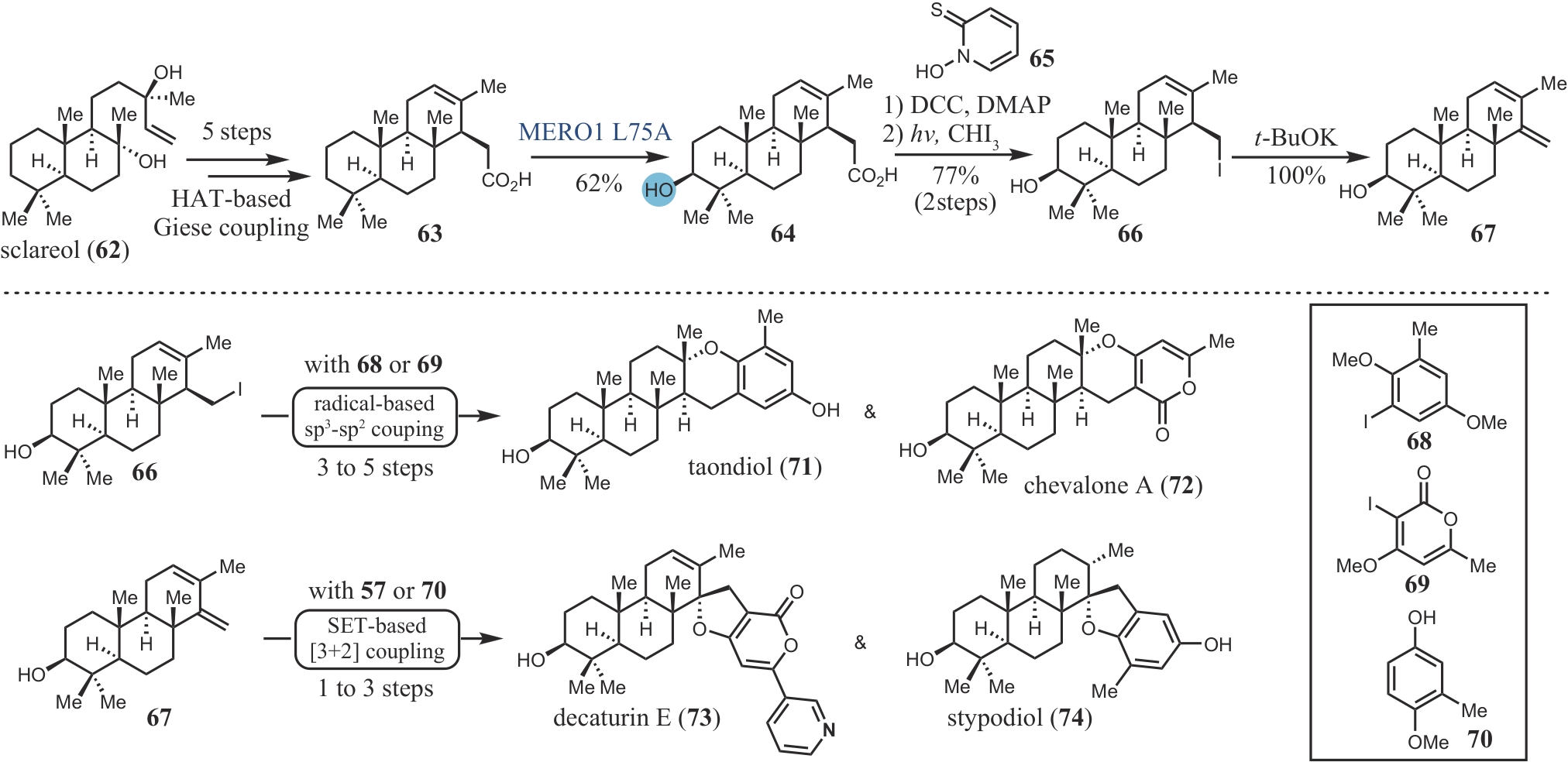
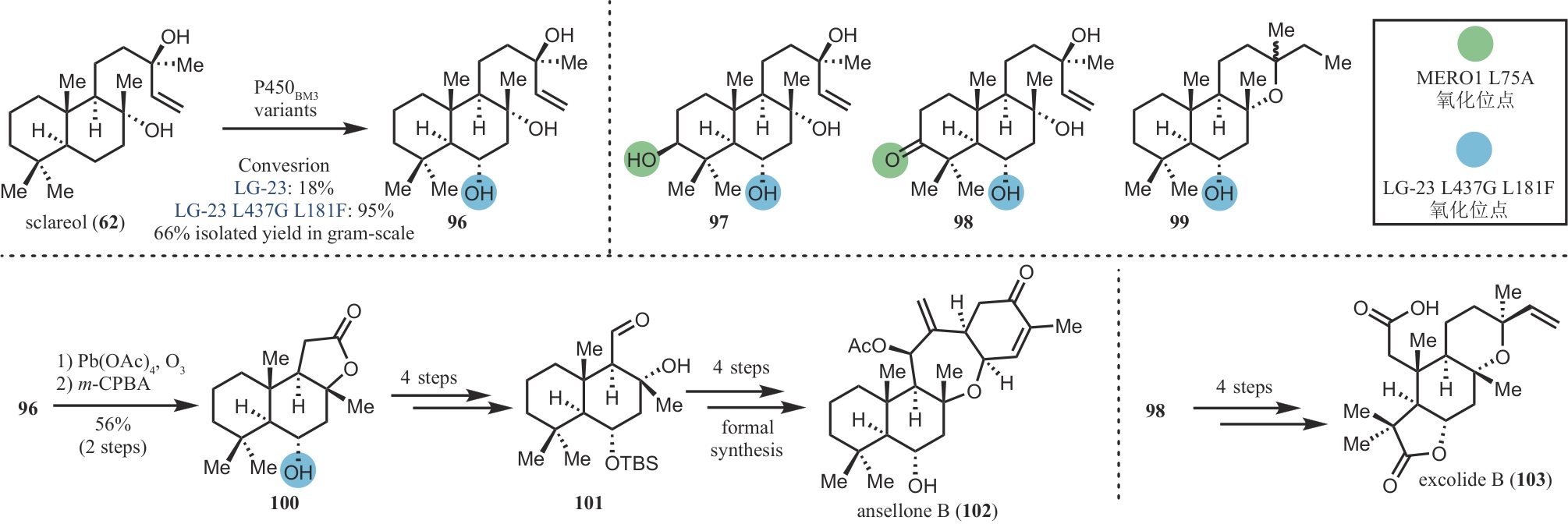
| 48 | KUMANIRENG A S, KATO T, KITAHARA Y. Cyclization of polyenes X. Biogenetic type synthesis of dl-taondiol[J]. Chemistry Letters, 1973, 2(10): 1045-1047. |
| 49 | ABAD A, AGULLÓ C, ARNÓ M, et al. An efficient stereoselective synthesis of stypodiol and epistypodiol[J]. The Journal of Organic Chemistry, 1998, 63(15): 5100-5106. |
| 50 | TAKIKAWA H, IMAMURA Y, SASAKI M. Synthesis and absolute configuration of brevione B, an allelochemical isolated from Penicillium sp[J]. Tetrahedron, 2006, 62(1): 39-48. |
| 51 | LI J, LI F Z, KING-SMITH E, et al. Merging chemoenzymatic and radical-based retrosynthetic logic for rapid and modular synthesis of oxidized meroterpenoids[J]. Nature Chemistry, 2020, 12: 173-179. |
| 52 | EVERSON D A, SHRESTHA R, WEIX D J. Nickel-catalyzed reductive cross-coupling of aryl halides with alkyl halides[J]. Journal of the American Chemical Society, 2010, 132(3): 920-921. |
| 53 | ANADA M, HANARI T, KAKITA K, et al. Total synthesis of brasilicardins A and C[J]. Organic Letters, 2017, 19(20): 5581-5584. |
| 54 | BOYKO Y D, HUCK C J, NING S, et al. Synthetic studies on selective, proapoptotic isomalabaricane triterpenoids aided by computational techniques[J]. Journal of the American Chemical Society, 2021, 143(4): 2138-2155. |
| 55 | BOYKO Y D, HUCK C J, SARLAH D. Total synthesis of isomalabaricane triterpenoids[J]. Journal of the American Chemical Society, 2019, 141(36): 14131-14135. |
| 56 | POWERS Z, SCHARF A, CHENG A, et al. Biomimetic synthesis of meroterpenoids by dearomatization-driven polycyclization[J]. Angewandte Chemie International Edition, 2019, 58(45): 16141-16146. |
| 57 | YOSHIMURA F, ITOH R, TORIZUKA M, et al. Asymmetric total synthesis of brasilicardins[J]. Angewandte Chemie International Edition, 2018, 57(52): 17161-17167. |
| 58 | MITSUHASHI T, BARRA L, POWERS Z, et al. Exploiting the potential of meroterpenoid cyclases to expand the chemical space of fungal meroterpenoids[J]. Angewandte Chemie International Edition, 2020, 59(52): 23772-23781. |
| 59 | VAN TAMELEN E E, SHARPLESS K B, HANZLIK R P, et al. Enzymic cyclization of trans, trans, trans-18,19-dehydrosqualene 2,3-oxide[J]. Journal of the American Chemical Society, 1967, 89(26): 7150-7151. |
| 60 | LI F Z, RENATA H. A Chiral-pool-based strategy to access trans-syn-fused drimane meroterpenoids: chemoenzymatic total syntheses of polysin, N-acetyl-polyveoline and the chrodrimanins[J]. Journal of the American Chemical Society, 2021, 143(43): 18280-18286. |
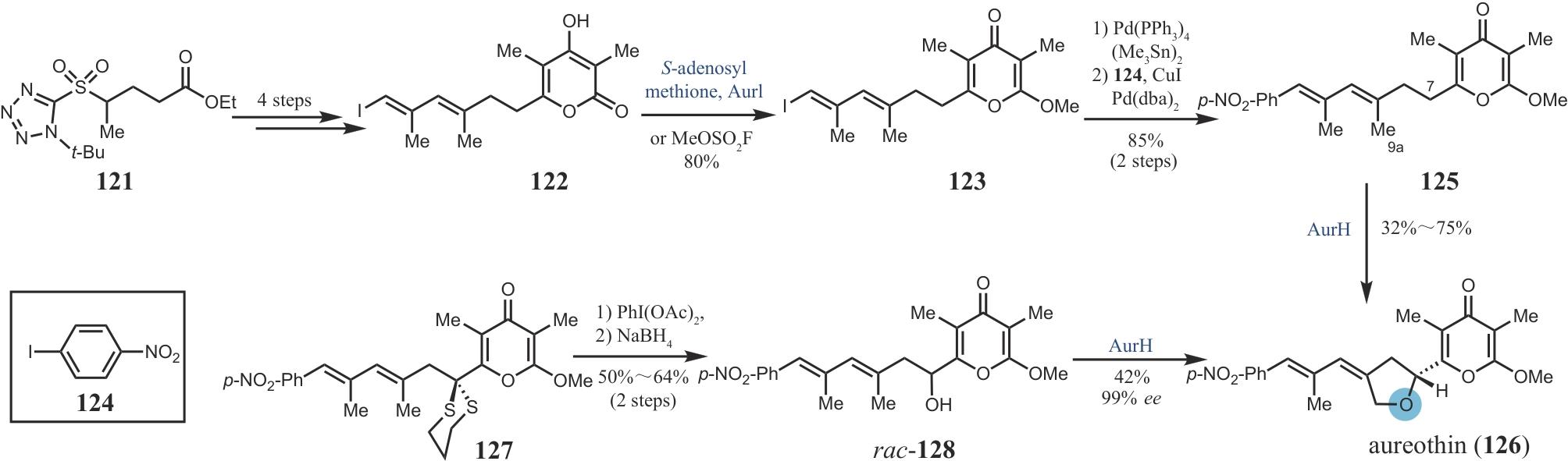
| 61 | KILLE S, ZILLY F E, ACEVEDO J P, et al. Regio- and stereoselectivity of P450-catalysed hydroxylation of steroids controlled by laboratory evolution[J]. Nature Chemistry, 2011, 3(9): 738-743. |
| 62 | SMITH A B, VISNICK M, HASELTINE J N, et al. Organometallic reagents in synthesis: a new protocol for construction of the indole nucleus[J]. Tetrahedron, 1986, 42(11): 2957-2969. |
| 63 | FIER P S, MALONEY K M. Synthesis of complex phenols enabled by a rationally designed hydroxide surrogate[J]. Angewandte Chemie International Edition, 2017, 56(16): 4478-4482. |
| 64 | FU S M, LIU B. Recent progress in the synthesis of limonoids and limonoid-like natural products[J]. Organic Chemistry Frontiers, 2020, 7(14): 1903-1947. |
| 65 | BRANDT G E L, SCHMIDT M D, PRISINZANO T E, et al. Gedunin, a novel Hsp90 inhibitor: semisynthesis of derivatives and preliminary structure-activity relationships[J]. Journal of Medicinal Chemistry, 2008, 51(20): 6495-6502. |
| 66 | ENGLISH A W, LIU K, NICOLINI J M, et al. Small-molecule trkB agonists promote axon regeneration in cut peripheral nerves[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(40): 16217-16222. |
| 67 | GORANTLA N V, DAS R, CHIDAMBARAM H, et al. Basic limonoid modulates chaperone-mediated proteostasis and dissolve Tau fibrils[J]. Scientific Reports, 2020, 10(1): 4023. |
| 68 | BEHENNA D C, COREY E J. Simple enantioselective approach to synthetic limonoids[J]. Journal of the American Chemical Society, 2008, 130(21): 6720-6721. |
| 69 | COREY E J, HAHL R W. Synthesis of a limonoid, azadiradione[J]. Tetrahedron Letters, 1989, 30(23): 3023-3026. |
| 70 | YAMASHITA S, NARUKO A, NAKAZAWA Y, et al. Total synthesis of limonin[J]. Angewandte Chemie International Edition, 2015, 54(29): 8538-8541. |
| 71 | LI J, CHEN F, RENATA H. Concise chemoenzymatic synthesis of gedunin[J]. Journal of the American Chemical Society, 2022, 144(42): 19238-19242. |
| 72 | FENG J J, GARZA V J, KRISCHE M J. Redox-triggered C—C coupling of alcohols and vinyl epoxides: diastereo- and enantioselective formation of all-carbon quaternary centers via tert-(hydroxy)-prenylation[J]. Journal of the American Chemical Society, 2014, 136(25): 8911-8914. |
| 73 | BRILL Z G, CONDAKES M L, TING C P, et al. Navigating the chiral pool in the total synthesis of complex terpene natural products[J]. Chemical Reviews, 2017, 117(18): 11753-11795. |
| 74 | LI F Z, DENG H P, RENATA H. Remote B-ring oxidation of sclareol with an engineered P450 facilitates divergent access to complex terpenoids[J]. Journal of the American Chemical Society, 2022, 144(17): 7616-7621. |
| 75 | ZHANG W, YAO H L, YU J X, et al. Total syntheses of sesterterpenoid ansellones A and B, and phorbadione[J]. Angewandte Chemie International Edition, 2017, 56(17): 4787-4791. |
| 76 | HE H B, JIANG H, CHEN Y, et al. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity[J]. Nature Communications, 2018, 9(1): 2550. |
| 77 | LIAO Y J, BAI H Y, LI Z H, et al. Longikaurin A, a natural ent-kaurane, induces G2/M phase arrest via downregulation of Skp2 and apoptosis induction through ROS/JNK/c-Jun pathway in hepatocellular carcinoma cells[J]. Cell Death & Disease, 2014, 5(3): e1137. |
| 78 | HONG Y J, TANTILLO D J. Formation of beyerene, kaurene, trachylobane, and atiserene diterpenes by rearrangements that avoid secondary carbocations[J]. Journal of the American Chemical Society, 2010, 132(15): 5375-5386. |
| 79 | LAZARSKI K E, MORITZ B J, THOMSON R J. The total synthesis of Isodon diterpenes[J]. Angewandte Chemie International Edition, 2014, 53(40): 10588-10599. |
| 80 | RIEHL P S, DEPORRE Y C, ARMALY A M, et al. New avenues for the synthesis of ent-kaurene diterpenoids[J]. Tetrahedron, 2015, 71(38): 6629-6650. |
| 81 | CHERNEY E C, LOPCHUK J M, GREEN J C, et al. A unified approach to ent-atisane diterpenes and related alkaloids: synthesis of (-)-methyl atisenoate, (-)-isoatisine, and the hetidine skeleton[J]. Journal of the American Chemical Society, 2014, 136(36): 12592-12595. |
| 82 | KENNY M J, MANDER L N, SETHI S P. Synthetic studies on rabdosia diterpene lactones Ⅱ: the synthesis of 15-desoxyeffusin[J]. Tetrahedron Letters, 1986, 27(33): 3927-3930. |
| 83 | KOBAYASHI S, SHIBUKAWA K, HAMADA Y, et al. Syntheses of (-)-tripterifordin and (-)-neotripterifordin from stevioside[J]. The Journal of Organic Chemistry, 2018, 83(3): 1606-1613. |
| 84 | DONG L B, ZHANG X, RUDOLF J D, et al. Cryptic and stereospecific hydroxylation, oxidation, and reduction in platensimycin and platencin biosynthesis[J]. Journal of the American Chemical Society, 2019, 141(9): 4043-4050. |
| 85 | RUDOLF J D, DONG L B, ZHANG X, et al. Cytochrome P450-catalyzed hydroxylation initiating ether formation in platensimycin biosynthesis[J]. Journal of the American Chemical Society, 2018, 140(39): 12349-12353. |
| 86 | ZHANG X, KING-SMITH E, DONG L B, et al. Divergent synthesis of complex diterpenes through a hybrid oxidative approach[J]. Science, 2020, 369(6505): 799-806. |
| 87 | OISHI H, HOSOKAWA T, OKUTOMI T, et al. Pesticidal activity of aureothin[J]. Agricultural and Biological Chemistry, 1969, 33(12): 1790-1791. |
| 88 | JACOBSEN M F, MOSES J E, ADLINGTON R M, et al. A short total synthesis of aureothin and N-acetylaureothamine[J]. Organic Letters, 2005, 7(4): 641-644. |
| 89 | LIANG G X, SEIPLE I B, TRAUNER D. Stereoselective syntheses of the bioactive polypropionates aureothin, N-acetylaureothamine, and aureonitrile[J]. Organic Letters, 2005, 7(14): 2837-2839. |
| 90 | ISHIBASHI Y, OHBA S, NISHIYAMA S, et al. Absolute configuration of (+)-aureothin: a toxic metabolite possessing γ-pyrone unit[J]. Bulletin of the Chemical Society of Japan, 1995, 68(12): 3643-3649. |
| 91 | HE J, HERTWECK C. Biosynthetic origin of the rare nitroaryl moiety of the polyketide antibiotic aureothin: involvement of an unprecedented N-oxygenase[J]. Journal of the American Chemical Society, 2004, 126(12): 3694-3695. |
| 92 | HE J, MÜLLER M, HERTWECK C. Formation of the aureothin tetrahydrofuran ring by a bifunctional cytochrome P450 monooxygenase[J]. Journal of the American Chemical Society, 2004, 126(51): 16742-16743. |
| 93 | RICHTER M E, TRAITCHEVA N, KNÜPFER U, et al. Sequential asymmetric polyketide heterocyclization catalyzed by a single cytochrome P450 monooxygenase (AurH)[J]. Angewandte Chemie International Edition, 2008, 47(46): 8872-8875. |
| 94 | WERNEBURG M, HERTWECK C. Chemoenzymatic total synthesis of the antiproliferative polyketide (+)-(R)-aureothin[J]. ChemBioChem, 2008, 9(13): 2064-2066. |
| 95 | HENROT M, RICHTER M E A, MADDALUNO J, et al. Convergent asymmetric synthesis of (+)-aureothin employing an oxygenase-mediated resolution step[J]. Angewandte Chemie International Edition, 2012, 51(38): 9587-9591. |
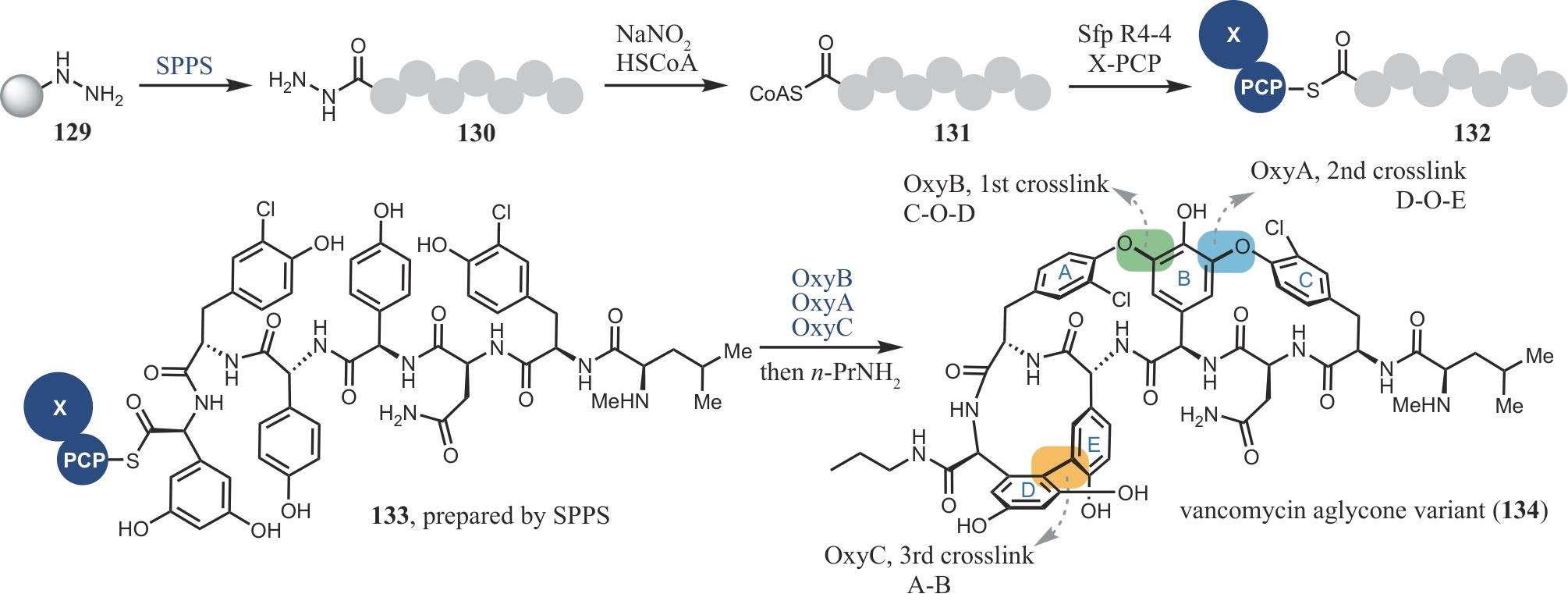
| 96 | LEVINE D P. Vancomycin: a history[J]. Clinical Infectious Diseases, 2006, 42(): S5-S12. |
| 97 | SHUGRUE C R, MILLER S J. Applications of nonenzymatic catalysts to the alteration of natural products[J]. Chemical Reviews, 2017, 117(18): 11894-11951. |
| 98 | HUBBARD B K, WALSH C T. Vancomycin assembly: nature’s way[J]. Angewandte Chemie International Edition, 2003, 42(7): 730-765. |
| 99 | HUBBARD B K, WALSH C T. Der Aufbau von Vancomycin: so macht es die Natur[J]. Angewandte Chemie, 2003, 115(7): 752-789. |
| 100 | OKANO A, ISLEY N A, BOGER D L. Total syntheses of vancomycin-related glycopeptide antibiotics and key analogues[J]. Chemical Reviews, 2017, 117(18): 11952-11993. |
| 101 | STEGMANN E, PELZER S, BISCHOFF D, et al. Genetic analysis of the balhimycin (vancomycin-type) oxygenase genes[J]. Journal of Biotechnology, 2006, 124(4): 640-653. |
| 102 | FORNERIS C C, SEYEDSAYAMDOST M R. In vitro reconstitution of OxyC activity enables total chemoenzymatic syntheses of vancomycin aglycone variants[J]. Angewandte Chemie International Edition, 2018, 57(27): 8048-8052. |
| 103 | HAUSER N, IRELAND K A, CHIOTI V T, et al. Robust chemoenzymatic synthesis of keratinimicin aglycone analogues facilitated by the structure and selectivity of OxyB[J]. ACS Chemical Biology, 2023, 18(7): 1473-1479. |
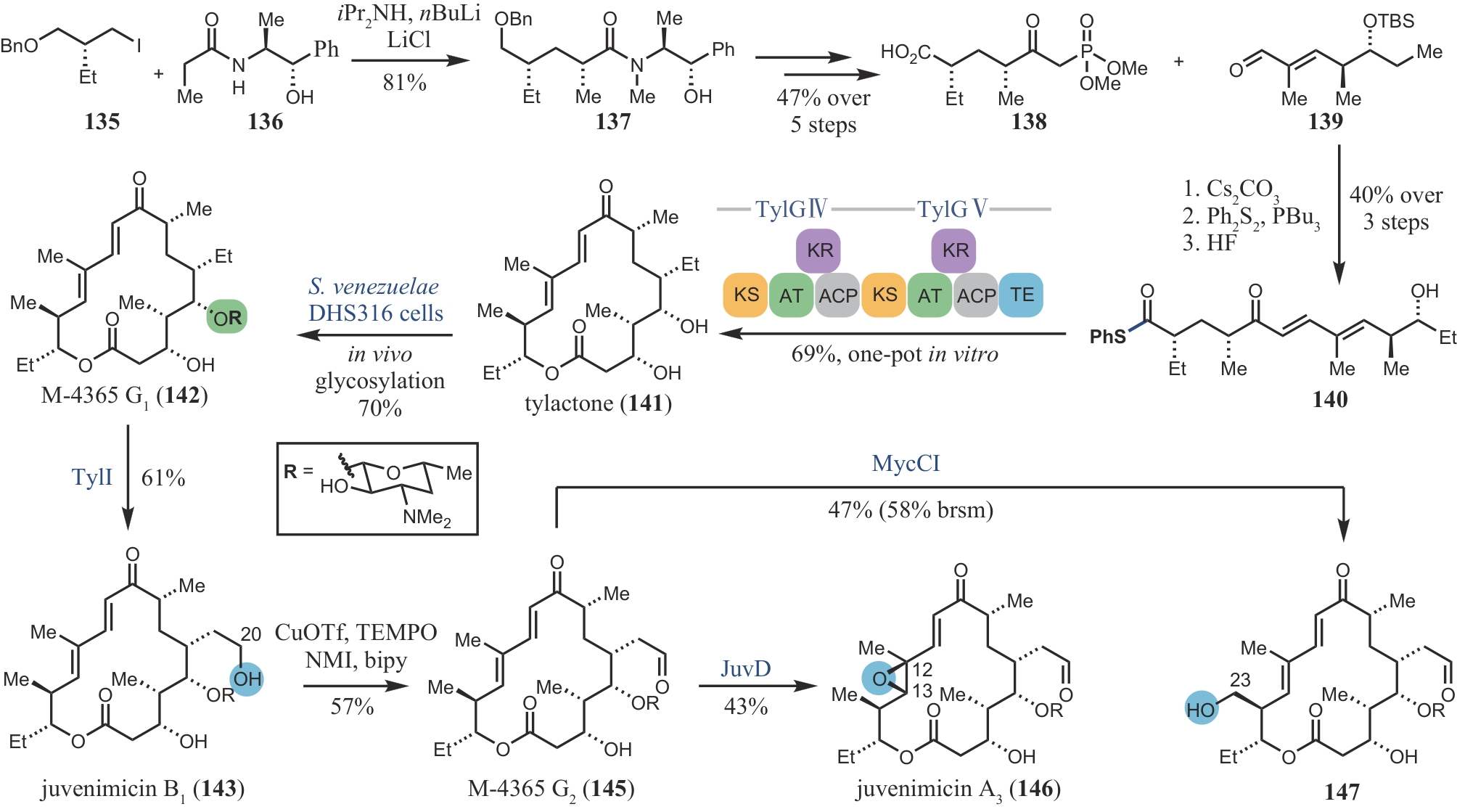
| 104 | LOWELL A N, M D Ⅱ DEMARS, SLOCUM S T, et al. Chemoenzymatic total synthesis and structural diversification of tylactone-based macrolide antibiotics through late-stage polyketide assembly, tailoring, and C—H functionalization[J]. Journal of the American Chemical Society, 2017, 139(23): 7913-7920. |
| 105 | CHAGANTY S, GOLAKOTI T, HELTZEL C, et al. Isolation and structure determination of cryptophycins 38, 326, and 327 from the terrestrial cyanobacterium Nostoc sp. GSV 224[J]. Journal of Natural Products, 2004, 67(8): 1403-1406. |
| 106 | SMITH C D, ZHANG X Q, MOOBERRY S L, et al. Cryptophycin: a new antimicrotubule agent active against drug-resistant cells[J]. Cancer Research, 1994, 54(14): 3779-3784. |
| 107 | EDELMAN M J, GANDARA D R, HAUSNER P, et al. Phase 2 study of cryptophycin 52 (LY355703) in patients previously treated with platinum based chemotherapy for advanced non-small cell lung cancer[J]. Lung Cancer, 2003, 39(2): 197-199. |
| 108 | MAGARVEY N A, BECK Z Q, GOLAKOTI T, et al. Biosynthetic characterization and chemoenzymatic assembly of the cryptophycins. Potent anticancer agents from Nostoc cyanobionts[J]. ACS Chemical Biology, 2006, 1(12): 766-779. |
| 109 | SUN H H, ZHANG H F, ANG E L, et al. Biocatalysis for the synthesis of pharmaceuticals and pharmaceutical intermediates[J]. Bioorganic & Medicinal Chemistry, 2018, 26(7): 1275-1284. |
| 110 | YANG Y, ARNOLD F H. Navigating the unnatural reaction space: directed evolution of heme proteins for selective carbene and nitrene transfer[J]. Accounts of Chemical Research, 2021, 54(5): 1209-1225. |
| 111 | ZHOU Q, CHIN M, FU Y, et al. Stereodivergent atom-transfer radical cyclization by engineered cytochromes P450[J]. Science, 2021, 374(6575): 1612-1616. |
| 112 | MILLER D C, ATHAVALE S V, ARNOLD F H. Combining chemistry and protein engineering for new-to-nature biocatalysis[J]. Nature Synthesis, 2022, 1(1): 18-23. |
| 113 | 杨谦, 程伯涛, 汤志军, 等. 基因组挖掘在天然产物发现中的应用和前景[J]. 合成生物学, 2021, 2(5): 697-715. |
| YANG Q, CHENG B T, TANG Z J, et al. Applications and prospects of genome mining in the discovery of natural products[J]. Synthetic Biology Journal, 2021, 2(5): 697-715. | |
| 114 | WU Z, JENNIFER KAN S B J, LEWIS R D, et al. Machine learning-assisted directed protein evolution with combinatorial libraries[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(18): 8852-8858. |
| 115 | JACQUES P, BÉCHET M, BIGAN M, et al. High-throughput strategies for the discovery and engineering of enzymes for biocatalysis[J]. Bioprocess and Biosystems Engineering, 2017, 40(2): 161-180. |
| 116 | KING-SMITH E, FABER F A, REILLY U, et al. Predictive Minisci late stage functionalization with transfer learning[J]. Nature Communications, 2024, 15(1): 426. |
| 117 | FINNIGAN W, LUBBERINK M, HEPWORTH L J, et al. RetroBioCat database: a platform for collaborative curation and automated meta-analysis of biocatalysis data[J]. ACS Catalysis, 2023, 13(17): 11771-11780. |
| [1] | 张守祺, 王涛, 孔尧, 邹家胜, 刘元宁, 徐正仁. 天然产物的化学-酶法合成:方法与策略的演进[J]. 合成生物学, 2024, 5(5): 913-940. |
| [2] | 张发光, 曲戈, 孙周通, 马军安. 从化学合成到生物合成——天然产物全合成新趋势[J]. 合成生物学, 2021, 2(5): 674-696. |
| [3] | 刘裕, 韦惠玲, 刘骥翔, 王少杰, 苏海佳. 人工多菌体系的设计与构建:合成生物学研究新前沿[J]. 合成生物学, 2021, 2(4): 635-650. |
| [4] | 钱秀娟, 陈琳, 章文明, 周杰, 董维亮, 信丰学, 姜岷. 人工多细胞体系设计与构建研究进展[J]. 合成生物学, 2020, 1(3): 267-284. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||