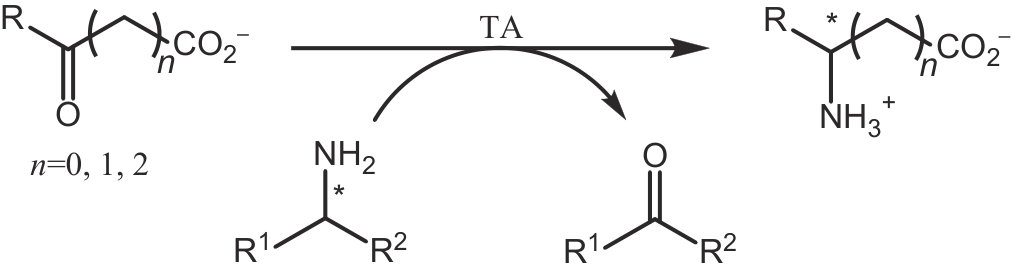
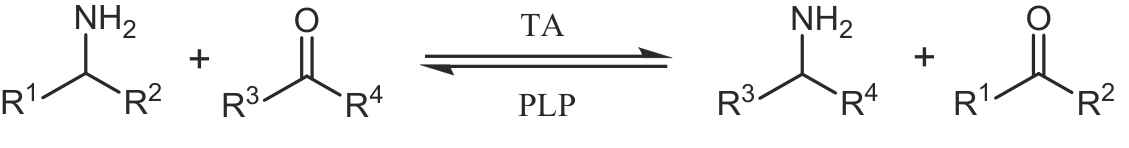
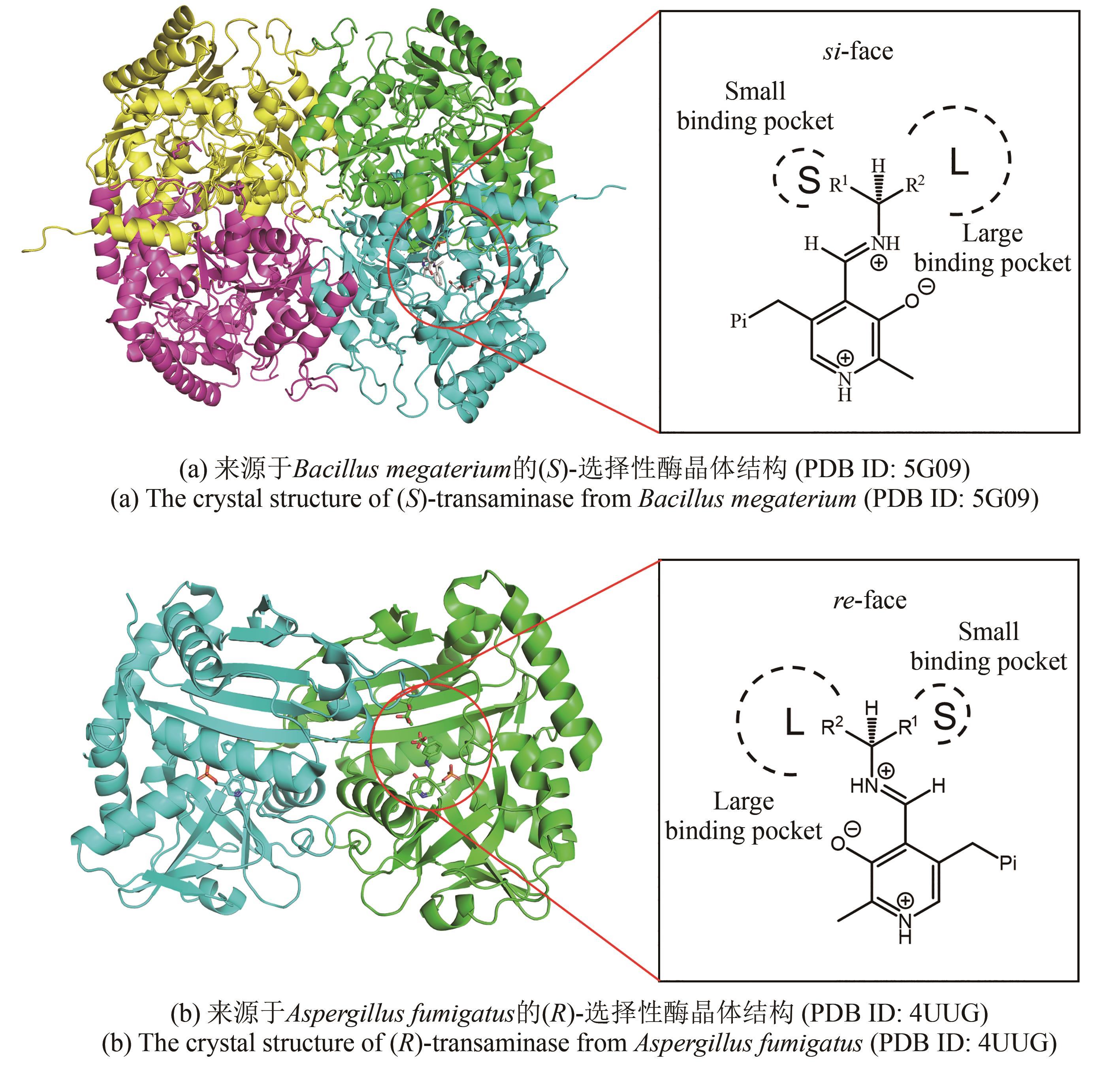
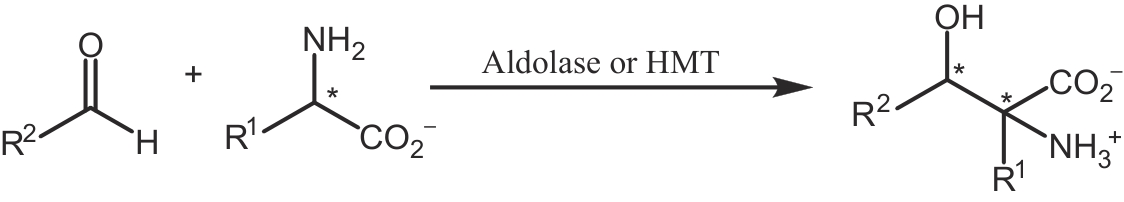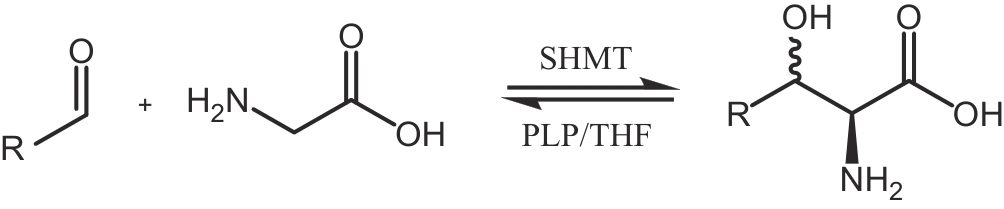合成生物学 ›› 2024, Vol. 5 ›› Issue (6): 1319-1349.DOI: 10.12211/2096-8280.2024-015
酶促合成手性氨基酸的研究进展
王子渊1, 杨立荣1,2, 吴坚平1,2, 郑文隆1
- 1.浙江大学杭州国际科创中心,生物与分子智造研究院,浙江 杭州 311215
2.浙江大学化学工程与生物工程学院,浙江 杭州 310058
-
收稿日期:2024-02-04修回日期:2024-05-16出版日期:2024-12-31发布日期:2025-01-10 -
通讯作者:郑文隆 -
作者简介:王子渊 (1995—),女,博士后。研究方向为蛋白工程、生物化工。E-mail:ziyuanwang@zju.edu.cn郑文隆 (1991—),男,研究员。研究方向为生物催化与转化、蛋白质智能设计等。E-mail:per@zju.edu.cn -
基金资助:国家自然科学基金(22308317);国家重点研发计划(2019YFA0905000);浙江省“尖兵”“领雁”研发攻关计划(2024C03013)
A review on enzyme-catalyzed synthesis of chiral amino acids
WANG Ziyuan1, YANG Lirong1,2, WU Jianping1,2, ZHENG Wenlong1
- 1.Institute for Intelligent Bio/Chem Manufacturing,ZJU-Hangzhou Global Scientific and Technological Innovation Center,Zhejiang University,Hangzhou 311215,Zhejiang,China
2.College of Chemical and Biological Engineering,Zhejiang University,Hangzhou 310058,Zhejiang,China
-
Received:2024-02-04Revised:2024-05-16Online:2024-12-31Published:2025-01-10 -
Contact:ZHENG Wenlong
摘要:
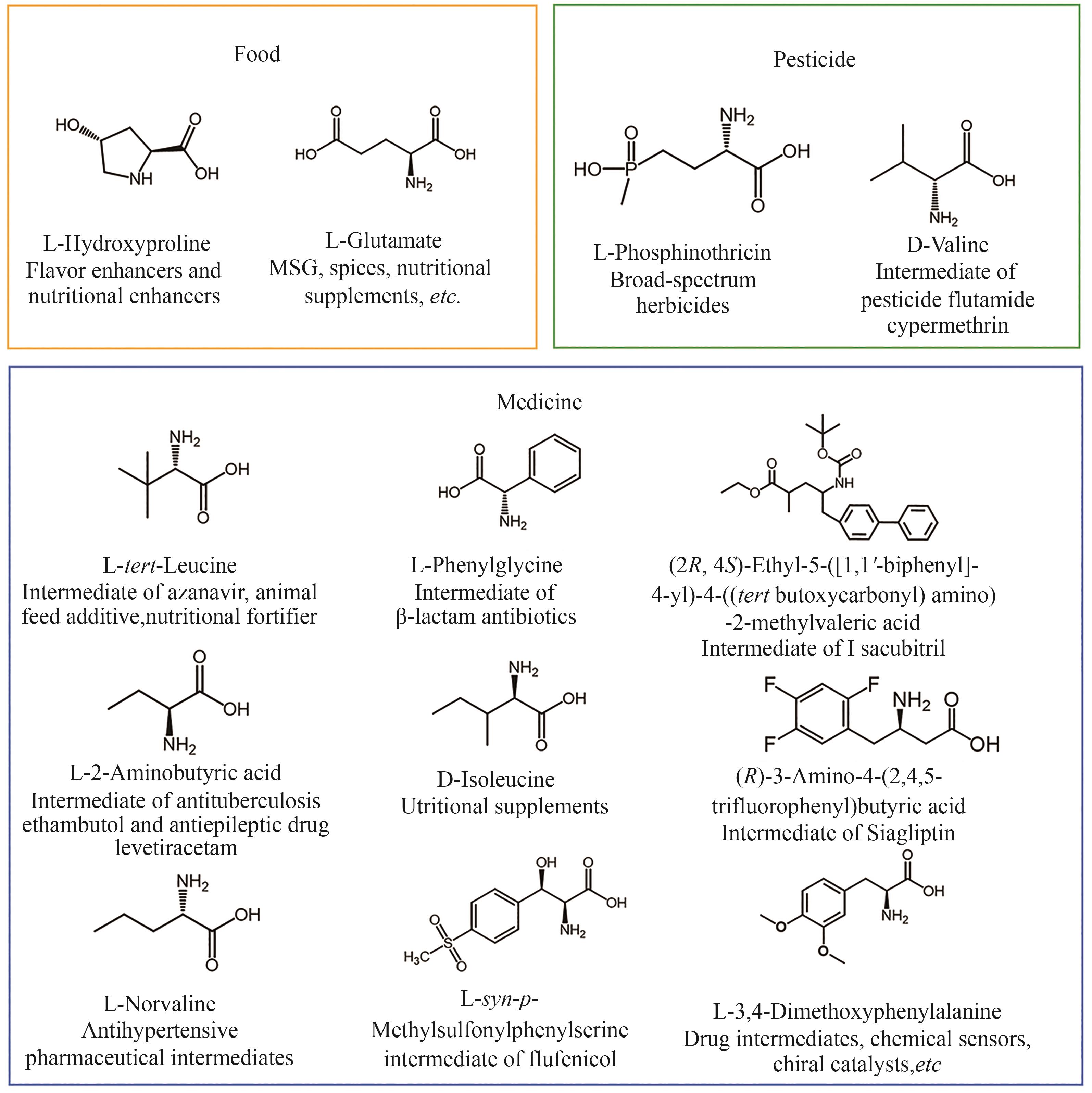
手性氨基酸是一类重要的高价值化学品,广泛应用于食品、医药、化工、农药等多个领域。手性氨基酸常用的制备方法可以分为四类,包括化学合成、蛋白质水解、发酵和酶促合成。其中,酶促合成手性氨基酸以其反应条件温和、立体选择性高、步骤简单、应用范围广等优势备受关注。近年来,得益于生物信息学和蛋白质工程等技术的快速发展,大量性能优异的酶制剂被开发,并成功应用于多种手性氨基酸的制备。本文重点综述了酶促不对称合成和去消旋化合成两种路径在手性氨基酸合成中的应用,包括关键酶制剂氨基酸脱氢酶、转氨酶、氨裂解酶、醛缩酶、氨基酸氧化酶、氨基酸脱氨酶等的开发与改造,及其在草铵膦、叔亮氨酸、西格列汀中间体等高价值手性氨基酸合成中的应用。同时,总结了酶促合成手性氨基酸领域面临的主要困境,如关键酶元件缺乏,以及野生酶非对映体选择性低、底物谱窄、催化活性低、稳定性差、反应条件局限等。最后,展望了自动化实验装置、机器学习和人工智能等前沿技术在酶改造领域的应用,以及通过反应器设计和反应过程控制,开发更为高效和环境友好的催化工艺,推动酶促合成手性氨基酸技术更广泛的工业应用。
中图分类号:
引用本文
王子渊, 杨立荣, 吴坚平, 郑文隆. 酶促合成手性氨基酸的研究进展[J]. 合成生物学, 2024, 5(6): 1319-1349.
WANG Ziyuan, YANG Lirong, WU Jianping, ZHENG Wenlong. A review on enzyme-catalyzed synthesis of chiral amino acids[J]. Synthetic Biology Journal, 2024, 5(6): 1319-1349.
方法 Methods | 酶制剂 Enzyme | 底物 Substrate | 立体选择性 Stereosele-ctivity | 理论产率 Theoretical yield | 原子经济性 Atomic economy | 典型案例 Typical examples |
|---|---|---|---|---|---|---|
| Asymmetric synthesis | Amino acid dehydrogenase, Transaminase, Ammonia lyase, Amino mutase, Aldolase, Hydroxymethyltransferase, etc. | Keto acids, α,β-unsaturated carboxylic acids, Amino acids and Aldehydes | High | 100% | High | L-tert-Leucine [ (R)-3-Amino-4-(2,4,5-trifluorophenyl)butyric acid [ |
| Racemization synthesis | Amino acid dehydrogenase, Transaminase, Ammonia lyase, Amino mutases, Aldolase, Hydroxymethyltransferase, Amino acid oxidase, Amino acid deaminase, Amino acid racemase, etc. | Racemic amino acids | High | 100% | High | |
| Dynamic kinetic resolution | Amino acid oxidase, Amino acid deaminase, Amino acid dehydrogenase, Amino acid racemase, etc. | Racemic amino acids | High | 50% | Low | — |
表1 酶促合成手性氨基酸的三种常用方法比较
Table 1 Comparison of three common methods for enzyme-catalyzed synthesis of chiral amino acids
方法 Methods | 酶制剂 Enzyme | 底物 Substrate | 立体选择性 Stereosele-ctivity | 理论产率 Theoretical yield | 原子经济性 Atomic economy | 典型案例 Typical examples |
|---|---|---|---|---|---|---|
| Asymmetric synthesis | Amino acid dehydrogenase, Transaminase, Ammonia lyase, Amino mutase, Aldolase, Hydroxymethyltransferase, etc. | Keto acids, α,β-unsaturated carboxylic acids, Amino acids and Aldehydes | High | 100% | High | L-tert-Leucine [ (R)-3-Amino-4-(2,4,5-trifluorophenyl)butyric acid [ |
| Racemization synthesis | Amino acid dehydrogenase, Transaminase, Ammonia lyase, Amino mutases, Aldolase, Hydroxymethyltransferase, Amino acid oxidase, Amino acid deaminase, Amino acid racemase, etc. | Racemic amino acids | High | 100% | High | |
| Dynamic kinetic resolution | Amino acid oxidase, Amino acid deaminase, Amino acid dehydrogenase, Amino acid racemase, etc. | Racemic amino acids | High | 50% | Low | — |
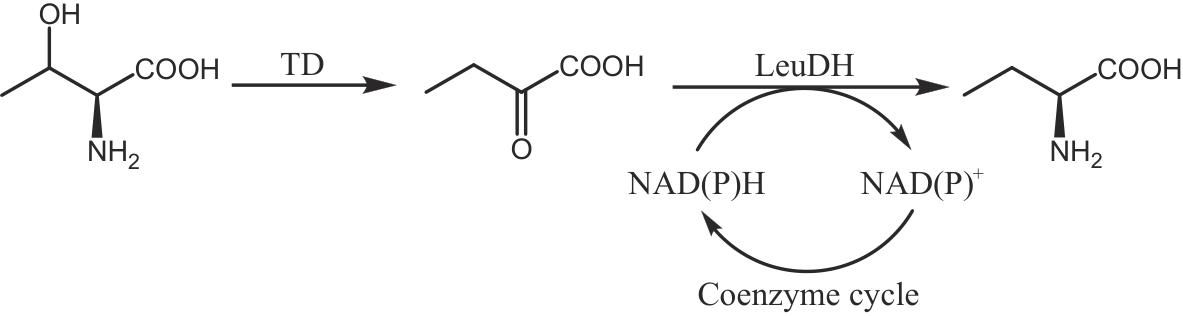
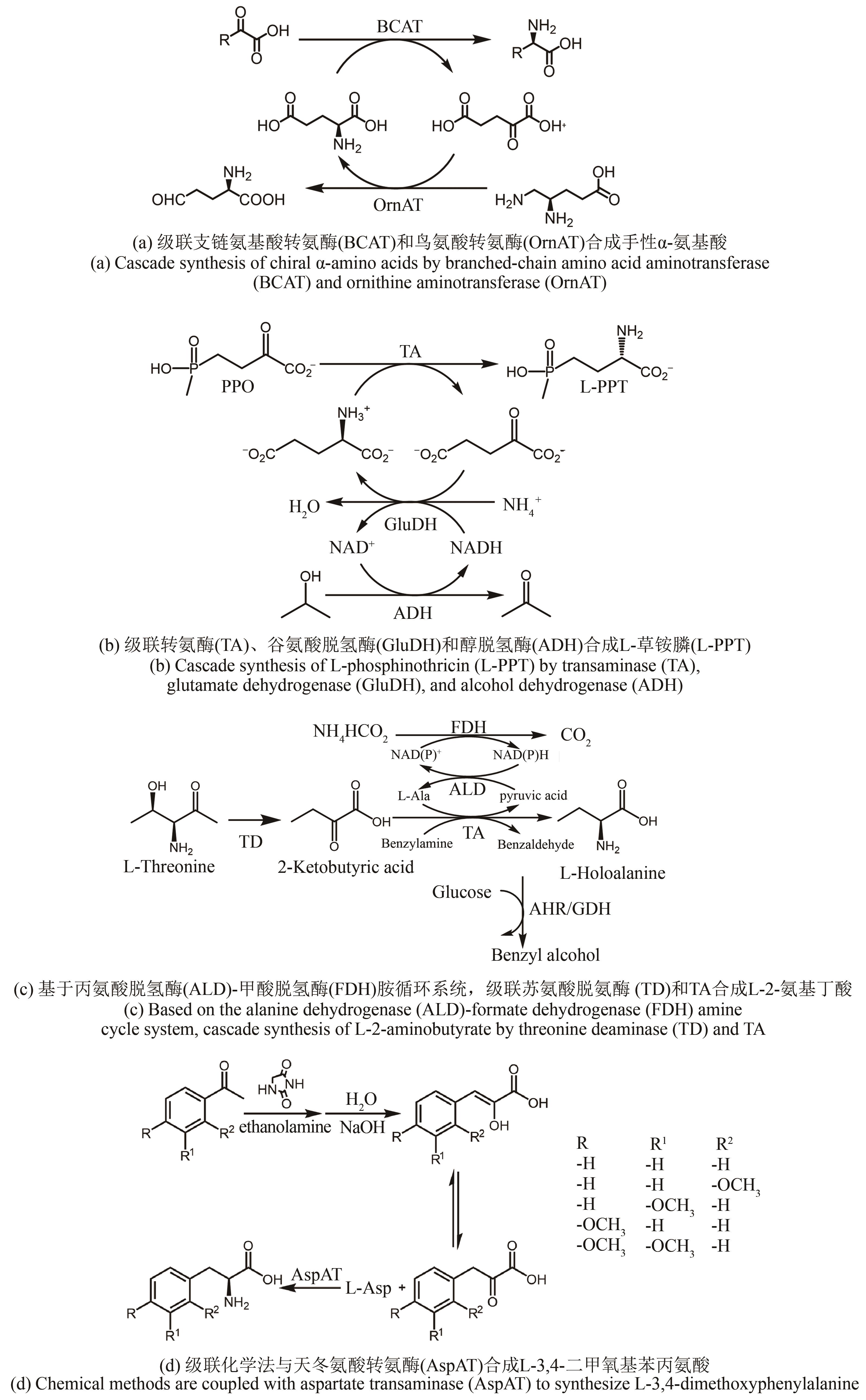
图5 利用L-苏氨酸合成L-2-氨基丁酸的多酶级联体系TD—苏氨酸脱氨酶;LeuDH—亮氨酸脱氢酶
Fig. 5 Multi-enzymatic cascade system for synthesizing L-2-aminobutyric acid from L-threonineTD—Threonine deaminase; LeuDH—Leucine dehydrogenase
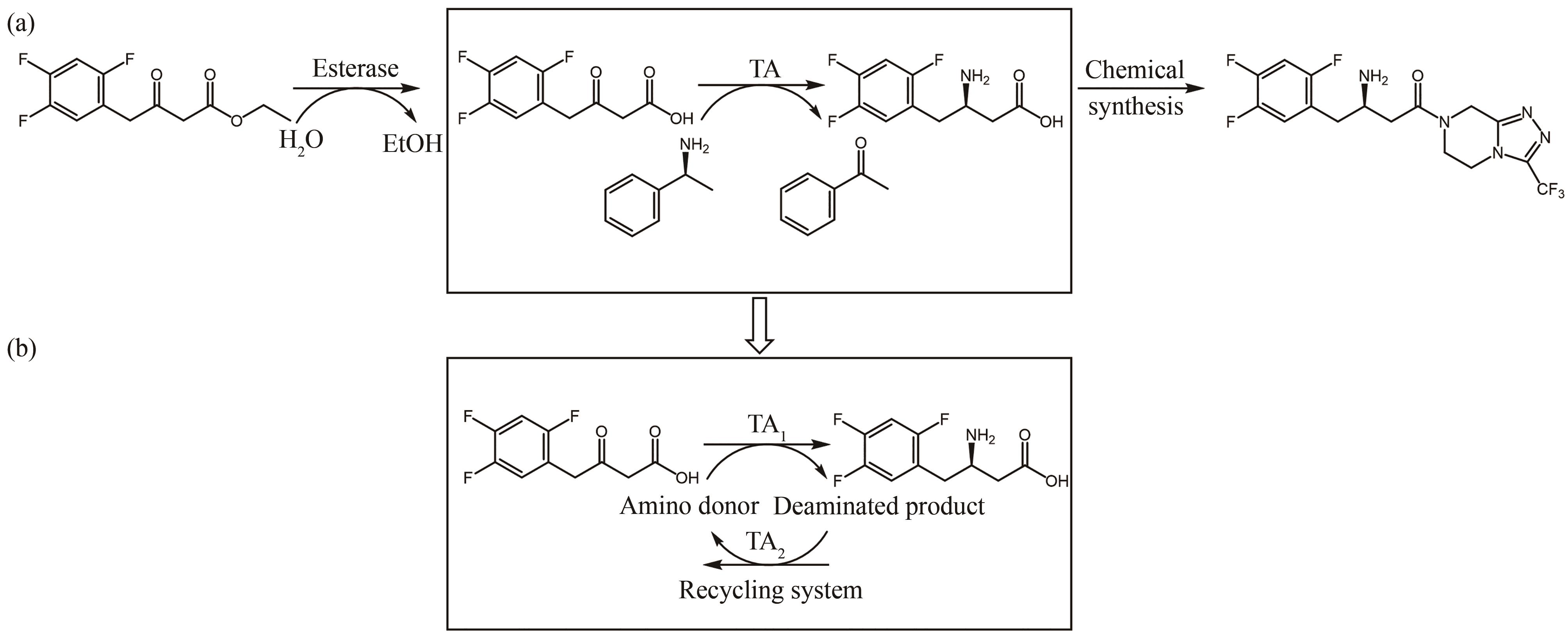
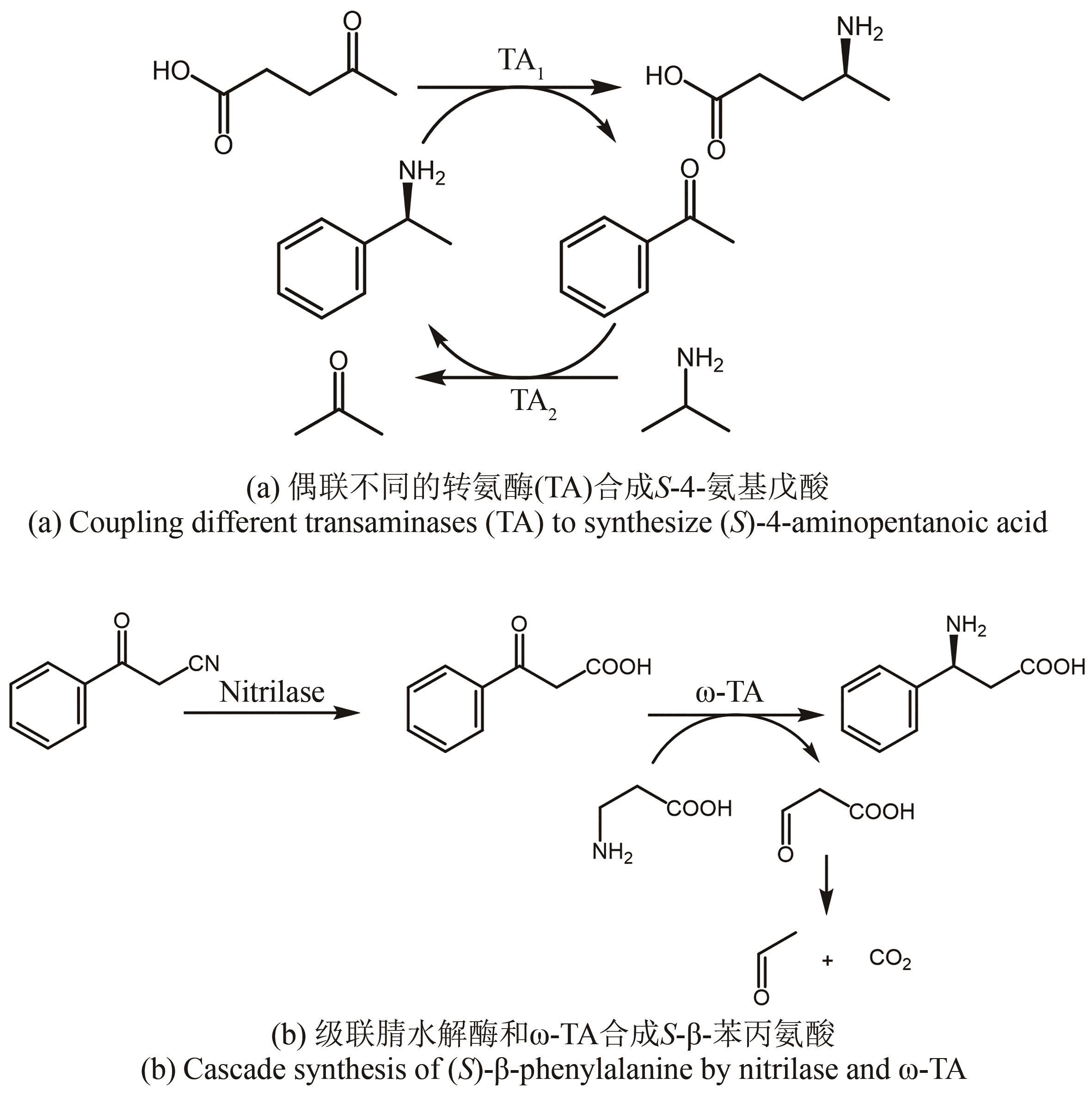
图11 氨基不对称转移反应合成西格列汀中间体(a)及TA-转氨酶(b)
Fig. 11 Synthesis of intermediate of sitagliptin by asymmetric transfer of amino groups to keto acids(a) and TA-transaminase(b)

图13 解氨酶(AL)或氨基变位酶(AM)催化α,β-不饱和羧酸的选择性胺化加成反应
Fig. 13 Enantioselective addition of ammonia to α,β-unsaturated acids by ammonia lyase (AL) or amino mutase (AM)

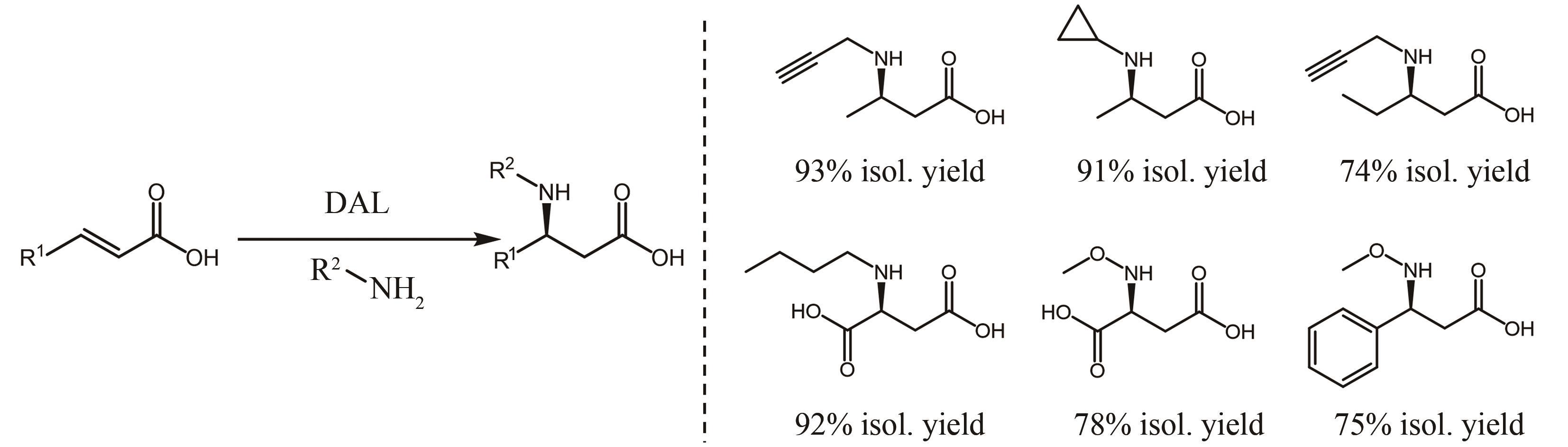
图14 用于手性氨基酸合成的解氨酶的催化反应一般通式DAL—天冬氨酸解氨酶;MAL—甲基天冬氨酸解氨酶;PAL—苯丙氨酸解氨酶;HAL—组氨酸解氨酶;TAL—酪氨酸解氨酶
Fig. 14 General catalytic reaction formula for ammonia lyases used for chiral amino acid synthesisDAL—Aspartate ammonia-lyase; MAL—Methylaspartate ammonia-lyase; PAL—Phenylalanine ammonia-lyase; HAL—Histidine ammonia-lyase; TAL—Tyrosine ammonia-lyase
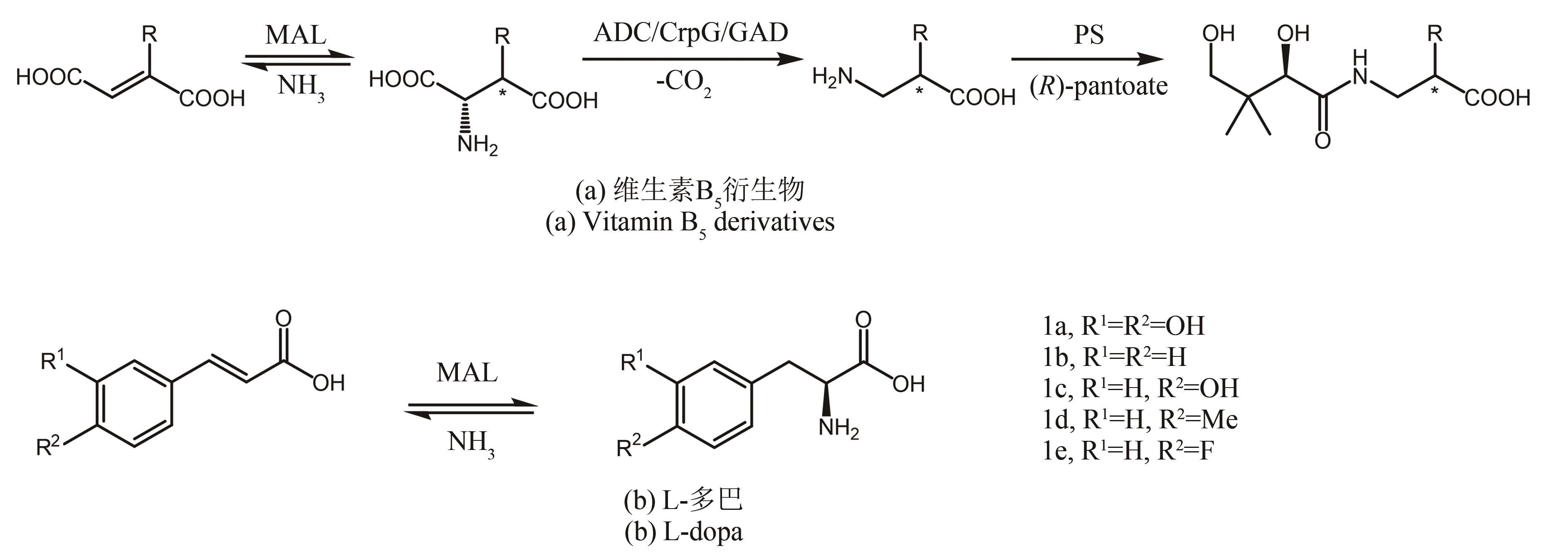
图18 甲基天冬氨酸裂解酶(MAL)催化α,β-不饱和羧酸选择性胺化ADC—天冬氨酸-α-脱羧酶;CrpG—β-甲基天冬氨酸-α-脱羧酶;GAD—谷氨酸脱羧酶;PS—泛酸盐合成酶
Fig. 18 Enantioselective addition of ammonia to α,β-unsaturated acids by Methylaspartate ammonia lyases (MAL)ADC—Aspartate-α-decarboxylase; CrpG—β-Methylaspartate-α-decarboxylase; GAD—Glutamate decarboxylase; PS—Pantothenate synthetase
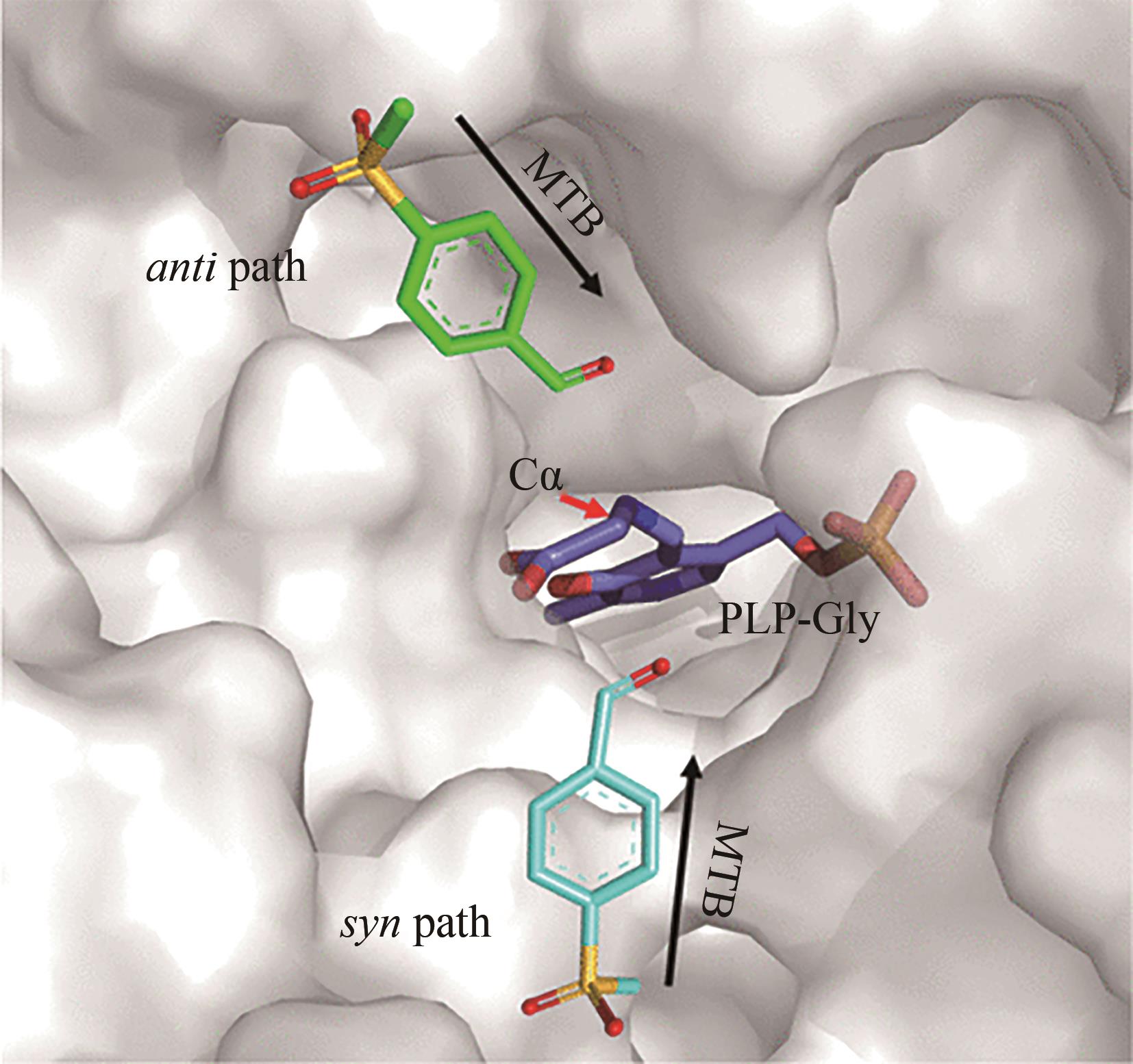
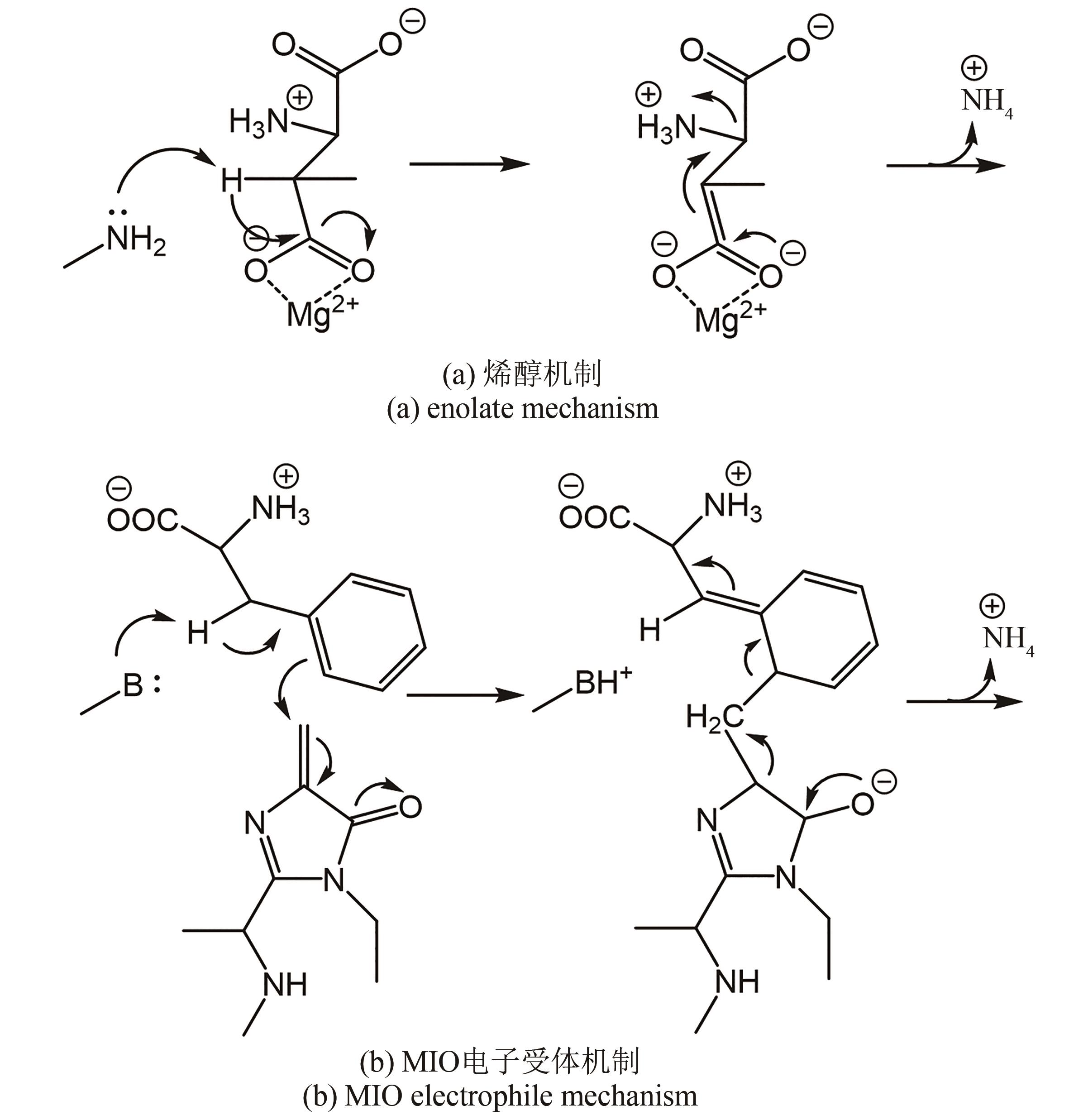
图 23 “路径假说”的示意图[92][醛类(MTB)通过顺式路径或反式路径攻击醛胺PLP-Gly的Cα形成相应的产品构型]
Fig. 23 Schematic diagram of the path hypothesis[92][Aldehydes (MTB) attack Cα of aldimine PLP-Gly through the syn path or anti path to form the corresponding configuration of products]
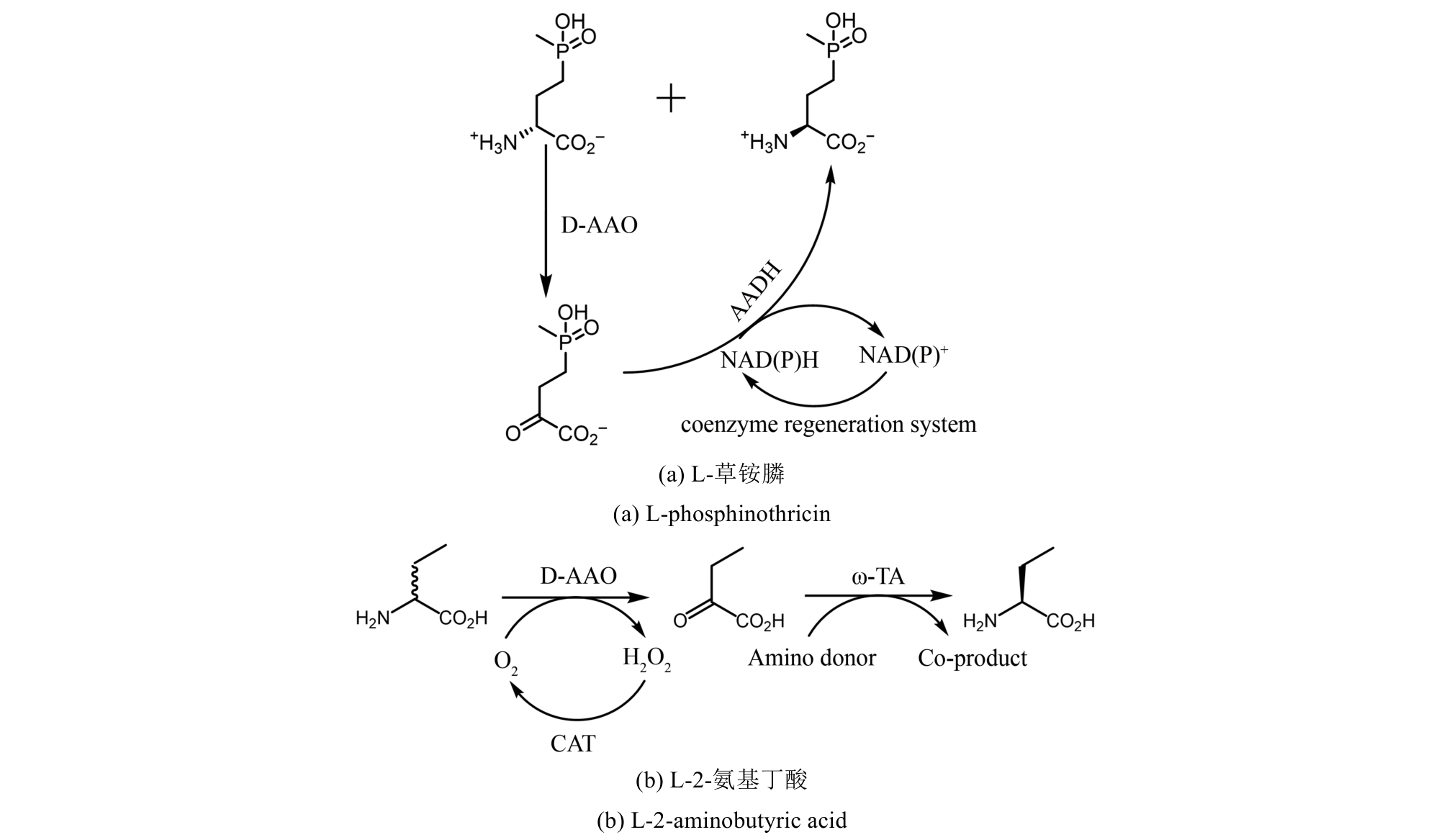
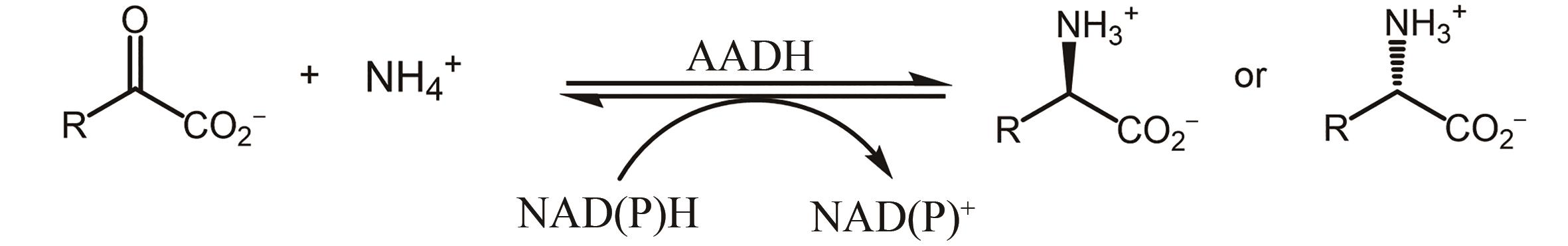
图27 氨基酸氧化酶参与去消旋化合成手性氨基酸AAO—氨基酸氧化酶;AADH—氨基酸脱氢酶;CAT—过氧化氢酶;TA-转氨酶
Fig. 27 Amino acid oxidase is involved in the deracemization synthesis of chiral amino acidsAAO—Amino acid oxidase; AADH—Amino acid dehydrogenase; CAT—Catalase; TA—Transaminase
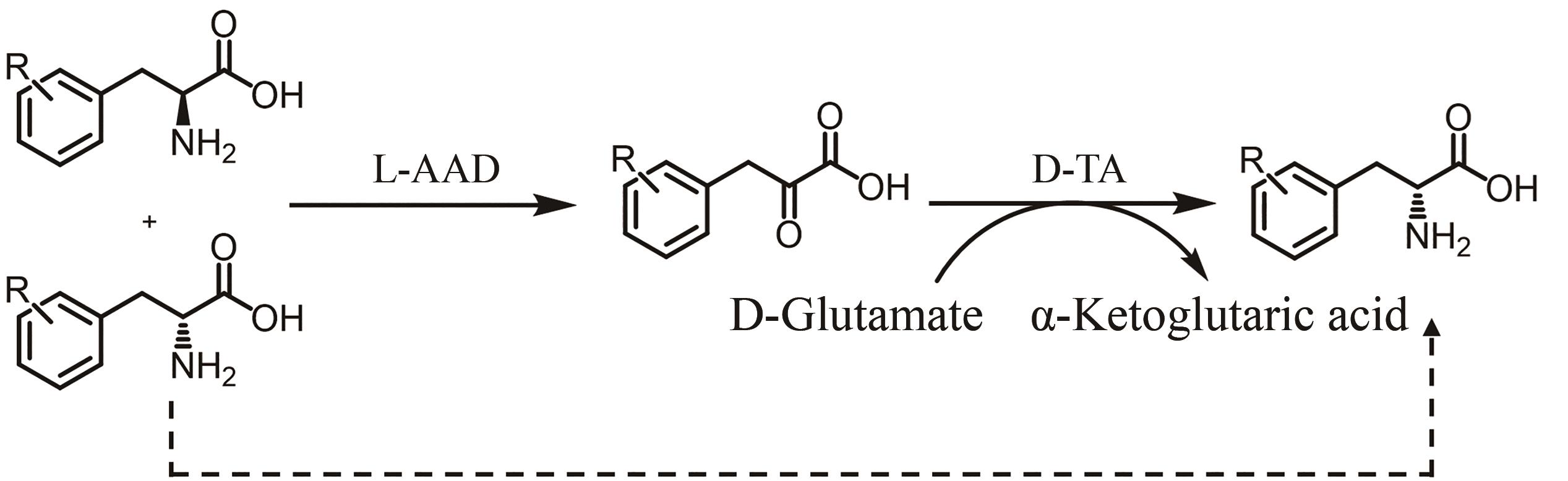
图29 氨基酸脱氨酶参与去消旋化合成手性氨基酸AAD—氨基酸脱氨酶;TA—转氨酶
Fig. 29 Amino acid deaminase is involved in the deracemization synthesis of chiral amino acidsAAD—Amino acid deaminase; TA—Transaminase
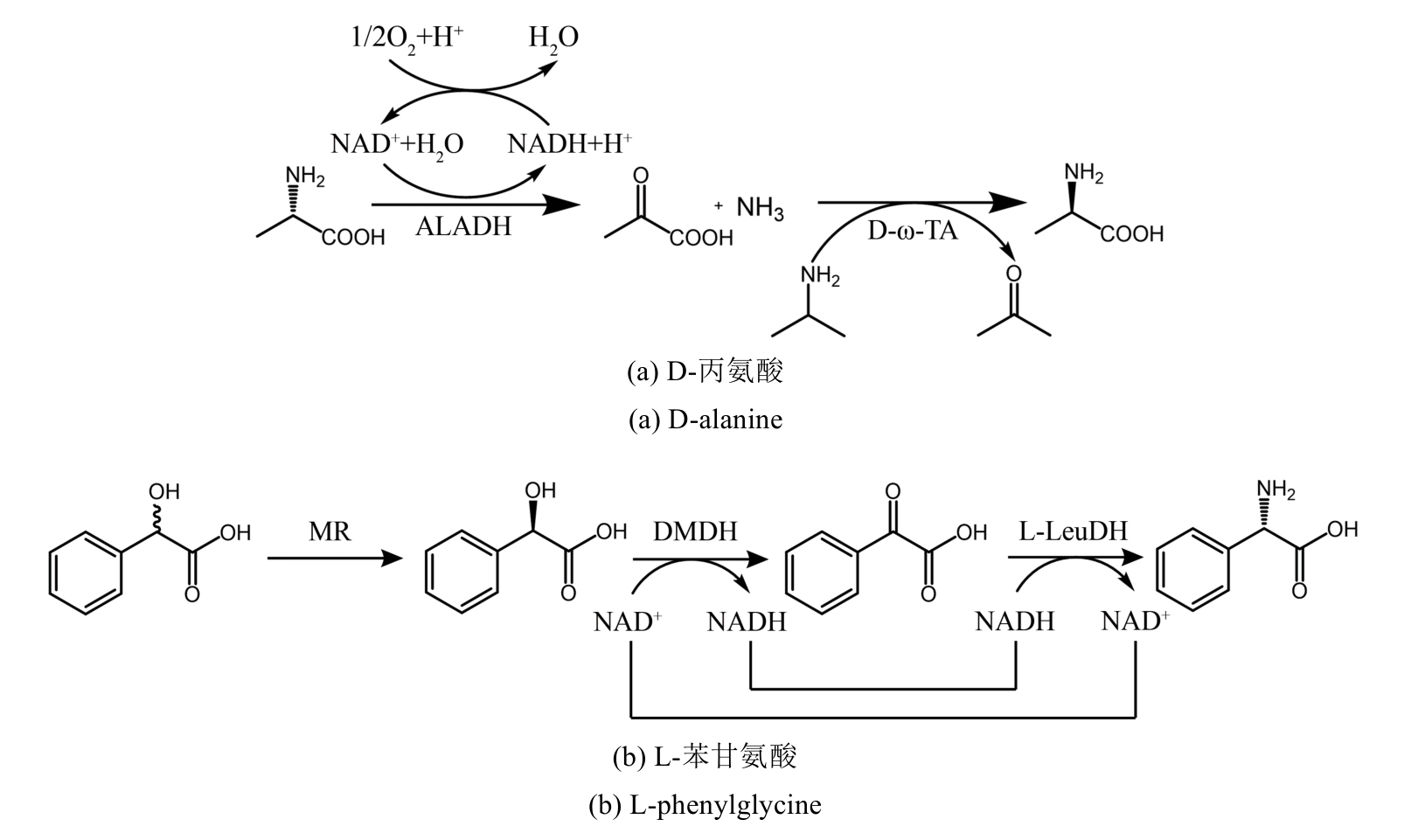
图30 氨基酸脱氢酶参与去消旋化合成手性氨基酸ALADH—丙氨酸脱氢酶;TA—转氨酶;MR—扁桃酸消旋酶;DMDH—D-扁桃酸脱氢酶;LeuDH—亮氨酸脱氢酶
Fig. 30 Amino acid dehydrogenase is involved in the deracemization synthesis of chiral amino acidsALADH—Alanine dehydrogenase; TA—Transaminase; MR—Mandelate racemase; DMDH—D-Mandelate dehydrogenase; LeuDH—Leucine dehydrogenase
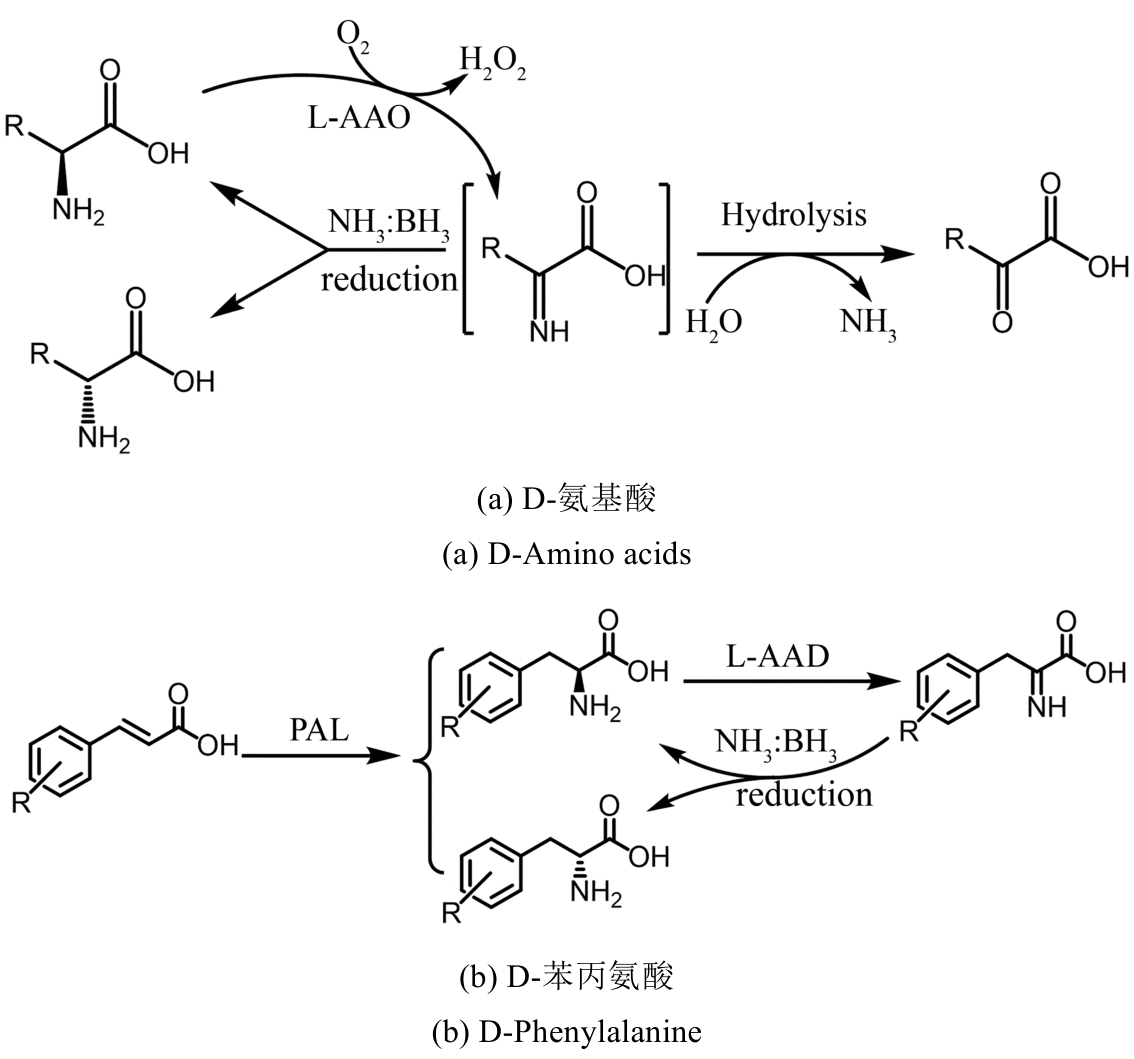
图31 化学酶法去消旋化合成手性氨基酸AAO—氨基酸氧化酶;PAL—苯丙氨酸解氨酶;AAD—氨基酸脱氨酶
Fig. 31 Chemo-enzymatic deracemization synthesis of chiral amino acidsAAO—Amino acid oxidase; PAL—Phenylalanine ammonia-lyase; AAD—Amino acid deaminase
产品 Products | 应用 Applications | 合成路线 Synthetic routes | 酶制剂 Enzyme | 参考文献 References |
|---|---|---|---|---|
| Broad-spectrum herbicides | Asymmetric reductive amination of keto acids | Glutamate dehydrogenase, Alcohol dehydrogenase/Glucose dehydrogenase/Formate dehydrogenase | [ | |
| Deracemization synthesis | D-amino acid oxidase, catalase, glutamate dehydrogenase, Alcohol dehydrogenase/Glucose dehydrogenase/Formate dehydrogenase | [ | ||
| Intermediate of azanavir, animal feed additive, nutritional fortifier | Asymmetric reductive amination of keto acids | Leucine dehydrogenase,Alcohol dehydrogenase/Glucose dehydrogenase/Formate dehydrogenase | [ | |
| Asymmetric transfer of amino groups to keto acids | Transaminase | [ | ||
| Intermediate of antituberculosis ethambutol and antiepileptic drug levetiracetam | Asymmetric reductive amination of keto acids | Leucine dehydrogenase,threonine deaminase,Glucose dehydrogenase | [ | |
| Asymmetric transfer of amino groups to keto acids | Transaminase,Glutamate dehydrogenase,Alcohol dehydrogenase | [ | ||
| Deracemization synthesis | [ | |||
| L-phenylglycine | Intermediate of β-lactam antibiotics | Asymmetric reductive amination of keto acids | Amino acid dehydrogenase,Alcohol dehydrogenase/Glucose dehydrogenase/Formate dehydrogenase | [ |
| Deracemization synthesis | Mandelate racemase, | [ | ||
| (R)-3-amino-4-(2,4,5-trifluorophenyl)butyric acid | Intermediate of siagliptin | Asymmetric transfer of amino groups to keto acids | Transaminase | [ |
| L-norvaline | Intermediate of perindopril | Asymmetric transfer of amino groups to keto acids | Transaminase | [ |
| (2R,4S)-ethyl-5-([1,1'- biphenyl]-4-yl) -4- ((tert butoxycarbonyl) amino)-2-methylvaleric acid | Intermediate of sacubitril | Asymmetric transfer of amino groups to keto acids | Transaminase | [ |
| Drug intermediates, chemical sensors, chiral catalysts, etc | Asymmetric transfer of amino groups to keto acids | Transaminase | [ | |
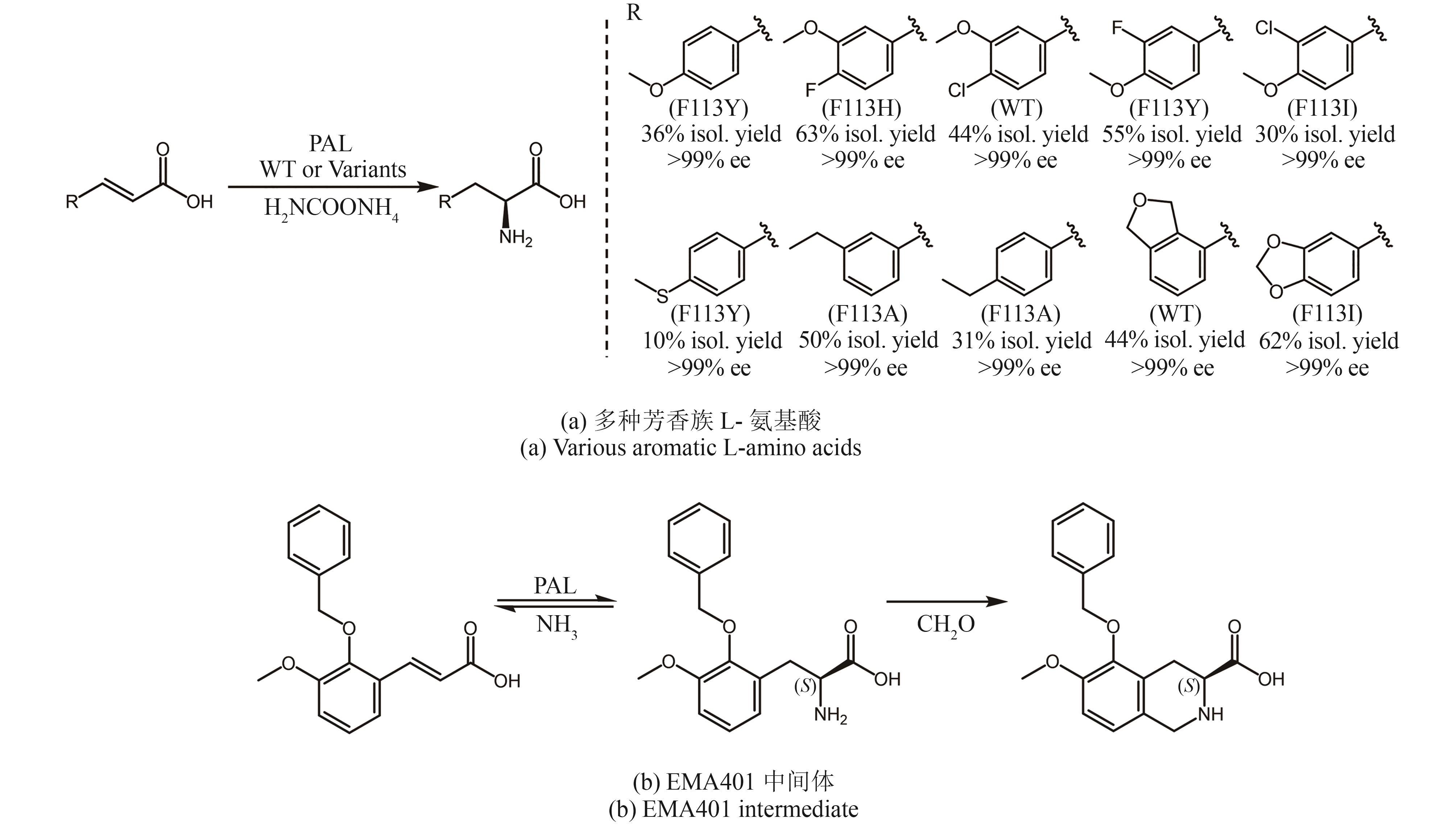
| (3S)-5-(benzyloxy)-6-methoxy-1,2,3,4-tetrahydroisoquinoline-3-carboxylic Acid | Intermediate of olodanrigan (EMA401) | Enantioselective addition of ammonia to α,β-unsaturated acids | Phenylalanine ammonia-lyase | [ |
| (R)-pantothenic acid | Intermediate of antimicrobials against plasmodium falciparum and multidrug-resistant staphylococcus aureus | Enantioselective addition of ammonia to α,β-unsaturated acids | 3-Methylaspartate ammonia lyase, Aspartate-α-decarboxylase, β-methylaspartate-α- decarboxylase/glutamate decarboxylase, Pantothenate synthetase | [ |
| Intermediate of flufenicol | Aldol condensation of an amino acid to aldehydes | [ | ||
| β-(2-furyl)serine | Intermediate of furan antibiotic and 2-amino-1-(2-furanyl)ethanol | Aldol condensation of an amino acid to aldehydes | [ |
表2 部分应用于生产实践的酶促合成路线
Table 2 Examples of enzymatic synthetic routes applied in the production
产品 Products | 应用 Applications | 合成路线 Synthetic routes | 酶制剂 Enzyme | 参考文献 References |
|---|---|---|---|---|
| Broad-spectrum herbicides | Asymmetric reductive amination of keto acids | Glutamate dehydrogenase, Alcohol dehydrogenase/Glucose dehydrogenase/Formate dehydrogenase | [ | |
| Deracemization synthesis | D-amino acid oxidase, catalase, glutamate dehydrogenase, Alcohol dehydrogenase/Glucose dehydrogenase/Formate dehydrogenase | [ | ||
| Intermediate of azanavir, animal feed additive, nutritional fortifier | Asymmetric reductive amination of keto acids | Leucine dehydrogenase,Alcohol dehydrogenase/Glucose dehydrogenase/Formate dehydrogenase | [ | |
| Asymmetric transfer of amino groups to keto acids | Transaminase | [ | ||
| Intermediate of antituberculosis ethambutol and antiepileptic drug levetiracetam | Asymmetric reductive amination of keto acids | Leucine dehydrogenase,threonine deaminase,Glucose dehydrogenase | [ | |
| Asymmetric transfer of amino groups to keto acids | Transaminase,Glutamate dehydrogenase,Alcohol dehydrogenase | [ | ||
| Deracemization synthesis | [ | |||
| L-phenylglycine | Intermediate of β-lactam antibiotics | Asymmetric reductive amination of keto acids | Amino acid dehydrogenase,Alcohol dehydrogenase/Glucose dehydrogenase/Formate dehydrogenase | [ |
| Deracemization synthesis | Mandelate racemase, | [ | ||
| (R)-3-amino-4-(2,4,5-trifluorophenyl)butyric acid | Intermediate of siagliptin | Asymmetric transfer of amino groups to keto acids | Transaminase | [ |
| L-norvaline | Intermediate of perindopril | Asymmetric transfer of amino groups to keto acids | Transaminase | [ |
| (2R,4S)-ethyl-5-([1,1'- biphenyl]-4-yl) -4- ((tert butoxycarbonyl) amino)-2-methylvaleric acid | Intermediate of sacubitril | Asymmetric transfer of amino groups to keto acids | Transaminase | [ |
| Drug intermediates, chemical sensors, chiral catalysts, etc | Asymmetric transfer of amino groups to keto acids | Transaminase | [ | |
| (3S)-5-(benzyloxy)-6-methoxy-1,2,3,4-tetrahydroisoquinoline-3-carboxylic Acid | Intermediate of olodanrigan (EMA401) | Enantioselective addition of ammonia to α,β-unsaturated acids | Phenylalanine ammonia-lyase | [ |
| (R)-pantothenic acid | Intermediate of antimicrobials against plasmodium falciparum and multidrug-resistant staphylococcus aureus | Enantioselective addition of ammonia to α,β-unsaturated acids | 3-Methylaspartate ammonia lyase, Aspartate-α-decarboxylase, β-methylaspartate-α- decarboxylase/glutamate decarboxylase, Pantothenate synthetase | [ |
| Intermediate of flufenicol | Aldol condensation of an amino acid to aldehydes | [ | ||
| β-(2-furyl)serine | Intermediate of furan antibiotic and 2-amino-1-(2-furanyl)ethanol | Aldol condensation of an amino acid to aldehydes | [ |
| 19 | ZHOU F, XU Y, NIE Y, et al. Substrate-specific engineering of amino acid dehydrogenase superfamily for synthesis of a variety of chiral amines and amino acids[J]. Catalysts, 2022, 12(4): 380. |
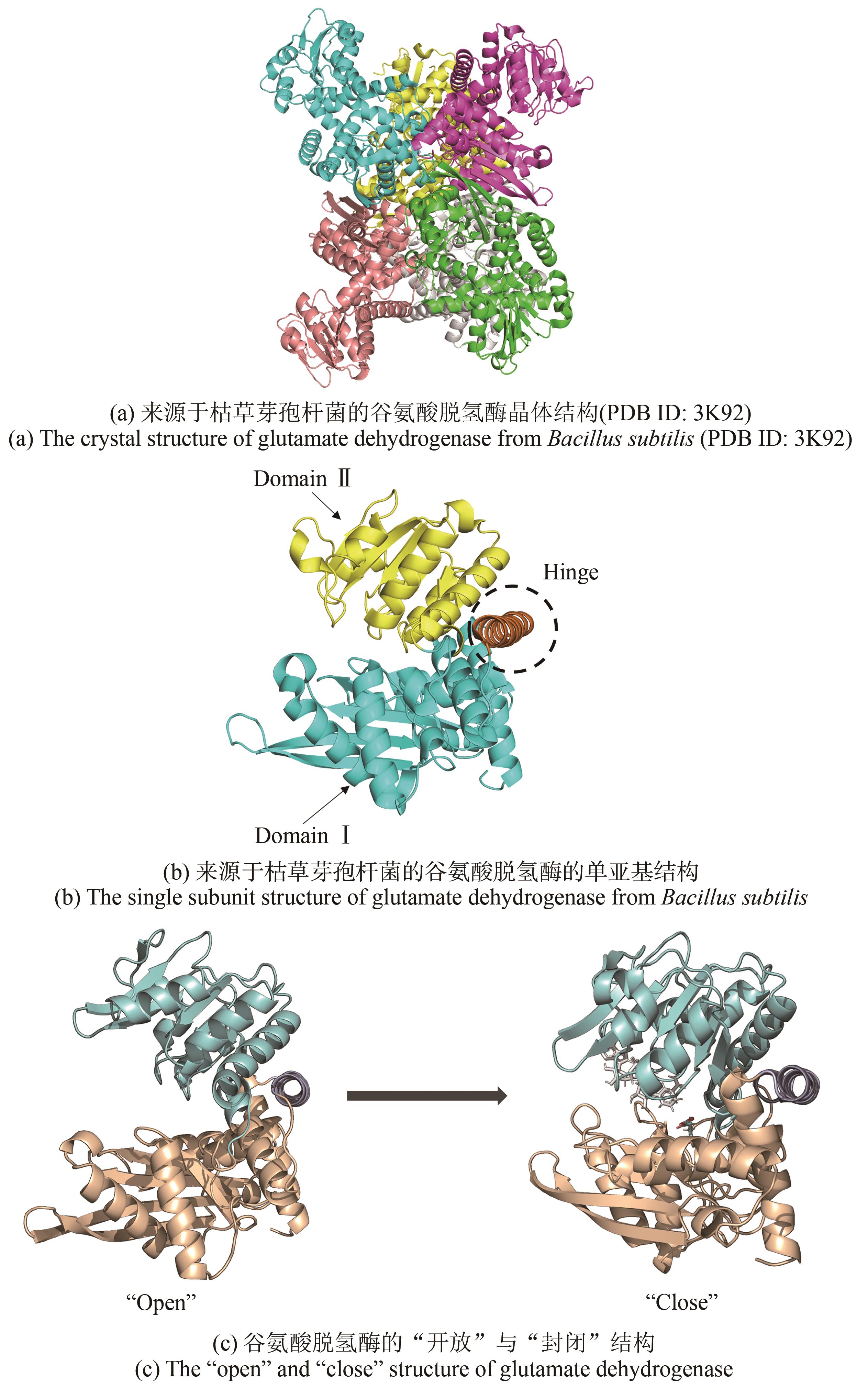
| 20 | TOMITA T, YIN L L, NAKAMURA S, et al. Crystal structure of the 2-iminoglutarate-bound complex of glutamate dehydrogenase from Corynebacterium glutamicum [J]. FEBS Letters, 2017, 591(11): 1611-1622. |
| 21 | OLIVEIRA T, PANJIKAR S, CARRIGAN J B, et al. Crystal structure of NAD+-dependent Peptoniphilus asaccharolyticus glutamate dehydrogenase reveals determinants of cofactor specificity[J]. Journal of Structural Biology, 2012, 177(2): 543-552. |
| 22 | YIN X J, LIU Y Y, MENG L J, et al. Semi-rational hinge engineering: modulating the conformational transformation of glutamate dehydrogenase for enhanced reductive amination activity towards non-natural substrates[J]. Catalysis Science & Technology, 2020, 10(10): 3376-3386. |
| 23 | SON H F, KIM I K, KIM K J. Structural insights into domain movement and cofactor specificity of glutamate dehydrogenase from Corynebacterium glutamicum [J]. Biochemical and Biophysical Research Communications, 2015, 459(3): 387-392. |
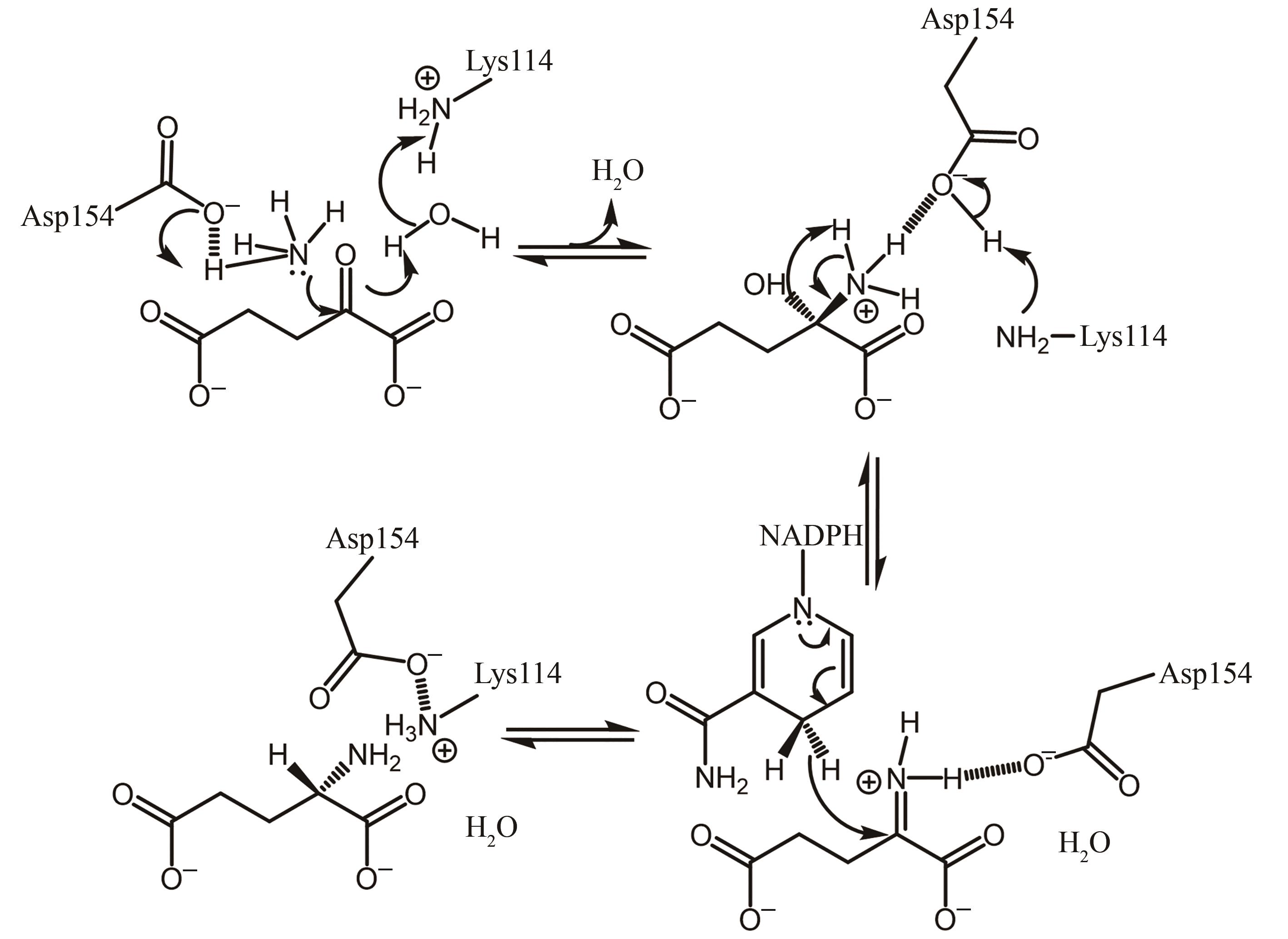
| 24 | PRAKASH P, PUNEKAR N S, BHAUMIK P. Structural basis for the catalytic mechanism and α-ketoglutarate cooperativity of glutamate dehydrogenase[J]. The Journal of Biological Chemistry, 2018, 293(17): 6241-6258. |
| 25 | WU T, WANG Y M, ZHANG N X, et al. Reshaping substrate-binding pocket of leucine dehydrogenase for bidirectionally accessing structurally diverse substrates[J]. ACS Catalysis, 2023, 13(1): 158-168. |
| 26 | WANG Z Y, QU H J, LI W Q, et al. Semi-rational design of diaminopimelate dehydrogenase from Symbiobacterium thermophilum improved its activity toward hydroxypyruvate for D-serine synthesis[J]. Catalysts, 2023, 13(3): 576. |
| 27 | LIU N, WU L, FENG J H, et al. Crystal structures and catalytic mechanism of L-erythro-3,5-diaminohexanoate dehydrogenase and rational engineering for asymmetric synthesis of β-amino acids[J]. Angewandte Chemie International Edition, 2021, 60(18): 10203-10210. |
| 28 | WANG Z Y, ZHOU H S, YU H R, et al. Computational redesign of the substrate binding pocket of glutamate dehydrogenase for efficient synthesis of noncanonical L-amino acids[J]. ACS Catalysis, 2022, 12(21): 13619-13629. |
| 29 | YIN X J, ZENG Y J, CHEN J, et al. Combined active pocket and hinge region engineering to develop an NADPH-dependent phenylglycine dehydrogenase[J]. Bioorganic Chemistry, 2022, 120: 105601. |
| 30 | LUO W, ZHU J, ZHAO Y Z, et al. Cloning and expression of a novel leucine dehydrogenase: characterization and L-tert-leucine production[J]. Frontiers in Bioengineering and Biotechnology, 2020, 8: 186. |
| 31 | MENG X Q, YANG L, LIU Y, et al. Identification and rational engineering of a high substrate-tolerant leucine dehydrogenase effective for the synthesis of L-tert-leucine[J]. ChemCatChem, 2021, 13(14): 3340-3349. |
| 32 | LIAO L X, ZHANG Y H, WANG Y L, et al. Construction and characterization of a novel glucose dehydrogenase-leucine dehydrogenase fusion enzyme for the biosynthesis of L-tert-leucine[J]. Microbial Cell Factories, 2021, 20(1): 3. |
| 33 | LIU Y, ZHONG X Z, LUO Z, et al. The identification of a robust leucine dehydrogenase from a directed soil metagenome for efficient synthesis of L-2-aminobutyric acid[J]. Biotechnology Journal, 2023, 18(8): e2200590. |
| 34 | 付妍, 张君轩, 付雪蓉, 等. 三酶级联催化L-苏氨酸生产L-2-氨基丁酸[J]. 生物工程学报,2020,36(4): 782-791. |
| FU Y, ZHANG J X, FU X R, et al. Production of L-2-aminobutyric acid from L-threonine using a trienzyme cascade[J]. Chinese Journal of Biotechnology, 2020, 36(4): 782-791. | |
| 35 | CHENG F, LI H, ZHANG K, et al. Tuning amino acid dehydrogenases with featured sequences for L-phosphinothricin synthesis by reductive amination[J]. Journal of Biotechnology, 2020, 312: 35-43. |
| 36 | TANG C D, ZHANG Z H, SHI H L, et al. Directed evolution of formate dehydrogenase and its application in the biosynthesis of L-phenylglycine from phenylglyoxylic acid[J]. Molecular Catalysis, 2021, 513: 111666. |
| 37 | MENG X Q, LIU Y, YANG L, et al. Rational identification of a high catalytic efficiency leucine dehydrogenase and process development for efficient synthesis of l-phenylglycine[J]. Biotechnology Journal, 2023, 18(5): e2200465. |
| 38 | SCHNEIDER G, KÄCK H, LINDQVIST Y. The manifold of vitamin B6 dependent enzymes[J]. Structure, 2000, 8(1): R1-R6. |
| 39 | ELIOT A C, KIRSCH J F. Pyridoxal phosphate enzymes: mechanistic, structural, and evolutionary considerations[J]. Annual Review of Biochemistry, 2004, 73: 383-415. |
| 40 | ROCHA J F, PINA A F, SOUSA S F, et al. PLP-dependent enzymes as important biocatalysts for the pharmaceutical, chemical and food industries: a structural and mechanistic perspective[J]. Catalysis Science & Technology, 2019, 9(18): 4864-4876. |
| 41 | BEZSUDNOVA E Y, POPOV V O, BOYKO K M. Structural insight into the substrate specificity of PLP fold type Ⅳ transaminases[J]. Applied Microbiology and Biotechnology, 2020, 104(6): 2343-2357. |
| 1 | BOMMARIUS A S, SCHWARM M, STINGL K, et al. Synthesis and use of enantiomerically pure tert-leucine[J]. Tetrahedron: Asymmetry, 1995, 6(12): 2851-2888. |
| 2 | SHIN J S, KIM B G. Transaminase-catalyzed asymmetric synthesis of L-2-aminobutyric acid from achiral reactants[J]. Biotechnology Letters, 2009, 31(10): 1595-1599. |
| 3 | ZHU L, TAO R S, WANG Y, et al. Removal of L-alanine from the production of L-2-aminobutyric acid by introduction of alanine racemase and D-amino acid oxidase[J]. Applied Microbiology and Biotechnology, 2011, 90(3): 903-910. |
| 4 | TAO R S, JIANG Y, ZHU F Y, et al. A one-pot system for production of L-2-aminobutyric acid from L-threonine by L-threonine deaminase and a NADH-regeneration system based on L-leucine dehydrogenase and formate dehydrogenase[J]. Biotechnology Letters, 2014, 36(4): 835-841. |
| 5 | BAO Z X, SUN Y, RAI K, et al. The promising indicators of the thermal and mechanical properties of collagen from bass and tilapia: synergistic effects of hydroxyproline and cysteine[J]. Biomaterials Science, 2018, 6(11): 3042-3052. |
| 6 | ZHOU H S, MENG L J, YIN X J, et al. Biocatalytic asymmetric synthesis of L-phosphinothricin using a one-pot three enzyme system and a continuous substrate fed-batch strategy[J]. Applied Catalysis A: General, 2020, 589: 117239. |
| 7 | MANANDHAR M, CHUN E, ROMESBERG F E. Genetic code expansion: inception, development, commercialization[J]. Journal of the American Chemical Society, 2021, 143(13): 4859-4878. |
| 8 | DU Y H, LI L, ZHENG Y, et al. Incorporation of non-canonical amino acids into antimicrobial peptides: advances, challenges, and perspectives[J]. Applied and Environmental Microbiology, 2022, 88(23): e0161722. |
| 9 | UGWUMBA I N, OZAWA K, XU Z Q, et al. Improving a natural enzyme activity through incorporation of unnatural amino acids[J]. Journal of the American Chemical Society, 2011, 133(2): 326-333. |
| 10 | WILKINSON H C, DALBY P A. Fine-tuning the activity and stability of an evolved enzyme active-site through noncanonical amino-acids[J]. The FEBS Journal, 2021, 288(6): 1935-1955. |
| 11 | WANG L X, ZHU W J, GAO Z, et al. Biosynthetic L-tert-leucine using Escherichia coli co-expressing a novel NADH-dependent leucine dehydrogenase and a formate dehydrogenase[J]. Electronic Journal of Biotechnology, 2020, 47: 83-88. |
| 12 | ZHOU F, MU X Q, NIE Y, et al. Enhanced catalytic efficiency and coenzyme affinity of leucine dehydrogenase by comprehensive screening strategy for L-tert-leucine synthesis[J]. Applied Microbiology and Biotechnology, 2021, 105(9): 3625-3634. |
| 13 | SAVILE C K, JANEY J M, MUNDORFF E C, et al. Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture[J]. Science, 2010, 329(5989): 305-309. |
| 42 | SHIN J S, KIM B G. Exploring the active site of amine: pyruvate aminotransferase on the basis of the substrate structure-reactivity relationship: how the enzyme controls substrate specificity and stereoselectivity[J]. The Journal of Organic Chemistry, 2002, 67(9): 2848-2853. |
| 43 | MATHEW S, YUN H. ω-Transaminases for the production of optically pure amines and unnatural amino acids[J]. ACS Catalysis, 2012, 2(6): 993-1001. |
| 44 | SHILOVA S A, MATYUTA I O, KHRENOVA M G, et al. In search for structural targets for engineering D-amino acid transaminase: modulation of pH optimum and substrate specificity[J]. The Biochemical Journal, 2023, 480(16): 1267-1284. |
| 45 | SHILOVA S A, MATYUTA I O, PETROVA E S, et al. Expanded substrate specificity in D-amino acid transaminases: a case study of transaminase from Blastococcus saxobsidens [J]. International Journal of Molecular Sciences, 2023, 24(22): 16194. |
| 46 | VOSS M, XIANG C, ESQUE J, et al. Creation of (R)-amine transaminase activity within an α-amino acid transaminase scaffold[J]. ACS Chemical Biology, 2020, 15(2): 416-424. |
| 47 | MOORE J C, RODRIGUEZ-GRANILLO A, CRESPO A, et al. “Site and mutation”-specific predictions enable minimal directed evolution libraries[J]. ACS Synthetic Biology, 2018, 7(7): 1730-1741. |
| 48 | JIA D X, PENG C, LI J L, et al. Redesign of (R)-omega-transaminase and its application for synthesizing amino acids with bulky side chain[J]. Applied Biochemistry and Biotechnology, 2021, 193(11): 3624-3640. |
| 49 | CHENG F, CHEN X L, XIANG C, et al. Fluorescence-based high-throughput screening system for R-ω-transaminase engineering and its substrate scope extension[J]. Applied Microbiology and Biotechnology, 2020, 104(7): 2999-3009. |
| 50 | VOSS M, DAS D, GENZ M, et al. In silico based engineering approach to improve transaminases for the conversion of bulky substrates[J]. ACS Catalysis, 2018, 8(12): 11524-11533. |
| 51 | WANG Y G, FENG J H, DONG W Y, et al. Improving catalytic activity and reversing enantio-specificity of ω-transaminase by semi-rational engineering en route to chiral bulky β-amino esters[J]. ChemCatChem, 2021, 13(15): 3396-3400. |
| 52 | WALTON C J W, THIEBAUT F, BRUNZELLE J S, et al. Structural determinants of the stereoinverting activity of Pseudomonas stutzeri D-phenylglycine aminotransferase[J]. Biochemistry, 2018, 57(37): 5437-5446. |
| 53 | NOVICK S J, DELLAS N, GARCIA R, et al. Engineering an amine transaminase for the efficient production of a chiral sacubitril precursor[J]. ACS Catalysis, 2021, 11(6): 3762-3770. |
| 54 | ST-JACQUES A D, EYAHPAISE M È C, CHICA R A. Computational design of multisubstrate enzyme specificity[J]. ACS Catalysis, 2019, 9(6): 5480-5485. |
| 55 | JEON H, PAGAR A D, KANG H, et al. Creation of a (R)-β- transaminase by directed evolution of D-amino acid aminotransferase[J]. ACS Catalysis, 2022, 12(21): 13207-13214. |
| 56 | LAND H, CAMPILLO-BROCAL J C, SVEDENDAHL HUMBLE M, et al. B-factor guided proline substitutions in Chromobacterium violaceum amine transaminase: evaluation of the proline rule as a method for enzyme stabilization[J]. ChemBioChem, 2019, 20(10): 1297-1304. |
| 57 | XIE Z H, ZHAI L X, MENG D, et al. Improving the catalytic thermostability of Bacillus altitudinis W3 ω-transaminase by proline substitutions[J]. 3 Biotech, 2020, 10(7): 323. |
| 58 | MARCHINI V, BENÍTEZ-MATEOS A I, HUTTER S L, et al. Fusion of formate dehydrogenase and alanine dehydrogenase as an amino donor regenerating system coupled to transaminases[J]. ChemBioChem, 2022, 23(21): e202200428. |
| 59 | KELEFIOTIS-STRATIDAKIS P, TYRIKOS-ERGAS T, PAVLIDIS I V. The challenge of using isopropylamine as an amine donor in transaminase catalysed reactions[J]. Organic & Biomolecular Chemistry, 2019, 17(7): 1634-1642. |
| 60 | DAWOOD A W H, WEIß M S, SCHULZ C, et al. Isopropylamine as amine donor in transaminase-catalyzed reactions: better acceptance through reaction and enzyme engineering[J]. ChemCatChem, 2018, 10(18): 3943-3949. |
| 61 | KHOBRAGADE T P, SARAK S, PAGAR A D, et al. Synthesis of sitagliptin intermediate by a multi-enzymatic cascade system using lipase and transaminase with benzylamine as an amino donor[J]. Frontiers in Bioengineering and Biotechnology, 2021, 9: 757062. |
| 62 | ZHENG X X, CUI Y L, LI T, et al. Biochemical and structural characterization of a highly active branched-chain amino acid aminotransferase from Pseudomonas sp. for efficient biosynthesis of chiral amino acids[J]. Applied Microbiology and Biotechnology, 2019, 103(19): 8051-8062. |
| 63 | LUO W, HU J G, LU J P, et al. One pot cascade synthesis of L-2-aminobutyric acid employing ω-transaminase from Paracoccus pantotrophus [J]. Molecular Catalysis, 2021, 515: 111890. |
| 64 | YU J H, LI J, CAO S Y, et al. Chemoenzymatic synthesis of L-3,4-dimethoxyphenyl-alanine and its analogues using aspartate aminotransferase as a key catalyst[J]. Catalysis Communications, 2019, 120: 28-32. |
| 65 | KHOBRAGADE T P, YU S, JUNG H, et al. Promoter engineering-mediated Tuning of esterase and transaminase expression for the chemoenzymatic synthesis of sitagliptin phosphate at the kilogram-scale[J]. Biotechnology & Bioengineering, 2021, 118(8): 3263-3268. |
| 66 | KHOBRAGADE T P, PAGAR A D, GIRI P, et al. Biocatalytic cascade for synthesis of sitagliptin intermediate employing coupled transaminase[J]. Biotechnology and Bioprocess Engineering, 2023, 28(2): 300-309. |
| 67 | RODA S, FERNANDEZ-LOPEZ L, BENEDENS M, et al. A plurizyme with transaminase and hydrolase activity catalyzes cascade reactions[J]. Angewandte Chemie International Edition, 2022, 61(37): e202207344. |
| 14 | 杨立荣, 周海胜, 居述云, 等. 一种生物酶法去消旋化制备L‑草铵膦的方法: CN201710834958.4[P]. 2017-09-15. |
| YANG L R, ZHOU H S, JU S Y, et al. A method of enzymatic racemization for preparing L-phosphinothricin: CN201710834958.4[P]. 2017-09-15. | |
| 15 | CAO C H, GONG H, DONG Y, et al. Enzyme cascade for biocatalytic deracemization of D,L-phosphinothricin[J]. Journal of Biotechnology, 2021, 325: 372-379. |
| 16 | TANG C D, SHI H L, JIA Y Y, et al. High level and enantioselective production of L-phenylglycine from racemic mandelic acid by engineered Escherichia coli using response surface methodology[J]. Enzyme and Microbial Technology, 2020, 136: 109513. |
| 17 | HEINKS T, PAULUS J, KOOPMEINERS S, et al. Recombinant L-amino acid oxidase with broad substrate spectrum for co-substrate recycling in (S)-selective transaminase-catalyzed kinetic resolutions[J]. ChemBioChem, 2022, 23(16): e202200329. |
| 18 | WEGNER U, MATTHES F, VON WIRÉN N, et al. Enhancing a Sphaerobacter thermophilus ω-transaminase for kinetic resolution of β- and γ-amino acids[J]. AMB Express, 2023, 13(1): 117. |
| 68 | KHOBRAGADE T P, GIRI P, PAGAR A D, et al. Dual-function transaminases with hybrid nanoflower for the production of value-added chemicals from biobased levulinic acid[J]. Frontiers in Bioengineering and Biotechnology, 2023, 11: 1280464. |
| 69 | ZHANG Z J, CAI R F, XU J H. Characterization of a new nitrilase from Hoeflea phototrophica DFL-43 for a two-step one-pot synthesis of (S)-β-amino acids[J]. Applied Microbiology and Biotechnology, 2018, 102(14): 6047-6056. |
| 70 | PARMEGGIANI F, WEISE N J, AHMED S T, et al. Synthetic and therapeutic applications of ammonia-lyases and aminomutases[J]. Chemical Reviews, 2018, 118(1): 73-118. |
| 71 | POPPE L, PAIZS C, KOVÁCS K, et al. Preparation of unnatural amino acids with ammonia-lyases and 2,3-aminomutases[J]. Methods in Molecular Biology, 2012, 794: 3-19. |
| 72 | FIBRIANSAH G, VEETIL V P, POELARENDS G J, et al. Structural basis for the catalytic mechanism of aspartate ammonia lyase[J]. Biochemistry, 2011, 50(27): 6053-6062. |
| 73 | LEVY C W, BUCKLEY P A, SEDELNIKOVA S, et al. Insights into enzyme evolution revealed by the structure of methylaspartate ammonia lyase[J]. Structure, 2002, 10(1): 105-113. |
| 74 | VIOLA R E. The ammonia-lyases: enzymes that use a wide range of approaches to catalyze the same type of reaction[J]. Critical Reviews in Biochemistry and Molecular Biology, 2019, 54(6): 467-483. |
| 75 | WALKER K D, KLETTKE K, AKIYAMA T, et al. Cloning, heterologous expression, and characterization of a phenylalanine aminomutase involved in taxol biosynthesis[J]. Journal of Biological Chemistry, 2004, 279(52): 53947-53954. |
| 76 | KALKREUTER E, SHEN B. MIO-containing aminomutases for α- to β-amino acids[J]. Trends in Chemistry, 2022, 4(1): 91-92. |
| 77 | ATTANAYAKE G, WALTER T, WALKER K D. Understanding which residues of the active site and loop structure of a tyrosine aminomutase define its mutase and lyase activities[J]. Biochemistry, 2018, 57(25): 3503-3514. |
| 78 | BATA Z, MOLNÁR Z, MADARAS E, et al. Substrate tunnel engineering aided by X-ray crystallography and functional dynamics swaps the function of MIO-enzymes[J]. ACS Catalysis, 2021, 11(8): 4538-4549. |
| 79 | CHESTERS C, WILDING M, GOODALL M, et al. Thermal bifunctionality of bacterial phenylalanine aminomutase and ammonia lyase enzymes[J]. Angewandte Chemie International Edition, 2012, 51(18): 4344-4348. |
| 80 | AHMED S T, PARMEGGIANI F, WEISE N J, et al. Engineered ammonia lyases for the production of challenging electron-rich L-phenylalanines[J]. ACS Catalysis, 2018, 8(4): 3129-3132. |
| 81 | HARDEGGER L A, BENEY P, BIXEL D, et al. Toward a scalable synthesis and process for EMA401, part Ⅲ: Using an engineered phenylalanine ammonia lyase enzyme to synthesize a non-natural phenylalanine derivative[J]. Organic Process Research & Development, 2020, 24(9): 1763-1771. |
| 82 | BOROS K, MOISĂ M E, NAGY C L, et al. Robust, site-specifically immobilized phenylalanine ammonia-lyases for the enantioselective ammonia addition of cinnamic acids[J]. Catalysis Science & Technology, 2021, 11(16): 5553-5563. |
| 83 | CUI Y L, WANG Y H, TIAN W Y, et al. Development of a versatile and efficient C—N lyase platform for asymmetric hydroamination via computational enzyme redesign[J]. Nature Catalysis, 2021, 4(5): 364-373. |
| 84 | BRACK Y, SUN C H, YI D, et al. Exploring the substrate switch motif of aromatic ammonia lyases[J]. ChemBioChem, 2023, 24(23): e202300584. |
| 85 | ABIDIN M Z, SARAVANAN T, ZHANG J L, et al. Modular enzymatic cascade synthesis of vitamin B5 and its derivatives[J]. Chemistry, 2018, 24(66): 17434-17438. |
| 86 | NI Z F, XU P, ZONG M H, et al. Structure-guided protein engineering of ammonia lyase for efficient synthesis of sterically bulky unnatural amino acids[J]. Bioresources and Bioprocessing, 2021, 8(1): 103. |
| 87 | SZYMANSKI W, WU B, WEINER B, et al. Phenylalanine aminomutase-catalyzed addition of ammonia to substituted cinnamic acids: a route to enantiopure alpha- and beta-amino acids[J]. The Journal of Organic Chemistry, 2009, 74(23): 9152-9157. |
| 88 | WU B, SZYMAŃSKI W, WYBENGA G G, et al. Mechanism-inspired engineering of phenylalanine aminomutase for enhanced β-regioselective asymmetric amination of cinnamates[J]. Angewandte Chemie International Edition, 2012, 51(2): 482-486. |
| 89 | AHMED S T, PARMEGGIANI F, WEISE N J, et al. Synthesis of enantiomerically pure ring-substituted L-pyridylalanines by biocatalytic hydroamination[J]. Organic Letters, 2016, 18(21): 5468-5471. |
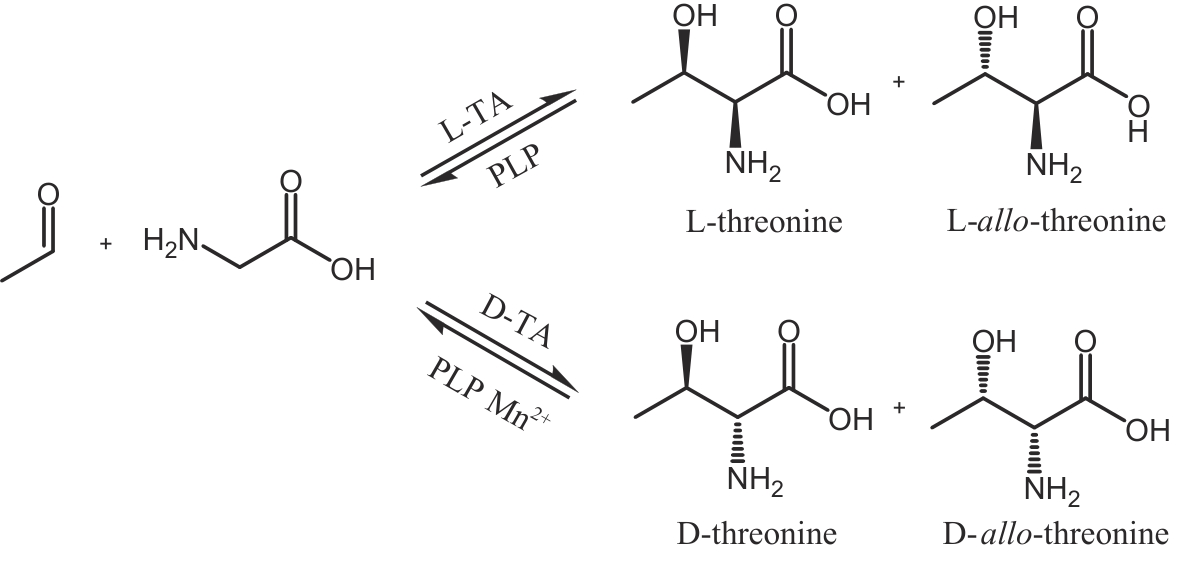
| 90 | DÜCKERS N, BAER K, SIMON S, et al. Threonine aldolases—screening, properties and applications in the synthesis of non-proteinogenic β-hydroxy-α-amino acids[J]. Applied Microbiology and Biotechnology, 2010, 88(2): 409-424. |
| 91 | DI SALVO M L, REMESH S G, VIVOLI M, et al. On the catalytic mechanism and stereospecificity of Escherichia coli L-threonine aldolase[J]. The FEBS Journal, 2014, 281(1): 129-145. |
| 92 | 何远志, 冯雁. 苏氨酸醛缩酶的结构与功能及其在药物合成中的应用[J]. 生物化学与生物物理进展, 2023, 50(5): 962-977. |
| HE Y Z, FENG Y. Structure and function of threonine aldolase and its application in pharmaceutical synthesis[J]. Progress in Biochemistry and Biophysics, 2023, 50(5): 962-977. | |
| 93 | WANG L C, XU L, SU B M, et al. Improving the Cβ stereoselectivity of L-threonine aldolase for the synthesis of L-threo-4-methylsulfonylphenylserine by modulating the substrate-binding pocket to control the orientation of the substrate entrance[J]. Chemistry, 2021, 27(37): 9654-9660. |
| 94 | LI L H, ZHANG R Z, XU Y, et al. Comprehensive screening strategy coupled with structure-guided engineering of L-threonine aldolase from Pseudomonas putida for enhanced catalytic efficiency towards L-threo-4-methylsulfonylphenylserine[J]. Frontiers in Bioengineering and Biotechnology, 2023, 11: 1117890. |
| 95 | LIU Z C, CHEN X, CHEN Q J, et al. Engineering of L-threonine aldolase for the preparation of 4-(methylsulfonyl)phenylserine, an important intermediate for the synthesis of florfenicol and thiamphenicol[J]. Enzyme and Microbial Technology, 2020, 137: 109551. |
| 96 | CHEN Q J, CHEN X, FENG J H, et al. Improving and inverting Cβ-stereoselectivity of threonine aldolase via substrate-binding-guided mutagenesis and a stepwise visual screening[J]. ACS Catalysis, 2019, 9(5): 4462-4469. |
| 97 | ZHENG W L, PU Z J, XIAO L X, et al. Mutability-landscape-guided engineering of L-threonine aldolase revealing the prelog rule in mediating diastereoselectivity of C—C bond formation[J]. Angewandte Chemie International Edition, 2023, 62(2): e202213855. |
| 98 | ZHENG W L, YU H R, FANG S, et al. Directed evolution of L-threonine aldolase for the diastereoselective synthesis of β-hydroxy-α-amino acids[J]. ACS Catalysis, 2021, 11(6): 3198-3205. |
| 99 | PARK S H, SEO H, SEOK J W, et al. Cβ-selective aldol addition of D-threonine aldolase by spatial constraint of aldehyde binding[J]. ACS Catalysis, 2021, 11(12): 6892-6899. |
| 100 | ZHENG W L, CHEN K T, WANG Z, et al. Construction of a highly diastereoselective aldol reaction system with L-threonine aldolase by computer-assisted rational molecular modification and medium engineering[J]. Organic Letters, 2020, 22(15): 5763-5767. |
| 101 | FANG S, YU H R, XIAO L X, et al. Counteracting the activity-diastereoselectivity trade-off of L-threonine aldolase by regulating the proton transfer microenvironment[J]. Advanced Synthesis & Catalysis, 2022, 364(24): 4363-4370. |
| 102 | HE Y Z, LI S Y, WANG J, et al. Discovery and engineering of the L-threonine aldolase from Neptunomonas marine for the efficient synthesis of β-hydroxy-α-amino acids via C—C formation[J]. ACS Catalysis, 2023, 13(11): 7210-7220. |
| 103 | FERNANDES H S, RAMOS M J, CERQUEIRA N M F S A. Catalytic mechanism of the serine hydroxymethyltransferase: a computational ONIOM QM/MM study[J]. ACS Catalysis, 2018, 8(11): 10096-10110. |
| 104 | SANTATIWONGCHAI J, GLEESON D, GLEESON M P. Theoretical evaluation of the reaction mechanism of serine hydroxymethyltransferase[J]. The Journal of Physical Chemistry B, 2019, 123(2): 407-418. |
| 105 | HERNANDEZ K, ZELEN I, PETRILLO G, et al. Engineered L-serine hydroxymethyltransferase from Streptococcus thermophilus for the synthesis of α, α-dialkyl-α-amino acids[J]. Angewandte Chemie International Edition, 2015, 54(10): 3013-3017. |
| 106 | ANGELACCIO S, FLORIO R, CONSALVI V, et al. Serine hydroxymethyltransferase from the cold adapted microorganism Psychromonas ingrahamii: a low temperature active enzyme with broad substrate specificity[J]. International Journal of Molecular Sciences, 2012, 13(2): 1314-1326. |
| 107 | MA′RUF I F, RESTIAWATY E, SYIHAB S F, et al. Characterization of thermostable serine hydroxymethyltransferase for β-hydroxy amino acids synthesis[J]. Amino Acids, 2023, 55(1): 75-88. |
| 108 | KUMAR A, WU G B, WU Z, et al. Improved catalytic properties of a serine hydroxymethyl transferase from Idiomarina loihiensis by site directed mutagenesis[J]. International Journal of Biological Macromolecules, 2018, 117: 1216-1223. |
| 109 | ZUO Z Y, ZHENG Z L, LIU Z G, et al. Cloning, DNA shuffling and expression of serine hydroxymethyltransferase gene from Escherichia coli strain AB90054[J]. Enzyme and Microbial Technology, 2007, 40(4): 569-577. |
| 110 | TENG Z X, PAN X W, LIU Y R, et al. Engineering serine hydroxymethyltransferases for efficient synthesis of L-serine in Escherichia coli [J]. Bioresource Technology, 2024, 393: 130153. |
| 111 | GWON H J, YOSHIOKA H, SONG N E, et al. Optimal production of L-threo-2,3-dihydroxyphenylserine (L-threo-DOPS) on a large scale by diastereoselectivity-enhanced variant of L-threonine aldolase expressed in Escherichia coli [J]. Preparative Biochemistry & Biotechnology, 2012, 42(2): 143-154. |
| 112 | TENG H D, CHEN K T, WANG L, et al. Rational immobilization of L-threonine aldolase from Bacillus nealsonii for efficiently synthesis of L-syn-3-[4-(methylsulfonyl)] phenylserine[J]. Process Biochemistry, 2023, 130: 685-694. |
| 113 | CAI B Q, BOCOLA M, ZHOU A M, et al. Computer-aided directed evolution of L-threonine aldolase for asymmetric biocatalytic synthesis of a chloramphenicol intermediate[J]. Bioorganic & Medicinal Chemistry, 2022, 68: 116880. |
| 114 | XI Z W, LI L H, ZHANG X Y, et al. Expanding the L-threonine transaldolase toolbox for the diastereomeric synthesis of β-hydroxy-α-amino acids[J]. Molecular Catalysis, 2023, 543: 113139. |
| 115 | WANG L C, XU L, SU B M, et al. An effective chemo-enzymatic method with an evolved L-threonine aldolase for preparing L-threo-4-methylsulfonylphenylserine ethyl ester of high optical purity[J]. Molecular Catalysis, 2022, 525: 112355. |
| 116 | ZHENG W L, CHEN K T, FANG S, et al. Construction and application of PLP self-sufficient biocatalysis system for threonine aldolase[J]. Enzyme and Microbial Technology, 2020, 141: 109667. |
| 117 | GONG L, XIU Y S, DONG J J, et al. Sustainable one-pot chemo-enzymatic synthesis of chiral furan amino acid from biomass via magnetic solid acid and threonine aldolase[J]. Bioresource Technology, 2021, 337: 125344. |
| 118 | SEO Y M, MATHEW S, BEA H S, et al. Deracemization of unnatural amino acid: homoalanine using D-amino acid oxidase and ω-transaminase[J]. Organic & Biomolecular Chemistry, 2012, 10(12): 2482-2485. |
| 119 | MOLLA G, MELIS R, POLLEGIONI L. Breaking the mirror: L-amino acid deaminase, a novel stereoselective biocatalyst[J]. Biotechnology Advances, 2017, 35(6): 657-668. |
| 120 | WU L C, GUO X L, WU G B, et al. Efficient enzymatic synthesis of α-keto acids by redesigned substrate-binding pocket of the L-amino acid deaminase (PmiLAAD)[J]. Enzyme and Microbial Technology, 2020, 132: 109393. |
| 121 | WALTON C J W, PARMEGGIANI F, BARBER J E B, et al. Engineered aminotransferase for the production of D-phenylalanine derivatives using biocatalytic cascades[J]. ChemCatChem, 2018, 10(2): 470-474. |
| 122 | HAN S W, SHIN J S. One-pot preparation of d-amino acids through biocatalytic deracemization using alanine dehydrogenase and ω-transaminase[J]. Catalysis Letters, 2018, 148(12): 3678-3684. |
| 123 | ISHIDA C, MIYATA R, HASEBE F, et al. Reconstruction of hyper-thermostable ancestral L-amino acid oxidase to perform deracemization to D-amino acids[J]. ChemCatChem, 2021, 13(24): 5228-5235. |
| 124 | NAKANO S, KOZUKA K, MINAMINO Y, et al. Ancestral L-amino acid oxidases for deracemization and stereoinversion of amino acids[J]. Communications Chemistry, 2020, 3(1): 181. |
| 125 | ZHU L B, FENG G Q, GE F, et al. One-pot enzymatic synthesis of D-arylalanines using phenylalanine ammonia lyase and L-amino acid deaminase[J]. Applied Biochemistry and Biotechnology, 2019, 187(1): 75-89. |
| [1] | 温艳华, 刘合栋, 曹春来, 巫瑞波. 蛋白质工程在医药产业中的应用[J]. 合成生物学, 2025, 6(1): 65-86. |
| [2] | 程峰, 邹树平, 徐建妙, 汤恒, 薛亚平, 郑裕国. 生物高纯精草:高光学纯L-草铵膦生物制造的创新与发展[J]. 合成生物学, 2024, 5(6): 1404-1418. |
| [3] | 夏孔晨, 徐维华, 吴起. 光酶催化混乱性反应的研究进展[J]. 合成生物学, 2024, 5(5): 997-1020. |
| [4] | 陈志航, 季梦麟, 戚逸飞. 人工智能蛋白质结构设计算法研究进展[J]. 合成生物学, 2023, 4(3): 464-487. |
| [5] | 梁丽亚, 刘嵘明. 靶向DNA的Ⅱ类CRISPR/Cas系统的蛋白工程化改造[J]. 合成生物学, 2023, 4(1): 86-101. |
| [6] | 祁延萍, 朱晋, 张凯, 刘彤, 王雅婕. 定向进化在蛋白质工程中的应用研究进展[J]. 合成生物学, 2022, 3(6): 1081-1108. |
| [7] | 涂涛, 罗会颖, 姚斌. 蛋白质工程在饲料用酶研发中的应用研究进展[J]. 合成生物学, 2022, 3(3): 487-499. |
| [8] | 王汇滨, 车昌丽, 游松. Fe/α-酮戊二酸依赖型卤化酶在绿色卤化反应中的研究进展[J]. 合成生物学, 2022, 3(3): 545-566. |
| [9] | 后佳琦, 姜楠, 马莲菊, 卢元. 无细胞蛋白质合成:从基础研究到工程应用[J]. 合成生物学, 2022, 3(3): 465-486. |
| [10] | 卞佳豪, 杨广宇. 人工智能辅助的蛋白质工程[J]. 合成生物学, 2022, 3(3): 429-444. |
| [11] | 万逸尘, 许孔亮, 郑仁朝, 郑裕国. 化学品体外生物合成途径设计、元件组装和应用[J]. 合成生物学, 2021, 2(6): 886-901. |
| [12] | 吴淑可, 周颐, 王文, 张巍, 高鹏飞, 李智. 从单酶催化到多酶级联催化——从王义翘教授在酶技术领域的贡献说开去[J]. 合成生物学, 2021, 2(4): 543-558. |
| [13] | 刘美霞, 李强子, 孟冬冬, 魏欣蕾, 游淳. 烟酰胺类辅酶依赖型氧化还原酶的辅酶偏好性改造及其在合成生物学中的应用[J]. 合成生物学, 2020, 1(5): 570-582. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||