合成生物学 ›› 2021, Vol. 2 ›› Issue (4): 543-558.DOI: 10.12211/2096-8280.2021-004
从单酶催化到多酶级联催化——从王义翘教授在酶技术领域的贡献说开去
吴淑可1, 周颐1, 王文2, 张巍3, 高鹏飞4, 李智5
- 1.华中农业大学生命科学技术学院,湖北 武汉 430070
2.雀巢新加坡研发中心,新加坡 619625
3.新加坡科技研究局(A*STAR)生物过程技术研究所,新加坡 138668
4.葛兰素史克中国投资有限公司,北京 100025
5.新加坡国立大学化学与分子工程系,新加坡 117585
-
收稿日期:2021-01-12修回日期:2021-03-17出版日期:2021-08-31发布日期:2021-09-10 -
通讯作者:吴淑可,李智 -
作者简介:吴淑可 (1987—),男,博士,特聘教授,主要从事酶工程及微生物催化研究。E-mail:shukewu@mail.hzau.edu.cn李智 ,男,博士,新加坡国立大学化学与生物分子工程系副教授,主要从事酶催化与生物化工研究。E-mail:chelz@nus.edu.sg
From single-enzyme catalysis to multienzyme cascade: inspired from Professor Daniel I.C. Wang’s pioneer work in enzyme technology
WU Shuke1, ZHOU Yi1, WANG Wen2, ZHANG Wei3, GAO Pengfei4, LI Zhi5
- 1.College of Life Science and Technology,Huazhong Agricultural University,Wuhan 430070,Hubei,China
2.Nestlé R&D Center,Singapore 619625
3.Bioprocessing Technology Institute,Agency for Science,Technology and Research,Singapore 138668
4.GlaxoSmithKline (China) Investment Co. Ltd,Beijing 100025,China
5.Department of Chemical and Biomolecular Engineering,National University of Singapore,Singapore 117585
-
Received:2021-01-12Revised:2021-03-17Online:2021-08-31Published:2021-09-10 -
Contact:WU Shuke, LI Zhi
摘要:
作为国际生物工程和生物技术领域的奠基人和业界泰斗,麻省理工学院王义翘教授几十年来的研究涵盖了生化工程的上、中、下游的众多方向。在酶技术领域,他在早期就完成了几项标志性的工作,例如鱼蛋白的酶消化、甲醇氧化酶的固定化、无细胞多酶合成、非水相酶催化等。从2005年开始,王教授在十年间通过新加坡-麻省理工联盟(Singapore-MIT Alliance)项目和新加坡国立大学李智教授联合指导了数名博士生,并在酶技术的几个子方向上取得了重要成果,包括:①开发了酶固定化的方法用于构建高活力可回收的磁性纳米生物催化剂;②探索了P450单加氧化酶在水相-离子液体体系中的不对称亚砜化反应;③开发了基于通透全细胞的辅酶NADPH再生系统;④成功开发了模块化的多酶级联催化合成高值手性化合物,显著拓展了多酶级联催化的复杂度。其中,多酶级联催化因其“一锅法”的合成特性,避免额外的单元操作,节省人力、物力的投入和废弃物的产生等特点,近年来已经成为酶技术领域的研究热点。在介绍王教授相关工作之余,本文还总结了多酶级联催化在合成手性化合物和大宗化学品方面的最新进展,讨论了其未来的发展方向,并就其进一步整合合成生物学以及王教授所践行的定量化和工程化的理念进行了展望。
中图分类号:
引用本文
吴淑可, 周颐, 王文, 张巍, 高鹏飞, 李智. 从单酶催化到多酶级联催化——从王义翘教授在酶技术领域的贡献说开去[J]. 合成生物学, 2021, 2(4): 543-558.
WU Shuke, ZHOU Yi, WANG Wen, ZHANG Wei, GAO Pengfei, LI Zhi. From single-enzyme catalysis to multienzyme cascade: inspired from Professor Daniel I.C. Wang’s pioneer work in enzyme technology[J]. Synthetic Biology Journal, 2021, 2(4): 543-558.
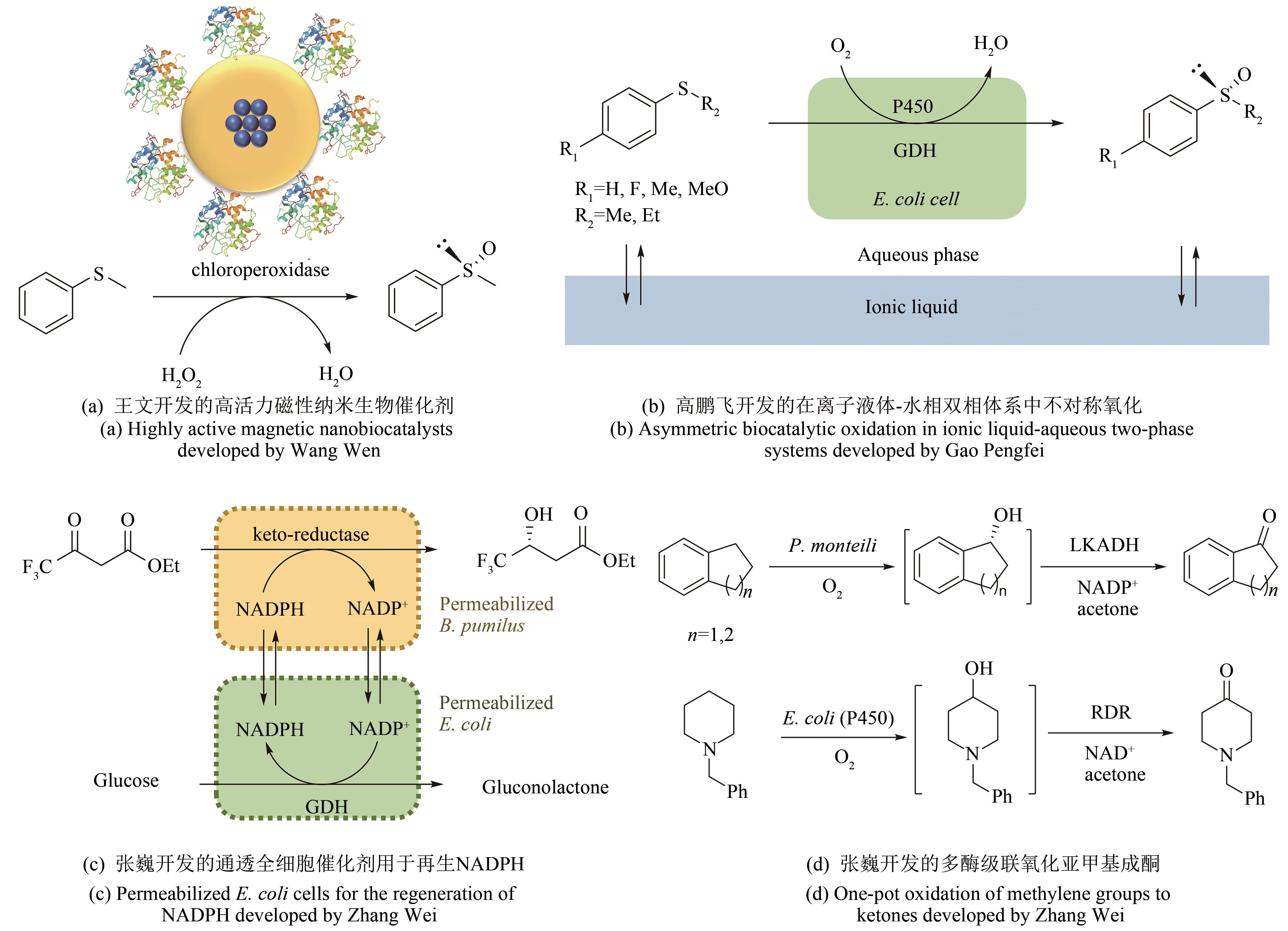
图2 王义翘教授在新加坡-麻省理工联盟项目里联合指导本文作者的四项酶技术领域的工作
Fig. 2 Four enzyme technology projects co-supervised by Prof. Wang through the Singapore-MIT Alliance Program
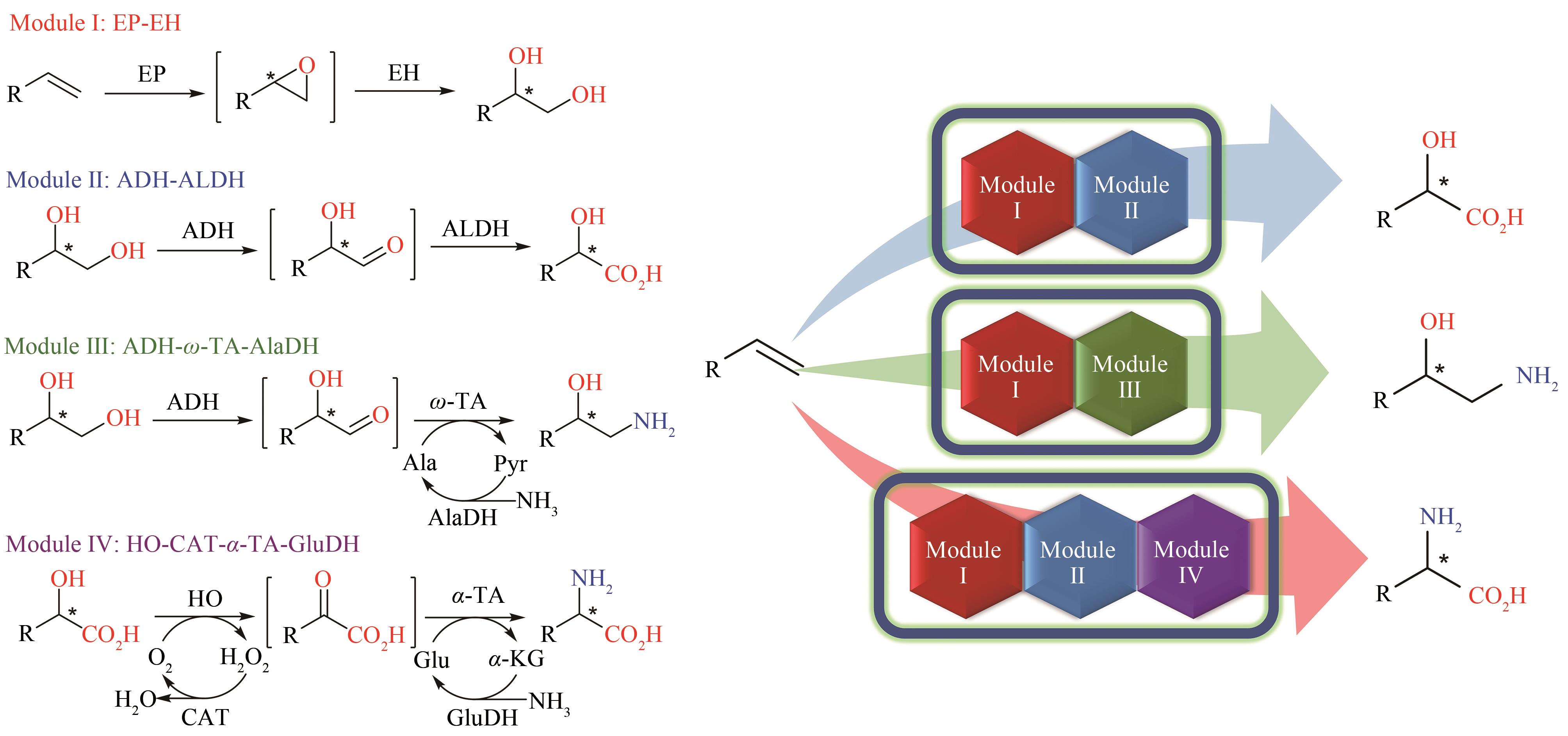
图3 王义翘教授在新加坡-麻省理工联盟项目里联合指导本文作者吴淑可开发的模块化多酶级联催化转化烯烃生成三类手性分子EP—环氧化酶;EH—环氧水解酶;ADH—醇脱氢酶; ALDH—醛脱氢酶;ω-TA—ω-转氨酶;AlaDH—丙氨酸脱氢酶;HO—羟基酸氧化酶;α-TA—α-转氨酶;CAT—过氧化氢酶;GluDH—谷氨酸脱氢酶
Fig. 3 Modular multi-enzyme cascade catalysis for the transformation of alkenes into three types of chiral molecules developed by Wu Shuke and co-supervised by Prof. Wang through the Singapore-MIT Alliance.EP—epoxidase; EH—epoxide hydrolase; ADH—alcohol dehydrogenase; ALDH—aldehyde dehydrogenase; ω-TA—ω-transaminase; AlaDH—alanine dehydrogenase; HO—hydroxy acid oxidase; α-TA—α-transaminase; CAT—catalase; GluDH—glutamate dehydrogenase
| 1 | AFEYAN N B, COONEY C L. Professor Daniel IC Wang: a legacy of education, innovation, publication, and leadership [J]. Biotechnology and Bioengineering, 2006, 95(2): 206-217. |
| 2 | 丁明珠, 李炳志, 王颖, 等. 合成生物学重要研究方向进展[J]. 合成生物学, 2020, 1(1) :7-28. |
| DING M, LI B, WANG Y, et al. Significant research progress in synthetic biology [J]. Synthetic Biology Journal, 2020, 1(1): 7-28. | |
| 3 | SCHMID A, DORDICK J S, HAUER B, et al. Industrial biocatalysis today and tomorrow [J]. Nature, 2001, 409(6817): 258-268. |
| 4 | BORNSCHEUER U T, HUISMAN G W, KAZLAUSKAS R J, et al. Engineering the third wave of biocatalysis [J]. Nature, 2012, 485(7397): 185-194. |
| 5 | KIRK O, BORCHERT T V, FUGLSANG C C. Industrial enzyme applications [J]. Current Opinion in Biotechnology, 2002, 13(4): 345-351. |
| 6 | REETZ M T. Biocatalysis in organic chemistry and biotechnology: past, present, and future [J]. Journal of the American Chemical Society, 2013, 135(34): 12480-12496. |
| 7 | WU S, SNAJDROVA R, MOORE J C, et al. Biocatalysis: enzymatic synthesis for industrial applications [J]. Angewandte Chemie International Edition, 2021, 60(1): 88-119. |
| 8 | ARCHER M C, RAGNARSSON J O, TANNENBAUM S R, et al. Enzymatic solubilization of an insoluble substrate, fish protein concentrate: process and kinetic considerations [J]. Biotechnology and Bioengineering, 1973, 15(1): 181-196. |
| 9 | ARCHER M C, STILLINGS B R, TANNENBAUM S R, et al. Reduction in mercury content of fish protein concentrate by enzymic digestion [J]. Journal of Agricultural and Food Chemistry, 1973, 21(6): 1116-1117. |
| 10 | BARATTI J, COUDERC R, COONEY C L, et al. Preparation and properties of immobilized methanol oxidase [J]. Biotechnology and Bioengineering, 1978, 20(3): 333-348. |
| 11 | HAMILTON B K, MONTGOMERY J P, WANG D I C. Enzyme reactions for preparative scale synthesis [M]. Enzyme Engineering Volume 2. Boston, MA: Springer, 1974, 153-159. |
| 12 | GARDNER C R, COLTON C K, LANGER R S, et al. Enzymatic regeneration of ATP from AMP and ADP part i. thermodynamics, kinetics, and process development [M]. Enzyme Engineering Volume 2. Boston, MA: Springer, 1974, 209-216. |
| 13 | TZENG C H, THRASHER K D, MONTGOMERY J P, et al. High productivity tank fermentation for gramicidin S synthetases [J]. Biotechnology and Bioengineering, 1975, 17(1): 143-152. |
| 14 | WANG D I C, HAMILTON B K. Kinetics of the enzymatic synthesis of peptide antibiotics [J]. Biotechnology and Bioengineering, 1977, 19(8): 1225-1232. |
| 15 | BOMMARIUS A S, HATTON T A, WANG D I C. Xanthine oxidase reactivity in reversed micellar systems: a contribution to the prediction of enzymic activity in organized media [J]. Journal of the American Chemical Society, 1995, 117(16): 4515-4523. |
| 16 | LASKO D R, WANG D I C. In situ fermentation monitoring with recombinant firefly luciferase [J]. Biotechnology and Bioengineering, 1993, 42(1): 30-36. |
| 17 | lASKO D R, WANG D I C. On-line monitoring of intracellular ATP concentration in Escherichia coli fermentations [J]. Biotechnology and Bioengineering, 1996, 52(3): 364-372. |
| 18 | FASAN R, JENNIFER KAN S B, ZHAO H. A continuing career in biocatalysis: Frances H. Arnold [J]. ACS Catalysis, 2019, 9(11): 9775-9788. |
| 19 | ACEVEDO-ROCHA C G, HOLLMANN F, SANCHIS J, et al. A pioneering career in catalysis: Manfred T. Reetz [J]. ACS Catalysis, 2020, 10(24): 15123-15139. |
| 20 | 刘延峰,周景文,刘龙, 等. 合成生物学与食品制造[J]. 合成生物学, 2020, 1(1): 84-91. |
| LIU Y F, ZHOU J W, LIU L, et al. Synthetic biology and food manufacturing [J]. Synthetic Biology Journal, 2020, 1(1): 84-91. | |
| 21 | 史硕博,孟琼宇,乔玮博,等. 塑造低碳经济的第三代固碳生物炼制[J]. 合成生物学, 2020, 1(1): 44-59. |
| SHI S B, MENG Q Y, QIAO W B, et al. Establishing carbon dioxide-based third-generation biorefinery for a sustainable low-carbon economy [J]. Synthetic Biology Journal, 2020, 1(1): 44-59. | |
| 22 | 王凯, 刘子鹤, 陈必强, 等. 微生物利用二氧化碳合成燃料及化学品——第三代生物炼制[J]. 合成生物学, 2020, 1(1): 60-70. |
| WANG K, LIU Z H, CHEN B Q, et al. Microbial utilization of carbon dioxide to synthesize fuels and chemicals——third-generation biorefineries [J]. Synthetic Biology Journal, 2020, 1(1): 60-70. | |
| 23 | 高教琪, 周雍进. 甲醇生物转化的机遇与挑战[J]. 合成生物学, 2020, 1(2): 158-173. |
| GAO J Q, ZHOU Y J. Advances in methanol bio-transformation [J]. Synthetic Biology Journal, 2020, 1(2): 158-173. | |
| 24 | 贺俊斌, 孟松, 潘海学, 等. 多酶催化串联策略在复杂天然产物合成中的应用[J]. 合成生物学, 2020, 1(2): 226-246. |
| HE J B, MENG S, PAN H X, et al. Applications of the multienzyme-catalyzed tandem strategy in the synthesis of complex natural products [J]. Synthetic Biology Journal, 2020, 1(2): 226-246. | |
| 25 | WANG W, YAO L, CHENG C Y, et al. Harnessing the hygroscopic and biofluorescent behaviors of genetically tractable microbial cells to design biohybrid wearables [J]. Science Advances, 2017, 3(5): e1601984. |
| 26 | MATEO C, PALOMO J M, FERNANDEZ-LORENTE G, et al. Improvement of enzyme activity, stability and selectivity via immobilization techniques [J]. Enzyme and Microbial Technology, 2007, 40(6): 1451-1463. |
| 27 | SHELDON R A. Enzyme immobilization: the quest for optimum performance [J]. Advanced Synthesis & Catalysis, 2007, 349(8-9): 1289-1307. |
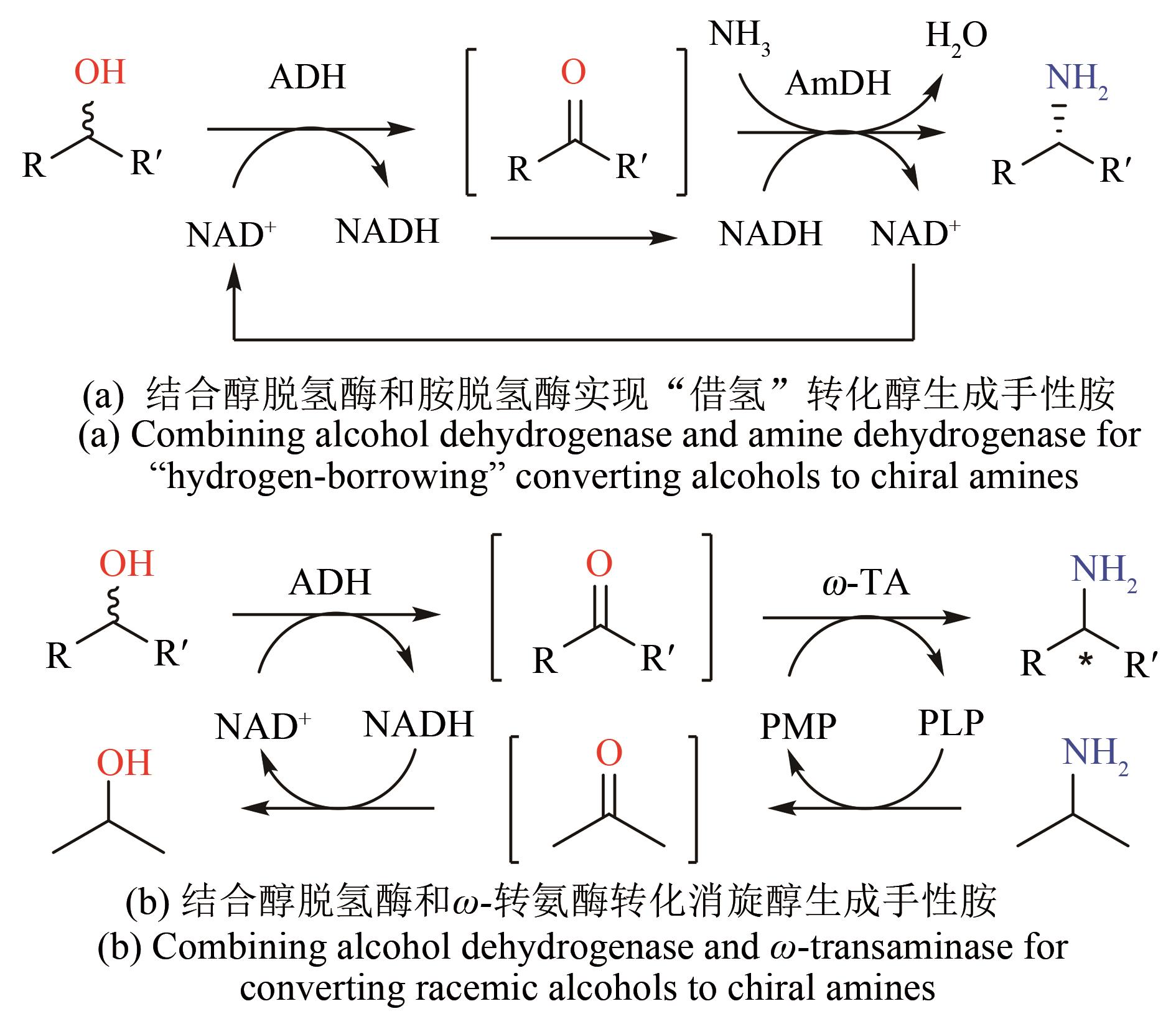
| 28 | WANG W, XU Y, WANG D I C, et al. Recyclable nanobiocatalyst for enantioselective sulfoxidation: facile fabrication and high performance of chloroperoxidase-coated magnetic nanoparticles with iron oxide core and polymer shell [J]. Journal of the American Chemical Society, 2009, 131(36): 12892-12893. |
| 29 | WANG W, WANG D I C, LI Z. Facile fabrication of recyclable and active nanobiocatalyst: purification and immobilization of enzyme in one pot with Ni-NTA functionalized magnetic nanoparticle [J]. Chemical Communications, 2011; 47(28): 8115-8117. |
| 30 | NGO T P, ZHANG W, WANG W, et al. Reversible clustering of magnetic nanobiocatalysts for high-performance biocatalysis and easy catalyst recycling [J]. Chemical Communications, 2012, 48(38): 4585-4587. |
| 31 | VAHIDI A K, YANG Y, NGO T P, et al. Simple and efficient immobilization of extracellular his-tagged enzyme directly from cell culture supernatant as active and recyclable nanobiocatalyst: high-performance production of biodiesel from waste grease [J]. ACS Catalysis, 2015, 5(6): 3157-3161. |
| 32 | VAHIDI A K, WANG Z, LI Z. Facile synthesis of S-substituted L-cysteines with nano-sized immobilized O-acetylserine sulfhydrylase [J]. ChemCatChem, 2018, 10(17): 3671-3674. |
| 33 | RANTWIJK F VAN, SHELDON R A. Biocatalysis in ionic liquids [J]. Chemical Reviews, 2007,107(6): 2757-2785. |
| 34 | XU P, ZHENG G W, ZONG M H, et al. Recent progress on deep eutectic solvents in biocatalysis [J]. Bioresources and Bioprocessing, 2017, 4(1): 34. |
| 35 | GAO P, LI A, LEE H H, et al. Enhancing enantioselectivity and productivity of P450-catalyzed asymmetric sulfoxidation with an aqueous/ionic liquid biphasic system [J]. ACS Catalysis, 2014, 4(10): 3763-3771. |
| 36 | REETZ M T. Laboratory evolution of stereoselective enzymes: a prolific source of catalysts for asymmetric reactions [J]. Angewandte Chemie International Edition, 2011, 50(1): 138-174. |
| 37 | TANG W L, LI Z, ZHAO H. Inverting the enantioselectivity of P450pyr monooxygenase by directed evolution [J]. Chemical Communications, 2010, 46(30): 5461-5463. |
| 38 | PHAM S Q, POMPIDOR G, LIU J, et al. Evolving P450pyr hydroxylase for highly enantioselective hydroxylation at non-activated carbon atom [J]. Chemical Communications, 2012, 48(38): 4618-4620. |
| 39 | YANG Y, LIU J, LI Z. Engineering of P450pyr hydroxylase for the highly regio- and enantioselective subterminal hydroxylation of alkanes [J]. Angewandte Chemie International Edition, 2014, 53(12): 3120-3124. |
| 40 | LIU W, WANG P. Cofactor regeneration for sustainable enzymatic biosynthesis [J]. Biotechnology Advances, 2007, 25(4): 369-384. |
| 41 | ZHANG J, DUETZ W A, WITHOLT B, et al. Rapid identification of new bacterial alcohol dehydrogenases for (R)-and (S)-enantioselective reduction of β-ketoesters [J]. Chemical Communications, 2004(18): 2120-2121. |
| 42 | ZHANG W, O'CONNOR K, WANG D I C, et al. Bioreduction with efficient recycling of NADPH by coupled permeabilized microorganisms[J]. Applied and Environmental Microbiology, 2009, 75(3): 687-694. |
| 43 | SCHRITTWIESER J H, VELIKOGNE S, HALL M, et al. Artificial biocatalytic linear cascades for preparation of organic molecules [J]. Chemical Reviews, 2018, 118(1): 270-348. |
| 44 | FRANCE S P, HEPWORTH L J, TURNER N J, et al. Constructing biocatalytic cascades: in vitro and in vivo approaches to de novo multi-enzyme pathways [J]. ACS Catalysis, 2017, 7(1): 710-724. |
| 45 | WU S K, LI Z. Whole-cell cascade biotransformations for one-pot multistep organic synthesis [J]. ChemCatChem, 2018, 10(10): 2164-2178. |
| 46 | KUSKA J, O'REILLY E. Engineered biosynthetic pathways and biocatalytic cascades for sustainable synthesis [J]. Current Opinion in Chemical Biology, 2020, 58: 146-54. |
| 47 | LI R J, ZHANG Z, ACEVEDO-ROCHA C G, et al. Biosynthesis of organic molecules via artificial cascade reactions based on cytochrome P450 monooxygenases [J]. Green Synthesis and Catalysis, 2020, 1(1): 52-59. |
| 48 | VÁZQUEZ-GONZÁLEZ M, WANG C, WILLNER I. Biocatalytic cascades operating on macromolecular scaffolds and in confined environments [J]. Nature Catalysis, 2020, 3(3): 256-273. |
| 49 | WANG Z, SEKAR B S, LI Z. Recent advances in artificial enzyme cascades for the production of value-added chemicals [J]. Bioresource Technology, 2021, 323: 124551. |
| 50 | ZHANG W, TANG W L, WANG D I C, et al. Concurrent oxidations with tandem biocatalysts in one pot: green, selective and clean oxidations of methylene groups to ketones [J]. Chemical Communications, 2011, 47(11): 3284-3286. |
| 51 | WU S K, CHEN Y, XU Y, et al. Enantioselective trans-dihydroxylation of aryl olefins by cascade biocatalysis with recombinant Escherichia coli coexpressing monooxygenase and epoxide hydrolase [J]. ACS Catalysis, 2014, 4(2): 409-420. |
| 52 | WU S K, ZHOU Y, WANG T, et al. Highly regio- and enantioselective multiple oxy-and amino-functionalizations of alkenes by modular cascade biocatalysis [J]. Nature Communications, 2016, 7: 11917. |
| 53 | ZHOU Y, WU S K, LI Z. One-pot enantioselective synthesis of d-phenylglycines from racemic mandelic acids, styrenes, or biobased l-phenylalanine via cascade biocatalysis [J]. Advanced Synthesis & Catalysis, 2017, 359(24): 4305-4316. |
| 54 | WU S K, LIU J, LI Z. Biocatalytic formal anti-Markovnikov hydroamination and hydration of aryl alkenes [J]. ACS Catalysis, 2017, 7(8): 5225-5233. |
| 55 | WU S K, ZHOU Y, SEET D, et al. Regio-and stereoselective oxidation of styrene derivatives to arylalkanoic acids via one-pot cascade biotransformations [J]. Advanced Synthesis & Catalysis, 2017, 359(12): 2132-2141. |
| 56 | ZHOU Y, WU S K, MAO J, et al. Bioproduction of benzylamine from renewable feedstocks via a nine-step artificial enzyme cascade and engineered metabolic pathways [J]. ChemSusChem, 2018, 11(13): 2221-2228. |
| 57 | ZHOU Y, SEKAR B S, WU S K, et al. Benzoic acid production via cascade biotransformation and coupled fermentation-biotransformation [J]. Biotechnology and Bioengineering, 2020, 117(8): 2340-2350. |
| 58 | SEKAR B S, MAO J, LUKITO B R, et al. Bioproduction of enantiopure (R)- and (S)-2-phenylglycinols from styrenes and renewable feedstocks [J]. Advanced Synthesis & Catalysis, 2021, 363(7): 1892-1903. |
| 59 | ZHANG J, YANG X, DONG R, et al. Cascade biocatalysis for regio- and stereoselective aminohydroxylation of styrenyl olefins to enantiopure arylglycinols [J]. ACS Sustainable Chemistry & Engineering, 2020, 8(49): 18277-18285. |
| 60 | ZHOU Y, WU S K, LI Z. Cascade biocatalysis for sustainable asymmetric synthesis: from biobased l-phenylalanine to high-value chiral chemicals [J]. Angewandte Chemie International Edition, 2016, 55(38): 11647-11650. |
| 61 | SEKAR B S, LUKITO B R, LI Z. Production of natural 2-phenylethanol from glucose or glycerol with coupled Escherichia coli strains expressing l-phenylalanine biosynthesis pathway and artificial biocascades [J]. ACS Sustainable Chemistry & Engineering. 2019, 7(14): 12231-12239. |
| 62 | NUGENT T C, EL-SHAZLY M. Chiral amine synthesis-recent developments and trends for enamide reduction, reductive amination, and imine reduction [J]. Advanced Synthesis & Catalysis, 2010, 352(5): 753-819. |
| 63 | SAVILE C K, JANEY J M, MUNDORFF E C, et al. Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture [J]. Science, 2010, 329(5989): 305-309. |
| 64 | ABRAHAMSON M J, VAZQUEZ-FIGUEROA E, WOODALL N B, et al. Development of an amine dehydrogenase for synthesis of chiral amines [J]. Angewandte Chemie International Edition, 2012, 51(16): 3969-3972. |
| 65 | MUTTI F G, KNAUS T, SCRUTTON N S, et al. Conversion of alcohols to enantiopure amines through dual-enzyme hydrogen-borrowing cascades [J]. Science, 2015, 349(6255): 1525-1529. |
| 66 | CHEN F F, LIU Y Y, ZHENG G W, et al. Asymmetric amination of secondary alcohols by using a redox-neutral two-enzyme cascade [J]. ChemCatChem, 2015, 7(23): 3838-3841. |
| 67 | YU H L, LI T, CHEN F F, et al. Bioamination of alkane with ammonium by an artificially designed multienzyme cascade [J]. Metabolic Engineering, 2018, 47: 184-189. |
| 68 | TAUBER K, FUCHS M, SATTLER J H, et al. Artificial multi-enzyme networks for the asymmetric amination of sec-alcohols [J]. Chemistry-A European Journal, 2013, 19(12): 4030-4035. |
| 69 | TIAN K, LI Z. A simple biosystem for the high-yielding cascade conversion of racemic alcohols to enantiopure amines [J]. Angewandte Chemie International Edition, 2020, 59(48): 21745-21751. |
| 70 | ALEKU G A, FRANCE S P, MAN H, et al. A reductive aminase from Aspergillus oryzae [J]. Nature Chemistry, 2017, 9(10): 961-969. |
| 71 | RAMSDEN J I, HEATH R S, DERRINGTON S R, et al. Biocatalytic N-alkylation of amines using either primary alcohols or carboxylic acids via reductive aminase cascades [J]. Journal of the American Chemical Society, 2019, 141(3): 1201-1206. |
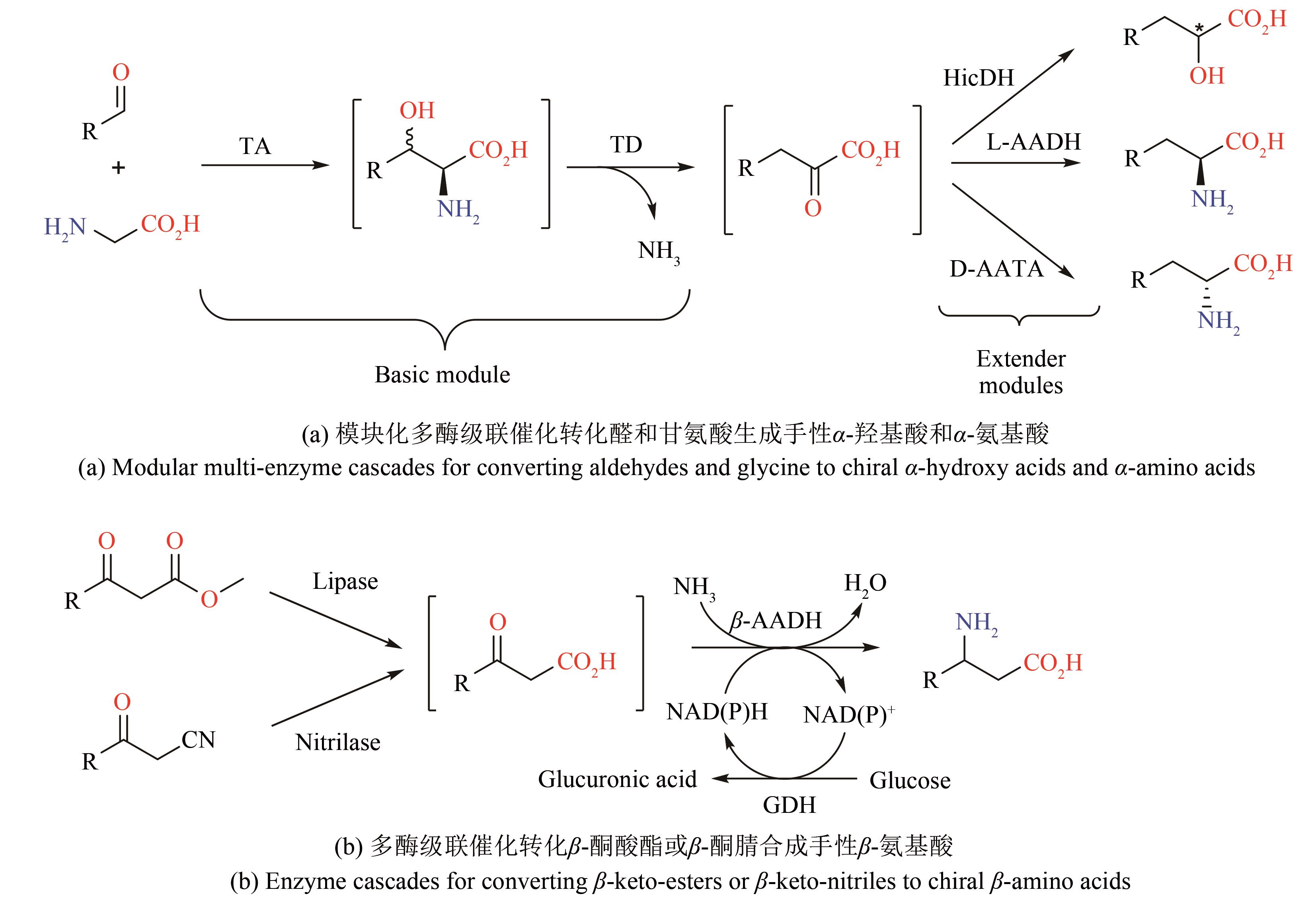
| 72 | XUE Y P, CAO C H, ZHENG Y G. Enzymatic asymmetric synthesis of chiral amino acids [J]. Chemical Society Reviews, 2018, 47(4): 1516-1561. |
| 73 | ALMHJELL P J, BOVILLE C E, ARNOLD F H. Engineering enzymes for noncanonical amino acid synthesis [J]. Chemical Society Reviews, 2018, 47(24): 8980-8997. |
| 74 | ALTENBUCHNER J, SIEMANN-HERZBERG M, SYLDATK C. Hydantoinases and related enzymes as biocatalysts for the synthesis of unnatural chiral amino acids [J]. Current Opinion in Biotechnology, 2001, 12(6): 559-563. |
| 75 | LIU F, ZHOU J, XU M, et al. One-pot biocatalytic preparation of enantiopure unusual α-amino acids from α-hydroxy acids via a hydrogen-borrowing dual-enzyme cascade [J]. Catalysts, 2020, 10(12): 1470. |
| 76 | SONG W, WANG J H, WU J, et al. Asymmetric assembly of high-value α-functionalized organic acids using a biocatalytic chiral-group-resetting process [J]. Nature Communications, 2018, 9: 3818. |
| 77 | LI R, WIJMA H J, SONG L, et al. Computational redesign of enzymes for regio- and enantioselective hydroamination [J]. Nature Chemical Biology, 2018, 14(7): 664-670. |
| 78 | WANG J, SONG W, WU J, et al. Efficient production of phenylpropionic acids by an amino-group-transformation biocatalytic cascade [J]. Biotechnology and Bioengineering, 2020, 117(3): 614-625. |
| 79 | ZHANG D, CHEN X, ZHANG R, et al. Development of β-amino acid dehydrogenase for the synthesis of β-amino acids via reductive amination of β-keto acids [J]. ACS Catalysis, 2015, 5(4): 2220-2224. |
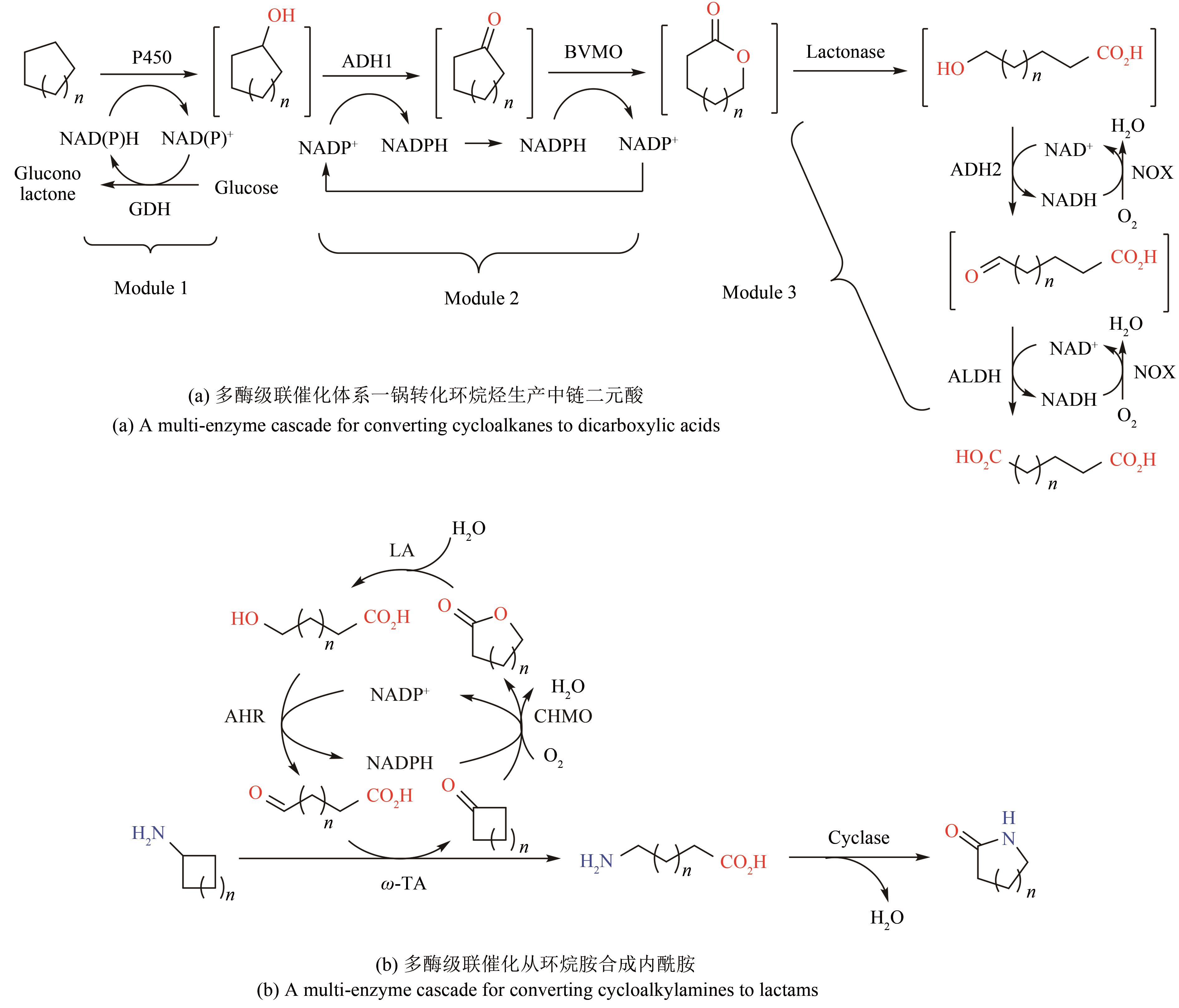
| 80 | AHMED S T, LEFERINK N G, SCRUTTON N S. Chemo-enzymatic routes towards the synthesis of bio-based monomers and polymers [J]. Molecular Catalysis, 2019, 467: 95-110. |
| 81 | GE J, YANG X, YU H, et al. High-yield whole cell biosynthesis of Nylon 12 monomer with self-sufficient supply of multiple cofactors [J]. Metabolic Engineering, 2020, 62: 172-185. |
| 82 | WANG F, ZHAO J, LI Q, et al. One-pot biocatalytic route from cycloalkanes to α-ω-dicarboxylic acids by designed Escherichia coli consortia [J]. Nature Communications, 2020, 11: 5035. |
| 83 | ZHANG Z, LI Q, WANG F, et al. One-pot biosynthesis of 1,6-hexanediol from cyclohexane by de novo designed cascade biocatalysis [J]. Green Chemistry, 2020, 22(21): 7476-7483. |
| 84 | SARAK S, SUNG S, JEON H, et al. An integrated cofactor/co-product recycling cascade for the biosynthesis of nylon monomers from cycloalkylamines [J]. Angewandte Chemie International Edition, 2021, 60(7): 3481-3486. |
| 85 | 张建志, 付立豪, 唐婷, 等. 酶蛋白元件的规模化挖掘[J]. 合成生物学, 2020,1 (3): 267-284. |
| ZHANG J Z, FU L H, TANG T, et al. Scalable enzyme mining via synthetic biology [J]. Synthetic Biology Journal, 2020, 1(3): 267-284. | |
| 86 | 曲戈,朱彤,蒋迎迎, 等. 蛋白质工程:从定向进化到计算设计[J]. 生物工程学报, 2019, 35(10): 1843-1856. |
| QU G, ZHU T, JIANG Y Y, et al. Protein engineering: from directed evolution to computational design [J]. Chinese Journal of Biotechnology, 2019, 35(10): 1843-1856. | |
| 87 | 于勇, 朱欣娜, 张学礼. 大宗化学品细胞工厂的构建与应用[J]. 合成生物学,2020,1(6):674-684. |
| YU Y, ZHU X N, ZHANG X L. Construction and application of microbial cell factories for production of bulk chemicals [J]. Synthetic Biology Journal, 2020, 1(6): 674-684. | |
| 88 | WALSH C T, MOORE B S. Enzymatic cascade reactions in biosynthesis [J]. Angewandte Chemie International Edition, 2019, 58(21): 6846-6879. |
| 89 | HUFFMAN M A, FRYSZKOWSKA A, ALVIZO O, et al. Design of an in vitro biocatalytic cascade for the manufacture of islatravir [J]. Science, 2019, 366(6470): 1255-1259. |
| 90 | RUDROFF F, MIHOVILOVIC M D, GROEGER H, et al. Opportunities and challenges for combining chemo- and biocatalysis [J]. Nature Catalysis, 2018, 1(1): 12-22. |
| 91 | HUANG X, CAO M, ZHAO H. Integrating biocatalysis with chemocatalysis for selective transformations [J]. Current Opinion in Chemical Biology, 2020, 55: 161-170. |
| 92 | LITMAN ZC, WANG Y, ZHAO H, et al. Cooperative asymmetric reactions combining photocatalysis and enzymatic catalysis [J]. Nature, 2018, 560(7718): 355-359. |
| 93 | WU S K, ZHOU Y, GERNGROSS D, et al. Chemo-enzymatic cascades to produce cycloalkenes from bio-based resources [J]. Nature Communications, 2019, 10: 5060. |
| 94 | ZHANG W, LEE J H, YOUNES S H, et al. Photobiocatalytic synthesis of chiral secondary fatty alcohols from renewable unsaturated fatty acids [J]. Nature Communications, 2020, 11: 2258. |
| 95 | WEI X, HAN P, YOU C. Facilitation of cascade biocatalysis by artificial multi-enzyme complexes—a review [J]. Chinese Journal of Chemical Engineering, 2020, 28(11): 2799-2809. |
| 96 | KANG W, MA T, LIU M, et al. Modular enzyme assembly for enhanced cascade biocatalysis and metabolic flux [J]. Nature Communications, 2019, 10: 4248. |
| 97 | NI J, GAO Y Y, TAO F, et al. Temperature-directed biocatalysis for the sustainable production of aromatic aldehydes or alcohols [J]. Angewandte Chemie International Edition, 2018, 57(5): 1214-1217. |
| 98 | NI J, WU Y T, TAO F, et al. A coenzyme-free biocatalyst for the value-added utilization of lignin-derived aromatics [J]. Journal of the American Chemical Society, 2018, 140(47): 16001-16005. |
| 99 | SCHWANDER T, BORZYSKOWSKI L S VON, BURGENER S, et al. A synthetic pathway for the fixation of carbon dioxide in vitro [J]. Science, 2016, 354(6314): 900-904. |
| 100 | LU X, LIU Y, YANG Y, et al. Constructing a synthetic pathway for acetyl-coenzyme A from one-carbon through enzyme design [J]. Nature Communications, 2019, 10: 1378. |
| [1] | 温艳华, 刘合栋, 曹春来, 巫瑞波. 蛋白质工程在医药产业中的应用[J]. 合成生物学, 2025, 6(1): 65-86. |
| [2] | 程峰, 邹树平, 徐建妙, 汤恒, 薛亚平, 郑裕国. 生物高纯精草:高光学纯L-草铵膦生物制造的创新与发展[J]. 合成生物学, 2024, 5(6): 1404-1418. |
| [3] | 付雨, 钟芳锐. 化学原理驱动的光生物不对称催化研究进展[J]. 合成生物学, 2024, 5(5): 1021-1049. |
| [4] | 夏孔晨, 徐维华, 吴起. 光酶催化混乱性反应的研究进展[J]. 合成生物学, 2024, 5(5): 997-1020. |
| [5] | 张守祺, 王涛, 孔尧, 邹家胜, 刘元宁, 徐正仁. 天然产物的化学-酶法合成:方法与策略的演进[J]. 合成生物学, 2024, 5(5): 913-940. |
| [6] | 叶伟, 李芮, 姜卫红, 顾阳. 二氧化碳微生物转化与体外酶催化体系研究进展[J]. 合成生物学, 2023, 4(6): 1223-1245. |
| [7] | 明阳, 陈彬, 黄小强. 光酶催化合成进展[J]. 合成生物学, 2023, 4(4): 651-675. |
| [8] | 曾涛, 巫瑞波. 数据驱动的酶反应预测与设计[J]. 合成生物学, 2023, 4(3): 535-550. |
| [9] | 祁延萍, 朱晋, 张凯, 刘彤, 王雅婕. 定向进化在蛋白质工程中的应用研究进展[J]. 合成生物学, 2022, 3(6): 1081-1108. |
| [10] | 王汇滨, 车昌丽, 游松. Fe/α-酮戊二酸依赖型卤化酶在绿色卤化反应中的研究进展[J]. 合成生物学, 2022, 3(3): 545-566. |
| [11] | 王盼盼, 于洪巍. 酶催化在维生素及其衍生物制备中的应用[J]. 合成生物学, 2022, 3(3): 500-515. |
| [12] | 楼玉姣, 徐鉴, 吴起. 生物催化惰性碳氢键的氘代反应研究进展[J]. 合成生物学, 2022, 3(3): 530-544. |
| [13] | 杨璐, 瞿旭东. 亚胺还原酶在手性胺合成中的应用[J]. 合成生物学, 2022, 3(3): 516-529. |
| [14] | 熊亮斌, 宋璐, 赵云秋, 刘坤, 刘勇军, 王风清, 魏东芝. 甾体化合物绿色生物制造:从生物转化到微生物从头合成[J]. 合成生物学, 2021, 2(6): 942-963. |
| [15] | 张发光, 曲戈, 孙周通, 马军安. 从化学合成到生物合成——天然产物全合成新趋势[J]. 合成生物学, 2021, 2(5): 674-696. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||