Synthetic Biology Journal ›› 2025, Vol. 6 ›› Issue (4): 899-919.DOI: 10.12211/2096-8280.2025-004
• Invited Review • Previous Articles Next Articles
Advancements in the study of probiotics for adjunctive prevention and treatment of malignancies
ZHU Xinyue1, CHEN Tiantian1, SHAO Hengxuan1, TANG Manyu1, HUA Wei1,2, CHENG Yanling1,2
- 1.Biochemical Engineering College,Beijing Union University,Beijing 100023,China
2.Beijing Key Laboratory for Utilization of Biomass Wastes,Beijing 100023,China
-
Received:2025-01-08Revised:2025-04-23Online:2025-09-03Published:2025-08-31 -
Contact:CHENG Yanling
益生菌辅助防治恶性肿瘤的研究进展
朱欣悦1, 陈恬恬1, 邵恒煊1, 唐曼玉1, 华威1,2, 程艳玲1,2
- 1.北京联合大学生物化学工程学院,北京 100023
2.生物质废弃物资源化利用北京市重点实验室,北京 100023
-
通讯作者:程艳玲 -
作者简介:朱欣悦 (2000—),女,硕士研究生。研究方向为农副产品高值化利用。 E-mail:zss89661@163.com程艳玲 (1972—),女,博士,教授。研究方向为食品、农产品、生物制品等加工副产物与废弃物的高值化利用;生活垃圾与污水的高能效转化、治理与利用。 E-mail:cheng1012cn@aliyun.com -
基金资助:美国农业部加州巴旦木委员会“Safety Assessment of Almond Hull as a Novel Food and Food Ingredient”(BIO-22-01-YC)
CLC Number:
Cite this article
ZHU Xinyue, CHEN Tiantian, SHAO Hengxuan, TANG Manyu, HUA Wei, CHENG Yanling. Advancements in the study of probiotics for adjunctive prevention and treatment of malignancies[J]. Synthetic Biology Journal, 2025, 6(4): 899-919.
朱欣悦, 陈恬恬, 邵恒煊, 唐曼玉, 华威, 程艳玲. 益生菌辅助防治恶性肿瘤的研究进展[J]. 合成生物学, 2025, 6(4): 899-919.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2025-004
| 功能 | 菌种 | 剂量 | 疾病 | 研究对象 | 研究结果 | 参考文献 |
|---|---|---|---|---|---|---|
| 抑制致癌物质生成 | 嗜酸乳杆菌 | 1010个/(mL·d) | — | 人 | β-葡萄糖醛酸苷酶活性↓、硝基还原酶活性↓ | [ |
| 调节肠道菌群 | 罗伊氏乳杆菌KUB-AC5 | 109 CFU/d | 沙门氏菌感染 | C57BL/6小鼠 | 沙门氏菌数量↓、Kc、IL6、Nos2、IFN-γ↓ | [ |
| 嗜酸乳杆菌、鼠李糖乳杆菌 | 6×109 CFU/d | Hp感染 | 人 | 幽门螺杆菌丰度↓ | [ | |
| 提升抗氧化能力,预防肿瘤发生 | 嗜酸乳杆菌、鼠李糖乳杆菌 | 1×109 CFU/(0.1mL·d) | CRC | CRC大鼠感染前服用益生菌 | SOD、GPx、GSH↑、MDA↓、肿瘤发生率↓ | [ |
| 发酵黏液乳杆菌GR-3 | 1×109 CFU/d | CRC | C57BL/6小鼠 | SOD、GSH↑、MDA↓,肿瘤发生率↓ | [ | |
| 调节免疫细胞,发挥抗炎作用 | 双歧杆菌与乳酸菌混合 | 1×109 CFU/(kg·d) | AIH | C57BL/6小鼠 | Treg↑、ALT↓、AST↓、IL-17A↓、IFN-γ↓、TGF-β↑ | [ |
| 植物乳杆菌 | 3×109 CFU/mL | 结肠炎 | Wistar大鼠 | TNF-α↑,IL-6↓ | [ |
Table 1 The role of probiotics in tumor prevention
| 功能 | 菌种 | 剂量 | 疾病 | 研究对象 | 研究结果 | 参考文献 |
|---|---|---|---|---|---|---|
| 抑制致癌物质生成 | 嗜酸乳杆菌 | 1010个/(mL·d) | — | 人 | β-葡萄糖醛酸苷酶活性↓、硝基还原酶活性↓ | [ |
| 调节肠道菌群 | 罗伊氏乳杆菌KUB-AC5 | 109 CFU/d | 沙门氏菌感染 | C57BL/6小鼠 | 沙门氏菌数量↓、Kc、IL6、Nos2、IFN-γ↓ | [ |
| 嗜酸乳杆菌、鼠李糖乳杆菌 | 6×109 CFU/d | Hp感染 | 人 | 幽门螺杆菌丰度↓ | [ | |
| 提升抗氧化能力,预防肿瘤发生 | 嗜酸乳杆菌、鼠李糖乳杆菌 | 1×109 CFU/(0.1mL·d) | CRC | CRC大鼠感染前服用益生菌 | SOD、GPx、GSH↑、MDA↓、肿瘤发生率↓ | [ |
| 发酵黏液乳杆菌GR-3 | 1×109 CFU/d | CRC | C57BL/6小鼠 | SOD、GSH↑、MDA↓,肿瘤发生率↓ | [ | |
| 调节免疫细胞,发挥抗炎作用 | 双歧杆菌与乳酸菌混合 | 1×109 CFU/(kg·d) | AIH | C57BL/6小鼠 | Treg↑、ALT↓、AST↓、IL-17A↓、IFN-γ↓、TGF-β↑ | [ |
| 植物乳杆菌 | 3×109 CFU/mL | 结肠炎 | Wistar大鼠 | TNF-α↑,IL-6↓ | [ |
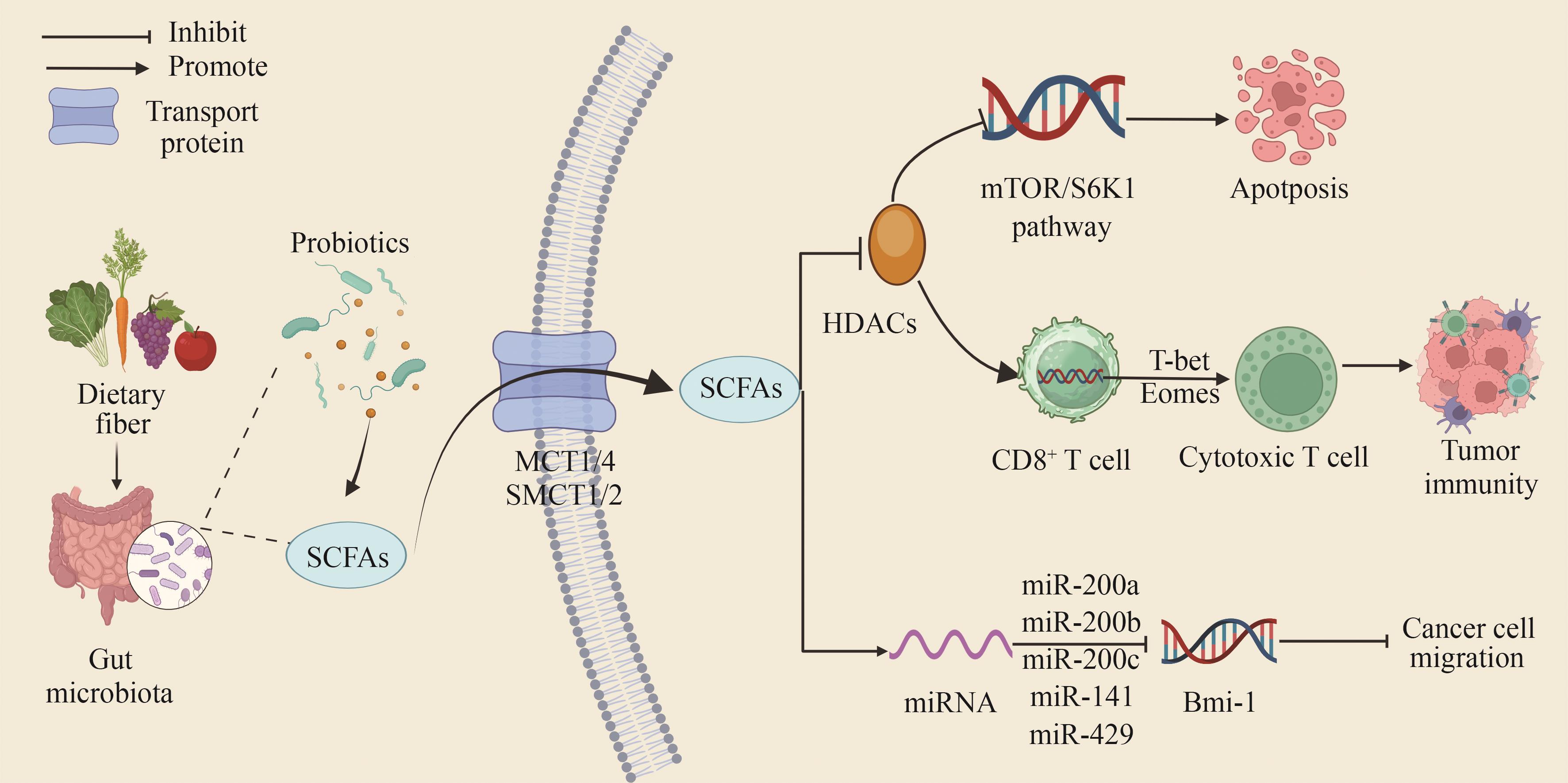
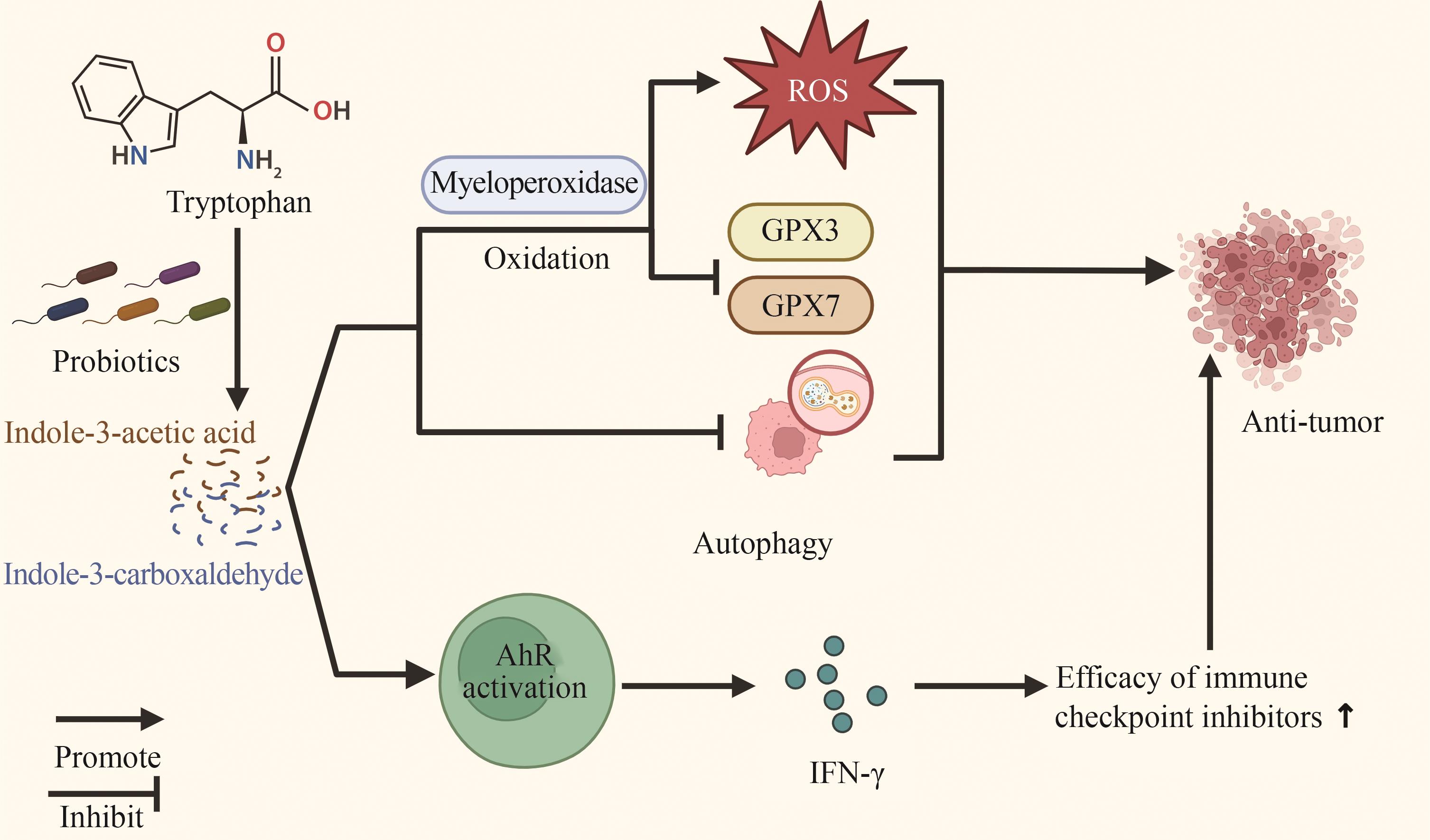
Fig. 1 Mechanism of SCFAs produced by probiotic metabolism acting on the tumor microenvironment[Short-chain fatty acids (SCFAs) are the main metabolites produced by gut microbiota through dietary fermentation. They enter cells primarily via monocarboxylate transporters (MCTs) and sodium-coupled monocarboxylate transporters (SMCTs), directly inhibiting the activity of histone deacetylases (HDACs) and promoting tumor cell apoptosis through the mTOR/S6K1 signaling pathway. Meanwhile, inhibition of HDAC activity can also promote the differentiation of CD8+ T cells into cytotoxic T lymphocytes (CTLs), thereby enhancing anti-tumor immune capacity.]
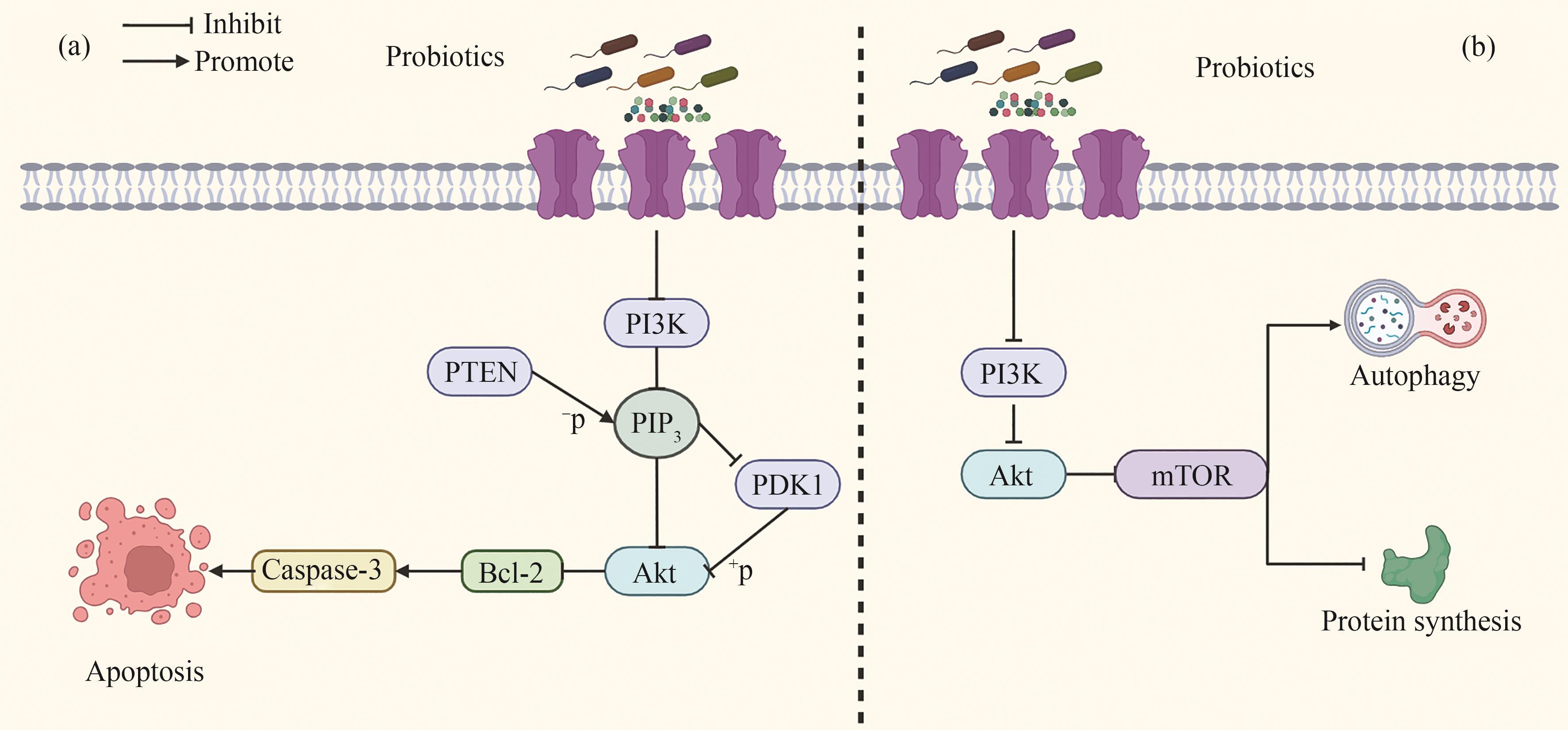
Fig. 2 The mechanism of anti-tumor activity of probiotics and their metabolites through the PI3K-AKT signaling pathway[(a) Probiotics regulate the expression of PI3K, PTEN, and PDK1, thereby downregulating AKT expression and inhibiting the production of Bcl-2. This promotes an increase in the expression of Caspase-3, a protease that plays a central role in cell apoptosis, and thus induces apoptosis of tumor cells. (b) Probiotics exert anti-tumor effects by promoting autophagy of tumor cells and inhibiting protein synthesis in tumor cells through the PI3K/AKT/mTOR signaling pathway.]
| 肿瘤类型 | 菌种/代谢产物 | 研究结果 | 参考文献 |
|---|---|---|---|
| 结直肠癌 | 副干酪乳杆菌PC-H1 | PDK1和AKT↓,Bcl-2↓,细胞凋亡率↑ | [ |
| 肠道罗斯拜瑞氏菌 | 肿瘤发生率↓,ZO-1↑,Claudin-3↑,CDK6↓ | [ | |
| 11种从粪便中分离的细菌 | MHC-Ⅰ↑,IFN-γ+ CD8+ T细胞↑ | [ | |
| 双歧杆菌 | CD3⁺、CD4⁺、CD4⁺/CD8⁺↑,D-乳酸、二胺氧化酶↓ | [ | |
| 副干酪乳杆菌SD1与鼠李糖乳杆菌 SD11 | IL-1β、TNF-α、IL-6、IL-8、IL-17A↓,IL-10、IL-12↑,SCFA↑ | [ | |
| 胃癌 | 布氏乳杆菌/无细胞上清液 | BAX、Caspase-3、Caspase-9↑,细胞凋亡率↑ | [ |
| 健康人群的粪便菌群/乙酸盐、丁酸盐 | SNU-216、MGC-803、HGC-27凋亡率↑,GES-1细胞中BAX、caspase-3、Bcl-2↑ | [ | |
| 口腔癌 | 鼠李糖乳杆菌 | Caspase-3、Caspase-8、Caspase-9、BAX、p53、p21、Fas↑,Bcl-2、Bcl-xL↓,HSC-3抑制率↑ | [ |
| 植物乳杆菌 | PTEN↑,MAPK↓ | [ | |
| 植物乳杆菌ATCC 8014 | Bcl-2、TLR4、NFκB↓ | [ | |
| 肝细胞癌 | 罗伊氏乳杆菌 | ALT、AST↓,IL-17A↓,乙酸盐↑ | [ |
| 嗜酸乳杆菌ATCC 4356 | ALT↓,TLR2、STAT-3、P38-MAPK↓,IL-17↓ | [ | |
| 鼠李糖乳杆菌Probio-M9 | 客观缓解率、疾病控制率、无进展生存期、总生存期、手术转化率↑ | [ |
Table 2 Inhibitory effect of probiotics on common digestive system tumors
| 肿瘤类型 | 菌种/代谢产物 | 研究结果 | 参考文献 |
|---|---|---|---|
| 结直肠癌 | 副干酪乳杆菌PC-H1 | PDK1和AKT↓,Bcl-2↓,细胞凋亡率↑ | [ |
| 肠道罗斯拜瑞氏菌 | 肿瘤发生率↓,ZO-1↑,Claudin-3↑,CDK6↓ | [ | |
| 11种从粪便中分离的细菌 | MHC-Ⅰ↑,IFN-γ+ CD8+ T细胞↑ | [ | |
| 双歧杆菌 | CD3⁺、CD4⁺、CD4⁺/CD8⁺↑,D-乳酸、二胺氧化酶↓ | [ | |
| 副干酪乳杆菌SD1与鼠李糖乳杆菌 SD11 | IL-1β、TNF-α、IL-6、IL-8、IL-17A↓,IL-10、IL-12↑,SCFA↑ | [ | |
| 胃癌 | 布氏乳杆菌/无细胞上清液 | BAX、Caspase-3、Caspase-9↑,细胞凋亡率↑ | [ |
| 健康人群的粪便菌群/乙酸盐、丁酸盐 | SNU-216、MGC-803、HGC-27凋亡率↑,GES-1细胞中BAX、caspase-3、Bcl-2↑ | [ | |
| 口腔癌 | 鼠李糖乳杆菌 | Caspase-3、Caspase-8、Caspase-9、BAX、p53、p21、Fas↑,Bcl-2、Bcl-xL↓,HSC-3抑制率↑ | [ |
| 植物乳杆菌 | PTEN↑,MAPK↓ | [ | |
| 植物乳杆菌ATCC 8014 | Bcl-2、TLR4、NFκB↓ | [ | |
| 肝细胞癌 | 罗伊氏乳杆菌 | ALT、AST↓,IL-17A↓,乙酸盐↑ | [ |
| 嗜酸乳杆菌ATCC 4356 | ALT↓,TLR2、STAT-3、P38-MAPK↓,IL-17↓ | [ | |
| 鼠李糖乳杆菌Probio-M9 | 客观缓解率、疾病控制率、无进展生存期、总生存期、手术转化率↑ | [ |
| 菌属 | 菌种 |
|---|---|
| 双歧杆菌属(Bifidobacterium) | 青春双歧杆菌(B. adolescentis)、动物双歧杆菌动物亚种(B.animalis subsp. animalis)、动物双歧杆菌乳亚种(B.animalis subsp. lactis)、两歧双歧杆菌(B.bifidum)、长双歧杆菌长亚种(B.longum subsp. longum)、长双歧杆菌婴儿亚种(B.longum subsp. infantis)、短双歧杆菌(B.breve) |
| 乳杆菌属(Lactobacillus) | 嗜酸乳杆菌(L.acidophilus)、卷曲乳杆菌(L.crispatus)、德氏乳杆菌保加利亚亚种(L.delbrueckii subsp. bulgaricus)、德氏乳杆菌乳亚种(L.delbrueckii subsp. lactis)、格氏乳杆菌(L.gasseri)、瑞士乳杆菌(L.helveticus)、约氏乳杆菌(L.johnsonii)、马乳酒样乳杆菌马乳酒样亚种(L.kefiranofaciens subsp. kefiranofaciens) |
| 乳酪杆菌属(Lacticaseibacillus) | 干酪乳酪杆菌(L.casei)、副干酪乳酪杆菌(L.paracasei)、鼠李糖乳酪杆菌(L.rhamnosus) |
| 黏液乳杆菌属(Limosilactobacillus) | 发酵黏液乳杆菌(L.fermentum)、罗伊氏黏液乳杆菌(L.reuteri) |
| 乳植杆菌属(Lactiplantibacillus) | 植物乳植杆菌(L.plantarum) |
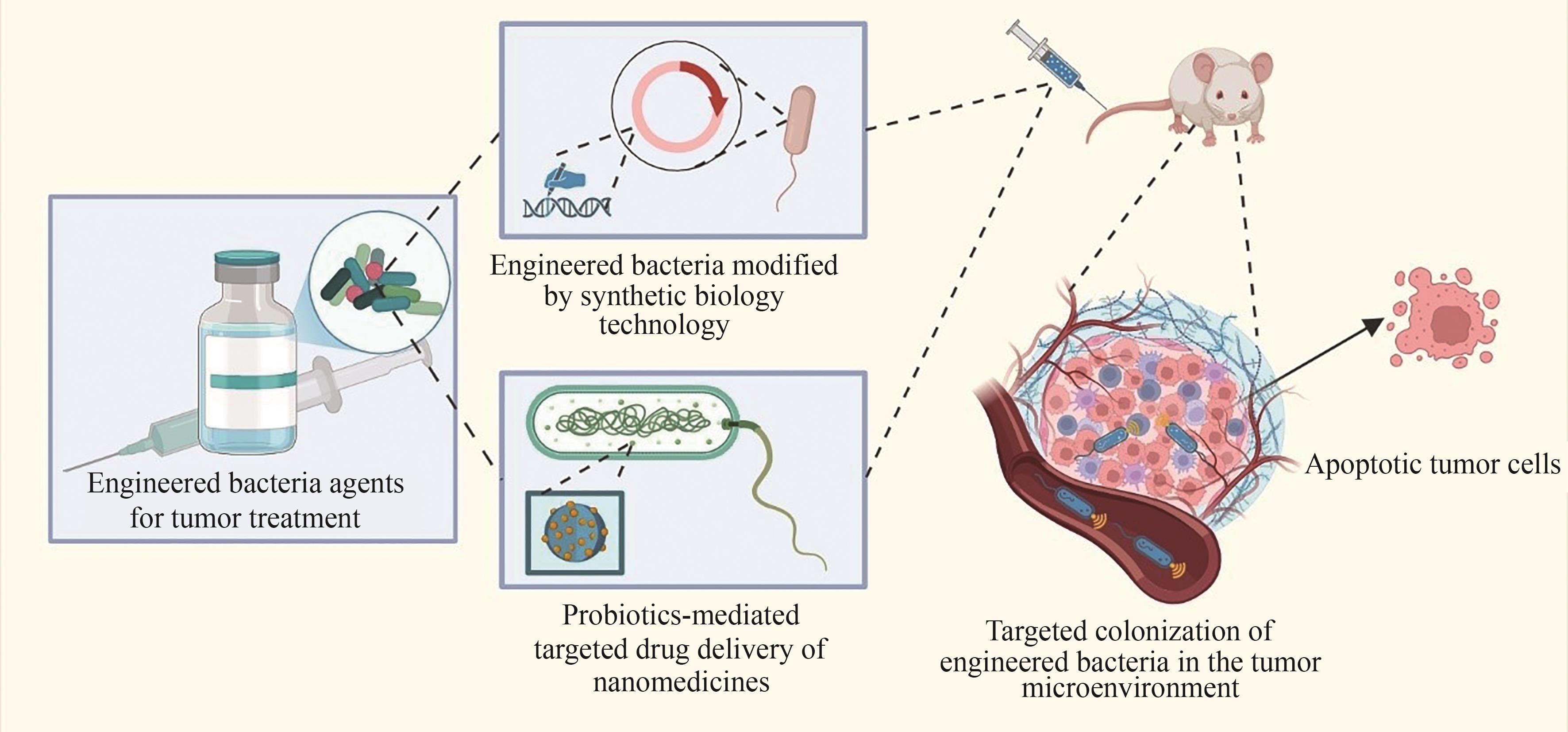
| 联合乳杆菌属(Ligilactobacillus) | 唾液联合乳杆菌(L.salivarius) |
| 广布乳杆菌属(Latilactobacillus) | 弯曲广布乳杆菌(L.curvatus)、清酒广布乳杆菌(L.sakei) |
| 链球菌属(Streptococcus) | 唾液链球菌嗜热亚种(S.salivarius subsp.thermophilus) |
| 乳球菌属(Lactococcus) | 乳酸乳球菌乳亚种(L.lactis subsp.lactis)、乳酸乳球菌乳亚种(双乙酰型)(L.lactis subsp.lactisbiovar diacetylactis)、乳脂乳球菌(L.cremori) |
| 丙酸杆菌属(Propionibacterium) | 费氏丙酸杆菌谢氏亚种(P.freudenreichii subsp.shermanii) |
| 丙酸菌属(Acidipropionibacterium) | 产丙酸丙酸菌(A.acidipropionici) |
| 明串珠菌属(Leuconostoc) | 肠膜明串珠菌肠膜亚种(L.mesenteroides subsp.mesenteroides) |
| 片球菌属(Pediococcus) | 乳酸片球菌(P.acidilactici)、戊糖片球菌(P.pentosaceus) |
| 魏茨曼氏菌属(Weizmannia) | 凝结魏茨曼氏菌(W.coagulans) |
| 动物球菌属(Mammaliicoccus) | 小牛动物球菌(M.vitulinus) |
| 葡萄球菌属(Staphylococcus) | 木糖葡萄球菌(S.xylosus)、肉葡萄球菌(S.carnosus) |
| 克鲁维酵母属(Kluyveromyces) | 马克斯克鲁维酵母(K.marxianus) |
Table 3 List of probiotic strains approved by countries for use in food
| 菌属 | 菌种 |
|---|---|
| 双歧杆菌属(Bifidobacterium) | 青春双歧杆菌(B. adolescentis)、动物双歧杆菌动物亚种(B.animalis subsp. animalis)、动物双歧杆菌乳亚种(B.animalis subsp. lactis)、两歧双歧杆菌(B.bifidum)、长双歧杆菌长亚种(B.longum subsp. longum)、长双歧杆菌婴儿亚种(B.longum subsp. infantis)、短双歧杆菌(B.breve) |
| 乳杆菌属(Lactobacillus) | 嗜酸乳杆菌(L.acidophilus)、卷曲乳杆菌(L.crispatus)、德氏乳杆菌保加利亚亚种(L.delbrueckii subsp. bulgaricus)、德氏乳杆菌乳亚种(L.delbrueckii subsp. lactis)、格氏乳杆菌(L.gasseri)、瑞士乳杆菌(L.helveticus)、约氏乳杆菌(L.johnsonii)、马乳酒样乳杆菌马乳酒样亚种(L.kefiranofaciens subsp. kefiranofaciens) |
| 乳酪杆菌属(Lacticaseibacillus) | 干酪乳酪杆菌(L.casei)、副干酪乳酪杆菌(L.paracasei)、鼠李糖乳酪杆菌(L.rhamnosus) |
| 黏液乳杆菌属(Limosilactobacillus) | 发酵黏液乳杆菌(L.fermentum)、罗伊氏黏液乳杆菌(L.reuteri) |
| 乳植杆菌属(Lactiplantibacillus) | 植物乳植杆菌(L.plantarum) |
| 联合乳杆菌属(Ligilactobacillus) | 唾液联合乳杆菌(L.salivarius) |
| 广布乳杆菌属(Latilactobacillus) | 弯曲广布乳杆菌(L.curvatus)、清酒广布乳杆菌(L.sakei) |
| 链球菌属(Streptococcus) | 唾液链球菌嗜热亚种(S.salivarius subsp.thermophilus) |
| 乳球菌属(Lactococcus) | 乳酸乳球菌乳亚种(L.lactis subsp.lactis)、乳酸乳球菌乳亚种(双乙酰型)(L.lactis subsp.lactisbiovar diacetylactis)、乳脂乳球菌(L.cremori) |
| 丙酸杆菌属(Propionibacterium) | 费氏丙酸杆菌谢氏亚种(P.freudenreichii subsp.shermanii) |
| 丙酸菌属(Acidipropionibacterium) | 产丙酸丙酸菌(A.acidipropionici) |
| 明串珠菌属(Leuconostoc) | 肠膜明串珠菌肠膜亚种(L.mesenteroides subsp.mesenteroides) |
| 片球菌属(Pediococcus) | 乳酸片球菌(P.acidilactici)、戊糖片球菌(P.pentosaceus) |
| 魏茨曼氏菌属(Weizmannia) | 凝结魏茨曼氏菌(W.coagulans) |
| 动物球菌属(Mammaliicoccus) | 小牛动物球菌(M.vitulinus) |
| 葡萄球菌属(Staphylococcus) | 木糖葡萄球菌(S.xylosus)、肉葡萄球菌(S.carnosus) |
| 克鲁维酵母属(Kluyveromyces) | 马克斯克鲁维酵母(K.marxianus) |
| 菌种 | 拉丁名 |
|---|---|
| 双歧双歧杆菌 | B.bifidum |
| 婴儿双歧杆菌 | B.infantis |
| 长双歧杆菌 | B.longum |
| 短双歧杆菌 | B.breve |
| 青春双歧杆菌 | B.adolescentis |
| 德氏乳杆菌保加利亚种 | Lactobacillusdelbrueckii subsp. bulgaricus |
| 嗜酸乳杆菌 | L.acidophilus |
| 干酪乳杆菌干酪亚种 | L.casei subsp. casei |
| 嗜热链球菌 | Streptococcusthermophilus |
| 罗伊氏乳杆菌 | Lactobacillusreuteri |
Table 4 List of probiotic strains approved by countries for use in health foods
| 菌种 | 拉丁名 |
|---|---|
| 双歧双歧杆菌 | B.bifidum |
| 婴儿双歧杆菌 | B.infantis |
| 长双歧杆菌 | B.longum |
| 短双歧杆菌 | B.breve |
| 青春双歧杆菌 | B.adolescentis |
| 德氏乳杆菌保加利亚种 | Lactobacillusdelbrueckii subsp. bulgaricus |
| 嗜酸乳杆菌 | L.acidophilus |
| 干酪乳杆菌干酪亚种 | L.casei subsp. casei |
| 嗜热链球菌 | Streptococcusthermophilus |
| 罗伊氏乳杆菌 | Lactobacillusreuteri |
| 菌种 | 肿瘤类型 | 研究结果 | 参考文献 |
|---|---|---|---|
| 副干酪乳杆菌SD1、LGG SD11 | 结直肠癌 | IL-1β、TNF-α、IL-6、IL-8、IL-17A↓,IL-10、IL-12↑,乙酸、丙酸、丁酸↑ | [ |
| 鼠李糖乳杆菌Probio-M9 | 肝癌 | 总生存期、客观缓解率、疾病控制率、手术转化率及无进展生存期↑ | [ |
| 海氏肠球菌、肠巴氏杆菌 | 肺癌 | 无进展生存时间、总生存时间↑ | [ |
| 双歧杆菌 | 肝癌 | D-乳酸、降钙素原、二胺氧化镁↑ | [ |
| 结直肠癌 | CA19-9、CA72-4↓,卡氏功能状态评分↑,胃肠道不良反应发生率↓ | [ | |
| 胃癌 | Hp根除率↑,IL-6、hs-CRP、TNF-α↓,DL、MIVP、IMSP↑ | [ | |
| 食管癌 | CD4+、CD4+/CD8+↑,NKG2A↓,NKG2D↑,D⁃乳酸↓,二胺氧化酶↑ | [ | |
| 乳腺癌 | CD4+、CD4+/CD8+↑,CD8+↓,CRP、IL-6、TNF-α↓ | [ | |
| 宫颈癌 | CD4+、CD3+、CD4+/CD8+↑,CD8+↓,IL-6、IL-1β、TNF-α↓ | [ |
Table 5 Research progress on probiotics as an adjunctive treatment for malignant tumors in clinical studies
| 菌种 | 肿瘤类型 | 研究结果 | 参考文献 |
|---|---|---|---|
| 副干酪乳杆菌SD1、LGG SD11 | 结直肠癌 | IL-1β、TNF-α、IL-6、IL-8、IL-17A↓,IL-10、IL-12↑,乙酸、丙酸、丁酸↑ | [ |
| 鼠李糖乳杆菌Probio-M9 | 肝癌 | 总生存期、客观缓解率、疾病控制率、手术转化率及无进展生存期↑ | [ |
| 海氏肠球菌、肠巴氏杆菌 | 肺癌 | 无进展生存时间、总生存时间↑ | [ |
| 双歧杆菌 | 肝癌 | D-乳酸、降钙素原、二胺氧化镁↑ | [ |
| 结直肠癌 | CA19-9、CA72-4↓,卡氏功能状态评分↑,胃肠道不良反应发生率↓ | [ | |
| 胃癌 | Hp根除率↑,IL-6、hs-CRP、TNF-α↓,DL、MIVP、IMSP↑ | [ | |
| 食管癌 | CD4+、CD4+/CD8+↑,NKG2A↓,NKG2D↑,D⁃乳酸↓,二胺氧化酶↑ | [ | |
| 乳腺癌 | CD4+、CD4+/CD8+↑,CD8+↓,CRP、IL-6、TNF-α↓ | [ | |
| 宫颈癌 | CD4+、CD3+、CD4+/CD8+↑,CD8+↓,IL-6、IL-1β、TNF-α↓ | [ |
| [1] | 赵文静, 尹周一, 王裕新, 等. 2024美国癌症统计报告解读及中美癌症流行情况对比[J]. 肿瘤防治研究, 2024, 51(8): 630-641. |
| ZHAO W J, YIN Z Y, WANG Y X, et al. Interpretation on cancer statistics, 2024 and comparison of cancer prevalence between China and America[J]. Cancer Research on Prevention and Treatment, 2024, 51(8): 630-641. | |
| [2] | World Health Organization. WHO report on cancer: setting priorities, investing wisely and providing care for all[R/OL]. (2020-02-03)[2024-12-01]. . |
| [3] | MAROOF H, HASSAN Z M, MOBAREZ A M, et al. Lactobacillus acidophilus could modulate the immune response against breast cancer in murine model[J]. Journal of Clinical Immunology, 2012, 32(6): 1353-1359. |
| [4] | HASSAN Z. Anti-cancer and biotherapeutic potentials of probiotic bacteria[J]. Journal of Cancer Science & Therapy, 2019, 11(1): 9-13. |
| [5] | GÓRSKA A, PRZYSTUPSKI D, NIEMCZURA M J, et al. Probiotic bacteria: a promising tool in cancer prevention and therapy[J]. Current Microbiology, 2019, 76(8): 939-949. |
| [6] | TRUFFI M, SORRENTINO L, CORSI F. Fibroblasts in the tumor microenvironment[M/OL]//Advances in experimental medicine and biology: tumor microenvironment. Cham: Springer International Publishing, 2020: 15-29. (2020-02-11)[2024-12-01]. . |
| [7] | PETROVA V, ANNICCHIARICO-PETRUZZELLI M, MELINO G, et al. The hypoxic tumour microenvironment[J]. Oncogenesis, 2018, 7: 10. |
| [8] | ANDERSEN M H. Tumor microenvironment antigens[J]. Seminars in Immunopathology, 2023, 45(2): 253-264. |
| [9] | O’TOOLE P W, JEFFERY I B. Gut microbiota and aging[J]. Science, 2015, 350(6265): 1214-1215. |
| [10] | KESEN M A, AIYEGORO O A. Beneficial characteristics and evaluation criteria of probiotics[J]. International Journal of Food and Bioscience, 2018, 1(1): 19-26. |
| [11] | JAVID H, KARIMI-SHAHRI M, KHORRAMDEL M, et al. Probiotics as an adjuvant for management of gastrointestinal cancers through their anti-inflammatory effects: a mechanistic review[J]. Current Medicinal Chemistry, 2023, 30(4): 390-406. |
| [12] | MATTILA-SANDHOLM T, MYLLÄRINEN P, CRITTENDEN R, et al. Technological challenges for future probiotic foods[J]. International Dairy Journal, 2002, 12(2/3): 173-182. |
| [13] | CHEN X H, LIU X M, TIAN F W, et al. Antagonistic activities of lactobacilli against Helicobacter pylori growth and infection in human gastric epithelial cells[J]. Journal of Food Science, 2012, 77(1): M9-M14. |
| [14] | LI J, SUNG C Y J, LEE N, et al. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(9): E1306-E1315. |
| [15] | MAKRAS L, TRIANTAFYLLOU V, FAYOL-MESSAOUDI D, et al. Kinetic analysis of the antibacterial activity of probiotic lactobacilli towards Salmonella enterica serovar Typhimurium reveals a role for lactic acid and other inhibitory compounds[J]. Research in Microbiology, 2006, 157(3): 241-247. |
| [16] | MAFE A N, IRUOGHENE EDO G, AKPOGHELIE P O, et al. Probiotics and food bioactives: unraveling their impact on gut microbiome, inflammation, and metabolic health[J/OL]. Probiotics and Antimicrobial Proteins, 2025. (2025-01-14)[2025-02-01]. . |
| [17] | ROWLAND I, GIBSON G, HEINKEN A, et al. Gut microbiota functions: metabolism of nutrients and other food components[J]. European Journal of Nutrition, 2018, 57(1): 1-24. |
| [18] | ROY S, TRINCHIERI G. Microbiota: a key orchestrator of cancer therapy[J]. Nature Reviews Cancer, 2017, 17(5): 271-285. |
| [19] | LE LEU R K, BROWN I L, HU Y, et al. A synbiotic combination of resistant starch and Bifidobacterium lactis facilitates apoptotic deletion of carcinogen-damaged cells in rat colon[J]. The Journal of Nutrition, 2005, 135(5): 996-1001. |
| [20] | URIBE-HERRANZ M, BITTINGER K, RAFAIL S, et al. Gut microbiota modulates adoptive cell therapy via CD8α dendritic cells and IL-12[J]. JCI Insight, 2018, 3(4): e94952. |
| [21] | NGUYEN C L, DOCAMPO M D, VAN DEN BRINK M R M, et al. The role of the intestinal microbiota in allogeneic HCT: clinical associations and preclinical mechanisms[J]. Current Opinion in Genetics & Development, 2021, 66: 25-35. |
| [22] | KHOSRAVI-DARANI K, BARZEGAR F, BAGHDADI M. Detoxification of heterocyclic aromatic amines by probiotic to inhibit medical hazards[J]. Mini Reviews in Medicinal Chemistry, 2019, 19(15): 1196-1203. |
| [23] | BLAKE S J, WOLF Y, BOURSI B, et al. Role of the microbiota in response to and recovery from cancer therapy[J]. Nature Reviews Immunology, 2024, 24(5): 308-325. |
| [24] | KWON S Y, THI-THU NGO H, SON J, et al. Exploiting bacteria for cancer immunotherapy[J]. Nature Reviews Clinical Oncology, 2024, 21(8): 569-589. |
| [25] | GOLDIN B R, GORBACH S L. The relationship between diet and rat fecal bacterial enzymes implicated in colon cancer[J]. Journal of the National Cancer Institute, 1976, 57(2): 371-375. |
| [26] | GOLDIN B R, SWENSON L, DWYER J, et al. Effect of diet and Lactobacillus acidophilus supplements on human fecal bacterial enzymes[J]. Journal of the National Cancer Institute, 1980, 64(2): 255-261. |
| [27] | MUGHINI-GRAS L, SCHAAPVELD M, KRAMERS J, et al. Increased colon cancer risk after severe Salmonella infection[J]. PLoS One, 2018, 13(1): e0189721. |
| [28] | ZHA L, GARRETT S, SUN J. Salmonella infection in chronic inflammation and gastrointestinal cancer[J]. Diseases, 2019, 7(1): 28. |
| [29] | VAN ELSLAND D M, DUIJSTER J W, ZHANG J L, et al. Repetitive non-typhoidal Salmonella exposure is an environmental risk factor for colon cancer and tumor growth[J]. Cell Reports Medicine, 2022, 3(12): 100852. |
| [30] | BUDDHASIRI S, SUKJOI C, KAEWSAKHORN T, et al. Anti-inflammatory effect of probiotic Limosilactobacillus reuteri KUB-AC5 against Salmonella infection in a mouse colitis model[J]. Frontiers in Microbiology, 2021, 12: 716761. |
| [31] | CHEN M J, CHEN C C, HUANG Y C, et al. The efficacy of Lactobacillus acidophilus and rhamnosus in the reduction of bacterial load of Helicobacter pylori and modification of gut microbiota: a double-blind, placebo-controlled, randomized trial[J]. Helicobacter, 2021, 26(6): e12857. |
| [32] | BEDADA T L, FETO T K, AWOKE K S, et al. Probiotics for cancer alternative prevention and treatment[J]. Biomedicine & Pharmacotherapy, 2020, 129: 110409. |
| [33] | VERMA A, SHUKLA G. Synbiotic (Lactobacillus rhamnosus+Lactobacillus acidophilus+inulin) attenuates oxidative stress and colonic damage in 1,2-dimethylhydrazine dihydrochloride-induced colon carcinogenesis in Sprague-Dawley rats: a long-term study[J]. European Journal of Cancer Prevention, 2014, 23(6): 550-559. |
| [34] | ZHOU T Y, WU J Y, KHAN A, et al. A probiotic Limosilactobacillus fermentum GR-3 mitigates colitis-associated tumorigenesis in mice via modulating gut microbiome[J]. NPJ Science of Food, 2024, 8: 61. |
| [35] | GRIVENNIKOV S I, GRETEN F R, KARIN M. Immunity, inflammation, and cancer[J]. Cell, 2010, 140(6): 883-899. |
| [36] | MUNN L L. Cancer and inflammation[J]. Wiley Interdisciplinary Reviews: Systems Biology and Medicine, 2017, 9(2): e1370. |
| [37] | KORNILUK A, KOPER O, KEMONA H, et al. From inflammation to cancer[J]. Irish Journal of Medical Science, 2017, 186(1): 57-62. |
| [38] | RITTER B, GRETEN F R. Modulating inflammation for cancer therapy[J]. Journal of Experimental Medicine, 2019, 216(6): 1234-1243. |
| [39] | JENSEN M D, JEPSEN P, VILSTRUP H, et al. Increased cancer risk in autoimmune hepatitis: a Danish nationwide cohort study[J]. The American Journal of Gastroenterology, 2022, 117(1): 129-137. |
| [40] | LIU Q Q, TIAN H X, KANG Y B, et al. Probiotics alleviate autoimmune hepatitis in mice through modulation of gut microbiota and intestinal permeability[J]. The Journal of Nutritional Biochemistry, 2021, 98: 108863. |
| [41] | RABBENOU W, ULLMAN T A. Risk of colon cancer and recommended surveillance strategies in patients with ulcerative colitis[J]. Gastroenterology Clinics of North America, 2020, 49(4): 791-807. |
| [42] | BERTKOVA I, HIJOVA E, CHMELAROVA A, et al. The effect of probiotic microorganisms and bioactive compounds on chemically induced carcinogenesis in rats[J]. Neoplasma, 2010, 57(5): 422-428. |
| [43] | LI Q X, LI Y K, WANG Y F, et al. Oral administration of Bifidobacterium breve promotes antitumor efficacy via dendritic cells-derived interleukin 12[J]. OncoImmunology, 2021, 10(1): 1868122. |
| [44] | WANG T, WANG P P, GE W P, et al. The probiotic Companilactobacillus crustorum MN047 alleviates colitis-associated tumorigenesis via modulating the intestinal microenvironment[J]. Food & Function, 2021, 12(22): 11331-11342. |
| [45] | YUE Y C, YE K, LU J, et al. Probiotic strain Lactobacillus plantarum YYC-3 prevents colon cancer in mice by regulating the tumour microenvironment[J]. Biomedicine & Pharmacotherapy, 2020, 127: 110159. |
| [46] | YANG X J, CAO Q, MA B, et al. Probiotic powder ameliorates colorectal cancer by regulating Bifidobacterium animalis, Clostridium cocleatum, and immune cell composition[J]. PLoS One, 2023, 18(3): e0277155. |
| [47] | DELEU S, MACHIELS K, RAES J, et al. Short chain fatty acids and its producing organisms: an overlooked therapy for IBD?[J]. eBioMedicine, 2021, 66: 103293. |
| [48] | FUSCO W, LORENZO M B, CINTONI M, et al. Short-chain fatty-acid-producing bacteria: key components of the human gut microbiota[J]. Nutrients, 2023, 15(9): 2211. |
| [49] | ZENG H W, LAZAROVA D L, BORDONARO M. Mechanisms linking dietary fiber, gut microbiota and colon cancer prevention[J]. World Journal of Gastrointestinal Oncology, 2014, 6(2): 41-51. |
| [50] | PERRIN P, PIERRE F, PATRY Y, et al. Only fibres promoting a stable butyrate producing colonic ecosystem decrease the rate of aberrant crypt foci in rats[J]. Gut, 2001, 48(1): 53-61. |
| [51] | REDDY B S, HIROSE Y, COHEN L A, et al. Preventive potential of wheat bran fractions against experimental colon carcinogenesis: implications for human colon cancer prevention[J]. Cancer Research, 2000, 60(17): 4792-4797. |
| [52] | MAGLIOCCA G, MONE P, DI IORIO B R, et al. Short-chain fatty acids in chronic kidney disease: focus on inflammation and oxidative stress regulation[J]. International Journal of Molecular Sciences, 2022, 23(10): 5354. |
| [53] | SCHEPERJANS F, AHO V, PEREIRA P A B, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype[J]. Movement Disorders, 2015, 30(3): 350-358. |
| [54] | DEN BESTEN G, VAN EUNEN K, GROEN A K, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism[J]. Journal of Lipid Research, 2013, 54(9): 2325-2340. |
| [55] | RUSSELL W R, GRATZ S W, DUNCAN S H, et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health[J]. The American Journal of Clinical Nutrition, 2011, 93(5): 1062-1072. |
| [56] | MAFE A N, BÜSSELBERG D. Modulation of the neuro-cancer connection by metabolites of gut microbiota[J]. Biomolecules, 2025, 15(2): 270. |
| [57] | MIRZAEI H, GHORBANI S, KHANIZADEH S, et al. Histone deacetylases in virus-associated cancers[J]. Reviews in Medical Virology, 2020, 30(1): e2085. |
| [58] | HAI R H, HE L E, SHU G, et al. Characterization of histone deacetylase mechanisms in cancer development[J]. Frontiers in Oncology, 2021, 11: 700947. |
| [59] | CHENG B B, PAN W, XIAO Y, et al. HDAC-targeting epigenetic modulators for cancer immunotherapy[J]. European Journal of Medicinal Chemistry, 2024, 265: 116129. |
| [60] | CAO M M, ZHANG Z R, HAN S, et al. Butyrate inhibits the proliferation and induces the apoptosis of colorectal cancer HCT116 cells via the deactivation of mTOR/S6K1 signaling mediated partly by SIRT1 downregulation[J]. Molecular Medicine Reports, 2019, 19(5): 3941-3947. |
| [61] | ZHOU Z H, ZHENG J R, LU Y, et al. Optimizing CD8+ T cell-based immunotherapy via metabolic interventions: a comprehensive review of intrinsic and extrinsic modulators[J]. Experimental Hematology & Oncology, 2024, 13(1): 103. |
| [62] | LUU M, RIESTER Z, BALDRICH A, et al. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer[J]. Nature Communications, 2021, 12: 4077. |
| [63] | BISHOP K S, XU H W, MARLOW G. Epigenetic regulation of gene expression induced by butyrate in colorectal cancer: involvement of microRNA[J]. Genetics & Epigenetics, 2017, 9: 1179237X17729900. |
| [64] | KLICKA K, GRZYWA T M, MIELNICZUK A, et al. The role of miR-200 family in the regulation of hallmarks of cancer[J]. Frontiers in Oncology, 2022, 12: 965231. |
| [65] | XIE H, WU Z Y, LI Z H, et al. Significance of ZEB2 in the immune microenvironment of colon cancer[J]. Frontiers in Genetics, 2022, 13: 995333. |
| [66] | STEELE J C, TORR E E, NOAKES K L, et al. The polycomb group proteins, BMI-1 and EZH2, are tumour-associated antigens[J]. British Journal of Cancer, 2006, 95(9): 1202-1211. |
| [67] | ONCEL S, SAFRATOWICH B D, LINDLAUF J E, et al. Efficacy of butyrate to inhibit colonic cancer cell growth is cell type-specific and apoptosis-dependent[J]. Nutrients, 2024, 16(4): 529. |
| [68] | FAES S, DORMOND O. PI3K and AKT: unfaithful partners in cancer[J]. International Journal of Molecular Sciences, 2015, 16(9): 21138-21152. |
| [69] | JIANG N N, DAI Q J, SU X R, et al. Role of PI3K/AKT pathway in cancer: the framework of malignant behavior[J]. Molecular Biology Reports, 2020, 47(6): 4587-4629. |
| [70] | ALIPOUR S, ABDOLALIZADEH M M, AMIRKHIZ M B, et al. Effects of probiotic bacteria Lactobacillus rhamnosus on SKT-PI3K signaling pathways and PTEN gene expression in CAOV-4 ovarian cancer cells Lactobacillus rhamnosus effects on ovarian cancer[J]. Cancer Plus, 2023, 5(4): 1968. |
| [71] | WANG L, LI S, FAN H L, et al. Bifidobacterium lactis combined with Lactobacillus plantarum inhibit glioma growth in mice through modulating PI3K/AKT pathway and gut microbiota[J]. Frontiers in Microbiology, 2022, 13: 986837. |
| [72] | SUN L L, TIAN W L, GUO X J, et al. Lactobacillus gasseri JM1 with potential probiotic characteristics alleviates inflammatory response by activating the PI3K/AKT signaling pathway in vitro [J]. Journal of Dairy Science, 2020, 103(9): 7851-7864. |
| [73] | SHI Y Q, MENG L Y, ZHANG C L, et al. Extracellular vesicles of Lacticaseibacillus paracasei PC-H1 induce colorectal cancer cells apoptosis via PDK1/AKT/Bcl-2 signaling pathway[J]. Microbiological Research, 2022, 255: 126921. |
| [74] | DONG Y Y, ZHU J, ZHANG M, et al. Probiotic Lactobacillus salivarius Ren prevent dimethylhydrazine-induced colorectal cancer through protein kinase B inhibition[J]. Applied Microbiology and Biotechnology, 2020, 104(17): 7377-7389. |
| [75] | MOHSENI A H, CASOLARO V, BERMÚDEZ-HUMARÁN L G, et al. Modulation of the PI3K/AKT/mTOR signaling pathway by probiotics as a fruitful target for orchestrating the immune response[J]. Gut Microbes, 2021, 13(1): 1886844. |
| [76] | WANG Z X, CHEN H Q, XIONG S S, et al. Lactobacillus plantarum SMUM211204 exopolysaccharides have tumor-suppressive effects on colorectal cancer by regulating autophagy via the mTOR pathway[J]. Journal of Agricultural and Food Chemistry, 2025, 73(10): 5931-5946. |
| [77] | JIA D, KUANG Z, WANG L J. The role of microbial indole metabolites in tumor[J]. Gut Microbes, 2024, 16(1): 2409209. |
| [78] | TINTELNOT J, XU Y, LESKER T R, et al. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer[J]. Nature, 2023, 615(7950): 168-174. |
| [79] | ZHU Q C, ZHANG G H, CAO M, et al. Microbiota-shaped neutrophil senescence regulates sexual dimorphism in bladder cancer[J]. Nature Immunology, 2025: 1-15. |
| [80] | NOZARI S, FARIDVAND Y, ETESAMI A, et al. Potential anticancer effects of cell wall protein fractions from Lactobacillus paracasei on human intestinal Caco‐2 cell line[J]. Letters in Applied Microbiology, 2019, 69(3): 148-154. |
| [81] | KANG X, LIU C G, DING Y Q, et al. Roseburia intestinalis generated butyrate boosts anti-PD-1 efficacy in colorectal cancer by activating cytotoxic CD8+ T cells[J]. Gut, 2023, 72(11): 2112-2122. |
| [82] | KAŹMIERCZAK-SIEDLECKA K, MARANO L, MEROLA E, et al. Sodium butyrate in both prevention and supportive treatment of colorectal cancer[J]. Frontiers in Cellular and Infection Microbiology, 2022, 12: 1023806. |
| [83] | 陈家文, 黄建东, 孙海涛. 工程菌在肿瘤治疗方面的应用进展[J]. 合成生物学, 2023, 4(4): 690-702. |
| CHEN J W, HUANG J D, SUN H T. Current developments in the use of engineered bacteria for cancer therapy[J]. Synthetic Biology Journal, 2023, 4(4): 690-702. | |
| [84] | ENGEVIK M A, LUK B, CHANG-GRAHAM A L, et al. Bifidobacterium dentium fortifies the intestinal mucus layer via autophagy and calcium signaling pathways[J]. mBio, 2019, 10(3): e01087-19. |
| [85] | WANG X L, HUANG Y X, YANG Z, et al. Effect of probiotics combined with immune checkpoint suppressors and chemotherapeutic agents on digestive system function, intestinal immunity and prognosis in patients with metastatic colorectal carcinoma: a quasi-experimental study[J]. BMC Gastroenterology, 2025, 25(1): 38. |
| [86] | WANITSUWAN W, PAHUMUNTO N, SURACHAT K, et al. Comparison of the effects of postbiotics and live-probiotics containing Lacticaseibacillus paracasei SD1 and Lacticaseibacillus rhamnosus SD11 in patients with previous colorectal cancer: a randomized controlled trial[J]. Journal of Functional Foods, 2024, 123: 106576. |
| [87] | LÜ Z Y, ZHANG Y, SHENG C, et al. Global burden of thyroid cancer in 2022: incidence and mortality estimates from GLOBOCAN[J]. Chinese Medical Journal, 2024, 137(21): 2567-2576. |
| [88] | SENCHUKOVA M A. Helicobacter pylori and gastric cancer progression[J]. Current Microbiology, 2022, 79(12): 383. |
| [89] | ABEDI A, TAFVIZI F, AKBARI N, et al. Cell-free supernatant of L. buchneri probiotic bacteria enhancing apoptosis activity in AGS gastric cancer cells[J]. Iranian Journal of Science, 2023, 47(4): 1071-1079. |
| [90] | YU X, OU J Z, WANG L Z, et al. Gut microbiota modulate CD8+ T cell immunity in gastric cancer through butyrate/GPR109A/HOPX[J]. Gut Microbes, 2024, 16(1): 2307542. |
| [91] | HAN Z H, CHENG S Y, DAI D, et al. The gut microbiome affects response of treatments in HER2-negative advanced gastric cancer[J]. Clinical and Translational Medicine, 2023, 13(7): e1312. |
| [92] | ANTONY J V M, RAMANI P, RAMASUBRAMANIAN A, et al. Particle size, penetration rate and effects of smoke and smokeless tobacco products-an in vitro analysis[J]. Heliyon, 2021, 7(3): e06455. |
| [93] | CHENG Z, XU H, WANG X P, et al. Lactobacillus raises in vitro anticancer effect of geniposide in HSC-3 human oral squamous cell carcinoma cells[J]. Experimental and Therapeutic Medicine, 2017, 14(5): 4586-4594. |
| [94] | ASOUDEH-FARD A, BARZEGARI A, DEHNAD A, et al. Lactobacillus plantarum induces apoptosis in oral cancer KB cells through upregulation of PTEN and downregulation of MAPK signalling pathways[J]. BioImpacts, 2017, 7(3): 193-198. |
| [95] | ZADEH V F, SOLEIMANI N A, KHOSRAVI A, et al. Investigating the effects of Lactobacillus plantarum strain ATCC 8014 on gene expression of NF-κB, TLR-4, and BCL-2 in oral rat cancer induced by 4-nitroquioline 1-oxide[J]. Jorjani Biomedicine Journal, 2022, 10(4): 12-20. |
| [96] | 李照, 朱继业. 《原发性肝癌诊疗指南(2024年版)》解读[J]. 临床肝胆病杂志, 2024, 40(7): 1324-1327. |
| LI Z, ZHU J Y. Interpretation of guidelines for the diagnosis and treatment of primary liver cancer (2024 edition)[J]. Journal of Clinical Hepatology, 2024, 40(7): 1324-1327. | |
| [97] | HU C P, XU B Q, WANG X D, et al. Gut microbiota-derived short-chain fatty acids regulate group 3 innate lymphoid cells in HCC[J]. Hepatology, 2023, 77(1): 48-64. |
| [98] | SRIKHAM K, DAENGPROK W, NIAMSUP P, et al. Characterization of Streptococcus salivarius as new probiotics derived from human breast milk and their potential on proliferative inhibition of liver and breast cancer cells and antioxidant activity[J]. Frontiers in Microbiology, 2021, 12: 797445. |
| [99] | WU Y J, CHENG G Y, CHEN H, et al. IL-17 predicts the effect of TACE combined with apatinib in hepatocellular carcinoma[J]. Clinical Hemorheology and Microcirculation, 2021, 77(1): 37-47. |
| [100] | WANG W, WANG Z, QIN Y, et al. Th17, synchronically increased with Tregs and Bregs, promoted by tumour cells via cell-contact in primary hepatic carcinoma[J]. Clinical & Experimental Immunology, 2018, 192(2): 181-192. |
| [101] | KHEDR O M S, EL-SONBATY S M, MOAWED F S M, et al. Lactobacillus acidophilus ATCC 4356 exopolysaccharides suppresses mediators of inflammation through the inhibition of TLR2/STAT-3/P38-MAPK pathway in DEN-induced hepatocarcinogenesis in rats[J]. Nutrition and Cancer, 2022, 74(3): 1037-1047. |
| [102] | 陈野. 益生菌联合PD-1抑制剂在治疗不可切除肝癌中的有效性及安全性分析[D]. 南昌: 南昌大学, 2023. |
| CHEN Y. Efficacy and safety of probiotics combined with PD-1 inhibitor in the treatment of unresectable hepatocellular carcinoma[D]. Nanchang: Nanchang University, 2023 | |
| [103] | CASTLEP E, EINSTEINM H, SAHASRABUDDHEV V. Cervical cancer prevention and control in women living with human immunodeficiency virus[J]. CA: A Cancer Journal for Clinicians, 2021, 71(6): 505-526. |
| [104] | COHEN P A, JHINGRAN A, OAKNIN A, et al. Cervical cancer[J]. The Lancet, 2019, 393(10167): 169-182. |
| [105] | ASHIQUE S, FARUK A, AHMAD F J, et al. It is all about probiotics to control cervical cancer[J]. Probiotics and Antimicrobial Proteins, 2024, 16(3): 979-992. |
| [106] | EMEREONYE C F, ARELOEGBE S E, OLADELE C A, et al. The role of cervicovaginal microbiome in the pathogenesis of cervical cancer[J]. Indian Journal of Gynecologic Oncology, 2025, 23(1): 32. |
| [107] | LIU Y J, ZHAO X M, WU F, et al. Effectiveness of vaginal probiotics Lactobacillus crispatus Chen-01 in women with high-risk HPV infection: a prospective controlled pilot study[J]. Aging, 2024, 16(14): 11446-11459. |
| [108] | RIAZ RAJOKA M S, ZHAO H B, LU Y, et al. Anticancer potential against cervix cancer (HeLa) cell line of probiotic Lactobacillus casei and Lactobacillus paracasei strains isolated from human breast milk[J]. Food & Function, 2018, 9(5): 2705-2715. |
| [109] | SUNGUR T, ASLIM B, KARAASLAN C, et al. Impact of Exopolysaccharides (EPSs) of Lactobacillus gasseri strains isolated from human vagina on cervical tumor cells (HeLa)[J]. Anaerobe, 2017, 47: 137-144. |
| [110] | JAHANSHAHI M, MALEKI DANA P, BADEHNOOSH B, et al. Anti-tumor activities of probiotics in cervical cancer[J]. Journal of Ovarian Research, 2020, 13(1): 68. |
| [111] | ABDOLALIPOUR E, MAHOOTI M, GORJI A, et al. Synergistic therapeutic effects of probiotic Lactobacillus casei TD-2 consumption on GM-CSF-induced immune responses in a murine model of cervical cancer[J]. Nutrition and Cancer, 2022, 74(1): 372-382. |
| [112] | 曹毛毛, 陈万青. GLOBOCAN 2020全球癌症统计数据解读[J]. 中国医学前沿杂志(电子版), 2021, 13(3): 63-69. |
| CAO M M, CHEN W Q. Interpretation on the global cancer statistics of GLOBOCAN 2020[J]. Chinese Journal of the Frontiers of Medical Science (Electronic Version), 2021, 13(3): 63-69. | |
| [113] | ROSA L S, SANTOS M L, ABREU J P, et al. Antiproliferative and apoptotic effects of probiotic whey dairy beverages in human prostate cell lines[J]. Food Research International, 2020, 137: 109450. |
| [114] | CELEBIOGLU H U. Effects of potential synbiotic interaction between Lactobacillus rhamnosus GG and salicylic acid on human colon and prostate cancer cells[J]. Archives of Microbiology, 2021, 203(3): 1221-1229. |
| [115] | LI Y T, YAN B S, HE S M. Advances and challenges in the treatment of lung cancer[J]. Biomedicine & Pharmacotherapy, 2023, 169: 115891. |
| [116] | GUI Q F, LU H F, ZHANG C X, et al. Well-balanced commensal microbiota contributes to anti-cancer response in a lung cancer mouse model[J]. Genetics and Molecular Research, 2015, 14(2): 5642-5651. |
| [117] | DAILLÈRE R, VÉTIZOU M, WALDSCHMITT N, et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects[J]. Immunity, 2016, 45(4): 931-943. |
| [118] | TOMITA Y, IKEDA T, SAKATA S, et al. Association of probiotic Clostridium butyricum therapy with survival and response to immune checkpoint blockade in patients with lung cancer[J]. Cancer Immunology Research, 2020, 8(10): 1236-1242. |
| [119] | MILLER K D, SIEGEL R L, LIN C C, et al. Cancer treatment and survivorship statistics, 2016[J]. CA: A Cancer Journal for Clinicians, 2016, 66(4): 271-289. |
| [120] | AYYASH M, ABU-JDAYIL B, ITSARANUWAT P, et al. Characterization, bioactivities, and rheological properties of exopolysaccharide produced by novel probiotic Lactobacillus plantarum C70 isolated from camel milk[J]. International Journal of Biological Macromolecules, 2020, 144: 938-946. |
| [121] | HASSAN Z, MUSTAFA S, RAHIM R A, et al. Anti-breast cancer effects of live, heat-killed and cytoplasmic fractions of Enterococcus faecalis and Staphylococcus hominis isolated from human breast milk[J]. In Vitro Cellular & Developmental Biology - Animal, 2016, 52(3): 337-348. |
| [122] | 易永华. 益生菌对乳腺癌化疗患者免疫功能及肠道微生态稳态的干预作用[D]. 兰州: 甘肃中医药大学, 2022. |
| YI Y H. The intervention effect of probiotics on immune function and intestinal microbiome homeostasis in breast cancer chemotherapy patients[D]. Lanzhou: Gansu University of Chinese Medicine, 2022. | |
| [123] | 周文献, 谭爱花, 王洪学, 等. 益生菌在卡培他滨联合吡咯替尼治疗晚期乳腺癌中的作用探讨[J]. 河北医学, 2024, 30(11): 1918-1924. |
| ZHOU W X, TAN A H, WANG H X, et al. The effect of probiotics on the efficacy and adverse reactions of breast cancer chemotherapy combined with targeted therapy[J]. Hebei Medicine, 2024, 30(11): 1918-1924. | |
| [124] | WANG A P, LING Z X, YANG Z X, et al. Gut microbial dysbiosis may predict diarrhea and fatigue in patients undergoing pelvic cancer radiotherapy: a pilot study[J]. PLoS One, 2015, 10(5): e0126312. |
| [125] | GREGORY T A, WEATHERS S P. The microbiome and central nervous system tumors[J]. Advances in Oncology, 2023, 3(1): 97-105. |
| [126] | THU M S, ONDEE T, NOPSOPON T, et al. Effect of probiotics in breast cancer: a systematic review and meta-analysis[J]. Biology, 2023, 12(2): 280. |
| [127] | GARCZYK A, KALICIAK I, DROGOWSKI K, et al. Influence of probiotics in prevention and treatment of patients who undergo chemotherapy or/and radiotherapy and suffer from mucositis, diarrhoea, constipation, nausea and vomiting[J]. Journal of Clinical Medicine, 2022, 11(12): 3412. |
| [128] | XIA C F, JIANG C L, LI W Y, et al. A phase Ⅱ randomized clinical trial and mechanistic studies using improved probiotics to prevent oral mucositis induced by concurrent radiotherapy and chemotherapy in nasopharyngeal carcinoma[J]. Frontiers in Immunology, 2021, 12: 618150. |
| [129] | 冯柳, 蒋春灵, 李道靖, 等. 益生菌对头颈部肿瘤患者放化疗引起的口腔黏膜炎的作用分析[J]. 肿瘤学杂志, 2021, 27(2): 136-141. |
| FENG L, JIANG C L, LI D J, et al. Efficacy of probiotics for radiotherapy/chemotherapy-induced oral mucositis in patients with head and neck cancer[J]. Journal of Chinese Oncology, 2021, 27(2): 136-141. | |
| [130] | LINN Y H, THU K K, WIN N H H. Effect of probiotics for the prevention of acute radiation-induced diarrhoea among cervical cancer patients: a randomized double-blind placebo-controlled study[J]. Probiotics and Antimicrobial Proteins, 2019, 11(2): 638-647. |
| [131] | MOTOORI M, YANO M, MIYATA H, et al. Randomized study of the effect of synbiotics during neoadjuvant chemotherapy on adverse events in esophageal cancer patients[J]. Clinical Nutrition, 2017, 36(1): 93-99. |
| [132] | LOBO D N, GIANOTTI L, ADIAMAH A, et al. Perioperative nutrition: recommendations from the ESPEN expert group[J]. Clinical Nutrition, 2020, 39(11): 3211-3227. |
| [133] | JIANG M Y, ZHANG X Y, ZHANG Y Q, et al. The effects of perioperative probiotics on postoperative gastrointestinal function in patients with brain tumors: a randomized, placebo-controlled study[J]. Nutrition and Cancer, 2023, 75(4): 1132-1142. |
| [134] | LIU W D, ZHENG C H, LI Q, et al. Preoperative oral probiotics relieve insulin resistance and gut dysbacteriosis in patients with gastric cancer after gastrectomy[J]. Journal of Functional Foods, 2023, 101: 105426. |
| [135] | 史新龙, 陈小婕, 李晶晶, 等. 益生菌酪酸梭菌胶囊在直肠癌患者围手术期的保护作用研究[J]. 现代消化及介入诊疗, 2020, 25(10): 1306-1310. |
| SHI X L, CHEN X J, LI J J, et al. Protective effect of probiotics Clostridium butyricum capsules in perioperative period of rectal cancer patients[J]. Modern Digestion & Intervention, 2020, 25(10): 1306-1310. | |
| [136] | 陈潇宇, 龚小波. 益生菌辅助早期肠内营养治疗腹腔镜肝癌术后胃肠功能障碍的临床疗效[C]//第五届全国医药研究论坛论文集(一). 榆林市医学会, 2024: 509-515. |
| CHEN X Y, GONG X B. Clinical efficacy of probiotics-assisted early enteral nutrition in the treatment of gastrointestinal dysfunction after laparoscopic liver cancer surgery[C]// Proceedings of the Fifth National Pharmaceutical Research Forum (Volume 1). Yulin Medical Association, 2024: 509-515. | |
| [137] | 席杨, 梁媛媛, 孙丽萍, 等. 益生菌的耐药性及其安全性的研究进展[J]. 食品安全导刊, 2024(23): 172-177, 181. |
| XI Y, LIANG Y Y, SUN L P, et al. Research progress on antibiotic resistance and safety of probiotic[J]. China Food Safety Magazine, 2024(23): 172-177, 181. | |
| [138] | 叶凡, 李琢, 黄兴, 等. 益生菌辅助化疗对晚期大肠癌患者的临床疗效和安全性评价[J]. 中国药师, 2024, 27(2): 295-301. |
| YE F, LI Z, HUANG X, et al. Clinical efficacy and safety evaluation of probiotic-assisted chemotherapy in patients with advanced colorectal cancer[J]. China Pharmacist, 2024, 27(2): 295-301. | |
| [139] | 何泉. 益生菌辅助四联方案治疗幽门螺杆菌阳性早期胃癌患者的疗效及对患者肠道菌群的影响[J]. 山西医药杂志, 2024, 53(14): 1081-1085. |
| HE Q. Efficacy of probiotic-assisted quadruple regimen in the treatment of Helicobacter pylori-positive early gastric cancer patients and its effect on intestinal flora[J]. Shanxi Medical Journal, 2024, 53(14): 1081-1085. | |
| [140] | 徐晓萌, 张盼盼, 宋佳, 等. 谷氨酰胺联合益生菌强化肠内营养对食管癌化疗患者的影响[J]. 华夏医学, 2023, 36(4): 52-56. |
| XU X M, ZHANG P P, SONG J, et al. Effects of glutamine combined with probiotics fortifying enteral nutrition on patients with esophageal cancer undergoing chemotherapy[J]. Acta Medicinae Sinica, 2023, 36(4): 52-56. | |
| [141] | 陆琼, 王洁. 肠道益生菌在人表皮生长因子受体-2阳性晚期乳腺癌治疗中对患者的腹泻反应及抗肿瘤疗效的影响[J]. 现代医学与健康研究电子杂志, 2023, 7(17): 47-49. |
| LU Q, WANG J. Effects of intestinal probiotics on diarrheal response and antitumor efficacy in patients with advanced human epidermal growth factor receptor-2 positive breast cancer[J]. Modern Medicine and Health Research Electronic Journal, 2023, 7(17): 47-49. | |
| [142] | 刘双姣, 张亚彬, 周巧巧, 等. 鸦胆子油乳注射液结合益生菌对宫颈癌术后放疗患者肠道菌群和免疫炎症的影响[J]. 环境与健康杂志, 2025, 42(3): 203-207. |
| LIU S J, ZHANG Y B, ZHOU Q Q, et al. Effects of Brucea oil emulsion injection and probiotics in combination on intestinal flora and immune inflammation in patients with cervical cancer postoperative radiotherapy[J]. Journal of Environment and Health, 2025, 42(3): 203-207. | |
| [143] | RUTTER J W, DEKKER L, OWEN K A, et al. Microbiome engineering: engineered live biotherapeutic products for treating human disease[J]. Frontiers in Bioengineering and Biotechnology, 2022, 10: 1000873. |
| [144] | MENG J, LIU S F, WU X. Engineered probiotics as live biotherapeutics for diagnosis and treatment of human diseases[J]. Critical Reviews in Microbiology, 2024, 50(3): 300-314. |
| [145] | RAEISI H, LEEFLANG J, HASAN S, et al. Bioengineered probiotics for Clostridioides difficile infection: an overview of the challenges and potential for this new treatment approach[J]. Probiotics and Antimicrobial Proteins, 2025, 17(2): 763-780. |
| [146] | SONNENBORN U. Escherichia coli strain Nissle 1917: from bench to bedside and back: history of a special Escherichia coli strain with probiotic properties[J]. FEMS Microbiology Letters, 2016, 363(19): fnw212. |
| [147] | BEHNSEN J, DERIU E, SASSONE-CORSI M, et al. Probiotics: properties, examples, and specific applications[J]. Cold Spring Harbor Perspectives in Medicine, 2013, 3(3): a010074. |
| [148] | CORRALES L, GAJEWSKI T F. Molecular pathways: targeting the stimulator of interferon genes (STING) in the immunotherapy of cancer[J]. Clinical Cancer Research, 2015, 21(21): 4774-4779. |
| [149] | LUKE J J, PIHA-PAUL S A, MEDINA T, et al. Phase Ⅰ study of SYNB1891, an engineered E. coli nissle strain expressing STING agonist, with and without atezolizumab in advanced malignancies[J]. Clinical Cancer Research, 2023, 29(13): 2435-2444. |
| [150] | 韩雨衡, 来兴欢, 乐子薇, 等. 肿瘤靶向性沙门氏菌VNP20009抗肿瘤作用及其对肿瘤免疫微环境的影响[J]. 药学学报, 2016, 51(9): 1417-1422. |
| HAN Y H, LAI X H, LE Z W, et al. Anti-tumor effect and impact on tumor immune microenvironment of tumor-targeted Salmonella VNP20009[J]. Acta Pharmaceutica Sinica, 2016, 51(9): 1417-1422. | |
| [151] | WU L Y, LI L, LI S F, et al. Macrophage-mediated tumor-targeted delivery of engineered Salmonella typhimurium VNP20009 in anti-PD1 therapy against melanoma[J]. Acta Pharmaceutica Sinica B, 2022, 12(10): 3952-3971. |
| [152] | PAN H Z, LI L Y, PANG G J, et al. Engineered NIR light-responsive bacteria as anti-tumor agent for targeted and precise cancer therapy[J]. Chemical Engineering Journal, 2021, 426: 130842. |
| [153] | XIE T Q, YAN X, QIN Y T, et al. Lactate/cysteine dual-consuming probiotic-nanomedicine biohybrid system for enhanced cancer chemo-immunotherapy[J]. Nano Letters, 2024, 24(50): 16132-16142. |
| [154] | LI W H, ZHANG Z F, LIU J, et al. Nanodrug-loaded Bifidobacterium bifidum conjugated with anti-death receptor antibody for tumor-targeted photodynamic and sonodynamic synergistic therapy[J]. Acta Biomaterialia, 2022, 146: 341-356. |
| [155] | TANG S, NING Q, YANG L, et al. Mechanisms of immune escape in the cancer immune cycle[J]. International Immunopharmacology, 2020, 86: 106700. |
| [156] | CHEN C, WANG Z H, DING Y, et al. Tumor microenvironment-mediated immune evasion in hepatocellular carcinoma[J]. Frontiers in Immunology, 2023, 14: 1133308. |
| [157] | RIEMER A B. Bacterial peptides presented on tumour cells could be immunotherapy targets[J]. Nature, 2021, 592(7852): 28-29. |
| [158] | KUMAR D, SHARMA-WALIA N, KAPOOR S, et al. Antibody-targeted nanoparticles for cancer treatment[M/OL]// SAXENA S, KHURANA S. NanoBioMedicine. Singapore: Springer Singapore, 2020: 35-65. (2020-02-04)[2024-01-01]. . |
| [159] | HARIMOTO T, HAHN J, CHEN Y Y, et al. A programmable encapsulation system improves delivery of therapeutic bacteria in mice[J]. Nature Biotechnology, 2022, 40(8): 1259-1269. |
| [160] | CAO Z P, CHENG S S, WANG X Y, et al. Camouflaging bacteria by wrapping with cell membranes[J]. Nature Communications, 2019, 10: 3452. |
| [1] | SHI Xinjie, DU Yiling. Research advances in the biosynthesis of nonribosomal peptides within the bisintercalator family as anticancer drugs [J]. Synthetic Biology Journal, 2024, 5(3): 593-611. |
| [2] | TAN Zibin, LIANG Kang, CHEN Youhai. Applications of synthetic biology in developing microbial-vectored cancer vaccines [J]. Synthetic Biology Journal, 2024, 5(2): 221-238. |
| [3] | LIN Sisi, PAN Chao, ZHANG Yifan, LIU Jinyao. Coated probiotic-based drug carriers for oral delivery of tumor antigens [J]. Synthetic Biology Journal, 2022, 3(4): 810-820. |
| [4] | GAO Mengxue, WANG Lina, HUANG He. Advances in synthetic biology assisted intestinal microecological therapy [J]. Synthetic Biology Journal, 2022, 3(1): 35-52. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||