Synthetic Biology Journal ›› 2022, Vol. 3 ›› Issue (3): 587-601.DOI: 10.12211/2096-8280.2021-053
• Research Article • Previous Articles Next Articles
Studies on the functional modulating effect of redox partners on the cytochrome P450 enzyme MycG
YANG Chaofan, JIANG Yuchao, SANG Moli, LI Shengying, ZHANG Wei
- Microbial Technology Institute,State key Laboratory of Microbial Technology,Shandong University,Qingdao 266237,Shandong,China
-
Received:2021-04-30Revised:2021-06-08Online:2022-07-13Published:2022-06-30 -
Contact:ZHANG Wei
还原伴侣对细胞色素P450酶MycG功能调控的研究
杨超凡, 姜玉超, 桑茉莉, 李盛英, 张伟
- 山东大学微生物技术研究院,山东大学微生物技术国家重点实验室,山东 青岛 266237
-
通讯作者:张伟 -
作者简介:杨超凡 (1997—),女,硕士研究生。主要研究方向为酶工程、合成生物学。 E-mail:yangchaofan@mail.sdu.edu.cn张伟 (1982—),男,博士,教授。主要研究方向为合成生物学、天然产物生物合成、酶工程。 E-mail:zhang_wei@sdu.edu.cn -
基金资助:国家重点研发计划(2019YFA0905700);国家自然科学基金(82022066)
CLC Number:
Cite this article
YANG Chaofan, JIANG Yuchao, SANG Moli, LI Shengying, ZHANG Wei. Studies on the functional modulating effect of redox partners on the cytochrome P450 enzyme MycG[J]. Synthetic Biology Journal, 2022, 3(3): 587-601.
杨超凡, 姜玉超, 桑茉莉, 李盛英, 张伟. 还原伴侣对细胞色素P450酶MycG功能调控的研究[J]. 合成生物学, 2022, 3(3): 587-601.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2021-053
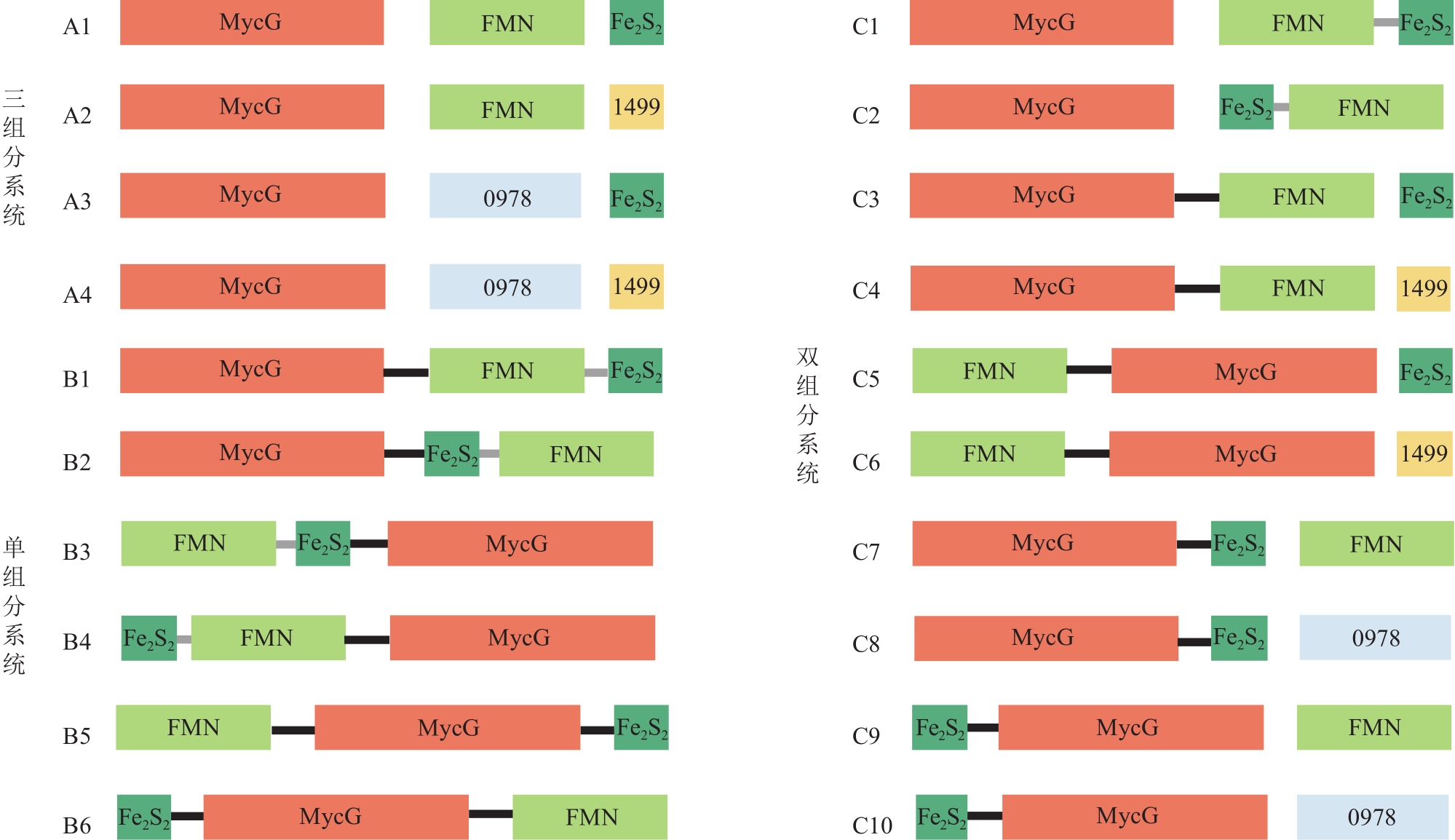
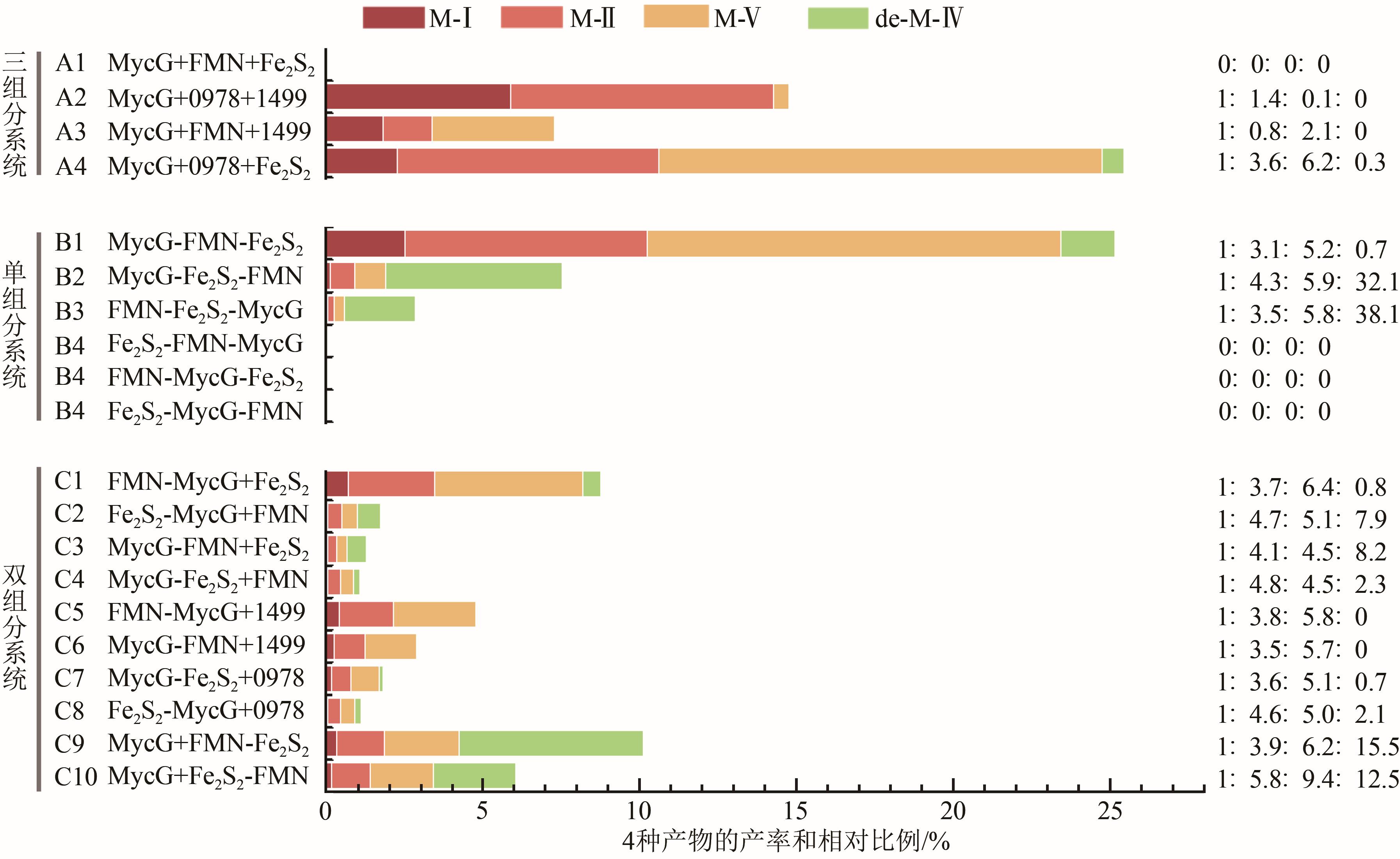
Fig. 3 Constructed P450 systems based on the recombination and reorganization of MycG and different redox partners(0978 and 1499 represent the ferredoxin reductase SelFdR0978 and ferredoxin SelFdx1499 from Synechococcus elongates PCC 7942, respectively; Black linker: L21, HQVAMLRDGDSFGGGPRHGAG; Gray linker: L9, GERREIRVD)
| 菌株 | 用途 | 来源 |
|---|---|---|
| E. coli DH5α | 用于本研究重组质粒克隆 | 擎科生物公司 |
| E. coli BL21(DE3) | 用于本研究重组蛋白表达 | 擎科生物公司 |
| BL21/pACYC-MycG | 用于表达质粒pACYC-MycG | 本实验室 |
| BL21/pET28b-RhFRED | 用于表达质粒pET28b-RhFRED | 本实验室 |
| BL21/pET28b-MycG-RhFRED | 用于表达质粒pET28b-MycG-RhFRED | 本实验室 |
| BL21/pET28b-SelFdR0978 | 用于表达质粒pET28b-SelFdR0978 | 本实验室 |
| BL21/pET28b-SelFdx1499 | 用于表达质粒pET28b-SelFdx1499 | 本实验室 |
| DH5α/BL21/pET28b-FMN | 用于克隆/表达质粒pET28b-FMN | 本研究 |
| DH5α/BL21/pET28b-Fe2S2 | 用于克隆/表达质粒pET28b-Fe2S2 | 本研究 |
| DH5α/BL21/pET28b-Fe2S2-FMN | 用于克隆/表达质粒pET28b-Fe2S2-FMN | 本研究 |
| DH5α/BL21/pET28b-MycG-FMN | 用于克隆/表达质粒pET28b-MycG-FMN | 本研究 |
| DH5α/BL21/pET28b-MycG-Fe2S2 | 用于克隆/表达质粒pET28b-MycG-Fe2S2 | 本研究 |
| DH5α/BL21/pET28b-FMN-MycG | 用于克隆/表达质粒pET28b-FMN-MycG | 本研究 |
| DH5α/BL21/pET28b-Fe2S2-MycG | 用于克隆/表达质粒pET28b-Fe2S2-MycG | 本研究 |
| DH5α/BL21/pET28b-MycG-Fe2S2-FMN | 用于克隆/表达质粒pET28b-MycG-Fe2S2-FMN | 本研究 |
| DH5α/BL21/pET28b-Fe2S2-FMN-MycG | 用于克隆/表达质粒pET28b-Fe2S2-FMN-MycG | 本研究 |
| DH5α/BL21/pET28b-FMN-Fe2S2-MycG | 用于克隆/表达质粒pET28b-FMN-Fe2S2-MycG | 本研究 |
| DH5α/BL21/pET28b-Fe2S2-MycG-FMN | 用于克隆/表达质粒pET28b-Fe2S2-MycG-FMN | 本研究 |
| DH5α/BL21/pET28b-FMN-MycG-Fe2S2 | 用于克隆/表达质粒pET28b-FMN-MycG-Fe2S2 | 本研究 |
Tab. 1 Strains and plasmids used in this study
| 菌株 | 用途 | 来源 |
|---|---|---|
| E. coli DH5α | 用于本研究重组质粒克隆 | 擎科生物公司 |
| E. coli BL21(DE3) | 用于本研究重组蛋白表达 | 擎科生物公司 |
| BL21/pACYC-MycG | 用于表达质粒pACYC-MycG | 本实验室 |
| BL21/pET28b-RhFRED | 用于表达质粒pET28b-RhFRED | 本实验室 |
| BL21/pET28b-MycG-RhFRED | 用于表达质粒pET28b-MycG-RhFRED | 本实验室 |
| BL21/pET28b-SelFdR0978 | 用于表达质粒pET28b-SelFdR0978 | 本实验室 |
| BL21/pET28b-SelFdx1499 | 用于表达质粒pET28b-SelFdx1499 | 本实验室 |
| DH5α/BL21/pET28b-FMN | 用于克隆/表达质粒pET28b-FMN | 本研究 |
| DH5α/BL21/pET28b-Fe2S2 | 用于克隆/表达质粒pET28b-Fe2S2 | 本研究 |
| DH5α/BL21/pET28b-Fe2S2-FMN | 用于克隆/表达质粒pET28b-Fe2S2-FMN | 本研究 |
| DH5α/BL21/pET28b-MycG-FMN | 用于克隆/表达质粒pET28b-MycG-FMN | 本研究 |
| DH5α/BL21/pET28b-MycG-Fe2S2 | 用于克隆/表达质粒pET28b-MycG-Fe2S2 | 本研究 |
| DH5α/BL21/pET28b-FMN-MycG | 用于克隆/表达质粒pET28b-FMN-MycG | 本研究 |
| DH5α/BL21/pET28b-Fe2S2-MycG | 用于克隆/表达质粒pET28b-Fe2S2-MycG | 本研究 |
| DH5α/BL21/pET28b-MycG-Fe2S2-FMN | 用于克隆/表达质粒pET28b-MycG-Fe2S2-FMN | 本研究 |
| DH5α/BL21/pET28b-Fe2S2-FMN-MycG | 用于克隆/表达质粒pET28b-Fe2S2-FMN-MycG | 本研究 |
| DH5α/BL21/pET28b-FMN-Fe2S2-MycG | 用于克隆/表达质粒pET28b-FMN-Fe2S2-MycG | 本研究 |
| DH5α/BL21/pET28b-Fe2S2-MycG-FMN | 用于克隆/表达质粒pET28b-Fe2S2-MycG-FMN | 本研究 |
| DH5α/BL21/pET28b-FMN-MycG-Fe2S2 | 用于克隆/表达质粒pET28b-FMN-MycG-Fe2S2 | 本研究 |
| 引物名称 | 核苷酸序列(5′~3′) |
|---|---|
| arm-FP | catatggctgccgcgcg |
| arm-RP | tagctcgagcaccaccacc |
| Fe2S2-FP | gagcacgccttcgacctcga |
| Fe2S2-RP | tcagagtcgcagggccagcc |
| FMN-FP | gtcaccgtcgagcgcctgga |
| FMN-RP | cgaggtgaagtgctcgacgt |
| MycG-FMN-FP | ggcagccatatgacttc |
| MycG-FMN-RP | cgaggtgaagtgctcgacgt |
| Fe2S2-arm-FP | cgcgcggcagccatatggagcacgccttcgacctc |
| Fe2S2-arm-RP | ggtggtggtgctcgagtcagagtcgcagggccag |
| FMN-arm-FP | cgcgcggcagccatatggtcaccgtcgagcgcctg |
| FMN-arm-RP | ggtggtggtgctcgagctacgaggtgaagtgctcgacg |
| MycG-FMN-arm-FP | cgcgcggcagccatatgacttcagctgaacctagggc |
| MycG-FMN-arm-RP | ggtggtggtgctcgagctacgaggtgaagtgctcgacg |
| MycG-Fe2S2-WL-FP | ttcagctgaagtcatcatatggctgccgcg |
| MycG-Fe2S2-WL-RP | gcggtgtcccgcaccgagcacgccttcgacctcgaa |
| MycG-L9-FP | atgacttcagctgaacctagggc |
| MycG-L9-RP | ggtgcgggacaccg |
| Fe2S2-L9-FP | gcgctcgacggtgacgacgtccggatcgagcgccgcgagggagagtcgcagg |
| Fe2S2-L9-RP | acgtcgagcacttcacctcgtgactcgagcaccaccacca |
| FMN-WL-FP | catatggctgccgcgcggcacc |
| FMN-WL-RP | gtcaccgtcgagcgcctggacc |
| MycG-FP | acttcagctgaacctagggc |
| MycG-FP2 | accatcggagaacccgccgcccgggcggtgtcccgcaccacttcagctgaacctaggg |
| MycG-RP | ccacacgaccggcagctcga |
| MycG-Fe2S2-FMN-WL-FP | aggttcagctgaagtcatatggctgccgcgcgg |
| MycG-Fe2S2-FMN-WL-RP | gcggtgtcccgcaccgagcacgccttcgacc |
| Fe2S2-FMN-G-WL-FP | gggttctccgatggtgaccggttgatggcggtgcagcaccgaggtgaagtgctcgacg |
| Fe2S2-FMN-G-WL-RP | ctgccggtcgtgtggtgactcgagcaccaccac |
| FMN-L9-FP | gtcaccgtcgagcgcctgga |
| FMN-L9-RP | gacgtccggatcgagcgccg |
| FMN-L21-FP | gtcaccgtcgagcgcctgga |
| FMN-L21-RP | taggttcagctgaagtggtgc |
| Fe2S2-L21-FP | gagcacgccttcgacctcga |
| Fe2S2-L21-RP | taggttcagctgaagtggtg |
| FMN-Fe2S2-G-WL-FP | ctcgatccggacgtcgagcacgccttcgac |
| FMN-Fe2S2-G-WL-RP | gcgctcgacggtgaccatatggctgccgcgcg |
| FMN-G-Fe2S2-WL-FP | gcgctcgacggtgaccatatggctgccgcgc |
| FMN-G-Fe2S2-WL-RP | acttcagctgaacctagggc |
| Fe2S2-G-FMN-WL-FP | aggtcgaaggcgtgctccatatggctgccgcg |
| Fe2S2-G-FMN-WL-RP | acttcagctgaacctagggc |
| FMN-MycG-WL-FP | ctgccggtcgtgtggtagctcgagcaccaccac |
| FMN-MycG-WL-RP | gggttctccgatggtgaccggttgatggcggtgcagcaccgaggtgaagtgctcgacg |
| Fe2S2-MycG-WL-FP | ctgccggtcgtgtggtagctcgagcaccaccac |
| Fe2S2-MycG-WL-RP | tgatggcggtgcagcacgagtcgcagggccagcc |
Tab. 2 Oligos used in this study
| 引物名称 | 核苷酸序列(5′~3′) |
|---|---|
| arm-FP | catatggctgccgcgcg |
| arm-RP | tagctcgagcaccaccacc |
| Fe2S2-FP | gagcacgccttcgacctcga |
| Fe2S2-RP | tcagagtcgcagggccagcc |
| FMN-FP | gtcaccgtcgagcgcctgga |
| FMN-RP | cgaggtgaagtgctcgacgt |
| MycG-FMN-FP | ggcagccatatgacttc |
| MycG-FMN-RP | cgaggtgaagtgctcgacgt |
| Fe2S2-arm-FP | cgcgcggcagccatatggagcacgccttcgacctc |
| Fe2S2-arm-RP | ggtggtggtgctcgagtcagagtcgcagggccag |
| FMN-arm-FP | cgcgcggcagccatatggtcaccgtcgagcgcctg |
| FMN-arm-RP | ggtggtggtgctcgagctacgaggtgaagtgctcgacg |
| MycG-FMN-arm-FP | cgcgcggcagccatatgacttcagctgaacctagggc |
| MycG-FMN-arm-RP | ggtggtggtgctcgagctacgaggtgaagtgctcgacg |
| MycG-Fe2S2-WL-FP | ttcagctgaagtcatcatatggctgccgcg |
| MycG-Fe2S2-WL-RP | gcggtgtcccgcaccgagcacgccttcgacctcgaa |
| MycG-L9-FP | atgacttcagctgaacctagggc |
| MycG-L9-RP | ggtgcgggacaccg |
| Fe2S2-L9-FP | gcgctcgacggtgacgacgtccggatcgagcgccgcgagggagagtcgcagg |
| Fe2S2-L9-RP | acgtcgagcacttcacctcgtgactcgagcaccaccacca |
| FMN-WL-FP | catatggctgccgcgcggcacc |
| FMN-WL-RP | gtcaccgtcgagcgcctggacc |
| MycG-FP | acttcagctgaacctagggc |
| MycG-FP2 | accatcggagaacccgccgcccgggcggtgtcccgcaccacttcagctgaacctaggg |
| MycG-RP | ccacacgaccggcagctcga |
| MycG-Fe2S2-FMN-WL-FP | aggttcagctgaagtcatatggctgccgcgcgg |
| MycG-Fe2S2-FMN-WL-RP | gcggtgtcccgcaccgagcacgccttcgacc |
| Fe2S2-FMN-G-WL-FP | gggttctccgatggtgaccggttgatggcggtgcagcaccgaggtgaagtgctcgacg |
| Fe2S2-FMN-G-WL-RP | ctgccggtcgtgtggtgactcgagcaccaccac |
| FMN-L9-FP | gtcaccgtcgagcgcctgga |
| FMN-L9-RP | gacgtccggatcgagcgccg |
| FMN-L21-FP | gtcaccgtcgagcgcctgga |
| FMN-L21-RP | taggttcagctgaagtggtgc |
| Fe2S2-L21-FP | gagcacgccttcgacctcga |
| Fe2S2-L21-RP | taggttcagctgaagtggtg |
| FMN-Fe2S2-G-WL-FP | ctcgatccggacgtcgagcacgccttcgac |
| FMN-Fe2S2-G-WL-RP | gcgctcgacggtgaccatatggctgccgcgcg |
| FMN-G-Fe2S2-WL-FP | gcgctcgacggtgaccatatggctgccgcgc |
| FMN-G-Fe2S2-WL-RP | acttcagctgaacctagggc |
| Fe2S2-G-FMN-WL-FP | aggtcgaaggcgtgctccatatggctgccgcg |
| Fe2S2-G-FMN-WL-RP | acttcagctgaacctagggc |
| FMN-MycG-WL-FP | ctgccggtcgtgtggtagctcgagcaccaccac |
| FMN-MycG-WL-RP | gggttctccgatggtgaccggttgatggcggtgcagcaccgaggtgaagtgctcgacg |
| Fe2S2-MycG-WL-FP | ctgccggtcgtgtggtagctcgagcaccaccac |
| Fe2S2-MycG-WL-RP | tgatggcggtgcagcacgagtcgcagggccagcc |
| 组合 | 铁氰化钾/[μmol/(L·min)) | 细胞色素C/[μmol/(L·min)) | |
|---|---|---|---|
| FMN-Fe2S2 | 34.38±0.05 | 13.57±0.01 | |
| Fe2S2-FMN | 24.37±0.02 | 10.21±0.01 | |
| MycG-FMN-Fe2S2 | 21.82±0.02 | 10.45±0.01 | |
| FMN-MycG-Fe2S2 | 6.92±0.003 | 8.42±0.01 | |
| Fe2S2-MycG-FMN | 6.64±0.02 | 3.23±0.01 | |
| FMN-Fe2S2-MycG | 26.07±0.03 | 10.22±0.01 | |
| MycG-Fe2S2-FMN | 5.85±0.006 | 5.04±0.009 | |
| Fe2S2-FMN-MycG | 25.36±0.02 | 6.0±0.01 | |
| MycG-FMN | 33.97±0.03 | Fe2S2:3.94±0.06 | SelFdx1499:7.04±0.03 |
| FMN-MycG | 19.81±0.03 | Fe2S2:10.37±0.02 | SelFdx1499:9.56±0.02 |
| SelFdR0978 | 24.23±0.04 | Fe2S2:1.63±0.02 | SelFdx1499:3.75±0.01 |
| FMN | 5.04±0.07 | Fe2S2:0.46±0.03 | SelFdx1499:1.86±0.02 |
Tab. 3 Electron transfer efficiencies of different recombinant proteins
| 组合 | 铁氰化钾/[μmol/(L·min)) | 细胞色素C/[μmol/(L·min)) | |
|---|---|---|---|
| FMN-Fe2S2 | 34.38±0.05 | 13.57±0.01 | |
| Fe2S2-FMN | 24.37±0.02 | 10.21±0.01 | |
| MycG-FMN-Fe2S2 | 21.82±0.02 | 10.45±0.01 | |
| FMN-MycG-Fe2S2 | 6.92±0.003 | 8.42±0.01 | |
| Fe2S2-MycG-FMN | 6.64±0.02 | 3.23±0.01 | |
| FMN-Fe2S2-MycG | 26.07±0.03 | 10.22±0.01 | |
| MycG-Fe2S2-FMN | 5.85±0.006 | 5.04±0.009 | |
| Fe2S2-FMN-MycG | 25.36±0.02 | 6.0±0.01 | |
| MycG-FMN | 33.97±0.03 | Fe2S2:3.94±0.06 | SelFdx1499:7.04±0.03 |
| FMN-MycG | 19.81±0.03 | Fe2S2:10.37±0.02 | SelFdx1499:9.56±0.02 |
| SelFdR0978 | 24.23±0.04 | Fe2S2:1.63±0.02 | SelFdx1499:3.75±0.01 |
| FMN | 5.04±0.07 | Fe2S2:0.46±0.03 | SelFdx1499:1.86±0.02 |
| 1 | GIRVAN H M, MUNRO A W. Applications of microbial cytochrome P450 enzymes in biotechnology and synthetic biology[J]. Current Opinion in Chemical Biology, 2016, 31: 136-145. |
| 2 | GUENGERICH F P, MUNRO A W. Unusual cytochrome P450 enzymes and reactions[J]. Journal of Biological Chemistry, 2013, 288(24): 17065-17073. |
| 3 | LAMB D C, WATERMAN M R. Unusual properties of the cytochrome P450 superfamily[J]. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences, 2013, 368(1612): 20120434. |
| 4 | GUENGERICH F P. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity[J]. Chemical Research in Toxicology, 2001, 14(6): 611-650. |
| 5 | SEVRIOUKOVA I F, GARCIA C, LI H Y, et al. Crystal structure of putidaredoxin, the [2Fe-2S] component of the P450cam monooxygenase system from Pseudomonas putida [J]. Journal of Molecular Biology, 2003, 333(2): 377-392. |
| 6 | KEIZERS P H J, MERSINLI B, REINLE W, et al. A solution model of the complex formed by adrenodoxin and adrenodoxin reductase determined by paramagnetic NMR spectroscopy[J]. Biochemistry, 2010, 49(32): 6846-6855. |
| 7 | MATSUBARA H, SASAKI R M, CHAIN R K. Spinach ferredoxin (Ⅰ): Amino acid composition and terminal sequences[J]. Journal of Biological Chemistry, 1968, 243(8): 1725-1731. |
| 8 | RUETTINGER R T, FULCO A J. Epoxidation of unsaturated fatty acids by a soluble cytochrome P450-dependent system from Bacillus megaterium [J]. Journal of Biological Chemistry, 1981, 256(11): 5728-5734. |
| 9 | JACKSON C J, LAMB D C, MARCZYLO T H, et al. A novel sterol 14α-demethylase/ferredoxin fusion protein (MCCYP51FX) from Methylococcus capsulatus represents a new class of the cytochrome P450 superfamily[J]. Journal of Biological Chemistry, 2002, 277(49): 46959-46965. |
| 10 | JACKSON R G, RYLOTT E L, FOURNIER D, et al. Exploring the biochemical properties and remediation applications of the unusual explosive-degrading P450 system XplA/B[J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(43): 16822-16827. |
| 11 | MUNRO A W, DAFF S, COGGINS J R, et al. Probing electron transfer in flavocytochrome P-450 BM3 and its component domains[J]. European Journal of Biochemistry, 1996, 239(2): 403-409. |
| 12 | MCLEAN K J, LUCIAKOVA D, BELCHER J, et al. Biological diversity of cytochrome P450 redox partner systems[J]. Advances in Experimental Medicine and Biology, 2015, 851: 299-317. |
| 13 | LI S Y, PODUST L M, SHERMAN D H. Engineering and analysis of a self-sufficient biosynthetic cytochrome P450 PikC fused to the RhFRED reductase domain[J]. Journal of the American Chemical Society, 2007, 129(43): 12940-12941. |
| 14 | NODATE M, KUBOTA M, MISAWA N. Functional expression system for cytochrome P450 genes using the reductase domain of self-sufficient P450RhF from Rhodococcus sp. NCIMB 9784[J]. Applied Microbiology and Biotechnology, 2006, 71(4): 455-462. |
| 15 | MCLEAN K J, HANS M, MEIJRINK B, et al. Single-step fermentative production of the cholesterol-lowering drug pravastatin via reprogramming of Penicillium chrysogenum [J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(9): 2847-2852. |
| 16 | ANZAI Y, LI S Y, CHAULAGAIN M R, et al. Functional analysis of MycCI and MycG, cytochrome P450 enzymes involved in biosynthesis of mycinamicin macrolide antibiotics[J]. Chemistry & Biology, 2008, 15(9): 950-959. |
| 17 | ZHANG W, LIU Y, YAN J Y, et al. New reactions and products resulting from alternative interactions between the P450 enzyme and redox partners[J]. Journal of the American Chemical Society, 2014, 136(9): 3640-3646. |
| 18 | GUENGERICH F P, MARTIN M V, SOHL C D, et al. Measurement of cytochrome P450 and NADPH-Cytochrome P450 reductase[J]. Nature Protocols, 2009, 4(9): 1245-1251. |
| 19 | ZHANG W, DU L, LI F W, et al. Mechanistic insights into interactions between bacterial class I P450 enzymes and redox partners[J]. ACS Catalysis, 2018, 8(11): 9992-10003. |
| 20 | TASNIM H, LANDRY A P, FONTENOT C R, et al. Exploring the FMN binding site in the mitochondrial outer membrane protein mitoNEET[J]. Free Radical Biology and Medicine, 2020, 156: 11-19. |
| 21 | HEIKAL A A. Intracellular coenzymes as natural biomarkers for metabolic activities and mitochondrial anomalies[J]. Biomarkers in Medicine, 2010, 4(2): 241-263. |
| 22 | LACOUR T, OHKAWA H. Engineering and biochemical characterization of the rat microsomal cytochrome P4501A1 fused to ferredoxin and ferredoxin-NADP+ reductase from plant chloroplasts[J]. Biochimica et Biophysica Acta-Protein Structure and Molecular Enzymology, 1999, 1433(1/2): 87-102. |
| 23 | WANG Q, HUANG X N, ZHANG J J, et al. Engineering self-sufficient aldehyde deformylating oxygenases fused to alternative electron transfer systems for efficient conversion of aldehydes into alkanes[J]. Chemical Communications, 2014, 50(33): 4299. |
| 24 | HUNTER D J B, ROBERTS G A, OST T W B, et al. Analysis of the domain properties of the novel cytochrome P450 RhF[J]. FEBS Letters, 2005, 579(10): 2215-2220. |
| 25 | TRIPATHI S, LI H Y, POULOS T L. Structural basis for effector control and redox partner recognition in cytochrome P450[J]. Science, 2013, 340(6137): 1227-1230. |
| 26 | ZHANG L L, XIE Z Z, LIU Z W, et al. Structural insight into the electron transfer pathway of a self-sufficient P450 monooxygenase[J]. Nature Communications, 2020, 11: 2676. |
| 27 | SADEGHI S J, MEHARENNA Y T, FANTUZZI A, et al. Engineering artificial redox chains by molecular 'LEGO'[J]. Faraday Discussions, 2000(116): 135-153. |
| 28 | LI Z, JIANG Y Y, GUENGERICH F P, et al. Engineering cytochrome P450 enzyme systems for biomedical and biotechnological applications[J]. Journal of Biological Chemistry, 2020, 295(3): 833-849. |
| [1] | XIE Xiangqian, GUO Wen, WANG Huan, LI Jin. Biosynthesis and chemical synthesis of ribosomally synthesized and post-translationally modified peptides containing aminovinyl cysteine [J]. Synthetic Biology Journal, 2024, 5(5): 981-996. |
| [2] | SHI Xinjie, DU Yiling. Research advances in the biosynthesis of nonribosomal peptides within the bisintercalator family as anticancer drugs [J]. Synthetic Biology Journal, 2024, 5(3): 593-611. |
| [3] | Haibo ZHOU, Qiyao SHEN, Hanna CHEN, Zongjie WANG, Yuezhong LI, Youming ZHANG, Xiaoying BIAN. Genome mining for novel natural products in Sorangium cellulosum So0157-2 by heterologous expression [J]. Synthetic Biology Journal, 2021, 2(5): 837-849. |
| [4] | Yuanjun HAN, Tianlu MO, Zixin DENG, Qi ZHANG, Wei DING. Study on the post-translational modification of RiPPs Xye catalyzed by CyFE PacB [J]. Synthetic Biology Journal, 2021, 2(5): 826-836. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||