Synthetic Biology Journal ›› 2024, Vol. 5 ›› Issue (5): 997-1020.DOI: 10.12211/2096-8280.2024-012
• Invited Review • Previous Articles Next Articles
Recent advances in photo-induced promiscuous enzymatic reactions
XIA Kongchen, XU Weihua, WU Qi
- Department of Chemistry,Zhejiang University,Hangzhou 310058,Zhejiang,China
-
Received:2024-01-25Revised:2024-04-28Online:2024-11-20Published:2024-10-31 -
Contact:XU Weihua, WU Qi
光酶催化混乱性反应的研究进展
夏孔晨, 徐维华, 吴起
- 浙江大学化学系,浙江 杭州 310058
-
通讯作者:徐维华,吴起 -
作者简介:夏孔晨 (2000—),女,硕士研究生。研究方向为酶定向进化与催化多功能性。 E-mail:22337042@zju.edu.cn徐维华 (1991—),女,博士后。研究方向为酶定向进化、光酶催化多功能性、酶促混乱性反应。 E-mail:xuwh@zju.edu.cn吴起 (1976—),男,教授,博士生导师,研究方向为酶定向进化、合成生物学、生物催化等。 E-mail:wuqi1000@163.com,llc123@zju.edu.cn -
基金资助:国家重点研发计划(2021YFC2102000);国家自然科学基金(22277105);浙江省自然科学基金(LZ24B020003)
CLC Number:
Cite this article
XIA Kongchen, XU Weihua, WU Qi. Recent advances in photo-induced promiscuous enzymatic reactions[J]. Synthetic Biology Journal, 2024, 5(5): 997-1020.
夏孔晨, 徐维华, 吴起. 光酶催化混乱性反应的研究进展[J]. 合成生物学, 2024, 5(5): 997-1020.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2024-012
| 1 | CIAMICIAN G. The photochemistry of the future[J]. Science, 1912, 36(926): 385-394. |
| 2 | SCHULTZ D M, YOON T P. Solar synthesis: prospects in visible light photocatalysis[J]. Science, 2014, 343(6174): 1239176. |
| 3 | WEI H L, CHEN H, CHEN J Z, et al. Nickel-catalyzed asymmetric hydrogenation of α-substituted vinylphosphonates and diarylvinylphosphine oxides[J]. Angewandte Chemie International Edition, 2023, 62(6): e202214990. |
| 4 | SPRAGUE-KLEIN E A, HE X, MARA M W, et al. Photo-electrochemical effect in the amorphous cobalt oxide water oxidation catalyst cobalt-phosphate (CoPi)[J]. ACS Energy Letters, 2022, 7(9): 3129-3138. |
| 5 | JOHNSTON C P, SMITH R T, ALLMENDINGER S, et al. Metallaphotoredox-catalysed sp3-sp3 cross-coupling of carboxylic acids with alkyl halides[J]. Nature, 2016, 536(7616): 322-325. |
| 6 | CAO D W, ATAYA M, CHEN Z P, et al. Light-driven transition-metal-free direct decarbonylation of unstrained diaryl ketones via a dual C-C bond cleavage[J]. Nature Communications, 2022, 13(1): 1805. |
| 7 | QUIRÓS I, MARTÍN M, GOMEZ-MENDOZA M, et al. Isonitriles as alkyl radical precursors in visible light mediated hydro- and deuterodeamination reactions[J]. Angewandte Chemie International Edition, 2024, 63(7): e202317683. |
| 8 | ZHANG L, PFUND B, WENGER O S, et al. Oxidase-type C-H/C-H coupling using an isoquinoline-derived organic photocatalyst[J]. Angewandte Chemie International Edition, 2022, 61(20): e202202649. |
| 9 | BACKUS E H G, HOSSEINPOUR S, RAMANAN C, et al. Ultrafast surface-specific spectroscopy of water at a photoexcited TiO2 model water-splitting photocatalyst[J]. Angewandte Chemie International Edition, 2024, 63(8): e202312123. |
| 10 | HUANG J, KANG Y Y, LIU J N, et al. Gradient tungsten-doped Bi3TiNbO9 ferroelectric photocatalysts with additional built-in electric field for efficient overall water splitting[J]. Nature Communications, 2023, 14(1): 7948. |
| 11 | QU G, LI A T, ACEVEDO-ROCHA C G, et al. The crucial role of methodology development in directed evolution of selective enzymes[J]. Angewandte Chemie International Edition, 2020, 59(32): 13204-13231. |
| 12 | SHELDON R A, WOODLEY J M. Role of biocatalysis in sustainable chemistry[J]. Chemical Reviews, 2018, 118(2): 801-838. |
| 13 | MAESTRE-REYNA M, WANG P H, NANGO E, et al. Visualizing the DNA repair process by a photolyase at atomic resolution[J]. Science, 2023, 382(6674): eadd7795. |
| 14 | ZHANG S W, HEYES D J, FENG L L, et al. Structural basis for enzymatic photocatalysis in chlorophyll biosynthesis[J]. Nature, 2019, 574(7780): 722-725. |
| 15 | SORIGUÉ D, LÉGERET B, CUINÉ S, et al. An algal photoenzyme converts fatty acids to hydrocarbons[J]. Science, 2017, 357(6354): 903-907. |
| 16 | SUN N N, HUANG J J, QIAN J Y, et al. Enantioselective [2+2]-cycloadditions with triplet photoenzymes[J]. Nature, 2022, 611(7937): 715-720. |
| 17 | TRIMBLE J S, CRAWSHAW R, HARDY F J, et al. A designed photoenzyme for enantioselective [2+2] cycloadditions[J]. Nature, 2022, 611(7937): 709-714. |
| 18 | ALPHAND V, VAN BERKEL W J H, JURKAŠ V, et al. Exciting enzymes: current state and future perspective of photobiocatalysis[J]. ChemPhotoChem, 2023, 7(7): e202200325. |
| 19 | SINGH P P, SINHA S, NAINWAL P, et al. Novel applications of photobiocatalysts in chemical transformations[J]. RSC Advances, 2024, 14(4): 2590-2601. |
| 20 | LI S H, SHI J F, LIU S S, et al. Molecule-electron-proton transfer in enzyme-photo-coupled catalytic system[J]. Chinese Journal of Catalysis, 2023, 44: 96-110. |
| 21 | YANG N, TIAN Y, ZHANG M, et al. Photocatalyst-enzyme hybrid systems for light-driven biotransformation[J]. Biotechnology Advances, 2022, 54: 107808. |
| 22 | ROTH S, NIESE R, MÜLLER M, et al. Redox out of the box: catalytic versatility across NAD(P)H-dependent oxidoreductases[J]. Angewandte Chemie International Edition, 2024, 63(13): e202314740. |
| 23 | SELLÉS VIDAL L, KELLY C L, MORDAKA P M, et al. Review of NAD(P)H-dependent oxidoreductases: properties, engineering and application[J]. Biochimica et Biophysica Acta Proteins and Proteomics, 2018, 1866(2): 327-347. |
| 24 | PAUL C E, EGGERICHS D, WESTPHAL A H, et al. Flavoprotein monooxygenases: versatile biocatalysts[J]. Biotechnology Advances, 2021, 51: 107712. |
| 25 | LEE S H, CHOI D S, KUK S K, et al. Photobiocatalysis: activating redox enzymes by direct or indirect transfer of photoinduced electrons[J]. Angewandte Chemie International Edition, 2018, 57(27): 7958-7985. |
| 26 | EMMANUEL M A, GREENBERG N R, OBLINSKY D G, et al. Accessing non-natural reactivity by irradiating nicotinamide-dependent enzymes with light[J]. Nature, 2016, 540(7633): 414-417. |
| 27 | EMMANUEL M A, BENDER S G, BILODEAU C, et al. Photobiocatalytic strategies for organic synthesis[J]. Chemical Reviews, 2023, 123(9): 5459-5520. |
| 28 | HARRISON W, HUANG X Q, ZHAO H M. Photobiocatalysis for abiological transformations[J]. Accounts of Chemical Research, 2022, 55(8): 1087-1096. |
| 29 | 明阳, 陈彬, 黄小强. 光酶催化合成进展[J]. 合成生物学, 2023, 4(4): 651-675. |
| MING Y, CHEN B, HUANG X Q. Recent advances in photoenzymatic synthesis[J]. Synthetic Biology Journal, 2023, 4(4): 651-675. | |
| 30 | SANDOVAL B A, MEICHAN A J, HYSTER T K. Enantioselective hydrogen atom transfer: discovery of catalytic promiscuity in flavin-dependent ‘ene’-reductases[J]. Journal of the American Chemical Society, 2017, 139(33): 11313-11316. |
| 31 | BIEGASIEWICZ K F, COOPER S J, EMMANUEL M A, et al. Catalytic promiscuity enabled by photoredox catalysis in nicotinamide-dependent oxidoreductases[J]. Nature Chemistry, 2018, 10(7): 770-775. |
| 32 | PENG Y Z, WANG Z G, CHEN Y, et al. Photoinduced promiscuity of cyclohexanone monooxygenase for the enantioselective synthesis of α-fluoroketones[J]. Angewandte Chemie International Edition, 2022, 61(50): e202211199. |
| 33 | XU J, CEN Y X, SINGH W, et al. Stereodivergent protein engineering of a lipase to access all possible stereoisomers of chiral esters with two stereocenters[J]. Journal of the American Chemical Society, 2019, 141(19): 7934-7945. |
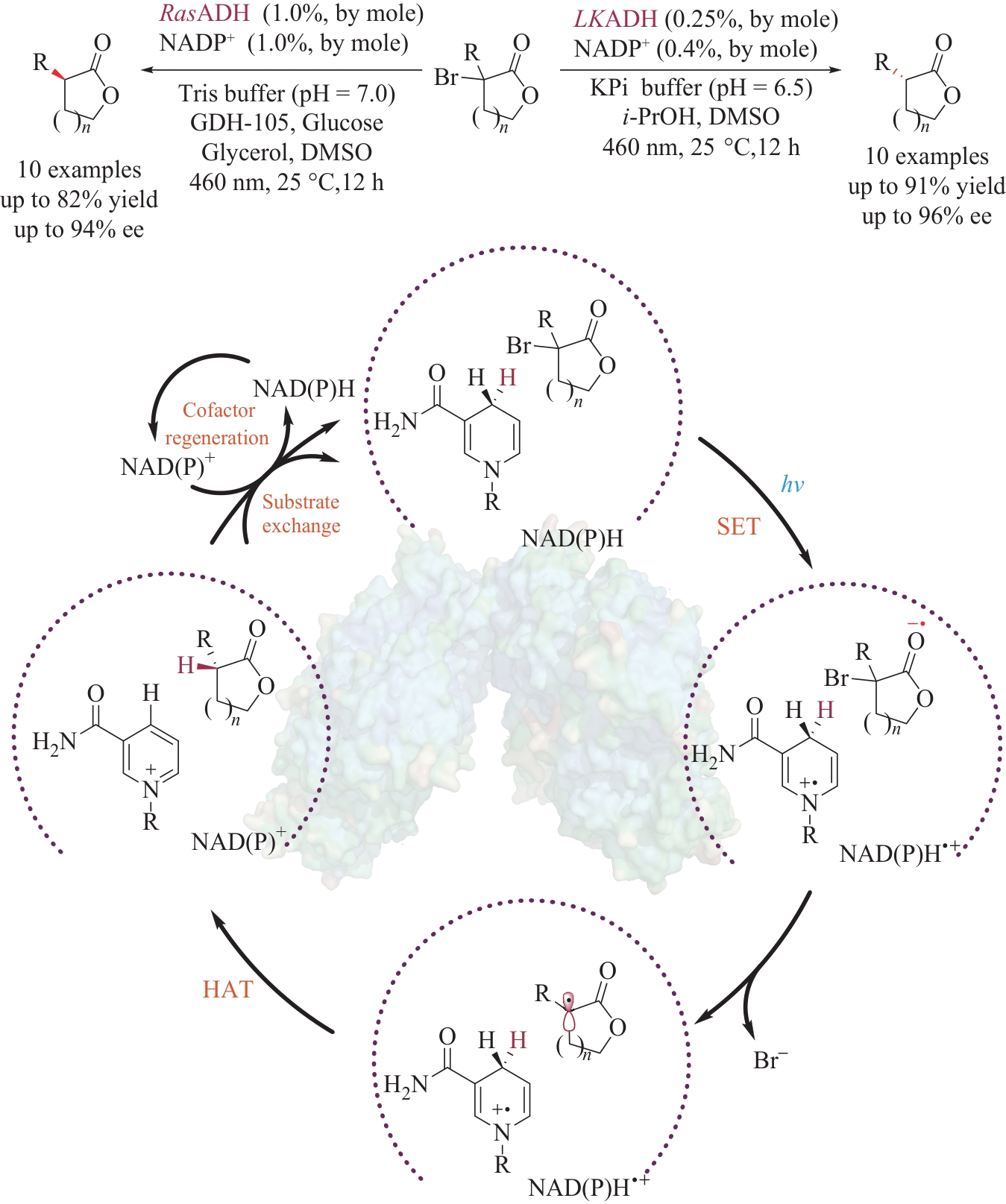
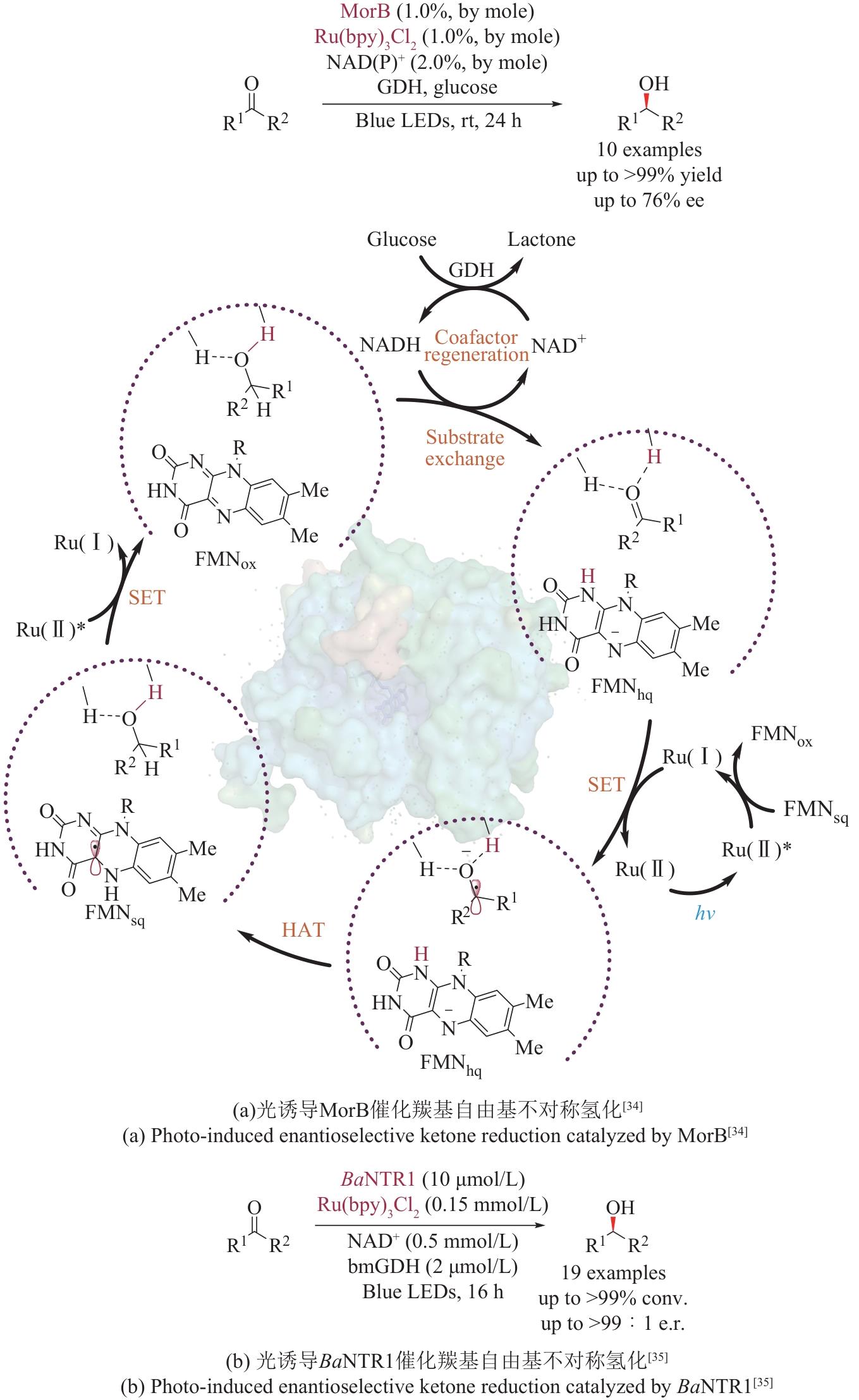
| 34 | SANDOVAL B A, KURTOIC S I, CHUNG M M, et al. Photoenzymatic catalysis enables radical-mediated ketone reduction in ene-reductases[J]. Angewandte Chemie International Edition, 2019, 58(26): 8714-8718. |
| 35 | PRATS LUJÁN A, BHAT M F, SARAVANAN T, et al. Chemo- and enantioselective photoenzymatic ketone reductions using a promiscuous flavin-dependent nitroreductase[J]. ChemCatChem, 2022, 14(8): e202200043. |
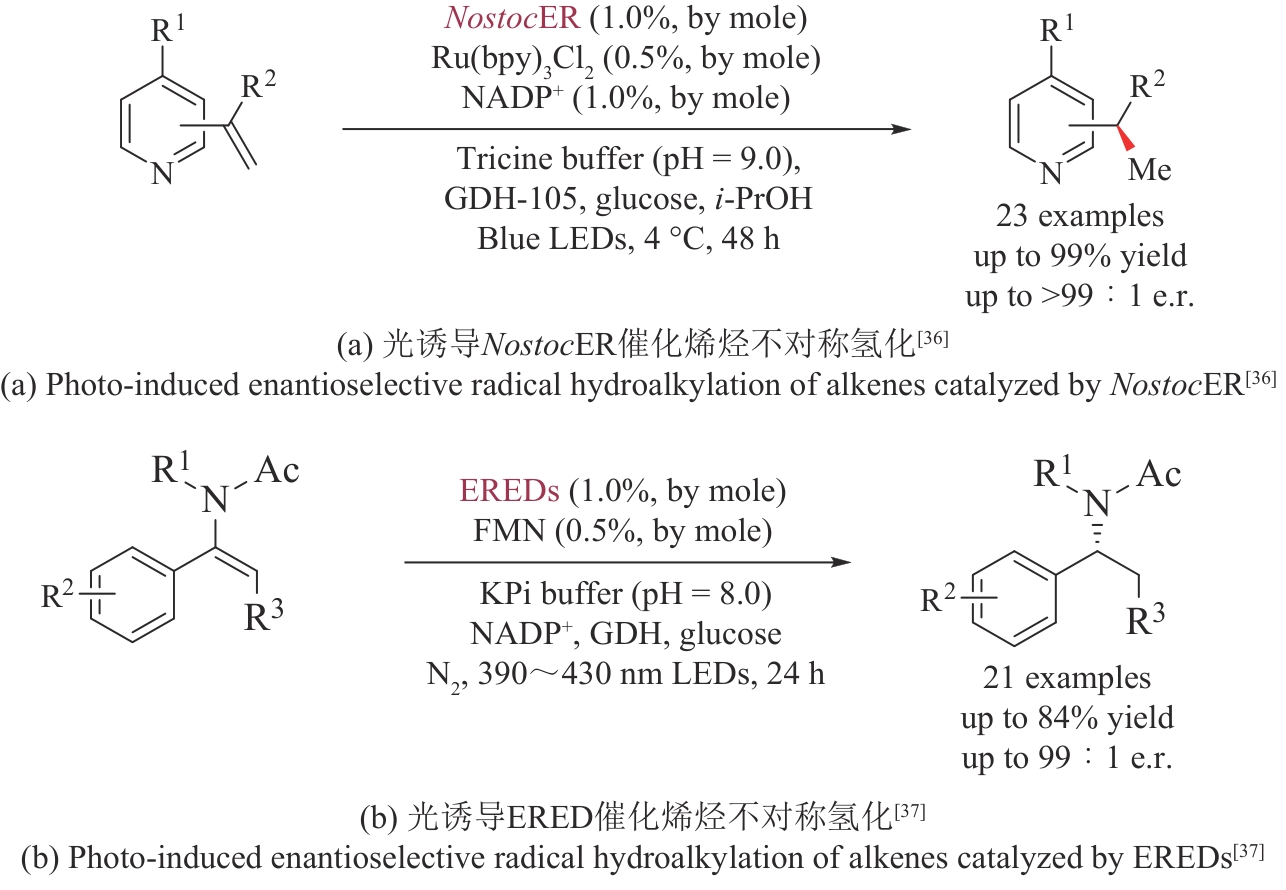
| 36 | NAKANO Y, BLACK M J, MEICHAN A J, et al. Photoenzymatic hydrogenation of heteroaromatic olefins using ‘ene’-reductases with photoredox catalysts[J]. Angewandte Chemie International Edition, 2020, 59(26): 10484-10488. |
| 37 | ZHANG J W, ZHANG Q Y, CHEN B, et al. Photoenzymatic conversion of enamides to enantioenriched benzylic amines enabled by visible-light-induced single-electron reduction[J]. ACS Catalysis, 2023, 13(24): 15682-15690. |
| 38 | BIEGASIEWICZ K F, COOPER S J, GAO X, et al. Photoexcitation of flavoenzymes enables a stereoselective radical cyclization[J]. Science, 2019, 364(6446): 1166-1169. |
| 39 | MONDAL S, DUMUR F, GIGMES D, et al. Enantioselective radical reactions using chiral catalysts[J]. Chemical Reviews, 2022, 122(6): 5842-5976. |
| 40 | CLAYMAN P D, HYSTER T K. Photoenzymatic generation of unstabilized alkyl radicals: an asymmetric reductive cyclization[J]. Journal of the American Chemical Society, 2020, 142(37): 15673-15677. |
| 41 | ZHU C T, YUAN Z B, DENG Z W, et al. Photoenzymatic enantioselective synthesis of oxygen-containing benzo-fused heterocycles[J]. Angewandte Chemie International Edition, 2023, 62(50): e202311762. |
| 42 | CAPONE M, DELL’ORLETTA G, NICHOLLS B T, et al. Evidence of a distinctive enantioselective binding mode for the photoinduced radical cyclization of α-chloroamides in ene-reductases[J]. ACS Catalysis, 2023, 13(23): 15310-15321. |
| 43 | COLEMAN T, KIRK A M, CHAO R R, et al. Understanding the mechanistic requirements for efficient and stereoselective alkene epoxidation by a cytochrome P450 enzyme[J]. ACS Catalysis, 2021, 11(4): 1995-2010. |
| 44 | SCHMERMUND L, VALENTINA JURKAŠ V, ÖZGEN F F. Photo-biocatalysis: biotransformations in the presence of light[J]. ACS Catalysis, 2019, 9(5): 4115-4144. |
| 45 | HUANG X Q, WANG B J, WANG Y J, et al. Photoenzymatic enantioselective intermolecular radical hydroalkylation[J]. Nature, 2020, 584(7819): 69-74. |
| 46 | OUYANG Y, TUREK-HERMAN J, QIAO T Z, et al. Asymmetric carbohydroxylation of alkenes using photoenzymatic catalysis[J]. Journal of the American Chemical Society, 2023, 145(31): 17018-17022. |
| 47 | LI M L, HARRISON W, ZHANG Z Y, et al. Remote stereocontrol with azaarenes via enzymatic hydrogen atom transfer[J]. Nature Chemistry, 2024, 16(2): 277-284. |
| 48 | BEST D, LAM H W. C-N-containing azaarenes as activating groups in enantioselective catalysis[J]. The Journal of Organic Chemistry, 2014, 79(3): 831-845. |
| 49 | GUO J, XIE Y, LAI Z M, et al. Enantioselective hydroalkylation of alkenylpyridines enabled by merging photoactive electron donor-acceptor complexes with chiral bifunctional organocatalysis[J]. ACS Catalysis, 2022, 12(20): 13065-13074. |
| 50 | BENDER S G, HYSTER T K. Pyridylmethyl radicals for enantioselective alkene hydroalkylation using “ene”-reductases[J]. ACS Catalysis, 2023, 13(22): 14680-14684. |
| 51 | DUAN X Y, CUI D, WANG Z G, et al. A photoenzymatic strategy for radical-mediated stereoselective hydroalkylation with diazo compounds[J]. Angewandte Chemie International Edition, 2023, 62(5): e202214135. |
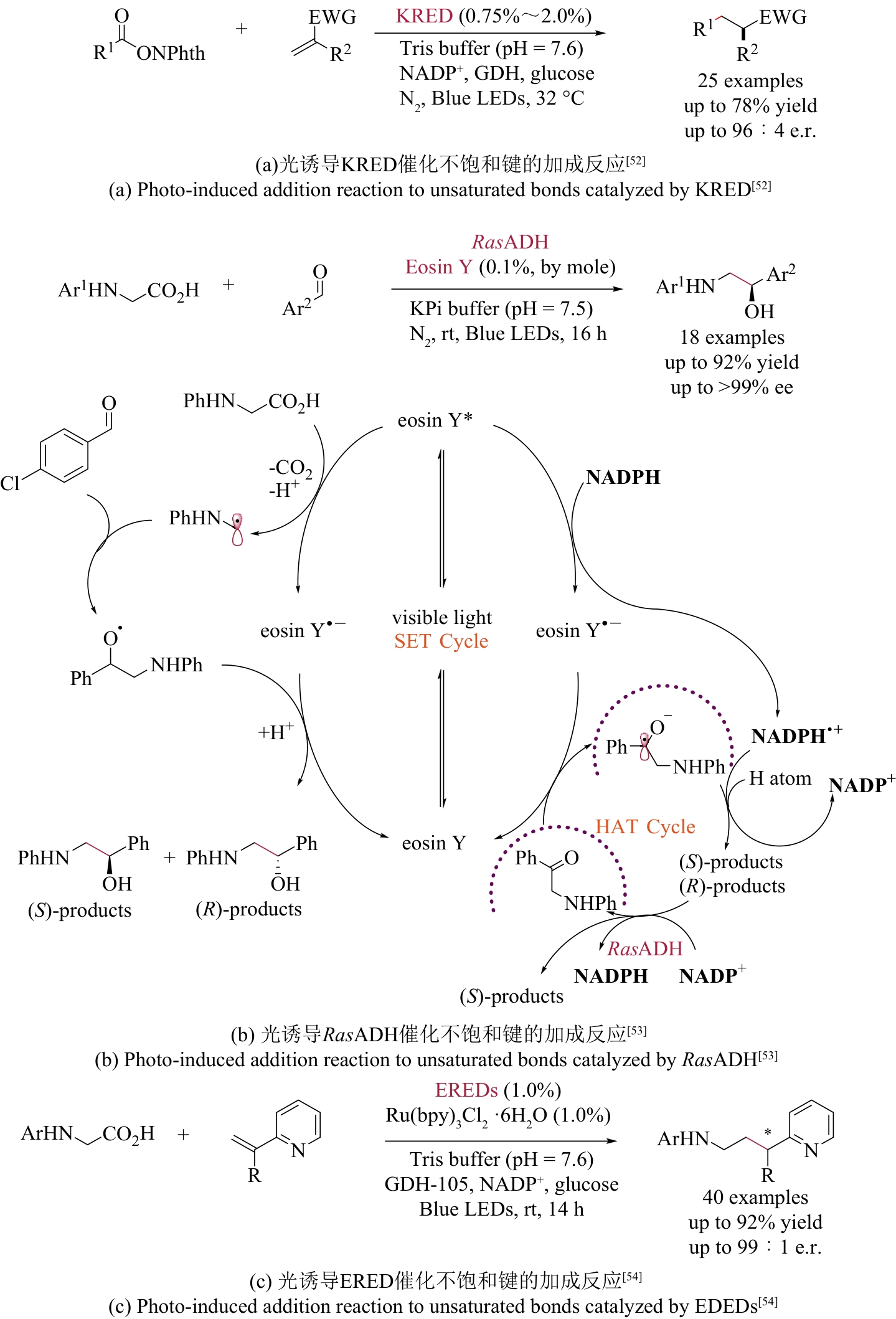
| 52 | HUANG X Q, FENG J Q, CUI J W, et al. Photoinduced chemomimetic biocatalysis for enantioselective intermolecular radical conjugate addition[J]. Nature Catalysis, 2022, 5: 586-593. |
| 53 | LIU Y Y, ZHU L Y, LI X M, et al. Photoredox/enzymatic catalysis enabling redox-neutral decarboxylative asymmetric C-C coupling for asymmetric synthesis of chiral 1,2-amino alcohols[J]. JACS Au, 2023, 3(11): 3005-3013. |
| 54 | SUN S Z, NICHOLLS B T, BAIN D, et al. Enantioselective decarboxylative alkylation using synergistic photoenzymatic catalysis[J]. Nature Catalysis, 2024, 7: 35-42. |
| 55 | XUE Y P, CAO C H, ZHENG Y G. Enzymatic asymmetric synthesis of chiral amino acids[J]. Chemical Society Reviews, 2018, 47(4): 1516-1561. |
| 56 | BLASKOVICH M A T. Unusual amino acids in medicinal chemistry[J]. Journal of Medicinal Chemistry, 2016, 59(24): 10807-10836. |
| 57 | WANG Y W, DENG L F, ZHANG X, et al. A radical approach to making unnatural amino acids: conversion of C-S bonds in cysteine derivatives into C-C bonds[J]. Angewandte Chemie International Edition, 2021, 60(4): 2155-2159. |
| 58 | DUMAS A, LERCHER L, SPICER C D, et al. Designing logical codon reassignment — expanding the chemistry in biology[J]. Chemical Science, 2015, 6(1): 50-69. |
| 59 | ELIOT A C, KIRSCH J F. Pyridoxal phosphate enzymes: mechanistic, structural, and evolutionary considerations[J]. Annual Review of Biochemistry, 2004, 73: 383-415. |
| 60 | NAJERA C, SANSANO J M. Catalytic asymmetric synthesis of alpha-amino acids[J]. Chemical Reviews, 2007, 107(11): 4584-4671. |
| 61 | CHENG L, LI D, MAI B K, et al. Stereoselective amino acid synthesis by synergistic photoredox-pyridoxal radical biocatalysis[J]. Science, 2023, 381(6656): 444-451. |
| 62 | ZHANG W, LU L X, ZHANG W, et al. Electrochemically driven cross-electrophile coupling of alkyl halides[J]. Nature, 2022, 604(7905): 292-297. |
| 63 | FU H G, CAO J Z, QIAO T Z, et al. An asymmetric sp3-sp3 cross-electrophile coupling using ‘ene’-reductases[J]. Nature, 2022, 610(7931): 302-307. |
| 64 | FU H G, QIAO T Z, CARCELLER J M, et al. Asymmetric C-alkylation of nitroalkanes via enzymatic photoredox catalysis[J]. Journal of the American Chemical Society, 2023, 145(2): 787-793. |
| 65 | BALAKRISHNAN A, PARAMASIVAM S, CHAKRABORTY S, et al. Solid-state nuclear magnetic resonance studies delineate the role of the protein in activation of both aromatic rings of thiamin[J]. Journal of the American Chemical Society, 2012, 134(1): 665-672. |
| 66 | BALAKRISHNAN A, GAO Y H, MOORJANI P, et al. Bifunctionality of the thiamin diphosphate cofactor: assignment of tautomeric/ionization states of the 4′-aminopyrimidine ring when various intermediates occupy the active sites during the catalysis of yeast pyruvate decarboxylase[J]. Journal of the American Chemical Society, 2012, 134(8): 3873-3885. |
| 67 | GAN M M, LIU J Q, ZHANG L, et al. Preparation and post-assembly modification of metallosupramolecular assemblies from poly(N-heterocyclic carbene) ligands[J]. Chemical Reviews, 2018, 118(19): 9587-9641. |
| 68 | XU Y Y, CHEN H W, YU L, et al. A light-driven enzymatic enantioselective radical acylation[J]. Nature, 2024, 625(7993): 74-78. |
| 69 | XIONG T, ZHANG Q. New amination strategies based on nitrogen-centered radical chemistry[J]. Chemical Society Reviews, 2016, 45(11): 3069-3087. |
| 70 | KWON K, SIMONS R T, NANDAKUMAR M, et al. Strategies to generate nitrogen-centered radicals that may rely on photoredox catalysis: development in reaction methodology and applications in organic synthesis[J]. Chemical Reviews, 2022, 122(2): 2353-2428. |
| 71 | YE Y X, CAO J Z, OBLINSKY D G, et al. Using enzymes to tame nitrogen-centred radicals for enantioselective hydroamination[J]. Nature Chemistry, 2023, 15(2): 206-212. |
| 72 | ZHANG Z Y, FENG J Q, YANG C, et al. Photoenzymatic enantioselective intermolecular radical hydroamination[J]. Nature Catalysis, 2023, 6(8): 687-694. |
| 73 | CHEN X Y, ZHENG D N, JIANG L Y, et al. Photoenzymatic hydrosulfonylation for the stereoselective synthesis of chiral sulfones[J]. Angewandte Chemie International Edition, 2023, 62(23): e202218140. |
| 74 | SHI Q L, KANG X W, LIU Z Y, et al. Single-electron oxidation-initiated enantioselective hydrosulfonylation of olefins enabled by photoenzymatic catalysis[J]. Journal of the American Chemical Society, 2024, 146(4): 2748-2756. |
| 75 | DUTTA U, MAITI S, BHATTACHARYA T, et al. Arene diversification through distal C(sp2)-H functionalization[J]. Science, 2021, 372(6543): eabd5992. |
| 76 | WANG Y J, CHANG W J, QIN S M, et al. Diversification of aryl sulfonyl compounds through ligand-controlled meta- and para-C-H borylation[J]. Angewandte Chemie International Edition, 2022, 61(34): e202206797. |
| 77 | LAUDER K, TOSCANI A, QI Y Y, et al. Photo-biocatalytic one-pot cascades for the enantioselective synthesis of 1,3-mercaptoalkanol volatile sulfur compounds[J]. Angewandte Chemie International Edition, 2018, 57(20): 5803-5807. |
| 78 | ZHAO B B, FENG J Q, YU L, et al. Direct visible-light-excited flavoproteins for redox-neutral asymmetric radical hydroarylation[J]. Nature Catalysis, 2023, 6(11): 996-1004. |
| 79 | COLGAN A C, PROCTOR R S J, GIBSON D C, et al. Hydrogen atom transfer driven enantioselective Minisci reaction of alcohols[J]. Angewandte Chemie International Edition, 2022, 61(25): e202200266. |
| 80 | PAGE C G, CAO J Z, OBLINSKY D G, et al. Regioselective radical alkylation of arenes using evolved photoenzymes[J]. Journal of the American Chemical Society, 2023, 145(21): 11866-11874. |
| 81 | LUJÁN A P, BHAT M F, TSATURYAN S, et al. Tailored photoenzymatic systems for selective reduction of aliphatic and aromatic nitro compounds fueled by light[J]. Nature Communications, 2023, 14(1): 5442. |
| 82 | CHEN L, ZHANG Z Y, HOSHINO A, et al. NADPH production by the oxidative pentose-phosphate pathway supports folate metabolism[J]. Nature Metabolism, 2019, 1(3): 404-415. |
| 83 | BAUMANN S, PAUL W, CHOI T Y, et al. Electron paramagnetic resonance of individual atoms on a surface[J]. Science, 2015, 350(6259): 417-420. |
| 84 | WANG Y J, XUE P, CAO M F, et al. Directed evolution: methodologies and applications[J]. Chemical Reviews, 2021, 121(20): 12384-12444. |
| 85 | QIN Z Y, ZHOU Y, LI Z, et al. Production of biobased ethylbenzene by cascade biocatalysis with an engineered photodecarboxylase[J]. Angewandte Chemie International Edition, 2024, 63(8): e202314566. |
| 86 | FU Y, LIU X H, XIA Y, et al. Whole-cell-catalyzed hydrogenation/deuteration of aryl halides with a genetically repurposed photodehalogenase[J]. Chem, 2023, 9(7): 1897-1909. |
| 87 | YU T H, CUI H Y, LI J C, et al. Enzyme function prediction using contrastive learning[J]. Science, 2023, 379(6639): 1358-1363. |
| 88 | CHONG W Y, QI Y, JI L R, et al. Computer-aided tunnel engineering: a promising strategy for improving lipase applications in esterification reactions[J]. ACS Catalysis, 2024, 14(1): 67-83. |
| 89 | BENINCÁ L A D, FRANÇA A S, BRÊDA G C, et al. Continuous-flow CvFAP photodecarboxylation of palmitic acid under environmentally friendly conditions[J]. Molecular Catalysis, 2022, 528: 112469. |
| 90 | TAN C L, TAO F, XU P. Direct carbon capture for the production of high-performance biodegradable plastics by cyanobacterial cell factories[J]. Green Chemistry, 2022, 24(11): 4470-4483. |
| 91 | ÖZGEN F F, RUNDA M E, SCHMIDT S. Photo-biocatalytic cascades: combining chemical and enzymatic transformations fueled by light[J]. ChemBioChem, 2021, 22(5): 790-806. |
| 92 | TAYLOR A, HEYES D J, SCRUTTON N S. Catalysis by nature′s photoenzymes[J]. Current Opinion in Structural Biology, 2022, 77: 102491. |
| 93 | COLLINS K D, GENSCH T, GLORIUS F. Contemporary screening approaches to reaction discovery and development[J]. Nature Chemistry, 2014, 6(10): 859-871. |
| 94 | VOLK M J, TRAN V G, TAN S I, et al. Metabolic engineering: methodologies and applications[J]. Chemical Reviews, 2023, 123(9): 5521-5570. |
| 95 | BULLER R, LUTZ S, KAZLAUSKAS R J, et al. From nature to industry: harnessing enzymes for biocatalysis[J]. Science, 2023, 382(6673): eadh8615. |
| [1] | WANG Ziyuan, YANG Lirong, WU Jianping, ZHENG Wenlong. A review on enzyme-catalyzed synthesis of chiral amino acids [J]. Synthetic Biology Journal, 2024, 5(6): 1319-1349. |
| [2] | Zhen ZHU, Jing TIAN, Jing JIANG, Wangyin WANG, Xupeng CAO. Progress in microalgae chloroplast organelle factory development [J]. Synthetic Biology Journal, 2022, 3(6): 1218-1234. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||