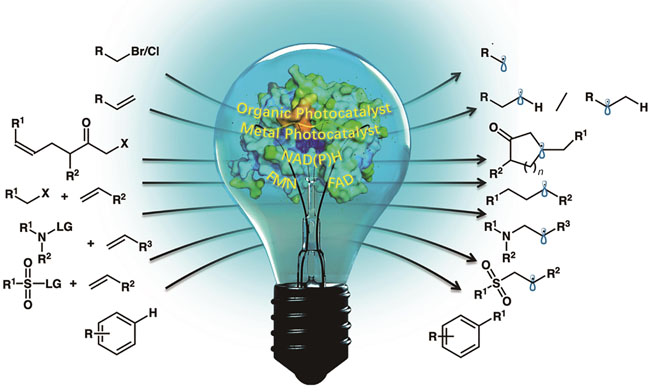
Photocatalysis has the advantages of mild reaction conditions, renewability, and strong reactivity, but the poor selectivity limits its further application in asymmetric synthesis. Enzymatic catalysis shows unique advantages of high selectivity and specificity, but it leads to some defects such as limited reaction types and relatively narrow substrate scope. Photoenzymatic catalysis combines the advantages of high reactivity of photocatalysis with high selectivity of enzymatic catalysis, providing a novel synthesis model, that is more in line with the requirements of modern green organic synthesis. The term “photoenzyme reactions” narrowly refers to the synergistic catalysis involving photoenzymes, which can be classified into the following four categories: natural photoenzymactic reactions, artificial photoenzymatic reactions, photo-biocatalysis cascade reactions, and photo-induced promiscuous enzymatic reactions. However, natural photoenzymes are rarely found in nature, the stringent substrate scope further hinders their application. Artificial photoenzymes integrate photosensitizers into the scaffold of natural enzymes, which have been well summarized in previous reviews. Photo-biocatalysis cascade reactions by combining photochemical steps and enzymatic steps can realize some complex organic synthesis processes. Since the first report on NAD(P)H-dependent KREDs-catalyzed enantioselective radical dehalogenation of lactones, photosensitive cofactor-dependent unnatural photoenzymatic catalysis demonstrated its great potential in the field of organic synthesis, and continues to thrive to date, which has addressed many problems difficult to be achieved in traditional organic synthesis. Since 2023, research into the promiscuity of photoenzyme catalysis has witnessed continuous breakthroughs, reporting diverse novel types of photoenzyme catalytic reactions and mechanisms. The precise control over stereoselectivity and even regioselectivity directly addresses the longstanding challenges in the field of organic synthesis. While there have been many publications summarizing the related research, yet rarely focused on this rapidly evolving field. In this review, we summarize the recent and representative reports of photo-induced promiscuous enzymatic reactions, and classify them according to asymmetric dehalogenation, hydrogenation, intramolecular cyclization, intermolecular C—C/C—N/C—S cross-coupling reactions through free radical pathways, etc. These reactions exhibit different mechanisms due to different enzymes and substrates. For example, in the process of redox initiation, there are two types: single-electron reduction initiation and single-electron oxidation initiation. In the radical termination process, single-electron reduction termination and single-electron oxidation termination may be used. The diversity of mechanisms also makes it possible to develop more photoenzyme-catalyzed promiscuous reactions. In the future, new photoenzymatic methods will be promoted by rapidly developing technologies such as genetic engineering, synthetic biology, enzyme engineering, flow chemistry, and artificial intelligence, and more efficient and highly selective new-to-nature reactions will emerge, significantly expanding the application range of photoenzyme catalysis in the field of green asymmetric synthesis.