Synthetic Biology Journal ›› 2024, Vol. 5 ›› Issue (5): 913-940.DOI: 10.12211/2096-8280.2024-028
• Invited Review • Previous Articles Next Articles
Chemoenzymatic synthesis of natural products: evolution of synthetic methodology and strategy
ZHANG Shouqi, WANG Tao, KONG Yao, ZOU Jiasheng, LIU Yuanning, XU Zhengren
- State Key Laboratory of Natural and Biomimetic Drugs,School of Pharmaceutical Sciences,Peking University,Beijing 100191,China
-
Received:2024-03-25Revised:2024-05-28Online:2024-11-20Published:2024-10-31 -
Contact:XU Zhengren
天然产物的化学-酶法合成:方法与策略的演进
张守祺, 王涛, 孔尧, 邹家胜, 刘元宁, 徐正仁
- 北京大学药学院,天然药物及仿生药物全国重点实验室,北京 100191
-
通讯作者:徐正仁 -
作者简介:张守祺 (1998—),男,博士研究生。研究方向为活性二萜的化学-酶法合成。 E-mail:shouqizhang_pku@pku.edu.cn王涛 (1991—),男,博士研究生。研究方向为活性二萜的化学-酶法合成。 E-mail:1005704167@qq.com徐正仁 (1983—),男,研究员,博士生导师。研究方向为活性天然产物的化学-酶法合成、新颖生物催化反应的发现与应用、天然药物化学等。 E-mail:zhengrenxu@bjmu.edu.cn -
基金资助:国家自然科学基金(81991525)
CLC Number:
Cite this article
ZHANG Shouqi, WANG Tao, KONG Yao, ZOU Jiasheng, LIU Yuanning, XU Zhengren. Chemoenzymatic synthesis of natural products: evolution of synthetic methodology and strategy[J]. Synthetic Biology Journal, 2024, 5(5): 913-940.
张守祺, 王涛, 孔尧, 邹家胜, 刘元宁, 徐正仁. 天然产物的化学-酶法合成:方法与策略的演进[J]. 合成生物学, 2024, 5(5): 913-940.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2024-028
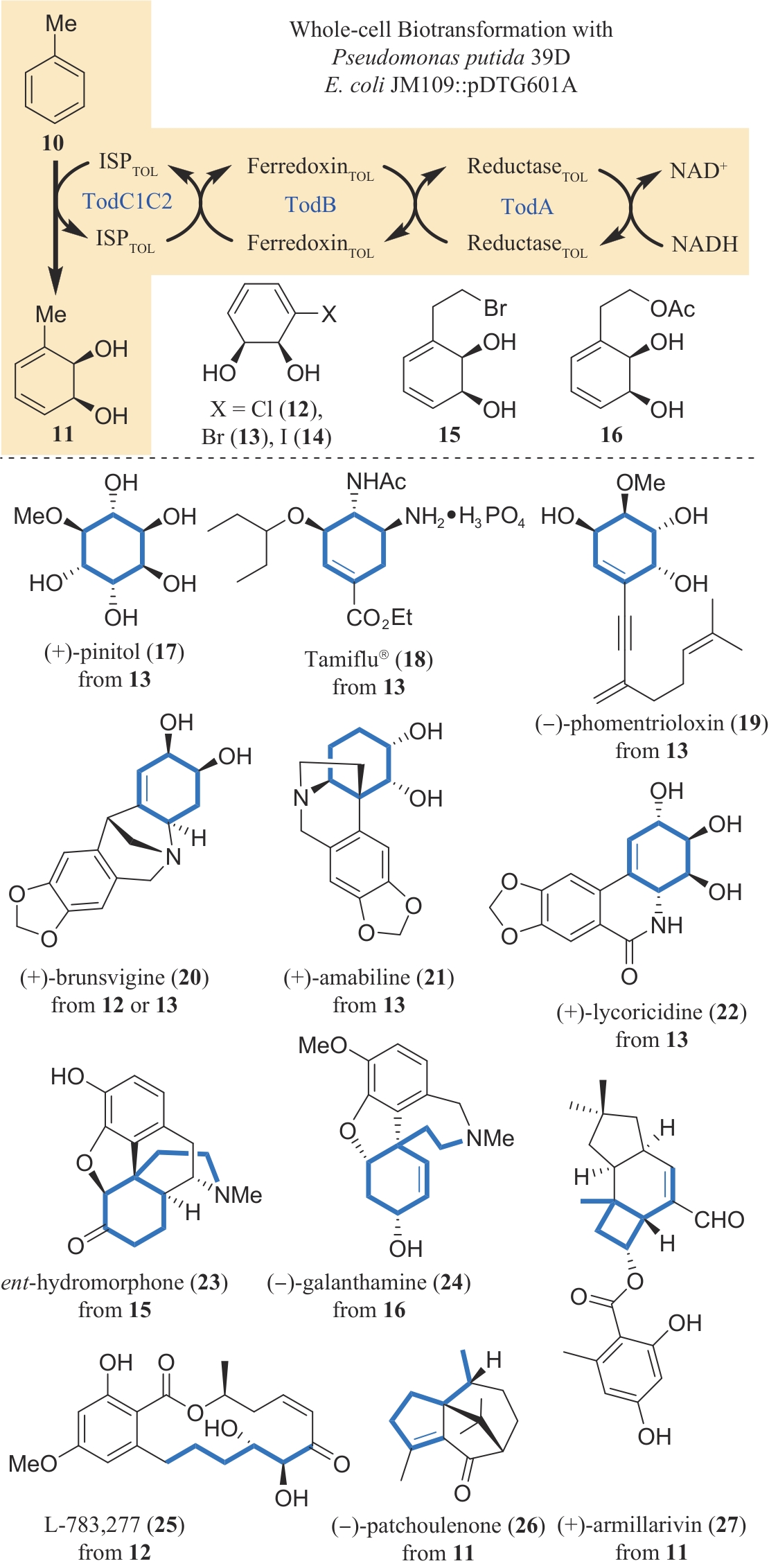
Fig. 3 Introduction of the key chiral center(s) to a simple substrate by the action of toluene dioxygenase and its application in the synthesis of various natural products
| 1 | NEWMAN D J, CRAGG G M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019[J]. Journal of Natural Products, 2020, 83(3): 770-803. |
| 2 | CRAGG G M, NEWMAN D J. Natural products: a continuing source of novel drug leads[J]. Biochimica et Biophysica Acta (BBA) - General Subjects, 2013, 1830(6): 3670-3695. |
| 3 | CARLSON E E. Natural products as chemical probes[J]. ACS Chemical Biology, 2010, 5(7): 639-653. |
| 4 | PYE C R, BERTIN M J, LOKEY R S, et al. Retrospective analysis of natural products provides insights for future discovery trends[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(22): 5601-5606. |
| 5 | BUTLER M S. The role of natural product chemistry in drug discovery[J]. Journal of Natural Products, 2004, 67(12): 2141-2153. |
| 6 | KOEHN F E, CARTER G T. The evolving role of natural products in drug discovery[J]. Nature Reviews Drug Discovery, 2005, 4(3): 206-220. |
| 7 | ATANASOV A G, ZOTCHEV S B, DIRSCH V M, et al. Natural products in drug discovery: advances and opportunities[J]. Nature Reviews Drug Discovery, 2021, 20(3): 200-216. |
| 8 | WILSON B A P, THORNBURG C C, HENRICH C J, et al. Creating and screening natural product libraries[J]. Natural Product Reports, 2020, 37(7): 893-918. |
| 9 | NEWMAN D J. Natural products as leads to potential drugs: an old process or the new hope for drug discovery?[J]. Journal of Medicinal Chemistry, 2008, 51(9): 2589-2599. |
| 10 | LI L, CHEN Z, ZHANG X W, et al. Divergent strategy in natural product total synthesis[J]. Chemical Reviews, 2018, 118(7): 3752-3832. |
| 11 | MULZER J. Trying to rationalize total synthesis[J]. Natural Product Reports, 2014, 31(4): 595-603. |
| 12 | WENDER P A. Toward the ideal synthesis and molecular function through synthesis-informed design[J]. Natural Product Reports, 2014, 31(4): 433-440. |
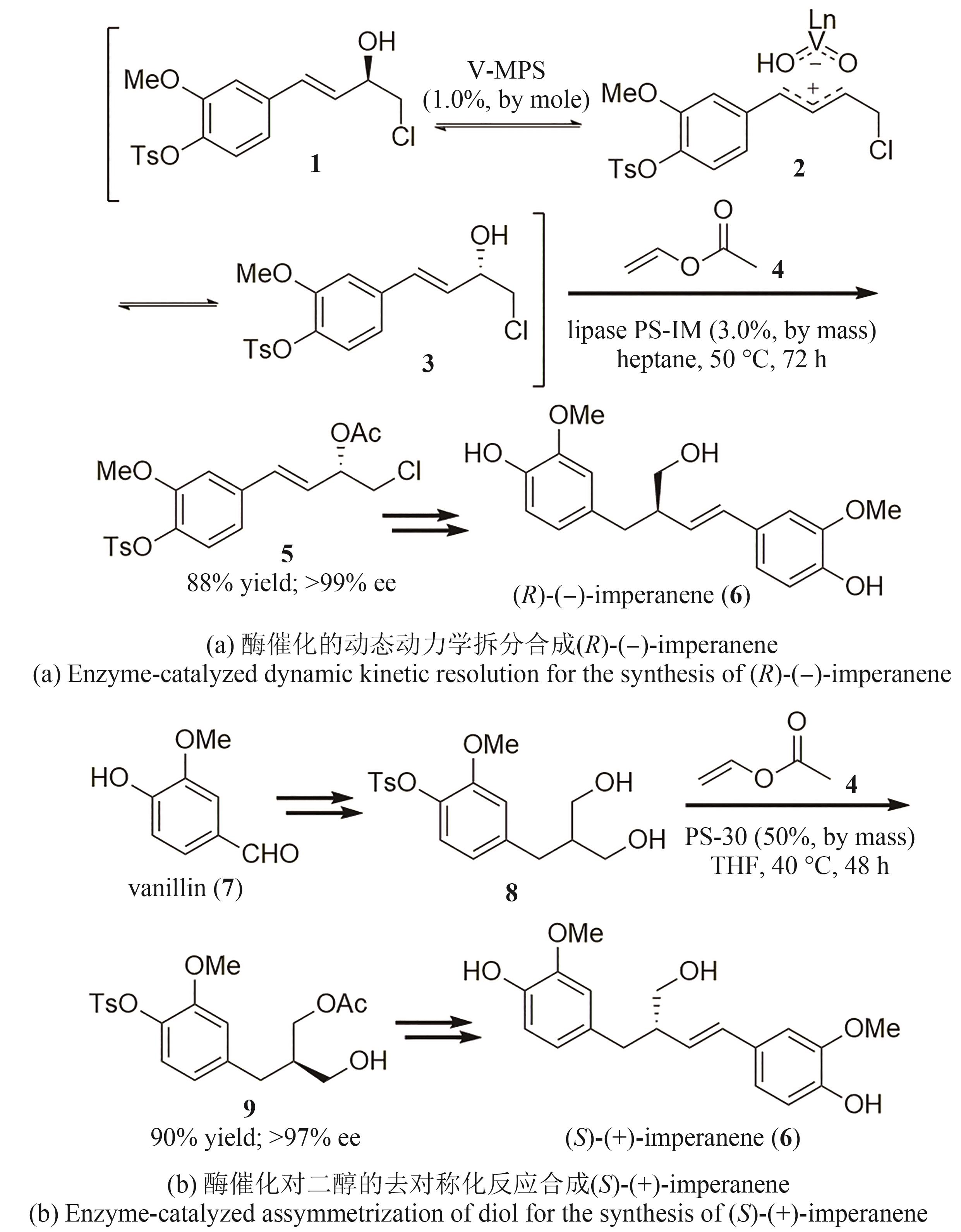
| 13 | DRAUZ K . GRÖGER H . MAY O. Enzyme catalysis in organic synthesis[M/OL]. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA, 2012. (2012-02-22)[2024-03-01]. . |
| 14 | RODRÍGUEZ BENÍTEZ A, NARAYAN A R H. Frontiers in biocatalysis: profiling function across sequence space[J]. ACS Central Science, 2019, 5(11): 1747-1749. |
| 119 | NAKAMURA H, SCHULTZ E E, BALSKUS E P. A new strategy for aromatic ring alkylation in cylindrocyclophane biosynthesis[J]. Nature Chemical Biology, 2017, 13(8): 916-921. |
| 120 | WANG H Q, MOU S B, XIAO W, et al. Structural basis for the Friedel-Crafts alkylation in cyclindrocyclophane biosynthesis [J]. ACS Catalysis, 2022, 12(3): 2108-2117. |
| 121 | CHEN K Y, WANG H Q, YUAN Y, et al. Chemoenzymatic synthesis of cylindrocyclophanes A and F and merocyclophanes A and D[J]. Angewandte Chemie International Edition, 2023, 62(46): e202307602. |
| 122 | GAO J M, LIU S N, ZHOU C, et al. A pyridoxal 5'-phosphate-dependent Mannich cyclase[J]. Nature Catalysis, 2023, 6: 476-486. |
| 123 | LIU S N, HAI Y. Chemoenzymatic approaches to izidine alkaloids: an efficient total synthesis of (+)-absouline and laburnamine[J]. ACS Catalysis, 2023, 13(24): 15725-15729. |
| 124 | WU X M, GUAN Q Y, HAN Y B, et al. Regeneration of phytochemicals by structure-driven organization of microbial biosynthetic steps[J]. Angewandte Chemie International Edition, 2022, 61(8): e202114919. |
| 125 | KIEFER A F, LIU Y C, GUMMERER R, et al. An artificial in vitro metabolism to angiopterlactone B inspired by traditional retrosynthesis[J]. Angewandte Chemie International Edition, 2023, 62(23): e202301178. |
| 126 | YI D, BAYER T, BADENHORST C P S, et al. Recent trends in biocatalysis[J]. Chemical Society Reviews, 2021, 50(14): 8003-8049. |
| 127 | YANG Y, ARNOLD F H. Navigating the unnatural reaction space: directed evolution of heme proteins for selective carbene and nitrene transfer[J]. Accounts of Chemical Research, 2021, 54(5): 1209-1225. |
| 128 | PAN Y J, LI G B, LIU R X, et al. Unnatural activities and mechanistic insights of cytochrome P450 PikC gained from site-specific mutagenesis by non-canonical amino acids[J]. Nature Communications, 2023, 14(1): 1669. |
| 129 | ROMERO E O, SAUCEDO A T, HERNÁNDEZ-MELÉNDEZ J R, et al. Enabling broader adoption of biocatalysis in organic chemistry[J]. Journal of the American Chemical Society Au, 2023, 3(8): 2073-2085. |
| 15 | LEWIS R D, FRANCE S P, MARTINEZ C A. Emerging technologies for biocatalysis in the pharmaceutical industry[J]. ACS Catalysis, 2023, 13(8): 5571-5577. |
| 16 | TRUPPO M D. Biocatalysis in the pharmaceutical industry: the need for speed[J]. ACS Medicinal Chemistry Letters, 2017, 8(5): 476-480. |
| 17 | REED J H, SEEBECK F P. Reagent engineering for group transfer biocatalysis[J]. Angewandte Chemie International Edition, 2024, 63(7): e202311159. |
| 18 | WU S K, SNAJDROVA R, MOORE J C, et al. Biocatalysis: enzymatic synthesis for industrial applications[J]. Angewandte Chemie International Edition, 2021, 60(1): 88-119. |
| 19 | TURNER N J, O’REILLY E. Biocatalytic retrosynthesis[J]. Nature Chemical Biology, 2013, 9(5): 285-288. |
| 20 | KIRSCHNING A, HAHN F. Merging chemical synthesis and biosynthesis: a new chapter in the total synthesis of natural products and natural product libraries[J]. Angewandte Chemie International Edition, 2012, 51(17): 4012-4022. |
| 21 | FRIEDRICH S, HAHN F. Opportunities for enzyme catalysis in natural product chemistry[J]. Tetrahedron, 2015, 71(10): 1473-1508. |
| 22 | MURRAY L A M, MCKINNIE S M K, MOORE B S, et al. Meroterpenoid natural products from Streptomyces bacteria- the evolution of chemoenzymatic syntheses[J]. Natural Product Reports, 2020, 37(10): 1334-1366. |
| 23 | CHAKRABARTY S, ROMERO E O, PYSER J B, et al. Chemoenzymatic total synthesis of natural products[J]. Accounts of Chemical Research, 2021, 54(6): 1374-1384. |
| 24 | ZHANG H L, TANG X Y. Combining microbial and chemical syntheses for the production of complex natural products[J]. Chinese Journal of Natural Medicines, 2022, 20(10): 729-736. |
| 25 | RODDAN R, CARTER E M, THAIR B, et al. Chemoenzymatic approaches to plant natural product inspired compounds[J]. Natural Product Reports, 2022, 39(7): 1375-1382. |
| 26 | STOUT C N, WASFY N M, CHEN F, et al. Charting the evolution of chemoenzymatic strategies in the syntheses of complex natural products[J]. Journal of the American Chemical Society, 2023, 145(33): 18161-18181. |
| 27 | BRILL Z G, CONDAKES M L, TING C P, et al. Navigating the chiral pool in the total synthesis of complex terpene natural products[J]. Chemical Reviews, 2017, 117(18): 11753-11795. |
| 28 | BREUER M, DITRICH K, HABICHER T, et al. Industrial methods for the production of optically active intermediates[J]. Angewandte Chemie International Edition, 2004, 43(7): 788-824. |
| 29 | FACIN B R, MELCHIORS M S, VALÉRIO A, et al. Driving immobilized lipases as biocatalysts: 10 years state of the art and future prospects[J]. Industrial & Engineering Chemistry Research, 2019, 58(14): 5358-5378. |
| 30 | WANG H H, ZHANG Q, YU X, et al. Application of lipase B from Candida antarctica in the pharmaceutical industry [J]. Industrial & Engineering Chemistry Research, 2023, 62(39): 15733-15751. |
| 31 | SLABU I, GALMAN J L, LLOYD R C, et al. Discovery, engineering, and synthetic application of transaminase biocatalysts[J]. ACS Catalysis, 2017, 7(12): 8263-8284. |
| 32 | TOOGOOD H S, SCRUTTON N S. Discovery, characterization, engineering and applications of ene-reductases for industrial biocatalysis[J]. ACS Catalysis, 2018, 8(4): 3532-3549. |
| 33 | YANG L C, DENG H P, RENATA H. Recent progress and developments in chemoenzymatic and biocatalytic dynamic kinetic resolution[J]. Organic Process Research & Development, 2022, 26(7): 1925-1943. |
| 34 | EGI M, SUGIYAMA K, SANETO M, et al. A mesoporous-silica-immobilized oxovanadium cocatalyst for the lipase-catalyzed dynamic kinetic resolution of racemic alcohols[J]. Angewandte Chemie International Edition, 2013, 52(13): 3654-3658. |
| 35 | MATSUNAGA K, SHIBUYA M, Imperanene OHIZUMI Y., a novel phenolic compound with platelet aggregation inhibitory activity from Imperata cylindrica [J]. Journal of Natural Products, 1995, 58(1): 138-139. |
| 36 | CARR J A, BISHT K S. Enantioselective synthesis of imperanene via enzymatic asymmetrization of an intermediary 1,3-diol[J]. Organic Letters, 2004, 6(19): 3297-3300. |
| 37 | GIBSON D T, KOCH J R, KALLIO R E. Oxidative degradation of aromatic hydrocarbons by microorganisms.Ⅰ. Enzymatic formation of catechol from benzene[J]. Biochemistry, 1968, 7(7): 2653-2662. |
| 38 | GIBSON D T, KOCH J R, SCHULD C L, et al. Oxidative degradation of aromatic hydrocarbons by microorganisms.Ⅱ. Metabolism of halogenated aromatic hydrocarbons[J]. Biochemistry, 1968, 7(11): 3795-3802. |
| 39 | GIBSON D T, HENSLEY M, YOSHIOKA H, et al. Formation of (+)-cis-2,3-dihydroxy-1-methylcyclohexa-4,6-diene from toluene by Pseudomonas putida [J]. Biochemistry, 1970, 9(7): 1626-1630. |
| 40 | ZYLSTRA G J, GIBSON D T. Toluene degradation by Pseudomonas putida F1. Nucleotide sequence of the todC1C2BADE genes and their expression in Escherichia coli [J]. The Journal of Biological Chemistry, 1989, 264(25): 14940-14946. |
| 41 | HUDLICKY T, GONZALEZ D, GIBSON D T. Enzymatic dihydroxylation of aromatics in enantioselective synthesis: expanding asymmetric methodology [J]. Aldrichimica Acta, 1999, 32(2): 35-62. |
| 42 | HUDLICKY T, REED J. Celebrating 20 years of SYNLETT- special account on the merits of biocatalysis and the impact of arene cis-dihydrodiolson enantioselective synthesis[J]. Synlett, 2009(5): 685-703. |
| 43 | LEWIS S E. Applications of biocatalytic arene ipso, ortho cis-dihydroxylation in synthesis[J]. Chemical Communications, 2014, 50(22): 2821-2830. |
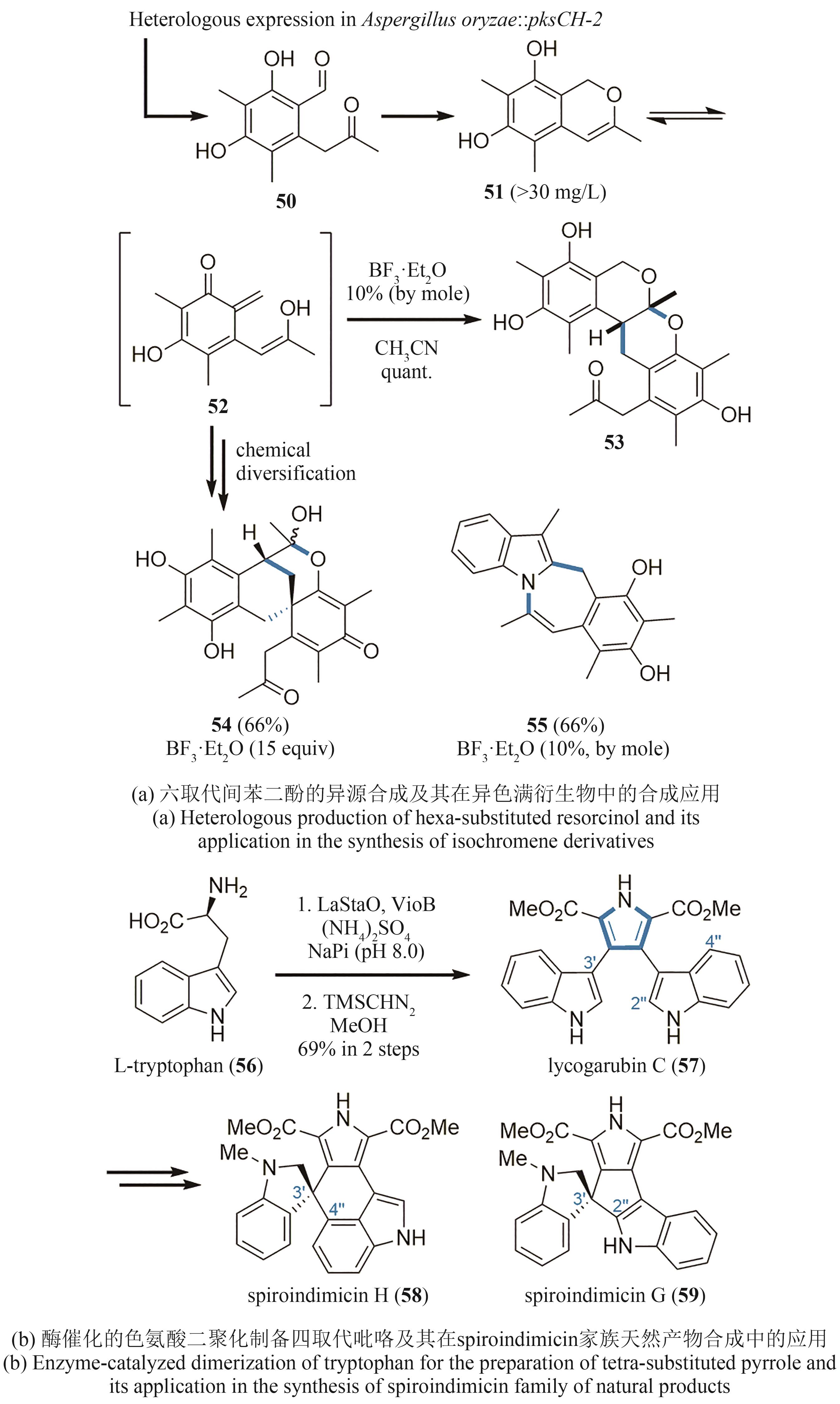
| 44 | TAHER E S, BANWELL M G, BUCKLER J N, et al. The exploitation of enzymatically-derived cis-1,2-dihydrocatechols and related compounds in the synthesis of biologically active natural products[J]. Chemical Record, 2018, 18(2): 239-264. |
| 45 | LAN P, YE S, BANWELL M G. The application of dioxygenase-based chemoenzymatic processes to the total synthesis of natural products[J]. Chemistry, an Asian Journal, 2019, 14(22): 4001-4012. |
| 46 | HUDLICKY T, PRICE J D, FAN R L, et al. Efficient and enantiodivergent synthesis of (+)- and (-)-pinitol[J]. Journal of the American Chemical Society, 1990, 112(25): 9439-9440. |
| 47 | MATVEENKO M, WILLIS A C, BANWELL M G. A chemoenzymatic synthesis of the anti-influenza agent Tamiflu®[J]. Tetrahedron Letters, 2008, 49(49): 7018-7020. |
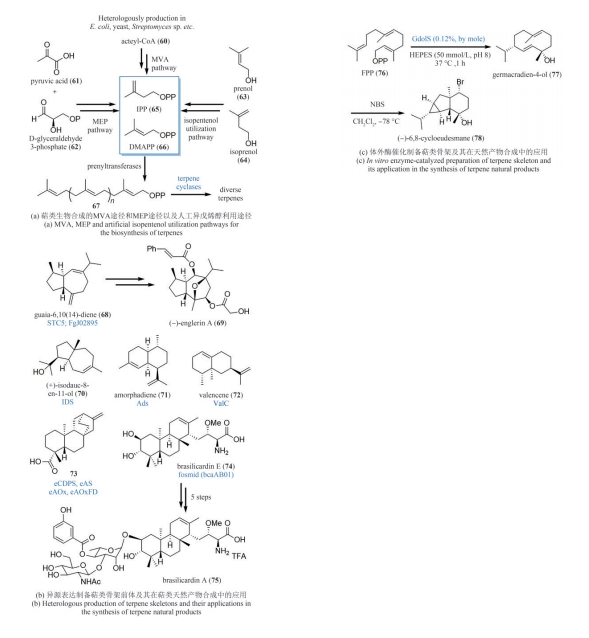
| 48 | MA X H, BANWELL M G, WILLIS A C. Chemoenzymatic total synthesis of the phytotoxic geranylcyclohexentriol (-)-phomentrioloxin[J]. Journal of Natural Products, 2013, 76(8): 1514-1518. |
| 49 | BANWELL M G, KOKAS O J, WILLIS A C. Chemoenzymatic approaches to the montanine alkaloids: a total synthesis of (+)-brunsvigine[J]. Organic Letters, 2007, 9(18): 3503-3506. |
| 50 | FINDLAY A D, BANWELL M G. A chemoenzymatic total synthesis of (+)-amabiline[J]. Organic Letters, 2009, 11(14): 3160-3162. |
| 51 | HUDLICKY T, OLIVO H F. A short synthesis of (+)- lycoricidine[J]. Journal of the American Chemical Society, 1992, 114(24): 9694-9696. |
| 52 | ENDOMA-ARIAS M A A, HUDLICKY T. Chemoenzymatic total synthesis of (+)-galanthamine and (+)-narwedine from phenethyl acetate[J]. Chemistry, 2016, 22(41): 14540-14543. |
| 53 | VARGHESE V, HUDLICKY T. Short chemoenzymatic total synthesis of ent-hydromorphone: an oxidative dearomatization/intramolecular [4+2] cycloaddition/amination sequence[J]. Angewandte Chemie International Edition, 2014, 53(17): 4355-4358. |
| 54 | LIN A, WILLIS A C, BANWELL M G. A chemoenzymatic and enantioselective total synthesis of the resorcylic acid lactone L-783, 290, the trans-isomer of L-783, 277[J]. Tetrahedron Letters, 2010, 51(7): 1044-1047. |
| 55 | BANWELL M G, HOCKLESS D C R, MCLEOD M D. Chemoenzymatic total syntheses of the sesquiterpene (-)-patchoulenone[J]. New Journal of Chemistry, 2003, 27(1): 50-59. |
| 56 | SCHWARTZ B D, MATOUŠOVÁ E, WHITE R, et al. A chemoenzymatic total synthesis of the protoilludane aryl ester (+)-armillarivin[J]. Organic Letters, 2013, 15(8): 1934-1937. |
| 57 | BAKER DOCKREY S A, NARAYAN A R H. Flavin-dependent biocatalysts in synthesis[J]. Tetrahedron, 2019, 75(9): 1115-1121. |
| 58 | FAHAD A A, ABOOD A, FISCH K M, et al. Oxidative dearomatisation: the key step of sorbicillinoid biosynthesis[J]. Chemical Science, 2014, 5(2): 523-527. |
| 59 | SIB A, GULDER T A M. Stereoselective total synthesis of bisorbicillinoid natural products by enzymatic oxidative dearomatization/dimerization[J]. Angewandte Chemie International Edition, 2017, 56(42): 12888-12891. |
| 60 | BAKER DOCKREY S A, LUKOWSKI A L, BECKER M R, et al. Biocatalytic site- and enantioselective oxidative dearomatization of phenols[J]. Nature Chemistry, 2018, 10(2): 119-125. |
| 61 | DAVISON J, FAHAD A A, CAI M H, et al. Genetic, molecular, and biochemical basis of fungal tropolone biosynthesis[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(20): 7642-7647. |
| 62 | ZABALA A O, XU W, CHOOI Y H, et al. Characterization of a silent azaphilone gene cluster from Aspergillus niger ATCC 1015 reveals a hydroxylation-mediated pyran-ring formation[J]. Chemistry & Biology, 2012, 19(8): 1049-1059. |
| 63 | SOMOZA A D, LEE K H, CHIANG Y M, et al. Reengineering an azaphilone biosynthesis pathway in Aspergillus nidulans to create lipoxygenase inhibitors[J]. Organic Letters, 2012, 14(4): 972-975. |
| 64 | PYSER J B, BAKER DOCKREY S A, BENÍTEZ A R, et al. Stereodivergent, chemoenzymatic synthesis of azaphilone natural products[J]. Journal of the American Chemical Society, 2019, 141(46): 18551-18559. |
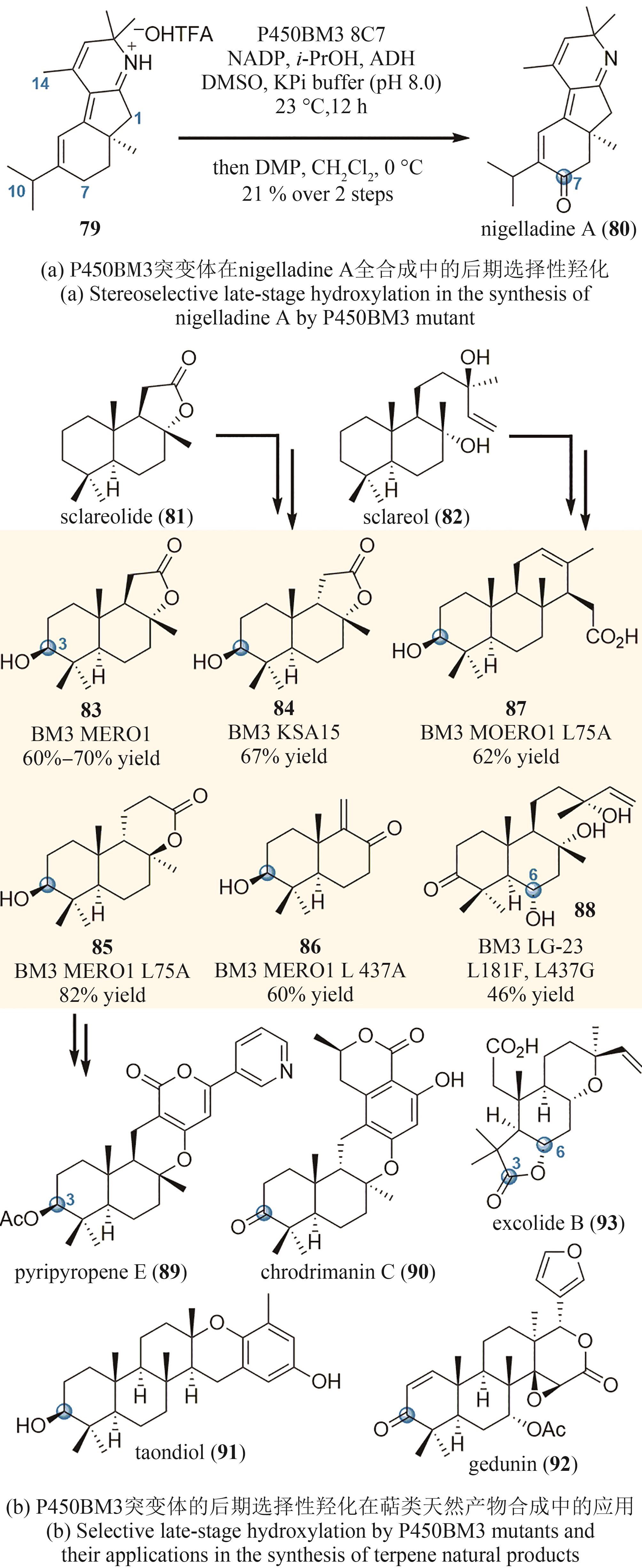
| 65 | HERR C Q, HAUSINGER R P. Amazing diversity in biochemical roles of Fe(Ⅱ)/2-oxoglutarate oxygenases[J]. Trends in Biochemical Sciences, 2018, 43(7): 517-532. |
| 66 | ITOH H, INOUE M. Comprehensive structure-activity relationship studies of macrocyclic natural products enabled by their total syntheses[J]. Chemical Reviews, 2019, 119(17): 10002-10031. |
| 67 | BAUD D, SAAIDI P L, MONFLEUR A, et al. Synthesis of mono- and dihydroxylated amino acids with new α-ketoglutarate-dependent dioxygenases: biocatalytic oxidation of C—H bonds[J]. ChemCatChem, 2014, 6(10): 3012-3017. |
| 68 | ZHANG X, KING-SMITH E, RENATA H. Total synthesis of tambromycin by combining chemocatalytic and biocatalytic C—H functionalization[J]. Angewandte Chemie International Edition, 2018, 57(18): 5037-5041. |
| 69 | MATTAY J, HÜTTEL W. Pipecolic acid hydroxylases: a monophyletic clade among cis-selective bacterial proline hydroxylases that discriminates L-proline[J]. ChemBioChem, 2017, 18(15): 1523-1528. |
| 70 | C R Ⅲ ZWICK, SOSA M B, RENATA H. Characterization of a citrulline 4-hydroxylase from nonribosomal peptide GE81112 biosynthesis and engineering of its substrate specificity for the chemoenzymatic synthesis of enduracididine[J]. Angewandte Chemie International Edition, 2019, 58(52): 18854-18858. |
| 71 | C R Ⅲ ZWICK, SOSA M B, RENATA H. Modular chemoenzymatic synthesis of GE81112 B1 and related analogues enables elucidation of its key pharmacophores[J]. Journal of the American Chemical Society, 2021, 143(3): 1673-1679. |
| 72 | FAN J, LIAO G, KINDINGER F, et al. Peniphenone and penilactone formation in Penicillium crustosum via 1,4-Michael additions of ortho-quinone methide from hydroxyclavatol to γ-butyrolactones from crustosic acid[J]. Journal of the American Chemical Society, 2019, 141(10): 4225-4229. |
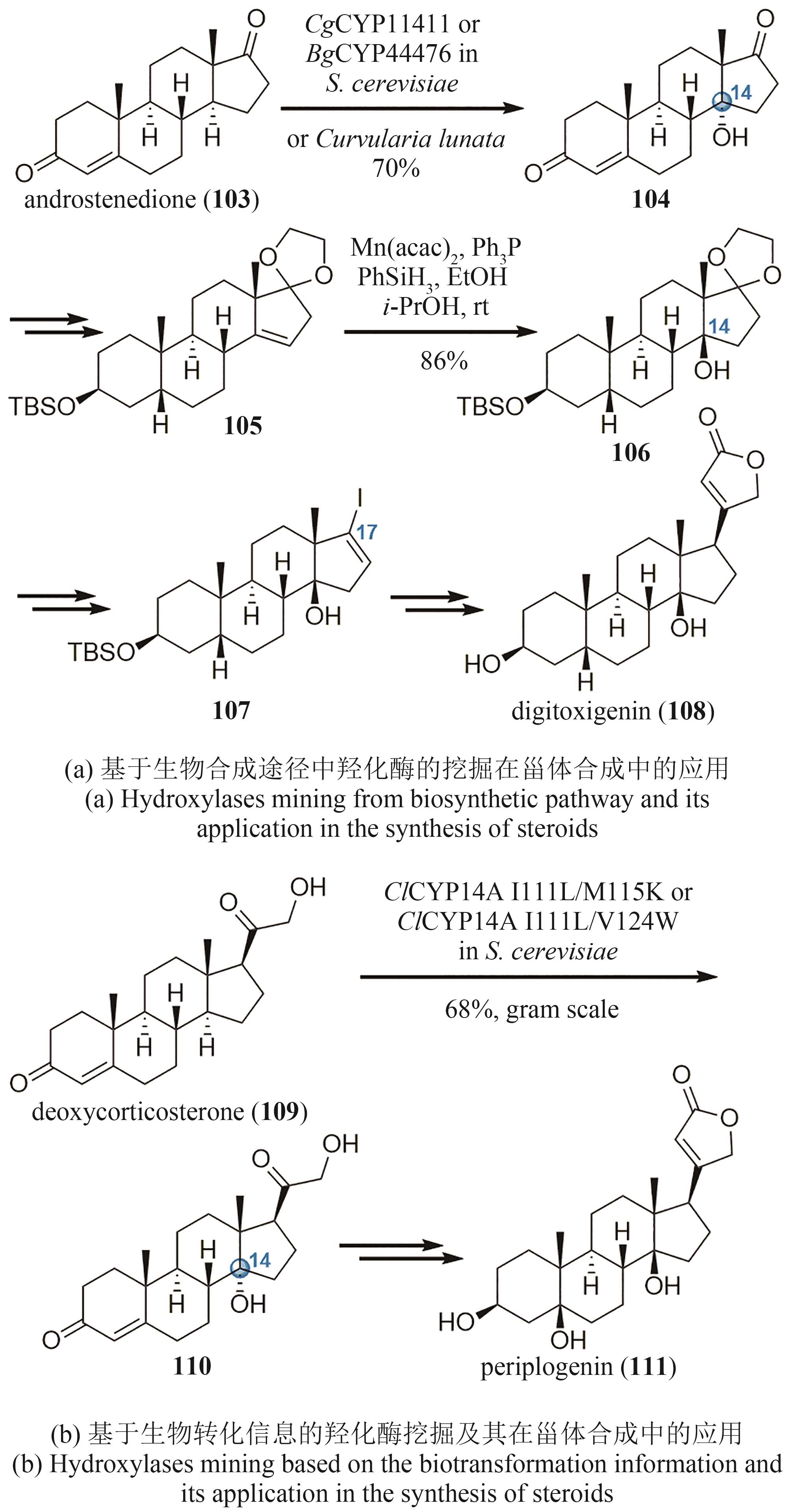
| 73 | DOYON T J, PERKINS J C, BAKER DOCKREY S A, et al. Chemoenzymatic o-quinone methide formation[J]. Journal of the American Chemical Society, 2019, 141(51): 20269-20277. |
| 74 | ROMERO E O, PERKINS J C, BURCH J E, et al. Chemoenzymatic synthesis of (+)-xyloketal B[J]. Organic Letters, 2023, 25(9): 1547-1552. |
| 75 | ASAI T, YAMAMOTO T, SHIRATA N, et al. Structurally diverse chaetophenol productions induced by chemically mediated epigenetic manipulation of fungal gene expression[J]. Organic Letters, 2013, 15(13): 3346-3349. |
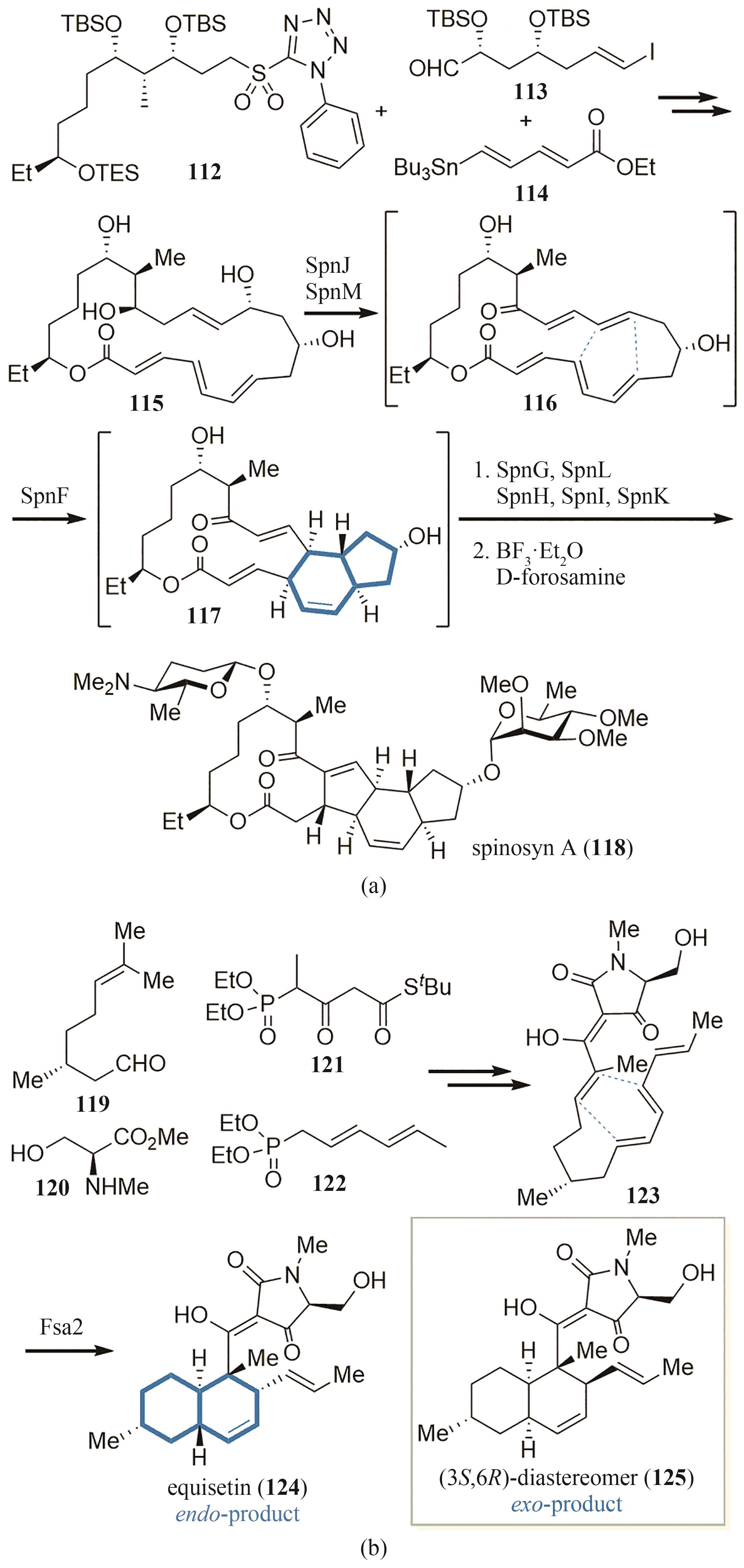
| 76 | ASAI T, TSUKADA K, ISE S, et al. Use of a biosynthetic intermediate to explore the chemical diversity of pseudo-natural fungal polyketides[J]. Nature Chemistry, 2015, 7(9): 737-743. |
| 77 | SÁNCHEZ C, MÉNDEZ C, SALAS J A. Indolocarbazole natural products: occurrence, biosynthesis, and biological activity[J]. Natural Product Reports, 2006, 23(6): 1007-1045. |
| 78 | BLAIR L M, SPERRY J. Total syntheses of (±)- spiroindimicins B and C enabled by a late-stage Schöllkopf-Magnus-Barton-Zard (SMBZ) reaction[J]. Chemical Communications, 2016, 52(4): 800-802. |
| 79 | ZHANG Z, RAY S, IMLAY L, et al. Total synthesis of (+)-spiroindimicin A and congeners unveils their antiparasitic activity[J]. Chemical Science, 2021, 12(30): 10388-10394. |
| 80 | ZHENG X K, LI Y, GUAN M T, et al. Biomimetic total synthesis of the spiroindimicin family of natural products[J]. Angewandte Chemie International Edition, 2022, 61(38): e202208802. |
| 81 | BUREAU J A, OLIVA M E, DONG Y M, et al. Engineering yeast for the production of plant terpenoids using synthetic biology approaches[J]. Natural Product Reports, 2023, 40(12): 1822-1848. |
| 82 | SIEMON T, WANG Z Q, BIAN G K, et al. Semisynthesis of plant-derived englerin A enabled by microbe engineering of guaia-6,10(14)-diene as building block[J]. Journal of the American Chemical Society, 2020, 142(6): 2760-2765. |
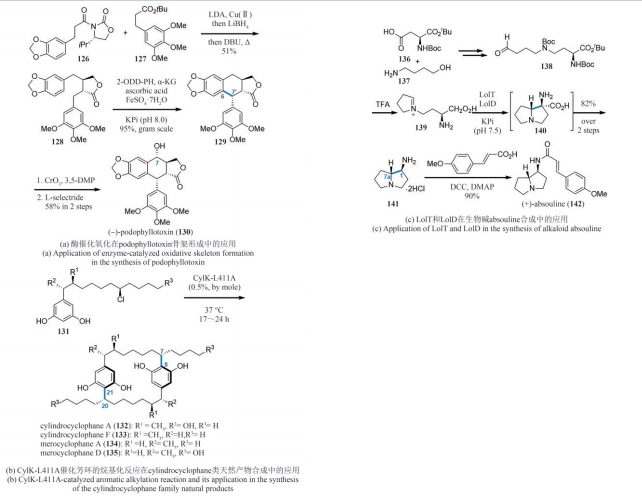
| 83 | MOU S B, XIAO W, WANG H Q, et al. Syntheses of epoxyguaiane sesquiterpenes (-)-englerin A, (-)-oxyphyllol, (+)-orientalol E, and (+)-orientalol F: a synthetic biology approach[J]. Organic Letters, 2020, 22(5): 1976-1979. |
| 84 | MOU S B, XIAO W, WANG H Q, et al. Syntheses of the carotane-type terpenoids (+)-schisanwilsonene A and (+)-tormesol via a two-stage approach[J]. Organic Letters, 2021, 23(2): 400-404. |
| 85 | ZHOU Q H, CHEN X F, MA D W. Asymmetric, protecting-group-free total synthesis of (-)-englerin A[J]. Angewandte Chemie International Edition, 2010, 49(20): 3513-3516. |
| 86 | MOLAWI K, DELPONT N, ECHAVARREN A M. Enantioselective synthesis of (-)-englerins A and B[J]. Angewandte Chemie International Edition, 2010, 49(20): 3517-3519. |
| 87 | GAYDOU M, MILLER R E, DELPONT N, et al. Synthesis of (+)-schisanwilsonene A by tandem gold-catalyzed cyclization/1,5-migration/cyclopropanation[J]. Angewandte Chemie International Edition, 2013, 52(25): 6396-6399. |
| 88 | LIU C G, CUI X Y, CHEN W, et al. Synthesis of oxygenated sesquiterpenoids enabled by combining metabolic engineering and visible-light photocatalysis[J]. Chemistry, 2022, 28(46): e202201230. |
| 89 | HSU S Y, PERUSSE D, HOUGARD T, et al. Semisynthesis of the neuroprotective metabolite, serofendic acid[J]. ACS Synthetic Biology, 2019, 8(10): 2397-2403. |
| 90 | BOTAS A, EITEL M, SCHWARZ P N, et al. Genetic engineering in combination with semi-synthesis leads to a new route for gram-scale production of the immunosuppressive natural product brasilicardin A[J]. Angewandte Chemie International Edition, 2021, 60(24): 13536-13541. |
| 91 | NAKANO C, KUDO F, EGUCHI T, et al. Genome mining reveals two novel bacterial sesquiterpene cyclases: (-)-germacradien-4-ol and (-)-epi-α-bisabolol synthases from Streptomyces citricolor [J]. ChemBioChem, 2011, 12(15): 2271-2275. |
| 92 | GRANT P S, MEYRELLES R, GAJSEK O, et al. Biomimetic cationic cyclopropanation enables an efficient chemoenzymatic synthesis of 6,8-cycloeudesmanes[J]. Journal of the American Chemical Society, 2023, 145(10): 5855-5863. |
| 93 | HONG B K, LUO T P, LEI X G. Late-stage diversification of natural products[J]. ACS Central Science, 2020, 6(5): 622-635. |
| 94 | DECORTE B L. Underexplored opportunities for natural products in drug discovery[J]. Journal of Medicinal Chemistry, 2016, 59(20): 9295-9304. |
| 95 | FASAN R D. Tuning P450 enzymes as oxidation catalysts[J]. ACS Catalysis, 2012, 2(4): 647-666. |
| 96 | MÜNCH J, PÜLLMANN P, ZHANG W Y, et al. Enzymatic hydroxylations of sp3-carbons[J]. ACS Catalysis, 2021, 11(15): 9168-9203. |
| 97 | WHITEHOUSE C J C, BELL S G, WONG L L. P450BM3(CYP102A1): connecting the dots[J]. Chemical Society Reviews, 2012, 41(3): 1218-1260. |
| 98 | LOSKOT S A, ROMNEY D K, ARNOLD F H, et al. Enantioselective total synthesis of nigelladine A via late-stage C—H oxidation enabled by an engineered P450 enzyme[J]. Journal of the American Chemical Society, 2017, 139(30): 10196-10199. |
| 99 | LI J, LI F Z, KING-SMITH E, et al. Merging chemoenzymatic and radical-based retrosynthetic logic for rapid and modular synthesis of oxidized meroterpenoids[J]. Nature Chemistry, 2020, 12(2): 173-179. |
| 100 | LI F Z, RENATA H. A Chiral-Pool-Based strategy to access trans-syn-fused drimane meroterpenoids: chemoenzymatic total syntheses of polysin, N-acetyl-polyveoline and the chrodrimanins[J]. Journal of the American Chemical Society, 2021, 143(43): 18280-18286. |
| 101 | LI J, CHEN F, RENATA H. Concise chemoenzymatic synthesis of gedunin[J]. Journal of the American Chemical Society, 2022, 144(42): 19238-19242. |
| 102 | LI F Z, DENG H P, RENATA H. Remote B-ring oxidation of sclareol with an engineered P450 facilitates divergent access to complex terpenoids[J]. Journal of the American Chemical Society, 2022, 144(17): 7616-7621. |
| 103 | DONG L B, ZHANG X, RUDOLF J D, et al. Cryptic and stereospecific hydroxylation, oxidation, and reduction in platensimycin and platencin biosynthesis[J]. Journal of the American Chemical Society, 2019, 141(9): 4043-4050. |
| 104 | RUDOLF J D, DONG L B, MANOOGIAN K, et al. Biosynthetic origin of the ether ring in platensimycin[J]. Journal of the American Chemical Society, 2016, 138(51): 16711-16721. |
| 105 | ZHANG X, KING-SMITH E, DONG L B, et al. Divergent synthesis of complex diterpenes through a hybrid oxidative approach[J]. Science, 2020, 369(6505): 799-806. |
| 106 | ZHAO Y, ZHANG B, SUN Z Q, et al. Biocatalytic C14-hydroxylation on androstenedione enabled modular synthesis of cardiotonic steroids[J]. ACS Catalysis, 2022, 12(16): 9839-9845. |
| 107 | SONG F Z, ZHENG M M, WANG J L, et al. Chemoenzymatic synthesis of C14-functionalized steroids[J]. Nature Synthesis, 2023, 2(8): 729-739. |
| 108 | JAMIESON C S, OHASHI M, LIU F, et al. The expanding world of biosynthetic pericyclases: cooperation of experiment and theory for discovery[J]. Natural Product Reports, 2019, 36(5): 698-713. |
| 109 | KIM H J, RUSZCZYCKY M W, CHOI S H, et al. Enzyme-catalysed [4+2] cycloaddition is a key step in the biosynthesis of spinosyn A[J]. Nature, 2011, 473(7345): 109-112. |
| 110 | KIM H J, CHOI S H, JEON B S, et al. Chemoenzymatic synthesis of spinosyn A[J]. Angewandte Chemie International Edition, 2014, 53(49): 13553-13557. |
| 111 | LI X J, ZHENG Q F, YIN J, et al. Chemo-enzymatic synthesis of equisetin[J]. Chemical Communications, 2017, 53(34): 4695-4697. |
| 112 | KATO N, NOGAWA T, HIROTA H, et al. A new enzyme involved in the control of the stereochemistry in the decalin formation during equisetin biosynthesis[J]. Biochemical and Biophysical Research Communications, 2015, 460(2): 210-215. |
| 113 | GAO L, SU C, DU X X, et al. FAD-dependent enzyme-catalysed intermolecular [4+2] cycloaddition in natural product biosynthesis[J]. Nature Chemistry, 2020, 12(7): 620-628. |
| 114 | LIU X J, YANG J, GAO L, et al. Chemoenzymatic total syntheses of artoninⅠwith an intermolecular Diels-Alderase[J]. Biotechnology Journal, 2020, 15(11): e2000119. |
| 115 | GAO L, ZOU Y K, LIU X J, et al. Enzymatic control of endo- and exo-stereoselective Diels-Alder reactions with broad substrate scope[J]. Nature Catalysis, 2021, 4(12): 1059-1069. |
| 116 | LAU W, SATTELY E S. Six enzymes from mayapple that complete the biosynthetic pathway to the etoposide aglycone[J]. Science, 2015, 349(6253): 1224-1228. |
| 117 | LI J, ZHANG X, RENATA H. Asymmetric chemoenzymatic synthesis of (-)-podophyllotoxin and related aryltetralin lignans[J]. Angewandte Chemie International Edition, 2019, 58(34): 11657-11660. |
| 118 | LAZZAROTTO M, HAMMERER L, HETMANN M, et al. Chemoenzymatic total synthesis of deoxy-, epi-, and podophyllotoxin and a biocatalytic kinetic resolution of dibenzylbutyrolactones[J]. Angewandte Chemie International Edition, 2019, 58(24): 8226-8230. |
| [1] | WEN Yanhua, LIU Hedong, CAO Chunlai, WU Ruibo. Applications of protein engineering in pharmaceutical industry [J]. Synthetic Biology Journal, 2025, 6(1): 65-86. |
| [2] | ZHONG Quanzhou, SHAN Yiyi, PEI Qingyun, JIN Yanyun, WANG Yihan, MENG Luyuan, WANG Xinyun, ZHANG Yuxin, LIU Kunyuan, WANG Huizhong, FENG Shangguo. Research progress in the production of α-arbutin through biosynthesis [J]. Synthetic Biology Journal, 2025, 6(1): 118-135. |
| [3] | ZHU Fanghuan, CEN Xuecong, CHEN Zhen. Research progress of diols production by microbes [J]. Synthetic Biology Journal, 2024, 5(6): 1367-1385. |
| [4] | LIU Yining, PU Wei, YANG Jinxing, WANG Yu. Recent advances in the biosynthesis of ω-amino acids and lactams [J]. Synthetic Biology Journal, 2024, 5(6): 1350-1366. |
| [5] | CHENG Feng, ZOU Shuping, XU Jianmiao, TANG Heng, XUE Yaping, ZHENG Yuguo. BioHPP®: a benchmark of biomanufacturing for high optically pure L-phosphinothricin [J]. Synthetic Biology Journal, 2024, 5(6): 1404-1418. |
| [6] | FU Yu, ZHONG Fangrui. Recent advances in chemically driven enantioselective photobiocatalysis [J]. Synthetic Biology Journal, 2024, 5(5): 1021-1049. |
| [7] | ZHENG Haotian, LI Chaofeng, LIU Liangxu, WANG Jiawei, LI Hengrun, NI Jun. Design, optimization and application of synthetic carbon-negative phototrophic community [J]. Synthetic Biology Journal, 2024, 5(5): 1189-1210. |
| [8] | CHENG Xiaolei, LIU Tiangang, TAO Hui. Recent research progress in non-canonical biosynthesis of terpenoids [J]. Synthetic Biology Journal, 2024, 5(5): 1050-1071. |
| [9] | YANG Haoran, YE Farong, HUANG Ping, WANG Ping. Recent advances in glycoprotein synthesis [J]. Synthetic Biology Journal, 2024, 5(5): 1072-1101. |
| [10] | CHENG Zhongyu, LI Fuzhuo. Recent advances in chemoenzymatic synthesis of natural products via site- selective P450 oxidation [J]. Synthetic Biology Journal, 2024, 5(5): 960-980. |
| [11] | LIU Zijian, MU Baiyang, DUAN Zhiqiang, WANG Xuan, LU Xiaojie. Advances in the development of DNA-compatible chemistries [J]. Synthetic Biology Journal, 2024, 5(5): 1102-1124. |
| [12] | XIE Xiangqian, GUO Wen, WANG Huan, LI Jin. Biosynthesis and chemical synthesis of ribosomally synthesized and post-translationally modified peptides containing aminovinyl cysteine [J]. Synthetic Biology Journal, 2024, 5(5): 981-996. |
| [13] | TANG Zhijun, HU Youcai, LIU Wen. Enzymatic (4+2)- and (2+2)-cycloaddition reactions: fundamentals and applications of regio- and stereoselectivity [J]. Synthetic Biology Journal, 2024, 5(3): 401-407. |
| [14] | ZHANG Jun, JIN Shixue, YUN Qian, QU Xudong. Biosynthesis of the unnatural extender units with polyketides and their structural modifications for applications in medicines [J]. Synthetic Biology Journal, 2024, 5(3): 561-570. |
| [15] | CHEN Xiwei, ZHANG Huaran, ZOU Yi. Biosynthesis and metabolic engineering of fungal non-ribosomal peptides [J]. Synthetic Biology Journal, 2024, 5(3): 571-592. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||