Synthetic Biology Journal ›› 2024, Vol. 5 ›› Issue (5): 1102-1124.DOI: 10.12211/2096-8280.2024-008
• Invited Review • Previous Articles Next Articles
Advances in the development of DNA-compatible chemistries
LIU Zijian1,2, MU Baiyang3, DUAN Zhiqiang1, WANG Xuan1, LU Xiaojie1,2
- 1.State Key Laboratory of Drug Research,Shanghai Institute of Materia Medica,Chinese Academic of Sciences,Shanghai 201203,China
2.University of Chinese Academy of Sciences,Beijing 100049,China
3.Shandong Second Medical University,Weifang 261053,Shandong,China
-
Received:2024-01-17Revised:2024-05-30Online:2024-11-20Published:2024-10-31 -
Contact:LU Xiaojie
与核酸兼容的化学反应开发进展
刘子健1,2, 穆柏杨3, 段志强1, 王璇1, 陆晓杰1,2
- 1.中国科学院上海药物研究所,原创新药研究全国重点实验室,上海 201203
2.中国科学院大学,北京 100049
3.山东第二医科大学,山东 潍坊 261053
-
通讯作者:陆晓杰 -
作者简介:刘子健 (2000—),男,硕士研究生。研究方向为on-DNA化学反应开发和DNA编码化合物库的构建和筛选。 E-mail:liuzijian@simm.ac.cn穆柏杨 (2000—),男,硕士研究生。研究方向为on-DNA化学反应开发和苗头化合物发现和优化。 E-mail:mubaiyang@simm.ac.cn陆晓杰 (1983—),男,博士,研究员,博士生导师。研究方向为DNA编码化合库技术的开发和应用。 E-mail:xjlu@simm.ac.cn -
基金资助:国家自然科学基金(22377139);国家重点研发计划(91953203)
CLC Number:
Cite this article
LIU Zijian, MU Baiyang, DUAN Zhiqiang, WANG Xuan, LU Xiaojie. Advances in the development of DNA-compatible chemistries[J]. Synthetic Biology Journal, 2024, 5(5): 1102-1124.
刘子健, 穆柏杨, 段志强, 王璇, 陆晓杰. 与核酸兼容的化学反应开发进展[J]. 合成生物学, 2024, 5(5): 1102-1124.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2024-008
| 条目 | 反应式 | 反应条件 | 参考文献 |
|---|---|---|---|
| 1 | 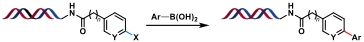 | Pd (PPh3)4, Na2CO3, H2O/DMA/CH3CN, 80 ℃ | [ |
| 2 |  | POPd/sSPhos, KOH, H2O/DMA, 80 ℃ | [ |
| 3 | 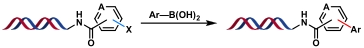 | Pd(OAc)2, Et3N H2O/DMA, rt, 2 h | [ |
| 4 | 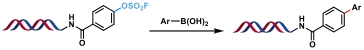 | Pd(OAc)2, Et3N H2O/DMA, rt, 2 h | [ |
| 5 |  | Pd(OAc)2, (rac)-BIDIME, K2CO3, DMA/H2O, 95℃, 2 h | [ |
| 6 | 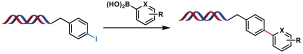 | Pd(OAc)2/TPPTS, K2CO3, H2O/DMA, 70℃, 2 h | [ |
| 7 | 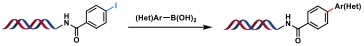 | Na2PdCl4/sSPhos, K2CO3, H2O/ACN, 37℃, 28 h | [ |
| 8 | 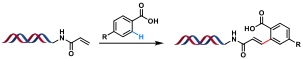 | [Ru], KOAc, DMF/H2O, 60℃, 10 h | [ |
| 9 |  | 1: PdCl2(dppf)DCM, K2CO3, DMSO/H2O, 80 ℃ 2: PdCl2(COD), NaOAc, DMA/H2O, 80 ℃ | [ |
Table 1 Metal-catalyzed C(sp2)—C(sp2) bond formation reactions
| 条目 | 反应式 | 反应条件 | 参考文献 |
|---|---|---|---|
| 1 |  | Pd (PPh3)4, Na2CO3, H2O/DMA/CH3CN, 80 ℃ | [ |
| 2 |  | POPd/sSPhos, KOH, H2O/DMA, 80 ℃ | [ |
| 3 | 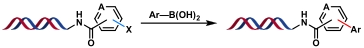 | Pd(OAc)2, Et3N H2O/DMA, rt, 2 h | [ |
| 4 |  | Pd(OAc)2, Et3N H2O/DMA, rt, 2 h | [ |
| 5 |  | Pd(OAc)2, (rac)-BIDIME, K2CO3, DMA/H2O, 95℃, 2 h | [ |
| 6 |  | Pd(OAc)2/TPPTS, K2CO3, H2O/DMA, 70℃, 2 h | [ |
| 7 |  | Na2PdCl4/sSPhos, K2CO3, H2O/ACN, 37℃, 28 h | [ |
| 8 |  | [Ru], KOAc, DMF/H2O, 60℃, 10 h | [ |
| 9 |  | 1: PdCl2(dppf)DCM, K2CO3, DMSO/H2O, 80 ℃ 2: PdCl2(COD), NaOAc, DMA/H2O, 80 ℃ | [ |
| 条目 | DNA兼容反应 | 反应条件 | 参考文献 |
|---|---|---|---|
| 1 | 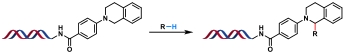 | CuOTf, TBHP ACN/H2O, 50/70 ℃, 10 h | [ |
| 2 | 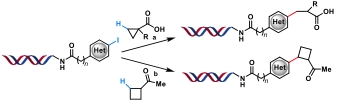 | a: Pd(OAc)2, AgOAc, Li2CO3, H2O/DMA, 80℃, 20 h b: Pd(OAc)2/ligand AgTFA, NaOAc H2O/DMA, 80℃, 20 h | [ |
Table 2 Metal-catalyzed C(sp2)—C(sp3) and C(sp2)—C(sp) bond formation reactions
| 条目 | DNA兼容反应 | 反应条件 | 参考文献 |
|---|---|---|---|
| 1 |  | CuOTf, TBHP ACN/H2O, 50/70 ℃, 10 h | [ |
| 2 |  | a: Pd(OAc)2, AgOAc, Li2CO3, H2O/DMA, 80℃, 20 h b: Pd(OAc)2/ligand AgTFA, NaOAc H2O/DMA, 80℃, 20 h | [ |
| 条目 | DNA兼容反应 | 反应条件 | 参考文献 |
|---|---|---|---|
| 1 | 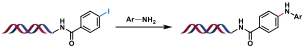 | t-BuXPhos Pd G1, CsOH, H2O/DMA, 100 ℃, 3 h | [ |
| 2 | 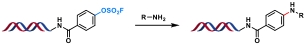 | t-Brettphos Pd G3, Et3N, H2O/DMA, 60 ℃, 2 h | [ |
| 3 | 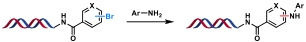 | t-BuXPhos-Pd-G3, NaOH, H2O/DMA, 60 ℃, 2 h | [ |
| 4 | 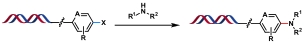 | Pd-PEPPSI-iPentCl-pyr, Na ascorbate, CsOH, DMA/H2O, 95 ℃, 15 min | [ |
| 5 |  | Pd(OAc)2/BippyPhos, Na ascorbate, K3PO4, DMA/H2O, 95 ℃, 15 min | [ |
| 6 |  | t-BuXPhos-Pd-G1, NaOH, H2O/DMA, 80 ℃, 3 h | [ |
| 7 | 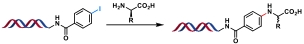 | 1: CuSO4·5H2O, Na ascorbate, H2O/DMA, 100 ℃, 2 h 2: CuSO4·5H2O, Proline, KOH, Na ascorbate, H2O/DMA, 100 ℃, 2 h | [ |
| 8 | 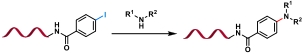 | Cu(OAc)2 /ligand, Na ascorbate, K3PO4, DMSO/H2O, 40℃, 3 h | [ |
| 9 | 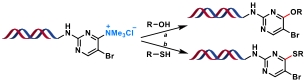 | a: K2CO3 or KOH, DMA/H2O, rt, or 60 ℃, 10 h b: K2CO3, DMA/H2O, rt, or 80 ℃, 10 h | [ |
| 10 | 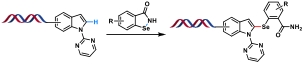 | [Rh], PBS (pH 4.2)-DMA (7∶1) 80 ℃, 6 h | [ |
| 11 | 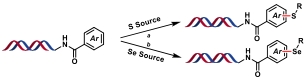 | a: I2, BSA, MeOH/H2O, 40, ℃, 150 min b: (1) BME, RT, 10 min (2) I2, BSA, MeOH/H2O, 40, ℃, 150 min | [ |
Table 3 Metal-catalyzed C—X coupling reactions
| 条目 | DNA兼容反应 | 反应条件 | 参考文献 |
|---|---|---|---|
| 1 |  | t-BuXPhos Pd G1, CsOH, H2O/DMA, 100 ℃, 3 h | [ |
| 2 |  | t-Brettphos Pd G3, Et3N, H2O/DMA, 60 ℃, 2 h | [ |
| 3 |  | t-BuXPhos-Pd-G3, NaOH, H2O/DMA, 60 ℃, 2 h | [ |
| 4 |  | Pd-PEPPSI-iPentCl-pyr, Na ascorbate, CsOH, DMA/H2O, 95 ℃, 15 min | [ |
| 5 |  | Pd(OAc)2/BippyPhos, Na ascorbate, K3PO4, DMA/H2O, 95 ℃, 15 min | [ |
| 6 |  | t-BuXPhos-Pd-G1, NaOH, H2O/DMA, 80 ℃, 3 h | [ |
| 7 |  | 1: CuSO4·5H2O, Na ascorbate, H2O/DMA, 100 ℃, 2 h 2: CuSO4·5H2O, Proline, KOH, Na ascorbate, H2O/DMA, 100 ℃, 2 h | [ |
| 8 |  | Cu(OAc)2 /ligand, Na ascorbate, K3PO4, DMSO/H2O, 40℃, 3 h | [ |
| 9 |  | a: K2CO3 or KOH, DMA/H2O, rt, or 60 ℃, 10 h b: K2CO3, DMA/H2O, rt, or 80 ℃, 10 h | [ |
| 10 |  | [Rh], PBS (pH 4.2)-DMA (7∶1) 80 ℃, 6 h | [ |
| 11 |  | a: I2, BSA, MeOH/H2O, 40, ℃, 150 min b: (1) BME, RT, 10 min (2) I2, BSA, MeOH/H2O, 40, ℃, 150 min | [ |
| 条目 | DNA兼容反应 | 反应条件 | 参考文献 |
|---|---|---|---|
| 1 | 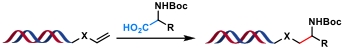 | [Ir], blue light, K2HPO4, DMSO/H2O, N2, rt, 6 h | [ |
| 2 | 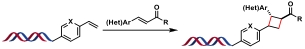 | [Ir], blue light, DMSO/H2O, glycerol, rt, 2 h | [ |
| 3 | 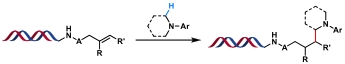 | [Ir], blue light, K2HPO4, DMSO/H2O, N2, rt, 2 h | [ |
| 4 | 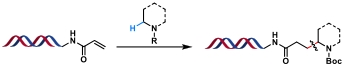 | [Ir], blue light, quinuclidine, DMSO/H2O, N2, rt, 45 min | [ |
| 5 | 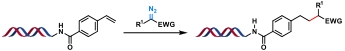 | [Ru], blue light, Hantzsch ester, 4-methylbenzenethiol DMA/H2O, rt, 3 h | [ |
| 6 | 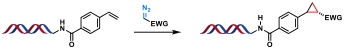 | [Ru], blue light, I2, DMA/H2O, rt, 3 h | [ |
| 7 | 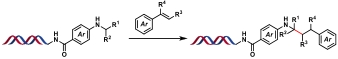 | [Ir], blue light, quinuclidine, DMF/H2O, N2, rt, 1.5 h | [ |
| 8 | 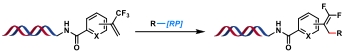 | [Ir], blue light, 2,6-lutidine, DMSO/H2O, rt, 10 min | [ |
| 9 | 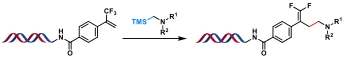 | [Ir], Kessil lamp, DMSO/H2O, rt, 5 min | [ |
| 10 | 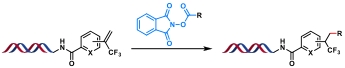 | Hantzsch ester, blue Kessil, DMSO/H2O, rt, 5 min | [ |
Table 4 Photocatalytic C(sp3)—C(sp3) bond formation reactions
| 条目 | DNA兼容反应 | 反应条件 | 参考文献 |
|---|---|---|---|
| 1 |  | [Ir], blue light, K2HPO4, DMSO/H2O, N2, rt, 6 h | [ |
| 2 |  | [Ir], blue light, DMSO/H2O, glycerol, rt, 2 h | [ |
| 3 |  | [Ir], blue light, K2HPO4, DMSO/H2O, N2, rt, 2 h | [ |
| 4 |  | [Ir], blue light, quinuclidine, DMSO/H2O, N2, rt, 45 min | [ |
| 5 |  | [Ru], blue light, Hantzsch ester, 4-methylbenzenethiol DMA/H2O, rt, 3 h | [ |
| 6 |  | [Ru], blue light, I2, DMA/H2O, rt, 3 h | [ |
| 7 |  | [Ir], blue light, quinuclidine, DMF/H2O, N2, rt, 1.5 h | [ |
| 8 |  | [Ir], blue light, 2,6-lutidine, DMSO/H2O, rt, 10 min | [ |
| 9 |  | [Ir], Kessil lamp, DMSO/H2O, rt, 5 min | [ |
| 10 |  | Hantzsch ester, blue Kessil, DMSO/H2O, rt, 5 min | [ |
| 条目 | DNA兼容反应 | 反应条件 | 参考文献 |
|---|---|---|---|
| 1 | 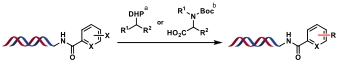 | a: 4CzIPN, Ni(TMHD)2, blue LED, DMSO/H2O, 45 min b: [Ir], Ni(TMHD)2, blue LED, TMG, MOPS pH 8 buffer, DMSO/H2O, 10 min | [ |
| 2 | 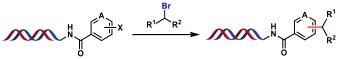 | [Ir], [Ni], blue Kessil, MgCl2, Et3N, DMSO/H2O, rt, 45 min | [ |
| 3 | 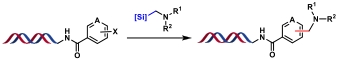 | [Ir], [Ni], blue Kessil, DMSO/H2O, rt, 15 min | [ |
| 4 | 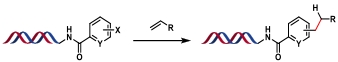 | [Ir], Hantzsch ester, blue Kessil, DMSO/H2O, rt, 5 min | [ |
| 5 | 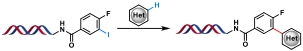 | [Ir], DIPEA, blue Kessil, DMSO/H2O, rt, 5 min | [ |
Table 5 Photocatalytic C(sp2)—C bond formation reactions
| 条目 | DNA兼容反应 | 反应条件 | 参考文献 |
|---|---|---|---|
| 1 |  | a: 4CzIPN, Ni(TMHD)2, blue LED, DMSO/H2O, 45 min b: [Ir], Ni(TMHD)2, blue LED, TMG, MOPS pH 8 buffer, DMSO/H2O, 10 min | [ |
| 2 |  | [Ir], [Ni], blue Kessil, MgCl2, Et3N, DMSO/H2O, rt, 45 min | [ |
| 3 |  | [Ir], [Ni], blue Kessil, DMSO/H2O, rt, 15 min | [ |
| 4 |  | [Ir], Hantzsch ester, blue Kessil, DMSO/H2O, rt, 5 min | [ |
| 5 |  | [Ir], DIPEA, blue Kessil, DMSO/H2O, rt, 5 min | [ |
| 条目 | 杂环结构 | 合成策略 | 参考文献 |
|---|---|---|---|
| 1 | 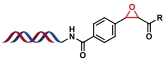 | 醛和α-氯代酮缩合环化 | [ |
| 2 | 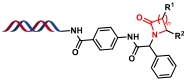 | 醛、氨基酸和异腈之间多组分缩合环化 | [ |
| 3 |  | Clauson-Kaas反应合成吡咯核心;再通过碘代和交叉偶联实现功能化 | [ |
| 4 | 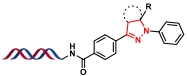 | 醛、苯磺酰肼和重氮盐环化生成二取代四唑;再与末端烯烃进行环加成反应 | [ |
| 5 | 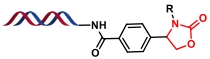 | 环氧化物开环生成β-氨基醇,再与氯甲酸酯环化 | [ |
| 6 | 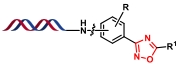 | 羟胺和腈生成偕胺肟,再由羧酸酰化后发生脱水环化 | [ |
| 7 | 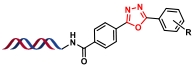 | 苯甲酰肼与醛缩合环化 | [ |
| 8 | 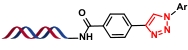 | 芳基硼酸先转化芳基叠氮化物,再与炔进行环加成 | [ |
| 9 |  | 腈与叠氮化物之间的环加成反应 | [ |
| 10 | 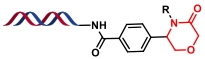 | 环氧化物开环生成β-氨基醇,再与氯乙酰氯环化 | [ |
| 11 | 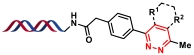 | 四嗪与烯基/羰基化合物进行IEDDA反应(烯基产物需要额外氧化) | [ |
| 12 | 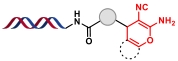 | 烯醇前体与羰基化合物和丙二腈之间的多组分缩合环化反应 | [ |
Table 6 Single-ring synthesis reactions
| 条目 | 杂环结构 | 合成策略 | 参考文献 |
|---|---|---|---|
| 1 |  | 醛和α-氯代酮缩合环化 | [ |
| 2 |  | 醛、氨基酸和异腈之间多组分缩合环化 | [ |
| 3 |  | Clauson-Kaas反应合成吡咯核心;再通过碘代和交叉偶联实现功能化 | [ |
| 4 |  | 醛、苯磺酰肼和重氮盐环化生成二取代四唑;再与末端烯烃进行环加成反应 | [ |
| 5 |  | 环氧化物开环生成β-氨基醇,再与氯甲酸酯环化 | [ |
| 6 |  | 羟胺和腈生成偕胺肟,再由羧酸酰化后发生脱水环化 | [ |
| 7 |  | 苯甲酰肼与醛缩合环化 | [ |
| 8 |  | 芳基硼酸先转化芳基叠氮化物,再与炔进行环加成 | [ |
| 9 |  | 腈与叠氮化物之间的环加成反应 | [ |
| 10 |  | 环氧化物开环生成β-氨基醇,再与氯乙酰氯环化 | [ |
| 11 |  | 四嗪与烯基/羰基化合物进行IEDDA反应(烯基产物需要额外氧化) | [ |
| 12 |  | 烯醇前体与羰基化合物和丙二腈之间的多组分缩合环化反应 | [ |
| 条目 | 杂环结构 | 合成策略 | 参考文献 |
|---|---|---|---|
| 1 | 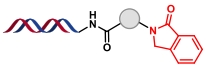 | 伯胺与邻苯二甲醛缩合环化反应 | [ |
| 2 | 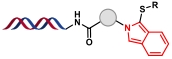 | 伯胺、邻苯二甲醛和4-叔丁基苯硫醇之间的多组分缩合环化反应 | |
| 3 | 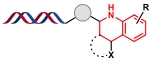 | 苯胺与醛缩合生成的亚胺,由布朗斯特酸活化后进行烯烃亲电加成、苯环亲电取代环化和消除反应 | [ |
| 4 | 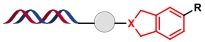 | 苯乙炔和1,6-庚二炔之间的环加成反应 | [ |
| 5 | 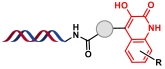 | 靛红与醛在苯甲酰肼促进下的环加成反应 | [ |
| 6 | 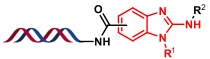 | 芳基伯胺与TCDI反应生成异硫氰酸酯,再与邻位仲胺生成硫脲;最后脱硫环化 | [ |
| 7 | 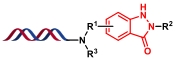 | 硝基芳烃经硼酸还原为亚硝基中间体,再与邻位酰胺氮原子亲核加成 | [ |
| 8 | 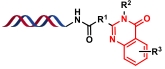 | 醛与邻氨基苯甲酰胺或醛与靛红酸酐和伯胺进行环化反应,再对环化产物进行氧化 | [ |
| 9 | 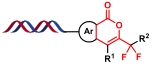 | 苯甲酸C—H活化后与炔发生环加成反应 | [ |
| 10 | 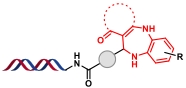 | 醛、邻苯二胺和1,3-二羰基化合物之间的多组分缩合环化反应 | [ |
Table 7 Fused-ring synthesis reactions
| 条目 | 杂环结构 | 合成策略 | 参考文献 |
|---|---|---|---|
| 1 |  | 伯胺与邻苯二甲醛缩合环化反应 | [ |
| 2 |  | 伯胺、邻苯二甲醛和4-叔丁基苯硫醇之间的多组分缩合环化反应 | |
| 3 |  | 苯胺与醛缩合生成的亚胺,由布朗斯特酸活化后进行烯烃亲电加成、苯环亲电取代环化和消除反应 | [ |
| 4 |  | 苯乙炔和1,6-庚二炔之间的环加成反应 | [ |
| 5 |  | 靛红与醛在苯甲酰肼促进下的环加成反应 | [ |
| 6 |  | 芳基伯胺与TCDI反应生成异硫氰酸酯,再与邻位仲胺生成硫脲;最后脱硫环化 | [ |
| 7 |  | 硝基芳烃经硼酸还原为亚硝基中间体,再与邻位酰胺氮原子亲核加成 | [ |
| 8 |  | 醛与邻氨基苯甲酰胺或醛与靛红酸酐和伯胺进行环化反应,再对环化产物进行氧化 | [ |
| 9 |  | 苯甲酸C—H活化后与炔发生环加成反应 | [ |
| 10 |  | 醛、邻苯二胺和1,3-二羰基化合物之间的多组分缩合环化反应 | [ |
| 条目 | 杂环结构 | 合成策略 | 参考文献 |
|---|---|---|---|
| 1 | 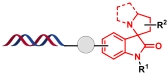 | 丙烯酰胺、靛红和脯氨酸之间的三组分环化反应 | [ |
| 2 | 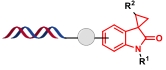 | 3-重氮吲哚酮和缺电子烯烃之间的环加成反应 | [ |
| 3 | 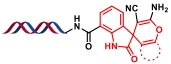 | 靛红、烯醇化物和丙二腈之间的环化反应 | [ |
| 4 | 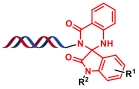 | 靛红和邻氨基苯甲酰胺之间的缩合环化反应 | [ |
| 5 | 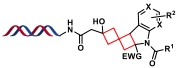 | 亚甲基环丁烷与不饱和杂环分子间的光环加成反应 | [ |
Table 8 Spiro-ring synthesis reactions
| 条目 | 杂环结构 | 合成策略 | 参考文献 |
|---|---|---|---|
| 1 |  | 丙烯酰胺、靛红和脯氨酸之间的三组分环化反应 | [ |
| 2 |  | 3-重氮吲哚酮和缺电子烯烃之间的环加成反应 | [ |
| 3 |  | 靛红、烯醇化物和丙二腈之间的环化反应 | [ |
| 4 |  | 靛红和邻氨基苯甲酰胺之间的缩合环化反应 | [ |
| 5 |  | 亚甲基环丁烷与不饱和杂环分子间的光环加成反应 | [ |
| 条目 | 多功能核心 | 衍生杂环 | 参考文献 |
|---|---|---|---|
| 1 | 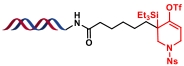 | 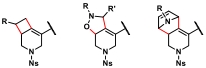 | [ |
| 2 | 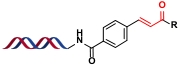 | 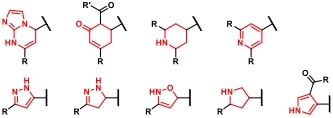 | [ |
| 3 |  | 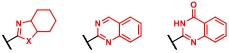 | [ |
| 4 |  | 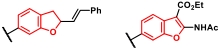 | [ |
Table 9 DOS-directed privileged heterocycles synthesis reactions
| 条目 | 多功能核心 | 衍生杂环 | 参考文献 |
|---|---|---|---|
| 1 |  |  | [ |
| 2 |  |  | [ |
| 3 |  |  | [ |
| 4 |  | 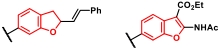 | [ |
| 1 | FOLMER R H A. Integrating biophysics with HTS-driven drug discovery projects[J]. Drug Discovery Today, 2016, 21(3): 491-498. |
| 2 | STARK J L, POWERS R. Application of NMR and molecular docking in structure-based drug discovery[J]. Topics in Current Chemistry, 2012, 326: 1-34. |
| 3 | ERLANSON D A, FESIK S W, HUBBARD R E, et al. Twenty years on: the impact of fragments on drug discovery[J]. Nature Reviews Drug Discovery, 2016, 15(9): 605-619. |
| 4 | BRENNER S, LERNER R A. Encoded combinatorial chemistry[J]. Proceedings of the National Academy of Sciences of the United States of America, 1992, 89(12): 5381-5383. |
| 5 | CASTAÑÓN J, ROMÁN J P, JESSOP T C, et al. Design and development of a technology platform for DNA-encoded library production and affinity selection[J]. SLAS Discovery, 2018, 23(5): 387-396. |
| 6 | DECURTINS W, WICHERT M, FRANZINI R M, et al. Automated screening for small organic ligands using DNA-encoded chemical libraries[J]. Nature Protocols, 2016, 11(4): 764-780. |
| 7 | LITOVCHICK A, DUMELIN C E, HABESHIAN S, et al. Encoded library synthesis using chemical ligation and the discovery of sEH inhibitors from a 334-million member library[J]. Scientific Reports, 2015, 5: 10916. |
| 8 | WANG J, LUNDBERG H, ASAI S, et al. Kinetically guided radical-based synthesis of C(sp3)—C(sp3) linkages on DNA[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(28): E6404-E6410. |
| 9 | BURROWS C J, MULLER J G. Oxidative nucleobase modifications leading to strand scission[J]. Chemical Reviews, 1998, 98(3): 1109-1152. |
| 10 | MA P X, ZHANG S N, HUANG Q P, et al. Evolution of chemistry and selection technology for DNA-encoded library[J]. Acta Pharmaceutica Sinica B, 2024, 14(2): 492-516. |
| 11 | KUNIG V, POTOWSKI M, GOHLA A, et al. DNA-encoded libraries—an efficient small molecule discovery technology for the biomedical sciences[J]. Biological Chemistry, 2018, 399(7): 691-710. |
| 12 | SHI Y, WU Y R, YU J Q, et al. DNA-encoded libraries (DELs): a review of on-DNA chemistries and their output[J]. RSC Advances, 2021, 11(4): 2359-2376. |
| 13 | FAIR R J, WALSH R T, HUPP C D. The expanding reaction toolkit for DNA-encoded libraries[J]. Bioorganic & Medicinal Chemistry Letters, 2021, 51: 128339. |
| 14 | SAHU R, YADAV S, NATH S, et al. DNA-encoded libraries via late-stage functionalization strategies: a review[J]. Chemical Communications, 2023, 59(41): 6128-6147. |
| 15 | FRANZINI R M, RANDOLPH C. Chemical space of DNA-encoded libraries[J]. Journal of Medicinal Chemistry, 2016, 59(14): 6629-6644. |
| 16 | MATSUO B, GRANADOS A, LEVITRE G, et al. Photochemical methods applied to DNA encoded library (DEL) synthesis[J]. Accounts of Chemical Research, 2023, 56(3): 385-401. |
| 17 | ADAMIK R, BUCHHOLCZ B, DARVAS F, et al. The potential of micellar media in the synthesis of DNA-encoded libraries[J]. Chemistry, 2022, 28(20): e202103967. |
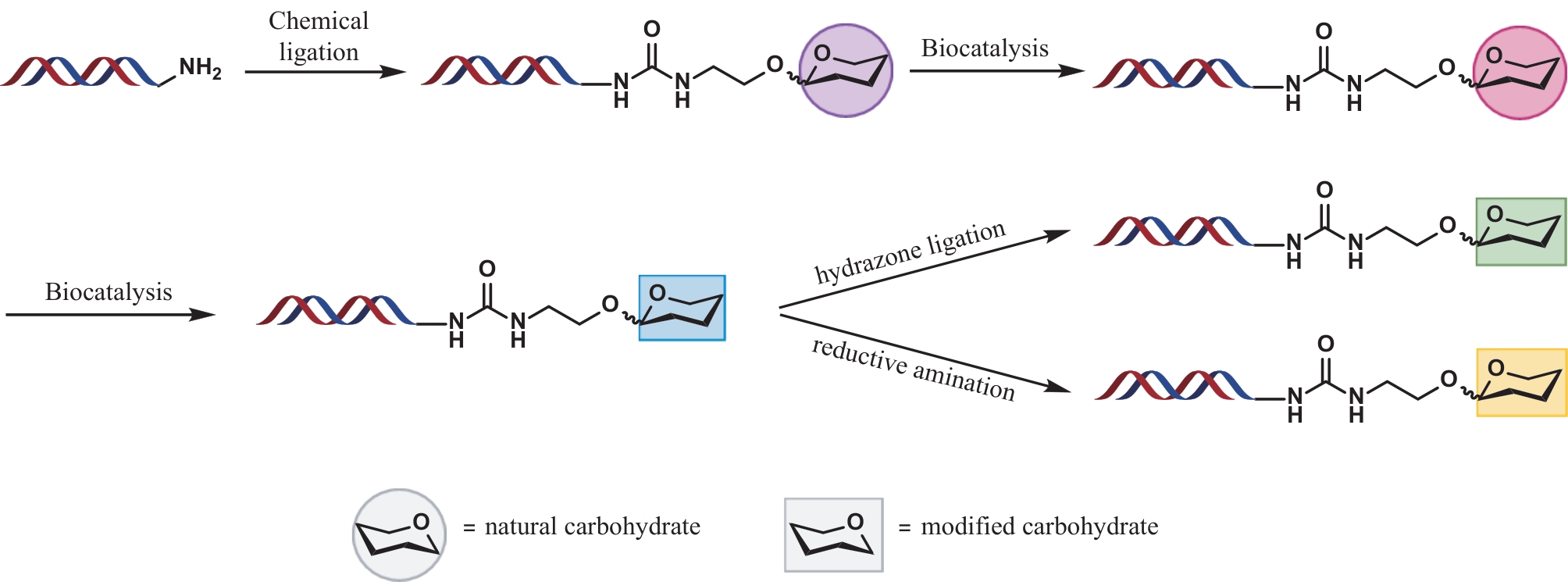
| 18 | THOMAS B, LU X J, BIRMINGHAM W R, et al. Application of biocatalysis to on-DNA carbohydrate library synthesis[J]. ChemBioChem, 2017, 18(9): 858-863. |
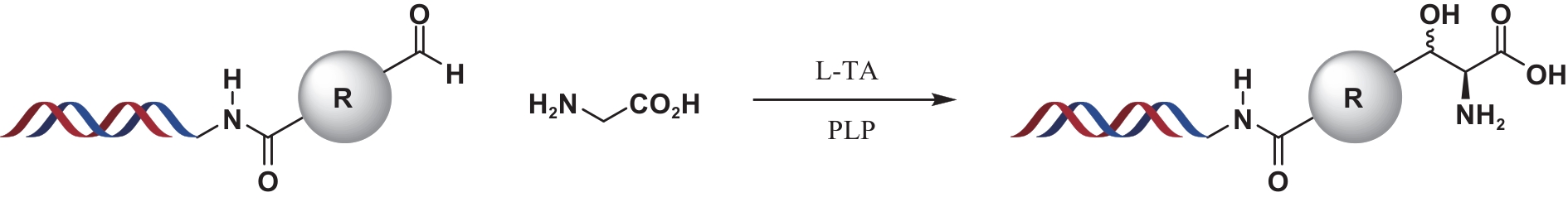
| 19 | CHAI J, LU X J, ARICO-MUENDEL C C, et al. Application of l-threonine aldolase to on-DNA reactions[J]. Bioconjugate Chemistry, 2021, 32(9): 1973-1978. |
| 20 | KADU B S. Suzuki-Miyaura cross coupling reaction: recent advancements in catalysis and organic synthesis[J]. Catalysis Science & Technology, 2021, 11(4): 1186-1221. |
| 21 | OMUMI A, BEACH D G, BAKER M, et al. Postsynthetic guanine arylation of DNA by Suzuki-Miyaura cross-coupling[J]. Journal of the American Chemical Society, 2011, 133(1): 42-50. |
| 22 | DING Y, CLARK M A. Robust Suzuki-Miyaura cross-coupling on DNA-linked substrates[J]. ACS Combinatorial Science, 2015, 17(1): 1-4. |
| 23 | DING Y, DELOREY J L, CLARK M A. Novel catalyst system for Suzuki-Miyaura coupling of challenging DNA-linked aryl chlorides[J]. Bioconjugate Chemistry, 2016, 27(11): 2597-2600. |
| 24 | LI J Y, HUANG H B. Development of DNA-compatible Suzuki-Miyaura reaction in aqueous media[J]. Bioconjugate Chemistry, 2018, 29(11): 3841-3846. |
| 25 | XU H T, MA F, WANG N, et al. DNA-encoded libraries: aryl fluorosulfonates as versatile electrophiles enabling facile on-DNA Suzuki, Sonogashira, and Buchwald reactions[J]. Advanced Science, 2019, 6(23): 1901551. |
| 26 | QU Y, LIU S X, WEN H N, et al. Palladium-mediated Suzuki-Miyaura cross-coupling reaction of potassium Boc-protected aminomethyltrifluoroborate with DNA-conjugated aryl bromides for DNA-encoded chemical library synthesis[J]. Biochemical and Biophysical Research Communications, 2020, 533(2): 209-214. |
| 27 | FAVALLI N, BASSI G, BIANCHI D, et al. Large screening of DNA-compatible reaction conditions for Suzuki and Sonogashira cross-coupling reactions and for reverse amide bond formation[J]. Bioorganic & Medicinal Chemistry, 2021, 41: 116206. |
| 28 | SIRIPURAM V K, SUNKARI Y K, NGUYEN T L, et al. DNA-compatible Suzuki-Miyaura cross-coupling reaction of aryl iodides with (hetero)aryl boronic acids for DNA-encoded libraries[J]. Frontiers in Chemistry, 2022, 10: 894603. |
| 29 | WANG X, SUN H, LIU J X, et al. Ruthenium-promoted C—H activation reactions between DNA-conjugated acrylamide and aromatic acids[J]. Organic Letters, 2018, 20(16): 4764-4768. |
| 30 | WANG X, SUN H, LIU J X, et al. Palladium-promoted DNA-compatible Heck reaction[J]. Organic Letters, 2019, 21(3): 719-723. |
| 31 | KRANTHIKUMAR R. Recent advances in C(sp3)—C(sp3) cross-coupling chemistry: a dominant performance of nickel catalysts[J]. Organometallics, 2022, 41(6): 667-679. |
| 32 | WEN X, DUAN Z Q, LIU J X, et al. On-DNA cross-dehydrogenative coupling reaction toward the synthesis of focused DNA-encoded tetrahydroisoquinoline libraries[J]. Organic Letters, 2020, 22(15): 5721-5725. |
| 33 | FAN Z L, ZHAO S, LIU T, et al. Merging C(sp3)—H activation with DNA-encoding[J]. Chemical Science, 2020, 11(45): 12282-12288. |
| 34 | LU X J, ROBERTS S E, FRANKLIN G J, et al. On-DNA Pd and Cu promoted C—N cross-coupling reactions[J]. MedChemComm, 2017, 8(8): 1614-1617. |
| 35 | FORERO-CORTÉS P A, HAYDL A M. The 25th anniversary of the Buchwald-Hartwig amination: development, applications, and outlook[J]. Organic Process Research & Development, 2019, 23(8): 1478-1483. |
| 36 | DE PEDRO BEATO E, PRIEGO J, GIRONDA-MARTÍNEZ A, et al. Mild and efficient palladium-mediated C—N cross-coupling reaction between DNA-conjugated aryl bromides and aromatic amines[J]. ACS Combinatorial Science, 2019, 21(2): 69-74. |
| 37 | CHEN Y C, FAVER J C, KU A F, et al. C—N coupling of DNA-conjugated (hetero)aryl bromides and chlorides for DNA-encoded chemical library synthesis[J]. Bioconjugate Chemistry, 2020, 31(3): 770-780. |
| 38 | CHHEDA P R, SIMMONS N, SCHUMAN D P, et al. Palladium-mediated C—N coupling of DNA-conjugated (hetero)aryl halides with aliphatic and (hetero)aromatic amines[J]. Organic Letters, 2022, 24(18): 3401-3406. |
| 39 | YANG J, XIA S D, LIU J X, et al. DNA-encoded focused indazole library synthesis by a palladium-mediated CN(sp2) cross-coupling reaction between DNA-linked (hetero)aryl halides and aromatic nitrogen heterocycles[J]. Tetrahedron Letters, 2022, 96: 153732. |
| 40 | YANG Q, ZHAO Y S, MA D W. Cu-mediated ullmann-type cross-coupling and industrial applications in route design, process development, and scale-up of pharmaceutical and agrochemical processes[J]. Organic Process Research & Development, 2022, 26(6): 1690-1750. |
| 41 | RUFF Y, BERST F. Efficient copper-catalyzed amination of DNA-conjugated aryl iodides under mild aqueous conditions[J]. MedChemComm, 2018, 9(7): 1188-1193. |
| 42 | WANG D Y, WEN X, XIONG C D, et al. Non-transition metal-mediated diverse aryl-heteroatom bond formation of arylammonium salts[J]. iScience, 2019, 15: 307-315. |
| 43 | XU H T, GU Y A, ZHANG S N, et al. A chemistry for incorporation of selenium into DNA-encoded libraries[J]. Angewandte Chemie International Edition, 2020, 59(32): 13273-13280. |
| 44 | YANG S L, ZHAO G X, GAO Y T, et al. In-solution direct oxidative coupling for the integration of sulfur/selenium into DNA-encoded chemical libraries[J]. Chemical Science, 2022, 13(9): 2604-2613. |
| 45 | TELLIS J C, PRIMER D N, MOLANDER G A. Dual catalysis. Single-electron transmetalation in organoboron cross-coupling by photoredox/nickel dual catalysis[J]. Science, 2014, 345(6195): 433-436. |
| 46 | PATEL S, BADIR S O, MOLANDER G A. Developments in photoredox-mediated alkylation for DNA-encoded libraries[J]. Trends in Chemistry, 2021, 3(3): 161-175. |
| 47 | KÖLMEL D K, LOACH R P, KNAUBER T, et al. Employing photoredox catalysis for DNA-encoded chemistry: decarboxylative alkylation of α-amino acids[J]. ChemMedChem, 2018, 13(20): 2159-2165. |
| 48 | KÖLMEL D K, RATNAYAKE A S, FLANAGAN M E, et al. Photocatalytic [2 + 2] cycloaddition in DNA-encoded chemistry[J]. Organic Letters, 2020, 22(8): 2908-2913. |
| 49 | WU R F, DU T, SUN W B, et al. Functionalization of DNA-tagged alkenes enabled by visible-light-induced C—H activation of N-aryl tertiary amines[J]. Organic Letters, 2021, 23(9): 3486-3490. |
| 50 | SHAN J M, LING X, LIU J X, et al. DNA-encoded CH functionality via photoredox-mediated hydrogen atom transformation catalysis[J]. Bioorganic & Medicinal Chemistry, 2021, 42: 116234. |
| 51 | FU X, TANG J, HUA R Y, et al. Functionalization of DNA-tagged alkenes with diazo compounds via photocatalysis[J]. Organic Letters, 2022, 24(11): 2208-2213. |
| 52 | MAHDAVI-AMIRI Y, HU M S J, FRIAS N, et al. Photoredox-catalysed hydroaminoalkylation of on-DNA N-arylamines[J]. Organic & Biomolecular Chemistry, 2023, 21(7): 1463-1467. |
| 53 | MÜLLER K, FAEH C, DIEDERICH F. Fluorine in pharmaceuticals: looking beyond intuition[J]. Science, 2007, 317(5846): 1881-1886. |
| 54 | PURSER S, MOORE P R, SWALLOW S, et al. Fluorine in medicinal chemistry[J]. Chemical Society Reviews, 2008, 37(2): 320-330. |
| 55 | PHELAN J P, LANG S B, SIM J H, et al. Open-air alkylation reactions in photoredox-catalyzed DNA-encoded library synthesis[J]. Journal of the American Chemical Society, 2019, 141(8): 3723-3732. |
| 56 | BADIR S O, SIM J H, BILLINGS K, et al. Multifunctional building blocks compatible with photoredox-mediated alkylation for DNA-encoded library synthesis[J]. Organic Letters, 2020, 22(3): 1046-1051. |
| 57 | BADIR S O, LIPP A, KRUMB M, et al. Photoredox-mediated hydroalkylation and hydroarylation of functionalized olefins for DNA-encoded library synthesis[J]. Chemical Science, 2021, 12(36): 12036-12045. |
| 58 | CHENG J P, LU Y, ZHU X Q, et al. Heterolytic and homolytic N—H bond dissociation energies of 4-substituted Hantzsch 2,6-dimethyl-1,4-dihydropyridines and the effect of one-electron transfer on the N—H bond activation[J]. The Journal of Organic Chemistry, 2000, 65(12): 3853-3857. |
| 59 | DING H, GREENBERG M M. DNA damage and interstrand cross-link formation upon irradiation of aryl iodide C-nucleotide analogues[J]. The Journal of Organic Chemistry, 2010, 75(3): 535-544. |
| 60 | KRUMB M, KAMMER L M, BADIR S O, et al. Photochemical C—H arylation of heteroarenes for DNA-encoded library synthesis[J]. Chemical Science, 2022, 13(4): 1023-1029. |
| 61 | JAMPILEK J. Heterocycles in medicinal chemistry[J]. Molecules, 2019, 24(21): 3839. |
| 62 | LIPINSKI C A, LOMBARDO F, DOMINY B W, et al. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings[J]. Advanced Drug Delivery Reviews, 2001, 46(1-3): 3-26. |
| 63 | WEN X, WU X Y, JIN R, et al. Privileged heterocycles for DNA-encoded library design and hit-to-lead optimization[J]. European Journal of Medicinal Chemistry, 2023, 248: 115079. |
| 64 | GAO Y T, ZHAO G X, HE P Y, et al. DNA-compatible synthesis of α,β-epoxyketones for DNA-encoded chemical libraries[J]. Bioconjugate Chemistry, 2022, 33(1): 105-110. |
| 65 | MANTELL M A, MARCAURELLE L, DING Y. One reaction served three ways: the on-DNA ugi 4C-3C reaction for the formation of lactams[J]. Organic Letters, 2023, 25(8): 1241-1245. |
| 66 | QI J J, LIU S X, SEYDIMEMET M, et al. A general set of DNA-compatible reactions for preparing DNA-tagged multisubstituted pyrroles[J]. Bioconjugate Chemistry, 2021, 32(11): 2290-2294. |
| 67 | ZHANG J, LI X F, WEI H M, et al. Sequential DNA-encoded building block fusion for the construction of polysubstituted pyrazoline core libraries[J]. Organic Letters, 2021, 23(21): 8429-8433. |
| 68 | FAN L J, DAVIE C P. Zirconium(Ⅳ)-catalyzed ring opening of on-DNA epoxides in water[J]. Chembiochem, 2017, 18(9): 843-847. |
| 69 | DU H C, BANGS M C, SIMMONS N, et al. Multistep synthesis of 1,2,4-oxadiazoles via DNA-conjugated aryl nitrile substrates[J]. Bioconjugate Chemistry, 2019, 30(5): 1304-1308. |
| 70 | MA F, LI J, ZHANG S N, et al. DNA-encoded libraries: hydrazide as a pluripotent precursor for on-DNA synthesis of various azole derivatives[J]. Chemistry, 2021, 27(31): 8214-8220. |
| 71 | COSTA M S, BOECHAT N, RANGEL E A, et al. Synthesis, tuberculosis inhibitory activity, and SAR study of N-substituted-phenyl-1,2,3-triazole derivatives[J]. Bioorganic & Medicinal Chemistry, 2006, 14(24): 8644-8653. |
| 72 | GIFFIN M J, HEASLET H, BRIK A, et al. A copper(Ⅰ)- catalyzed 1,2,3-triazole azide-alkyne click compound is a potent inhibitor of a multidrug-resistant HIV-1 protease variant[J]. Journal of Medicinal Chemistry, 2008, 51(20): 6263-6270. |
| 73 | MELDAL M, TORNØE C W. Cu-catalyzed azide-alkyne cycloaddition[J]. Chemical Reviews, 2008, 108(8): 2952-3015. |
| 74 | HEIN J E, TRIPP J C, KRASNOVA L B, et al. Copper (Ⅰ)-catalyzed cycloaddition of organic azides and 1-iodoalkynes[J]. Angewandte Chemie International Edition, 2009, 48(43): 8018-8021. |
| 75 | GIRONDA-MARTÍNEZ A, NERI D, SAMAIN F, et al. DNA-compatible diazo-transfer reaction in aqueous media suitable for DNA-encoded chemical library synthesis[J]. Organic Letters, 2019, 21(23): 9555-9558. |
| 76 | KABOUDIN B, ABEDI Y, YOKOMATSU T. One-pot synthesis of 1,2,3-triazoles from boronic acids in water using Cu(Ⅱ)-β-cyclodextrin complex as a nanocatalyst[J]. Organic & Biomolecular Chemistry, 2012, 10(23): 4543-4548. |
| 77 | QU Y, WEN H N, GE R, et al. Copper-mediated DNA-compatible one-pot click reactions of alkynes with aryl borates and TMS-N3 [J]. Organic Letters, 2020, 22(11): 4146-4150. |
| 78 | SINGH H, CHAWLA A S, KAPOOR V K, et al. Medicinal chemistry of tetrazoles[J]. Progress in Medicinal Chemistry, 1980, 17: 151-183. |
| 79 | DU H C, MATZUK M M, CHEN Y C. Synthesis of 5-substituted tetrazoles via DNA-conjugated nitrile[J]. Organic & Biomolecular Chemistry, 2020, 18(45): 9221-9226. |
| 80 | LI H L, SUN Z, WU W T, et al. Inverse-electron-demand Diels-Alder reactions for the synthesis of pyridazines on DNA[J]. Organic Letters, 2018, 20(22): 7186-7191. |
| 81 | GAO Y T, SUN Y, ZHAO G X, et al. On-DNA synthesis of functionalized 4H-pyran scaffolds for focused DNA-encoded chemical libraries[J]. Organic Letters, 2022, 24(36): 6664-6669. |
| 82 | NIE Q G, FANG X F, LIU C Y, et al. DNA-compatible ortho-phthalaldehyde (OPA)-mediated 2-substituted isoindole core formation and applications[J]. The Journal of Organic Chemistry, 2022, 87(5): 2551-2558. |
| 83 | ŠKOPIĆ M K, GÖTTE K, GRAMSE C, et al. Micellar Brønsted acid mediated synthesis of DNA-tagged heterocycles[J]. Journal of the American Chemical Society, 2019, 141(26): 10546-10555. |
| 84 | SUO Y R, XU M, SUN M M, et al. Ruthenium-mediated [2+2+2]cyclization: a route to forge indane and isoindoline core and its application in DNA-encoded library technology[J]. Organic Letters, 2022, 24(49): 9092-9096. |
| 85 | FANG X F, LIAO H L, FAN X H, et al. Incorporation of viridicatin alkaloid-like scaffolds into DNA-encoded chemical libraries[J]. Organic & Biomolecular Chemistry, 2023, 21(10): 2162-2166. |
| 86 | SU L Q, FENG J, PENG T, et al. Synthesis of multifunctional 2-aminobenzimidazoles on DNA via iodine-promoted cyclization[J]. Organic Letters, 2020, 22(4): 1290-1294. |
| 87 | BAO Y P, DENG Z F, FENG J, et al. A B2(OH)4-mediated synthesis of 2-substituted indazolone and its application in a DNA-encoded library[J]. Organic Letters, 2020, 22(16): 6277-6282. |
| 88 | WEN X, ZHANG M M, DUAN Z Q, et al. Discovery, SAR study of GST inhibitors from a novel quinazolin-4(1H)-one focused DNA-encoded library[J]. Journal of Medicinal Chemistry, 2023, 66(16): 11118-11132. |
| 89 | GAO H, LIN S, ZHANG S N, et al. Gem-difluoromethylene alkyne-enabled diverse C—H functionalization and application to the on-DNA synthesis of difluorinated isocoumarins[J]. Angewandte Chemie International Edition, 2021, 60(4): 1959-1966. |
| 90 | ZHAO G X, WANG H H, LUO J, et al. Multicomponent DNA-compatible synthesis of an annelated benzodiazepine scaffold for focused chemical libraries[J]. Organic Letters, 2023, 25(4): 665-670. |
| 91 | HIESINGER K, DAR’IN D, PROSCHAK E, et al. Spirocyclic scaffolds in medicinal chemistry[J]. Journal of Medicinal Chemistry, 2021, 64(1): 150-183. |
| 92 | WANG X, LIU J X, YAN Z Q, et al. Diversified strategy for the synthesis of DNA-encoded oxindole libraries[J]. Chemical Science, 2021, 12(8): 2841-2847. |
| 93 | NIE Q G, SUN J, FANG X F, et al. Antimony salt-promoted cyclization facilitating on-DNA syntheses of dihydroquinazolinone derivatives and its applications[J]. Chinese Chemical Letters, 2023, 34(8): 108132. |
| 94 | LI L B, MATSUO B, LEVITRE G, et al. Dearomative intermolecular [2+2] photocycloaddition for construction of C(sp3)-rich heterospirocycles on-DNA[J]. Chemical Science, 2023, 14(10): 2713-2720. |
| 95 | GALLOWAY W R J D, ISIDRO-LLOBET A, SPRING D R. Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules[J]. Nature Communications, 2010, 1: 80. |
| 96 | SPRING D R. Diversity-oriented synthesis; a challenge for synthetic chemists[J]. Organic & Biomolecular Chemistry, 2003, 1(22): 3867-3870. |
| 97 | WESTPHAL M V, HUDSON L, MASON J W, et al. Water-compatible cycloadditions of oligonucleotide-conjugated strained allenes for DNA-encoded library synthesis[J]. Journal of the American Chemical Society, 2020, 142(17): 7776-7782. |
| 98 | LIU S X, QI J J, LU W W, et al. Synthetic studies toward DNA-encoded heterocycles based on the on-DNA formation of α,β-unsaturated ketones[J]. Organic Letters, 2021, 23(3): 908-913. |
| 99 | FANG X F, WANG Y T, HE P Y, et al. Visible light-promoted divergent benzoheterocyclization from aldehydes for DNA-encoded chemical libraries[J]. Organic Letters, 2022, 24(17): 3291-3296. |
| 100 | ZHANG S L, ZHANG H M, LIU X W, et al. Mask and release strategy-enabled diversity-oriented synthesis for DNA-encoded library[J]. Advanced Science, 2024, 11(6): e2307049. |
| 101 | TRUPPO M D. Biocatalysis in the pharmaceutical industry: the need for speed[J]. ACS Medicinal Chemistry Letters, 2017, 8(5): 476-480. |
| [1] | ZHONG Quanzhou, SHAN Yiyi, PEI Qingyun, JIN Yanyun, WANG Yihan, MENG Luyuan, WANG Xinyun, ZHANG Yuxin, LIU Kunyuan, WANG Huizhong, FENG Shangguo. Research progress in the production of α-arbutin through biosynthesis [J]. Synthetic Biology Journal, 2025, 6(1): 118-135. |
| [2] | ZHU Fanghuan, CEN Xuecong, CHEN Zhen. Research progress of diols production by microbes [J]. Synthetic Biology Journal, 2024, 5(6): 1367-1385. |
| [3] | LIU Yining, PU Wei, YANG Jinxing, WANG Yu. Recent advances in the biosynthesis of ω-amino acids and lactams [J]. Synthetic Biology Journal, 2024, 5(6): 1350-1366. |
| [4] | ZHENG Haotian, LI Chaofeng, LIU Liangxu, WANG Jiawei, LI Hengrun, NI Jun. Design, optimization and application of synthetic carbon-negative phototrophic community [J]. Synthetic Biology Journal, 2024, 5(5): 1189-1210. |
| [5] | CHENG Xiaolei, LIU Tiangang, TAO Hui. Recent research progress in non-canonical biosynthesis of terpenoids [J]. Synthetic Biology Journal, 2024, 5(5): 1050-1071. |
| [6] | ZHANG Shouqi, WANG Tao, KONG Yao, ZOU Jiasheng, LIU Yuanning, XU Zhengren. Chemoenzymatic synthesis of natural products: evolution of synthetic methodology and strategy [J]. Synthetic Biology Journal, 2024, 5(5): 913-940. |
| [7] | XIE Xiangqian, GUO Wen, WANG Huan, LI Jin. Biosynthesis and chemical synthesis of ribosomally synthesized and post-translationally modified peptides containing aminovinyl cysteine [J]. Synthetic Biology Journal, 2024, 5(5): 981-996. |
| [8] | TANG Zhijun, HU Youcai, LIU Wen. Enzymatic (4+2)- and (2+2)-cycloaddition reactions: fundamentals and applications of regio- and stereoselectivity [J]. Synthetic Biology Journal, 2024, 5(3): 401-407. |
| [9] | ZHANG Jun, JIN Shixue, YUN Qian, QU Xudong. Biosynthesis of the unnatural extender units with polyketides and their structural modifications for applications in medicines [J]. Synthetic Biology Journal, 2024, 5(3): 561-570. |
| [10] | CHEN Xiwei, ZHANG Huaran, ZOU Yi. Biosynthesis and metabolic engineering of fungal non-ribosomal peptides [J]. Synthetic Biology Journal, 2024, 5(3): 571-592. |
| [11] | FENG Jin, PAN Haixue, TANG Gongli. Research advances in biosynthesis of natural product drugs within the past decade [J]. Synthetic Biology Journal, 2024, 5(3): 408-446. |
| [12] | XI Mengyu, HU Yiling, GU Yucheng, GE Huiming. Genome mining-directed discovery for natural medicinal products [J]. Synthetic Biology Journal, 2024, 5(3): 447-473. |
| [13] | SHI Xinjie, DU Yiling. Research advances in the biosynthesis of nonribosomal peptides within the bisintercalator family as anticancer drugs [J]. Synthetic Biology Journal, 2024, 5(3): 593-611. |
| [14] | SONG Yongxiang, ZHANG Xiufeng, LI Yanqin, XIAO Hua, YAN Yan. Resistance-gene directed discovery of bioactive natural products [J]. Synthetic Biology Journal, 2024, 5(3): 474-491. |
| [15] | TU Huiyang, HAN Weidong, ZHANG Bin. Strategies for the design and optimization of tumor neoantigen vaccines [J]. Synthetic Biology Journal, 2024, 5(2): 254-266. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||