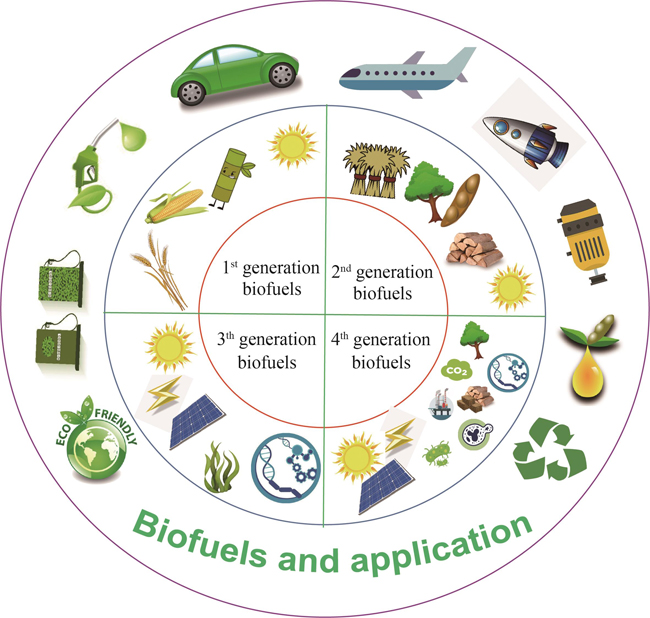
With the socioeconomic development, the dependence of human beings on fossil fuels has led to their shortage and climate change. This has created an urgent need for alternatives that are renewable and environmentally friendly, and biofuels are one of them. Nowadays, widely recognized biofuels like fuel ethanol and biodiesel face challenges in terms of their production capacity due to limitation on raw materials such as grains and edible oils and high cost as well. Hence, the integration of metabolic engineering and synthetic biology has opened avenues for utilizing diverse substrates from other renewable sources, such as solar energy, light energy, electric energy, and waste biomass. Microbial cell factories, including microalgae, bacteria, and yeast, play a crucial role in synthesizing biofuels. The review comments on the evolution of the four generations of biofuels, encompassing fuel ethanol, biodiesel, bio-gasoline, jet and aviation fuels. We also discuss how microorganisms can be explored for producing the third- and fourth-generation biofuels from a variety of unconventional substrates such as carbon dioxide, methanol, and methane, multi-energy coupling to synthesize biofuels from lignocellulose by bacterial or yeast, CO2 conversion by microalgae or electrochemical-biological systems, the conversion of methanol and methane by methyltrophic microbes, and the application of synthetic biology. Furthermore, we overview biosynthetic pathways and engineering strategies for optimizing biofuels production. These strategies can convert raw materials to various fuel products, including fatty acids and esters, advanced alcohols and esters, isoprenoids, and polyketides. Finally, we highlight some challenges in biofuels production, including raw material supply and cost issue, low production yield, and limited product variety. Meanwhile, to address these challenges, we propose corresponding solutions. For example, by optimizing carbon fixation pathways, and converting carbon dioxide into low-carbon substrates like methanol, autotrophic microorganisms, methylotrophic microorganisms, and other cell factories can utilize carbon dioxide as the major raw material to synthesize various biofuels, which can benefit the application of biofuels and further promote their industrial production.