Synthetic Biology Journal ›› 2025, Vol. 6 ›› Issue (2): 334-356.DOI: 10.12211/2096-8280.2024-057
• Invited Review • Previous Articles Next Articles
Synthetic biology drives the sustainable production of terpenoid fragrances and flavors
ZHANG Mengyao1,2,3, CAI Peng1,2, ZHOU Yongjin1,2
- 1.Division of Biotechnology,Dalian Institute of Chemical Physics,Chinese Academy of Sciences,Dalian 116023,Liaoning,China
2.Dalian Key Laboratory of Energy Biotechnology,Dalian 116023,Liaoning,China
3.University of Chinese Academy of Sciences,Beijing 100049,China
-
Received:2024-07-31Revised:2024-09-18Online:2025-05-20Published:2025-04-30 -
Contact:ZHOU Yongjin
合成生物学助力萜类香精香料可持续生产
张梦瑶1,2,3, 蔡鹏1,2, 周雍进1,2
- 1.中国科学院大连化学物理研究所生物技术研究部,辽宁 大连 116023
2.大连市能源生物技术重点实验室,辽宁 大连 116023
3.中国科学院大学,北京 100049
-
通讯作者:周雍进 -
作者简介:张梦瑶 (1999—),女,博士研究生。研究方向为天然产物的生物合成。E-mail:myzhang@dicp.ac.cn周雍进 (1984—),男,博士,研究员。研究方向为基于甲醇生物转化与天然产物生物合成。E-mail:zhouyongjin@dicp.ac.cn -
基金资助:国家重点研发计划(2022YFC2105900);中国科学院特别研究助理资助项目
CLC Number:
Cite this article
ZHANG Mengyao, CAI Peng, ZHOU Yongjin. Synthetic biology drives the sustainable production of terpenoid fragrances and flavors[J]. Synthetic Biology Journal, 2025, 6(2): 334-356.
张梦瑶, 蔡鹏, 周雍进. 合成生物学助力萜类香精香料可持续生产[J]. 合成生物学, 2025, 6(2): 334-356.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2024-057
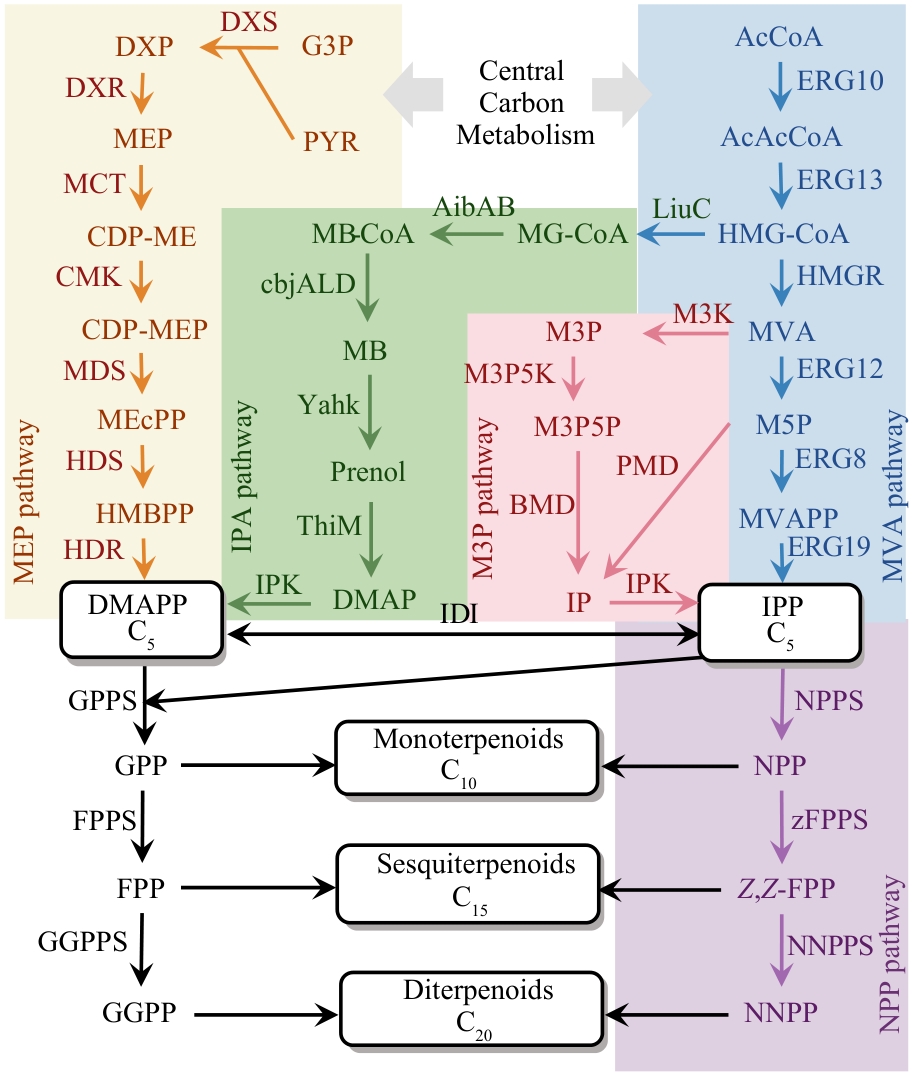
Fig. 2 Classical terpene biosynthetic pathways and alternative chemical synthetic routes(G3P—D-glyceraldehyde 3-phosphate; PYR—pyruvate; DXP—1-deoxy-D-xylulose 5-phosphate; MEP—2-C-methyl-D-erythritol 4-phosphate; CDP-ME—4-diphophocytidyl-2-C-methyl-D-erythrito; CDP-MEP—4-diphophocytidyl-2-C-methyl-D-erythritol 2phosphate; MEcPP—2-C-methyl-D-erythritol 2,4-cyclodiphosphate; HMBPP—4-hydroxy-3-methyl-butenyl diphosphate; DMAPP—dimethylallyl diphosphate; GPP—geranyl diphosphate; FPP—farnesyl diphosphate; GGPP—geranylgeranyl diphosphate; MG-CoA—3-methylglutaconyl-CoA; MB-CoA—3-methyl-2-butenoyl-CoA; MB—3-methyl-2-butenal; DMAP—dimethylallyl phosphate; M3P—mevalonate 3-phosphate; M3P5P—mevalonate 3,5-biphosphate; IP—isopentenyl phosphate; AcCoA—acetyl-CoA; AcAcCoA—acetoacetyl-CoA; HMGCoA—3-hydroxy-3-methylglutaryl-CoA; MVA—mevalonate; M5P—mevalonate 5-phosphate; MVAPP—mevalonate diphosphate; IPP—isopentenyl diphosphate; NPP—neryl diphosphate; Z,Z-FPP—Z,Z-farnesyl diphosphate; NNPP—nerylneryl diphosphate; DXS—DXP synthase; DXR—DXP reductoisomerase; MCT—MEP cytidylyltransferase; CMK—CDP-ME kinase; MDS—ME-CPP synthase; HDS—HMB-PP synthase; HDR—HMB-PP reductase; ERG10—ACCT acetyl-CoA C-acetyl transferase; ERG13—HMGS HMG-CoA synthase; HMGR—HMG-CoA reductase; ERG12—MK MVA kinase; ERG8—PMVK phosphomevalonate kinase; ERG19—MVD diphosphomevalonate decarboxylase; LiuC—enoyl-CoA hydratase; AibAB—glutaconyl-CoA decarboxylase; cbjALD—acyl-CoA reductase; YahK—alcohol dehydrogenase; ThiM—hydroxyethylthiazole kinase; IPK—isopentenyl phosphate kinase; IDI—isopentenyl-diphosphate isomerase; GPPS—geranyl pyrophosphate synthase; FPPS—farnesyl pyrophosphate synthase; GGPPS—geranylgeranyl diphosphate synthase; NPPS—nerol pyrophosphate synthase; zFPPS—Z,Z-Farnesyl diphosphate synthase; NNPPS—nerylneryl diphosphate synthase; M3K—mevalonate 3-kinase; M3P5K—mevalonate 3-phosphate 5-kinase; BMD—mevalonate biphosphate decarboxylase; IPK—isopentenyl phosphate kinase; PMD—mevalonate 5-phosphate decarboxylase.)
| 结构 | 化合物 | 气味特征 | 底盘菌株 | 培养条件 | 产量/(g/L) | 参考文献 |
|---|---|---|---|---|---|---|
| 链状单萜 | 香叶醇 Geraniol | 温和、甜香、花果香气 | 大肠杆菌 | 摇瓶发酵 | 2.1 | [ |
| 巴斯德毕赤酵母 | 24孔板发酵 | 1.2 | [ | |||
月桂烯 Myrcene | 甜香脂香气 | 大肠杆菌 | 摇瓶发酵 | 1.2 | [ | |
香茅醇 Citronellol | 甜润玫瑰花香 | 酿酒酵母 | 5 L生物反应器 | 8.3 | [ | |
芳樟醇 Linalool | 铃兰清香 | 菠萝潘托氏菌 | 5 mL试管 | S型5.6 R型3.7 | [ | |
| 单环单萜 | 柠檬烯 Limonene | 柠檬香气 | 大肠杆菌 | 3 L生物反应器 | 3.6 | [ |
α-松油醇 α-Terpineol | 丁香花气味 | 酿酒酵母 | 5 L生物反应器 | 21.8 | [ | |
左旋香芹酮 (-)-Carvone | 兰花香气、类清新薄荷气味 | 大肠杆菌 | 摇瓶发酵 柠檬烯为底物 | 44.3 | [ | |
| 双环单萜 | 龙脑 Borneol | 极强的樟脑和松木香气 | 酿酒酵母 | 摇瓶补料 | 0.75 | [ |
α-蒎烯 α-Pinene | 松木芳香,清新草本气味 | 大肠杆菌 | 摇瓶发酵 | 0.17 | [ | |
香桧烯 Sabinene | 湿泥土气味与木质香气 | 酿酒酵母 | 摇瓶发酵 | 0.15 | [ | |
| 链状倍半萜 | 橙花叔醇 Nerolidol | 清新柔和的木质香及花果香 | 酿酒酵母 | 摇瓶发酵 | 3.5 | [ |
β-法尼烯 β-Farnesol | 青草与柑橘混合的清新香气 | 酿酒酵母 | 200 t生物反应器 | 130 | [ | |
法尼醇 Farnesol | 温和的花香调 | 大肠杆菌 | 试管 | 0.53 | [ | |
| 单环倍半萜 | 红没药烯 Bisabolene | 果香与香脂香 | 酿酒酵母 | 5 L生物反应器 | 9.8 | [ |
α-葎草烯 α-Humulene | 丁香香型 | 热带念珠菌 | 30 L生物反应器 | 4.1 | [ | |
右旋大根香叶烯D (-)-Germacrene D | 辛辣的胡椒香气 | 酿酒酵母 | 5 L生物反应器 | 7.9 | [ | |
| 双环倍半萜 | 右旋瓦伦烯 (+)-Valencene | 愉悦的柑橘香 | 酿酒酵母 | 15 L生物反应器 | 16.6 | [ |
右旋诺卡酮 (+)-Nootkatone | 葡萄柚的香气 | 酿酒酵母 | 5 L生物反应器 | 0.80 | [ | |
檀香醇 Santalol | 柔和温暖的木质香型 | 酿酒酵母 | 5 L生物反应器 | 1.3 | [ | |
| 三环倍半萜 | α-檀香烯 α-Santalene | 温暖细腻的檀香木香气 | 巴斯德毕赤酵母 | 1 L生物反应器 | 21.5 | [ |
长叶烯 Longifolene | 鸢尾花与木质香 | 大肠杆菌 | 5 L生物反应器 | 0.38 | [ | |
广藿香醇 Patchoulol | 广藿芳香 | 酿酒酵母 | 5 L生物反应器 | 1.6 | [ | |
| 单环降异戊二烯 | α-紫罗酮 α-Ionone | 强烈的紫罗兰花与鸢尾根香 | 酿酒酵母 | 摇瓶补料 | 0.48 | [ |
β-紫罗酮 β-Ionone | 紫罗兰与玫瑰香,含木质香 | 解脂耶氏酵母 | 3 L生物反应器 | 0.98 | [ |
Table 1 Summary of cell factories for producing terpene fragrances
| 结构 | 化合物 | 气味特征 | 底盘菌株 | 培养条件 | 产量/(g/L) | 参考文献 |
|---|---|---|---|---|---|---|
| 链状单萜 | 香叶醇 Geraniol | 温和、甜香、花果香气 | 大肠杆菌 | 摇瓶发酵 | 2.1 | [ |
| 巴斯德毕赤酵母 | 24孔板发酵 | 1.2 | [ | |||
月桂烯 Myrcene | 甜香脂香气 | 大肠杆菌 | 摇瓶发酵 | 1.2 | [ | |
香茅醇 Citronellol | 甜润玫瑰花香 | 酿酒酵母 | 5 L生物反应器 | 8.3 | [ | |
芳樟醇 Linalool | 铃兰清香 | 菠萝潘托氏菌 | 5 mL试管 | S型5.6 R型3.7 | [ | |
| 单环单萜 | 柠檬烯 Limonene | 柠檬香气 | 大肠杆菌 | 3 L生物反应器 | 3.6 | [ |
α-松油醇 α-Terpineol | 丁香花气味 | 酿酒酵母 | 5 L生物反应器 | 21.8 | [ | |
左旋香芹酮 (-)-Carvone | 兰花香气、类清新薄荷气味 | 大肠杆菌 | 摇瓶发酵 柠檬烯为底物 | 44.3 | [ | |
| 双环单萜 | 龙脑 Borneol | 极强的樟脑和松木香气 | 酿酒酵母 | 摇瓶补料 | 0.75 | [ |
α-蒎烯 α-Pinene | 松木芳香,清新草本气味 | 大肠杆菌 | 摇瓶发酵 | 0.17 | [ | |
香桧烯 Sabinene | 湿泥土气味与木质香气 | 酿酒酵母 | 摇瓶发酵 | 0.15 | [ | |
| 链状倍半萜 | 橙花叔醇 Nerolidol | 清新柔和的木质香及花果香 | 酿酒酵母 | 摇瓶发酵 | 3.5 | [ |
β-法尼烯 β-Farnesol | 青草与柑橘混合的清新香气 | 酿酒酵母 | 200 t生物反应器 | 130 | [ | |
法尼醇 Farnesol | 温和的花香调 | 大肠杆菌 | 试管 | 0.53 | [ | |
| 单环倍半萜 | 红没药烯 Bisabolene | 果香与香脂香 | 酿酒酵母 | 5 L生物反应器 | 9.8 | [ |
α-葎草烯 α-Humulene | 丁香香型 | 热带念珠菌 | 30 L生物反应器 | 4.1 | [ | |
右旋大根香叶烯D (-)-Germacrene D | 辛辣的胡椒香气 | 酿酒酵母 | 5 L生物反应器 | 7.9 | [ | |
| 双环倍半萜 | 右旋瓦伦烯 (+)-Valencene | 愉悦的柑橘香 | 酿酒酵母 | 15 L生物反应器 | 16.6 | [ |
右旋诺卡酮 (+)-Nootkatone | 葡萄柚的香气 | 酿酒酵母 | 5 L生物反应器 | 0.80 | [ | |
檀香醇 Santalol | 柔和温暖的木质香型 | 酿酒酵母 | 5 L生物反应器 | 1.3 | [ | |
| 三环倍半萜 | α-檀香烯 α-Santalene | 温暖细腻的檀香木香气 | 巴斯德毕赤酵母 | 1 L生物反应器 | 21.5 | [ |
长叶烯 Longifolene | 鸢尾花与木质香 | 大肠杆菌 | 5 L生物反应器 | 0.38 | [ | |
广藿香醇 Patchoulol | 广藿芳香 | 酿酒酵母 | 5 L生物反应器 | 1.6 | [ | |
| 单环降异戊二烯 | α-紫罗酮 α-Ionone | 强烈的紫罗兰花与鸢尾根香 | 酿酒酵母 | 摇瓶补料 | 0.48 | [ |
β-紫罗酮 β-Ionone | 紫罗兰与玫瑰香,含木质香 | 解脂耶氏酵母 | 3 L生物反应器 | 0.98 | [ |
| 1 | BOM S, JORGE J, RIBEIRO H M, et al. A step forward on sustainability in the cosmetics industry: a review[J]. Journal of Cleaner Production, 2019, 225: 270-290. |
| 2 | EL-OTMANI N, ZEOUK I, HAMMANI O, et al. Analysis and quality control of bio-actives and herbal cosmetics: the case of traditional cooperatives from Fes-Meknes Region[J]. Tropical Journal of Natural Product Research, 2024, 8(5): 7181-7195. |
| 3 | MICHAILIDOU D F. The scent of change: sustainable fragrances through industrial biotechnology[J]. ChemBioChem, 2023, 24(19): e202300309. |
| 4 | WESTON-GREEN K, CLUNAS H, JIMENEZ NARANJO C. A review of the potential use of pinene and linalool as terpene-based medicines for brain health: discovering novel therapeutics in the flavours and fragrances of Cannabis [J]. Frontiers in Psychiatry, 2021, 12: 583211. |
| 5 | JIANG H, WANG X. Biosynthesis of monoterpenoid and sesquiterpenoid as natural flavors and fragrances[J]. Biotechnology Advances, 2023, 65: 108151. |
| 6 | ZHANG C B, LI M, ZHAO G R, et al. Harnessing yeast peroxisomes and cytosol acetyl-CoA for sesquiterpene α-humulene production[J]. Journal of Agricultural and Food Chemistry, 2020, 68(5): 1382-1389. |
| 7 | MASYITA A, MUSTIKA SARI R, ASTUTI A DWI, et al. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives[J]. Food Chemistry: X, 2022, 13: 100217. |
| 8 | CAO X, YU W, CHEN Y, et al. Engineering yeast for high-level production of diterpenoid sclareol[J]. Metabolic Engineering, 2023, 75: 19-28. |
| 9 | Linkedin. Terpenes Market Research Report: Company Snapshot 2031[EB/OL]. LinkedIn Pulse. [2024-03-01]. . |
| 10 | CAO C Y, CAO X, YU W, et al. Global metabolic rewiring of yeast enables overproduction of sesquiterpene (+)-valencene[J]. Journal of Agricultural and Food Chemistry, 2022, 70(23): 7180-7187. |
| 11 | VOIGT C A. Synthetic biology 2020—2030: six commercially-available products that are changing our world[J]. Nature Communications, 2020, 11(1): 6379. |
| 12 | KHALIL A S, COLLINS J J. Synthetic biology: applications come of age[J]. Nature Reviews Genetics, 2010, 11(5): 367-379. |
| 13 | VOLK M J, TRAN V G, TAN S I, et al. Metabolic engineering: methodologies and applications[J]. Chemical Reviews, 2023, 123(9): 5521-5570. |
| 14 | CIMERMANCIC P, MEDEMA M H, CLAESEN J, et al. Insights into secondary metabolism from a global analysis of prokaryotic biosynthetic gene clusters[J]. Cell, 2014, 158(2): 412-421. |
| 15 | BLIN K, SHAW S, AUGUSTIJN H E, et al. antiSMASH 7.0: new and improved predictions for detection, regulation, chemical structures and visualisation[J]. Nucleic Acids Research, 2023, 51(W1): W46-W50. |
| 16 | SKINNIDER M A, JOHNSTON C W, GUNABALASINGAM M, et al. Comprehensive prediction of secondary metabolite structure and biological activity from microbial genome sequences[J]. Nature Communications, 2020, 11(1): 6058. |
| 17 | WANG X R, CHEN N X, CRUZ-MORALES P, et al. Elucidation of genes enhancing natural product biosynthesis through co-evolution analysis[J]. Nature Metabolism, 2024, 6(5): 933-946. |
| 18 | ARKIN A P, COTTINGHAM R W, HENRY C S, et al. KBase: the United States Department of Energy systems biology knowledgebase[J]. Nature Biotechnology, 2018, 36(7): 566-569. |
| 19 | SEAVER S M D, LIU F, ZHANG Q Z, et al. The ModelSEED Biochemistry Database for the integration of metabolic annotations and the reconstruction, comparison and analysis of metabolic models for plants, fungi and microbes[J]. Nucleic Acids Research, 2021, 49(D1): D575-D588. |
| 20 | AGREN R, LIU L M, SHOAIE S, et al. The RAVEN toolbox and its use for generating a genome-scale metabolic model for Penicillium chrysogenum [J]. PLoS Computational Biology, 2013, 9(3): e1002980. |
| 21 | BURGARD A P, PHARKYA P, MARANAS C D. Optknock: a bilevel programming framework for identifying gene knockout strategies for microbial strain optimization[J]. Biotechnology and Bioengineering, 2003, 84(6): 647-657. |
| 22 | JUMPER J, EVANS R, PRITZEL A, et al. Highly accurate protein structure prediction with AlphaFold[J]. Nature, 2021, 596(7873): 583-589. |
| 23 | ABRAMSON J, ADLER J, DUNGER J, et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3[J]. Nature, 2024, 630(8016): 493-500. |
| 24 | GREENING C, CABOTAJE P R, VALENTIN ALVARADO L E, et al. Minimal and hybrid hydrogenases are active from Archaea[J]. Cell, 2024, 187(13): 3357-3372. e19. |
| 25 | TRIVEDI V, RAMESH A, WHEELDON I. Analyzing CRISPR screens in non-conventional microbes[J]. Journal of Industrial Microbiology & Biotechnology, 2023, 50(1): kuad006. |
| 26 | LIU X Q, CUI Z Y, SU T Y, et al. Identification of genome integration sites for developing a CRISPR-based gene expression toolkit in Yarrowia lipolytica [J]. Microbial Biotechnology, 2022, 15(8): 2223-2234. |
| 27 | HOLKENBRINK C, DAM M I, KILDEGAARD K R, et al. EasyCloneYALI: CRISPR/Cas9-based synthetic toolbox for engineering of the yeast Yarrowia lipolytica [J]. Biotechnology Journal, 2018, 13(9): 1700543. |
| 28 | CUI Z Y, JIANG X, ZHENG H H, et al. Homology-independent genome integration enables rapid library construction for enzyme expression and pathway optimization in Yarrowia lipolytica [J]. Biotechnology and Bioengineering, 2019, 116(2): 354-363. |
| 29 | LIU Q, SHI X N, SONG L L, et al. CRISPR-Cas9-mediated genomic multiloci integration in Pichia pastoris [J]. Microbial Cell Factories, 2019, 18(1): 144. |
| 30 | CAI P, DUAN X P, WU X Y, et al. Recombination machinery engineering facilitates metabolic engineering of the industrial yeast Pichia pastoris [J]. Nucleic Acids Research, 2021, 49(13): 7791-7805. |
| 31 | GAO J C, JIANG L H, LIAN J Z. Development of synthetic biology tools to engineer Pichia pastoris as a chassis for the production of natural products[J]. Synthetic and Systems Biotechnology, 2021, 6(2): 110-119. |
| 32 | GAO J Q, GAO N, ZHAI X X, et al. Recombination machinery engineering for precise genome editing in methylotrophic yeast Ogataea polymorpha [J]. iScience, 2021, 24(3): 102168. |
| 33 | YU W, GAO J Q, ZHAI X X, et al. Screening neutral sites for metabolic engineering of methylotrophic yeast Ogataea polymorpha [J]. Synthetic and Systems Biotechnology, 2021, 6(2): 63-68. |
| 34 | FATMA Z, TAN S I, BOOB A G, et al. A landing pad system for multicopy gene integration in Issatchenkia orientalis [J]. Metabolic Engineering, 2023, 78: 200-208. |
| 35 | KIM S K, HAN G H, SEONG W, et al. CRISPR interference-guided balancing of a biosynthetic mevalonate pathway increases terpenoid production[J]. Metabolic Engineering, 2016, 38: 228-240. |
| 36 | LIAN J Z, HAMEDIRAD M, HU S M, et al. Combinatorial metabolic engineering using an orthogonal tri-functional CRISPR system[J]. Nature Communications, 2017, 8(1): 1688. |
| 37 | DE MUNTER S, VAN PARYS A, BRAL L, et al. Rapid and effective generation of nanobody based CARs using PCR and Gibson assembly[J]. International Journal of Molecular Sciences, 2020, 21(3): 883. |
| 38 | SORIDA M, BONASIO R. An efficient cloning method to expand vector and restriction site compatibility of Golden Gate Assembly[J]. Cell Reports Methods, 2023, 3(8): 100564. |
| 39 | MA Y, SU S X, FU Z H, et al. Convenient synthesis and delivery of a megabase-scale designer accessory chromosome empower biosynthetic capacity[J]. Cell Research, 2024, 34(4): 309-322. |
| 40 | CARBONELL P, RADIVOJEVIC T, GARCÍA MARTÍN H. Opportunities at the intersection of synthetic biology, machine learning, and automation[J]. ACS Synthetic Biology, 2019, 8(7): 1474-1477. |
| 41 | IGNEA C, RAADAM M H, MOTAWIA M S, et al. Orthogonal monoterpenoid biosynthesis in yeast constructed on an isomeric substrate[J]. Nature Communications, 2019, 10(1): 3799. |
| 42 | LIPKO A, PĄCZKOWSKI C, PEREZ-FONS L, et al. Divergent contribution of the MVA and MEP pathways to the formation of polyprenols and dolichols in Arabidopsis [J]. Biochemical Journal, 2023, 480(8): 495-520. |
| 43 | PAN X M, DU W Y, ZHANG X W, et al. Discovery, structure, and mechanism of a class Ⅱ sesquiterpene cyclase[J]. Journal of the American Chemical Society, 2022, 144(48): 22067-22074. |
| 44 | ZHOU F, PICHERSKY E. More is better: the diversity of terpene metabolism in plants[J]. Current Opinion in Plant Biology, 2020, 55: 1-10. |
| 45 | ZENG L P, DEHESH K. The eukaryotic MEP-pathway genes are evolutionarily conserved and originated from Chlaymidia and cyanobacteria[J]. BMC Genomics, 2021, 22(1): 137. |
| 46 | ALLAMAND A, PIECHOWIAK T, LIÈVREMONT D, et al. The multifaceted MEP pathway: towards new therapeutic perspectives[J]. Molecules, 2023, 28(3): 1403. |
| 47 | BANERJEE A, WU Y, BANERJEE R, et al. Feedback inhibition of deoxy-D-xylulose-5-phosphate synthase regulates the methylerythritol 4-phosphate pathway[J]. Journal of Biological Chemistry, 2013, 288(23): 16926-16936. |
| 48 | DIAO J J, SONG X Y, ZHANG L, et al. Tailoring cyanobacteria as a new platform for highly efficient synthesis of astaxanthin[J]. Metabolic Engineering, 2020, 61: 275-287. |
| 49 | VOLKE D C, ROHWER J, FISCHER R, et al. Investigation of the methylerythritol 4-phosphate pathway for microbial terpenoid production through metabolic control analysis[J]. Microbial Cell Factories, 2019, 18(1): 192. |
| 50 | DU F L, YU H L, XU J H, et al. Enhanced limonene production by optimizing the expression of limonene biosynthesis and MEP pathway genes in E. coli [J]. Bioresources and Bioprocessing, 2014, 1(1): 10. |
| 51 | MA Y W, MCCLURE D D, SOMERVILLE M V, et al. Metabolic engineering of the MEP pathway in Bacillus subtilis for increased biosynthesis of menaquinone-7[J]. ACS Synthetic Biology, 2019, 8(7): 1620-1630. |
| 52 | BRÖKER J N, MÜLLER B, VAN DEENEN N, et al. Upregulating the mevalonate pathway and repressing sterol synthesis in Saccharomyces cerevisiae enhances the production of triterpenes[J]. Applied Microbiology and Biotechnology, 2018, 102(16): 6923-6934. |
| 53 | LIU F, LIU S C, QI Y K, et al. Enhancing trans-nerolidol productivity in Yarrowia lipolytica by improving precursor supply and optimizing nerolidol synthase activity[J]. Journal of Agricultural and Food Chemistry, 2022, 70(48): 15157-15165. |
| 54 | MUKHERJEE M, BLAIR R H, WANG Z Q. Machine-learning guided elucidation of contribution of individual steps in the mevalonate pathway and construction of a yeast platform strain for terpenoid production[J]. Metabolic Engineering, 2022, 74: 139-149. |
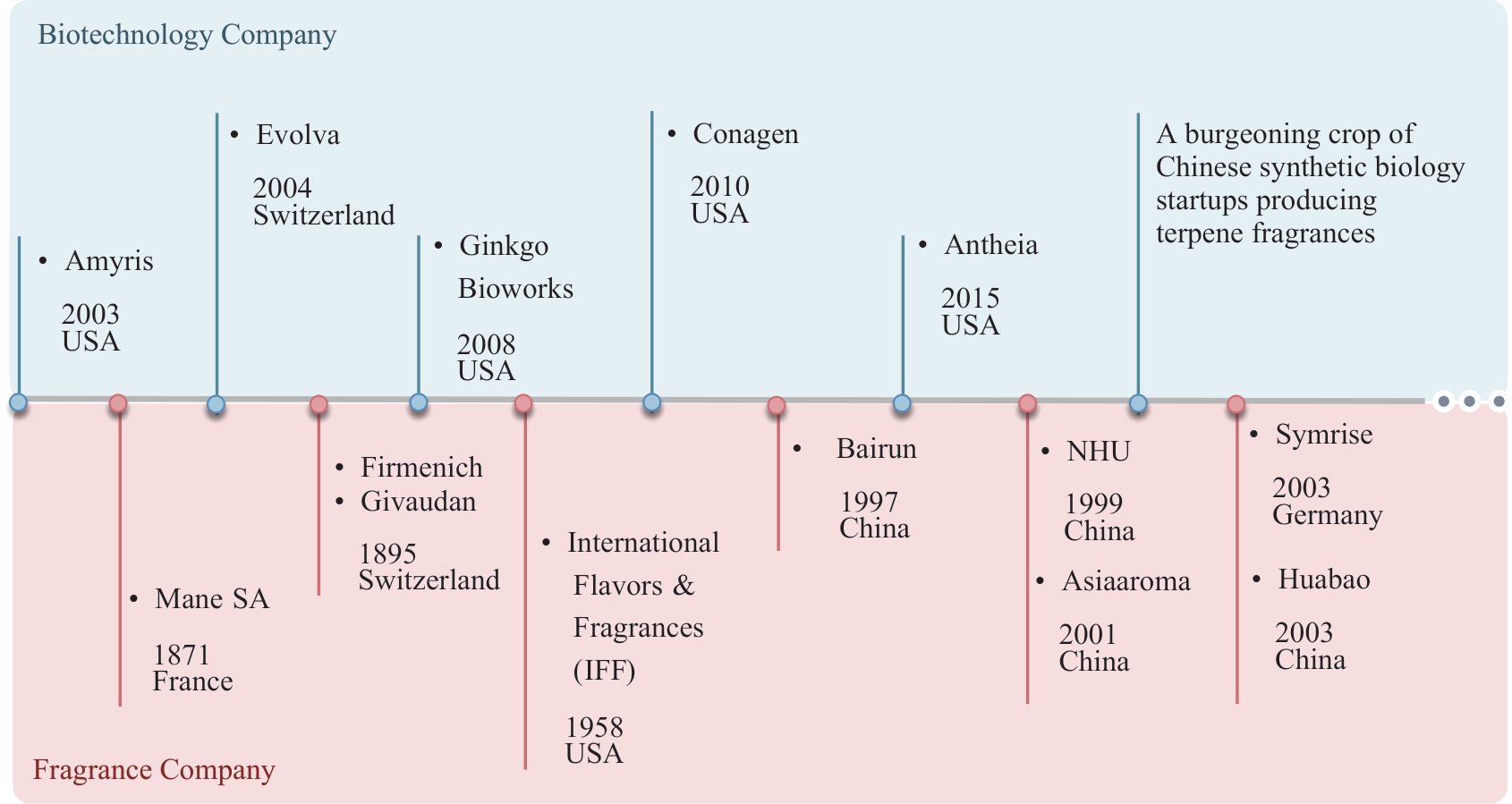
| 55 | RINALDI M A, FERRAZ C A, SCRUTTON N S. Alternative metabolic pathways and strategies to high-titre terpenoid production in Escherichia coli [J]. Natural Product Reports, 2022, 39(1): 90-118. |
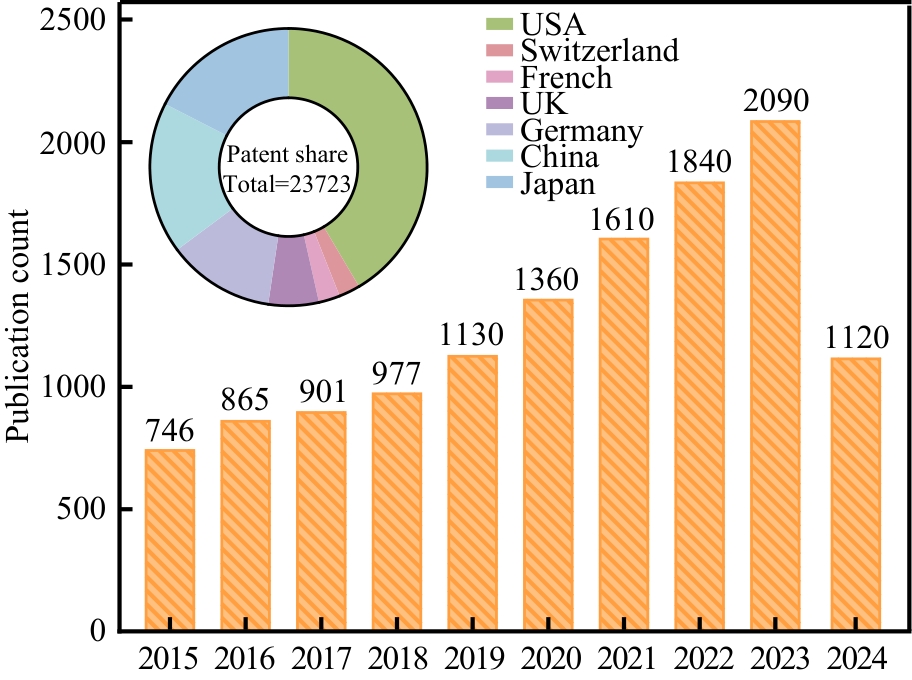
| 56 | CHEN H L, LIU C Q, LI M J, et al. Directed evolution of mevalonate kinase in Escherichia coli by random mutagenesis for improved lycopene[J]. RSC Advances, 2018, 8(27): 15021-15028. |
| 57 | CAO C Y, ZHANG H Y, CAO X, et al. Construction and optimization of nonclassical isoprenoid biosynthetic pathways in yeast peroxisomes for (+)-valencene production[J]. Journal of Agricultural and Food Chemistry, 2023, 71(29): 11124-11130. |
| 58 | CLOMBURG J M, QIAN S, TAN Z G, et al. The isoprenoid alcohol pathway, a synthetic route for isoprenoid biosynthesis[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(26): 12810-12815. |
| 59 | MA Y S, ZU Y X, HUANG S W, et al. Engineering a universal and efficient platform for terpenoid synthesis in yeast[J]. Proceedings of the National Academy of Sciences of the United States of America, 2023, 120(1): e2207680120. |
| 60 | LIU S S, ZHANG M Y, REN Y Y, et al. Engineering Rhodosporidium toruloides for limonene production[J]. Biotechnology for Biofuels, 2021, 14(1): 243. |
| 61 | SCHILMILLER A L, SCHAUVINHOLD I, LARSON M, et al. Monoterpenes in the glandular trichomes of tomato are synthesized from a neryl diphosphate precursor rather than geranyl diphosphate[J]. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(26): 10865-10870. |
| 62 | 程晓雷, 刘天罡, 陶慧. 萜类化合物的非常规生物合成研究进展[J]. 合成生物学, 2024, 5(5): 1050-1071. |
| CHENG X L, LIU T G, TAO H. Recent research progress in non-canonical biosynthesis of terpenoids[J]. Synthetic Biology Journal, 2024, 5(5): 1050-1071. | |
| 63 | LUO P, HUANG J H, LÜ J M, et al. Biosynthesis of fungal terpenoids[J]. Natural Product Reports, 2024, 41(5): 748-783. |
| 64 | LI Z, ZHANG L L, XU K W, et al. Molecular insights into the catalytic promiscuity of a bacterial diterpene synthase[J]. Nature Communications, 2023, 14(1): 4001. |
| 65 | ABE T, SHIRATORI H, KASHIWAZAKI K, et al. Structural-model-based genome mining can efficiently discover novel non-canonical terpene synthases hidden in genomes of diverse species[J]. Chemical Science, 2024, 15(27): 10402-10407. |
| 66 | DENG X M, YE Z L, DUAN J Y, et al. Complete pathway elucidation and heterologous reconstitution of (+)-nootkatone biosynthesis from Alpinia oxyphylla [J]. New Phytologist, 2024, 241(2): 779-792. |
| 67 | CHEN R, JIA Q D, MU X, et al. Systematic mining of fungal chimeric terpene synthases using an efficient precursor-providing yeast chassis[J]. Proceedings of the National Academy of Sciences of the United States of America, 2021, 118(29): e2023247118. |
| 68 | YANG D Y, ZHANG S S, MA K, et al. Discovery and characterization of a new family of diterpene cyclases in bacteria and fungi[J]. Angewandte Chemie, 2017, 129(17): 4827-4830. |
| 69 | DALETOS G, STEPHANOPOULOS G. Protein engineering strategies for microbial production of isoprenoids[J]. Metabolic Engineering Communications, 2020, 11: e00129. |
| 70 | YE Z L, HUANG Y L, SHI B, et al. Coupling cell growth and biochemical pathway induction in Saccharomyces cerevisiae for production of (+)-valencene and its chemical conversion to (+)-nootkatone[J]. Metabolic Engineering, 2022, 72: 107-115. |
| 71 | 祁延萍, 朱晋, 张凯, 等. 定向进化在蛋白质工程中的应用研究进展[J]. 合成生物学, 2022, 3(6): 1081-1108. |
| QI Y P, ZHU J, ZHANG K, et al. Recent development of directed evolution in protein engineering[J]. Synthetic Biology Journal, 2022, 3(6): 1081-1108. | |
| 72 | LAUCHLI D R, RABE D K S, KALBARCZYK K Z, et al. High-throughput screening for terpene-synthase-cyclization activity and directed evolution of a terpene synthase[J]. Angewandte Chemie International Edition, 2013, 52(21): 5571-5574. |
| 73 | WANG X, CHEN J M, ZHANG J, et al. Engineering Escherichia coli for production of geraniol by systematic synthetic biology approaches and laboratory-evolved fusion tags[J]. Metabolic Engineering, 2021, 66: 60-67. |
| 74 | YE C F, LI M X, GAO J C, et al. Metabolic engineering of Pichia pastoris for overproduction of cis-trans nepetalactol[J]. Metabolic Engineering, 2024, 84: 83-94. |
| 75 | WANG X, WANG J J, ZHANG X Y, et al. Efficient myrcene production using linalool dehydratase isomerase and rational biochemical process in Escherichia coli [J]. Journal of Biotechnology, 2023, 371: 33-40. |
| 76 | JIANG G Z, YAO M D, WANG Y, et al. A “push-pull-restrain” strategy to improve citronellol production in Saccharomyces cerevisiae [J]. Metabolic Engineering, 2021, 66: 51-59. |
| 77 | HOSHINO Y, MORIYA M, MATSUDAIRA A, et al. Stereospecific linalool production utilizing two-phase cultivation system in Pantoea ananatis [J]. Journal of Biotechnology, 2020, 324: 21-27. |
| 78 | ROLF J, JULSING M K, ROSENTHAL K, et al. A gram-scale limonene production process with engineered Escherichia coli [J]. Molecules, 2020, 25(8): 1881. |
| 79 | ZHANG C B, LI M, ZHAO G R, et al. α-Terpineol production from an engineered Saccharomyces cerevisiae cell factory[J]. Microbial Cell Factories, 2019, 18(1): 160. |
| 80 | YOSHIDA E, KOJIMA M, SUZUKI M, et al. Increased carvone production in Escherichia coli by balancing limonene conversion enzyme expression via targeted quantification concatamer proteome analysis[J]. Scientific Reports, 2021, 11(1): 22126. |
| 81 | ZHANG H Y, CAI P, GUO J, et. al . Engineering cellular dephosphorylation boosts (+)-borneol production in yeast[J]. Acta Pharmaceutica Sinica B. Unpublished. |
| 82 | NIU F X, HE X, WU Y Q, et al. Enhancing production of pinene in Escherichia coli by using a combination of tolerance, evolution, and modular co-culture engineering[J]. Frontiers in Microbiology, 2018, 9: 1623. |
| 83 | JIA H J, CHEN T H, QU J Z, et al. Collaborative subcellular compartmentalization to improve GPP utilization and boost sabinene accumulation in Saccharomyces cerevisiae [J]. Biochemical Engineering Journal, 2020, 164: 107768. |
| 84 | LU Z Y, PENG B Y, EBERT B E, et al. Auxin-mediated protein depletion for metabolic engineering in terpene-producing yeast[J]. Nature Communications, 2021, 12(1): 1051. |
| 85 | MEADOWS A L, HAWKINS K M, TSEGAYE Y, et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production[J]. Nature, 2016, 537(7622): 694-697. |
| 86 | WANG C L, PARK J E, CHOI E S, et al. Farnesol production in Escherichia coli through the construction of a farnesol biosynthesis pathway-application of PgpB and YbjG phosphatases[J]. Biotechnology Journal, 2016, 11(10): 1291-1297. |
| 87 | ZHANG W X, GUO J Q, WANG Z, et al. Improved production of germacrene A, a direct precursor of β-elemene, in engineered Saccharomyces cerevisiae by expressing a cyanobacterial germacrene A synthase[J]. Microbial Cell Factories, 2021, 20(1): 7. |
| 88 | ZHANG L H, YANG H Q, XIA Y Y, et al. Engineering the oleaginous yeast Candida tropicalis for α-humulene overproduction[J]. Biotechnology for Biofuels and Bioproducts, 2022, 15(1): 59. |
| 89 | LIU J J, CHEN C, WAN X K, et al. Identification of the sesquiterpene synthase AcTPS1 and high production of (–)-germacrene D in metabolically engineered Saccharomyces cerevisiae [J]. Microbial Cell Factories, 2022, 21(1): 89. |
| 90 | GUO J Y, ZHOU W, LI Y Y, et al. Combination of protein engineering and metabolic engineering to enhance (+)-nootkatone production in Saccharomyces cerevisiae [J]. Food Bioengineering, 2022, 1(2): 192-202. |
| 91 | ZHA W L, AN T Y, LI T, et al. Reconstruction of the biosynthetic pathway of santalols under control of the GAL regulatory system in yeast[J]. ACS Synthetic Biology, 2020, 9(2): 449-456. |
| 92 | ZUO Y M, XIAO F, GAO J C, et al. Establishing Komagataella phaffii as a cell factory for efficient production of sesquiterpenoid α-santalene[J]. Journal of Agricultural and Food Chemistry, 2022, 70(26): 8024-8031. |
| 93 | CAO Y J, ZHANG R B, LIU W, et al. Manipulation of the precursor supply for high-level production of longifolene by metabolically engineered Escherichia coli [J]. Scientific Reports, 2019, 9(1): 95. |
| 94 | LIU M, LIN Y C, GUO J J, et al. High-level production of sesquiterpene patchoulol in Saccharomyces cerevisiae [J]. ACS Synthetic Biology, 2021, 10(1): 158-172. |
| 95 | ZHANG C Q, CHEN X X, LINDLEY N D, et al. A “plug-n-play” modular metabolic system for the production of apocarotenoids[J]. Biotechnology and Bioengineering, 2018, 115(1): 174-183. |
| 96 | LU Y P, YANG Q Y, LIN Z L, et al. A modular pathway engineering strategy for the high-level production of β-ionone in Yarrowia lipolytica [J]. Microbial Cell Factories, 2020, 19(1): 49. |
| 97 | MAGNARD J L, ROCCIA A, CAISSARD J C, et al. Biosynthesis of monoterpene scent compounds in roses[J]. Science, 2015, 349(6243): 81-83. |
| 98 | ZHU K, KONG J, ZHAO B X, et al. Metabolic engineering of microbes for monoterpenoid production[J]. Biotechnology Advances, 2021, 53: 107837. |
| 99 | JIANG G Z, YAO M D, WANG Y, et al. Manipulation of GES and ERG20 for geraniol overproduction in Saccharomyces cerevisiae [J]. Metabolic Engineering, 2017, 41: 57-66. |
| 100 | CAO X, LÜ Y B, CHEN J, et al. Metabolic engineering of oleaginous yeast Yarrowia lipolytica for limonene overproduction[J]. Biotechnology for Biofuels, 2016, 9: 214. |
| 101 | JONGEDIJK E, CANKAR K, RANZIJN J, et al. Capturing of the monoterpene olefin limonene produced in Saccharomyces cerevisiae [J]. Yeast, 2015, 32(1): 159-171. |
| 102 | ZHAO Y, LIANG F Y, XIE Y M, et al. Oxetane ring formation in taxol biosynthesis is catalyzed by a bifunctional cytochrome P450 enzyme[J]. Journal of the American Chemical Society, 2024, 146(1): 801-810. |
| 103 | YUAN W, LÜ S, CHEN L Y, et al. Production of sesterterpene ophiobolin by a bifunctional terpene synthase in Escherichia coli [J]. Applied Microbiology and Biotechnology, 2019, 103(21-22): 8785-8797. |
| 104 | TANG N C, SU J C, SHMIDOV Y, et al. Synthetic intrinsically disordered protein fusion tags that enhance protein solubility[J]. Nature Communications, 2024, 15(1): 3727. |
| 105 | CHEAH L C, LIU L, STARK T, et al. Metabolic flux enhancement from the translational fusion of terpene synthases is linked to terpene synthase accumulation[J]. Metabolic Engineering, 2023, 77: 143-151. |
| 106 | GUO Q, SHI T Q, PENG Q Q, et al. Harnessing Yarrowia lipolytica peroxisomes as a subcellular factory for α-humulene overproduction[J]. Journal of Agricultural and Food Chemistry, 2021, 69(46): 13831-13837. |
| 107 | MILLER B R, KUNG Y. Structural features and domain movements controlling substrate binding and cofactor specificity in class Ⅱ HMG-CoA reductase[J]. Biochemistry, 2018, 57(5): 654-662. |
| 108 | KWAK S, KIM S R, XU H Q, et al. Enhanced isoprenoid production from xylose by engineered Saccharomyces cerevisiae [J]. Biotechnology and Bioengineering, 2017, 114(11): 2581-2591. |
| 109 | KIM T Y, PARK H, KIM S K, et al. Production of (-)-α-bisabolol in metabolically engineered Saccharomyces cerevisiae [J]. Journal of Biotechnology, 2021, 340: 13-21. |
| 110 | YUZBASHEVA E Y, AGRIMI G, YUZBASHEV T V, et al. The mitochondrial citrate carrier in Yarrowia lipolytica: its identification, characterization and functional significance for the production of citric acid[J]. Metabolic Engineering, 2019, 54: 264-274. |
| 111 | RODRIGUEZ S, DENBY C M, VAN VU T, et al. ATP citrate lyase mediated cytosolic acetyl-CoA biosynthesis increases mevalonate production in Saccharomyces cerevisiae [J]. Microbial Cell Factories, 2016, 15: 48. |
| 112 | HELLGREN J, GODINA A, NIELSEN J, et al. Promiscuous phosphoketolase and metabolic rewiring enables novel non-oxidative glycolysis in yeast for high-yield production of acetyl-CoA derived products[J]. Metabolic Engineering, 2020, 62: 150-160. |
| 113 | YE M, GAO J Q, LI J J, et al. Promoter engineering enables precise metabolic regulation towards efficient β-elemene production in Ogataea polymorpha [J]. Synthetic and Systems Biotechnology, 2024, 9(2): 234-241. |
| 114 | CHEN Y, DAVIET L, SCHALK M, et al. Establishing a platform cell factory through engineering of yeast acetyl-CoA metabolism[J]. Metabolic Engineering, 2013, 15: 48-54. |
| 115 | LI Q Y, FAN F Y, GAO X, et al. Balanced activation of IspG and IspH to eliminate MEP intermediate accumulation and improve isoprenoids production in Escherichia coli [J]. Metabolic Engineering, 2017, 44: 13-21. |
| 116 | WU T, LI S W, ZHANG B L, et al. Engineering Saccharomyces cerevisiae for the production of the valuable monoterpene ester geranyl acetate[J]. Microbial Cell Factories, 2018, 17(1): 85. |
| 117 | DUSSÉAUX S, WAJN W T, LIU Y X, et al. Transforming yeast peroxisomes into microfactories for the efficient production of high-value isoprenoids[J]. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(50): 31789-31799. |
| 118 | ZHAO J Z, LI C, ZHANG Y, et al. Dynamic control of ERG20 expression combined with minimized endogenous downstream metabolism contributes to the improvement of geraniol production in Saccharomyces cerevisiae [J]. Microbial Cell Factories, 2017, 16(1): 17. |
| 119 | ZHAO B X, ZHANG Y H, WANG Y P, et al. Biosynthesis of α-bisabolene from low-cost renewable feedstocks by peroxisome engineering and systems metabolic engineering of the yeast Yarrowia lipolytica [J]. Green Chemistry, 2023, 25(20): 8145-8159. |
| 120 | SON S H, KIM J E, PARK G R, et al. Metabolite trafficking enables membrane-impermeable-terpene secretion by yeast[J]. Nature Communications, 2022, 13(1): 2605. |
| 121 | QIN L, MA D S, LIN G Y, et al. Low temperature promotes the production and efflux of terpenoids in yeast[J]. Bioresource Technology, 2024, 395: 130376. |
| 122 | CHENG T, ZHANG K, GUO J, et al. Highly efficient biosynthesis of β-caryophyllene with a new sesquiterpene synthase from tobacco[J]. Biotechnology for Biofuels and Bioproducts, 2022, 15(1): 39. |
| 123 | SCANDIFFIO R, GEDDO F, COTTONE E, et al. Protective effects of (E)-β-caryophyllene (BCP) in Chronic inflammation [J]. Nutrients, 2020, 12(11): 3273. |
| 124 | TANG Q H, XU F, WEI X C, et al. Investigation of β-caryophyllene as terpene penetration enhancer: role of stratum corneum retention[J]. European Journal of Pharmaceutical Sciences, 2023, 183: 106401. |
| 125 | LIM H S, KIM S K, WOO S G, et al. (–)-α-Bisabolol production in engineered Escherichia coli expressing a novel (-)-α-bisabolol synthase from the globe artichoke Cynara cardunculus var. Scolymus [J]. Journal of Agricultural and Food Chemistry, 2021, 69(30): 8492-8503. |
| 126 | EDDIN L B, JHA N K, GOYAL S N, et al. Health benefits, pharmacological effects, molecular mechanisms, and therapeutic potential of α-bisabolol[J]. Nutrients, 2022, 14(7): 1370. |
| 127 | JIANG Y K, XIA L, GAO S, et al. Engineering Saccharomyces cerevisiae for enhanced (–)-α-bisabolol production[J]. Synthetic and Systems Biotechnology, 2023, 8(2): 187-195. |
| 128 | LIU S C, LIU Z J, WEI L J, et al. Pathway engineering and medium optimization for α-farnesene biosynthesis in oleaginous yeast Yarrowia lipolytica [J]. Journal of Biotechnology, 2020, 319: 74-81. |
| 129 | LI S, LUO S S, YIN X R, et al. Screening of ent-copalyl diphosphate synthase and metabolic engineering to achieve de novo biosynthesis of ent-copalol in Saccharomyces cerevisiae [J]. Synthetic and Systems Biotechnology, 2024, 9(4): 784-792. |
| 130 | ZHANG X Y, CHEN S T, LIN Y, et al. Metabolic engineering of Pichia pastoris for high-level production of lycopene[J]. ACS Synthetic Biology, 2023, 12(10): 2961-2972. |
| 131 | YE M, GAO J Q, ZHOU Y J. Global metabolic rewiring of the nonconventional yeast Ogataea polymorpha for biosynthesis of the sesquiterpenoid β-elemene[J]. Metabolic Engineering, 2023, 76: 225-231. |
| 132 | LUO G J, LIN Y, CHEN S T, et al. Overproduction of patchoulol in metabolically engineered Komagataella phaffii [J]. Journal of Agricultural and Food Chemistry, 2023, 71(4): 2049-2058. |
| 133 | WEI Y, MENG N, WANG Y C, et al. Transcription factor VvWRKY70 inhibits both norisoprenoid and flavonol biosynthesis in grape[J]. Plant Physiology, 2023, 193(3): 2055-2070. |
| 134 | MOSAFERI S, JELLEY R E, FEDRIZZI B, et al. Synthesis of d6-deuterated analogues of aroma molecules-β-damascenone, β-damascone and safranal[J]. Results in Chemistry, 2022, 4: 100264. |
| 135 | TOMASINO E, BOLMAN S. The potential effect of β-ionone and β-damascenone on sensory perception of pinot noir wine aroma[J]. Molecules, 2021, 26(5): 1288. |
| 136 | CHUDASAMA D. Importance of intellectual property rights[J]. Journal of Intellectual Property Rights Law, 2022, 4(2): 2582-9742. |
| 137 | 陈大明, 周光明, 刘晓, 等. 从全球专利分析看合成生物学技术发展趋势[J]. 合成生物学, 2020, 1(3): 372-384. |
| CHEN D M, ZHOU G M, LIU X, et al. Analysis of global patents for the trend of synthetic biology inventions[J]. Synthetic Biology Journal, 2020, 1(3): 372-384. | |
| 138 | WANG S H, SUN X, HAN Y Q, et al. Sustainable biosynthesis of squalene from waste cooking oil by the yeast Yarrowia lipolytica [J]. Metabolic Engineering Communications, 2024, 18: e00240. |
| 139 | PANG Y R, ZHAO Y K, LI S L, et al. Engineering the oleaginous yeast Yarrowia lipolytica to produce limonene from waste cooking oil[J]. Biotechnology for Biofuels, 2019, 12: 241. |
| 140 | ZHAO Y K, ZHU K, LI J, et al. High-efficiency production of bisabolene from waste cooking oil by metabolically engineered Yarrowia lipolytica [J]. Microbial Biotechnology, 2021, 14(6): 2497-2513. |
| [1] | WU Ke, LUO Jiahao, LI Feiran. Applications of machine learning in the reconstruction and curation of genome-scale metabolic models [J]. Synthetic Biology Journal, 2025, 6(3): 566-584. |
| [2] | TIAN Xiao-jun, ZHANG Rixin. “Economics Paradox” with cells in synthetic gene circuits [J]. Synthetic Biology Journal, 2025, 6(3): 532-546. |
| [3] | ZHANG Yiqing, LIU Gaowen. Exploration of gene functions and library construction for engineering strains from a synthetic biology perspective [J]. Synthetic Biology Journal, 2025, 6(3): 685-700. |
| [4] | YANG Ying, LI Xia, LIU Lizhong. Applications of synthetic biology to stem-cell-derived modeling of early embryonic development [J]. Synthetic Biology Journal, 2025, 6(3): 669-684. |
| [5] | HUANG Yi, SI Tong, LU Anjing. Standardization for biomanufacturing: global landscape, critical challenges, and pathways forward [J]. Synthetic Biology Journal, 2025, 6(3): 701-714. |
| [6] | SONG Chengzhi, LIN Yihan. AI-enabled directed evolution for protein engineering and optimization [J]. Synthetic Biology Journal, 2025, 6(3): 617-635. |
| [7] | GAO Qi, XIAO Wenhai. Advances in the biosynthesis of monoterpenes by yeast [J]. Synthetic Biology Journal, 2025, 6(2): 357-372. |
| [8] | ZHANG Lu’ou, XU Li, HU Xiaoxu, YANG Ying. Synthetic biology ushers cosmetic industry into the “bio-cosmetics” era [J]. Synthetic Biology Journal, 2025, 6(2): 479-491. |
| [9] | YI Jinhang, TANG Yulin, LI Chunyu, WU Heyun, MA Qian, XIE Xixian. Applications and advances in the research of biosynthesis of amino acid derivatives as key ingredients in cosmetics [J]. Synthetic Biology Journal, 2025, 6(2): 254-289. |
| [10] | WEI Lingzhen, WANG Jia, SUN Xinxiao, YUAN Qipeng, SHEN Xiaolin. Biosynthesis of flavonoids and their applications in cosmetics [J]. Synthetic Biology Journal, 2025, 6(2): 373-390. |
| [11] | XIAO Sen, HU Litao, SHI Zhicheng, WANG Fayin, YU Siting, DU Guocheng, CHEN Jian, KANG Zhen. Research advances in biosynthesis of hyaluronic acid with controlled molecular weights [J]. Synthetic Biology Journal, 2025, 6(2): 445-460. |
| [12] | WANG Qian, GUO Shiting, XIN Bo, ZHONG Cheng, WANG Yu. Advances in biosynthesis of L-arginine using engineered microorganisms [J]. Synthetic Biology Journal, 2025, 6(2): 290-305. |
| [13] | ZUO Yimeng, ZHANG Jiaojiao, LIAN Jiazhang. Enabling technology for the biosynthesis of cosmetic raw materials with Saccharomyces cerevisiae [J]. Synthetic Biology Journal, 2025, 6(2): 233-253. |
| [14] | TANG Chuan′gen, WANG Jing, ZHANG Shuo, ZHANG Haoning, KANG Zhen. Advances in synthesis and mining strategies for functional peptides [J]. Synthetic Biology Journal, 2025, 6(2): 461-478. |
| [15] | GUO Tingting, HAN Xiangning, HUANG Xiting, ZHANG Tingting, KONG Jian. Advances in synthetic biology tools for lactic acid bacteria and their application in the development of skin beneficial products [J]. Synthetic Biology Journal, 2025, 6(2): 320-333. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||