Synthetic Biology Journal ›› 2025, Vol. 6 ›› Issue (2): 320-333.DOI: 10.12211/2096-8280.2024-071
• Invited Review • Previous Articles Next Articles
Advances in synthetic biology tools for lactic acid bacteria and their application in the development of skin beneficial products
GUO Tingting, HAN Xiangning, HUANG Xiting, ZHANG Tingting, KONG Jian
- State Key Laboratory of Microbial Technology,Shandong University,Qingdao 266237,Shandong,China
-
Received:2024-09-11Revised:2024-11-14Online:2025-05-20Published:2025-04-30 -
Contact:KONG Jian
乳酸菌的合成生物学工具及在合成益肤因子中的应用
郭婷婷, 韩湘凝, 黄熙婷, 张婷婷, 孔健
- 山东大学微生物改造技术全国重点实验室,山东 青岛 266237
-
通讯作者:孔健 -
作者简介:郭婷婷 (1984—),女,副教授,硕士生导师。研究方向为乳酸菌遗传操作系统、乳酸菌合成生物学与活性产物的高效生产。E-mail:guotingting@sdu.edu.cn孔健 (1964—),女,教授,博士生导师,研究方向为乳酸菌生理遗传及其应用,乳酸菌生物活性物质的开发等。E-mail:kongjian@sdu.edu.cn -
基金资助:微生物改造技术全国重点实验室“揭榜挂帅”项目(SKLMTFCP-2023-05)
CLC Number:
Cite this article
GUO Tingting, HAN Xiangning, HUANG Xiting, ZHANG Tingting, KONG Jian. Advances in synthetic biology tools for lactic acid bacteria and their application in the development of skin beneficial products[J]. Synthetic Biology Journal, 2025, 6(2): 320-333.
郭婷婷, 韩湘凝, 黄熙婷, 张婷婷, 孔健. 乳酸菌的合成生物学工具及在合成益肤因子中的应用[J]. 合成生物学, 2025, 6(2): 320-333.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2024-071
| 1 | GRICE E A, SEGRE J A. The skin microbiome[J]. Nature Reviews Microbiology, 2011, 9(4): 244-253. |
| 2 | HARRIS-TRYON T A, GRICE E A. Microbiota and maintenance of skin barrier function[J]. Science, 2022, 376(6596): 940-945. |
| 3 | GEOGHEGAN J A, IRVINE A D, FOSTER T J. Staphylococcus aureus and atopic dermatitis: a complex and evolving relationship[J]. Trends in Microbiology, 2018, 26(6): 484-497. |
| 4 | BRANDWEIN M, FUKS G, ISRAEL A, et al. Skin microbiome compositional changes in atopic dermatitis accompany dead sea climatotherapy[J]. Photochemistry and Photobiology, 2019, 95(6): 1446-1453. |
| 5 | CHEN Y H, SONG Y P, CHEN Z G, et al. Early-life skin microbial biomarkers for eczema phenotypes in Chinese toddlers[J]. Pathogens, 2023, 12(5): 697. |
| 6 | WANG H L, CHAN M W M, CHAN H H, et al. Longitudinal changes in skin microbiome associated with change in skin status in patients with psoriasis[J]. Acta Dermato-Venereologica, 2020, 100(18): adv00329. |
| 7 | PLAVEC T V, BERLEC A. Safety aspects of genetically modified lactic acid bacteria[J]. Microorganisms, 2020, 8(2): 297. |
| 8 | MASOOD M I, QADIR M I, SHIRAZI J H, et al. Beneficial effects of lactic acid bacteria on human beings[J]. Critical Reviews in Microbiology, 2011, 37(1): 91-98. |
| 9 | DOU J X, FENG N, GUO F Y, et al. Applications of probiotic constituents in cosmetics[J]. Molecules, 2023, 28(19): 6765. |
| 10 | LEBEER S, OERLEMANS E F M, CLAES I, et al. Selective targeting of skin pathobionts and inflammation with topically applied lactobacilli[J]. Cell Reports Medicine, 2022, 3(2): 100521. |
| 11 | MOTTIN BPHARM V H M, SUYENAGA E S. An approach on the potential use of probiotics in the treatment of skin conditions: acne and atopic dermatitis[J]. International Journal of Dermatology, 2018, 57(12): 1425-1432. |
| 12 | CHRISTENSEN I B, VEDEL C, CLAUSEN M L, et al. Targeted screening of lactic acid bacteria with antibacterial activity toward Staphylococcus aureus clonal complex type 1 associated with atopic dermatitis[J]. Frontiers in Microbiology, 2021, 12: 733847. |
| 13 | ALSAHEB R A ABD, ALADDIN A, OTHMAN N Z, et al. Lactic acid applications in pharmaceutical and cosmeceutical industries[J]. Journal of Chemical and Pharmaceutical Research, 2015, 7(10): 729-735. |
| 14 | BOLOTIN A, WINCKER P, MAUGER S, et al. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403[J]. Genome Research, 2001, 11(5): 731-753. |
| 15 | KOK J, VAN GIJTENBEEK L A, DE JONG A, et al. The evolution of gene regulation research in Lactococcus lactis [J]. FEMS Microbiology Reviews, 2017, 41(Supp_1): S220-S243. |
| 16 | PEDERSEN M B, GARRIGUES C, TUPHILE K, et al. Impact of aeration and heme-activated respiration on Lactococcus lactis gene expression: identification of a heme-responsive operon[J]. Journal of Bacteriology, 2008, 190(14): 4903-4911. |
| 17 | LIU J M, WANG Z H, KANDASAMY V, et al. Harnessing the respiration machinery for high-yield production of chemicals in metabolically engineered Lactococcus lactis [J]. Metabolic Engineering, 2017, 44: 22-29. |
| 18 | NEEF J, KOEDIJK D G A M, BOSMA T, et al. Efficient production of secreted staphylococcal antigens in a non-lysing and proteolytically reduced Lactococcus lactis strain[J]. Applied Microbiology and Biotechnology, 2014, 98(24): 10131-10141. |
| 19 | CAMPOS G M, AMÉRICO M F, DOS SANTOS FREITAS A, et al. Lactococcus lactis as an interleukin delivery system for prophylaxis and treatment of inflammatory and autoimmune diseases[J]. Probiotics and Antimicrobial Proteins, 2024, 16(2): 352-366. |
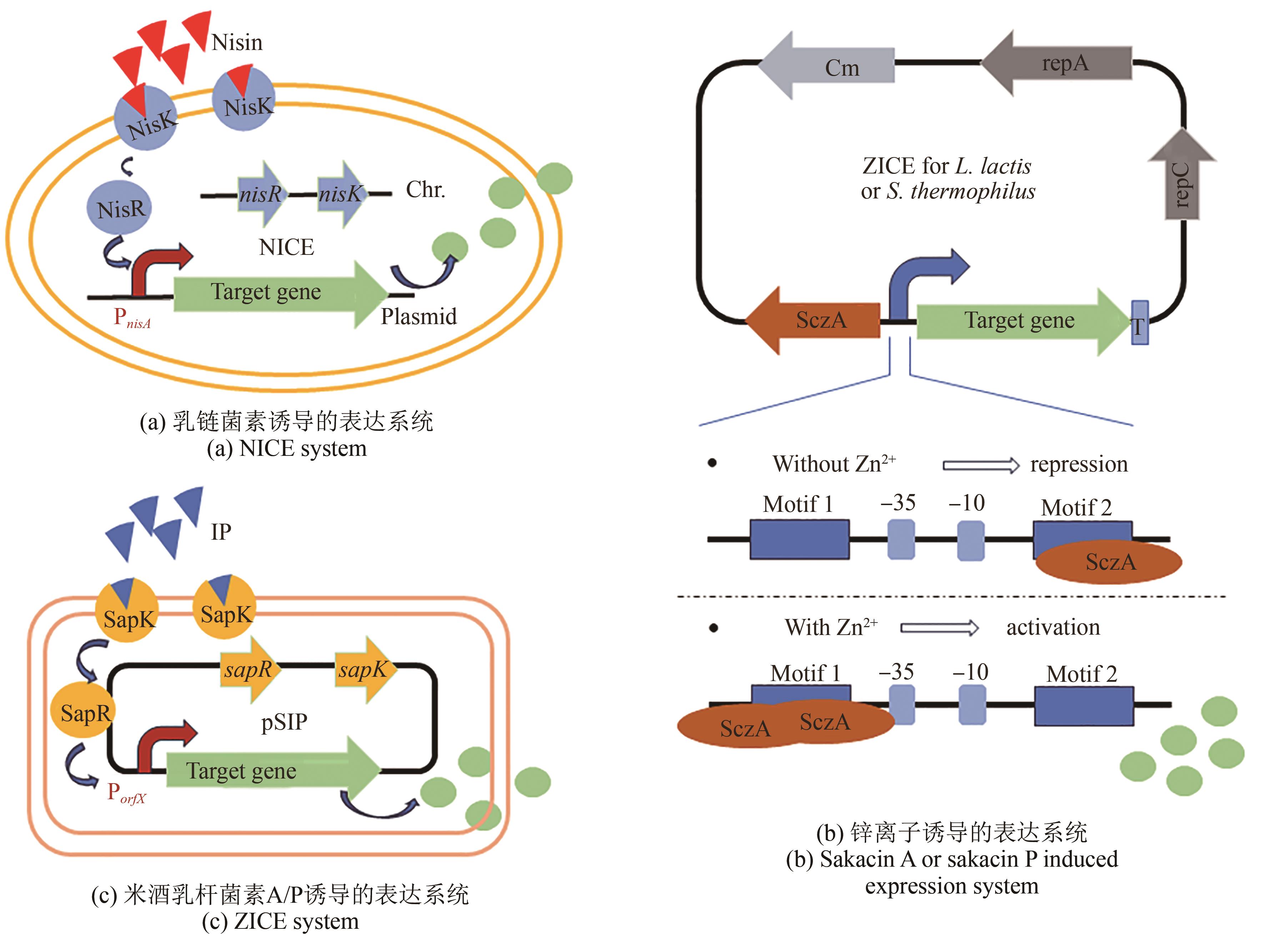
| 20 | URIOT O, DENIS S, JUNJUA M, et al. Streptococcus thermophilus: from yogurt starter to a new promising probiotic candidate?[J]. Journal of Functional Foods, 2017, 37: 74-89. |
| 21 | MARKAKIOU S, GASPAR P, JOHANSEN E, et al. Harnessing the metabolic potential of Streptococcus thermophilus for new biotechnological applications[J]. Current Opinion in Biotechnology, 2020, 61: 142-152. |
| 22 | SUN Z H, HARRIS H M B, MCCANN A, et al. Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated Genera[J]. Nature Communications, 2015, 6: 8322. |
| 23 | XIN Y P, MU Y L, KONG J, et al. Targeted and repetitive chromosomal integration enables high-level heterologous gene expression in Lactobacillus casei [J]. Applied and Environmental Microbiology, 2019, 85(9): e00033-19. |
| 24 | ZHOU D, JIANG Z N, PANG Q X, et al. CRISPR/Cas9-assisted seamless genome editing in Lactobacillus plantarum and its application in N-acetylglucosamine production[J]. Applied and Environmental Microbiology, 2019, 85(21): e01367-19. |
| 25 | BRON P A, MARCELLI B, MULDER J, et al. Renaissance of traditional DNA transfer strategies for improvement of industrial lactic acid bacteria[J]. Current Opinion in Biotechnology, 2019, 56: 61-68. |
| 26 | AMMANN A, NEVE H, GEIS A, et al. Plasmid transfer via transduction from Streptococcus thermophilus to Lactococcus lactis [J]. Journal of Bacteriology, 2008, 190(8): 3083-3087. |
| 27 | LAMPKOWSKA J, FELD L, MONAGHAN A, et al. A standardized conjugation protocol to asses antibiotic resistance transfer between lactococcal species[J]. International Journal of Food Microbiology, 2008, 127(1-2): 172-175. |
| 28 | DANDOY D, FREMAUX C, DE FRAHAN M H, et al. The fast milk acidifying phenotype of Streptococcus thermophilus can be acquired by natural transformation of the genomic island encoding the cell-envelope proteinase PrtS[J]. Microbial Cell Factories, 2011, 10(): S21. |
| 29 | MULDER J, WELS M, KUIPERS O P, et al. Unleashing natural competence in Lactococcus lactis by induction of the competence regulator ComX[J]. Applied and Environmental Microbiology, 2017, 83(20): e01320-17. |
| 30 | DAVID B, RADZIEJWOSKI A, TOUSSAINT F, et al. Natural DNA transformation is functional in Lactococcus lactis subsp. cremoris KW2[J]. Applied and Environmental Microbiology, 2017, 83(16): e01074-17. |
| 31 | MORAWSKA L P, KUIPERS O P. Cell-to-cell non-conjugative plasmid transfer between Bacillus subtilis and lactic acid bacteria[J]. Microbial Biotechnology, 2023, 16(4): 784-798. |
| 32 | HOLO H, NES I F. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media[J]. Applied and Environmental Microbiology, 1989, 55(12): 3119-3123. |
| 33 | NATORI Y, KANO Y, IMAMOTO F. Genetic transformation of Lactobacillus casei by electroporation[J]. Biochimie, 1990, 72(4): 265-269. |
| 34 | KONG L H, XIONG Z Q, XIA Y J, et al. High-efficiency transformation of Streptococcus thermophilus using electroporation[J]. Journal of the Science of Food and Agriculture, 2021, 101(15): 6578-6585. |
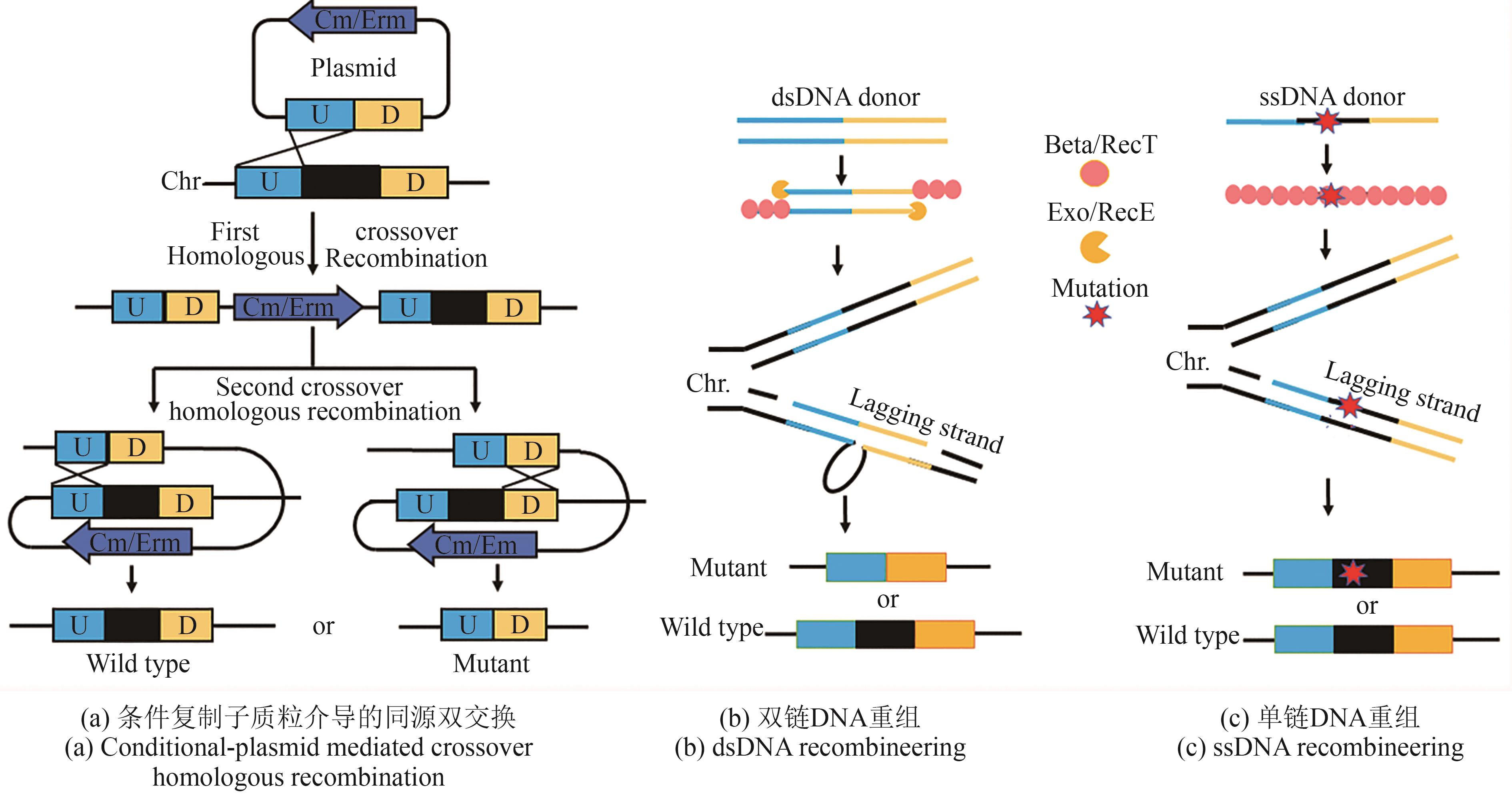
| 35 | MENG Q, YUAN Y X, LI Y Y, et al. Optimization of electrotransformation parameters and engineered promoters for Lactobacillus plantarum from wine[J]. ACS Synthetic Biology, 2021, 10(7): 1728-1738. |
| 36 | 陈韫慧, 夏永军, 宋馨, 等. 乳酸菌作为生物活性物质体内递送载体的研究进展[J]. 食品科学, 2023, 44(13): 193-202. |
| CHEN Y H, XIA Y J, SONG X, et al. Advances in the study of lactic acid bacteria as vehicles for the in vivo delivery of bioactive substances[J]. Food Science, 2023, 44(13): 193-202. | |
| 37 | ZHU D L, LIU F L, XU H J, et al. Isolation of strong constitutive promoters from Lactococcus lactis subsp. lactis N8[J]. FEMS Microbiology Letters, 2015, 362(16): fnv107. |
| 38 | NARITA J, ISHIDA S, OKANO K, et al. Improvement of protein production in lactic acid bacteria using 5′-untranslated leader sequence of slpA from Lactobacillus acidophilus. Improvement in protein production using UTLS[J]. Applied Microbiology and Biotechnology, 2006, 73(2): 366-373. |
| 39 | SUN W H, JIANG B, ZHANG Y, et al. Enabling the biosynthesis of malic acid in Lactococcus lactis by establishing the reductive TCA pathway and promoter engineering[J]. Biochemical Engineering Journal, 2020, 161: 107645. |
| 40 | PEIROTÉN Á, LANDETE J M. Natural and engineered promoters for gene expression in Lactobacillus species[J]. Applied Microbiology and Biotechnology, 2020, 104(9): 3797-3805. |
| 41 | KUIPERS O P, BEERTHUYZEN M M, DE RUYTER P G G A, et al. Autoregulation of nisin biosynthesis in Lactococcus lactis by signal transduction[J]. Journal of Biological Chemistry, 1995, 270(45): 27299-27304. |
| 42 | MIERAU I, KLEEREBEZEM M. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis [J]. Applied Microbiology and Biotechnology, 2005, 68(6): 705-717. |
| 43 | RENYE J A JR, SOMKUTI G A. Nisin-induced expression of pediocin in dairy lactic acid bacteria[J]. Journal of Applied Microbiology, 2010, 108(6): 2142-2151. |
| 44 | YANG P, WANG J, QI Q S. Prophage recombinases-mediated genome engineering in Lactobacillus plantarum [J]. Microbial Cell Factories, 2015, 14: 154. |
| 45 | LLULL D, POQUET I. New expression system tightly controlled by zinc availability in Lactococcus lactis [J]. Applied and Environmental Microbiology, 2004, 70(9): 5398-5406. |
| 46 | MIYOSHI A, JAMET E, COMMISSAIRE J, et al. A xylose-inducible expression system for Lactococcus lactis [J]. FEMS Microbiology Letters, 2004, 239(2): 205-212. |
| 47 | MADSEN S M, ARNAU J, VRANG A, et al. Molecular characterization of the pH-inducible and growth phase-dependent promoter P170 of Lactococcus lactis [J]. Molecular Microbiology, 1999, 32(1): 75-87. |
| 48 | XU X N, ZHANG L W, CUI Y, et al. Development of Zn2+-controlled expression system for lactic acid bacteria and its application in engineered probiotics[J]. Synthetic and Systems Biotechnology, 2024, 9(1): 152-158. |
| 49 | AXELSSON L, LINDSTAD G, NATERSTAD K. Development of an inducible gene expression system for Lactobacillus sakei [J]. Letters in Applied Microbiology, 2003, 37(2): 115-120. |
| 50 | JIMÉNEZ J J, DIEP D B, BORRERO J, et al. Cloning strategies for heterologous expression of the bacteriocin enterocin A by Lactobacillus sakei Lb790, Lb. plantarum NC8 and Lb. casei CECT475[J]. Microbial Cell Factories, 2015, 14: 166. |
| 51 | SØRVIG E, MATHIESEN G, NATERSTAD K, et al. High-level, inducible gene expression in Lactobacillus sakei and Lactobacillus plantarum using versatile expression vectors[J]. Microbiology, 2005, 151(Pt 7): 2439-2449. |
| 52 | FILSINGER G T, WANNIER T M, PEDERSEN F B, et al. Characterizing the portability of phage-encoded homologous recombination proteins[J]. Nature Chemical Biology, 2021, 17(4): 394-402. |
| 53 | MARKAKIOU S, NEVES A R, ZEIDAN A A, et al. Development of a tetracycline-inducible system for conditional gene expression in Lactococcus lactis and Streptococcus thermophilus [J]. Microbiology Spectrum, 2023, 11(3): e00668-23. |
| 54 | HEISS S, HÖRMANN A, TAUER C, et al. Evaluation of novel inducible promoter/repressor systems for recombinant protein expression in Lactobacillus plantarum [J]. Microbial Cell Factories, 2016, 15: 50. |
| 55 | BÖRNER R A, KANDASAMY V, AXELSEN A M, et al. Genome editing of lactic acid bacteria: opportunities for food, feed, pharma and biotech[J]. FEMS Microbiology Letters, 2019, 366(1): fny291. |
| 56 | LEENHOUTS K J, KOK J, VENEMA G. Lactococcal plasmid pWV01 as an integration vector for lactococci[J]. Applied and Environmental Microbiology, 1991, 57(9): 2562-2567. |
| 57 | MAGUIN E, DUWAT P, HEGE T, et al. New thermosensitive plasmid for Gram-positive bacteria[J]. Journal of Bacteriology, 1992, 174(17): 5633-5638. |
| 58 | XIN Y P, GUO T T, MU Y L, et al. Development of a counterselectable seamless mutagenesis system in lactic acid bacteria[J]. Microbial Cell Factories, 2017, 16(1): 116. |
| 59 | SOLEM C, DEFOOR E, JENSEN P R, et al. Plasmid pCS1966, a new selection/counterselection tool for lactic acid bacterium strain construction based on the oroP gene, encoding an orotate transporter from Lactococcus lactis [J]. Applied and Environmental Microbiology, 2008, 74(15): 4772-4775. |
| 60 | GOH Y J, AZCÁRATE-PERIL M A, O’FLAHERTY S, et al. Development and application of a upp-based counterselective gene replacement system for the study of the S-layer protein SlpX of Lactobacillus acidophilus NCFM[J]. Applied and Environmental Microbiology, 2009, 75(10): 3093-3105. |
| 61 | PETERSEN K V, MARTINUSSEN J, JENSEN P R, et al. Repetitive, marker-free, site-specific integration as a novel tool for multiple chromosomal integration of DNA[J]. Applied and Environmental Microbiology, 2013, 79(12): 3563-3569. |
| 62 | RAWSTHORNE H, TURNER K N, MILLS D A. Multicopy integration of heterologous genes, using the lactococcal group Ⅱ intron targeted to bacterial insertion sequences[J]. Applied and Environmental Microbiology, 2006, 72(9): 6088-6093. |
| 63 | ZHANG Y, BUCHHOLZ F, MUYRERS J P, et al. A new logic for DNA engineering using recombination in Escherichia coli [J]. Nature Genetics, 1998, 20(2): 123-128. |
| 64 | YU D G, ELLIS H M, LEE E C, et al. An efficient recombination system for chromosome engineering in Escherichia coli [J]. Proceedings of the National Academy of Sciences of the United States of America, 2000, 97(11): 5978-5983. |
| 65 | CHAI Y, SHAN S P, WEISSMAN K J, et al. Heterologous expression and genetic engineering of the tubulysin biosynthetic gene cluster using red/ET recombineering and inactivation mutagenesis[J]. Chemistry & Biology, 2012, 19(3): 361-371. |
| 66 | STRINGER A M, SINGH N, YERMAKOVA A, et al. FRUIT, a scar-free system for targeted chromosomal mutagenesis, epitope tagging, and promoter replacement in Escherichia coli and Salmonella enterica [J]. PLoS One, 2012, 7(9): e44841. |
| 67 | XIN Y P, GUO T T, MU Y L, et al. Identification and functional analysis of potential prophage-derived recombinases for genome editing in Lactobacillus casei [J]. FEMS Microbiology Letters, 2017, 364(24): fnx243. |
| 68 | HUANG H, SONG X, YANG S. Development of a RecE/T-assisted CRAISPR-Cas9 toolbox for Lactobacillus [J]. Biotechnology Journal, 2019, 14(7): 1800690. |
| 69 | VAN PIJKEREN J P, BRITTON R A. High efficiency recombineering in lactic acid bacteria[J]. Nucleic Acids Research, 2012, 40(10): e76. |
| 70 | HAO M Y, CUI Y H, QU X J. Analysis of CRISPR-Cas system in Streptococcus thermophilus and its application[J]. Frontiers in Microbiology, 2018, 9: 257. |
| 71 | YANG L, LI W X, UJIROGHENE O J, et al. Occurrence and diversity of CRISPR loci in Lactobacillus casei group[J]. Frontiers in Microbiology, 2020, 11: 624. |
| 72 | SCHUSTER J A, VOGEL R F, EHRMANN M A. Characterization and distribution of CRISPR-Cas systems in Lactobacillus sakei [J]. Archives of Microbiology, 2019, 201(3): 337-347. |
| 73 | MA S, WANG F Y, ZHANG X J, et al. Repurposing endogenous type Ⅱ CRISPR-Cas9 system for genome editing in Streptococcus thermophilus [J]. Biotechnology and Bioengineering, 2024, 121(2): 749-756. |
| 74 | MARTEL B, MOINEAU S. CRISPR-Cas: an efficient tool for genome engineering of virulent bacteriophages[J]. Nucleic Acids Research, 2014, 42(14): 9504-9513. |
| 75 | SONG X, HUANG H, XIONG Z Q, et al. CRISPR-Cas9D10A nickase-assisted genome editing in Lactobacillus casei [J]. Applied and Environmental Microbiology, 2017, 83(22): e01259-17. |
| 76 | OH J H, VAN PIJKEREN J P. CRISPR-Cas9-assisted recombineering in Lactobacillus reuteri [J]. Nucleic Acids Research, 2014, 42(17): e131. |
| 77 | GUO T T, XIN Y P, ZHANG Y, et al. A rapid and versatile tool for genomic engineering in Lactococcus lactis [J]. Microbial Cell Factories, 2019, 18(1): 22. |
| 78 | KONG L H, SONG X, XIA Y J, et al. Construction of a CRISPR/nCas9-assisted genome editing system for exopolysaccharide biosynthesis in Streptococcus thermophilus [J]. Food Research International, 2022, 158: 111550. |
| 79 | TIAN K R, HONG X, GUO M M, et al. Development of base editors for simultaneously editing multiple loci in Lactococcus lactis [J]. ACS Synthetic Biology, 2022, 11(11): 3644-3656. |
| 80 | VAN TILBURG A Y, CAO H J, VAN DER MEULEN S B, et al. Metabolic engineering and synthetic biology employing Lactococcus lactis and Bacillus subtilis cell factories[J]. Current Opinion in Biotechnology, 2019, 59: 1-7. |
| 81 | SHENG J Z, LING P X, ZHU X Q, et al. Use of induction promoters to regulate hyaluronan synthase and UDP-glucose-6-dehydrogenase of Streptococcus zooepidemicus expression in Lactococcus lactis: a case study of the regulation mechanism of hyaluronic acid polymer[J]. Journal of Applied Microbiology, 2009, 107(1): 136-144. |
| 82 | ZHONG Q, MA Y Q, XU D L, et al. Heterologous biosynthesis of hyaluronic acid using a new hyaluronic acid synthase derived from the probiotic Streptococcus thermophilus [J]. Fermentation, 2023, 9(6): 510. |
| 83 | MOHAMMED A A, NIAMAH A K. Identification and antioxidant activity of hyaluronic acid extracted from local isolates of Streptococcus thermophilus [J]. Materials Today: Proceedings, 2022, 60: 1523-1529. |
| 84 | LEW L C, GAN C Y, LIONG M T. Dermal bioactives from lactobacilli and bifidobacteria[J]. Annals of Microbiology, 2013, 63(3): 1047-1055. |
| 85 | SUNGUROĞLU C, SEZGIN D E, ÇELIK P A, et al. Higher titer hyaluronic acid production in recombinant Lactococcus lactis [J]. Preparative Biochemistry & Biotechnology, 2018, 48(8): 734-742. |
| 86 | PRASAD S B, JAYARAMAN G, RAMACHANDRAN K B. Hyaluronic acid production is enhanced by the additional co-expression of UDP-glucose pyrophosphorylase in Lactococcus lactis [J]. Applied Microbiology and Biotechnology, 2010, 86(1): 273-283. |
| 87 | HMAR R V, PRASAD S B, JAYARAMAN G, et al. Chromosomal integration of hyaluronic acid synthesis (has) genes enhances the molecular weight of hyaluronan produced in Lactococcus lactis [J]. Biotechnology Journal, 2014, 9(12): 1554-1564. |
| 88 | JEEVA P, SHANMUGA DOSS S, SUNDARAM V, et al. Production of controlled molecular weight hyaluronic acid by glucostat strategy using recombinant Lactococcus lactis cultures[J]. Applied Microbiology and Biotechnology, 2019, 103(11): 4363-4375. |
| 89 | 张少伦, 高聪, 李晓敏, 等. 代谢工程改造克雷伯氏菌生产1,3-丙二醇[J]. 生物工程学报, 2024, 40(8): 2386-2402. |
| ZHANG S L, GAO C, LI X M, et al. Metabolic engineering of Klebsiella pneumoniae for 1,3-propanediol production[J]. Chinese Journal of Biotechnology, 2024, 40(8): 2386-2402. | |
| 90 | 刘建明, 张炽坚, 张冰, 等. 巴氏梭菌作为工业底盘细胞高效生产1,3-丙二醇——从代谢工程和菌种进化到过程工程和产品分离[J]. 合成生物学, 2024, 5(6): 1386-1403. |
| LIU J M, ZHANG Z J, ZHANG B, et al. Clostridium pasteurianum as an industrial chassis for efficient productionof 1,3-propanediol: from metabolic engineering to fermentation andproduct separation[J]. Synthetic Biology Journal, 2024, 5(6): 1386-1403. | |
| 91 | 蒋欢, 马江山, 曾柏全, 等. 粗甘油发酵生产1,3-丙二醇的研究进展[J]. 生物技术通报, 2022, 38(10): 45-53. |
| JIANG H, MA J S, ZENG B Q, et al. Research progress in 1,3-propanediol production by fermenting crude glycerol[J]. Biotechnology Bulletin, 2022, 38(10): 45-53. | |
| 92 | WU Y X, LIN Y H. Fermentation redox potential control on the 1,3-propanediol production by Lactobacillus panis PM1[J]. Process Biochemistry, 2022, 114: 139-146. |
| 93 | JU J H, WANG D X, HEO S Y, et al. Enhancement of 1,3-propanediol production from industrial by-product by Lactobacillus reuteri CH53[J]. Microbial Cell Factories, 2020, 19(1): 6. |
| 94 | JU J H, HEO S Y, CHOI S W, et al. Effective bioconversion of 1,3-propanediol from biodiesel-derived crude glycerol using organic acid resistance-enhanced Lactobacillus reuteri JH83[J]. Bioresource Technology, 2021, 337: 125361. |
| 95 | SINGH K, AINALA S K, PARK S. Metabolic engineering of Lactobacillus reuteri DSM 20, 016 for improved 1,3-propanediol production from glycerol[J]. Bioresource Technology, 2021, 338: 125590. |
| 96 | SINGH K, PARK S. Construction of prophage-free and highly-transformable Limosilactobacillus reuteri strains and their use for production of 1,3-propanediol[J]. Biotechnology and Bioengineering, 2024, 121(1): 317-328. |
| 97 | HAWKINS C L, DAVIES M J. Detection, identification, and quantification of oxidative protein modifications[J]. Journal of Biological Chemistry, 2019, 294(51): 19683-19708. |
| 98 | KONG L H, XIONG Z Q, SONG X, et al. Characterization of a panel of strong constitutive promoters from Streptococcus thermophilus for fine-tuning gene expression[J]. ACS Synthetic Biology, 2019, 8(6): 1469-1472. |
| 99 | LIN J Z, ZOU Y X, CAO K L, et al. The impact of heterologous catalase expression and superoxide dismutase overexpression on enhancing the oxidative resistance in Lactobacillus casei [J]. Journal of Industrial Microbiology & Biotechnology, 2016, 43(5): 703-711. |
| 100 | AN H R, ZHAI Z Y, YIN S, et al. Coexpression of the superoxide dismutase and the catalase provides remarkable oxidative stress resistance in Lactobacillus rhamnosus [J]. Journal of Agricultural and Food Chemistry, 2011, 59(8): 3851-3856. |
| 101 | LI Y, HUGENHOLTZ J, SYBESMA W, et al. Using Lactococcus lactis for glutathione overproduction[J]. Applied Microbiology and Biotechnology, 2005, 67(1): 83-90. |
| 102 | XU C T, SHI Z W, SHAO J Q, et al. Metabolic engineering of Lactococcus lactis for high level accumulation of glutathione and S-adenosyl-L-methionine[J]. World Journal of Microbiology & Biotechnology, 2019, 35(12): 185. |
| 103 | WU J P, TIAN X F, XU X N, et al. Engineered probiotic Lactococcus lactis for lycopene production against ROS stress in intestinal epithelial cells[J]. ACS Synthetic Biology, 2022, 11(4): 1568-1576. |
| 104 | MA J, LI C Y, WANG J R, et al. Genetically engineered Escherichia coli nissle 1917 secreting GLP-1 analog exhibits potential antiobesity effect in high-fat diet-induced obesity mice[J]. Obesity, 2020, 28(2): 315-322. |
| 105 | CUBILLOS-RUIZ A, ALCANTAR M A, DONGHIA N M, et al. An engineered live biotherapeutic for the prevention of antibiotic-induced dysbiosis[J]. Nature Biomedical Engineering, 2022, 6(7): 910-921. |
| 106 | SCOTT B M, GUTIÉRREZ-VÁZQUEZ C, SANMARCO L M, et al. Self-tunable engineered yeast probiotics for the treatment of inflammatory bowel disease[J]. Nature Medicine, 2021, 27(7): 1212-1222. |
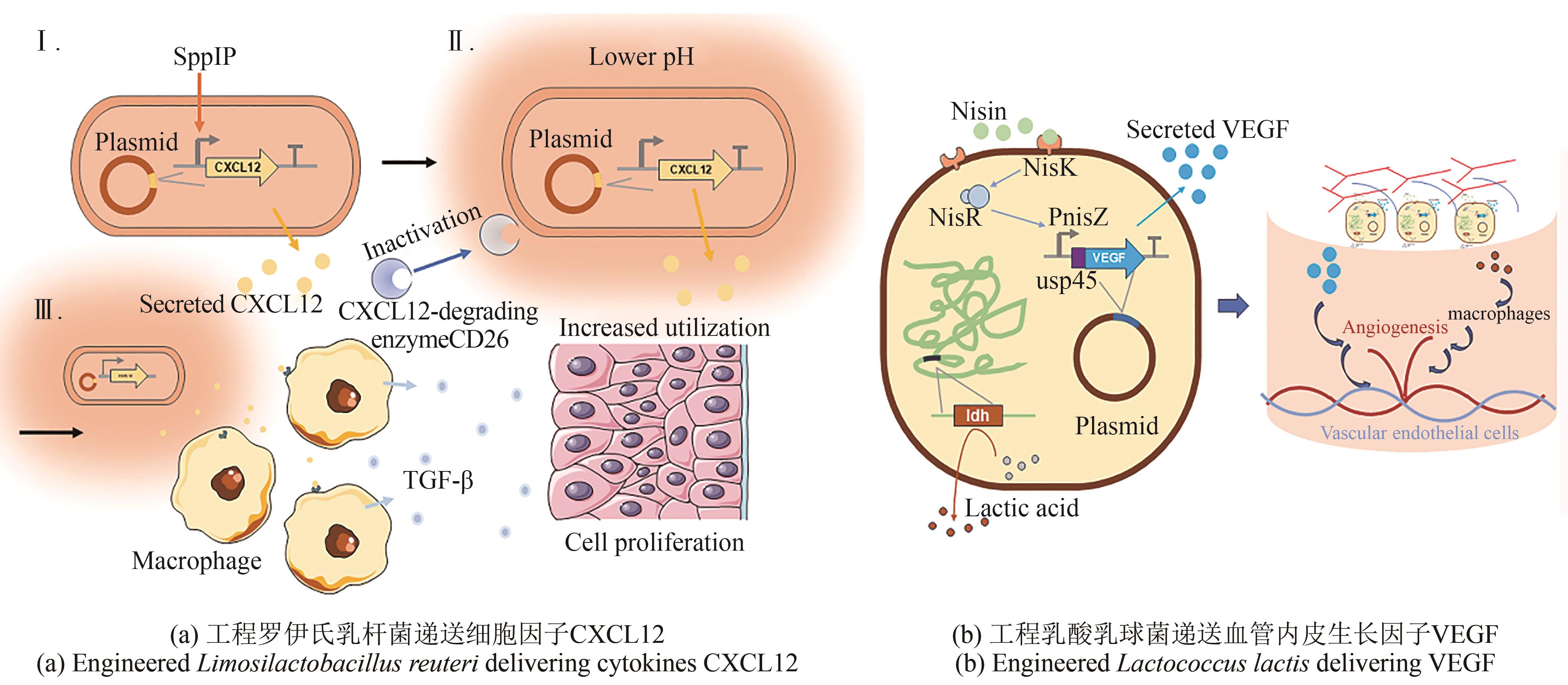
| 107 | VÅGESJÖ E, ÖHNSTEDT E, MORTIER A, et al. Accelerated wound healing in mice by on-site production and delivery of CXCL12 by transformed lactic acid bacteria[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(8): 1895-1900. |
| 108 | ÖHNSTEDT E, LOFTON TOMENIUS H, FRANK P, et al. Accelerated wound healing in minipigs by on-site production and delivery of CXCL12 by transformed lactic acid bacteria[J]. Pharmaceutics, 2022, 14(2): 229. |
| 109 | ÖHNSTEDT E, VÅGESJÖ E, FASTH A, et al. Engineered bacteria to accelerate wound healing: an adaptive, randomised, double-blind, placebo-controlled, first-in-human phase 1 trial[J]. EClinicalMedicine, 2023, 60: 102014. |
| 110 | ZHAO X X, LI S J, DING J N, et al. Combination of an engineered Lactococcus lactis expressing CXCL12 with light-emitting diode yellow light as a treatment for scalded skin in mice[J]. Microbial Biotechnology, 2021, 14(5): 2090-2100. |
| 111 | LI L Y, YANG C, MA B L, et al. Hydrogel-encapsulated engineered microbial consortium as a photoautotrophic “living material” for promoting skin wound healing[J]. ACS Applied Materials & Interfaces, 2023, 15(5): 6536-6547. |
| 112 | LU Y F, LI H S, WANG J, et al. Engineering bacteria-activated multifunctionalized hydrogel for promoting diabetic wound healing[J]. Advanced Functional Materials, 2021, 31(48): 2105749. |
| 113 | MING Z Z, HAN L, BAO M Y, et al. Living bacterial hydrogels for accelerated infected wound healing[J]. Advanced Science, 2021, 8(24): 2102545. |
| 114 | MIERAU I, LEIJ P, VAN SWAM I, et al. Industrial-scale production and purification of a heterologous protein in Lactococcus lactis using the nisin-controlled gene expression system NICE: the case of lysostaphin[J]. Microbial Cell Factories, 2005, 4: 15. |
| 115 | LUBKOWICZ D, HO C L, HWANG I Y, et al. Reprogramming probiotic Lactobacillus reuteri as a biosensor for Staphylococcus aureus derived AIP-I detection[J]. ACS Synthetic Biology, 2018, 7(5): 1229-1237. |
| [1] | WU Ke, LUO Jiahao, LI Feiran. Applications of machine learning in the reconstruction and curation of genome-scale metabolic models [J]. Synthetic Biology Journal, 2025, 6(3): 566-584. |
| [2] | TIAN Xiao-jun, ZHANG Rixin. “Economics Paradox” with cells in synthetic gene circuits [J]. Synthetic Biology Journal, 2025, 6(3): 532-546. |
| [3] | ZHANG Yiqing, LIU Gaowen. Exploration of gene functions and library construction for engineering strains from a synthetic biology perspective [J]. Synthetic Biology Journal, 2025, 6(3): 685-700. |
| [4] | YANG Ying, LI Xia, LIU Lizhong. Applications of synthetic biology to stem-cell-derived modeling of early embryonic development [J]. Synthetic Biology Journal, 2025, 6(3): 669-684. |
| [5] | HUANG Yi, SI Tong, LU Anjing. Standardization for biomanufacturing: global landscape, critical challenges, and pathways forward [J]. Synthetic Biology Journal, 2025, 6(3): 701-714. |
| [6] | SONG Chengzhi, LIN Yihan. AI-enabled directed evolution for protein engineering and optimization [J]. Synthetic Biology Journal, 2025, 6(3): 617-635. |
| [7] | ZHANG Mengyao, CAI Peng, ZHOU Yongjin. Synthetic biology drives the sustainable production of terpenoid fragrances and flavors [J]. Synthetic Biology Journal, 2025, 6(2): 334-356. |
| [8] | ZHANG Lu’ou, XU Li, HU Xiaoxu, YANG Ying. Synthetic biology ushers cosmetic industry into the “bio-cosmetics” era [J]. Synthetic Biology Journal, 2025, 6(2): 479-491. |
| [9] | YI Jinhang, TANG Yulin, LI Chunyu, WU Heyun, MA Qian, XIE Xixian. Applications and advances in the research of biosynthesis of amino acid derivatives as key ingredients in cosmetics [J]. Synthetic Biology Journal, 2025, 6(2): 254-289. |
| [10] | WEI Lingzhen, WANG Jia, SUN Xinxiao, YUAN Qipeng, SHEN Xiaolin. Biosynthesis of flavonoids and their applications in cosmetics [J]. Synthetic Biology Journal, 2025, 6(2): 373-390. |
| [11] | XIAO Sen, HU Litao, SHI Zhicheng, WANG Fayin, YU Siting, DU Guocheng, CHEN Jian, KANG Zhen. Research advances in biosynthesis of hyaluronic acid with controlled molecular weights [J]. Synthetic Biology Journal, 2025, 6(2): 445-460. |
| [12] | WANG Qian, GUO Shiting, XIN Bo, ZHONG Cheng, WANG Yu. Advances in biosynthesis of L-arginine using engineered microorganisms [J]. Synthetic Biology Journal, 2025, 6(2): 290-305. |
| [13] | ZUO Yimeng, ZHANG Jiaojiao, LIAN Jiazhang. Enabling technology for the biosynthesis of cosmetic raw materials with Saccharomyces cerevisiae [J]. Synthetic Biology Journal, 2025, 6(2): 233-253. |
| [14] | TANG Chuan′gen, WANG Jing, ZHANG Shuo, ZHANG Haoning, KANG Zhen. Advances in synthesis and mining strategies for functional peptides [J]. Synthetic Biology Journal, 2025, 6(2): 461-478. |
| [15] | ZHANG Ping, ZHANG Weijiao, XU Ruirui, LI Jianghua, CHEN Jian, KANG Zhen. Research advances on the biosynthesis of mycosporine-like amino acids [J]. Synthetic Biology Journal, 2025, 6(2): 306-319. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||