Synthetic Biology Journal ›› 2025, Vol. 6 ›› Issue (3): 701-714.DOI: 10.12211/2096-8280.2025-040
• Invited Review • Previous Articles
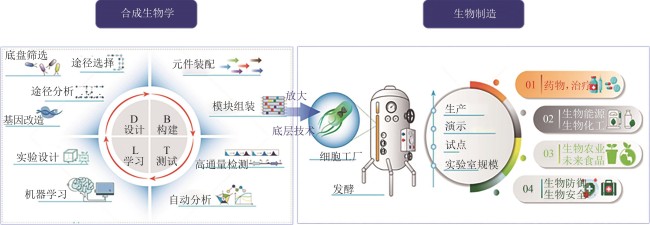
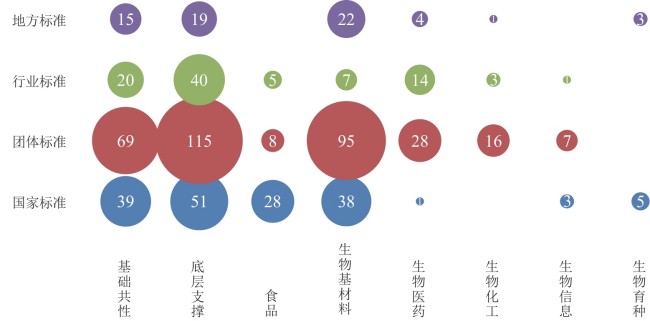
Standardization for biomanufacturing: global landscape, critical challenges, and pathways forward
HUANG Yi1, SI Tong1, LU Anjing2
- 1.State Key Laboratory of Quantitative Synthetic Biology,Shenzhen Institute of Synthetic Biology,Shenzhen Institutes of Advanced Technology,Chinese Academy of Sciences,Shenzhen 518055,Guangdong,China
2.China Center for Information Industry Development,Beijing 100081,China
-
Received:2025-04-29Revised:2025-06-04Online:2025-06-27Published:2025-06-30 -
Contact:LU Anjing
生物制造标准体系建设的现状、问题与建议
黄怡1, 司同1, 陆安静2
- 1.中国科学院深圳先进技术研究院,定量合成生物学全国重点实验室,深圳合成生物学创新研究院,广东 深圳 518055
2.中国电子信息产业发展研究院,北京 100081
-
通讯作者:陆安静 -
作者简介:黄怡 (1996—),女,助理研究员。研究方向为生物制造科技情报分析与政策研究。E-mail:y.huang2@siat.ac.cn陆安静 (1987—),男,副研究员。研究方向为生物制造产业分析、市场与政策研究。E-mail:legendblue@foxmail.com
CLC Number:
Cite this article
HUANG Yi, SI Tong, LU Anjing. Standardization for biomanufacturing: global landscape, critical challenges, and pathways forward[J]. Synthetic Biology Journal, 2025, 6(3): 701-714.
黄怡, 司同, 陆安静. 生物制造标准体系建设的现状、问题与建议[J]. 合成生物学, 2025, 6(3): 701-714.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2025-040
| 1 | 国家标准化管理委员会.标准和标准化[EB/OL]. (2017-11-06)[2025-06-03]. . |
| Standardization Administration of the People’s Republic of China. Standard and standardization[EB/OL]. (2017-11-06)[2025-06-03]. . | |
| 2 | MENG F K, ELLIS T. The second decade of synthetic biology: 2010-2020[J]. Nature Communications, 2020, 11: 5174. |
| 3 | 赵国屏. 合成生物学: 开启生命科学“会聚” 研究新时代[J]. 中国科学院院刊, 2018, 33(11): 1135-1149. |
| ZHAO G P. Synthetic biology: unsealing the convergence era of life science research[J]. Bulletin of Chinese Academy of Sciences, 2018, 33(11): 1135-1149. | |
| 4 | 刘陈立. 拥抱生物经济发展新机遇[N]. 经济日报, 2024-07-05(10). |
| LIU C L. Embrace the new opportunities for the development of the bio-economy[N]. Economic Daily, 2024-07-05(10). | |
| 5 | MEIGE A, EAGAR R, SEHLSTEDT U, et al. The brave new world of synthetic biology: major impacts, significant challenges[R/OL]. 2024[2025-06-03]. . |
| 6 | LUO N, ZHAO G P, LIU C L. Quantitative synthetic biology[J]. Nature Reviews Bioengineering, 2024, 2(11): 911-913. |
| 7 | MUNSON M, MUNRO S, SALIT M. Synthetic biology standards consortium kick-off workshop report[R]. U.S. National Institute of Standards and Technology, 2015. |
| 8 | The White House. National biotechnology and biomanufacturing initiative[EB/OL]. (2022-09-12)[2025-06-03]. . |
| 9 | THOMAS D. The U.S. biomanufacturing economy: value added, supply chains, cost, sustainability, and efficiency[R/OL]. National Institute of Standards and Technology, 2023[2025-06-04]. . |
| 10 | The White House. U.S. government national standards strategy for critical and emerging technologies (usg nsscet): implementation roadmap[EB/OL]. (2024-07-26)[2025-06-03]. . |
| 11 | FREEMONT P S, NI C, AURAND E, et al. Engineering biology metrics and technical standards for the global bioeconomy[R/OL]. London, UK, 2024[2025-06-03]. . |
| 12 | FREEMONT P, ADEOGUN M. Standards and metrics for engineering biology in the UK: driving growth, investment and engineering biology powered solutions for UK companies[R/OL]. London, UK, 2024[2025-06-03]. . |
| 13 | ORDOZGOITI E, PORCAR M, BALDWIN G, et al. Standardization in synthetic biology: a white book[R]. European Union: fostering synthetic biology standardization through international collaboration, 2015. |
| 14 | Commission European. Building the future with nature: boosting biotechnology and biomanufacturing in the EU[EB/OL]. (2024-03-27)[2025-06-03]. . |
| 15 | Standard terminology for industrial biotechnology and synthetic biology: [S/OL]. [2025-06-03]. . |
| 16 | Biotechnology — Cell counting — Part 1: General guidance on cell counting methods: [S/OL]. [2025-06-03]. . |
| 17 | Biotechnology — Cell counting — Part 2: Experimental design and statistical analysis to quantify counting method performance: [S/OL]. [2025-06-03]. . |
| 18 | 陈国强, 吴赴清, 郑爽, 等. 我国生物制造底盘菌种现状、问题及对策[J]. 中国科学院院刊, 2025, 40(1): 2-13. |
| CHEN G Q, WU F Q, ZHENG S, et al. Current status and applications of microbial chassis strains for Chinese biomanufacturing industry[J]. Bulletin of Chinese Academy of Sciences, 2025, 40(1): 2-13. | |
| 19 | Microbiology of food and animal feeding stuffs horizontal method for the detection and enumeration of presumptiveEscherichia coli-most probable number technique-AMENDMENT 1: inclusion of performance testing of culture media and reagents: Amd 1:2023[S/OL]. [2025-06-03]. . |
| 20 | Biotechnology — Biobanking — General requirements for biobanking: [S/OL]. [2025-06-03]. . |
| 21 | Biotechnology — Biobanking — Requirements for animal biological material: [S/OL]. [2025-06-03]. . |
| 22 | Biotechnology — Biobanking — Requirements for the biobanking of plant biological material for research and development: [S/OL]. [2025-06-03]. . |
| 23 | Biotechnology — Biobanking — General requirements for the validation and verification of processing methods for biological material in biobanks: [S/OL]. [2025-06-03]. . |
| 24 | Biotechnology — Biobanking — Requirements for human mesenchymal stromal cells derived from bone marrow: [S/OL]. [2025-06-03]. . |
| 25 | Biotechnology — Biobanking — Requirements for human neural stem cells derived from pluripotent stem cells: [S/OL]. [2025-06-03]. . |
| 26 | Biotechnology — Requirements for data formatting and description in the life sciences: [S/OL]. [2025-06-03]. . |
| 27 | ROSS D, TONNER P D, VASILYEVA O B. Method for reproducible automated bacterial cell culture and measurement[J]. Synthetic Biology, 2022, 7(1): ysac013. |
| 28 | HADDOCK-ANGELLI T, BEAL J, FARNY N, et al. Standard iGEM cell measurement protocol[R/OL]. (2019-07-21)[2025-06-03] . |
| 29 | Biotechnology — Nucleic acid synthesis — Part 2: Requirements for the production and quality control of synthesized gene fragments, genes, and genomes: [S/OL]. [2025-06-03]. . |
| 30 | 中国生物工程学会. 合成生物学路线图2030:驱动下一代生物制造的引擎[M]. 北京: 科学出版社, 2024. |
| Chinese Society of Biotechnology. Synthetic biology roadmap 2030: the engine driving the next generation of biomanufacturing[M].Beijing: Science Press, 2024. | |
| 31 | ROMANTSEVA E, STRYCHALSKI E A. CELL-FREE (Comparable engineered living lysates for research education and entrepreneurship) workshop report[R/OL]. U.S. National Institute of Standards and Technology, 2020 [2025-06-03]. . |
| 32 | ROMANTSEVA E. Metrology for cell-free expression systems[R/OL].U.S. National Institute of Standards and Technology, 2025 [2025-06-03]. . |
| 33 | Foodstuffs — Methods of analysis for the detection of genetically modified organisms and derived products — General requirements and definitions — Amendment 1: Amd 1:2013[S/OL]. [2025-06-03]. . |
| 34 | Foodstuffs — Methods of analysis for the detection of genetically modified organisms and derived products — Quantitative nucleic acid based methods — Amendment 1: Amd 1:2013[S/OL]. [2025-06-03]. . |
| 35 | Bio-based products-Vocabulary: EN 16575:2014 [S/OL]. [2025-06-03]. . |
| 36 | Rubber and rubber products — Determination of biobased content — Part 3: Biobased mass content: [S/OL]. [2025-06-03]. . |
| 37 | Bio-based products. Bio-based content-Determination of the bio-based content using the material balance method: [S/OL]. [2025-06-03]. . |
| 38 | Plastics — Biobased content — Part 4: Determination of biobased mass content: [S/OL]. [2025-06-03]. . |
| 39 | Bio-based products-Life Cycle Assessment: EN 16760:2015 [S/OL]. [2025-06-03]. . |
| 40 | Bio-based products-Guidelines for Life Cycle Inventory (LCI) for the End-of-life phase: CEN/TR 16957:2016 [S/OL]. [2025-06-03]. . |
| 41 | Determination of the ultimate biodegradation of plastics materials in an aqueous system under anoxic (denitrifying) conditions - Method by measurement of pressure increase: EN 17417:2020 [S/OL]. [2025-06-03]. . |
| 42 | Plastics-Biodegradable mulch films for use in agriculture and horticulture-Requirements and test methods: EN 17033:2018 [S/OL]. [2025-06-03]. . |
| 43 | Plastics-Determination of the ultimate anaerobic biodegradation of plastic materials in an aqueous system-Method by measurement of biogas production: EN [S/OL]. [2025-06-03]. . |
| 44 | Determination of the ultimate aerobic biodegradability of plastic materials in an aqueous medium — Method by measuring the oxygen demand in a closed respirometer: [S/OL]. [2025-06-03]. . |
| 45 | Plastics — Evaluation of the action of microorganisms: [S/OL]. [2025-06-03]. . |
| 46 | Biotechnology — Bioprocessing — General requirements for the design of packaging to contain cells for therapeutic use: [S/OL]. [2025-06-03]. . |
| 47 | Biotechnology — Ancillary materials present during the production of cellular therapeutic products and gene therapy products: [S/OL]. [2025-06-03]. . |
| 48 | Biotechnology. Bioprocessing. General requirements and considerations for equipment systems used in the manufacturing of cells for therapeutic use: [S/OL]. [2025-06-03]. . |
| 49 | Biotechnology — Analytical methods — General requirements and considerations for the testing and characterization of cellular therapeutic products: [S/OL]. [2025-06-03]. . |
| 50 | Biotechnology — General requirements for transportation of cells for therapeutic use: [S/OL]. [2025-06-03]. . |
| 51 | 中共中央 国务院.国家标准化发展纲要[EB/OL]. (2021-10-10)[2025-06-03]. . |
| Central Committee of the Communist Party of China and State Council. National standardization development outline [EB/OL]. (2021-10-10)[2025-06-03]. . | |
| 52 | 工业和信息化部等四部门. 新产业标准化领航工程实施方案(2023—2035年)[EB/OL].(2023-08-03)[2025-06-03]. . |
| Ministry of Industry and Information Technology of the People’s Republic of China, Ministry of Science and Technology of the People’s Republic of China, National Energy Administration, et al. Implementation plan for new industry standardization pilot project (2023—2035) [EB/OL]. (2023-08-03)[2025-06-03]. . | |
| 53 | 工业和信息化部,教育部,科学技术部,等. 工业和信息化部等七部门关于推动未来产业创新发展的实施意见[EB/OL]. (2024-01-18)[2025-06-03]. . |
| Ministry of Industry and Information Technology of the People’s Republic of China, Ministry of Education of the People’s Republic of China, Ministry of Science and Technology of the People’s Republic of China, et al. The guideline to support development of future industries released by the Ministry of Industry and Information Technology and six other ministry and agencies[EB/OL]. (2024-01-18)[2025-06-03]. . | |
| 54 | 市场监管总局, 中央网信办, 国家发展改革委等18部门. 贯彻实施〈国家标准化发展纲要〉行动计划(2024—2025年)[EB/OL]. (2024-03-27)[2025-06-03]. . |
| State Administration for Market Regulation, Office of the Central Cyberspace Affairs Commission, National Development and Reform Commission, et al. Implement the action plan for the implementation of the national standardization development outline (2024-2025)[EB/OL]. (2024-03-27)[2025-06-03]. . | |
| 55 | 国家市场监督管理总局, 国家标准化管理委员会. 生物技术 生物样本保藏 动物生物样本保藏要求: [S]. 北京: 中国标准出版社, 2024. |
| State Administration for Market Regulation, Standardization Administration of the People’s Republic of China. Biotechnology—Biobanking—Requirements for the biobanking of animal biological material: [S]. Beijing: Standards Press of China, 2024. | |
| 56 | 国家市场监督管理总局, 国家标准化管理委员会. 生物技术 生物样本保藏 用于研究和开发用途的植物生物样本保藏要求: [S]. 北京: 中国标准出版社, 2024. |
| State Administration for Market Regulation, Standardization Administration of the People’s Republic of China. Biotechnology—Biobanking—Requirements for the biobanking of plant biological material for research and development: [S]. Beijing: Standards Press of China, 2024. | |
| 57 | 国家市场监督管理总局, 国家标准化管理委员会. 人感染病原微生物与样本保藏通用要求: [S]. 北京: 中国标准出版社, 2023. |
| State Administration for Market Regulation, Standardization Administration of the People’s Republic of China. General requirements for preservation of human pathogenic microorganisms and samples: [S]. Beijing: Standards Press of China, 2023. | |
| 58 | 人胃癌类器官构建、质量控制与保藏: T/1984 [S].北京: 中国标准出版社, 2024. |
| Human gastric cancer organoid construction, quality control and preservation: T/1984 [S]. Beijing: Standards Press of China, 2024. | |
| 59 | 人肝祖细胞类器官构建、质量控制与保藏操作指南: T/CRHA 017—2023 [S]. 北京: 中国标准出版社, 2023. |
| Operational guidelines for construction, quality control and preservation of human liver progenitor cell organoids: T/CRHA 017—2023 [S]. Beijing: Standards Press of China, 2023. | |
| 60 | 人肝胆肿瘤细胞类器官构建、质量控制与保藏操作指南: T/CRHA 018—2023 [S]. 北京: 中国标准出版社, 2023. |
| Operational guidelines for construction, quality control and preservation of human hepatobiliary tumor cell organoids: T/CRHA 018—2023 [S]. Beijing: Standards Press of China, 2023. | |
| 61 | 人胆系上皮组织类器官构建、质量控制与保藏操作指南: T/CRHA 019—2023 [S]. 北京: 中国标准出版社, 2023. |
| Operational guidelines for construction, quality control and preservation of human biliary epithelial tissue organoids: T/CRHA 019—2023 [S]. Beijing: Standards Press of China, 2023. | |
| 62 | 原料药及中间体连续制造指导原则: T/PIAC 00001—2023 [S]. 北京: 中国标准出版社, 2023. |
| Guidelines for continuous manufacturing of drug substances and intermediates: T/PIAC 00001—2023 [S]. Beijing: Standards Press of China, 2023. | |
| 63 | 张春鹏, 范宇婷, 董红霞. 美国新药技术成熟度评价研究[J]. 中国药物警戒, 2020, 17(6): 353-356. |
| ZHANG C P, FAN Y T, DONG H X. Research on the evaluation method of new drug technology readiness level in the United States[J]. Chinese Journal of Pharmacovigilance, 2020, 17(6): 353-356. |
| [1] | WU Ke, LUO Jiahao, LI Feiran. Applications of machine learning in the reconstruction and curation of genome-scale metabolic models [J]. Synthetic Biology Journal, 2025, 6(3): 566-584. |
| [2] | TIAN Xiao-jun, ZHANG Rixin. “Economics Paradox” with cells in synthetic gene circuits [J]. Synthetic Biology Journal, 2025, 6(3): 532-546. |
| [3] | ZHANG Yiqing, LIU Gaowen. Exploration of gene functions and library construction for engineering strains from a synthetic biology perspective [J]. Synthetic Biology Journal, 2025, 6(3): 685-700. |
| [4] | YANG Ying, LI Xia, LIU Lizhong. Applications of synthetic biology to stem-cell-derived modeling of early embryonic development [J]. Synthetic Biology Journal, 2025, 6(3): 669-684. |
| [5] | SONG Chengzhi, LIN Yihan. AI-enabled directed evolution for protein engineering and optimization [J]. Synthetic Biology Journal, 2025, 6(3): 617-635. |
| [6] | ZHANG Mengyao, CAI Peng, ZHOU Yongjin. Synthetic biology drives the sustainable production of terpenoid fragrances and flavors [J]. Synthetic Biology Journal, 2025, 6(2): 334-356. |
| [7] | ZHANG Lu’ou, XU Li, HU Xiaoxu, YANG Ying. Synthetic biology ushers cosmetic industry into the “bio-cosmetics” era [J]. Synthetic Biology Journal, 2025, 6(2): 479-491. |
| [8] | YI Jinhang, TANG Yulin, LI Chunyu, WU Heyun, MA Qian, XIE Xixian. Applications and advances in the research of biosynthesis of amino acid derivatives as key ingredients in cosmetics [J]. Synthetic Biology Journal, 2025, 6(2): 254-289. |
| [9] | WEI Lingzhen, WANG Jia, SUN Xinxiao, YUAN Qipeng, SHEN Xiaolin. Biosynthesis of flavonoids and their applications in cosmetics [J]. Synthetic Biology Journal, 2025, 6(2): 373-390. |
| [10] | XIAO Sen, HU Litao, SHI Zhicheng, WANG Fayin, YU Siting, DU Guocheng, CHEN Jian, KANG Zhen. Research advances in biosynthesis of hyaluronic acid with controlled molecular weights [J]. Synthetic Biology Journal, 2025, 6(2): 445-460. |
| [11] | WANG Qian, GUO Shiting, XIN Bo, ZHONG Cheng, WANG Yu. Advances in biosynthesis of L-arginine using engineered microorganisms [J]. Synthetic Biology Journal, 2025, 6(2): 290-305. |
| [12] | ZUO Yimeng, ZHANG Jiaojiao, LIAN Jiazhang. Enabling technology for the biosynthesis of cosmetic raw materials with Saccharomyces cerevisiae [J]. Synthetic Biology Journal, 2025, 6(2): 233-253. |
| [13] | TANG Chuan′gen, WANG Jing, ZHANG Shuo, ZHANG Haoning, KANG Zhen. Advances in synthesis and mining strategies for functional peptides [J]. Synthetic Biology Journal, 2025, 6(2): 461-478. |
| [14] | GUO Tingting, HAN Xiangning, HUANG Xiting, ZHANG Tingting, KONG Jian. Advances in synthetic biology tools for lactic acid bacteria and their application in the development of skin beneficial products [J]. Synthetic Biology Journal, 2025, 6(2): 320-333. |
| [15] | ZHANG Ping, ZHANG Weijiao, XU Ruirui, LI Jianghua, CHEN Jian, KANG Zhen. Research advances on the biosynthesis of mycosporine-like amino acids [J]. Synthetic Biology Journal, 2025, 6(2): 306-319. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||