Synthetic Biology Journal ›› 2025, Vol. 6 ›› Issue (2): 445-460.DOI: 10.12211/2096-8280.2024-062
• Invited Review • Previous Articles Next Articles
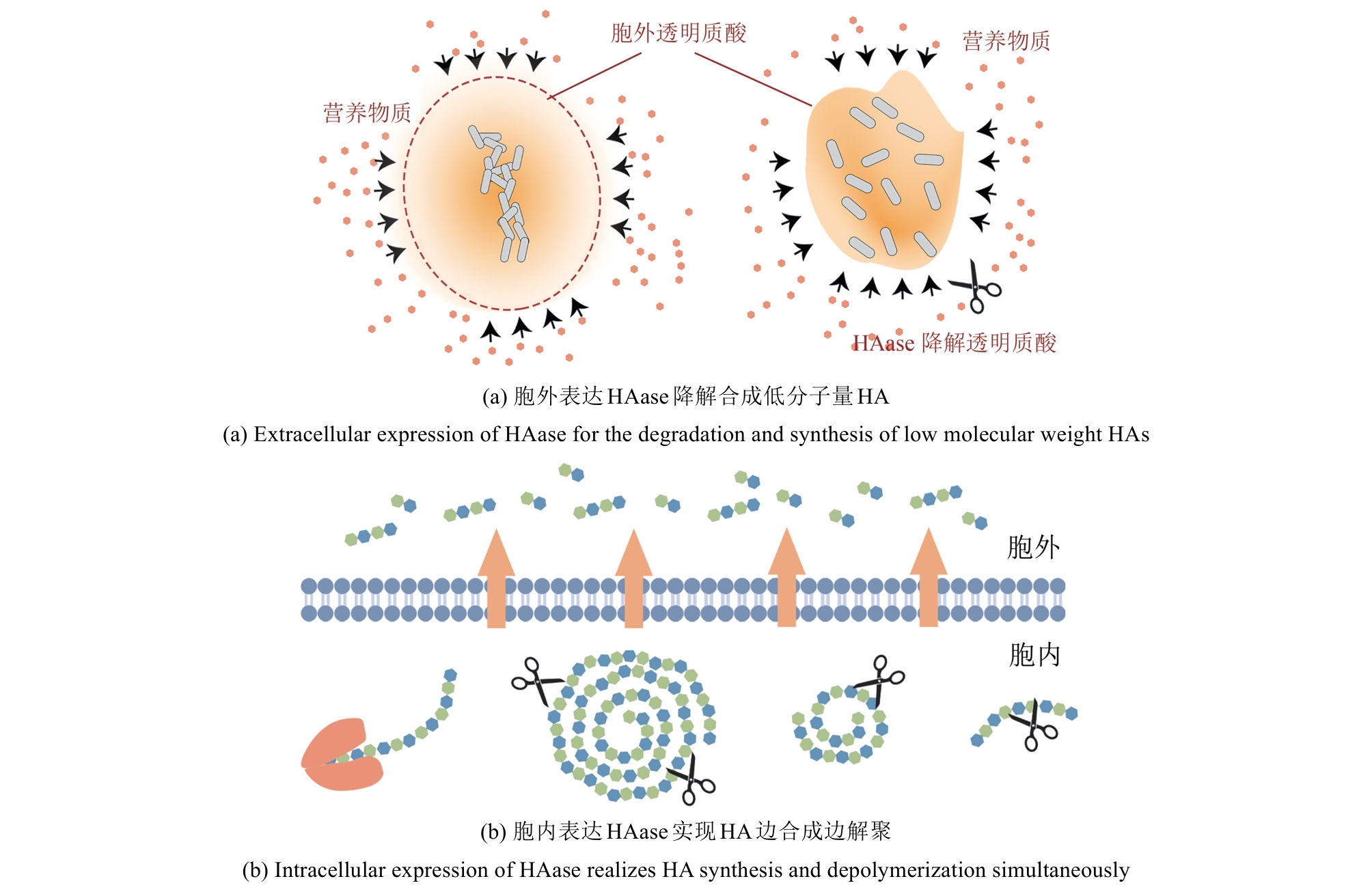
Research advances in biosynthesis of hyaluronic acid with controlled molecular weights
XIAO Sen1,2,3, HU Litao1,2,3, SHI Zhicheng1,2, WANG Fayin1,2, YU Siting1,2, DU Guocheng2,4, CHEN Jian2,3,4, KANG Zhen1,2,3
- 1.Key Laboratory of Carbohydrate Chemistry and Biotechnology,Ministry of Education,School of Biotechnology,Jiangnan University,Wuxi 214122,Jiangsu,China
2.Science Center for Future Foods,Jiangnan University,Wuxi 214122,Jiangsu,China
3.Institute of Future Food Technology,Jiangsu Industrial Technology Research Institute,Yixing 214200,Jiangsu,China
4.Key Laboratory of Industrial Biotechnology,Ministry of Education,School of Biotechnology,Jiangnan University,Wuxi 214122,Jiangsu,China
-
Received:2024-08-14Revised:2024-10-24Online:2025-05-20Published:2025-04-30 -
Contact:KANG Zhen
可控分子量透明质酸的生物合成研究进展
肖森1,2,3, 胡立涛1,2,3, 石智诚1,2, 王发银1,2, 余思婷1,2, 堵国成2,4, 陈坚2,3,4, 康振1,2,3
- 1.江南大学生物工程学院,糖化学与生物技术教育部重点实验室,江苏 无锡 214122
2.江南大学,未来食品科学中心,江苏 无锡 214122
3.江苏省产业技术研究院,江苏集萃未来食品技术研究所有限公司,江苏 宜兴 214200
4.江南大学生物工程学院,工业生物技术教育部重点实验室,江苏 无锡 214122
-
通讯作者:康振 -
作者简介:肖森 (2002—),男,硕士研究生。研究方向为透明质酸的生物合成与酶工程改造。 E-mail:6240201118@stu.jiangnan.edu.cn康振 (1982—),男,博士,教授,博士生导师。研究方向为食品微生物合成生物学与生物制造。E-mail:zkang@jiangnan.edu.cn -
基金资助:江南大学食品科学与资源国家重点实验室研发项目(5962060204240220);国家自然科学基金(32370066);中央高校基本科研基金(JUSRP622003);无锡市产业创新研究院先导技术预研项目(XD24006)
CLC Number:
Cite this article
XIAO Sen, HU Litao, SHI Zhicheng, WANG Fayin, YU Siting, DU Guocheng, CHEN Jian, KANG Zhen. Research advances in biosynthesis of hyaluronic acid with controlled molecular weights[J]. Synthetic Biology Journal, 2025, 6(2): 445-460.
肖森, 胡立涛, 石智诚, 王发银, 余思婷, 堵国成, 陈坚, 康振. 可控分子量透明质酸的生物合成研究进展[J]. 合成生物学, 2025, 6(2): 445-460.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2024-062
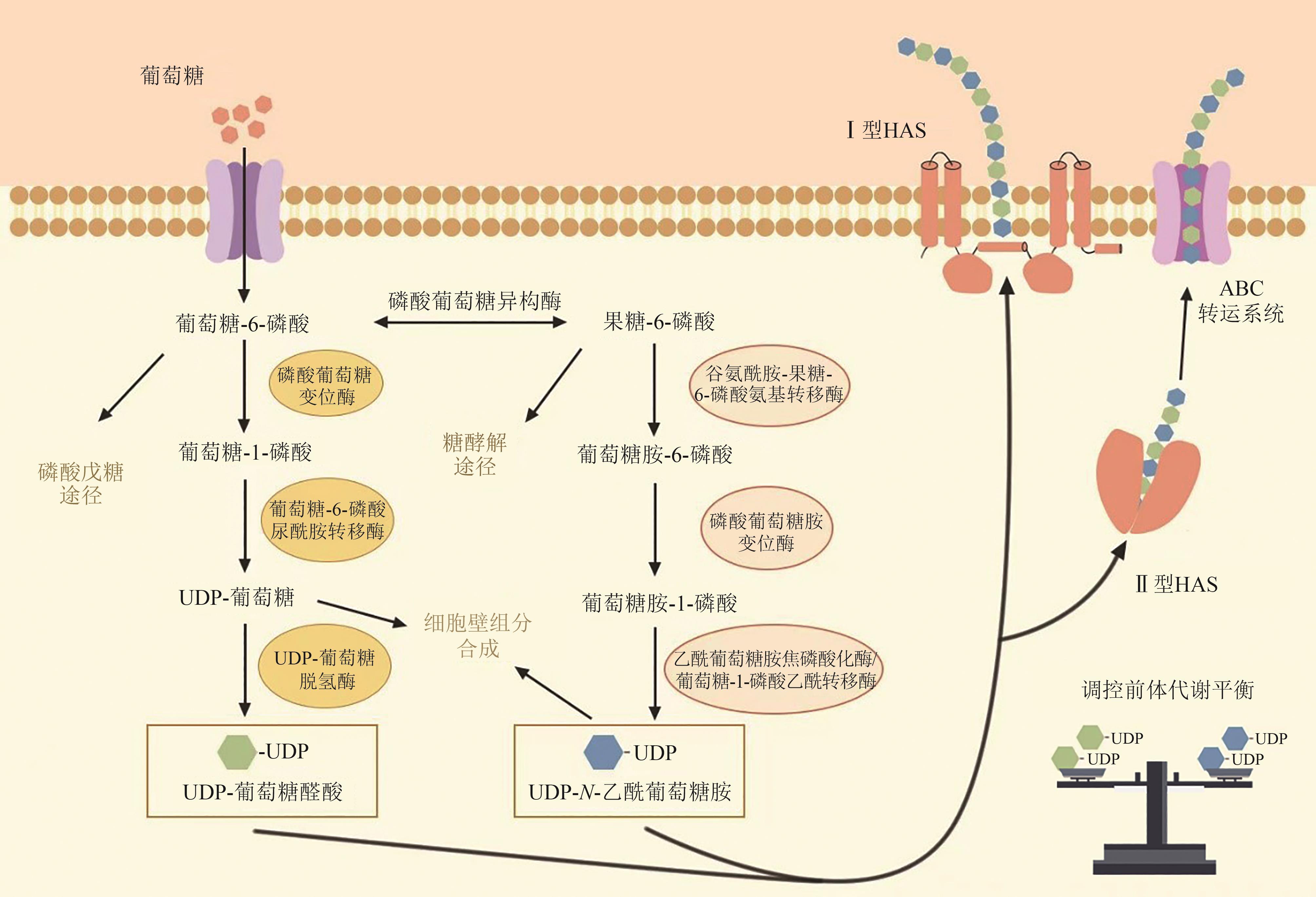
| 工程菌株 | 改造策略 | HA产量 /(g/L) | HA分子量 /Da | 参考文献 |
|---|---|---|---|---|
| S. zooepidemicus ATCC 39920 | 敲除HA裂解酶编码基因hylB | 3.40 | 2.28×106 | [ |
| S. zooepidemicus | 过表达HAS编码基因sehasA,提高碳源浓度 | 6.90 | 2.75×106 | [ |
| S. zooepidemicus MTCC 3523 | 调节溶解氧水平与N-乙酰葡萄糖胺供应水平 | 2.4 | 2.53×106 | [ |
| S. zooepidemicus | 添加铁纳米颗粒 | 0.435 | 1.48×106 | [ |
| S. zooepidemicus ATCC 39920 | 紫外诱变策略,开发两阶段半连续发酵工艺 | 29.38 | — | [ |
| E. coli K12 W3110 | 敲除6-磷酸果糖激酶Ⅰ编码基因pfkA和葡萄糖-6-磷酸脱氢酶编码基因zwf,表达HA合成途径基因簇galU-ugd和glmS-glmM-glmU | 0.02998 | — | [ |
| E. coli Top10 | 表达兽疫链球菌来源HAS编码基因sehasA和UDP-葡萄糖脱氢酶编码基因ugdA | 0.19 | 0.35×106~1.9×106 | [ |
| E. coli JM109 | 表达多杀巴斯德杆菌来源HAS编码基因pmhasA和大肠杆菌K5来源UDP-葡萄糖脱氢酶编码基因ugdA | 3.8 | — | [ |
| Lactobacillus acidophilus | 表达兽疫链球菌来源HAS编码基因sehasA和UDP-葡萄糖脱氢酶编码基因ugdA | 1.7 | — | [ |
| Lactococcus lactis CES15 | PnisA启动子调控兽疫链球菌来源HAS编码基因sehasA表达 | 6.09 | — | [ |
| Streptomyces albulus | 表达兽疫链球菌来源HAS编码基因sehasA和阿维米蒂利斯链霉菌来源UDP-葡萄糖脱氢酶编码基因ugdA、乙酰葡萄糖胺焦磷酸化酶/葡萄糖-1-磷酸乙酰转移酶双功能酶编码基因glmU、葡萄糖-6-磷酸尿酰胺转移酶编码基因gtaB | 6.2 | 2×106 | [ |
| Pichia pastoris | 表达非洲爪蟾来源HAS编码基因xhasA2和UDP-葡萄糖脱氢酶编码基因xhasB,毕赤酵母来源的葡萄糖-6-磷酸尿酰胺转移酶编码基因hasC,乙酰葡萄糖胺焦磷酸化酶/葡萄糖-1-磷酸乙酰转移酶双功能酶编码基因hasD,磷酸葡萄糖异构酶编码基因hasE | 1.7 | 1.2×106~2.5×106 | [ |
| B. subtilis 168 | 表达兽疫链球菌来源HAS编码基因sehasA,枯草芽孢杆菌来源UDP-葡萄糖脱氢酶编码基因tuaD,葡萄糖-6-磷酸尿酰胺转移酶编码基因gtaB,乙酰葡萄糖胺焦磷酸化酶/葡萄糖-1-磷酸乙酰转移酶双功能酶编码基因glmU,磷酸葡萄糖胺变位酶编码基因glmM和谷氨酰胺-果糖-6-磷酸氨基转移酶编码基因glmS;下调6-磷酸果糖激酶Ⅰ编码基因pfkA表达 | 3.16 | 1.4×106~1.83×106 | [ |
| B. subtilis BGSC 1A751 | 表达细菌血红蛋白编码基因vhb、C组链球菌来源HAS编码基因hasA、枯草芽孢杆菌来源UDP-葡萄糖脱氢酶编码基因tuaD | 1.8 | — | [ |
| C. glutamicum 13032 | 表达酿脓链球菌来源HAS编码基因spHasA,恶臭假单胞菌来源谷氨酰胺-果糖-6-磷酸氨基转移酶编码基因ptglmS,谷氨酸棒杆菌来源UDP-葡萄糖脱氢酶编码基因cgugdA2;敲除糖基转移酶编码基因cg0420和cg0424 | 34.2 | 3.21×105 | [ |
| C. glutamicum 13032 | 表达兽疫链球菌来源HAS编码基因sehasA,谷氨酸棒杆菌来源UDP-葡萄糖脱氢酶编码基因hasB | 8.3 | 1.3×106 | [ |
| C. glutamicum 13032 | 表达兽疫链球菌来源HAS编码基因seHasA,UDP-葡萄糖脱氢酶编码基因hasB,敲除果糖1,6-二磷酸醛缩酶编码基因fba,葡萄糖-6-磷酸脱氢酶编码基因zwf,阻断乳酸和乙酸合成 | 28.7 | 2.1×105 | [ |
Table 1 Metabolic engineered microorganisms to synthesize HAs
| 工程菌株 | 改造策略 | HA产量 /(g/L) | HA分子量 /Da | 参考文献 |
|---|---|---|---|---|
| S. zooepidemicus ATCC 39920 | 敲除HA裂解酶编码基因hylB | 3.40 | 2.28×106 | [ |
| S. zooepidemicus | 过表达HAS编码基因sehasA,提高碳源浓度 | 6.90 | 2.75×106 | [ |
| S. zooepidemicus MTCC 3523 | 调节溶解氧水平与N-乙酰葡萄糖胺供应水平 | 2.4 | 2.53×106 | [ |
| S. zooepidemicus | 添加铁纳米颗粒 | 0.435 | 1.48×106 | [ |
| S. zooepidemicus ATCC 39920 | 紫外诱变策略,开发两阶段半连续发酵工艺 | 29.38 | — | [ |
| E. coli K12 W3110 | 敲除6-磷酸果糖激酶Ⅰ编码基因pfkA和葡萄糖-6-磷酸脱氢酶编码基因zwf,表达HA合成途径基因簇galU-ugd和glmS-glmM-glmU | 0.02998 | — | [ |
| E. coli Top10 | 表达兽疫链球菌来源HAS编码基因sehasA和UDP-葡萄糖脱氢酶编码基因ugdA | 0.19 | 0.35×106~1.9×106 | [ |
| E. coli JM109 | 表达多杀巴斯德杆菌来源HAS编码基因pmhasA和大肠杆菌K5来源UDP-葡萄糖脱氢酶编码基因ugdA | 3.8 | — | [ |
| Lactobacillus acidophilus | 表达兽疫链球菌来源HAS编码基因sehasA和UDP-葡萄糖脱氢酶编码基因ugdA | 1.7 | — | [ |
| Lactococcus lactis CES15 | PnisA启动子调控兽疫链球菌来源HAS编码基因sehasA表达 | 6.09 | — | [ |
| Streptomyces albulus | 表达兽疫链球菌来源HAS编码基因sehasA和阿维米蒂利斯链霉菌来源UDP-葡萄糖脱氢酶编码基因ugdA、乙酰葡萄糖胺焦磷酸化酶/葡萄糖-1-磷酸乙酰转移酶双功能酶编码基因glmU、葡萄糖-6-磷酸尿酰胺转移酶编码基因gtaB | 6.2 | 2×106 | [ |
| Pichia pastoris | 表达非洲爪蟾来源HAS编码基因xhasA2和UDP-葡萄糖脱氢酶编码基因xhasB,毕赤酵母来源的葡萄糖-6-磷酸尿酰胺转移酶编码基因hasC,乙酰葡萄糖胺焦磷酸化酶/葡萄糖-1-磷酸乙酰转移酶双功能酶编码基因hasD,磷酸葡萄糖异构酶编码基因hasE | 1.7 | 1.2×106~2.5×106 | [ |
| B. subtilis 168 | 表达兽疫链球菌来源HAS编码基因sehasA,枯草芽孢杆菌来源UDP-葡萄糖脱氢酶编码基因tuaD,葡萄糖-6-磷酸尿酰胺转移酶编码基因gtaB,乙酰葡萄糖胺焦磷酸化酶/葡萄糖-1-磷酸乙酰转移酶双功能酶编码基因glmU,磷酸葡萄糖胺变位酶编码基因glmM和谷氨酰胺-果糖-6-磷酸氨基转移酶编码基因glmS;下调6-磷酸果糖激酶Ⅰ编码基因pfkA表达 | 3.16 | 1.4×106~1.83×106 | [ |
| B. subtilis BGSC 1A751 | 表达细菌血红蛋白编码基因vhb、C组链球菌来源HAS编码基因hasA、枯草芽孢杆菌来源UDP-葡萄糖脱氢酶编码基因tuaD | 1.8 | — | [ |
| C. glutamicum 13032 | 表达酿脓链球菌来源HAS编码基因spHasA,恶臭假单胞菌来源谷氨酰胺-果糖-6-磷酸氨基转移酶编码基因ptglmS,谷氨酸棒杆菌来源UDP-葡萄糖脱氢酶编码基因cgugdA2;敲除糖基转移酶编码基因cg0420和cg0424 | 34.2 | 3.21×105 | [ |
| C. glutamicum 13032 | 表达兽疫链球菌来源HAS编码基因sehasA,谷氨酸棒杆菌来源UDP-葡萄糖脱氢酶编码基因hasB | 8.3 | 1.3×106 | [ |
| C. glutamicum 13032 | 表达兽疫链球菌来源HAS编码基因seHasA,UDP-葡萄糖脱氢酶编码基因hasB,敲除果糖1,6-二磷酸醛缩酶编码基因fba,葡萄糖-6-磷酸脱氢酶编码基因zwf,阻断乳酸和乙酸合成 | 28.7 | 2.1×105 | [ |
| 1 | WEISSMANN B, MEYER K. The structure of hyalobiuronic acid and of hyaluronic acid from umbilical Cord1, 2 [J]. Journal of the American Chemical Society, 1954, 76(7): 1753-1757. |
| 2 | SERRA M, CASAS A E, TOUBARRO D, et al. Microbial hyaluronic acid production: a review[J]. Molecules, 2023, 28(5): 2084. |
| 3 | KOGAN G, SOLTÉS L, STERN R, et al. Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications[J]. Biotechnology Letters, 2007, 29(1): 17-25. |
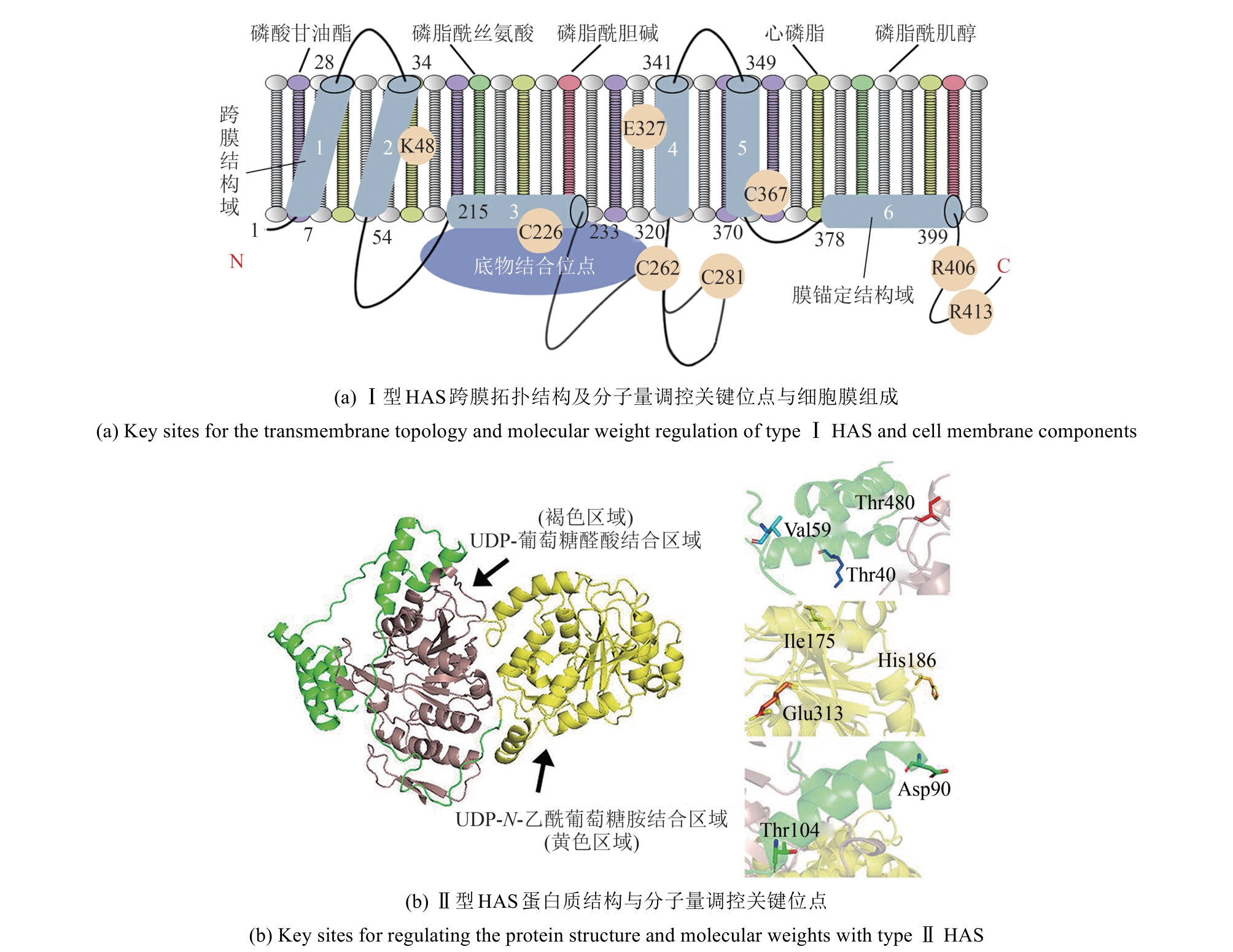
| 4 | KUMARI K, BAGGENSTOSS B A, PARKER A L, et al. Mutation of two intramembrane polar residues conserved within the hyaluronan synthase family alters hyaluronan product size[J]. Journal of Biological Chemistry, 2006, 281(17): 11755-11760. |
| 5 | NECAS J, BARTOSIKOVA L, BRAUNER P, et al. Hyaluronic acid (hyaluronan): a review[J]. Veterinární Medicína, 2008, 53(8): 397-411. |
| 6 | HYNNEKLEIV L, MAGNO M, VERNHARDSDOTTIR R R, et al. Hyaluronic acid in the treatment of dry eye disease[J]. Acta Ophthalmologica, 2022, 100(8): 844-860. |
| 7 | RODRIGUEZ-MARQUEZ C D, ARTEAGA-MARIN S, RIVAS-SÁNCHEZ A, et al. A review on current strategies for extraction and purification of hyaluronic acid[J]. International Journal of Molecular Sciences, 2022, 23(11): 6038. |
| 8 | CHONG B F, BLANK L M, MCLAUGHLIN R, et al. Microbial hyaluronic acid production[J]. Applied Microbiology and Biotechnology, 2005, 66(4): 341-351. |
| 9 | DEANGELIS P L, JING W, DRAKE R R, et al. Identification and molecular cloning of a unique hyaluronan synthase from Pasteurella multocida [J]. Journal of Biological Chemistry, 1998, 273(14): 8454-8458. |
| 10 | ZHANG Y J, DONG J J, XU G C, et al. Efficient production of hyaluronic acid by Streptococcus zooepidemicus using two-stage semi-continuous fermentation[J]. Bioresource Technology, 2023, 377: 128896. |
| 11 | LIU L, LIU Y F, LI J H, et al. Microbial production of hyaluronic acid: current state, challenges, and perspectives[J]. Microbial Cell Factories, 2011, 10: 99. |
| 12 | ZHANG X Z, WANG M, LI T J, et al. Construction of efficient Streptococcus zooepidemicus strains for hyaluoronic acid production based on identification of key genes involved in sucrose metabolism[J]. AMB Express, 2016, 6(1): 121. |
| 13 | PIRES A M B, SANTANA M H A. Metabolic effects of the initial glucose concentration on microbial production of hyaluronic acid[J]. Applied Biochemistry and Biotechnology, 2010, 162(6): 1751-1761. |
| 14 | WEI M M, HUANG Y, ZHU J Y, et al. Advances in hyaluronic acid production: biosynthesis and genetic engineering strategies based on Streptococcus: a review[J]. International Journal of Biological Macromolecules, 2024, 270: 132334. |
| 15 | MENAA F, MENAA A, MENAA B. Hyaluronic acid and derivatives for tissue engineering[J]. Journal of Biotechnology & Biomaterials, 2013, S3: 001. |
| 16 | BUFFA R, NEŠPOROVÁ K, BASARABOVÁ I, et al. Synthesis and study of branched hyaluronic acid with potential anticancer activity[J]. Carbohydrate Polymers, 2019, 223: 115047. |
| 17 | DOSIO F, ARPICCO S, STELLA B, et al. Hyaluronic acid for anticancer drug and nucleic acid delivery[J]. Advanced Drug Delivery Reviews, 2016, 97: 204-236. |
| 18 | KIM J H, MOON M J, KIM D Y, et al. Hyaluronic acid-based nanomaterials for cancer therapy[J]. Polymers, 2018, 10(10): 1133. |
| 19 | PEDRON S, WOLTER G L, CHEN J W E, et al. Hyaluronic acid-functionalized gelatin hydrogels reveal extracellular matrix signals temper the efficacy of erlotinib against patient-derived glioblastoma specimens[J]. Biomaterials, 2019, 219: 119371. |
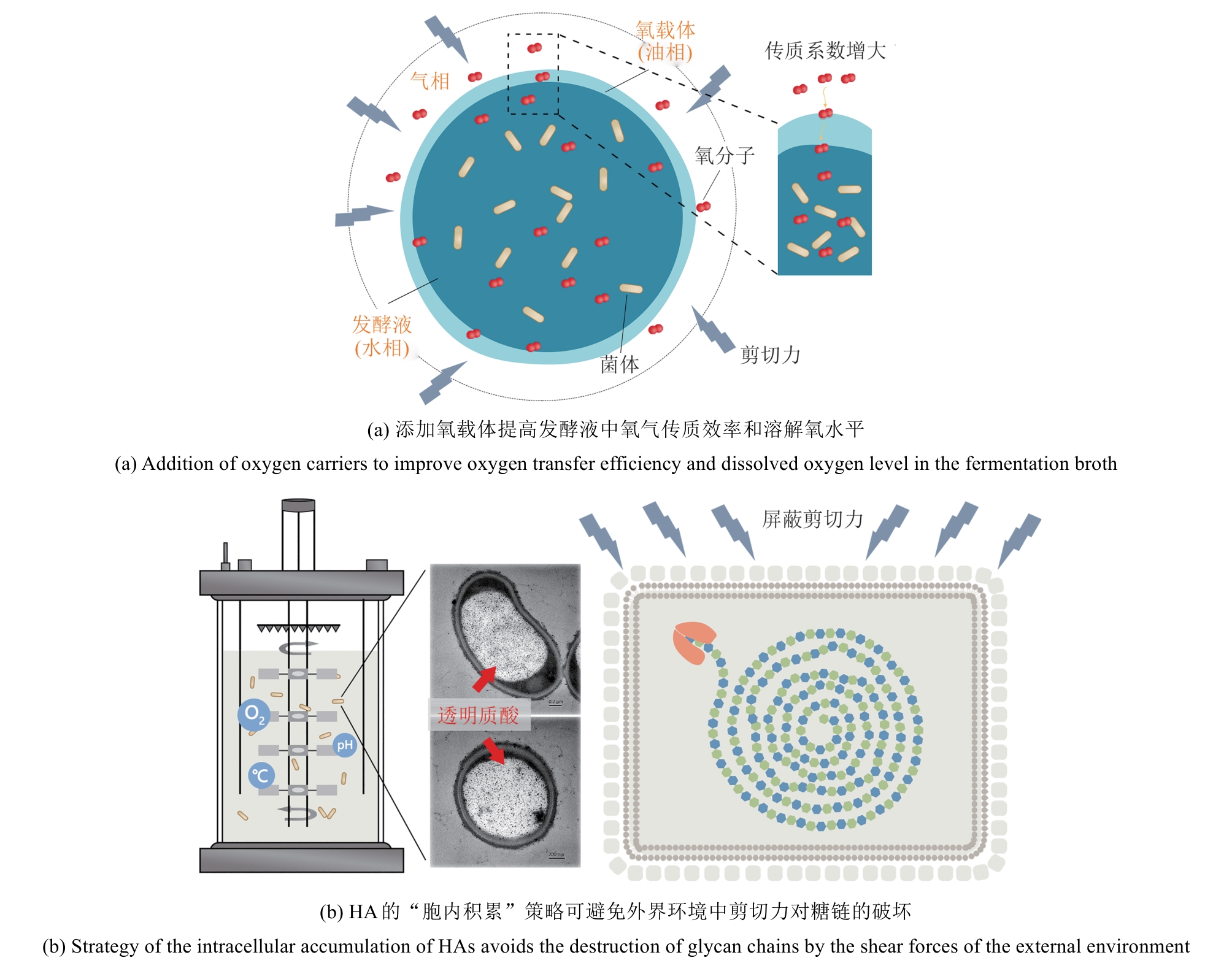
| 20 | LU J F, ZHU Y, SUN H L, et al. Highly efficient production of hyaluronic acid by Streptococcus zooepidemicus R42 derived from heterologous expression of bacterial haemoglobin and mutant selection[J]. Letters in Applied Microbiology, 2016, 62(4): 316-322. |
| 21 | KIMURA M, MAESHIMA T, KUBOTA T, et al. Absorption of orally administered hyaluronan[J]. Journal of Medicinal Food, 2016, 19(12): 1172-1179. |
| 22 | TIAN X, AZPURUA J, HINE C, et al. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat[J]. Nature, 2013, 499(7458): 346-349. |
| 23 | TAKASUGI M, FIRSANOV D, TOMBLINE G, et al. Naked mole-rat very-high-molecular-mass hyaluronan exhibits superior cytoprotective properties[J]. Nature Communications, 2020, 11(1): 2376. |
| 24 | LAGUNAS-RANGEL F A. Naked mole-rat hyaluronan[J]. Biochimie, 2024, 220: 58-66. |
| 25 | DEL MARMOL D, HOLTZE S, KICHLER N, et al. Abundance and size of hyaluronan in naked mole-rat tissues and plasma[J]. Scientific Reports, 2021, 11(1): 7951. |
| 26 | RANKIN K S, FRANKEL D. Hyaluronan in cancer-from the naked mole rat to nanoparticle therapy[J]. Soft Matter, 2016, 12(17): 3841-3848. |
| 27 | BUKHARI S N A, ROSWANDI N L, WAQAS M, et al. Hyaluronic acid, a promising skin rejuvenating biomedicine: a review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects[J]. International Journal of Biological Macromolecules, 2018, 120: 1682-1695. |
| 28 | DOUSE-DEAN T, JACOB C I. Fast and easy treatment for reduction of the Tyndall effect secondary to cosmetic use of hyaluronic acid[J]. Journal of Drugs in Dermatology, 2008, 7(3): 281-283. |
| 29 | XUE Y, CHEN H Y, XU C, et al. Synthesis of hyaluronic acid hydrogels by crosslinking the mixture of high-molecular-weight hyaluronic acid and low-molecular-weight hyaluronic acid with 1,4-butanediol diglycidyl ether[J]. RSC Advances, 2020, 10(12): 7206-7213. |
| 30 | ANDRADE DEL OLMO J, ALONSO J M, SÁEZ MARTÍNEZ V, et al. Biocompatible hyaluronic acid-divinyl sulfone injectable hydrogels for sustained drug release with enhanced antibacterial properties against Staphylococcus aureus [J]. Materials Science and Engineering: C, 2021, 125: 112102. |
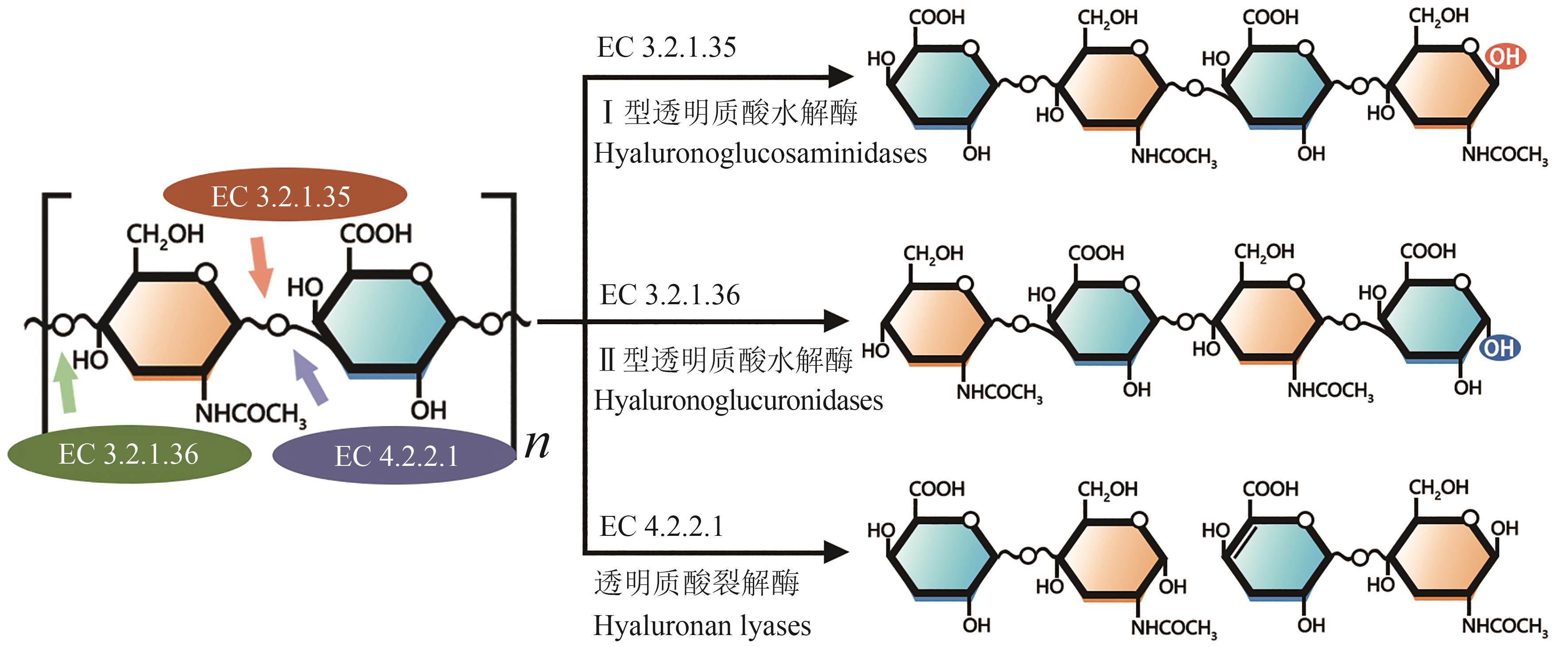
| 31 | HUERTA-ÁNGELES G, ONDREÁŠ F, BRANDEJSOVÁ M, et al. Formulation of hyaluronan grafted with dodecanoic acid as a potential ophthalmic treatment[J]. Carbohydrate Polymers, 2020, 246: 116578. |
| 32 | MARINHO A, NUNES C, REIS S. Hyaluronic acid: a key ingredient in the therapy of inflammation[J]. Biomolecules, 2021, 11(10): 1518. |
| 33 | DONG Y F, ARIF A, OLSSON M, et al. Endotoxin free hyaluronan and hyaluronan fragments do not stimulate TNF-α, interleukin-12 or upregulate co-stimulatory molecules in dendritic cells or macrophages[J]. Scientific Reports, 2016, 6: 36928. |
| 34 | AUDAM T N, DASSANAYAKA S, JURKOVIC A, et al. Abstract 823: the extracellular matrix component, hyaluronan, provokes a pro-inflammatory phenotype in macrophages[J]. Circulation Research, 2019, 125(S1): 823. |
| 35 | POHLIG F, GUELL F, LENZE U, et al. Hyaluronic acid suppresses the expression of metalloproteinases in osteoarthritic cartilage stimulated simultaneously by interleukin 1β and mechanical load[J]. PLoS One, 2016, 11(3): e0150020. |
| 36 | DU W L, YANG X P, HE S F, et al. Novel hyaluronic acid oligosaccharide-loaded and CD44v6-targeting oxaliplatin nanoparticles for the treatment of colorectal cancer[J]. Drug Delivery, 2021, 28(1): 920-929. |
| 37 | CUI Y L, WANG F, VOORHEES J J, et al. Rejuvenation of aged human skin by injection of cross-linked hyaluronic acid[J]. Plastic and Reconstructive Surgery, 2021, 147(1S-2): 43S-49S. |
| 38 | JANG M, BAEK S, KANG G, et al. Dissolving microneedle with high molecular weight hyaluronic acid to improve skin wrinkles, dermal density and elasticity[J]. International Journal of Cosmetic Science, 2020, 42(3): 302-309. |
| 39 | MISRA S, HASCALL V C, MARKWALD R R, et al. Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer[J]. Frontiers in Immunology, 2015, 6: 201. |
| 40 | BHATTACHARYA D, SVECHKAREV D, SOUCHEK J J, et al. Impact of structurally modifying hyaluronic acid on CD44 interaction[J]. Journal of Materials Chemistry B, 2017, 5(41): 8183-8192. |
| 41 | KHEGAI I I. Hyaluronan metabolism and tumor progression[J]. Russian Journal of Bioorganic Chemistry, 2022, 48(5): 896-905. |
| 42 | CAMACHO K M, MENEGATTI S, MITRAGOTRI S. Low-molecular-weight polymer-drug conjugates for synergistic anticancer activity of camptothecin and doxorubicin combinations[J]. Nanomedicine, 2016, 11(9): 1139-1151. |
| 43 | PASSI A, VIGETTI D. Hyaluronan as tunable drug delivery system[J]. Advanced Drug Delivery Reviews, 2019, 146: 83-96. |
| 44 | WOO J E, SEONG H J, LEE S Y, et al. Metabolic engineering of Escherichia coli for the production of hyaluronic acid from glucose and galactose[J]. Frontiers in Bioengineering and Biotechnology, 2019, 7: 351. |
| 45 | YU H M, STEPHANOPOULOS G. Metabolic engineering of Escherichia coli for biosynthesis of hyaluronic acid[J]. Metabolic Engineering, 2008, 10(1): 24-32. |
| 46 | LAI Z W, ABDUL RAHIM R, ARIFF A B, et al. Comparison of hyaluronic acid biosynthesis by the recombinant Escherichia coli strains in different mode of bioreactor operation[J]. Journal of Microbiology, Biotechnology and Food Sciences, 2016, 6(3): 905-910. |
| 47 | YU H M, TYO K, ALPER H, et al. A high-throughput screen for hyaluronic acid accumulation in recombinant Escherichia coli transformed by libraries of engineered sigma factors[J]. Biotechnology and Bioengineering, 2008, 101(4): 788-796. |
| 48 | MAO Z C, SHIN H D, CHEN R. A recombinant E. coli bioprocess for hyaluronan synthesis[J]. Applied Microbiology and Biotechnology, 2009, 84(1): 63-69. |
| 49 | SCHULTE S, DOSS S S, JEEVA P, et al. Exploiting the diversity of streptococcal hyaluronan synthases for the production of molecular weight-tailored hyaluronan[J]. Applied Microbiology and Biotechnology, 2019, 103(18): 7567-7581. |
| 50 | PUVENDRAN K, JAYARAMAN G. Enhancement of acetyl-CoA by acetate co-utilization in recombinant Lactococcus lactis cultures enables the production of high molecular weight hyaluronic acid[J]. Applied Microbiology and Biotechnology, 2019, 103(17): 6989-7001. |
| 51 | JEEVA P, SHANMUGA DOSS S, SUNDARAM V, et al. Production of controlled molecular weight hyaluronic acid by glucostat strategy using recombinant Lactococcus lactis cultures[J]. Applied Microbiology and Biotechnology, 2019, 103(11): 4363-4375. |
| 52 | SUNGUROĞLU C, SEZGIN D E, AYTAR ÇELIK P, et al. Higher titer hyaluronic acid production in recombinant Lactococcus lactis [J]. Preparative Biochemistry & Biotechnology, 2018, 48(8): 734-742. |
| 53 | LI Y Y, LI G Q, ZHAO X, et al. Regulation of hyaluronic acid molecular weight and titer by temperature in engineered Bacillus subtilis [J]. 3 Biotech, 2019, 9(6): 225. |
| 54 | WESTBROOK A W, REN X, OH J, et al. Metabolic engineering to enhance heterologous production of hyaluronic acid in Bacillus subtilis [J]. Metabolic Engineering, 2018, 47: 401-413. |
| 55 | WIDNER B, BEHR R, VON DOLLEN S, et al. Hyaluronic acid production in Bacillus subtilis [J]. Applied and Environmental Microbiology, 2005, 71(7): 3747-3752. |
| 56 | WESTBROOK A W, REN X, MOO-YOUNG M, et al. Engineering of cell membrane to enhance heterologous production of hyaluronic acid in Bacillus subtilis [J]. Biotechnology and Bioengineering, 2018, 115(1): 216-231. |
| 57 | JIA Y N, ZHU J, CHEN X F, et al. Metabolic engineering of Bacillus subtilis for the efficient biosynthesis of uniform hyaluronic acid with controlled molecular weights[J]. Bioresource Technology, 2013, 132: 427-431. |
| 58 | CHIEN L J, LEE C K. Enhanced hyaluronic acid production in Bacillus subtilis by coexpressing bacterial hemoglobin[J]. Biotechnology Progress, 2007, 23(5): 1017-1022. |
| 59 | JEONG E J, SHIM W Y, KIM J H. Metabolic engineering of Pichia pastoris for production of hyaluronic acid with high molecular weight[J]. Journal of Biotechnology, 2014, 185: 28-36. |
| 60 | CHENG F Y, YU H M, STEPHANOPOULOS G. Engineering Corynebacterium glutamicum for high-titer biosynthesis of hyaluronic acid[J]. Metabolic Engineering, 2019, 55: 276-289. |
| 61 | CHENG F Y, GONG Q Y, YU H M, et al. High-titer biosynthesis of hyaluronic acid by recombinant Corynebacterium glutamicum [J]. Biotechnology Journal, 2016, 11(4): 574-584. |
| 62 | HOFFMANN J, ALTENBUCHNER J. Hyaluronic acid production with Corynebacterium glutamicum: effect of media composition on yield and molecular weight[J]. Journal of Applied Microbiology, 2014, 117(3): 663-678. |
| 63 | CHENG F Y, LUOZHONG S J, GUO Z G, et al. Enhanced biosynthesis of hyaluronic acid using engineered Corynebacterium glutamicum via metabolic pathway regulation[J]. Biotechnology Journal, 2017, 12(10): 1700191. |
| 64 | WANG Y, HU L T, HUANG H, et al. Eliminating the capsule-like layer to promote glucose uptake for hyaluronan production by engineered Corynebacterium glutamicum [J]. Nature Communications, 2020, 11(1): 3120. |
| 65 | UCM R, AEM M, LHB Z, et al. Comprehensive review on biotechnological production of hyaluronic acid: status, innovation, market and applications[J]. Bioengineered, 2022, 13(4): 9645-9661. |
| 66 | WANG J, HE W, WANG T, et al. Sucrose-modified iron nanoparticles for highly efficient microbial production of hyaluronic acid by Streptococcus zooepidemicus [J]. Colloids and Surfaces B, Biointerfaces, 2021, 205: 111854. |
| 67 | SUN X Q, WANG Z, BI Y L, et al. Genetic and functional characterization of the hyaluronate lyase HylB and the beta-N-acetylglucosaminidase HylZ in Streptococcus zooepidemicus [J]. Current Microbiology, 2015, 70(1): 35-42. |
| 68 | ZAKERI A, RASAEE M J, POURZARDOSHT N. Enhanced hyluronic acid production in Streptococcus zooepidemicus by over expressing HasA and molecular weight control with niscin and glucose[J]. Biotechnology Reports, 2017, 16: 65-70. |
| 69 | MOHAN N, TADI S R R, PAVAN S S, et al. Deciphering the role of dissolved oxygen and N-acetyl glucosamine in governing higher molecular weight hyaluronic acid synthesis in Streptococcus zooepidemicus cell factory[J]. Applied Microbiology and Biotechnology, 2020, 104(8): 3349-3365. |
| 70 | CHAHUKI F F, AMINZADEH S, JAFARIAN V, et al. Hyaluronic acid production enhancement via genetically modification and culture medium optimization in Lactobacillus acidophilus [J]. International Journal of Biological Macromolecules, 2019, 121: 870-881. |
| 71 | YOSHIMURA T, SHIBATA N, HAMANO Y, et al. Heterologous production of hyaluronic acid in an ε-poly-L-lysine producer, Streptomyces albulus [J]. Applied and Environmental Microbiology, 2015, 81(11): 3631-3640. |
| 72 | JIN P, KANG Z, YUAN P H, et al. Production of specific-molecular-weight hyaluronan by metabolically engineered Bacillus subtilis 168[J]. Metabolic Engineering, 2016, 35: 21-30. |
| 73 | CHEN W Y, MARCELLIN E, HUNG J, et al. Hyaluronan molecular weight is controlled by UDP-N-acetylglucosamine concentration in Streptococcus zooepidemicus [J]. Journal of Biological Chemistry, 2009, 284(27): 18007-18014. |
| 74 | WEIGEL P H, HASCALL V C, TAMMI M. Hyaluronan synthases[J]. Journal of Biological Chemistry, 1997, 272(22): 13997-14000. |
| 75 | JOHNS M R, GOH L T, OEGGERLI A. Effect of pH, agitation and aeration on hyaluronic acid production by Streptococcus zooepidemicus [J]. Biotechnology Letters, 1994, 16(5): 507-512. |
| 76 | LI Y Y, SHI Z Z, SHAO Y Z, et al. Temperature-controlled molecular weight of hyaluronic acid produced by engineered Bacillus subtilis [J]. Biotechnology Letters, 2021, 43(1): 271-277. |
| 77 | HUANG W C, CHEN S J, CHEN T L. The role of dissolved oxygen and function of agitation in hyaluronic acid fermentation[J]. Biochemical Engineering Journal, 2006, 32(3): 239-243. |
| 78 | SKANDALIS S S, KARALIS T, HELDIN P. Intracellular hyaluronan: importance for cellular functions[J]. Seminars in Cancer Biology, 2020, 62: 20-30. |
| 79 | HMAR R V, PRASAD S B, JAYARAMAN G, et al. Chromosomal integration of hyaluronic acid synthesis (has) genes enhances the molecular weight of hyaluronan produced in Lactococcus lactis [J]. Biotechnology Journal, 2014, 9(12): 1554-1564. |
| 80 | AGARWAL G, K V K, PRASAD S B, et al. Biosynthesis of hyaluronic acid polymer: dissecting the role of sub structural elements of hyaluronan synthase[J]. Scientific Reports, 2019, 9(1): 12510. |
| 81 | MALONEY F P, KUKLEWICZ J, COREY R A, et al. Structure, substrate recognition and initiation of hyaluronan synthase[J]. Nature, 2022, 604(7904): 195-201. |
| 82 | HUBBARD C, MCNAMARA J T, AZUMAYA C, et al. The hyaluronan synthase catalyzes the synthesis and membrane translocation of hyaluronan[J]. Journal of Molecular Biology, 2012, 418(1-2): 21-31. |
| 83 | WEIGEL P H, BAGGENSTOSS B A. Hyaluronan synthase polymerizing activity and control of product size are discrete enzyme functions that can be uncoupled by mutagenesis of conserved cysteines[J]. Glycobiology, 2012, 22(10): 1302-1310. |
| 84 | YANG J, CHENG F Y, YU H M, et al. Key role of the carboxyl terminus of hyaluronan synthase in processive synthesis and size control of hyaluronic acid polymers[J]. Biomacromolecules, 2017, 18(4): 1064-1073. |
| 85 | MANDAWE J, INFANZON D B, EISELE A, et al. Directed evolution of hyaluronic acid synthase from Pasteurella multocida towards high-molecular-weight hyaluronic acid[J]. ChemBioChem, 2018, 19(13): 1414-1423. |
| 86 | DUAN X J, NIU H X, TAN W S, et al. Mechanism analysis of effect of oxygen on molecular weight of hyaluronic acid produced by Streptococcus zooepidemicus [J]. Journal of Microbiology and Biotechnology, 2009, 19(3): 299-306. |
| 87 | LAI Z W, RAHIM R A, ARIFF A B, et al. Biosynthesis of high molecular weight hyaluronic acid by Streptococcus zooepidemicus using oxygen vector and optimum impeller tip speed[J]. Journal of Bioscience and Bioengineering, 2012, 114(3): 286-291. |
| 88 | ABDULLAH THAIDI N I, MOHAMAD R, WASOH H, et al. Development of in situ product recovery (ISPR) system using amberlite IRA67 for enhanced biosynthesis of hyaluronic acid by Streptococcus zooepidemicus [J]. Life, 2023, 13(2): 558. |
| 89 | HU L T, WANG Y, HU Y X, et al. Biosynthesis of non-sulfated high-molecular-weight glycosaminoglycans and specific-sized oligosaccharides[J]. Carbohydrate Polymers, 2022, 295: 119829. |
| 90 | ZHANG X, DUAN X J, TAN W S. Mechanism for the effect of agitation on the molecular weight of hyaluronic acid produced by Streptococcus zooepidemicus [J]. Food Chemistry, 2010, 119(4): 1643-1646. |
| 91 | WU Y. Preparation of low-molecular-weight hyaluronic acid by ozone treatment[J]. Carbohydrate Polymers, 2012, 89(2): 709-712. |
| 92 | KARAMI M, SHAHRAKY M K, RANJBAR M, et al. Preparation, purification, and characterization of low-molecular-weight hyaluronic acid[J]. Biotechnology Letters, 2021, 43(1): 133-142. |
| 93 | PANG B, WANG H, HUANG H, et al. Enzymatic production of low-molecular-weight hyaluronan and its oligosaccharides: a review and prospects[J]. Journal of Agricultural and Food Chemistry, 2022, 70(44): 14129-14139. |
| 94 | GURA E, HÜCKEL M, MÜLLER P J. Specific degradation of hyaluronic acid and its rheological properties[J]. Polymer Degradation and Stability, 1998, 59(1-3): 297-302. |
| 95 | HUANG Y C, HUANG K Y, LEW W Z, et al. Gamma-irradiation-prepared low molecular weight hyaluronic acid promotes skin wound healing[J]. Polymers, 2019, 11(7): 1214. |
| 96 | CHEN H Y, QIN J, HU Y. Efficient degradation of high-molecular-weight hyaluronic acid by a combination of ultrasound, hydrogen peroxide, and copper ion[J]. Molecules, 2019, 24(3): 617. |
| 97 | HUANG H, LIANG Q X, WANG Y, et al. High-level constitutive expression of leech hyaluronidase with combined strategies in recombinant Pichia pastoris [J]. Applied Microbiology and Biotechnology, 2020, 104(4): 1621-1632. |
| 98 | JIN P, KANG Z, ZHANG N, et al. High-yield novel leech hyaluronidase to expedite the preparation of specific hyaluronan oligomers[J]. Scientific Reports, 2014, 4: 4471. |
| 99 | WANG L, LIU Q Q, GONG X, et al. Cloning and biochemical characterization of a hyaluronate lyase from Bacillus sp. CQMU-D[J]. Journal of Microbiology and Biotechnology, 2023, 33(2): 235-241. |
| 100 | LI Y J, ZHANG S L, WU H, et al. Biochemical characterization of a thermophilic hyaluronate lyase TcHly8C from Thermasporomyces composti DSM22891[J]. International Journal of Biological Macromolecules, 2020, 165: 1211-1218. |
| 101 | NGUYEN V A, OGURA K, MATSUE M, et al. Novel hyaluronate lyase involved in pathogenicity of Streptococcus dysgalactiae subsp. equisimilis [J]. Frontiers in Microbiology, 2020, 11: 552418. |
| 102 | NEHMÉ R, NASREDDINE R, ORLIC L, et al. Kinetic theory of hyaluronan cleavage by bovine testicular hyaluronidase in standard and crowded environments[J]. Biochimica et Biophysica Acta (BBA) - General Subjects, 2021, 1865(3): 129837. |
| 103 | LIU L, DU G C, CHEN J, et al. Influence of hyaluronidase addition on the production of hyaluronic acid by batch culture of Streptococcus zooepidemicus [J]. Food Chemistry, 2008, 110(4): 923-926. |
| 104 | HE J, HUANG H, ZOU X P, et al. Construction of saturated odd- and even-numbered hyaluronan oligosaccharide building block library[J]. Carbohydrate Polymers, 2020, 231: 115700. |
| [1] | WU Ke, LUO Jiahao, LI Feiran. Applications of machine learning in the reconstruction and curation of genome-scale metabolic models [J]. Synthetic Biology Journal, 2025, 6(3): 566-584. |
| [2] | TIAN Xiao-jun, ZHANG Rixin. “Economics Paradox” with cells in synthetic gene circuits [J]. Synthetic Biology Journal, 2025, 6(3): 532-546. |
| [3] | LI Yongzhu, CHEN Yu. Advances and prospects in genome-scale models of yeast [J]. Synthetic Biology Journal, 2025, 6(3): 585-602. |
| [4] | ZHANG Yiqing, LIU Gaowen. Exploration of gene functions and library construction for engineering strains from a synthetic biology perspective [J]. Synthetic Biology Journal, 2025, 6(3): 685-700. |
| [5] | YANG Ying, LI Xia, LIU Lizhong. Applications of synthetic biology to stem-cell-derived modeling of early embryonic development [J]. Synthetic Biology Journal, 2025, 6(3): 669-684. |
| [6] | HUANG Yi, SI Tong, LU Anjing. Standardization for biomanufacturing: global landscape, critical challenges, and pathways forward [J]. Synthetic Biology Journal, 2025, 6(3): 701-714. |
| [7] | SONG Chengzhi, LIN Yihan. AI-enabled directed evolution for protein engineering and optimization [J]. Synthetic Biology Journal, 2025, 6(3): 617-635. |
| [8] | GAO Qi, XIAO Wenhai. Advances in the biosynthesis of monoterpenes by yeast [J]. Synthetic Biology Journal, 2025, 6(2): 357-372. |
| [9] | SHENG Zhouhuang, CHEN Zhixian, ZHANG Yan. Research progress of yeast mannoproteins [J]. Synthetic Biology Journal, 2025, 6(2): 408-421. |
| [10] | ZHANG Mengyao, CAI Peng, ZHOU Yongjin. Synthetic biology drives the sustainable production of terpenoid fragrances and flavors [J]. Synthetic Biology Journal, 2025, 6(2): 334-356. |
| [11] | ZHANG Lu’ou, XU Li, HU Xiaoxu, YANG Ying. Synthetic biology ushers cosmetic industry into the “bio-cosmetics” era [J]. Synthetic Biology Journal, 2025, 6(2): 479-491. |
| [12] | LU Jinchang, WU Yaokang, LV Xueqin, LIU Long, CHEN Jian, LIU Yanfeng. Green biomanufacturing of ceramide sphingolipids [J]. Synthetic Biology Journal, 2025, 6(2): 422-444. |
| [13] | YI Jinhang, TANG Yulin, LI Chunyu, WU Heyun, MA Qian, XIE Xixian. Applications and advances in the research of biosynthesis of amino acid derivatives as key ingredients in cosmetics [J]. Synthetic Biology Journal, 2025, 6(2): 254-289. |
| [14] | WEI Lingzhen, WANG Jia, SUN Xinxiao, YUAN Qipeng, SHEN Xiaolin. Biosynthesis of flavonoids and their applications in cosmetics [J]. Synthetic Biology Journal, 2025, 6(2): 373-390. |
| [15] | WANG Qian, GUO Shiting, XIN Bo, ZHONG Cheng, WANG Yu. Advances in biosynthesis of L-arginine using engineered microorganisms [J]. Synthetic Biology Journal, 2025, 6(2): 290-305. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||