Synthetic Biology Journal ›› 2025, Vol. 6 ›› Issue (2): 391-407.DOI: 10.12211/2096-8280.2024-076
• Invited Review • Previous Articles Next Articles
Research progress in the biosynthesis of salidroside
HUANG Shuhan1, MA He1, LUO Yunzi1,2,3,4
- 1.Frontiers Science Center for Synthetic Biology (Ministry of Education),School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072,China
2.State Key Laboratory of Synthetic Biology,Tianjin University,Tianjin 300072,China
3.Haihe Laboratory of Sustainable Chemical Transformations,Tianjin 300192,China
4.Georgia Tech Shenzhen Institute,Tianjin University,Shenzhen 518071,Guangdong,China
-
Received:2024-10-18Revised:2025-03-09Online:2025-05-20Published:2025-04-30 -
Contact:LUO Yunzi
生物合成红景天苷的研究进展
黄姝涵1, 马赫1, 罗云孜1,2,3,4
- 1.天津大学化工学院,教育部合成生物学前沿科学中心,天津 300072
2.天津大学合成生物技术全国重点实验室,天津 300072
3.物质绿色创造与制造海河实验室,天津 300192
4.天津大学佐治亚理工大学深圳学院,广东 深圳 518071
-
通讯作者:罗云孜 -
作者简介:黄姝涵 (2002—),女,硕士研究生。研究方向为天然产物的生物合成。E-mail:huangshuhan@tju.edu.cn马赫 (1999—),男,博士研究生。研究方向为天然产物的生物合成。E-mail:2021207667@tju.edu.cn罗云孜 (1985—),女,博士,教授,博士生导师。研究方向为合成生物学。E-mail:yunzi.luo@tju.edu.cn
第一联系人:黄姝涵、马赫为共同第一作者 -
基金资助:国家自然科学基金(32471492);物质绿色创造与制造海河实验室(24HHWCSS00006);广东省重点研发计划(2020B0303070002)
CLC Number:
Cite this article
HUANG Shuhan, MA He, LUO Yunzi. Research progress in the biosynthesis of salidroside[J]. Synthetic Biology Journal, 2025, 6(2): 391-407.
黄姝涵, 马赫, 罗云孜. 生物合成红景天苷的研究进展[J]. 合成生物学, 2025, 6(2): 391-407.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2024-076
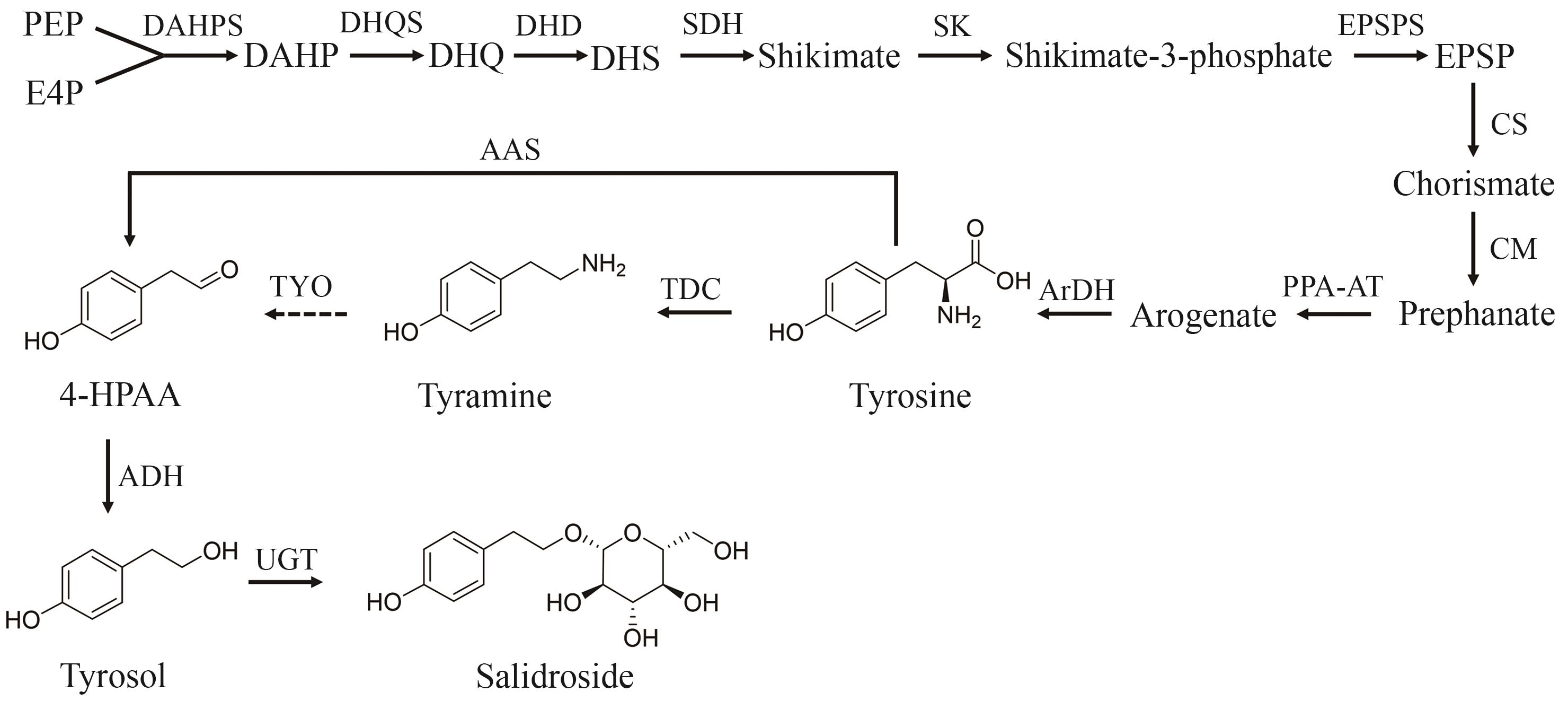
Fig. 1 Biosynthetic pathways of salidroside in plants(PEP—phosphoenolpyruvate; E4P—erythrose-4-phosphate; DAHP—3-deoxy-D-arabino-heptulosonate-7-phosphate; DHQ—3-dehydroquinate; DHS—3-dehydroshikimate; EPSP—5-enolpyruvylshikimate-3-phosphate; 4-HPAA—4-hydroxyphenylacetaldehyde; DAHPS—3-deoxy-D-arabino-heptulosonate-7-phosphate synthase; DHQS—3-dehydroquinate synthase; DHD—3-dehydroquinate dehydratase; SDH—shikimate dehydrogenase; SK—shikimate kinase; EPSPS—5-enolpyruvylshikimate-3-phosphate synthase; CS—chorismate synthase; CM—chorismate mutase; PPA-AT—prephenate aminotransferase; ArDH—arogenate dehydrogenase; TDC—tyrosine decarboxylase; TYO—tyramine oxidase; AAS—aromatic aldehyde synthase; ADH—alcohol dehydrogenase; UGT—uridine diphosphate glycosyltransferase)
| 酶的名称 | 酶的来源植物 | 酶的功能 | 红景天苷产量(mg/L)、干重(mg/g DW)或酶活Kcat/Km[L/(mmol·s)] | 参考文献 |
|---|---|---|---|---|
| Rr4HPAAS | 蔷薇红景天 | 4-羟基苯乙醛合酶 | 11.71 L/(mmol·s) | [ |
| RrUGT29 | 蔷薇红景天 | 糖基转移酶 | 316.04 L/(mmol·s) | [ |
| RrUGT32 | 蔷薇红景天 | 糖基转移酶 | NA | [ |
| RrUGT33 | 蔷薇红景天 | 糖基转移酶 | 420.60 L/(mmol·s) | [ |
| AtUGT73C5 | 拟南芥 | 糖基转移酶 | NA | [ |
| AtUGT73C6 | 拟南芥 | 糖基转移酶 | NA | [ |
| AtUGT85A1 | 拟南芥 | 糖基转移酶 | 288.00 mg/L | [ |
| RsUGT73B6 | 库页红景天 | 糖基转移酶 | 8.76 mg/g DW | [ |
| RsUGT72B14 | 库页红景天 | 糖基转移酶 | 19.81 mg/g DW | [ |
| RsUGT74R1 | 库页红景天 | 糖基转移酶 | 5.72 mg/g DW | [ |
Table 1 Names and sources of enzymes related to salidroside synthesis
| 酶的名称 | 酶的来源植物 | 酶的功能 | 红景天苷产量(mg/L)、干重(mg/g DW)或酶活Kcat/Km[L/(mmol·s)] | 参考文献 |
|---|---|---|---|---|
| Rr4HPAAS | 蔷薇红景天 | 4-羟基苯乙醛合酶 | 11.71 L/(mmol·s) | [ |
| RrUGT29 | 蔷薇红景天 | 糖基转移酶 | 316.04 L/(mmol·s) | [ |
| RrUGT32 | 蔷薇红景天 | 糖基转移酶 | NA | [ |
| RrUGT33 | 蔷薇红景天 | 糖基转移酶 | 420.60 L/(mmol·s) | [ |
| AtUGT73C5 | 拟南芥 | 糖基转移酶 | NA | [ |
| AtUGT73C6 | 拟南芥 | 糖基转移酶 | NA | [ |
| AtUGT85A1 | 拟南芥 | 糖基转移酶 | 288.00 mg/L | [ |
| RsUGT73B6 | 库页红景天 | 糖基转移酶 | 8.76 mg/g DW | [ |
| RsUGT72B14 | 库页红景天 | 糖基转移酶 | 19.81 mg/g DW | [ |
| RsUGT74R1 | 库页红景天 | 糖基转移酶 | 5.72 mg/g DW | [ |
| 名称 | 优点 | 缺点 |
|---|---|---|
| 大肠杆菌 | 应用广泛、适宜天然产物生产[ | 致病性大肠杆菌可能含有毒素[ |
| 成熟的高密度细胞培养技术[ | 缺乏对部分植物来源酶的转录和翻译功能[ | |
| 生长速率快,具有多种系统代谢工程工具和策略[ | ||
| 可以结合和转导转移DNA,遗传物质可以水平转移[ | ||
| 开发了各种蛋白质表达系统,可以通过质粒大规模生产重组蛋白[ | ||
| 酿酒酵母 | 易于基因操作,营养需求简单,无细胞内毒素,安全性高[ | 特征明确的启动子数量不足,动态范围差[ |
| 高分泌能力,在多种碳源上的高生长速率[ | 异源蛋白表达量较少[ | |
| 具有翻译后修饰能力,对噬菌体等传染性病原体不敏感[ | ||
| 遗传易处理性和整体易用性[ |
Table 2 Comparison of different microbial chassis for being engineered with salidroside synthesis
| 名称 | 优点 | 缺点 |
|---|---|---|
| 大肠杆菌 | 应用广泛、适宜天然产物生产[ | 致病性大肠杆菌可能含有毒素[ |
| 成熟的高密度细胞培养技术[ | 缺乏对部分植物来源酶的转录和翻译功能[ | |
| 生长速率快,具有多种系统代谢工程工具和策略[ | ||
| 可以结合和转导转移DNA,遗传物质可以水平转移[ | ||
| 开发了各种蛋白质表达系统,可以通过质粒大规模生产重组蛋白[ | ||
| 酿酒酵母 | 易于基因操作,营养需求简单,无细胞内毒素,安全性高[ | 特征明确的启动子数量不足,动态范围差[ |
| 高分泌能力,在多种碳源上的高生长速率[ | 异源蛋白表达量较少[ | |
| 具有翻译后修饰能力,对噬菌体等传染性病原体不敏感[ | ||
| 遗传易处理性和整体易用性[ |
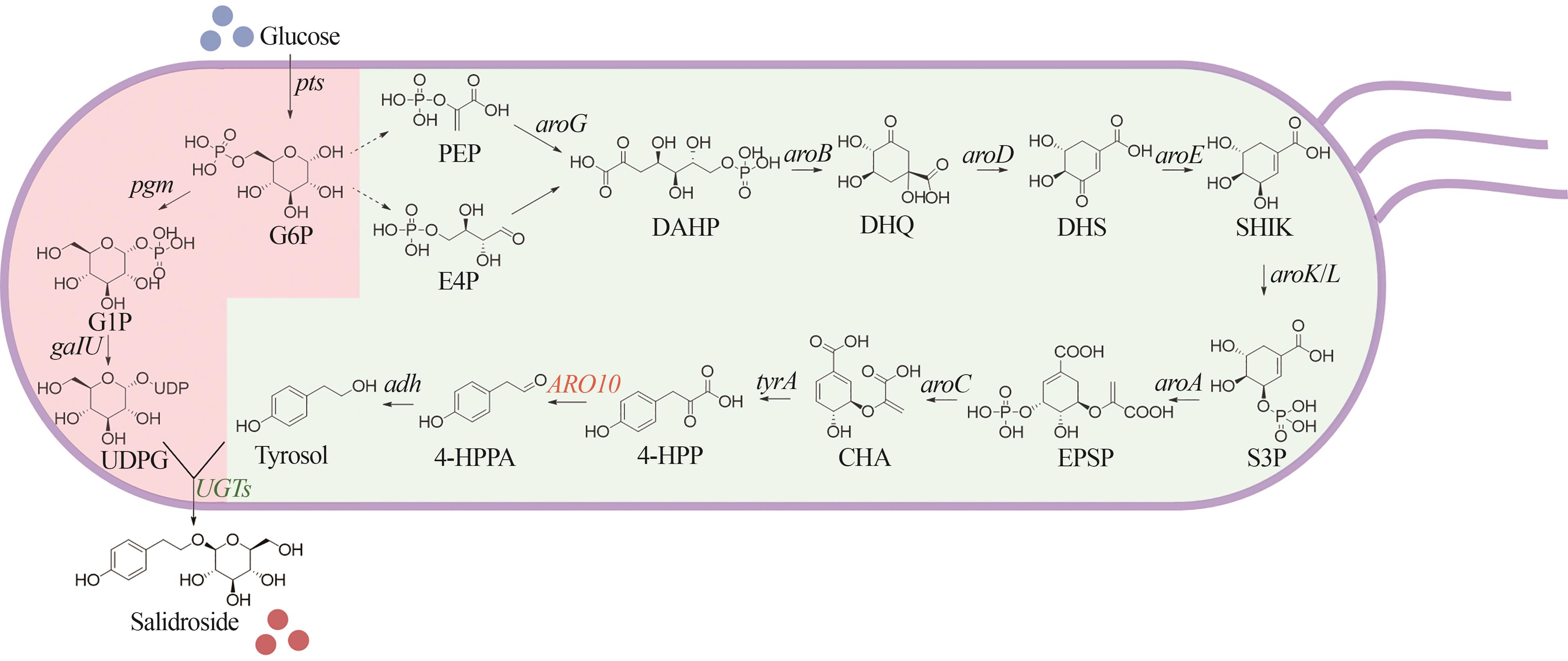
Fig. 2 Biosynthetic pathways of salidroside in Escherichia coli(Compounds and genes are represented by straight and italic letters, respectively. The black letters represent endogenous genes of Escherichia coli, the orange letters represent genes from Saccharomyces cerevisiae, and the green letters represent genes from plants.)
微生物 类别 | 产量 | 发酵方式 | 改造策略 | 参考文献 |
|---|---|---|---|---|
| 大肠杆菌 | 6.70 mg/L | 摇瓶培养 | 高效表达UGT72B14 | [ |
| 56.90 mg/L | 摇瓶培养 | 异源表达红景天来源的UGT73B6、酵母的ARO10,构建共养大肠杆菌,过表达tyrA(tyrA*syn )、aroG(aroG*syn )等酪醇内源合成途径基因,敲除tyrR、pykA等旁路基因 | [ | |
| 288.00 mg/L | 摇瓶培养 | 异源表达AtUGT85A1和PcAAS | [ | |
| 1.04 g/L | 摇瓶培养 | 异源表达地衣芽孢杆菌ZSP01来源的UGTBL1 | [ | |
| 8.17 g/L | 5 L发酵罐 | 构建了UDP-葡萄糖循环再生系统,整合糖基转移酶基因UGT33 | [ | |
| 9.48 g/L | 5 L发酵罐 | 增加大肠杆菌中UGT85A1的拷贝数 | [ | |
| 7.50 g/L | 摇瓶发酵 | 过表达UDP-葡萄糖合成路径中的基因pgm和galU,并利用酶工程策略对糖基转移酶UGT85A1进行改造,整合突变体基因UGT85A1A21G | [ | |
| 16.80 g/L | 5 L发酵罐 | |||
| 酿酒酵母 | 640.00 mg/L | 摇瓶培养 | 整合ARO4K229L 和ARO7T266I 到酵母菌上,过表达TYR1和ARO10,异源表达OsUGT13 | [ |
| 732.50 mg/L | 5 L发酵罐 | 过表达ARO4K229L 、ARO7G141S 、aroL,而后引入PcAASsyn 和AtUGT85A1syn | [ | |
| 1575.45 mg/L | 摇瓶培养 | 过表达RKI1、TKL1、ARO3K222L 、ARO4K229L 、ARO7G141S 突变体、分支酸合成酶ARO2与苯丙氨酸脱羧酶ARO10并敲除酪醇竞争路径中的PDC1、PHA2,整合RrU8GT33 | [ | |
| 26.55 g /L | 5 L发酵罐 | |||
| 1.82 g/L | 3 L发酵罐 | 敲除PDC1、PHA2和TRP3,异源表达PcAAS、EcTyrAM53I/A354V,异源表达Xfpk、UGT85A1 | [ | |
| 2.40 g/L | 摇瓶培养 | 将突变体ARO3D154N 整合到酿酒酵母工程菌中 | [ | |
植物内 生菌 | 2.34 mg/L | 摇瓶培养 | 筛选了347种内生菌,最终获得目标菌株Phialocephala fortinii Rac56,并在此基础上优化了其发酵条件 | [ |
| 混菌培养 | 3.80 g/L | 摇瓶培养 | 酿酒酵母中共表达GmSUS和RrUGT33,大肠杆菌中异源表达AAS,建立共培养体系并优化发酵条件 | [ |
| 6.03 g/L | 5 L发酵罐 | 在两株大肠杆菌中分别表达KDC4和UGT85A1构建酪醇生产菌株与红景天苷生产菌株,并对两种菌株的碳源利用进行优化 | [ |
Table 3 Production of salidroside through microbial biosynthesis and strategies for optimizing associated pathways
微生物 类别 | 产量 | 发酵方式 | 改造策略 | 参考文献 |
|---|---|---|---|---|
| 大肠杆菌 | 6.70 mg/L | 摇瓶培养 | 高效表达UGT72B14 | [ |
| 56.90 mg/L | 摇瓶培养 | 异源表达红景天来源的UGT73B6、酵母的ARO10,构建共养大肠杆菌,过表达tyrA(tyrA*syn )、aroG(aroG*syn )等酪醇内源合成途径基因,敲除tyrR、pykA等旁路基因 | [ | |
| 288.00 mg/L | 摇瓶培养 | 异源表达AtUGT85A1和PcAAS | [ | |
| 1.04 g/L | 摇瓶培养 | 异源表达地衣芽孢杆菌ZSP01来源的UGTBL1 | [ | |
| 8.17 g/L | 5 L发酵罐 | 构建了UDP-葡萄糖循环再生系统,整合糖基转移酶基因UGT33 | [ | |
| 9.48 g/L | 5 L发酵罐 | 增加大肠杆菌中UGT85A1的拷贝数 | [ | |
| 7.50 g/L | 摇瓶发酵 | 过表达UDP-葡萄糖合成路径中的基因pgm和galU,并利用酶工程策略对糖基转移酶UGT85A1进行改造,整合突变体基因UGT85A1A21G | [ | |
| 16.80 g/L | 5 L发酵罐 | |||
| 酿酒酵母 | 640.00 mg/L | 摇瓶培养 | 整合ARO4K229L 和ARO7T266I 到酵母菌上,过表达TYR1和ARO10,异源表达OsUGT13 | [ |
| 732.50 mg/L | 5 L发酵罐 | 过表达ARO4K229L 、ARO7G141S 、aroL,而后引入PcAASsyn 和AtUGT85A1syn | [ | |
| 1575.45 mg/L | 摇瓶培养 | 过表达RKI1、TKL1、ARO3K222L 、ARO4K229L 、ARO7G141S 突变体、分支酸合成酶ARO2与苯丙氨酸脱羧酶ARO10并敲除酪醇竞争路径中的PDC1、PHA2,整合RrU8GT33 | [ | |
| 26.55 g /L | 5 L发酵罐 | |||
| 1.82 g/L | 3 L发酵罐 | 敲除PDC1、PHA2和TRP3,异源表达PcAAS、EcTyrAM53I/A354V,异源表达Xfpk、UGT85A1 | [ | |
| 2.40 g/L | 摇瓶培养 | 将突变体ARO3D154N 整合到酿酒酵母工程菌中 | [ | |
植物内 生菌 | 2.34 mg/L | 摇瓶培养 | 筛选了347种内生菌,最终获得目标菌株Phialocephala fortinii Rac56,并在此基础上优化了其发酵条件 | [ |
| 混菌培养 | 3.80 g/L | 摇瓶培养 | 酿酒酵母中共表达GmSUS和RrUGT33,大肠杆菌中异源表达AAS,建立共培养体系并优化发酵条件 | [ |
| 6.03 g/L | 5 L发酵罐 | 在两株大肠杆菌中分别表达KDC4和UGT85A1构建酪醇生产菌株与红景天苷生产菌株,并对两种菌株的碳源利用进行优化 | [ |
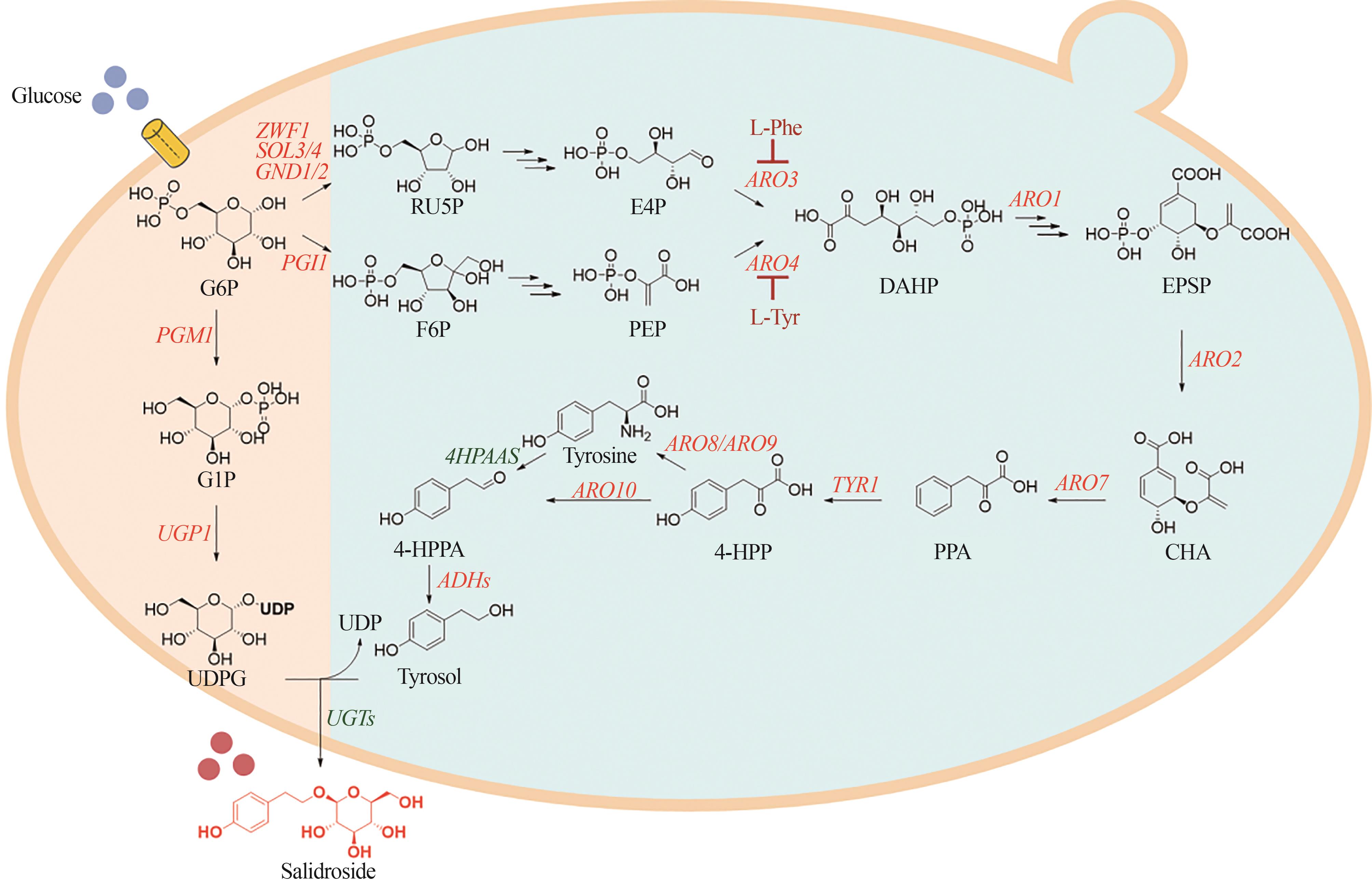
Fig.3 Biosynthetic pathways of salidroside in Saccharomyces cerevisiae(The orange letters represent the endogenous genes of S. cerevisiae, and the green letters represent the plant-derived genes.)
| 1 | PRZYBYŁ J L, WĘGLARZ Z, GESZPRYCH A. Quality of Rhodiola rosea cultivated in Poland[J]. Acta Horticulturae, 2008(765): 143-150. |
| 2 | TASHEVA K, KOSTURKOVA G. Bulgarian golden root in vitro cultures for micropropagation and reintroduction[J]. Central European Journal of Biology, 2010, 5(6): 853-863. |
| 3 | WĘGLARZ Z, PRZYBYŁ J L, GESZPRYCH A. Roseroot (Rhodiola rosea L.): effect of internal and external factors on accumulation of biologically active compounds[M/OL]//RAMAWAT K, MERILLON, J. Bioactive molecules and medicinal plants. Springer: Berlin, Heidelberg, 2008: 297-315 [2024-07-01]. . |
| 4 | XIONG Y L, WANG Y M, XIONG Y L, et al. Protective effect of salidroside on hypoxia-related liver oxidative stress and inflammation via Nrf2 and JAK2/STAT3 signaling pathways[J]. Food Science & Nutrition, 2021, 9(9): 5060-5069. |
| 5 | LIU Q, CHEN J Z, ZENG A Q, et al. Pharmacological functions of salidroside in renal diseases: facts and perspectives[J]. Frontiers in Pharmacology, 2024, 14: 1309598. |
| 6 | ZHANG X M, XIE L, LONG J Y, et al. Salidroside: a review of its recent advances in synthetic pathways and pharmacological properties[J]. Chemico-Biological Interactions, 2021, 339: 109268. |
| 7 | XU Z W, CHEN X, JIN X H, et al. SILAC-based proteomic analysis reveals that salidroside antagonizes cobalt chloride-induced hypoxic effects by restoring the tricarboxylic acid cycle in cardiomyocytes[J]. Journal of Proteomics, 2016, 130: 211-220. |
| 8 | CHEN X P, KOU Y B, LU Y S, et al. Salidroside ameliorated hypoxia-induced tumorigenesis of BxPC-3 cells via downregulating hypoxia-inducible factor (HIF)-1α and LOXL2[J]. Journal of Cellular Biochemistry, 2020, 121(1): 165-173. |
| 9 | PAN C L, DAI G L, ZHANG H W, et al. Salidroside ameliorates orthopedic surgery-induced cognitive dysfunction by activating adenosine 5′-monophosphate-activated protein kinase signaling in mice[J]. European Journal of Pharmacology, 2022, 929: 175148. |
| 10 | LAI W F, LUO R, TANG Y H, et al. Salidroside directly activates HSC70, revealing a new role for HSC70 in BDNF signalling and neurogenesis after cerebral ischemia[J]. Phytotherapy Research, 2024, 38(6): 2619-2640. |
| 11 | SHI T Y, CHEN H, JING L L, et al. Development of a kilogram-scale synthesis of salidroside and its analogs[J]. Synthetic Communications, 2011, 41(17): 2594-2600. |
| 12 | TROSHCHENKO A T, YUODVIRSHIS A M. Synthesis of glycosides of 2-(p-hydroxyphenyl)ethanol (tyrosol)[J]. Chemistry of Natural Compounds, 1969, 5(4): 217-220. |
| 13 | 史明明. 红景天苷的合成方法研究[D]. 长沙: 湖南师范大学, 2012. |
| SHI M M. Study on synthetic methods of salidroside[D]. Changsha: Hunan Normal University, 2012. | |
| 14 | JIANG J J, YIN H, WANG S, et al. Metabolic engineering of Saccharomyces cerevisiae for high-level production of salidroside from glucose[J]. Journal of Agricultural and Food Chemistry, 2018, 66(17): 4431-4438. |
| 15 | LI Y Z, WU A P, WANG D D, et al. Salidroside attenuates oxygen and glucose deprivation-induced neuronal injury by inhibiting ferroptosis[J]. Asian Pacific Journal of Tropical Biomedicine, 2023, 13(2): 70-79. |
| 16 | LIU Y J, WANG J Y, WANG L, et al. Biosynthesis and biotechnological production of salidroside from Rhodiola genus plants[J]. Phytochemistry Reviews, 2022, 21(5): 1605-1626. |
| 17 | BERNATONIENE J, JAKSTAS V, KOPUSTINSKIENE D M. Phenolic compounds of Rhodiola rosea L. as the potential alternative therapy in the treatment of chronic diseases[J]. International Journal of Molecular Sciences, 2023, 24(15): 12293. |
| 18 | YAO Y Y, REN Z C, YANG R H, et al. Salidroside reduces neuropathology in Alzheimer’s disease models by targeting NRF2/SIRT3 pathway[J]. Cell & Bioscience, 2022, 12(1): 180. |
| 19 | LI L P, FU W L, WANG J, et al. Salidroside improves cellular senescence in COPD by inhibiting the JAK2/STAT3 pathway[J]. Journal of Biological Regulators and Homeostatic Agents, 2024, 38(5): 4415-4426. |
| 20 | KIM K J, JUNG Y S, YOU D M, et al. Neuroprotective effects of ethanolic extract from dry Rhodiola rosea L. rhizomes[J]. Food Science and Biotechnology, 2021, 30(2): 287-297. |
| 21 | DING X R, WANG W L, CHEN J W, et al. Salidroside protects inner ear hair cells and spiral ganglion neurons from manganese exposure by regulating ROS levels and inhibiting apoptosis[J]. Toxicology Letters, 2019, 310: 51-60. |
| 22 | LIU B, WEI H L, LAN M, et al. microRNA-21 mediates the protective effects of salidroside against hypoxia/reoxygenation-induced myocardial oxidative stress and inflammatory response[J]. Experimental and Therapeutic Medicine, 2020, 19(3): 1655-1664. |
| 23 | LUO X P, LIAO H, PENG J, et al. Salidroside protects chondrocytes against IL-1β-induced injury and alleviates osteoarthritis progression by activating the Nrf2 pathway[J]. Discovery Medicine, 2024, 36(181): 266-277. |
| 24 | LIU S S, YU X W, HU B J, et al. Salidroside rescued mice from experimental sepsis through anti-inflammatory and anti-apoptosis effects[J]. Journal of Surgical Research, 2015, 195(1): 277-283. |
| 25 | MAO G X, DENG H B, YUAN L G, et al. Protective role of salidroside against aging in a mouse model induced by D-galactose[J]. Biomedical and Environmental Sciences, 2010, 23(2): 161-166. |
| 26 | RAMASAMY R, VANNUCCI S J, YAN S S D, et al. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation[J]. Glycobiology, 2005, 15(7): 16R-28R. |
| 27 | ALIKHANI M, MACLELLAN C M, RAPTIS M, et al. Advanced glycation end products induce apoptosis in fibroblasts through activation of ROS, MAP kinases, and the FOXO1 transcription factor[J]. American Journal of Physiology Cell Physiology, 2007, 292(2): C850-C856. |
| 28 | BUCALA R, CERAMI A. Advanced glycosylation: chemistry, biology, and implications for diabetes and aging[J]. Advances in Pharmacology, 1992, 23: 1-34. |
| 29 | MAO G X, WANG Y, QIU Q, et al. Salidroside protects human fibroblast cells from premature senescence induced by H2O2 partly through modulating oxidative status[J]. Mechanisms of Ageing and Development, 2010, 131(11/12): 723-731. |
| 30 | QIAN E W, GE D T, KONG S K. Salidroside promotes erythropoiesis and protects erythroblasts against oxidative stress by up-regulating glutathione peroxidase and thioredoxin[J]. Journal of Ethnopharmacology, 2011, 133(2): 308-314. |
| 31 | ZHONG X Y, LIN R H, LI Z F, et al. Effects of salidroside on cobalt chloride-induced hypoxia damage and mTOR signaling repression in PC12 cells[J]. Biological & Pharmaceutical Bulletin, 2014, 37(7): 1199-1206. |
| 32 | 陈努菊. 一种红景天保湿抗衰乳: CN108309915A[P]. 2018-07-24. |
| CHEN N J. Rhodiola rosea moisture-preserving anti-aging lotion: CN108309915A[P]. 2018-07-24. | |
| 33 | LU H, LI Y, ZHANG T, et al. Salidroside reduces high-glucose-induced podocyte apoptosis and oxidative stress via upregulating heme oxygenase-1 (HO-1) expression[J]. Medical Science Monitor, 2017, 23: 4067-4076. |
| 34 | LIN S Y, DAN X, DU X X, et al. Protective effects of salidroside against carbon tetrachloride (CCl4)-induced liver injury by initiating mitochondria to resist oxidative stress in mice[J]. International Journal of Molecular Sciences, 2019, 20(13): 3187. |
| 35 | XING S S, YANG X Y, LI W J, et al. Salidroside stimulates mitochondrial biogenesis and protects against H2O2-induced endothelial dysfunction[J]. Oxidative Medicine and Cellular Longevity, 2014, 2014(1): 904834. |
| 36 | 黎明华, 汤长发, 欧阳江琼.红景天苷对运动后自由基和能量代谢改变的影响[J]. 中国应用生理学杂志, 2012, 28(1): 53-56. |
| LI M H, TANG C F, OUYANG J Q. Influence of salidroside from Rhodiola sachalinensis A. Bor on some related indexes of free radical and energy metabolism after exercise in mice[J]. Chinese Journal of Applied Physiology, 2012, 28(1): 53-56. | |
| 37 | 胥鑫萌, 王文权, 孙洁怡, 等. 红景天苷在日化领域中的研究进展[J]. 中国洗涤用品工业, 2020(12): 74-79. |
| XU X M, WANG W Q, SUN J Y, et al. Research progress of salidroside in daily chemical field[J]. China Cleaning Industry, 2020(12): 74-79. | |
| 38 | FAN X J, WANG Y, WANG L, et al. Salidroside induces apoptosis and autophagy in human colorectal cancer cells through inhibition of PI3K/Akt/mTOR pathway[J]. Oncology Reports, 2016, 36(6): 3559-3567. |
| 39 | HU X L, ZHANG X Q, QIU S F, et al. Salidroside induces cell-cycle arrest and apoptosis in human breast cancer cells[J]. Biochemical and Biophysical Research Communications, 2010, 398(1): 62-67. |
| 40 | LIU Z B, LI X S, SIMONEAU A R, et al. Rhodiola rosea extracts and salidroside decrease the growth of bladder cancer cell lines via inhibition of the mTOR pathway and induction of autophagy[J]. Molecular Carcinogenesis, 2012, 51(3): 257-267. |
| 41 | RONG L, LI Z D, LENG X, et al. Salidroside induces apoptosis and protective autophagy in human gastric cancer AGS cells through the PI3K/Akt/mTOR pathway[J]. Biomedicine & Pharmacotherapy, 2020, 122: 109726. |
| 42 | HAO W W, LI N, MI C F, et al. Salidroside attenuates cardiac dysfunction in a rat model of diabetes[J]. Diabetic Medicine, 2022, 39(3): e14683. |
| 43 | GE Y L, ZHANG B, SONG J B, et al. Discovery of salidroside as a novel non-coding RNA modulator to delay cellular senescence and promote BK-dependent apoptosis in cerebrovascular smooth muscle cells of simulated microgravity rats[J]. International Journal of Molecular Sciences, 2023, 24(19): 14531. |
| 44 | XU N, HUANG F, JIAN C D, et al. Neuroprotective effect of salidroside against central nervous system inflammation-induced cognitive deficits: a pivotal role of sirtuin 1-dependent Nrf-2/HO-1/NF-κB pathway[J]. Phytotherapy Research, 2019, 33(5): 1438-1447. |
| 45 | YANG S X, WANG L S, ZENG Y, et al. Salidroside alleviates cognitive impairment by inhibiting ferroptosis via activation of the Nrf2/GPX4 axis in SAMP8 mice[J]. Phytomedicine, 2023, 114: 154762. |
| 46 | HEMWIMOL S, PAVASANT P, SHOTIPRUK A. Ultrasound-assisted extraction of anthraquinones from roots of Morinda citrifolia [J]. Ultrasonics Sonochemistry, 2006, 13(6): 543-548. |
| 47 | MAO Y, LI Y, YAO N. Simultaneous determination of salidroside and tyrosol in extracts of Rhodiola L. by microwave assisted extraction and high-performance liquid chromatography[J]. Journal of Pharmaceutical and Biomedical Analysis, 2007, 45(3): 510-515. |
| 48 | ĐUROVIĆ S, NIKOLIĆ B, LUKOVIĆ N, et al. The impact of high-power ultrasound and microwave on the phenolic acid profile and antioxidant activity of the extract from yellow soybean seeds[J]. Industrial Crops and Products, 2018, 122: 223-231. |
| 49 | ZHANG Y F, ZHOU Z L, ZOU L, et al. Imidazolium-based ionic liquids with inorganic anions in the extraction of salidroside and tyrosol from Rhodiola: the role of cations and anions on the extraction mechanism[J]. Journal of Molecular Liquids, 2019, 275: 136-145. |
| 50 | LI F J, YUAN Y, LI H, et al. Infrared-assisted extraction of salidroside from the root of Rhodiola crenulata with a novel ionic liquid that dissolves cellulose[J]. RSC Advances, 2015, 5(59): 47326-47333. |
| 51 | HU B, ZHOU K, LIU Y T, et al. Optimization of microwave-assisted extraction of oil from tiger nut (Cyperus esculentus L.) and its quality evaluation[J]. Industrial Crops and Products, 2018, 115: 290-297. |
| 52 | GÓMEZ A V, TADINI C C, BISWAS A, et al. Microwave-assisted extraction of soluble sugars from banana puree with natural deep eutectic solvents (NADES)[J]. LWT, 2019, 107: 79-88. |
| 53 | GYÖRGY Z, TOLONEN A, NEUBAUER P, et al. Enhanced biotransformation capacity of Rhodiola rosea callus cultures for glycosid production[J]. Plant Cell, Tissue and Organ Culture, 2005, 83(2): 129-135. |
| 54 | RATTAN S, KUMAR A, KUMAR D, et al. Enhanced production of phenylethanoids mediated through synergistic approach of precursor feeding and light regime in cell suspension culture of Rhodiola imbricata (Edgew.)[J]. Applied Biochemistry and Biotechnology, 2022, 194(7): 3242-3260. |
| 55 | XU J F, LIU C B, HAN A M, et al. Strategies for the improvement of salidroside production in cell suspension cultures of Rhodiola sachalinensis [J]. Plant Cell Reports, 1998, 17(4): 288-293. |
| 56 | WU S X, ZU Y G, WU M. High yield production of salidroside in the suspension culture of Rhodiola sachalinensis [J]. Journal of Biotechnology, 2003, 106(1): 33-43. |
| 57 | RATTAN S, WARGHAT A R. Integration of biotechnological approaches for the production of phenylethanoids and phenylpropanoids in in vitro cultures of Rhodiola sp. a comprehensive review[J]. Industrial Crops and Products, 2023, 206: 117625. |
| 58 | XIE H, SHEN C Y, JIANG J G. The sources of salidroside and its targeting for multiple chronic diseases[J]. Journal of Functional Foods, 2020, 64: 103648. |
| 59 | XU J F, SU Z G, FENG P S. Activity of tyrosol glucosyltransferase and improved salidroside production through biotransformation of tyrosol in Rhodiola sachalinensis cell cultures[J]. Journal of Biotechnology, 1998, 61(1): 69-73. |
| 60 | TORRENS-SPENCE M P, PLUSKAL T, LI F S, et al. Complete pathway elucidation and heterologous reconstitution of Rhodiola salidroside biosynthesis[J]. Molecular Plant, 2018, 11(1): 205-217. |
| 61 | CHUNG D, KIM S Y, AHN J H. Production of three phenylethanoids, tyrosol, hydroxytyrosol, and salidroside, using plant genes expressing in Escherichia coli [J]. Scientific Reports, 2017, 7(1): 2578. |
| 62 | GRECH-BARAN M, SYKŁOWSKA-BARANEK K, PIETROSIUK A. Biotechnological approaches to enhance salidroside, rosin and its derivatives production in selected Rhodiola spp. in vitro cultures[J]. Phytochemistry Reviews, 2015, 14(4): 657-674. |
| 63 | IACOMETTI C, MARX K, HÖNICK M, et al. Activating silent glycolysis bypasses in Escherichia coli [J]. BioDesign Research, 2022, 2022: 9859643. |
| 64 | ROSTAIN W, SHEN S S, CORDERO T, et al. Engineering a circular riboregulator in Escherichia coli [J]. BioDesign Research, 2020, 2020: 1916789. |
| 65 | PARK S Y, YANG D S, HA S H, et al. Metabolic engineering of microorganisms for the production of natural compounds[J]. Advanced Biosystems, 2018, 2(1): 1700190. |
| 66 | LEE S Y. High cell-density culture of Escherichia coli [J]. Trends in Biotechnology, 1996, 14(3): 98-105. |
| 67 | YANG D, PARK S Y, PARK Y S, et al. Metabolic engineering of Escherichia coli for natural product biosynthesis[J]. Trends in Biotechnology, 2020, 38(7): 745-765. |
| 68 | BRÜSSOW H, CANCHAYA C, HARDT W D. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion[J]. Microbiology and Molecular Biology Reviews, 2004, 68(3): 560-602, tableofcontents. |
| 69 | CORNELIS P. Expressing genes in different Escherichia coli compartments[J]. Current Opinion in Biotechnology, 2000, 11(5): 450-454. |
| 70 | PALMELA C, CHEVARIN C, XU Z L, et al. Adherent-invasive Escherichia coli in inflammatory bowel disease[J]. Gut, 2018, 67(3): 574-587. |
| 71 | PUTIGNANI L, MASSA O, ALISI A. Engineered Escherichia coli as new source of flavonoids and terpenoids[J]. Food Research International, 2013, 54(1): 1084-1095. |
| 72 | HAMMER S K, AVALOS J L. Harnessing yeast organelles for metabolic engineering[J]. Nature Chemical Biology, 2017, 13(8): 823-832. |
| 73 | WAGNER J M, ALPER H S. Synthetic biology and molecular genetics in non-conventional yeasts: current tools and future advances[J]. Fungal Genetics and Biology, 2016, 89: 126-136. |
| 74 | BLOUNT B A, WEENINK T, ELLIS T. Construction of synthetic regulatory networks in yeast[J]. FEBS Letters, 2012, 586(15): 2112-2121. |
| 75 | NIELSEN J, LARSSON C, VAN MARIS A, et al. Metabolic engineering of yeast for production of fuels and chemicals[J]. Current Opinion in Biotechnology, 2013, 24(3): 398-404. |
| 76 | 蒋慧慧, 王强, 饶志明, 等. 酿酒酵母启动子工程研究进展[J]. 中国生物工程杂志, 2023, 43(11): 78-91. |
| JIANG H H, WANG Q, RAO Z M, et al. Research progress of the promoter engineering in Saccharomyces cerevisiae [J]. China Biotechnology, 2023, 43(11): 78-91. | |
| 77 | THAK E J, YOO S J, MOON H Y, et al. Yeast synthetic biology for designed cell factories producing secretory recombinant proteins[J]. FEMS Yeast Research, 2020, 20(2): foaa009. |
| 78 | XUE Y X, CHEN X Z, YANG C, et al. Engineering Eschericha coli for enhanced tyrosol production[J]. Journal of Agricultural and Food Chemistry, 2017, 65(23): 4708-4714. |
| 79 | YANG C, CHEN X Z, CHANG J Z, et al. Reconstruction of tyrosol synthetic pathways in Escherichia coli [J]. Chinese Journal of Chemical Engineering, 2018, 26(12): 2615-2621. |
| 80 | SHEN N, SATOH Y, KOMA D, et al. Optimization of tyrosol-producing pathway with tyrosine decarboxylase and tyramine oxidase in high-tyrosine-producing Escherichia coli [J]. Journal of Bioscience and Bioengineering, 2024, 137(2): 115-123. |
| 81 | 沈玉平, 周紫微, 贺茜, 等. 群体感应动态调控促进大肠杆菌合成酪醇[J]. 生物工程学报, 2023, 39(8): 3379-3393. |
| SHEN Y P, ZHOU Z W, HE X, et al. Dynamic regulation using a quorum-sensing circuit enhances the production of tyrosol by Escherichia coli [J]. Chinese Journal of Biotechnology, 2023, 39(8): 3379-3393. | |
| 82 | XUE F Y, GUO H L, HU Y Y, et al. Expression of Codon-optimized plant glycosyltransferase UGT72B14 in Escherichia coli enhances salidroside production[J]. BioMed Research International, 2016, 2016(1): 9845927. |
| 83 | BAI Y F, BI H P, ZHUANG Y B, et al. Production of salidroside in metabolically engineered Escherichia coli [J]. Scientific Reports, 2014, 4: 6640. |
| 84 | FAN B, CHEN T Y, ZHANG S, et al. Mining of efficient microbial UDP-glycosyltransferases by motif evolution cross plant kingdom for application in biosynthesis of salidroside[J]. Scientific Reports, 2017, 7(1): 463. |
| 85 | 魏晨昱, 黄珠莹, 沈知行, 等. 基于尿苷二磷酸葡萄糖循环再生系统高效转化酪醇合成红景天苷[J]. 生物工程学报, 2024, 40(9): 3127-3141. |
| WEI C Y, HUANG Z Y, SHEN Z X, et al. Efficient synthesis of salidroside from tyrosol based on UDPG recycling system[J]. Chinese Journal of Biotechnology, 2024, 40(9): 3127-3141. | |
| 86 | LIU S S, XIA Y Y, YANG H Q, et al. Rational chromosome engineering of Escherichia coli for overproduction of salidroside[J]. Biochemical Engineering Journal, 2022, 184: 108474. |
| 87 | ZENG W Z, WANG H J, CHEN J B, et al. Engineering Escherichia coli for efficient de novo synthesis of salidroside[J]. Journal of Agricultural and Food Chemistry, 2024, 72(51): 28369-28377. |
| 88 | KALLSCHEUER N, MENEZES R, FOITO A, et al. Identification and microbial production of the raspberry phenol salidroside that is active against Huntington’s disease[J]. Plant Physiology, 2019, 179(3): 969-985. |
| 89 | LIU H Y, TIAN Y J, ZHOU Y, et al. Multi-modular engineering of Saccharomyces cerevisiae for high-titre production of tyrosol and salidroside[J]. Microbial Biotechnology, 2021, 14(6): 2605-2616. |
| 90 | GUO W, HUANG Q L, FENG Y H, et al. Rewiring central carbon metabolism for tyrosol and salidroside production in Saccharomyces cerevisiae [J]. Biotechnology and Bioengineering, 2020, 117(8): 2410-2419. |
| 91 | LIU H Y, XIAO Q J, WU X X, et al. Mechanistic investigation of a D to N mutation in DAHP synthase that dictates carbon flux into the shikimate pathway in yeast[J]. Communications Chemistry, 2023, 6(1): 152. |
| 92 | CUI J L, GUO T T, CHAO J B, et al. Potential of the endophytic fungus Phialocephala fortinii Rac56 found in Rhodiola plants to produce salidroside and p-tyrosol[J]. Molecules, 2016, 21(4): 502. |
| 93 | ZHOU X J, ZHANG X X, WANG D, et al. Efficient biosynthesis of salidroside via artificial in vivo enhanced UDP-glucose system using cheap sucrose as substrate[J]. ACS Omega, 2024, 9(20): 22386-22397. |
| 94 | LIU X, LI X B, JIANG J L, et al. Convergent engineering of syntrophic Escherichia coli coculture for efficient production of glycosides[J]. Metabolic Engineering, 2018, 47: 243-253. |
| 95 | BORNEMAN A R, PRETORIUS I S, CHAMBERS P J. Comparative genomics: a revolutionary tool for wine yeast strain development[J]. Current Opinion in Biotechnology, 2013, 24(2): 192-199. |
| 96 | GOU Y W, LI D F, ZHAO M H, et al. Intein-mediated temperature control for complete biosynthesis of sanguinarine and its halogenated derivatives in yeast[J]. Nature Communications, 2024, 15(1): 5238. |
| 97 | CHEN R B, GAO J Q, YU W, et al. Engineering cofactor supply and recycling to drive phenolic acid biosynthesis in yeast[J]. Nature Chemical Biology, 2022, 18(5): 520-529. |
| 98 | WANG X Q, YUAN B, ZHANG F L, et al. Novel roles of the greatwall kinase Rim15 in yeast oxidative stress tolerance through mediating antioxidant systems and transcriptional regulation[J]. Antioxidants, 2024, 13(3): 260. |
| 99 | GAO D, LIU T F, GAO J C, et al. De novo biosynthesis of vindoline and catharanthine in Saccharomyces cerevisiae [J]. BioDesign Research, 2022, 2022: 2. |
| 100 | SILVA N A DA, SRIKRISHNAN S. Introduction and expression of genes for metabolic engineering applications in Saccharomyces cerevisiae [J]. FEMS Yeast Research, 2012, 12(2): 197-214. |
| 101 | KRIVORUCHKO A, SIEWERS V, NIELSEN J. Opportunities for yeast metabolic engineering: lessons from synthetic biology[J]. Biotechnology Journal, 2011, 6(3): 262-276. |
| 102 | SIDDIQUI M S, THODEY K, TRENCHARD I, et al. Advancing secondary metabolite biosynthesis in yeast with synthetic biology tools[J]. FEMS Yeast Research, 2012, 12(2): 144-170. |
| 103 | ZHANG Q S, CAO H. Expression of chitosanase from Aspergillus fumigatus chitosanase in Saccharomyces cerevisiae by CRISPR-Cas9 tools[J]. Bioresources and Bioprocessing, 2024, 11(1): 20. |
| 104 | HAZELWOOD L A, DARAN J M, VAN MARIS A J A, et al. The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism[J]. Applied and Environmental Microbiology, 2008, 74(8): 2259-2266. |
| 105 | KÖNIG V, PFEIL A, BRAUS G H, et al. Substrate and metal complexes of 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase from Saccharomyces cerevisiae provide new insights into the catalytic mechanism[J]. Journal of Molecular Biology, 2004, 337(3): 675-690. |
| 106 | SUÁSTEGUI M, GUO W H, FENG X Y, et al. Investigating strain dependency in the production of aromatic compounds in Saccharomyces cerevisiae [J]. Biotechnology and Bioengineering, 2016, 113(12): 2676-2685. |
| 107 | CUI J L, GUO T T, REN Z X, et al. Diversity and antioxidant activity of culturable endophytic fungi from alpine plants of Rhodiola crenulata, R. angusta, and R. sachalinensis [J]. PLoS One, 2015, 10(3): e0118204. |
| 108 | 杨欣, 曾宪军, 丁仁芳, 等. 一株红景天内生细菌的筛选及初步研究[J]. 微生物学通报, 2015, 42(10): 1962-1970. |
| YANG X, ZENG X J, DING R F, et al. Screening and preliminary study on one endophytic bacterium of Rhodiola crenulata [J]. Microbiology China, 2015, 42(10): 1962-1970. | |
| 109 | 郭勇, 崔森. 红景天苷药理作用及机制的研究进展[J]. 临床医学进展, 2022, 12(8): 7202-7207. |
| GUO Y, CUI S. Research progress on the pharmacological effects and mechanisms of salidroside[J]. Advances in Clinical Medicine, 2022, 12(8): 7202-7207. | |
| 110 | LAI Y M, CHEN H F, LIU L R, et al. Engineering a synthetic pathway for tyrosol synthesis in Escherichia coli [J]. ACS Synthetic Biology, 2022, 11(1): 441-447. |
| 111 | ALIPIEVA K, KORKINA L, ORHAN I E, et al. Verbascoside: a review of its occurrence, (bio)synthesis and pharmacological significance[J]. Biotechnology Advances, 2014, 32(6): 1065-1076. |
| 112 | GEORGIEV M, PASTORE S, LULLI D, et al. Verbascum xanthophoeniceum-derived phenylethanoid glycosides are potent inhibitors of inflammatory chemokines in dormant and interferon-gamma-stimulated human keratinocytes[J]. Journal of Ethnopharmacology, 2012, 144(3): 754-760. |
| 113 | WU P T, LV Q G, WANG S, et al. Using a dual-disease target mapping network pharmacology approach, verbascoside ameliorates osteoporosis by activating estrogen signaling to alleviate oxidative stress[J]. Combinatorial Chemistry & High Throughput Screening, 2024. |
| 114 | LU R R, ZHANG L, WANG H H, et al. Echinacoside exerts antidepressant-like effects through enhancing BDNF-CREB pathway and inhibiting neuroinflammation via regulating microglia M1/M2 polarization and JAK1/STAT3 pathway[J]. Frontiers in Pharmacology, 2023, 13: 993483. |
| 115 | CHEN Y, LI Y Q, FANG J Y, et al. Establishment of the concurrent experimental model of osteoporosis combined with Alzheimer’s disease in rat and the dual-effects of echinacoside and acteoside from Cistanche tubulosa [J]. Journal of Ethnopharmacology, 2020, 257: 112834. |
| 116 | LIANG Y, CHEN C, XIA B M, et al. Neuroprotective effect of echinacoside in subacute mouse model of Parkinson’s disease[J]. BioMed Research International, 2019, 2019(1): 4379639. |
| 117 | GAI X Y, LIN P C, HE Y F, et al. Echinacoside prevents hypoxic pulmonary hypertension by regulating the pulmonary artery function[J]. Journal of Pharmacological Sciences, 2020, 144(4): 237-244. |
| 118 | DING L, YE H, GU L D, et al. Echinacoside alleviates cognitive impairment in cerebral ischemia rats through α 7nAChR-induced autophagy[J]. Chinese Journal of Integrative Medicine, 2022, 28(9): 809-816. |
| 119 | WEI W, LAN X B, LIU N, et al. Echinacoside alleviates hypoxic-ischemic brain injury in neonatal rat by enhancing antioxidant capacity and inhibiting apoptosis[J]. Neurochemical Research, 2019, 44(7): 1582-1592. |
| 120 | CHOO H J, KIM E J, KIM S Y, et al. Microbial synthesis of hydroxytyrosol and hydroxysalidroside[J]. Applied Biological Chemistry, 2018, 61(3): 295-301. |
| 121 | YANG Y H, XI D Y, WU Y N, et al. Complete biosynthesis of the phenylethanoid glycoside verbascoside[J]. Plant Communications, 2023, 4(4): 100592. |
| 122 | XU F X, CAO H, CUI X W, et al. Optimization of fermentation condition for echinacoside yield improvement with Penicillium sp. H1, an endophytic fungus isolated from Ligustrum lucidum Ait using response surface methodology[J]. Molecules, 2018, 23(10): 2586. |
| 123 | BAI P G, YANG Y H, TANG J, et al. High-level sustainable production of complex phenylethanoid glycosides from glucose through engineered yeast cell factories[J]. Metabolic Engineering, 2025, 87: 95-108. |
| 124 | 李彤, 杨雅茹, 闵清, 等. 红景天苷提取、含量测定及药理作用研究进展[J]. 药物化学, 2022(2): 190-197. |
| LI T, YANG Y R, MIN Q, et al. Extraction, determination and pharmacological action of salidroside[J]. Hans Journal of Medicinal Chemistry, 2022(2): 190-197. |
| [1] | WU Ke, LUO Jiahao, LI Feiran. Applications of machine learning in the reconstruction and curation of genome-scale metabolic models [J]. Synthetic Biology Journal, 2025, 6(3): 566-584. |
| [2] | TIAN Xiao-jun, ZHANG Rixin. “Economics Paradox” with cells in synthetic gene circuits [J]. Synthetic Biology Journal, 2025, 6(3): 532-546. |
| [3] | LI Yongzhu, CHEN Yu. Advances and prospects in genome-scale models of yeast [J]. Synthetic Biology Journal, 2025, 6(3): 585-602. |
| [4] | ZHANG Yiqing, LIU Gaowen. Exploration of gene functions and library construction for engineering strains from a synthetic biology perspective [J]. Synthetic Biology Journal, 2025, 6(3): 685-700. |
| [5] | YANG Ying, LI Xia, LIU Lizhong. Applications of synthetic biology to stem-cell-derived modeling of early embryonic development [J]. Synthetic Biology Journal, 2025, 6(3): 669-684. |
| [6] | HUANG Yi, SI Tong, LU Anjing. Standardization for biomanufacturing: global landscape, critical challenges, and pathways forward [J]. Synthetic Biology Journal, 2025, 6(3): 701-714. |
| [7] | SONG Chengzhi, LIN Yihan. AI-enabled directed evolution for protein engineering and optimization [J]. Synthetic Biology Journal, 2025, 6(3): 617-635. |
| [8] | GAO Qi, XIAO Wenhai. Advances in the biosynthesis of monoterpenes by yeast [J]. Synthetic Biology Journal, 2025, 6(2): 357-372. |
| [9] | ZHANG Mengyao, CAI Peng, ZHOU Yongjin. Synthetic biology drives the sustainable production of terpenoid fragrances and flavors [J]. Synthetic Biology Journal, 2025, 6(2): 334-356. |
| [10] | ZHANG Lu’ou, XU Li, HU Xiaoxu, YANG Ying. Synthetic biology ushers cosmetic industry into the “bio-cosmetics” era [J]. Synthetic Biology Journal, 2025, 6(2): 479-491. |
| [11] | YI Jinhang, TANG Yulin, LI Chunyu, WU Heyun, MA Qian, XIE Xixian. Applications and advances in the research of biosynthesis of amino acid derivatives as key ingredients in cosmetics [J]. Synthetic Biology Journal, 2025, 6(2): 254-289. |
| [12] | WEI Lingzhen, WANG Jia, SUN Xinxiao, YUAN Qipeng, SHEN Xiaolin. Biosynthesis of flavonoids and their applications in cosmetics [J]. Synthetic Biology Journal, 2025, 6(2): 373-390. |
| [13] | XIAO Sen, HU Litao, SHI Zhicheng, WANG Fayin, YU Siting, DU Guocheng, CHEN Jian, KANG Zhen. Research advances in biosynthesis of hyaluronic acid with controlled molecular weights [J]. Synthetic Biology Journal, 2025, 6(2): 445-460. |
| [14] | WANG Qian, GUO Shiting, XIN Bo, ZHONG Cheng, WANG Yu. Advances in biosynthesis of L-arginine using engineered microorganisms [J]. Synthetic Biology Journal, 2025, 6(2): 290-305. |
| [15] | ZUO Yimeng, ZHANG Jiaojiao, LIAN Jiazhang. Enabling technology for the biosynthesis of cosmetic raw materials with Saccharomyces cerevisiae [J]. Synthetic Biology Journal, 2025, 6(2): 233-253. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||