|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Advances in the biological utilization of one-carbon compounds
Synthetic Biology Journal
DOI: 10.12211/2096-8280.2025-077
Table 2
The hosts of C1 utilization pathways mentioned in this paper
Extracts from the Article
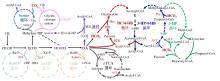
自养微生物驱动的CO2生物固定技术是实现负碳制造的核心路径,通过整合光合或化能自养代谢网络,可将温室气体直接转化为高附加值化学品。然而,现有自养底盘细胞的工业化应用仍面临多重瓶颈,例如,嗜极生理特性限制常规发酵工艺适配;倍增时间长(典型蓝藻>8 h vs 大肠杆菌<0.5 h);基因组注释完整度不足(如非编码RNA功能注释率<30%);基因编辑效率低下等。尽管如此,通过合成生物学工具创新与代谢模型优化,部分光合蓝藻、绿藻和化能异样菌的工程化研究获得了阶段性成功[45-49],其中细长聚球藻(Synechococcus elongatus,S. elongatus),莱茵衣藻(Chlamydomonas reinhardtii,C. reinhardtii)和钩虫贪铜菌(Cupriavidus necator,C. necator)具有较强的工程化应用前景[47,50,51],下面将对这三种底盘细胞进行简要介绍(表2)。
甲醇、甲烷、甲酸、甲醛等C1化合物均可由CO2直接制备,具有清洁可再生的优点。天然甲基营养菌内源的C1利用途径可高效利用这些C1底物作为唯一碳源,为负碳生物制造提供了天然底盘。基于CRISPR-Cas基因组编辑与合成基因线路设计,研究者已构建出多维度工程化的甲基营养菌,其代表性工业底盘包括:毕赤酵母、扭脱甲基杆菌(Methylorubrum extorquens, M. extorquens)、甲醇芽孢杆菌(Bacillus methanolicus, B. methanolicus)[60],此外,多形汉逊酵母凭借其出色的甲醇转化能力,逐步获得了合成生物学家的青睐[61]。下面将对上述底盘进行详细介绍(表2):
大肠杆菌与酿酒酵母作为合成生物学与工业生物制造的经典模式底盘(表2),凭借其高度成熟的基因编辑技术、规模化发酵体系及全基因组注释完整性,在代谢工程应用中展现出显著优势[35, 69]。相较于自养与天然甲基营养菌,其核心优势体现在:1)模块化遗传工具库完备;2)代谢网络可塑性高,支持超过200种化学品异源合成;3)工业适配性强,已建立FDA认证的大规模生产工艺[35, 70]。因此,基于大肠杆菌和酿酒酵母构建合成甲基营养菌,成为负碳生物制造的另一个热点研究领域[7, 71]。
以CO2为代表的温室气体的利用一直是科学研究的重要方向。然而,因其化学惰性、低能量密度及溶解性等固有特性使其直接生物利用面临诸多挑战。例如,CO2还原固定需要高昂的能量输入;经济高效的CO2捕集技术是前提条件;CO2的低溶解度限制了生物固碳速率。目前,CO2的直接生物利用主要依托两种策略:一是基于工程化自养菌。天然自养菌普遍存在生长缓慢、基因编辑工具匮乏等局限[115]。近年来,合成生物学工具的突破(如高效基因编辑系统与新型遗传工具的开发)为改造自养型细胞工厂提供了关键技术支撑[116,117],推动了以CO2为唯一碳源合成甘露醇、蔗糖、法尼烯等高价值化学品的进程[54,56,81,118]。此外,“异养底盘自养化”作为一种补充策略展现出巨大潜力,例如,Diethard Mattanovich教授团队将CBB循环引入毕赤酵母,成功实现了以CO2为碳源合成衣康酸和乳酸,开创了人工自养新范式[119]。二是依托于人工构建的固碳途径进行CO2的体外生物利用,例如,Tobias J. Erb团队设计的THETA循环,整合了来自9个物种的17种酶,可在体外将CO2高效转化为acetyl-CoA,其固碳效率优于天然系统[20],然而,该类多酶系统难以实现体内应用,我们猜测主要源于以下原因:途径中关键中间代谢物被宿主内源酶催化产生分流,导致固碳循环中断[20];细胞能量供应不足以支撑CO2的持续固定;体内环境导致固碳循环中特定酶的活性降低,固碳循环通量降低。
Other Images/Table from this Article
|