合成生物学 ›› 2024, Vol. 5 ›› Issue (2): 267-280.DOI: 10.12211/2096-8280.2023-078
流感病毒改造新策略及其应用
郭茜亚, 陈积, 董铭心
- 青岛大学药学院药物化学系,山东 青岛 266000
-
收稿日期:2023-11-09修回日期:2024-02-22出版日期:2024-04-30发布日期:2024-04-28 -
通讯作者:董铭心 -
作者简介:郭茜亚 (1997—),女,博士研究生。研究方向为溶瘤病毒的研发策略及应用。E-mail:guoxiya132@163.com董铭心 (1978—),男,教授,博士生导师。研究方向为抗病毒和神经系统小分子药物及疫苗。E-mail:Mxdong64@qdu.edu.cn -
基金资助:国家重点研发计划“合成生物学”重点专项(2018YFA0900804)
New strategies for engineering influenza viruses and their applications
GUO Xiya, CHEN Ji, DONG Mingxin
- Department of Medicinal Chemistry,College of Pharmacy,Qingdao University,Qingdao 266000,Shandong,China
-
Received:2023-11-09Revised:2024-02-22Online:2024-04-30Published:2024-04-28 -
Contact:DONG Mingxin
摘要:
流感病毒有着极强的变异性和传播性,常在全球范围内引起季节性的流感爆发。流感病毒的基因组序列、蛋白结构与功能、病毒的包装机制等环节研究相对清楚,也是一种重要的模式病毒,用于条件控制基因元件的发现和确证,构建智能响应型病毒等。随着反向遗传学与合成生物学的发展,通过基因工程改造的流感病毒能更好地控制病毒复制来提高疫苗的安全性,以及诱发机体产生强烈的免疫反应,在肿瘤免疫治疗领域引发广泛关注。本文描述了蛋白质水解靶向嵌合病毒、条件复制型流感减毒活病毒和高干扰素敏感病毒等三种新型减毒流感病毒改造策略,并对编码过早终止密码子的嵌合抗原肽的流感病毒、与PD-L1或CTLA4免疫检查点重组的流感病毒、截短的NS1片段表达GM-CSF的流感病毒分别对黑色素瘤、肝癌的溶瘤作用进行评述。未来,将通过创新性地运用不同策略、不同病毒来构建减毒活疫苗和溶瘤病毒,以便在临床上获得更加安全有效的治疗手段。
中图分类号:
引用本文
郭茜亚, 陈积, 董铭心. 流感病毒改造新策略及其应用[J]. 合成生物学, 2024, 5(2): 267-280.
GUO Xiya, CHEN Ji, DONG Mingxin. New strategies for engineering influenza viruses and their applications[J]. Synthetic Biology Journal, 2024, 5(2): 267-280.
| 1 | WOOLHOUSE M E J, BRIERLEY L. Epidemiological characteristics of human-infective RNA viruses[J]. Scientific Data, 2018, 5: 180017. |
| 2 | GOUNDER A P, BOON A C M. Influenza pathogenesis: the effect of host factors on severity of disease[J]. Journal of Immunology, 2019, 202(2): 341-350. |
| 3 | FUKUYAMA S, KAWAOKA Y. The pathogenesis of influenza virus infections: the contributions of virus and host factors[J]. Current Opinion in Immunology, 2011, 23(4): 481-486. |
| 4 | BELSHE R B, EDWARDS K M, VESIKARI T, et al. Live attenuated versus inactivated influenza vaccine in infants and young children[J]. The New England Journal of Medicine, 2007, 356(7): 685-696. |
| 5 | BELSHE R B, NEWMAN F K, WILKINS K, et al. Comparative immunogenicity of trivalent influenza vaccine administered by intradermal or intramuscular route in healthy adults[J]. Vaccine, 2007, 25(37-38): 6755-6763. |
| 6 | GORSE G J, BELSHE R B, MUNN N J. Superiority of live attenuated compared with inactivated influenza A virus vaccines in older, chronically ill adults[J]. Chest, 1991, 100(4): 977-984. |
| 7 | PICA N, PALESE P. Toward a universal influenza virus vaccine: prospects and challenges[J]. Annual Review of Medicine, 2013, 64: 189-202. |
| 8 | BUONAGURIO D A, BECHERT T M, YANG C F, et al. Genetic stability of live, cold-adapted influenza virus components of the FluMist/CAIV-T vaccine throughout the manufacturing process[J]. Vaccine, 2006, 24(12): 2151-2160. |
| 9 | AMBROSE C S, LUKE C, COELINGH K. Current status of live attenuated influenza vaccine in the United States for seasonal and pandemic influenza[J]. Influenza and Other Respiratory Viruses, 2008, 2(6): 193-202. |
| 10 | DE VILLIERS P J, STEELE A D, HIEMSTRA L A, et al. Efficacy and safety of a live attenuated influenza vaccine in adults 60 years of age and older[J]. Vaccine, 2009, 28(1): 228-234. |
| 11 | KRUG R M. Functions of the influenza A virus NS1 protein in antiviral defense[J]. Current Opinion in Virology, 2015, 12: 1-6. |
| 12 | AYLLON J, GARCÍA-SASTRE A. The NS1 protein: a multitasking virulence factor[J]. Current Topics in Microbiology and Immunology, 2015, 386: 73-107. |
| 13 | FERNANDEZ-SESMA A, MARUKIAN S, EBERSOLE B J, et al. Influenza virus evades innate and adaptive immunity via the NS1 protein[J]. Journal of Virology, 2006, 80(13): 6295-6304. |
| 14 | GEISS G K, SALVATORE M, TUMPEY T M, et al. Cellular transcriptional profiling in influenza A virus-infected lung epithelial cells: the role of the nonstructural NS1 protein in the evasion of the host innate defense and its potential contribution to pandemic influenza[J]. Proceedings of the National Academy of Sciences of the United States of America, 2002, 99(16): 10736-10741. |
| 15 | RICHT J A, GARCÍA-SASTRE A. Attenuated influenza virus vaccines with modified NS1 proteins[J]. Current Topics in Microbiology and Immunology, 2009, 333: 177-195. |
| 16 | MUELLER S N, LANGLEY W A, CARNERO E, et al. Immunization with live attenuated influenza viruses that express altered NS1 proteins results in potent and protective memory CD8+ T-cell responses[J]. Journal of Virology, 2010, 84(4): 1847-1855. |
| 17 | PICA N, LANGLOIS R A, KRAMMER F, et al. NS1-truncated live attenuated virus vaccine provides robust protection to aged mice from viral challenge[J]. Journal of Virology, 2012, 86(19): 10293-10301. |
| 18 | EGOROV A, BRANDT S, SEREINIG S, et al. Transfectant influenza A viruses with long deletions in the NS1 protein grow efficiently in Vero cells[J]. Journal of Virology, 1998, 72(8): 6437-6441. |
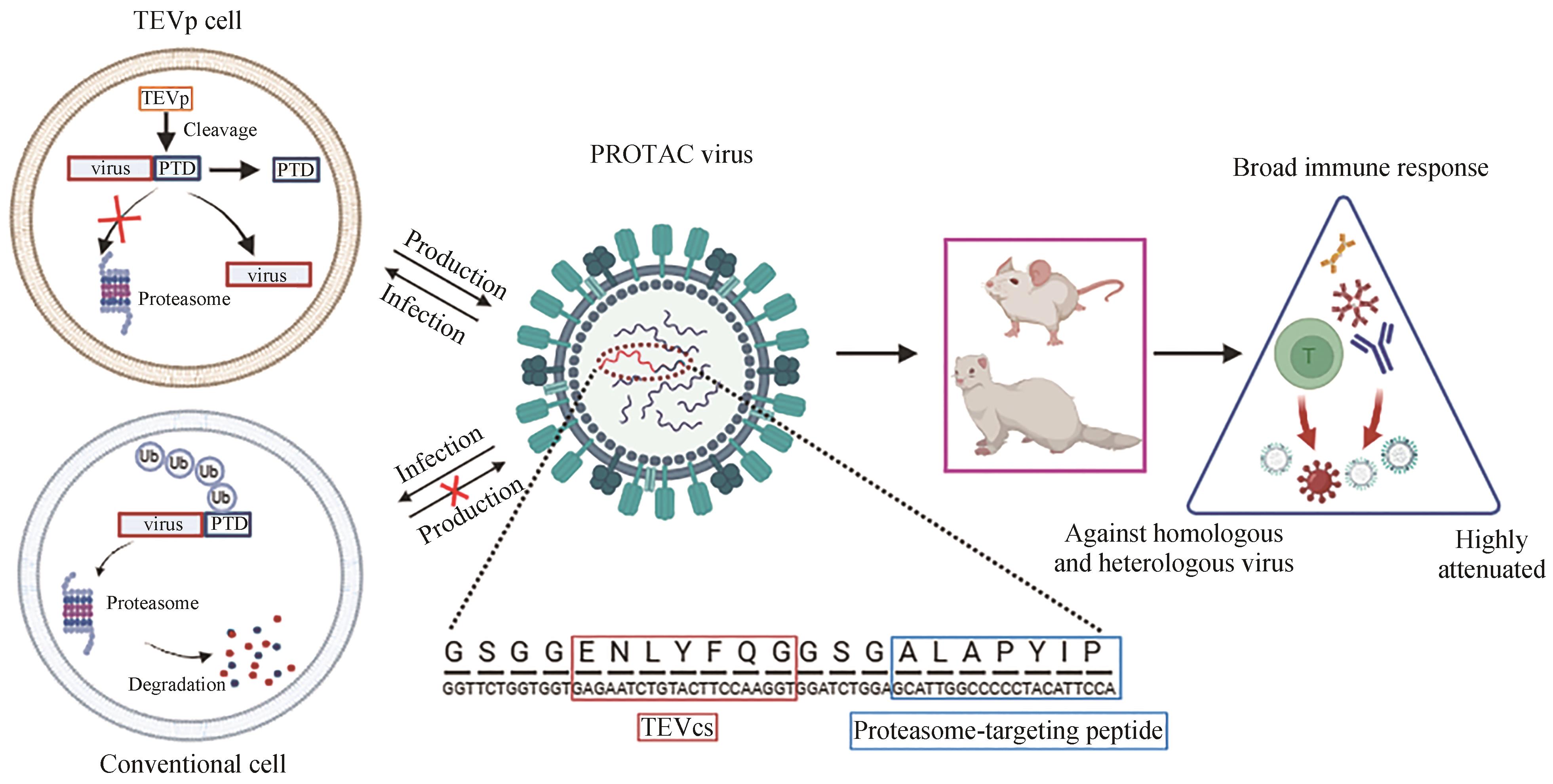
| 19 | SI L L, SHEN Q, LI J, et al. Generation of a live attenuated influenza A vaccine by proteolysis targeting[J]. Nature Biotechnology, 2022, 40(9): 1370-1377. |
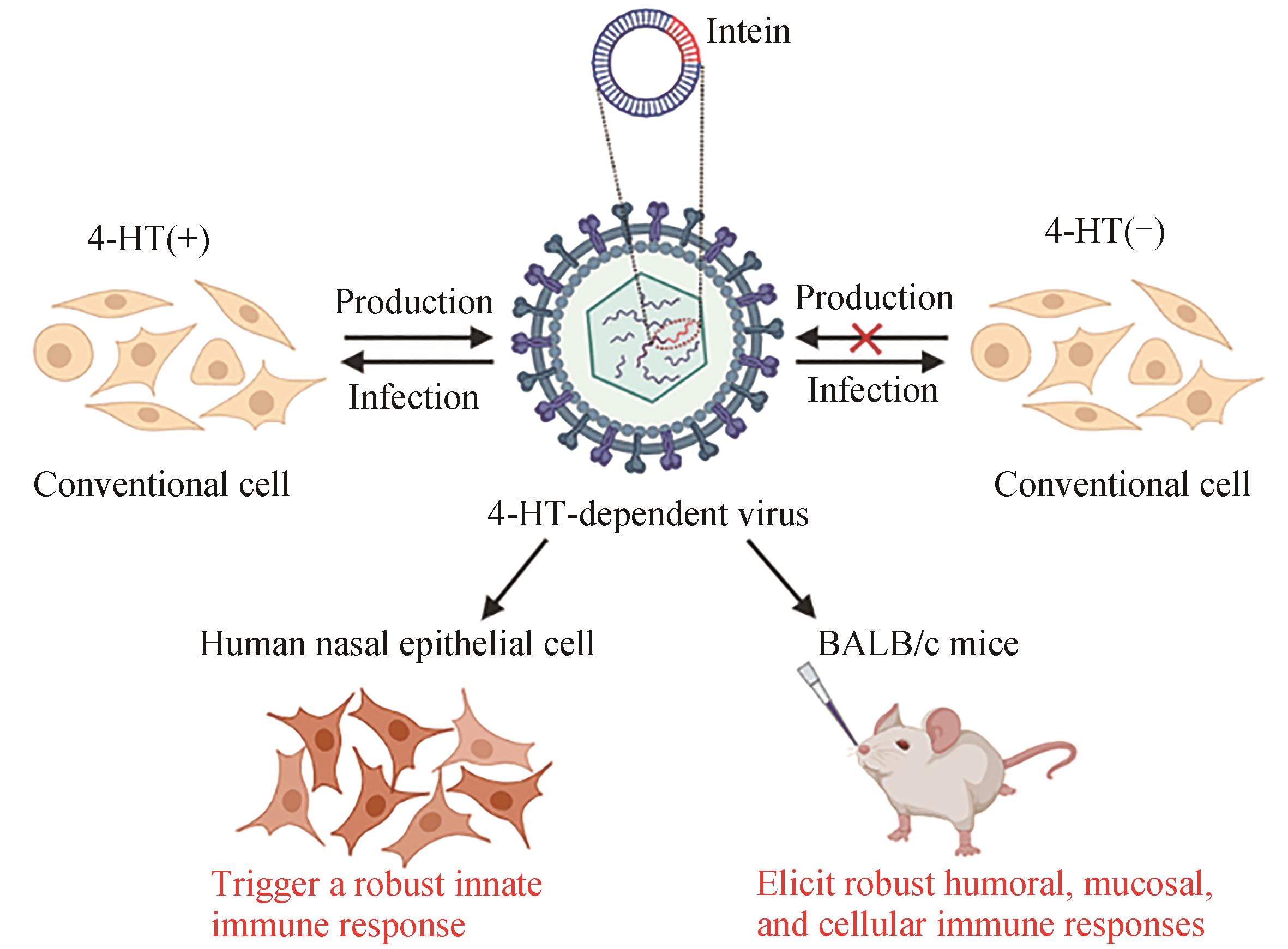
| 20 | CHEN J, WANG J Y, ZHU H Y, et al. Generation of a live attenuated influenza A vaccine using chemical-triggered intein[J]. ACS Synthetic Biology, 2023, 12(6): 1686-1695. |
| 21 | DU Y S, XIN L, SHI Y, et al. Genome-wide identification of interferon-sensitive mutations enables influenza vaccine design[J]. Science, 2018, 359(6373): 290-296. |
| 22 | ALSAAB H O, SAU S, ALZHRANI R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome[J]. Frontiers in Pharmacology, 2017, 8: 561. |
| 23 | MARIN-ACEVEDO J A, DHOLARIA B, SOYANO A E, et al. Next generation of immune checkpoint therapy in cancer: new developments and challenges[J]. Journal of Hematology & Oncology, 2018, 11(1): 39. |
| 24 | SCUDELLARI M. Protein-slaying drugs could be the next blockbuster therapies[J]. Nature, 2019, 567(7748): 298-300. |
| 25 | SALAMI J, CREWS C M. Waste disposal—an attractive strategy for cancer therapy[J]. Science, 2017, 355(6330): 1163-1167. |
| 26 | CROMM P M, CREWS C M. Targeted protein degradation: from chemical biology to drug discovery[J]. Cell Chemical Biology, 2017, 24(9): 1181-1190. |
| 27 | DESHAIES R J. Protein degradation: prime time for PROTACs[J]. Nature Chemical Biology, 2015, 11(9): 634-635. |
| 28 | YAU R, RAPE M. The increasing complexity of the ubiquitin code[J]. Nature Cell Biology, 2016, 18(6): 579-586. |
| 29 | FINLEY D. Recognition and processing of ubiquitin-protein conjugates by the proteasome[J]. Annual Review of Biochemistry, 2009, 78: 477-513. |
| 30 | HON W C, WILSON M I, HARLOS K, et al. Structural basis for the recognition of hydroxyproline in HIF-1 alpha by pVHL[J]. Nature, 2002, 417(6892): 975-978. |
| 31 | JAAKKOLA P, MOLE D R, TIAN Y M, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2 - regulated prolyl hydroxylation[J]. Science, 2001, 292(5516): 468-472. |
| 32 | IVAN M, KONDO K, YANG H, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing[J]. Science, 2001, 292(5516): 464-468. |
| 33 | GU S S, CUI D R, CHEN X Y, et al. PROTACs: an emerging targeting technique for protein degradation in drug discovery[J]. BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology, 2018, 40(4): e1700247. |
| 34 | ZHANG C, PENG Z H, ZHU M L, et al. USP9X destabilizes pVHL and promotes cell proliferation[J]. Oncotarget, 2016, 7(37): 60519-60534. |
| 35 | IWAI K, YAMANAKA K, KAMURA T, et al. Identification of the von Hippel-Lindau tumor-suppressor protein as part of an active E3 ubiquitin ligase complex[J]. Proceedings of the National Academy of Sciences of the United States of America, 1999, 96(22): 12436-12441. |
| 36 | LATIF F, TORY K, GNARRA J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene[J]. Science, 1993, 260(5112): 1317-1320. |
| 37 | LOS M, JANSEN G H, KAELIN W G, et al. Expression pattern of the von Hippel-Lindau protein in human tissues[J]. Laboratory Investigation, 1996, 75(2): 231-238. |
| 38 | PING J H, LOPES T J S, NEUMANN G, et al. Development of high-yield influenza B virus vaccine viruses[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(51): E8296-E8305. |
| 39 | MUELLER S, COLEMAN J R, PAPAMICHAIL D, et al. Live attenuated influenza virus vaccines by computer-aided rational design[J]. Nature Biotechnology, 2010, 28(7): 723-726. |
| 40 | WEI C J, CRANK M C, SHIVER J, et al. Next-generation influenza vaccines: opportunities and challenges[J]. Nature Reviews Drug Discovery, 2020, 19(4): 239-252. |
| 41 | BANIK S M, PEDRAM K, WISNOVSKY S, et al. Lysosome-targeting chimaeras for degradation of extracellular proteins[J]. Nature, 2020, 584(7820): 291-297. |
| 42 | TAKAHASHI D, MORIYAMA J, NAKAMURA T, et al. AUTACs: cargo-specific degraders using selective autophagy[J]. Molecular Cell, 2019, 76(5): 797-810.e10. |
| 43 | LI Z Y, WANG C, WANG Z Y, et al. Allele-selective lowering of mutant HTT protein by HTT-LC3 linker compounds[J]. Nature, 2019, 575(7781): 203-209. |
| 44 | BUSKIRK A R, ONG Y C, GARTNER Z J, et al. Directed evolution of ligand dependence: small-molecule-activated protein splicing[J]. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(29): 10505-10510. |
| 45 | YUEN C M, RODDA S J, VOKES S A, et al. Control of transcription factor activity and osteoblast differentiation in mammalian cells using an evolved small-molecule-dependent intein[J]. Journal of the American Chemical Society, 2006, 128(27): 8939-8946. |
| 46 | PECK S H, CHEN I, LIU D R. Directed evolution of a small-molecule-triggered intein with improved splicing properties in mammalian cells[J]. Chemistry & Biology, 2011, 18(5): 619-630. |
| 47 | LO C Y, TANG Y S, SHAW P C. Structure and function of influenza virus ribonucleoprotein[J]. Sub-Cellular Biochemistry, 2018, 88: 95-128. |
| 48 | LIU Q, YANG Y J, TAN X F, et al. Plasmodium parasite as an effective hepatocellular carcinoma antigen glypican-3 delivery vector[J]. Oncotarget, 2017, 8(15): 24785-24796. |
| 49 | YU R R, ZHU B, CHEN D G. Type Ⅰ interferon-mediated tumor immunity and its role in immunotherapy[J]. Cellular and Molecular Life Sciences, 2022, 79(3): 191. |
| 50 | WU N C, OLSON C A, DU Y S, et al. Functional constraint profiling of a viral protein reveals discordance of evolutionary conservation and functionality[J]. PLoS Genetics, 2015, 11(7): e1005310. |
| 51 | DU Y S, ZHANG T H, DAI L, et al. Effects of mutations on replicative fitness and major histocompatibility complex class Ⅰ binding affinity are among the determinants underlying cytotoxic-T-lymphocyte escape of HIV-1 gag epitopes[J]. mBio, 2017, 8(6): e01050-17. |
| 52 | HOFFMANN E, NEUMANN G, KAWAOKA Y, et al. A DNA transfection system for generation of influenza A virus from eight plasmids[J]. Proceedings of the National Academy of Sciences of the United States of America, 2000, 97(11): 6108-6113. |
| 53 | 夏忠军,常建华,张力. 基因工程腺病毒(H101)瘤内注射联合化疗治疗头颈部及食管鳞癌的Ⅲ期临床研究[J]. 癌症, 2004, 23(12): 1666-1670. |
| XIA Z J, CHANG J H, ZHANG L, et al. Phase Ⅲ randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus[J]. Chinese Journal of Cancer, 2004, 23(12): 1666-1670. | |
| 54 | GARBER K. China approves world’s first oncolytic virus therapy for cancer treatment[J]. Journal of the National Cancer Institute, 2006, 98(5): 298-300. |
| 55 | FERRUCCI P F, PALA L, CONFORTI F, et al. Talimogene laherparepvec (T-VEC): an intralesional cancer immunotherapy for advanced melanoma[J]. Cancers, 2021, 13(6): 1383. |
| 56 | WANG T, ZHANG J J, WANG Y L, et al. Influenza-trained mucosal-resident alveolar macrophages confer long-term antitumor immunity in the lungs[J]. Nature Immunology, 2023, 24(3): 423-438. |
| 57 | PASTORINO U. The development of an international registry[J]. Journal of Thoracic Oncology, 2010, 5(S2): S196-S197. |
| 58 | SITNIK S, MASEMANN D, LEITE DANTAS R, et al. PD-1 IC inhibition synergistically improves influenza A virus-mediated oncolysis of metastatic pulmonary melanoma[J]. Molecular Therapy Oncolytics, 2020, 17: 190-204. |
| 59 | ANDTBACKA R H I, KAUFMAN H L, COLLICHIO F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma[J]. Journal of Clinical Oncology, 2015, 33(25): 2780-2788. |
| 60 | ANDTBACKA R H, ROSS M, PUZANOV I, et al. Patterns of clinical response with talimogene laherparepvec (T-VEC) in patients with melanoma treated in the OPTiM phase Ⅲ clinical trial[J]. Annals of Surgical Oncology, 2016, 23(13): 4169-4177. |
| 61 | BOMMAREDDY P K, PATEL A, HOSSAIN S, et al. Talimogene laherparepvec (T-VEC) and other oncolytic viruses for the treatment of melanoma[J]. American Journal of Clinical Dermatology, 2017, 18(1): 1-15. |
| 62 | JOHNSON D B, PUZANOV I, KELLEY M C. Talimogene laherparepvec (T-VEC) for the treatment of advanced melanoma[J]. Immunotherapy, 2015, 7(6): 611-619. |
| 63 | ALCAZER V, BONAVENTURA P, TONON L, et al. Neoepitopes-based vaccines: challenges and perspectives[J]. European Journal of Cancer, 2019, 108: 55-60. |
| 64 | BLASS E, OTT P A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines[J]. Nature Reviews Clinical Oncology, 2021, 18(4): 215-229. |
| 65 | YARCHOAN M, JOHNSON B A 3 RD, LUTZ E R,et al. Targeting neoantigens to augment antitumour immunity[J]. Nature Reviews Cancer, 2017, 17(9): 569. |
| 66 | TWUMASI-BOATENG K, PETTIGREW J L, EUNICE KWOK Y Y E, et al. Oncolytic viruses as engineering platforms for combination immunotherapy[J]. Nature Reviews Cancer, 2018, 18(7): 419-432. |
| 67 | GERLACH T, ELBAHESH H, SALETTI G, et al. Recombinant influenza A viruses as vaccine vectors[J]. Expert Review of Vaccines, 2019, 18(4): 379-392. |
| 68 | JI D Z, ZHANG Y J, SUN J Q, et al. An engineered influenza virus to deliver antigens for lung cancer vaccination[J/OL]. Nature Biotechnology, 2023[2023-12-01]. . |
| 69 | SHARMA P, ALLISON J P. The future of immune checkpoint therapy[J]. Science, 2015, 348(6230): 56-61. |
| 70 | WEI S C, DUFFY C R, ALLISON J P. Fundamental mechanisms of immune checkpoint blockade therapy[J]. Cancer Discovery, 2018, 8(9): 1069-1086. |
| 71 | WANG G, KANG X, CHEN K S, et al. An engineered oncolytic virus expressing PD-L1 inhibitors activates tumor neoantigen-specific T cell responses[J]. Nature Communications, 2020, 11(1): 1395. |
| 72 | RIBAS A, DUMMER R, PUZANOV I, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy[J]. Cell, 2017, 170 (6): 1109-1119 e10. |
| 73 | HILDNER K, EDELSON B T, PURTHA W E, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity[J]. Science, 2008, 322(5904): 1097-1100. |
| 74 | BOMMAREDDY P K, SHETTIGAR M, KAUFMAN H L. Integrating oncolytic viruses in combination cancer immunotherapy[J]. Nature Reviews Immunology, 2018, 18(8): 498-513. |
| 75 | RUSSELL S J, BARBER G N. Oncolytic viruses as antigen-agnostic cancer vaccines[J]. Cancer Cell, 2018, 33(4): 599-605. |
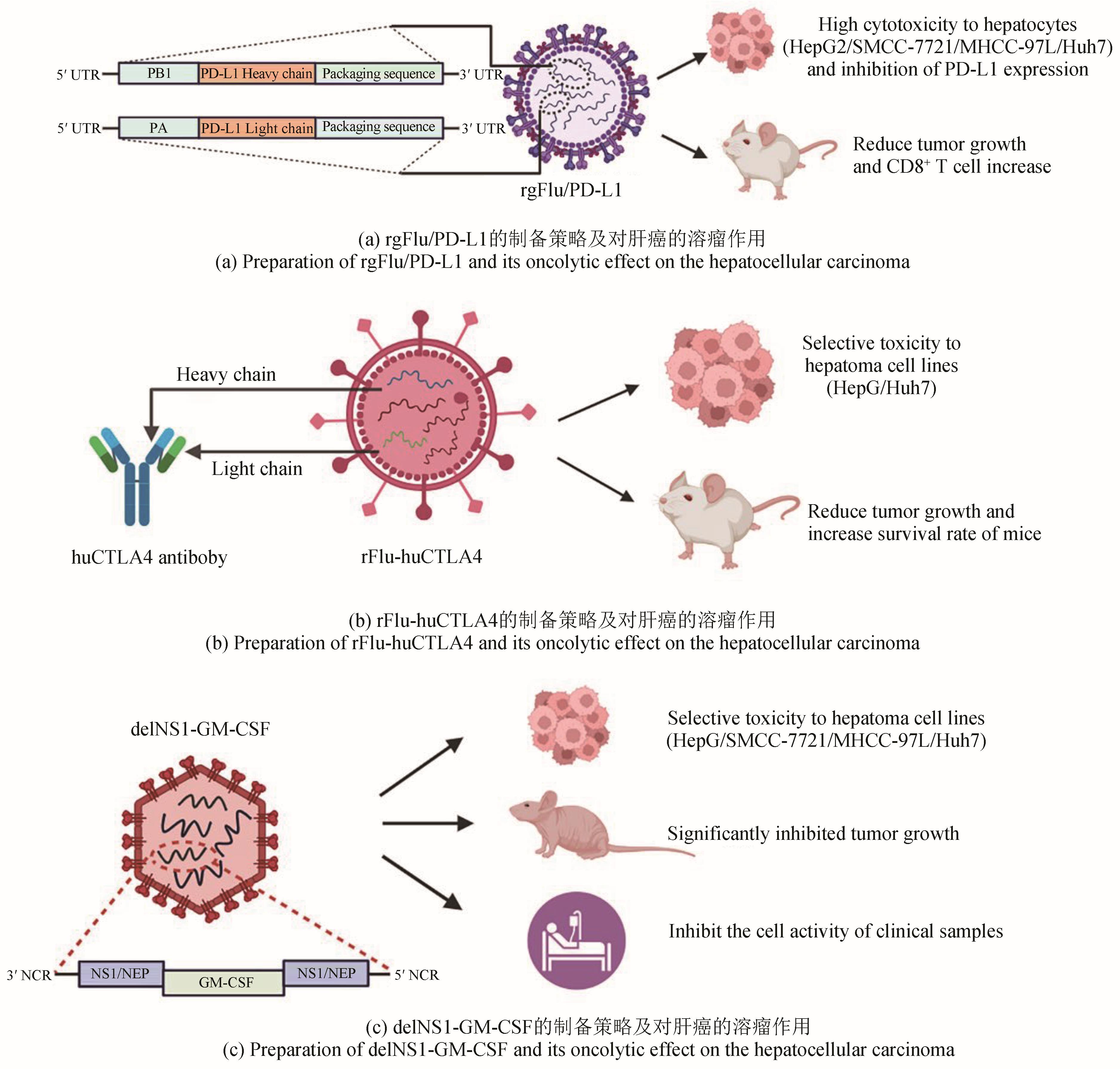
| 76 | SUN F, XU Y, DENG Z Y, et al. A recombinant oncolytic influenza virus expressing a PD-L1 antibody induces CD8+ T-cell activation via the cGas-STING pathway in mice with hepatocellular carcinoma[J]. International Immunopharmacology, 2023, 120: 110323. |
| 77 | HOSSEINI A, GHARIBI T, MAROFI F, et al. CTLA-4: from mechanism to autoimmune therapy[J]. International Immunopharmacology, 2020, 80: 106221. |
| 78 | YANG H, LEI G L, SUN F, et al. Oncolytic activity of a chimeric influenza A virus carrying a human CTLA4 antibody in hepatocellular carcinoma[J]. Frontiers in Oncology, 2022, 12: 875525. |
| 79 | LEI G L, WANG L P, DONG S H, et al. A recombinant influenza virus with a CTLA4-specific scFv inhibits tumor growth in a mouse model[J]. Cell Biology International, 2021, 45(6): 1202-1210. |
| 80 | HAMILTON J R, VIJAYAKUMAR G, PALESE P. A recombinant antibody-expressing influenza virus delays tumor growth in a mouse model[J]. Cell Reports, 2018, 22(1): 1-7. |
| 81 | BURDACH S E, MÜSCHENICH M, JOSEPHS W, et al. Granulocyte-macrophage-colony stimulating factor for prevention of neutropenia and infections in children and adolescents with solid tumors. Results of a prospective randomized study[J]. Cancer, 1995, 76(3): 510-516. |
| 82 | JAHAN N, TALAT H, CURRY W T. Agonist OX40 immunotherapy improves survival in glioma-bearing mice and is complementary with vaccination with irradiated GM-CSF-expressing tumor cells[J]. Neuro-Oncology, 2018, 20(1): 44-54. |
| 83 | YANG P H, SUN F, WANG R L, et al. Oncolytic activity of a novel influenza A virus carrying granulocyte-macrophage colony-stimulating factor in hepatocellular carcinoma[J]. Human Gene Therapy, 2019, 30(3): 330-338. |
| 84 | LIPKIN W I. Biocontainment in gain-of-function infectious disease research[J]. mBio, 2012, 3(5): e00290-12. |
| 85 | SEIDEL J A, OTSUKA A, KABASHIMA K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations[J]. Frontiers in Oncology, 2018, 8: 86. |
| [1] | 叶青, 秦成峰. “国际公共卫生紧急事件”下的mRNA疫苗研发[J]. 合成生物学, 2024, 5(2): 310-320. |
| [2] | 刘泽众, 周洁, 朱赟, 陆路, 姜世勃. 基于重组人Ⅲ型胶原蛋白的三聚体抗原疫苗策略在新冠和流感疫苗中的应用[J]. 合成生物学, 2024, 5(2): 385-395. |
| [3] | 周爱林, 刘奕, 巴方, 钟超. 细菌群体感应元件构建和工程应用[J]. 合成生物学, 2021, 2(2): 234-246. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||