合成生物学 ›› 2024, Vol. 5 ›› Issue (6): 1279-1299.DOI: 10.12211/2096-8280.2024-041
几丁质资源生物降解和高值转化的研究进展
张阿磊, 魏国光, 张弛, 陈磊, 周奚, 刘伟, 陈可泉
- 南京工业大学生物与制药工程学院,材料化学工程国家重点实验室,江苏 南京 211816
-
收稿日期:2024-05-13修回日期:2024-08-04出版日期:2024-12-31发布日期:2025-01-10 -
通讯作者:陈可泉 -
作者简介:张阿磊 (1993—),男,副教授,硕士生导师。研究方向为以酶催化技术转化低值含氮大分子(甲壳素、硝化纤维素等)合成高值含氮化学品如材料单体、功能糖等,涉及酶挖掘、酶改造及酶催化过程强化等技术。E-mail:zhangalei@njtech.edu.cn陈可泉 (1982—),男,教授,博士生导师。主要从事材料单体、医药中间体、营养化学品等生物制造研究,研究方向包括:①酶的挖掘、改造与固定化;②细胞工厂的构建与调控;③生物反应过程装备开发与过程强化;④生物基产品制造与工程化。E-mail:kqchen@njtech.edu.cn -
基金资助:国家重点研发计划(2021YFA0911400);国家自然科学基金(22278220);江苏省农业自主创新基金(CX(22)3070)
Research progress on bio-degradation and valuable bio-conversion of chitinous resources
ZHANG Alei, WEI Guoguang, ZHANG Chi, CHEN Lei, ZHOU Xi, LIU Wei, CHEN Kequan
- State Key Laboratory of Materials-Oriented Chemical Engineering,College of Biotechnology and Pharmaceutical Engineering,Nanjing Tech University,Nanjing 211816,Jiangsu,China
-
Received:2024-05-13Revised:2024-08-04Online:2024-12-31Published:2025-01-10 -
Contact:CHEN Kequan
摘要:
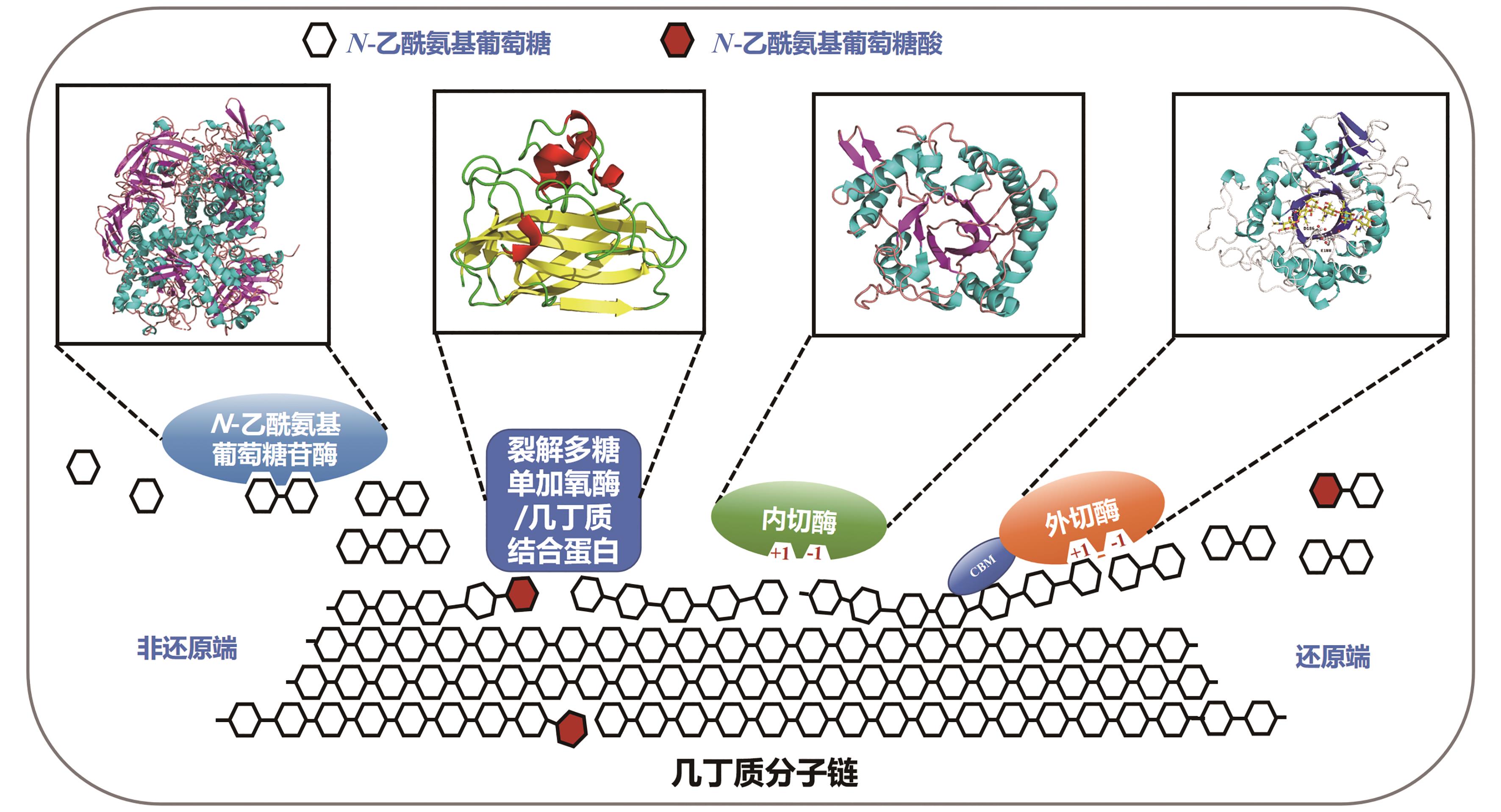
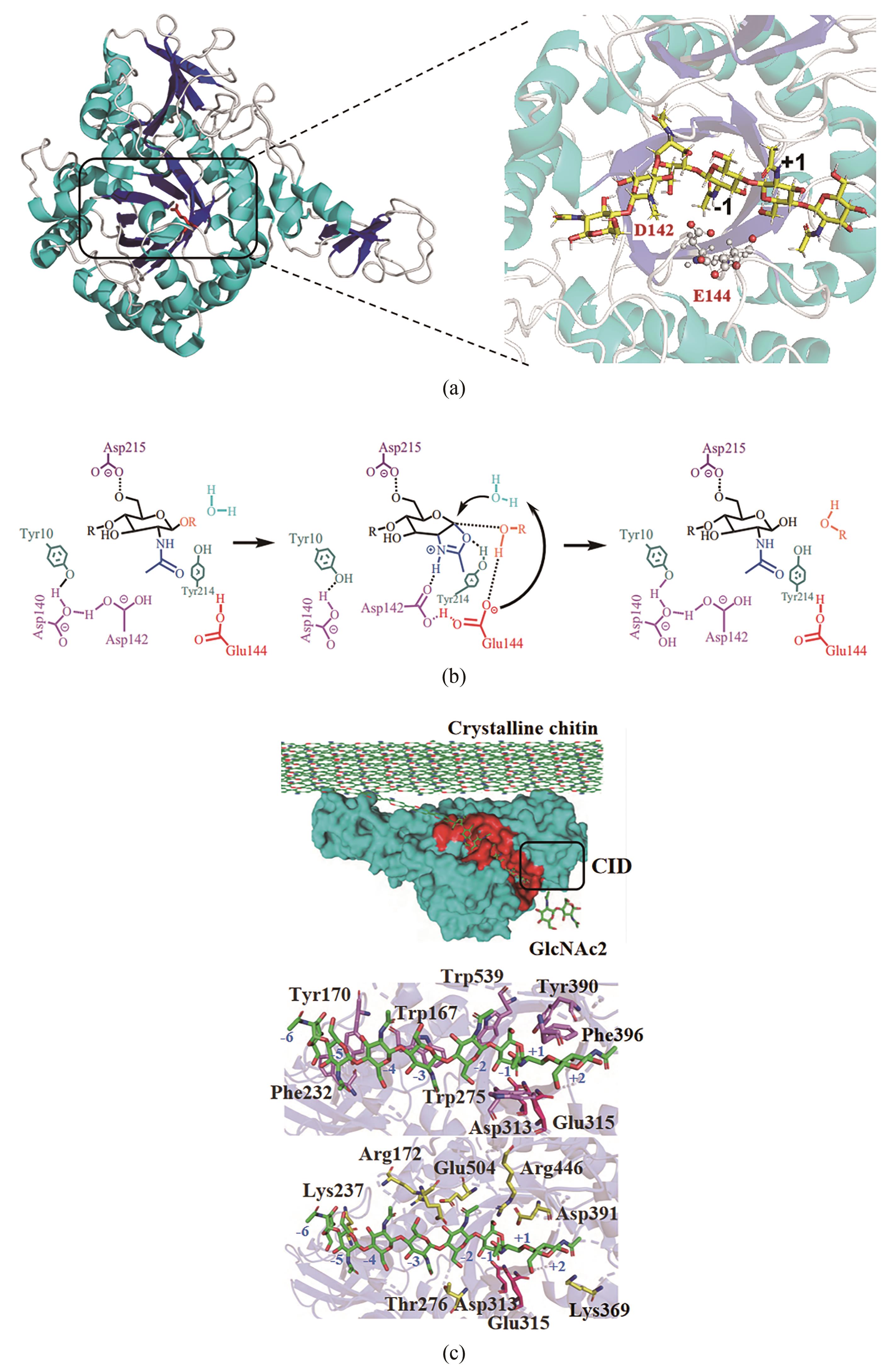
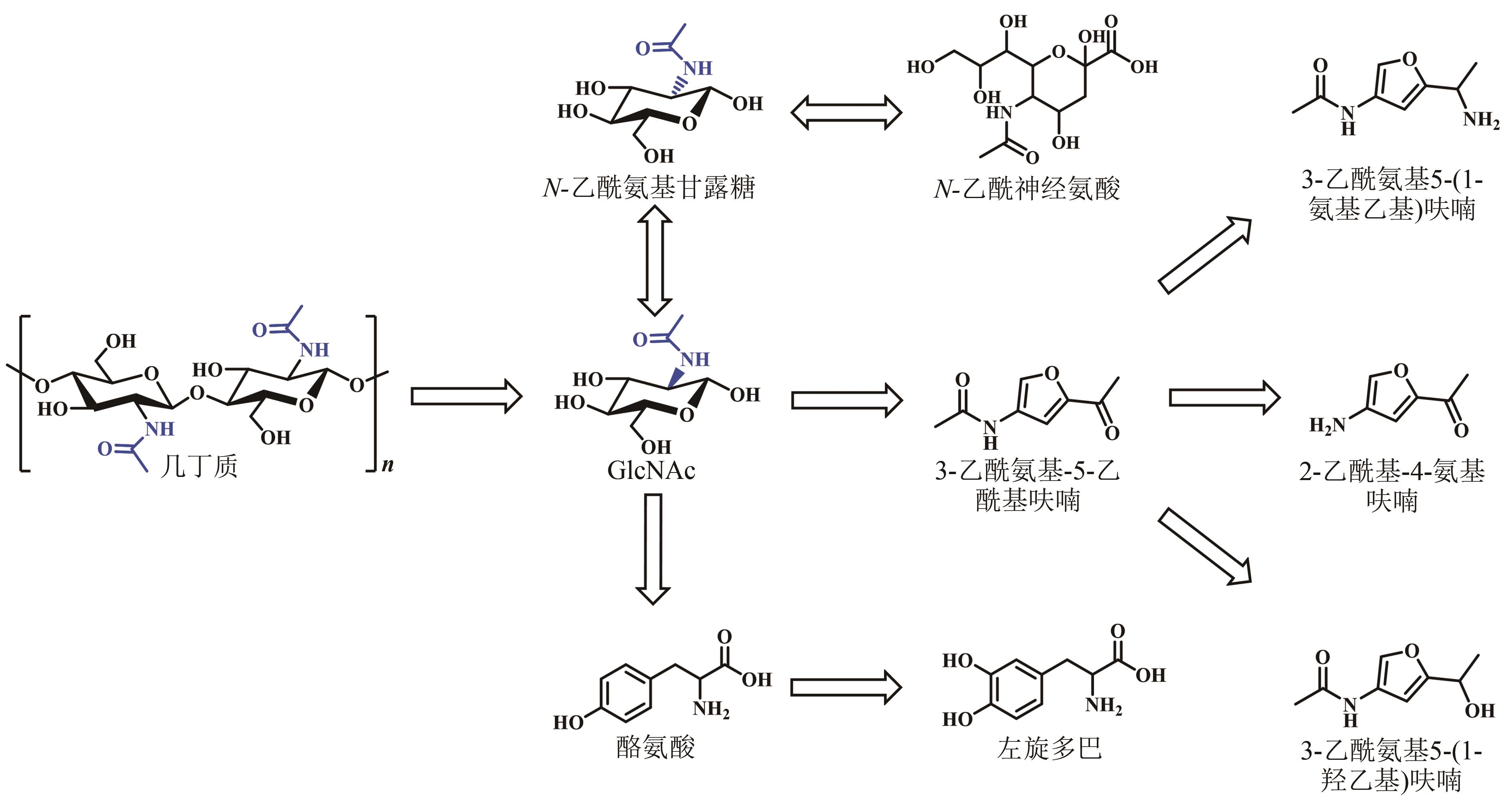
几丁质是由N-乙酰氨基葡萄糖(GlcNAc)通过β-1,4-糖苷键构成的高分子聚合物,是地球上储量最丰富的含氮生物质资源,在自然界分布广泛,主要存在于虾蟹外壳、昆虫外骨骼和真菌细胞壁中。由于几丁质含量巨大、可再生,特别是含有珍贵的氮元素,其资源化利用一直受到广泛关注。然而几丁质结构中丰富的氢键作用力与巨大的分子量,赋予了其高结晶度和不溶于水的特性,导致其降解和高值化利用受到挑战,因此常被作为垃圾丢弃或掩埋,污染环境的同时浪费资源。在几丁质降解利用的众多方法中,生物法因过程环保、反应条件温和等优点,在绿色可持续发展的大背景下展现出巨大潜力。本文首先系统介绍了自然界中催化几丁质降解关键酶的来源与分类、催化机制及特性。其次综述了生物法降解几丁质为单糖(GlcNAc和氨基葡萄糖)和寡糖(几丁寡糖和壳寡糖),以及进一步生物转化合成含氮化合物的现状。最后阐述了几丁质生物降解和高值转化过程中所面临的几丁质降解与转化酶活性低、效率差及成本高昂等诸多挑战,展望了发展迅速的合成生物学在几丁质生物转化中的重要作用,这将为几丁质资源的高效生物炼制提供助力。
中图分类号:
引用本文
张阿磊, 魏国光, 张弛, 陈磊, 周奚, 刘伟, 陈可泉. 几丁质资源生物降解和高值转化的研究进展[J]. 合成生物学, 2024, 5(6): 1279-1299.
ZHANG Alei, WEI Guoguang, ZHANG Chi, CHEN Lei, ZHOU Xi, LIU Wei, CHEN Kequan. Research progress on bio-degradation and valuable bio-conversion of chitinous resources[J]. Synthetic Biology Journal, 2024, 5(6): 1279-1299.
| 生物类型 | 来源分类 | 几丁质含量 |
|---|---|---|
| 节肢动物 | 甲壳纲:虾、蟹等 | 20%~85% |
| 昆虫纲:蝗虫/蝴蝶/蚊/蛾/蝇/蚕等蛹壳中 | 20%~60% | |
| 多足/蛛形纲:马陆、蜈蚣、蜘蛛、蝎子、螨虫等 | 4%~22% | |
| 软体动物 | 双神经/腹足/掘足/瓣鳃/头足纲:鲍鱼、蜗牛、角贝、牡蛎、乌贼等 | 3%~26% |
| 环节动物 | 原环虫/毛足纲:角涡虫、沙蚕、蚯蚓等 | 20%~38% |
| 原生动物 | 鞭毛虫/肉足/孢子虫/纤毛虫纲:锥体虫、变形虫、疟原虫、草履虫等 | 极少 |
| 腔肠动物 | 水螅虫/钵水母/珊瑚虫纲:水螅、筒螅、海月水母、海蜇、霞水母等 | 3%~30% |
| 海藻 | 主要是绿藻 | 少量 |
| 真菌 | 囊菌、担子菌、藻菌等 | 微量至45% |
| 动物关节 | 蹄、足的坚硬部分、动物肌肉、骨结合处等 | 少量 |
表1 几丁质的来源与含量[20]
Table 1 Sources and contents of chitin[20]
| 生物类型 | 来源分类 | 几丁质含量 |
|---|---|---|
| 节肢动物 | 甲壳纲:虾、蟹等 | 20%~85% |
| 昆虫纲:蝗虫/蝴蝶/蚊/蛾/蝇/蚕等蛹壳中 | 20%~60% | |
| 多足/蛛形纲:马陆、蜈蚣、蜘蛛、蝎子、螨虫等 | 4%~22% | |
| 软体动物 | 双神经/腹足/掘足/瓣鳃/头足纲:鲍鱼、蜗牛、角贝、牡蛎、乌贼等 | 3%~26% |
| 环节动物 | 原环虫/毛足纲:角涡虫、沙蚕、蚯蚓等 | 20%~38% |
| 原生动物 | 鞭毛虫/肉足/孢子虫/纤毛虫纲:锥体虫、变形虫、疟原虫、草履虫等 | 极少 |
| 腔肠动物 | 水螅虫/钵水母/珊瑚虫纲:水螅、筒螅、海月水母、海蜇、霞水母等 | 3%~30% |
| 海藻 | 主要是绿藻 | 少量 |
| 真菌 | 囊菌、担子菌、藻菌等 | 微量至45% |
| 动物关节 | 蹄、足的坚硬部分、动物肌肉、骨结合处等 | 少量 |
| 酶 | 活性 | 酶活 | 参考文献 |
|---|---|---|---|
| RFChiA | 持续性外切 | 6.9 U/mg | [ |
| Chit46 | 内切 | 9.5 U/mg | [ |
| ActChi | 持续性外切 | 3.7 U/mg | [ |
| Chi304 | 内切、外切 | ND | [ |
| Chit33 | 内切 | 2.7 U/mg | [ |
| R-SaChiA4 | 内切 | 28 U/mg | [ |
| CmChi1 | 内切、外切、N-乙酰氨基葡萄糖苷酶 | 1.1 U/mg | [ |
表2 可降解结晶几丁质的酶
Table 2 Enzymes capable of catalyzing the degradation of crystalline chitin
| 酶 | 活性 | 酶活 | 参考文献 |
|---|---|---|---|
| RFChiA | 持续性外切 | 6.9 U/mg | [ |
| Chit46 | 内切 | 9.5 U/mg | [ |
| ActChi | 持续性外切 | 3.7 U/mg | [ |
| Chi304 | 内切、外切 | ND | [ |
| Chit33 | 内切 | 2.7 U/mg | [ |
| R-SaChiA4 | 内切 | 28 U/mg | [ |
| CmChi1 | 内切、外切、N-乙酰氨基葡萄糖苷酶 | 1.1 U/mg | [ |
| 来源 | 酶 | 底物 | 产物 | 寡糖产率/产量 | 参考文献 |
|---|---|---|---|---|---|
| Salinivibrio sp. BAO-1801 | 野生几丁质酶 | 胶体几丁质 | 几丁二糖,少量GlcNAc | 71.5% | [ |
| T. gamsii R1 | 野生几丁质酶 | 胶体几丁质 | 几丁二糖、三糖 | 11.62 g/L,1.92 g/L | [ |
| S. marcescens | SmChiB | 胶体几丁质 | 几丁二糖 | 2.04 g/L | [ |
| 堆肥宏基因组 | ActChi | 粉粒几丁质 | 几丁二糖 | 17% | [ |
| T. harzianum | rChit46 | 胶体几丁质 | 几丁二糖,微量GlcNAc | 94.8% | [ |
| P. barengoltzii | PbChi70 | 胶体几丁质 | 几丁二糖,微量GlcNAc | 21.6 g/L,89.5% | [ |
| F. johnsoniae UW101 | FjChiB | 胶体几丁质 | 几丁二糖、三糖 | — | [ |
| S. marcescens | rCHI-2 | 胶体几丁质 | 几丁二糖,微量GlcNAc | — | [ |
| M. thermophila C1 | Chi1 | 溶胀几丁质 | 几丁二糖,微量GlcNAc | — | [ |
| A. fumigatus df347 | AfChi28 | 胶体几丁质 | 几丁二糖到几丁四糖 | — | [ |
| A. media CZW001 | AmChi | 粉粒几丁质 | 几丁五糖、六糖 | — | [ |
| B. aryabhattai | BaChiA | 粉粒几丁质 | 几丁二糖到几丁六糖 | — | [ |
| Corallococcus sp. EGB | CcCti1 | 胶体几丁质 | 几丁二糖到几丁六糖 | — | [ |
表3 酶法降解几丁质生产几丁寡糖
Table 3 Production of N-acetyl chitooligosaccharides from enzymatic degradation of chitin
| 来源 | 酶 | 底物 | 产物 | 寡糖产率/产量 | 参考文献 |
|---|---|---|---|---|---|
| Salinivibrio sp. BAO-1801 | 野生几丁质酶 | 胶体几丁质 | 几丁二糖,少量GlcNAc | 71.5% | [ |
| T. gamsii R1 | 野生几丁质酶 | 胶体几丁质 | 几丁二糖、三糖 | 11.62 g/L,1.92 g/L | [ |
| S. marcescens | SmChiB | 胶体几丁质 | 几丁二糖 | 2.04 g/L | [ |
| 堆肥宏基因组 | ActChi | 粉粒几丁质 | 几丁二糖 | 17% | [ |
| T. harzianum | rChit46 | 胶体几丁质 | 几丁二糖,微量GlcNAc | 94.8% | [ |
| P. barengoltzii | PbChi70 | 胶体几丁质 | 几丁二糖,微量GlcNAc | 21.6 g/L,89.5% | [ |
| F. johnsoniae UW101 | FjChiB | 胶体几丁质 | 几丁二糖、三糖 | — | [ |
| S. marcescens | rCHI-2 | 胶体几丁质 | 几丁二糖,微量GlcNAc | — | [ |
| M. thermophila C1 | Chi1 | 溶胀几丁质 | 几丁二糖,微量GlcNAc | — | [ |
| A. fumigatus df347 | AfChi28 | 胶体几丁质 | 几丁二糖到几丁四糖 | — | [ |
| A. media CZW001 | AmChi | 粉粒几丁质 | 几丁五糖、六糖 | — | [ |
| B. aryabhattai | BaChiA | 粉粒几丁质 | 几丁二糖到几丁六糖 | — | [ |
| Corallococcus sp. EGB | CcCti1 | 胶体几丁质 | 几丁二糖到几丁六糖 | — | [ |
| 酶(来源) | 底物 | GlcNAc浓度/(g/L) | 转化率 | 参考文献 |
|---|---|---|---|---|
C. meiyuanensis SYBC-H1发酵液 | 粉粒几丁质 | 39.3 | 98% | [ |
| 微生物发酵处理几丁质 | 19.2 | 96% | [ | |
| 超声处理几丁质 | 2.65 | 100% | [ | |
| 高压均质小龙虾壳 | 3.9 | — | [ | |
| 有机溶剂预处理几丁质 | 4.6~7.6 | 96% | [ | |
| 碱冻融处理几丁质 | 75 | 98% | [ | |
| A. caviae CH129发酵液 | 胶体几丁质 | — | 93% | [ |
| A. terreus 发酵液 | 膨胀几丁质 | 46 | 92% | [ |
| S. proteamaculans NJ303发酵液 | 高压均质小龙虾壳 | 3.9 | 78% | [ |
| T. harzianum发酵液 | 冻干几丁质粉 | 14 | 80% | [ |
| S. albolongus 发酵液 | 胶体几丁质 | 4.4 | 89% | [ |
| ScChiC, ScHEX | 粉粒几丁质 | 9.4 | 94% | [ |
| SaChiA4, SvNag2557 | 胶体几丁质 | 8.0 | 80% | [ |
| ChiA, BsNagZ | 胶体几丁质 | — | 88% | [ |
| BpChiA, BlNagZ | 胶体几丁质 | — | 64% | [ |
| CmChi1 | 胶体几丁质 | 9.8 | 98% | [ |
| ChiG | 胶体几丁质 | — | — | [ |
| AMCase | 胶体几丁质 | 1.2 | 87% | [ |
| PbChi70突变体, NAGase | 胶体几丁质 | — | 97% | [ |
| PbChi74, NAGase | 胶体几丁质 | 27.8 | 93% | [ |
表4 酶法降解几丁质生产GlcNAc
Table 4 Production of GlcNAc from enzymatic degradation of chitin
| 酶(来源) | 底物 | GlcNAc浓度/(g/L) | 转化率 | 参考文献 |
|---|---|---|---|---|
C. meiyuanensis SYBC-H1发酵液 | 粉粒几丁质 | 39.3 | 98% | [ |
| 微生物发酵处理几丁质 | 19.2 | 96% | [ | |
| 超声处理几丁质 | 2.65 | 100% | [ | |
| 高压均质小龙虾壳 | 3.9 | — | [ | |
| 有机溶剂预处理几丁质 | 4.6~7.6 | 96% | [ | |
| 碱冻融处理几丁质 | 75 | 98% | [ | |
| A. caviae CH129发酵液 | 胶体几丁质 | — | 93% | [ |
| A. terreus 发酵液 | 膨胀几丁质 | 46 | 92% | [ |
| S. proteamaculans NJ303发酵液 | 高压均质小龙虾壳 | 3.9 | 78% | [ |
| T. harzianum发酵液 | 冻干几丁质粉 | 14 | 80% | [ |
| S. albolongus 发酵液 | 胶体几丁质 | 4.4 | 89% | [ |
| ScChiC, ScHEX | 粉粒几丁质 | 9.4 | 94% | [ |
| SaChiA4, SvNag2557 | 胶体几丁质 | 8.0 | 80% | [ |
| ChiA, BsNagZ | 胶体几丁质 | — | 88% | [ |
| BpChiA, BlNagZ | 胶体几丁质 | — | 64% | [ |
| CmChi1 | 胶体几丁质 | 9.8 | 98% | [ |
| ChiG | 胶体几丁质 | — | — | [ |
| AMCase | 胶体几丁质 | 1.2 | 87% | [ |
| PbChi70突变体, NAGase | 胶体几丁质 | — | 97% | [ |
| PbChi74, NAGase | 胶体几丁质 | 27.8 | 93% | [ |
| 1 | ZHAO H B, HOLLADAY J E, BROWN H, et al. Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural[J]. Science, 2007, 316(5831): 1597-1600. |
| 2 | KUMARI S, RATH P, HARI KUMAR A SRI, et al. Extraction and characterization of chitin and chitosan from fishery waste by chemical method[J]. Environmental Technology & Innovation, 2015, 3: 77-85. |
| 3 | WAN A C A, TAI B C U. CHITIN—a promising biomaterial for tissue engineering and stem cell technologies[J]. Biotechnology Advances, 2013, 31(8): 1776-1785. |
| 4 | YAN N, CHEN X. Sustainability: don’t waste seafood waste[J]. Nature, 2015, 524(7564): 155-157. |
| 5 | 王爱勤. 甲壳素化学[M]. 北京: 科学出版社, 2008. |
| WANG A Q. Chitin chemistry[M]. Beijing: Science Press, 2008. | |
| 6 | CHEN X, YAN N. Novel catalytic systems to convert chitin and lignin into valuable chemicals[J]. Catalysis Surveys from Asia, 2014, 18(4): 164-176. |
| 7 | SHELDON R A. Green and sustainable manufacture of chemicals from biomass: state of the art[J]. Green Chemistry, 2014, 16(3): 950-963. |
| 8 | KAUR S, DHILLON G S. Recent trends in biological extraction of chitin from marine shell wastes: a review[J]. Critical Reviews in Biotechnology, 2015, 35(1): 44-61. |
| 9 | NASEEM S, PARRINO S M, BUENTEN D M, et al. Novel roles for GlcNAc in cell signaling[J]. Communicative & Integrative Biology, 2012, 5(2): 156-159. |
| 10 | RICHTER J, CAPKOVÁ K, HŘÍBALOVÁ V, et al. Collagen-induced arthritis: severity and immune response attenuation using multivalent N-acetyl glucosamine[J]. Clinical and Experimental Immunology, 2014, 177(1): 121-133. |
| 11 | 曹秀明. 壳寡糖及衍生物抗肿瘤作用、免疫调节作用及其机制的研究[D]. 青岛: 中国海洋大学, 2010. |
| CAO X M. Research on the anti-tumor and immunomodulatory effects and mechanisms of chitosan oligosaccharides and their derivatives [D]. Qingdao: Ocean University of China, 2010. | |
| 12 | 李兆申. N-乙酰氨基葡萄糖治疗腹泻型肠易激综合征多中心临床研究[J]. 中华消化杂志, 2009, 29(4): 267-270. |
| LI Z S. Treatment of diarrhea-predominant irritable bowel syndrome with N-acetyl-D-glucosamine: a randomized, double-blind, placebo-controlled multi-center study coorperative group[J]. Chinese Journal of Digestion, 2009, 29(4): 267-270. | |
| 13 | 张虎, 杜昱. 几丁寡糖与壳寡糖制备和功能[C]// 中国甲壳资源研究开发应用学术研讨会, 1997. |
| ZHANG H, DU Y. Preparation and function of chitosan oligosaccharides and chitosan oligosaccharides [C]// the academic symposium on the research, Development and Application of Chinese Crustacean Resources, 1997. | |
| 14 | SHAN J W, XUN J C. Preparation method of chitin oligosaccharide zinc borone magnesium fertilizer: CN1597634[P]. 2004-08-27. |
| 15 | DAI J H, LI F K, FU X. Towards shell biorefinery: advances in chemical-catalytic conversion of chitin biomass to organonitrogen chemicals[J]. ChemSusChem, 2020, 13(24): 6498-6508. |
| 16 | CHEN X, SONG S, LI H Y, et al. Expanding the boundary of biorefinery: organonitrogen chemicals from biomass[J]. Accounts of Chemical Research, 2021, 54(7): 1711-1722. |
| 17 | JI X L, ZHAO Y F, LUI M Y, et al. Catalytic conversion of chitin-based biomass to nitrogen-containing chemicals[J]. iScience, 2024, 27(6): 109857. |
| 18 | ILANKOVAN P, HEIN S, NG C H, et al. Production of N-acetyl chitobiose from various chitin substrates using commercial enzymes[J]. Carbohydrate Polymers, 2006, 63(2): 245-250. |
| 19 | IL’INA A V, O Yu ZUEVA, LOPATIN S A, et al. Enzymatic hydrolysis of α-chitin[J]. Applied Biochemistry and Microbiology, 2004, 40(1): 35-38. |
| 20 | MULISCH M. Chitin in protistan organisms: distribution, synthesis and deposition[J]. European Journal of Protistology, 1993, 29(1): 1-18. |
| 21 | 伍军, 毛宏辉. 从麻辣小龙虾虾壳中提取甲壳素的研究[J]. 粮油加工, 2008(10): 128-130. |
| WU J, MAO H H. Study on extracting chitin from shell of spicy crayfish[J]. Cereals and Oils Processing, 2008(10): 128-130. | |
| 22 | ZHANG J, XU W R, ZHANG Y C. Facile production of chitin from shrimp shells using a deep eutectic solvent and acetic acid[J]. RSC Advances, 2022, 12(35): 22631-22638. |
| 23 | SETOGUCHI T, KATO T, YAMAMOTO K, et al. Facile production of chitin from crab shells using ionic liquid and citric acid[J]. International Journal of Biological Macromolecules, 2012, 50(3): 861-864. |
| 24 | ZHU P, GU Z J, HONG S, et al. One-pot production of chitin with high purity from lobster shells using choline chloride-malonic acid deep eutectic solvent[J]. Carbohydrate Polymers, 2017, 177: 217-223. |
| 25 | SOROKULOVA I, KRUMNOW A, GLOBA L, et al. Efficient decomposition of shrimp shell waste using Bacillus cereus and Exiguobacterium acetylicum [J]. Journal of Industrial Microbiology & Biotechnology, 2009, 36(8): 1123-1126. |
| 26 | 李磊. 以嗜酸乳杆菌SWO1发酵虾头虾壳提取蛋白质和甲壳素的工艺研究[D]. 广州: 华南农业大学, 2011. |
| LI L. Study on the process of extracting protein and chitin from shrimp head shells using Lactobacillus acidophilus SWO1 fermentation[D]. Guangzhou: South China Agricultural University, 2011. | |
| 27 | 刘斯雅, 林瑞君, 庄泽娟, 等. 植物乳杆菌发酵虾头、虾壳回收蛋白质和甲壳素的研究[J]. 现代食品科技, 2011, 27(4): 408-411, 383. |
| LIU S Y, LIN R J, ZHUANG Z J, et al. Recovery of protein and chitin from shrimp waste by lactic acid fermentation with l.plantarum[J]. Modern Food Science and Technology, 2011, 27(4): 408-411, 383. | |
| 28 | KHANAFARI A, MARANDI R, SANATEI S. Recovery of chitin and chitosan from shrimp waste by chemical and microbial methods[J]. Iranian Journal of Enviromental Health Science & Engineering, 2008, 5(1): 19-24. |
| 29 | JANG M K, KONG B G, JEONG Y I, et al. Physicochemical characterization of α-chitin, β-chitin, and γ-chitin separated from natural resources[J]. Journal of Polymer Science Part A: Polymer Chemistry, 2004, 42(14): 3423-3432. |
| 30 | FENG F, LIU Y, HU K A. Influence of alkali-freezing treatment on the solid state structure of chitin[J]. Carbohydrate Research, 2004, 339(13): 2321-2324. |
| 31 | GHOSH M, SADHUKHAN S, DEY K K. Elucidating the internal structure and dynamics of α-chitin by 2DPASS-MAS-NMR and spin-lattice relaxation measurements[J]. Solid State Nuclear Magnetic Resonance, 2019, 97: 7-16. |
| 32 | GUGGOLZ T, HENNE S, POLITI Y, et al. Histochemical evidence of β-chitin in parapodial glandular organs and tubes of Spiophanes (Annelida, Sedentaria: Spionidae), and first studies on selected Annelida[J]. Journal of Morphology, 2015, 276(12): 1433-1447. |
| 33 | GARDNER K H, BLACKWELL J. Refinement of the structure of beta-chitin[J]. Biopolymers, 1975, 14(8): 1581-1595. |
| 34 | SUBRAMANI A K, RAVAL R, SUNDARESHAN S, et al. A marine chitinase from Bacillus aryabhattai with antifungal activity and broad specificity toward crystalline chitin degradation[J]. Preparative Biochemistry & Biotechnology, 2022, 52(10): 1160-1172. |
| 35 | AKRAM F, AKRAM R, UL HAQ I, et al. Biotechnological eminence of chitinases: a focus on thermophilic enzyme sources, production strategies and prominent applications[J]. Protein and Peptide Letters, 2021, 28(9): 1009-1022. |
| 36 | JUPATANAKUL N, PENGON J, SELISANA S M G, et al. Serratia marcescens secretes proteases and chitinases with larvicidal activity against Anopheles dirus [J]. Acta Tropica, 2020, 212: 105686. |
| 37 | JEONG H C, JU W T, JO K H, et al. Purification and characterization of a 34-kDa chitobiosidase from Aeromonas sp. GJ-18[J]. Journal of the Korean Society for Applied Biological Chemistry, 2012, 55(1): 7-12. |
| 38 | ZHANG A L, MO X F, WEI G G, et al. The draft genome sequence and analysis of an efficiently chitinolytic bacterium Chitinibacter sp. strain GC72[J]. Current Microbiology, 2020, 77(12): 3903-3908. |
| 39 | LI Z K, XIA C Y, WANG Y X, et al. Identification of an endo-chitinase from Corallococcus sp. EGB and evaluation of its antifungal properties[J]. International Journal of Biological Macromolecules, 2019, 132: 1235-1243. |
| 40 | HAO Z K, LI J S, WANG D H, et al. Efficient production of GlcNAc in an aqueous-organic system with a Chitinolyticbacter meiyuanensis SYBC-H1 mutant[J]. Biotechnology Letters, 2022, 44(4): 623-633. |
| 41 | NUERO O M. Production of chitinase by Fusarium species[J]. Current Microbiology, 1995, 30(5): 287-289. |
| 42 | BINOD P, PUSZTAHELYI T, NAGY V, et al. Production and purification of extracellular chitinases from Penicillium aculeatum NRRL 2129 under solid-state fermentation[J]. Enzyme and Microbial Technology, 2005, 36(7): 880-887. |
| 43 | KHAN F I, BISETTY K, SINGH S, et al. Chitinase from Thermomyces lanuginosus SSBP and its biotechnological applications[J]. Extremophiles, 2015, 19(6): 1055-1066. |
| 44 | PRABAVATHY V R, MATHIVANAN N, SAGADEVAN E, et al. Self-fusion of protoplasts enhances chitinase production and biocontrol activity in Trichoderma harzianum [J]. Bioresource Technology, 2006, 97(18): 2330-2334. |
| 45 | GAO F, ZHANG B S, ZHAO J H, et al. Deacetylation of chitin oligomers increases virulence in soil-borne fungal pathogens[J]. Nature Plants, 2019, 5(11): 1167-1176. |
| 46 | HANSEN L D, ØSTENSEN M, ARSTAD B, et al. 2-Naphthol impregnation prior to steam explosion promotes LPMO-assisted enzymatic saccharification of spruce and yields high-purity lignin[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(16): 5233-5242. |
| 47 | LIU F, SELIN C, ZOU Z W, et al. LmCBP1, a secreted chitin-binding protein, is required for the pathogenicity of Leptosphaeria maculans on Brassica napus [J]. Fungal Genetics and Biology, 2020, 136: 103320. |
| 48 | THOMPSON C E. Erratum: molecular evolution and transcriptional profile of GH3 and GH20 β-N-acetylglucosaminidases in the entomopathogenic fungus Metarhizium anisopliae [J]. Genetics and Molecular Biology, 2019, 42(1): 151. |
| 49 | HENRISSAT B, BAIROCH A. Updating the sequence-based classification of glycosyl hydrolases[J]. Biochemical Journal, 1996, 316(Pt 2): 695-696. |
| 50 | CAO S N, GAO P, XIA W S, et al. Cloning and characterization of a novel GH75 family chitosanase from Penicillium oxalicum M2[J]. Process Biochemistry, 2022, 120: 41-52. |
| 51 | 王治伟, 刘志敏. 微生物几丁质酶研究进展[J]. 生物技术通讯, 2006, 17(3): 439-442. |
| WANG Z W, LIU Z M. Advance in study and application on chitinase produced by microbes[J]. Letters in Biotechnology, 2006, 17(3): 439-442. | |
| 52 | CHEN Y, ZHOU N, CHEN X M, et al. Characterization of a new multifunctional GH20 β-N-acetylglucosaminidase from Chitinibacter sp. GC72 and its application in converting chitin into N-acetyl glucosamine[J]. Frontiers in Microbiology, 2022, 13: 874908. |
| 53 | SUN X M, LI Y J, TIAN Z N, et al. A novel thermostable chitinolytic machinery of Streptomyces sp. F-3 consisting of chitinases with different action modes[J]. Biotechnology for Biofuels, 2019, 12: 136. |
| 54 | DAHIYA D, PILLI A, CHIRRA P R R, et al. Morphological and structural characterization of chitin as a substrate for the screening, production, and molecular characterization of chitinase by Bacillus velezensis [J]. Environmental Science and Pollution Research, 2022, 29(57): 86550-86561. |
| 55 | CHAULAGAIN D, SHAMABADI N S, LESLIE S A, et al. From natural microbe screening to sustained chitinase activity in exogenous hosts[J]. ACS Synthetic Biology, 2024, 13(4): 1165-1176. |
| 56 | ZHANG A L, MO X F, ZHOU N, et al. Identification of chitinolytic enzymes in Chitinolyticbacter meiyuanensis and mechanism of efficiently hydrolyzing chitin to N-acetyl glucosamine[J]. Frontiers in Microbiology, 2020, 11: 572053. |
| 57 | 郝之奎. Chitinolyticbacter meiyuanensis的筛选鉴定及其发酵产几丁质酶研究[D]. 无锡: 江南大学, 2011. |
| HAO Z K. Screening and identification of Chitinolyticbacter meiyuanensis and its fermentation for chitinase production[D]. Wuxi: Jiangnan University, 2011. | |
| 58 | STAM M, LANGLOIS J, CHEVALIER C, et al. NetSyn: genomic context exploration of protein families[EB/OL]. bioRxiv, 2023, 2023.02.15.528638. (2023-02-15)[2024-03-01] .. |
| 59 | LIU H W, ZHANG B, LI C S, et al. Knock down of chitosanase expression in phytopathogenic fungus Fusarium solani and its effect on pathogenicity[J]. Current Genetics, 2010, 56(3): 275-281. |
| 60 | LACOMBE-HARVEY M È, BRZEZINSKI R, BEAULIEU C. Chitinolytic functions in Actinobacteria: ecology, enzymes, and evolution[J]. Applied Microbiology and Biotechnology, 2018, 102(17): 7219-7230. |
| 61 | LV C Y, GU T Y, MA R, et al. Biochemical characterization of a GH19 chitinase from Streptomyces alfalfae and its applications in crystalline chitin conversion and biocontrol[J]. International Journal of Biological Macromolecules, 2021, 167: 193-201. |
| 62 | 程爱丽, 唐文华, 王益民. 枯草芽孢杆菌B-908几丁质酶基因的转化及表达[J]. 植物病理学报, 1996, 26(3): 204. |
| CHENG A L, TANG W H, WANG Y M. The transformation and expression of chitinase gene from Bacillus subtilis B-908[J]. Acta Phytopathologica Sinica, 1996, 26(3): 204. | |
| 63 | VAIKUNTAPU P R, MALLAKUNTLA M K, DAS S N, et al. Applicability of endochitinase of Flavobacterium johnsoniae with transglycosylation activity in generating long-chain chitooligosaccharides[J]. International Journal of Biological Macromolecules, 2018, 117: 62-71. |
| 64 | SUN B, ZHAO X C, XU B R, et al. Discovering and designing a chimeric hyperthermophilic chitinase for crystalline chitin degradation[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(12): 4690-4698. |
| 65 | CHEN X M, ZHAI C, KANG L X, et al. High-level expression and characterization of a highly thermostable chitosanase from Aspergillus fumigatus in Pichia pastoris [J]. Biotechnology Letters, 2012, 34(4): 689-694. |
| 66 | WANG X H, CHI N Y, BAI F W, et al. Characterization of a cold-adapted and salt-tolerant exo-chitinase (ChiC) from Pseudoalteromonas sp. DL-6[J]. Extremophiles, 2016, 20(2): 167-176. |
| 67 | DAS S, DEY P, ROY D, et al. N-acetyl-D-glucosamine production by a chitinase of marine fungal origin: a case study of potential industrial significance for valorization of waste chitins[J]. Applied Biochemistry and Biotechnology, 2019, 187(1): 407-423. |
| 68 | YANG S Q, FU X, YAN Q J, et al. Cloning, expression, purification and application of a novel chitinase from a thermophilic marine bacterium Paenibacillus barengoltzii [J]. Food Chemistry, 2016, 192: 1041-1048. |
| 69 | XU Y, OUYANG B, DENG L Y, et al. Biochemical characterization of a novel hyperthermophilic chitinase from a deep-sea Thermotogae bacterium[J]. Process Biochemistry, 2024, 143: 60-72. |
| 70 | 王琳, 陈雅如, 程湄婕, 等. 微生物几丁质酶研究进展及应用[J]. 中国生物工程杂志, 2022, 42(12): 101-110. |
| WANG L, CHEN Y R, CHENG M J, et al. Research advances in microbial chitinase and its applications[J]. China Biotechnology, 2022, 42(12): 101-110. | |
| 71 | NOVIKOV V Y. Acid hydrolysis of chitin and chitosan[J]. Russian Journal of Applied Chemistry, 2004, 77(3): 484-487. |
| 72 | ROY I, MONDAL K, GUPTA M N. Accelerating enzymatic hydrolysis of chitin by microwave pretreatment[J]. Biotechnology Progress, 2003, 19(6): 1648-1653. |
| 73 | XING R E, LIU S, YU H H, et al. Salt-assisted acid hydrolysis of chitosan to oligomers under microwave irradiation[J]. Carbohydrate Research, 2005, 340(13): 2150-2153. |
| 74 | SU H P, GAO L, SUN J A, et al. Engineering a carbohydrate binding module to enhance chitinase catalytic efficiency on insoluble chitinous substrate[J]. Food Chemistry, 2021, 355: 129462. |
| 75 | 陈可泉, 周宁, 张阿磊, 等. 人工构建几丁质小体多酶复合体scaford-chiC-chiA-sg的方法及应用: CN112522246A[P]. 2021-03-19. |
| CHEN K Q, ZHOU N, ZHANG A L, et al. Artificial construction of scaford-chiC-chiA-sg, a multi-enzyme complex of chitinosomes, and its application: CN112522246A[P]. 2021-03-19. | |
| 76 | DENG J J, SHI D, MAO H H, et al. Heterologous expression and characterization of an antifungal chitinase (Chit46) from Trichoderma harzianum GIM 3.442 and its application in colloidal chitin conversion[J]. International Journal of Biological Macromolecules, 2019, 134: 113-121. |
| 77 | JIMÉNEZ-ORTEGA E, KIDIBULE P E, FERNÁNDEZ-LOBATO M, et al. Structure-function insights into the fungal endo-chitinase Chit33 depict its mechanism on chitinous material[J]. International Journal of Molecular Sciences, 2022, 23(14): 7599. |
| 78 | ZHANG A L, HE Y M, WEI G G, et al. Molecular characterization of a novel chitinase CmChi1 from Chitinolyticbacter meiyuanensis SYBC-H1 and its use in N-acetyl-D-glucosamine production[J]. Biotechnology for Biofuels, 2018, 11: 179. |
| 79 | GOUGERDCHI V, DORANI E, VALIZADEH M, et al. Overexpression of the chimeric chitinase (ChBD) gene in Lycopersicon esculentum L. enhanced resistance to Sclerotinia sclerotiorum [J]. Plant Cell, Tissue and Organ Culture, 2022, 151(1): 165-175. |
| 80 | ATAEI A, ZAMANI M, MOTALLEBI M, et al. Increased antifungal activity of Chit42 from Trichoderma atroviride by addition of a chitin binding domain[J]. Tropical Plant Pathology, 2016, 41(6): 350-356. |
| 81 | YAN J J, LIU W D, LI Y J, et al. Functional and structural analysis of Pichia pastoris-expressed Aspergillus niger 1,4-β- endoglucanase[J]. Biochemical and Biophysical Research Communications, 2016, 475(1): 8-12. |
| 82 | YANG Y, SOSSAH F L, LI Z, et al. Genome-wide identification and analysis of chitinase GH18 gene family in Mycogone perniciosa [J]. Frontiers in Microbiology, 2021, 11: 596719. |
| 83 | CHU F M, WANG D, LIU T, et al. An optimized cocktail of chitinolytic enzymes to produce N,N'-diacetylchitobiose and N-acetyl-D-glucosamine from defatted krill by-products[J]. International Journal of Biological Macromolecules, 2019, 133: 1029-1034. |
| 84 | NAKAMURA A, OKAZAKI K I, FURUTA T, et al. Processive chitinase is Brownian monorail operated by fast catalysis after peeling rail from crystalline chitin[J]. Nature Communications, 2018, 9(1): 3814. |
| 85 | SONGSIRIRITTHIGUL C, LAPBOONRUENG S, PECHSRICHUANG P, et al. Expression and characterization of Bacillus licheniformis chitinase (ChiA), suitable for bioconversion of chitin waste[J]. Bioresource Technology, 2010, 101(11): 4096-4103. |
| 86 | MENGHIU G, OSTAFE V, PRODANOVIC R, et al. Biochemical characterization of chitinase A from Bacillus licheniformis DSM8785 expressed in Pichia pastoris KM71H[J]. Protein Expression and Purification, 2019, 154: 25-32. |
| 87 | 潘梦妍, 徐显皓, 刘延峰, 等. 甲壳素酶Chisb的定向进化及生物转化合成几丁寡糖[J]. 生物工程学报, 2019, 35(9): 1787-1796. |
| PAN M Y, XU X H, LIU Y F, et al. Directed evolution of chitinase Chisb and biosynthesis of chitooligosaccharides[J]. Chinese Journal of Biotechnology, 2019, 35(9): 1787-1796. | |
| 88 | XU P, NI Z F, ZONG M H, et al. Improving the thermostability and activity of Paenibacillus pasadenensis chitinase through semi-rational design[J]. International Journal of Biological Macromolecules, 2020, 150: 9-15. |
| 89 | GÅSEIDNES S, SYNSTAD B, JIA X H, et al. Stabilization of a chitinase from Serratia marcescens by Gly→Ala and Xxx→Pro mutations[J]. Protein Engineering, 2003, 16(11): 841-846. |
| 90 | ZHAO S, LIU M Y, SUN X M, et al. Engineering the relatively conserved residues in active site architecture of thermophilic chitinase SsChi18A enhanced catalytic activity[J]. Biomacromolecules, 2024, 25(1): 238-247. |
| 91 | LIU J W, XU Q, WU Y, et al. Carbohydrate-binding modules of ChiB and ChiC promote the chitinolytic system of Serratia marcescens BWL1001[J]. Enzyme and Microbial Technology, 2023, 162: 110118. |
| 92 | DOAN C T, TRAN T N, WEN I H, et al. Conversion of shrimp head waste for production of a thermotolerant, detergent-stable, alkaline protease by Paenibacillus sp[J]. Catalysts, 2019, 9(10): 798. |
| 93 | 罗洒, 秦臻, 陈启明, 等. 解淀粉芽孢杆菌壳聚糖酶毕赤酵母高效表达[C]//中国食品科学技术学会第十五届年会论文集. 青岛, 2018: 436. |
| LUO S, QIN Z, CHEN Q M, et al. Efficient expression of chitosan enzyme from Bacillus amyloliquefaciens in Pichia pastoris [C]// Proceedings of the 15th Annual Conference of the Chinese Society for Food Science and Technology. Qingdao, 2018: 436. | |
| 94 | ZHOU J L, LIU X B, YUAN F, et al. Biocatalysis of heterogenously-expressed chitosanase for the preparation of desirable chitosan oligosaccharides applied against phytopathogenic fungi[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(12): 4781-4791. |
| 95 | 李茜茜, 杨云洁, 管世敏, 等. 壳寡糖的制备与应用研究进展[J]. 粮食与油脂, 2023, 36(9): 27-31. |
| LI Q Q, YANG Y J, GUAN S M, et al. Research progress on the preparation and application of chitosan oligosaccharides[J]. Cereals & Oils, 2023, 36(9): 27-31. | |
| 96 | KUROIWA T, NAKAGAWA Y, TAKAYANAGI R, et al. Chitosanase-immobilized magnetite-agar gel particles as a highly stable and reusable biocatalyst for enhanced production of physiologically active chitosan oligosaccharides[J]. Enzyme and Microbial Technology, 2024, 178: 110443. |
| 97 | LE B, YANG S H. Characterization of a chitinase from Salinivibrio sp. BAO-1801 as an antifungal activity and a biocatalyst for producing chitobiose[J]. Journal of Basic Microbiology, 2018, 58(10): 848-856. |
| 98 | WANG J R, ZHU M J, WANG P, et al. Biochemical properties of a cold-active chitinase from marine Trichoderma gamsii R1 and its application to preparation of chitin oligosaccharides[J]. Marine Drugs, 2023, 21(6): 332. |
| 99 | LI J C, ZHENG J M, LIANG Y H, et al. Expression and characterization of a chitinase from Serratia marcescens [J]. Protein Expression and Purification, 2020, 171: 105613. |
| 100 | KROLICKA M, HINZ S W A, KOETSIER M J, et al. β-N-Acetylglucosaminidase MthNAG from Myceliophthora thermophila C1, a thermostable enzyme for production of N-acetylglucosamine from chitin[J]. Applied Microbiology and Biotechnology, 2018, 102(17): 7441-7454. |
| 101 | WU Y L, WANG S, YANG D F, et al. The discovery, enzymatic characterization and functional analysis of a newly isolated chitinase from marine-derived fungus Aspergillus fumigatus df347[J]. Marine Drugs, 2022, 20(8): 520. |
| 102 | DING Z W, LI T, CHEN M, et al. Purification and characterization of a chitinase from Aeromonas media CZW001 as a biocatalyst for producing chitinpentaose and chitinhexaose[J]. Biotechnology and Applied Biochemistry, 2023, 70(1): 281-289. |
| 103 | SUBRAMANI A K, RAMACHANDRA R, THOTE S, et al. Engineering a recombinant chitinase from the marine bacterium Bacillus aryabhattai with targeted activity on insoluble crystalline chitin for chitin oligomer production[J]. International Journal of Biological Macromolecules, 2024, 264(Pt 2): 130499. |
| 104 | DENG J J, ZHANG M S, LI Z W, et al. One-step processing of shrimp shell waste with a chitinase fused to a carbohydrate-binding module[J]. Green Chemistry, 2020, 22(20): 6862-6873. |
| 105 | 鲁梦唯, 陈晟, 吴敬. 维氏气单胞菌来源几丁质酶的克隆表达及应用[J]. 食品与生物技术学报, 2022, 41(4): 55-63. |
| LU M W, CHEN S, WU J. Cloning, expression and application of chitinase from Aeromonas veronii [J]. Journal of Food Science and Biotechnology, 2022, 41(4): 55-63. | |
| 106 | BHUVANACHANDRA B, PODILE A R. A transglycosylating chitinase from Chitiniphilus shinanonensis (CsChiL) hydrolyzes chitin in a processive manner[J]. International Journal of Biological Macromolecules, 2020, 145: 1-10. |
| 107 | SINGH S, GALLAGHER R, DERRICK P J, et al. Glycosidase-catalysed oligosaccharide synthesis: preparation of the N-acetylchitooligosaccharidespenta-N-acetylchitopentaose and hexa-N-acetylchitohexaose using the β-N-acetylhexosaminidase of Aspergillus oryzae [J]. Tetrahedron: Asymmetry, 1995, 6(11): 2803-2810. |
| 108 | ZHANG A L, MO X F, ZHOU N, et al. A novel bacterial β-N-acetyl glucosaminidase from Chitinolyticbacter meiyuanensis possessing transglycosylation and reverse hydrolysis activities[J]. Biotechnology for Biofuels, 2020, 13: 115. |
| 109 | 黄晓月, 毕思远, 区家豪, 等. 木瓜蛋白酶法制备抗氧化活性壳寡糖的工艺优化[J]. 生物学杂志, 2022, 39(1): 104-109. |
| HUANG X Y, BI S Y, OU J H, et al. Process optimization of preparation of antioxidant chitooligosaccharides by papain[J]. Journal of Biology, 2022, 39(1): 104-109. | |
| 110 | 董惠忠. 聚合度6-8壳寡糖的制备关键技术研究[D]. 上海: 华东理工大学, 2014. |
| DONG H Z. Key technology research on the preparation of chitosan oligosaccharides with a polymerization degree of 6-8[D]. Shanghai: East China University of Science and Technology, 2014. | |
| 111 | ZHANG A L, GAO C, WANG J, et al. An efficient enzymatic production of N-acetyl-D-glucosamine from crude chitin powders[J]. Green Chemistry, 2016, 18(7): 2147-2154. |
| 112 | ZHANG A L, WEI G G, MO X F, et al. Enzymatic hydrolysis of chitin pretreated by bacterial fermentation to obtain pure N-acetyl-D-glucosamine[J]. Green Chemistry, 2018, 20(10): 2320-2327. |
| 113 | WANG Y Y, ZHANG A L, MO X F, et al. The effect of ultrasonication on enzymatic hydrolysis of chitin to N-acetyl glucosamine via sequential and simultaneous strategies[J]. Process Biochemistry, 2020, 99: 265-269. |
| 114 | WEI G G, ZHANG A L, CHEN K Q, et al. Enzymatic production of N-acetyl-D-glucosamine from crayfish shell wastes pretreated via high pressure homogenization[J]. Carbohydrate Polymers, 2017, 171: 236-241. |
| 115 | ZHOU N, YANG P F, CHEN J, et al. Effect of organic solvents treatment on structure of chitin and its enzymatic hydrolysis[J]. Polymer Degradation and Stability, 2022, 198: 109654. |
| 116 | ZHANG A L, WANG C Y, CHEN J, et al. Efficient enzymatic hydrolysis of chitin into N-acetyl glucosamine using alkali as a recyclable pretreatment reagent[J]. Green Chemistry, 2021, 23(8): 3081-3089. |
| 117 | CARDOZO F A, GONZALEZ J M, FEITOSA V A, et al. Bioconversion of α-chitin into N-acetyl-glucosamine using chitinases produced by marine-derived Aeromonas caviae isolates[J]. World Journal of Microbiology & Biotechnology, 2017, 33(11): 201. |
| 118 | DAS S, SEN R, ROY D. Enzymatic processing of chitinaceous wastes for N-acetyl-D-glucosamine production: an example of green and efficient environmental management[J]. Environmental Engineering and Management Journal, 2012, 11(10): 1849-1855. |
| 119 | LI J, HUANG W C, GAO L, et al. Efficient enzymatic hydrolysis of ionic liquid pretreated chitin and its dissolution mechanism[J]. Carbohydrate Polymers, 2019, 211: 329-335. |
| 120 | NGUYEN-THI N, DOUCET N. Combining chitinase C and N-acetylhexosaminidase from Streptomyces coelicolor A3(2) provides an efficient way to synthesize N-acetylglucosamine from crystalline chitin[J]. Journal of Biotechnology, 2016, 220: 25-32. |
| 121 | LI J, GAO K P, SECUNDO F, et al. Biochemical characterization of two β-N-acetylglucosaminidases from Streptomyces violascens for efficient production of N-acetyl-D-glucosamine[J]. Food Chemistry, 2021, 364: 130393. |
| 122 | SONG W, ZHANG N, YANG M, et al. Multiple strategies to improve the yield of chitinase a from Bacillus licheniformis in Pichia pastoris to obtain plant growth enhancer and GlcNAc[J]. Microbial Cell Factories, 2020, 19(1): 181. |
| 123 | DU C, ZHOU Y L, LIU L, et al. Bacterial surface-assembled chitinosome for dismantling chitin into N-acetyl glucosamine[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(30): 11239-11247. |
| 124 | OKAZAKI S, KOMATSU A, NAKANO M, et al. A novel endo-type chitinase possessing chitobiase activity derived from the chitinolytic bacterium, Chitiniphilus shinanonensis SAY3T[J]. Bioscience, Biotechnology, and Biochemistry, 2023, 87(12): 1543-1550. |
| 125 | DU C, JIANG S, JIANG S J, et al. A Bacillus pumilus originated β-N-acetylglucosaminidase for chitin combinatory hydrolysis and exploration of its thermostable mechanism[J]. International Journal of Biological Macromolecules, 2019, 132: 1282-1289. |
| 126 | HAN S S, XUE Y B, YAN Q J, et al. Development of a two-enzyme system in Aspergillus niger for efficient production of N-acetyl-β-D-glucosamine from powdery chitin[J]. Bioresource Technology, 2024, 393: 130024. |
| 127 | FU X, YAN Q J, YANG S Q, et al. An acidic, thermostable exochitinase with β-N-acetylglucosaminidase activity from Paenibacillus barengoltzii converting chitin to N-acetyl glucosamine[J]. Biotechnology for Biofuels, 2014, 7(1): 174. |
| 128 | SURESH P V, ANIL KUMAR P K. Enhanced degradation of α-chitin materials prepared from shrimp processing byproduct and production of N-acetyl-D-glucosamine by thermoactive chitinases from soil mesophilic fungi[J]. Biodegradation, 2012, 23(4): 597-607. |
| 129 | ZHU W X, WANG D, LIU T, et al. Production of N-acetyl-D-glucosamine from mycelial waste by a combination of bacterial chitinases and an insect N-acetyl-D-glucosaminidase[J]. Journal of Agricultural and Food Chemistry, 2016, 64(35): 6738-6744. |
| 130 | GAO C, ZHANG A L, CHEN K Q, et al. Characterization of extracellular chitinase from Chitinibacter sp. GC72 and its application in GlcNAc production from crayfish shell enzymatic degradation[J]. Biochemical Engineering Journal, 2015, 97: 59-64. |
| 131 | 吕永梅, 章晓洋, 高文博, 等. 一种几丁质脱乙酰基酶突变体及其编码基因与应用: CN116144636A[P]. 2023-05-23. |
| LV Y M, ZHANG X Y, GAO W B, et al. A chitin deacetylase mutant and its coding gene and application: CN116144636A[P]. 2023-05-23. | |
| 132 | INOKUMA K, TAKANO M, HOSHINO K. Direct ethanol production from N-acetylglucosamine and chitin substrates by Mucor species[J]. Biochemical Engineering Journal, 2013, 72: 24-32. |
| 133 | LI S W, ZENG R J, SHENG G P. An excellent anaerobic respiration mode for chitin degradation by Shewanella oneidensis MR-1 in microbial fuel cells[J]. Biochemical Engineering Journal, 2017, 118: 20-24. |
| 134 | LIU Q Z, WEI G G, YANG P F, et al. One-pot biosynthesis of N-acetylneuraminic acid from chitin via combination of chitin-degrading enzymes, N-acetylglucosamine-2-epimerase, and N-neuraminic acid aldolase[J]. Frontiers in Microbiology, 2023, 14: 1156924. |
| 135 | MA X Q, GÖZAYDIN G, YANG H Y, et al. Upcycling chitin-containing waste into organonitrogen chemicals via an integrated process[J]. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(14): 7719-7728. |
| 136 | HAO Y C, ZONG M H, WANG Z L, et al. Chemoenzymatic access to enantiopure N-containing furfuryl alcohol from chitin-derived N-acetyl-D-glucosamine[J]. Bioresources and Bioprocessing, 2021, 8(1): 80. |
| 137 | HAO Y C, ZONG M H, CHEN Q, et al. Engineering carbonyl reductase for one-pot chemobiocatalytic enantioselective synthesis of a value-added N-containing chiral alcohol from N-acetyl-D-glucosamine[J]. Green Chemistry, 2023, 25(13): 5051-5058. |
| 138 | 陈可泉, 魏国光, 张阿磊, 等. 一种利用N-乙酰氨基葡萄糖制备 3-氨基-5-(α-氨基乙基)四氢呋喃的方法: CN109824629B[P]. 2022-12-09. |
| CHEN K Q, WEI G G, ZHANG A L, et al. A method for preparing 3-amino-5-(α-aminoethyl) tetrahydrofuranusing from N-acetylglucosamine: CN109824629B[P]. 2022-12-09. | |
| 139 | WU C Q, ZHANG X, LIU W, et al. Biocatalytic synthesis of two furan-based amino compounds 2-acetyl-4-aminofuran and 3-acetylamino-5-(α-aminoethyl)-furan from chitin resources[J]. ACS Sustainable Chemistry & Engineering, 2024, 12(30): 11145-11154. |
| [1] | 石婷, 宋展, 宋世怡, 张以恒. 体外生物转化(ivBT):生物制造的新前沿[J]. 合成生物学, 2024, 5(6): 1437-1460. |
| [2] | 刘宽庆, 张以恒. 木质素的生物降解和生物利用[J]. 合成生物学, 2024, 5(6): 1264-1278. |
| [3] | 刘伟松, 张坤城, 崔会娟, 朱之光, 张以恒, 张玲玲. 电能辅助二氧化碳生物转化[J]. 合成生物学, 2023, 4(6): 1191-1222. |
| [4] | 明阳, 陈彬, 黄小强. 光酶催化合成进展[J]. 合成生物学, 2023, 4(4): 651-675. |
| [5] | 李磊, 高鑫, 齐宏斌, 李超, 路福平, 毛淑红, 秦慧民. 现代生物技术推动塑料中聚对苯二甲酸乙二酯绿色降解的研究进展[J]. 合成生物学, 2022, 3(4): 763-780. |
| [6] | 郭姝媛, 吴良焕, 刘香健, 王博, 于涛. 微生物中一碳代谢网络构建的进展与挑战[J]. 合成生物学, 2022, 3(1): 116-137. |
| [7] | 钱秀娟, 刘嘉唯, 薛瑞, 刘豪杰, 闻小红, 杨璐, 徐安明, 许斌, 信丰学, 周杰, 董维亮, 姜岷. 合成生物学助力废弃塑料资源生物解聚与升级再造[J]. 合成生物学, 2021, 2(2): 161-180. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||