合成生物学 ›› 2022, Vol. 3 ›› Issue (6): 1150-1173.DOI: 10.12211/2096-8280.2022-039
巴斯德毕赤酵母底盘细胞的工程化改造及应用
刘启, 钱芷兰, 宋丽丽, 要超颖, 徐名强, 任燕娜, 蔡孟浩
- 华东理工大学生物工程学院,生物反应器工程国家重点实验室,上海 200237
-
收稿日期:2022-07-04修回日期:2022-08-03出版日期:2022-12-31发布日期:2023-01-17 -
通讯作者:蔡孟浩 -
作者简介:刘启 (1991—),男,博士后。研究方向为酵母底盘的改造优化及合成遗传线路设计。E-mail:liuqi0496@ecust.edu.cn蔡孟浩 (1984—),男,博士,副教授,博士生导师。研究方向为合成生物学与发酵工程,通过遗传元件挖掘与工具开发、真核微生物底盘设计与改造、细胞代谢重构与发酵优化,实现药/食源功能分子的规模化发酵制备。E-mail:cmh022199@ecust.edu.cn -
基金资助:国家自然科学基金面上项目(31870073);国家重点研发计划(2018YFC1706202);上海市青年科技启明星项目(19QA1402700)
Rewiring and application of Pichia pastoris chassis cell
LIU Qi, QIAN Zhilan, SONG Lili, YAO Chaoying, XU Mingqiang, REN Yanna, CAI Menghao
- State Key Laboratory of Bioreactor Engineering,School of Biotechnology,East China University of Science and Technology,Shanghai 200237,China
-
Received:2022-07-04Revised:2022-08-03Online:2022-12-31Published:2023-01-17 -
Contact:CAI Menghao
摘要:
优质的微生物底盘宿主是实现绿色、可持续生物制造的重要平台。巴斯德毕赤酵母底盘宿主因其在蛋白表达和发酵生产中的诸多优势受到了广泛的关注和应用。而作为一种工业甲基营养酵母,其可以有效地利用来源广泛的甲醇作为唯一碳源,使其成为碳一化合物潜在的生物转化平台。近年来,随着合成生物技术和生物制药技术的快速发展,围绕毕赤酵母底盘的工程化改造研究逐渐增多,并取得了卓有成效的进展,促进了毕赤酵母底盘的发展和升级。本文简述了毕赤酵母底盘细胞的发展和应用现状,从基因操纵技术、基因表达调控、代谢工程改造等方面介绍了毕赤酵母的工程化改造策略及应用效果,总结了毕赤酵母中合成生物技术、调控元器件、新型表达平台和生物转化体系的建立与开发情况。在此基础上,进一步强调了毕赤酵母中CRISPR介导的基因编辑及调控、转录系统的重构及人工设计,介绍了其在蛋白表达和化合物合成方面的应用,并分析了其在实际应用中的优势和问题。最后,对毕赤酵母在后续研究中的底盘升级方向和应用场景进行了展望。
中图分类号:
引用本文
刘启, 钱芷兰, 宋丽丽, 要超颖, 徐名强, 任燕娜, 蔡孟浩. 巴斯德毕赤酵母底盘细胞的工程化改造及应用[J]. 合成生物学, 2022, 3(6): 1150-1173.
LIU Qi, QIAN Zhilan, SONG Lili, YAO Chaoying, XU Mingqiang, REN Yanna, CAI Menghao. Rewiring and application of Pichia pastoris chassis cell[J]. Synthetic Biology Journal, 2022, 3(6): 1150-1173.

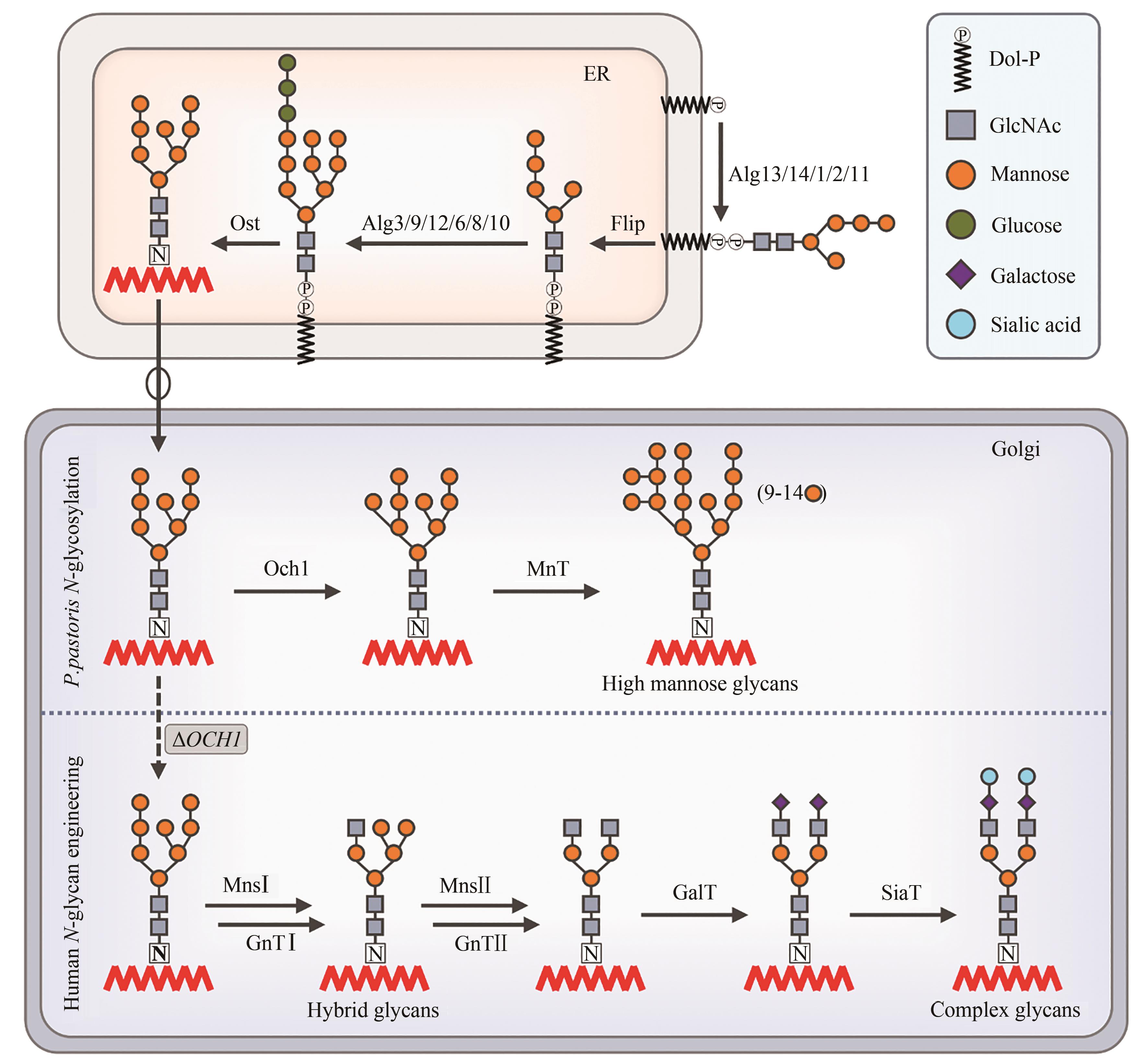
图1 毕赤酵母CRISPR介导的基因编辑过程及工程化改造策略[29-30, 41, 57](标注蓝色字体代表该蛋白敲除后可以有效抑制NHEJ作用;标注红色字体代表该蛋白过表达可以提升HDR介导的修复效率)
Fig. 1 CRISPR mediated gene editing process and engineering strategy in Pichia pastoris[29-30, 41, 57](Blue font indicates that the knockout of target protein can inhibit NHEJ; red font indicates that the overexpression of target protein can enhance HDR efficiency)
| 菌株改造策略 | Cas9/gRNA表达方式 | 游离质粒ARS | HDR效率 | 参考 文献 | |
|---|---|---|---|---|---|
| 敲除 | 敲入 | ||||
| Wild type | P HTX1 -Cas9 P HTX1 -HH-sgRNA-HDV | PARS1 | 100%(单基因) | — | [ |
| 敲除ku70 | P HTX1 -Cas9 P HTX1 -HH-sgRNA-HDV | PARS1 | 100%(单基因) | — | [ |
| 敲除ku70 | P HTX1 -Cas9 P HTX1 -HH-sgRNA-HDV | PARS1 | — | 75%~97.9%(单位点) 57.7%~70.0%(双位点) 12.5%~32.1%(三位点) | [ |
| Wild type | P GAP- Cas9 P SER -sgRNA | panARS | 80%(单基因) | — | [ |
| 敲除ku70 | P ENO1 -Cas9 P tRNA1 -tRNA1-sgRNA | PARS1 | — | 40%(三位点) | [ |
敲除MPH1和 过表达PpRAD52 | P HTX1 -Cas9 P HTX1 -HH-sgRNA-HDV | panARS | 90%(单基因) | 43%~70%(单位点) 25%(三位点) | [ |
| Wild type | P GAP -Cas9(整合) P SER -sgRNA | panARS | — | 98.6%(单位点) | [ |
| PARS1 | 33.3%~93.1%(双位点) 10%~75%(三位点) | ||||
| 敲除ku70 | P GAP -Cas9(整合) P SER -sgRNA | PARS1 | — | 33.3%~57%(三位点) | [ |
过表达ScRAD52 ScRAD59 ScMRE11 | P GAP -Cas9(整合) P SER -sgRNA | PARS1 | — | 100%(单位点) 95.7%~97.9%(双位点) 64.5%~80.9%(三位点) | [ |
| Wild type | P GAP -FnCpf1(整合) P SER -crRNA | panARS | 99%(单基因) 65%~80%(双基因) 30%(三基因) | — | [ |
表1 毕赤酵母中CRISPR介导的HDR效率及工程化改造策略
Tab. 1 CRISPR mediated HDR efficiency and engineering strategy in Pichia pastoris
| 菌株改造策略 | Cas9/gRNA表达方式 | 游离质粒ARS | HDR效率 | 参考 文献 | |
|---|---|---|---|---|---|
| 敲除 | 敲入 | ||||
| Wild type | P HTX1 -Cas9 P HTX1 -HH-sgRNA-HDV | PARS1 | 100%(单基因) | — | [ |
| 敲除ku70 | P HTX1 -Cas9 P HTX1 -HH-sgRNA-HDV | PARS1 | 100%(单基因) | — | [ |
| 敲除ku70 | P HTX1 -Cas9 P HTX1 -HH-sgRNA-HDV | PARS1 | — | 75%~97.9%(单位点) 57.7%~70.0%(双位点) 12.5%~32.1%(三位点) | [ |
| Wild type | P GAP- Cas9 P SER -sgRNA | panARS | 80%(单基因) | — | [ |
| 敲除ku70 | P ENO1 -Cas9 P tRNA1 -tRNA1-sgRNA | PARS1 | — | 40%(三位点) | [ |
敲除MPH1和 过表达PpRAD52 | P HTX1 -Cas9 P HTX1 -HH-sgRNA-HDV | panARS | 90%(单基因) | 43%~70%(单位点) 25%(三位点) | [ |
| Wild type | P GAP -Cas9(整合) P SER -sgRNA | panARS | — | 98.6%(单位点) | [ |
| PARS1 | 33.3%~93.1%(双位点) 10%~75%(三位点) | ||||
| 敲除ku70 | P GAP -Cas9(整合) P SER -sgRNA | PARS1 | — | 33.3%~57%(三位点) | [ |
过表达ScRAD52 ScRAD59 ScMRE11 | P GAP -Cas9(整合) P SER -sgRNA | PARS1 | — | 100%(单位点) 95.7%~97.9%(双位点) 64.5%~80.9%(三位点) | [ |
| Wild type | P GAP -FnCpf1(整合) P SER -crRNA | panARS | 99%(单基因) 65%~80%(双基因) 30%(三基因) | — | [ |
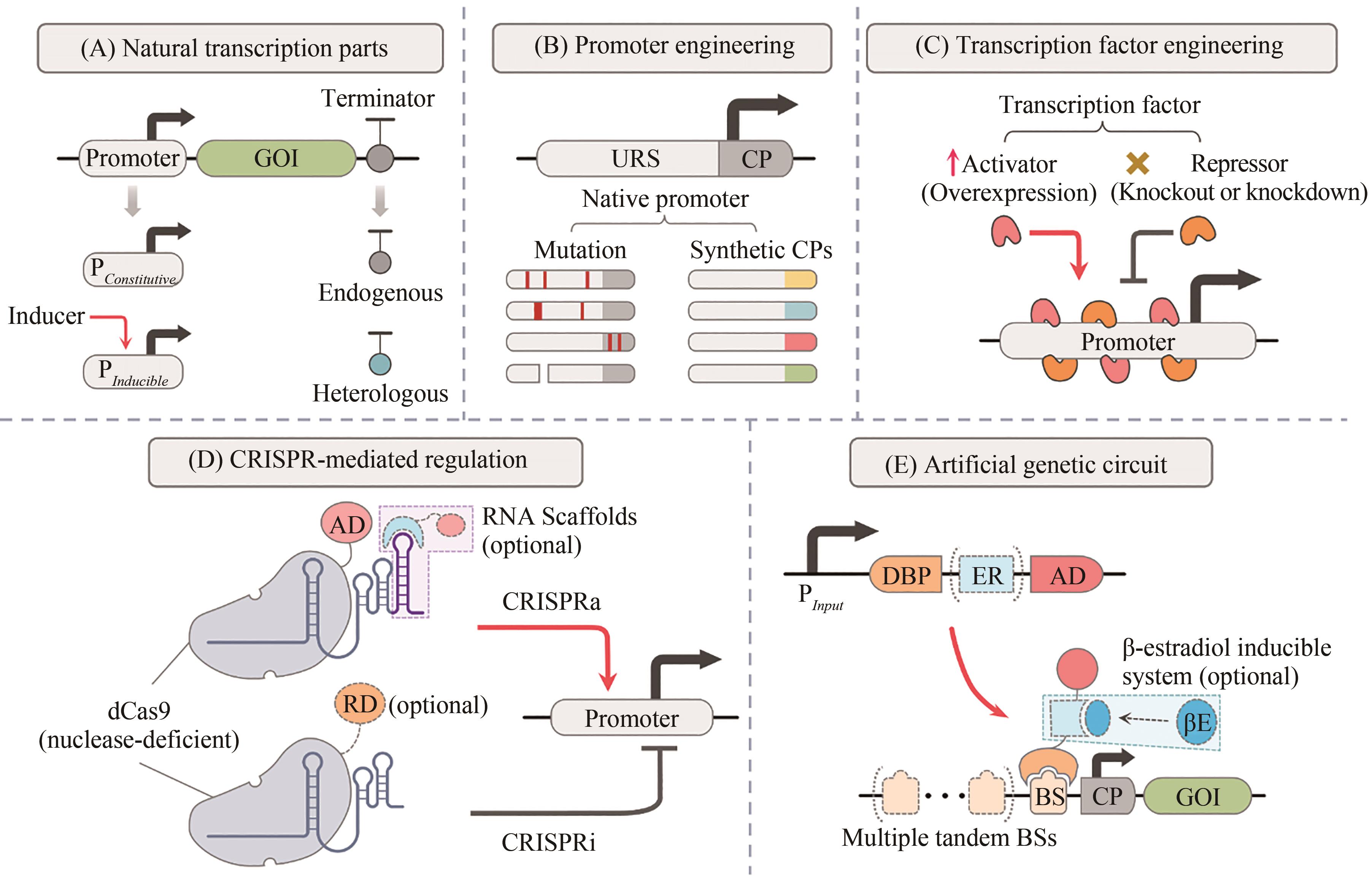
图2 毕赤酵母中转录调控系统的改造及工程化设计[24-28, 40, 59, 78, 82, 88, 94-103, 108-115]URS—上游调控序列;CP—核心启动子;AD—激活结构域;RD—阻遏结构域;DBP—DNA结合蛋白;BS—结合位点;ER—雌二醇受体;βE—β-雌二醇
Fig. 2 Rewiring and engineering design of transcription regulation system in Pichia pastoris[24-28, 40, 59, 78, 82, 88, 94-103, 108-115]URS—upstream regulatory sequence; CP—core promoter; AD—activation domain; RD—repression domain; DBP—DNA binding protein; BS—binding site; ER—estradiol receptor; βE—β-estradiol
| 外源蛋白 | 分子 伴侣 | 效价提升倍数 | 参考 文献 |
|---|---|---|---|
| 狂犬病病毒糖蛋白 | PDI1 ERO1 GPX1 | 9.6倍 3倍 8.2倍 | [ |
| 白介素-2-人血清白蛋白融合蛋白 | PDI1 KAR2 ERO1 | 2.2倍 1.9倍 2.3倍 | [ |
| 猪肽聚糖识别蛋白 | PDI1 | 5倍(高拷贝) | [ |
| 疏水蛋白HFBI | KAR2 | 14倍(单拷贝) 9.8倍(双拷贝) 22倍(三拷贝) | [ |
PDI ERO1 | 7.8倍(三拷贝) 30倍(三拷贝) | ||
| 人溶菌酶 | HAC1 | 1.2倍 | [ |
| 铜绿假单胞菌弹性蛋白酶 | HAC1 | 1.8~3.9倍 | [ |
| 大肠杆菌植酸酶 | HAC1 PDI1 | 1.36倍 1.40倍 | [ |
| 家蚕乙酰胆碱酯酶 | PDI1 | 5倍 | [ |
| 几丁质酶 | HAC1 | 1.3倍 | [ |
表2 分子伴侣共表达提高毕赤酵母蛋白表达量
Tab. 2 Co-expression of chaperone to enhance protein expression in Pichia pastoris
| 外源蛋白 | 分子 伴侣 | 效价提升倍数 | 参考 文献 |
|---|---|---|---|
| 狂犬病病毒糖蛋白 | PDI1 ERO1 GPX1 | 9.6倍 3倍 8.2倍 | [ |
| 白介素-2-人血清白蛋白融合蛋白 | PDI1 KAR2 ERO1 | 2.2倍 1.9倍 2.3倍 | [ |
| 猪肽聚糖识别蛋白 | PDI1 | 5倍(高拷贝) | [ |
| 疏水蛋白HFBI | KAR2 | 14倍(单拷贝) 9.8倍(双拷贝) 22倍(三拷贝) | [ |
PDI ERO1 | 7.8倍(三拷贝) 30倍(三拷贝) | ||
| 人溶菌酶 | HAC1 | 1.2倍 | [ |
| 铜绿假单胞菌弹性蛋白酶 | HAC1 | 1.8~3.9倍 | [ |
| 大肠杆菌植酸酶 | HAC1 PDI1 | 1.36倍 1.40倍 | [ |
| 家蚕乙酰胆碱酯酶 | PDI1 | 5倍 | [ |
| 几丁质酶 | HAC1 | 1.3倍 | [ |

图4 毕赤酵母中不同碳源底物的代谢途径及各类天然产物的合成[33-34, 60, 82, 171-184, 199-203]DHA—二羟丙酮;GAP—3-磷酸甘油醛;Xu5P—5-磷酸木酮糖;DHAP—磷酸二羟丙酮;XuMP cycle—单磷酸木酮糖循环;Aox—醇氧化酶;Cat—过氧化氢酶;Das—二羟丙酮合成酶;Dak—二羟丙酮激酶;Tpi—磷酸丙糖异构酶;Fld—甲醛脱氢酶;Fgh—S-甲酰基谷胱甘肽水解酶;Fdh—甲酸脱氢酶;Pdh—丙酮酸脱氢酶;Pdc—丙酮酸脱羧酶;Adh—乙醇脱氢酶;Ald—乙醛脱氢酶;Acs—乙酰辅酶A合成酶;Acc—乙酰辅酶A羧化酶;Fas—脂肪酸合成酶;表示酶催化反应;表示代谢物在细胞内外或不同细胞器间的穿梭;表示多步代谢途径
Fig. 4 Metabolic pathways of different carbon sources and synthesis of various natural products based on in Pichia pastoris[33-34, 60, 82, 171-184, 199- 203]DHA—dihydroxyacetone; GAP—glyceraldehyde-3-phosphate; Xu5P—xylulose 5-phosphate; DHAP—dihydroxyacetone phosphate; XuMP cycle—xylulose monophosphate cycle; Aox—alcohol oxidase; Cat—catalase; Das—dihydroxyacetone synthase; Dak—dihydroxyacetone kinase; Tpi—triosephosphate isomerase; Fld—formaldehyde dehydrogenase; Fgh—S-formylglutathione hydrolase; Fdh—formate dehydrogenase; Pdh—Pyruvate dehydrogenase; Pdc—pyruvate decarboxylase; Adh—alcohol dehydrogenase; Ald—acetaldehyde dehydrogenase; Acs—acetyl-CoA synthetase; Acc—acetyl-CoA carboxylase; Fas—fatty acid synthetase; indicate enzyme catalyzed reaction; indicate the shuttle of metabolites between extracellular and intracellular or between different organelles; indicate a multistep metabolic pathway.
| 种类 | 化合物 | 底物及培养方式 | 产量 | 参考文献 |
|---|---|---|---|---|
| 四碳有机酸 | 苹果酸 | 甲醇(摇瓶) | 2.79 g/L | [ |
| 葡萄糖(摇瓶) | 8.55 g/L | |||
| 甲醇(反应器) | 42.28 g/L | [ | ||
| 富马酸 | 甲醇(反应器) | 0.76 g/L | ||
| 琥珀酸 | 甲醇(反应器) | 9.42 g/L | ||
| 脂肪酸衍生物 | 蓖麻油酸 | 甲醇(摇瓶) | 171.44 mg/L | [ |
| 长链α-烯烃 | 甲醇(摇瓶) | 1.6 mg/L | [ | |
| 脂肪酸 | 甲醇(反应器) | 23.4 g/L | [ | |
| 脂肪醇 | 甲醇(反应器) | 2.0 g/L | ||
| 萜类化合物 | 番茄红素 | 葡萄糖(摇瓶) | 1.141 μg/g DCW | [ |
| 甲醇(反应器) | 714 mg/L | [ | ||
| β-胡萝卜素 | 葡萄糖(摇瓶) | 339 μg/g DCW | [ | |
| 虾青素 | 葡萄糖(摇瓶) | 3.7 μg/g DCW | [ | |
| 诺卡酮 | 甲醇(反应器) | 208 mg/L | [ | |
| 达玛烯二醇 | 甲醇(摇瓶) | 1.073 mg/g DCW | [ | |
| 聚酮类化合物 | 6-甲基水杨酸 | 甲醇(反应器) | 2.2 g/L | [ |
| 橘霉素 | 甲醇(摇瓶) | 0.6 mg/L | [ | |
| 洛伐他汀 | 甲醇(反应器) | 250.8 mg/L | [ | |
| 甲醇(反应器) | 419.0 mg/L | [ | ||
| 莫纳可林J | 甲醇(反应器) | 593.9 mg/L | [ | |
| 乙醇(反应器) | 3.2 g/L | [ | ||
| 黄酮类化合物 | 黄芩素 | 乙醇(摇瓶) | 401.9 mg/L | [ |
| 千层纸素 | 乙醇(摇瓶) | 339.5 mg/L |
表3 基于毕赤酵母底盘细胞的天然产物合成
Tab. 3 Production of natural products in Pichia pastoris chassis cell
| 种类 | 化合物 | 底物及培养方式 | 产量 | 参考文献 |
|---|---|---|---|---|
| 四碳有机酸 | 苹果酸 | 甲醇(摇瓶) | 2.79 g/L | [ |
| 葡萄糖(摇瓶) | 8.55 g/L | |||
| 甲醇(反应器) | 42.28 g/L | [ | ||
| 富马酸 | 甲醇(反应器) | 0.76 g/L | ||
| 琥珀酸 | 甲醇(反应器) | 9.42 g/L | ||
| 脂肪酸衍生物 | 蓖麻油酸 | 甲醇(摇瓶) | 171.44 mg/L | [ |
| 长链α-烯烃 | 甲醇(摇瓶) | 1.6 mg/L | [ | |
| 脂肪酸 | 甲醇(反应器) | 23.4 g/L | [ | |
| 脂肪醇 | 甲醇(反应器) | 2.0 g/L | ||
| 萜类化合物 | 番茄红素 | 葡萄糖(摇瓶) | 1.141 μg/g DCW | [ |
| 甲醇(反应器) | 714 mg/L | [ | ||
| β-胡萝卜素 | 葡萄糖(摇瓶) | 339 μg/g DCW | [ | |
| 虾青素 | 葡萄糖(摇瓶) | 3.7 μg/g DCW | [ | |
| 诺卡酮 | 甲醇(反应器) | 208 mg/L | [ | |
| 达玛烯二醇 | 甲醇(摇瓶) | 1.073 mg/g DCW | [ | |
| 聚酮类化合物 | 6-甲基水杨酸 | 甲醇(反应器) | 2.2 g/L | [ |
| 橘霉素 | 甲醇(摇瓶) | 0.6 mg/L | [ | |
| 洛伐他汀 | 甲醇(反应器) | 250.8 mg/L | [ | |
| 甲醇(反应器) | 419.0 mg/L | [ | ||
| 莫纳可林J | 甲醇(反应器) | 593.9 mg/L | [ | |
| 乙醇(反应器) | 3.2 g/L | [ | ||
| 黄酮类化合物 | 黄芩素 | 乙醇(摇瓶) | 401.9 mg/L | [ |
| 千层纸素 | 乙醇(摇瓶) | 339.5 mg/L |
| 1 | XU X H, LIU Y F, DU G C, et al. Microbial chassis development for natural product biosynthesis[J]. Trends in Biotechnology, 2020, 38(7): 779-796. |
| 2 | LIU J Y, WU X, YAO M D, et al. Chassis engineering for microbial production of chemicals: from natural microbes to synthetic organisms[J]. Current Opinion in Biotechnology, 2020, 66: 105-112. |
| 3 | GASSER B, MATTANOVICH D. A yeast for all seasons - is Pichia pastoris a suitable chassis organism for future bioproduction?[J]. FEMS Microbiology Letters, 2018, 365(17): fny181. |
| 4 | TRIPATHI N K, SHRIVASTAVA A. Recent developments in bioprocessing of recombinant proteins: expression hosts and process development[J]. Frontiers in Bioengineering and Biotechnology, 2019, 7: 420. |
| 5 | GAO J C, JIANG L H, LIAN J Z. Development of synthetic biology tools to engineer Pichia pastoris as a chassis for the production of natural products[J]. Synthetic and Systems Biotechnology, 2021, 6(2): 110-119. |
| 6 | SUN W, ZUO Y M, YAO Z Y, et al. Recent advances in synthetic biology applications of Pichia species [M]//Synthetic biology of yeasts. Cham: Springer International Publishing, 2022: 251-292. |
| 7 | KARBALAEI M, REZAEE S A, FARSIANI H. Pichia pastoris: a highly successful expression system for optimal synthesis of heterologous proteins[J]. Journal of Cellular Physiology, 2020, 235(9): 5867-5881. |
| 8 | ZHU T C, SUN H B, WANG M Y, et al. Pichia pastoris as a versatile cell factory for the production of industrial enzymes and chemicals: current status and future perspectives[J]. Biotechnology Journal, 2019, 14(6): e1800694. |
| 9 | ZAHRL R J, PEÑA D A, MATTANOVICH D, et al. Systems biotechnology for protein production in Pichia pastoris [J]. FEMS Yeast Research, 2017, 17(7): fox068. |
| 10 | YAMADA Y, MATSUDA M, MAEDA K, et al. The phylogenetic relationships of methanol-assimilating yeasts based on the partial sequences of 18S and 26S ribosomal RNAs: the proposal of Komagataella gen. nov. (Saccharomycetaceae)[J]. Bioscience, Biotechnology, and Biochemistry, 1995, 59(3): 439-444. |
| 11 | KURTZMAN C P. Description of Komagataella phaffii sp. nov. and the transfer of Pichia pseudopastoris to the methylotrophic yeast genus Komagataella [J]. International Journal of Systematic and Evolutionary Microbiology, 2005, 55(2): 973-976. |
| 12 | ZHU T C, ZHAO T X, BANKEFA O E, et al. Engineering unnatural methylotrophic cell factories for methanol-based biomanufacturing: challenges and opportunities[J]. Biotechnology Advances, 2020, 39: 107467. |
| 13 | PATRA P, DAS M, KUNDU P, et al. Recent advances in systems and synthetic biology approaches for developing novel cell-factories in non-conventional yeasts[J]. Biotechnology Advances, 2021, 47: 107695. |
| 14 | YANG Z L, ZHANG Z S. Engineering strategies for enhanced production of protein and bio-products in Pichia pastoris: a review[J]. Biotechnology Advances, 2018, 36(1): 182-195. |
| 15 | CIOFALO V, BARTON N, KREPS J, et al. Safety evaluation of a lipase enzyme preparation, expressed in Pichia pastoris, intended for use in the degumming of edible vegetable oil[J]. Regulatory Toxicology and Pharmacology, 2006, 45(1): 1-8. |
| 16 | SAFDER I, KJAN S, I-U ISLAM, et al. Pichia pastoris expression system: a potential candidate to express protein in industrial and biopharmaceutical domains[J]. Biomedical Letters, 2018, 4(1): 1-14. |
| 17 | DE SCHUTTER K, LIN Y C, TIELS P, et al. Genome sequence of the recombinant protein production host Pichia pastoris [J]. Nature Biotechnology, 2009, 27(6): 561-566. |
| 18 | KUBERL A, SCHNEIDER J, THALLINGER G G, et al. High-quality genome sequence of Pichia pastoris CBS7435[J]. Journal of Biotechnology, 2011, 154(4): 312-320. |
| 19 | STURMBERGER L, CHAPPELL T, GEIER M, et al. Refined Pichia pastoris reference genome sequence[J]. Journal of Biotechnology, 2016, 235: 121-131. |
| 20 | WANG X L, WANG Q, WANG J J, et al. Mit1 transcription factor mediates methanol signaling and regulates the alcohol oxidase 1 (AOX1) promoter in Pichia pastoris [J]. Journal of Biological Chemistry, 2016, 291(12): 6245-6261. |
| 21 | GUPTA A, KRISHNA RAO K, SAHU U, et al. Characterization of the transactivation and nuclear localization functions of Pichia pastoris zinc finger transcription factor Mxr1p[J]. Journal of Biological Chemistry, 2021, 297(4): 101247. |
| 22 | ATA O, REBNEGGER C, TATTO N E, et al. A single Gal4-like transcription factor activates the Crabtree effect in Komagataella phaffii [J]. Nature Communications, 2018, 9: 4911. |
| 23 | VOGL T, KICKENWEIZ T, PITZER J, et al. Engineered bidirectional promoters enable rapid multi-gene co-expression optimization[J]. Nature Communications, 2018, 9: 3589. |
| 24 | ITO Y, TERAI G, ISHIGAMI M, et al. Exchange of endogenous and heterogeneous yeast terminators in Pichia pastoris to tune mRNA stability and gene expression[J]. Nucleic Acids Research, 2020, 48(22): 13000-13012. |
| 25 | PORTELA R M C, VOGL T, KNIELY C, et al. Synthetic core promoters as universal parts for fine-tuning expression in different yeast species[J]. ACS Synthetic Biology, 2017, 6(3): 471-484. |
| 26 | RANTASALO A, LANDOWSKI C P, KUIVANEN J, et al. A universal gene expression system for fungi[J]. Nucleic Acids Research, 2018, 46(18): e111. |
| 27 | PEREZ-PINERA P, HAN N R, CLETO S, et al. Synthetic biology and microbioreactor platforms for programmable production of biologics at the point-of-care[J]. Nature Communications, 2016, 7: 12211. |
| 28 | LIU Q, SONG L L, PENG Q Q, et al. A programmable high-expression yeast platform responsive to user-defined signals[J]. Science Advances, 2022, 8(6): eabl5166. |
| 29 | CAI P, DUAN X P, WU X Y, et al. Recombination machinery engineering facilitates metabolic engineering of the industrial yeast Pichia pastoris [J]. Nucleic Acids Research, 2021, 49(13): 7791-7805. |
| 30 | GAO J C, YE C F, CHENG J T, et al. Enhancing homologous recombination efficiency in Pichia pastoris for multiplex genome integration using short homology arms[J]. ACS Synthetic Biology, 2022, 11(2): 547-553. |
| 31 | ZHANG X Y, GU S J, ZHENG X Y, et al. A novel and efficient genome editing tool assisted by CRISPR-Cas12a/Cpf1 for Pichia pastoris [J]. ACS Synthetic Biology, 2021, 10(11): 2927-2937. |
| 32 | GASSLER T, SAUER M, GASSER B, et al. The industrial yeast Pichia pastoris is converted from a heterotroph into an autotroph capable of growth on CO2 [J]. Nature Biotechnology, 2020, 38(2): 210-216. |
| 33 | LIU Y Q, BAI C X, LIU Q, et al. Engineered ethanol-driven biosynthetic system for improving production of acetyl-CoA derived drugs in Crabtree-negative yeast[J]. Metabolic Engineering, 2019, 54: 275-284. |
| 34 | QIAN Z L, YU J H, CHEN X J, et al. De novo production of plant 4'-deoxyflavones baicalein and oroxylin A from ethanol in Crabtree-negative yeast[J]. ACS Synthetic Biology, 2022, 11(4): 1600-1612. |
| 35 | SCHWARZHANS J P, LUTTERMANN T, GEIER M, et al. Towards systems metabolic engineering in Pichia pastoris [J]. Biotechnology Advances, 2017, 35(6): 681-710. |
| 36 | CAMATTARI A, GOH A, YIP L Y, et al. Characterization of a panARS-based episomal vector in the methylotrophic yeast Pichia pastoris for recombinant protein production and synthetic biology applications[J]. Microbial Cell Factories, 2016, 15(1): 139. |
| 37 | SCHWARZHANS J P, LUTTERMANN T, WIBBERG D, et al. A mitochondrial autonomously replicating sequence from Pichia pastoris for uniform high level recombinant protein production[J]. Frontiers in Microbiology, 2017, 8: 780. |
| 38 | NAKAMURA Y, NISHI T, NOGUCHI R, et al. A stable, autonomously replicating plasmid vector containing Pichia pastoris centromeric DNA[J]. Applied and Environmental Microbiology, 2018, 84(15): e02882-e02817. |
| 39 | GU Y, GAO J C, CAO M F, et al. Construction of a series of episomal plasmids and their application in the development of an efficient CRISPR/Cas9 system in Pichia pastoris [J]. World Journal of Microbiology & Biotechnology, 2019, 35(6): 79. |
| 40 | PRIELHOFER R, BARRERO J J, STEUER S, et al. GoldenPiCS: a Golden Gate-derived modular cloning system for applied synthetic biology in the yeast Pichia pastoris [J]. BMC Systems Biology, 2017, 11(1): 123. |
| 41 | NISHI T, ITO Y, NAKAMURA Y, et al. One-step in vivo assembly of multiple DNA fragments and genomic integration in Komagataella phaffii [J]. ACS Synthetic Biology, 2022, 11(2): 644-654. |
| 42 | JUTURU V, WU J C. Heterologous protein expression in Pichia pastoris: latest research progress and applications[J]. Chembiochem: a European Journal of Chemical Biology, 2018, 19(1): 7-21. |
| 43 | BAGHBAN R, FARAJNIA S, GHASEMI Y, et al. New developments in Pichia pastoris expression system, review and update[J]. Current Pharmaceutical Biotechnology, 2018, 19(6): 451-467. |
| 44 | NETT J H, HODEL N, RAUSCH S, et al. Cloning and disruption of the Pichia pastoris ARG1, ARG2, ARG3, HIS1, HIS2, HIS5, HIS6 genes and their use as auxotrophic markers[J]. Yeast, 2005, 22(4): 295-304. |
| 45 | YANG J J, NIE L, CHEN B, et al. Hygromycin-resistance vectors for gene expression in Pichia pastoris [J]. Yeast, 2014, 31(4): 115-125. |
| 46 | KANG Z, HUANG H, ZHANG Y F, et al. Recent advances of molecular toolbox construction expand Pichia pastoris in synthetic biology applications[J]. World Journal of Microbiology & Biotechnology, 2017, 33(1): 19. |
| 47 | SHIBUI T, HARA H. A new type of gene-disruption cassette with a rescue gene for Pichia pastoris [J]. Biotechnology Progress, 2017, 33(5): 1201-1208. |
| 48 | LI D, ZHANG B, LI S T, et al. A novel vector for construction of markerless multicopy overexpression transformants in Pichia pastoris [J]. Frontiers in Microbiology, 2017, 8: 1698. |
| 49 | LI C, LIN Y, ZHENG X Y, et al. Recycling of a selectable marker with a self-excisable plasmid in Pichia pastoris [J]. Scientific Reports, 2017, 7(1): 11113. |
| 50 | HAN M H, WANG W X, GONG X, et al. A modified method of gene disruption in Komagataella phaffii with Cre/loxP system[J]. Journal of Biotechnology, 2022, 347: 40-48. |
| 51 | ZIENTARA-RYTTER K, OZEKI K, NAZARKO T Y, et al. Pex3 and Atg37 compete to regulate the interaction between the pexophagy receptor, Atg30, and the Hrr25 kinase[J]. Autophagy, 2018, 14(3): 368-384. |
| 52 | PIVA L C, DE MARCO J L, DE MORAES L M P, et al. Acetamidase as a dominant recyclable marker for Komagataella phaffii strain engineering[J]. Applied Microbiology and Biotechnology, 2018, 102(6): 2753-2761. |
| 53 | YANG J J, JIANG W H, YANG S. mazF as a counter-selectable marker for unmarked genetic modification of Pichia pastoris [J]. FEMS Yeast Research, 2009, 9(4): 600-609. |
| 54 | CAI P, GAO J Q, ZHOU Y J. CRISPR-mediated genome editing in non-conventional yeasts for biotechnological applications[J]. Microbial Cell Factories, 2019, 18(1): 63. |
| 55 | LINO C A, HARPER J C, CARNEY J P, et al. Delivering CRISPR: a review of the challenges and approaches[J]. Drug Delivery, 2018, 25(1): 1234-1257. |
| 56 | WENINGER A, HATZL A M, SCHMID C, et al. Combinatorial optimization of CRISPR/Cas9 expression enables precision genome engineering in the methylotrophic yeast Pichia pastoris [J]. Journal of Biotechnology, 2016, 235: 139-149. |
| 57 | WENINGER A, FISCHER J E, RASCHMANOVÁ H, et al. Expanding the CRISPR/Cas9 toolkit for Pichia pastoris with efficient donor integration and alternative resistance markers[J]. Journal of Cellular Biochemistry, 2018, 119(4): 3183-3198. |
| 58 | DALVIE N C, LEAL J, WHITTAKER C A, et al. Host-informed expression of CRISPR guide RNA for genomic engineering in Komagataella phaffii [J]. ACS Synthetic Biology, 2020, 9(1): 26-35. |
| 59 | YANG Y K, LIU G Q, CHEN X, et al. High efficiency CRISPR/Cas9 genome editing system with an eliminable episomal sgRNA plasmid in Pichia pastoris [J]. Enzyme and Microbial Technology, 2020, 138: 109556. |
| 60 | GAO J C, XU J H, ZUO Y M, et al. Synthetic biology toolkit for marker-less integration of multigene pathways into Pichia pastoris via CRISPR/Cas9[J]. ACS Synthetic Biology, 2022, 11(2): 623-633. |
| 61 | ADIEGO-PÉREZ B, RANDAZZO P, DARAN J M, et al. Multiplex genome editing of microorganisms using CRISPR-Cas[J]. FEMS Microbiology Letters, 2019, 366(8): fnz086. |
| 62 | MCCARTY N S, GRAHAM A E, STUDENÁ L, et al. Multiplexed CRISPR technologies for gene editing and transcriptional regulation[J]. Nature Communications, 2020, 11: 1281. |
| 63 | RASCHMANOVÁ H, WENINGER A, GLIEDER A, et al. Implementing CRISPR-Cas technologies in conventional and non-conventional yeasts: current state and future prospects[J]. Biotechnology Advances, 2018, 36(3): 641-665. |
| 64 | YANG H, REN S L, YU S Y, et al. Methods favoring homology-directed repair choice in response to CRISPR/Cas9 induced-double strand breaks[J]. International Journal of Molecular Sciences, 2020, 21(18): 6461. |
| 65 | LIU Q, SHI X N, SONG L L, et al. CRISPR-Cas9-mediated genomic multiloci integration in Pichia pastoris [J]. Microbial Cell Factories, 2019, 18(1): 144. |
| 66 | LI T W, ZHU L W, XIAO B X, et al. CRISPR-Cpf1-mediated genome editing and gene regulation in human cells[J]. Biotechnology Advances, 2019, 37(1): 21-27. |
| 67 | CHE Z Q, CAO X Y, CHEN G G, et al. An effective combination of codon optimization, gene dosage, and process optimization for high-level production of fibrinolytic enzyme in Komagataella phaffii (Pichia pastoris)[J]. BMC Biotechnology, 2020, 20(1): 63. |
| 68 | HUANG M M, GAO Y Y, ZHOU X S, et al. Regulating unfolded protein response activator HAC1p for production of thermostable raw-starch hydrolyzingα-amylase in Pichia pastoris [J]. Bioprocess and Biosystems Engineering, 2017, 40(3): 341-350. |
| 69 | MAITY N, JASWAL A S, GAUTAM A, et al. High level production of stable human serum albumin in Pichia pastoris and characterization of the recombinant product[J]. Bioprocess and Biosystems Engineering, 2022, 45(2): 409-424. |
| 70 | SUNGA A J, TOLSTORUKOV I, CREGG J M. Posttransformational vector amplification in the yeast Pichia pastoris [J]. FEMS Yeast Research, 2008, 8(6): 870-876. |
| 71 | AW R, POLIZZI K M. Liquid PTVA: a faster and cheaper alternative for generating multi-copy clones in Pichia pastoris [J]. Microbial Cell Factories, 2016, 15: 29. |
| 72 | MARX H, MECKLENBRÄUKER A, GASSER B, et al. Directed gene copy number amplification in Pichia pastoris by vector integration into the ribosomal DNA locus[J]. FEMS Yeast Research, 2009, 9(8): 1260-1270. |
| 73 | SONG X P, SHAO C S, GUO Y G, et al. Improved the expression level of active transglutaminase by directional increasing copy of mtg gene in Pichia pastoris [J]. BMC Biotechnology, 2019, 19(1): 54. |
| 74 | SUN X W, LIU H, WANG P, et al. Construction of a novel MK-4 biosynthetic pathway in Pichia pastoris through heterologous expression of HsUBIAD1[J]. Microbial Cell Factories, 2019, 18(1): 169. |
| 75 | YAMADA R, OGURA K, KIMOTO Y, et al. Toward the construction of a technology platform for chemicals production from methanol: D-lactic acid production from methanol by an engineered yeast Pichia pastoris [J]. World Journal of Microbiology & Biotechnology, 2019, 35(2): 37. |
| 76 | MALCI K, WALLS L E, RIOS-SOLIS L. Multiplex genome engineering methods for yeast cell factory development[J]. Frontiers in Bioengineering and Biotechnology, 2020, 8: 589468. |
| 77 | WANG L Y, DENG A H, ZHANG Y, et al. Efficient CRISPR-Cas9 mediated multiplex genome editing in yeasts[J]. Biotechnology for Biofuels, 2018, 11: 277. |
| 78 | VOGL T, GLIEDER A. Regulation of Pichia pastoris promoters and its consequences for protein production[J]. New Biotechnology, 2013, 30(4): 385-404. |
| 79 | GASSER B, STEIGER M G, MATTANOVICH D. Methanol regulated yeast promoters: production vehicles and toolbox for synthetic biology[J]. Microbial Cell Factories, 2015, 14: 196. |
| 80 | CREGG J M, MADDEN K R, BARRINGER K J, et al. Functional characterization of the two alcohol oxidase genes from the yeast Pichia pastoris [J]. Molecular and Cellular Biology, 1989, 9(3): 1316-1323. |
| 81 | YAN C X, YU W, YAO L, et al. Expanding the promoter toolbox for metabolic engineering of methylotrophic yeasts[J]. Applied Microbiology and Biotechnology, 2022, 106(9/10): 3449-3464. |
| 82 | VOGL T, STURMBERGER L, KICKENWEIZ T, et al. A toolbox of diverse promoters related to methanol utilization: functionally verified parts for heterologous pathway expression in Pichia pastoris [J]. ACS Synthetic Biology, 2016, 5(2): 172-186. |
| 83 | LIU B, ZHANG Y W, ZHANG X, et al. Discovery of a rhamnose utilization pathway and rhamnose-inducible promoters in Pichia pastoris [J]. Scientific Reports, 2016, 6: 27352. |
| 84 | YAN C L, XU X X, ZHANG X, et al. Decreased rhamnose metabolic flux improved production of target proteins and cell flocculation in Pichia pastoris [J]. Frontiers in Microbiology, 2018, 9: 1771. |
| 85 | KARAOĞLAN M, ERDEN-KARAOĞLAN F, YıLMAZ S, et al. Identification of major ADH genes in ethanol metabolism of Pichia pastoris [J]. Yeast, 2020, 37(2): 227-236. |
| 86 | KARAOGLAN M, KARAOGLAN F E, INAN M. Comparison of ADH3 promoter with commonly used promoters for recombinant protein production in Pichia pastoris [J]. Protein Expression and Purification, 2016, 121: 112-117. |
| 87 | PRIELHOFER R, MAURER M, KLEIN J, et al. Induction without methanol: novel regulated promoters enable high-level expression in Pichia pastoris [J]. Microbial Cell Factories, 2013, 12: 5. |
| 88 | PRIELHOFER R, REICHINGER M, WAGNER N, et al. Superior protein titers in half the fermentation time: promoter and process engineering for the glucose-regulated GTH1 promoter of Pichia pastoris [J]. Biotechnology and Bioengineering, 2018, 115(10): 2479-2488. |
| 89 | LANDES N, GASSER B, VORAUER-UHL K, et al. The vitamin-sensitive promoter P THI11 enables pre-defined autonomous induction of recombinant protein production in Pichia pastoris [J]. Biotechnology and Bioengineering, 2016, 113(12): 2633-2643. |
| 90 | ÇALıK P, ATA Ö, GÜNEŞ H, et al. Recombinant protein production in Pichia pastoris under glyceraldehyde-3-phosphate dehydrogenase promoter: from carbon source metabolism to bioreactor operation parameters[J]. Biochemical Engineering Journal, 2015, 95: 20-36. |
| 91 | NIETO-TAYPE M A, GARCIA-ORTEGA X, ALBIOL J, et al. Continuous cultivation as a tool toward the rational bioprocess development with Pichia Pastoris cell factory[J]. Frontiers in Bioengineering and Biotechnology, 2020, 8: 632. |
| 92 | GARCÍA-ORTEGA X, CÁMARA E, FERRER P, et al. Rational development of bioprocess engineering strategies for recombinant protein production in Pichia pastoris (Komagataella phaffii) using the methanol-free GAP promoter. Where do we stand? [J]. New Biotechnology, 2019, 53: 24-34. |
| 93 | LIANG S L, ZOU C J, LIN Y, et al. Identification and characterization of P GCW14 : a novel, strong constitutive promoter of Pichia pastoris [J]. Biotechnology Letters, 2013, 35(11): 1865-1871. |
| 94 | HARTNER F S, RUTH C, LANGENEGGER D, et al. Promoter library designed for fine-tuned gene expression in Pichia pastoris [J]. Nucleic Acids Research, 2008, 36(12): e76. |
| 95 | XUAN Y J, ZHOU X S, ZHANG W W, et al. An upstream activation sequence controls the expression of AOX1 gene in Pichia pastoris [J]. FEMS Yeast Research, 2009, 9(8): 1271-1282. |
| 96 | YANG J, CAI H M, LIU J, et al. Controlling AOX1 promoter strength in Pichia pastoris by manipulating poly (dA:dT) tracts[J]. Scientific Reports, 2018, 8(1): 1401. |
| 97 | PORTELA R M C, VOGL T, EBNER K, et al. Pichia pastoris Alcohol Oxidase 1 (AOX1) core promoter engineering by high resolution systematic mutagenesis[J]. Biotechnology Journal, 2018, 13(3): e1700340. |
| 98 | ERGÜN B G, DEMIR İ, ÖZDAMAR T H, et al. Engineered deregulation of expression in yeast with designed hybrid-promoter architectures in coordination with discovered master regulator transcription factor[J]. Advanced Biosystems, 2020, 4(4): 1900172. |
| 99 | QIN X L, QIAN J C, YAO G F, et al. GAP promoter library for fine-tuning of gene expression in Pichia pastoris [J]. Applied and Environmental Microbiology, 2011, 77(11): 3600-3608. |
| 100 | ATA Ö, PRIELHOFER R, GASSER B, et al. Transcriptional engineering of the glyceraldehyde-3-phosphate dehydrogenase promoter for improved heterologous protein production in Pichia pastoris [J]. Biotechnology and Bioengineering, 2017, 114(10): 2319-2327. |
| 101 | NONG L Y, ZHANG Y M, DUAN Y H, et al. Engineering the regulatory site of the catalase promoter for improved heterologous protein production in Pichia pastoris [J]. Biotechnology Letters, 2020, 42(12): 2703-2709. |
| 102 | VOGL T, RUTH C, PITZER J, et al. Synthetic core promoters for Pichia pastoris [J]. ACS Synthetic Biology, 2014, 3(3): 188-191. |
| 103 | RAMAKRISHNAN K, PRATTIPATI M, SAMUEL P, et al. Transcriptional control of gene expression in Pichia pastoris by manipulation of terminators[J]. Applied Microbiology and Biotechnology, 2020, 104(18): 7841-7851. |
| 104 | ERGÜN B G, BERRIOS J, BINAY B, et al. Recombinant protein production in Pichia pastoris: from transcriptionally redesigned strains to bioprocess optimization and metabolic modelling[J]. FEMS Yeast Research, 2021, 21(7): foab057. |
| 105 | LIN-CEREGHINO G P, GODFREY L, DE LA CRUZ B J, et al. Mxr1p, a key regulator of the methanol utilization pathway and peroxisomal genes in Pichia pastoris [J]. Molecular and Cellular Biology, 2006, 26(3): 883-897. |
| 106 | PARUA P K, RYAN P M, TRANG K, et al. Pichia pastoris 14-3-3 regulates transcriptional activity of the methanol inducible transcription factor Mxr1 by direct interaction[J]. Molecular Microbiology, 2012, 85(2): 282-298. |
| 107 | SAHU U, KRISHNA RAO K, RANGARAJAN P N. Trm1p, a Zn(II)2Cys6-type transcription factor, is essential for the transcriptional activation of genes of methanol utilization pathway, in Pichia pastoris [J]. Biochemical and Biophysical Research Communications, 2014, 451(1): 158-164. |
| 108 | WANG X L, CAI M H, SHI L, et al. PpNrg1 is a transcriptional repressor for glucose and glycerol repression of AOX1 promoter in methylotrophic yeast Pichia pastoris [J]. Biotechnology Letters, 2016, 38(2): 291-298. |
| 109 | WANG J J, WANG X L, SHI L, et al. Methanol-independent protein expression by AOX1 promoter with trans-acting elements engineering and glucose-glycerol-shift induction in Pichia pastoris [J]. Scientific Reports, 2017, 7: 41850. |
| 110 | VOGL T, STURMBERGER L, FAULAND P C, et al. Methanol independent induction in Pichia pastoris by simple derepressed overexpression of single transcription factors[J]. Biotechnology and Bioengineering, 2018, 115(4): 1037-1050. |
| 111 | CHANG C H, HSIUNG H A, HONG K L, et al. Enhancing the efficiency of the Pichia pastoris AOX1 promoter via the synthetic positive feedback circuit of transcription factor Mxr1[J]. BMC Biotechnology, 2018, 18(1): 81. |
| 112 | YANG Y K, ZHENG Y T, WANG P C, et al. Characterization and application of a putative transcription factor (SUT2) in Pichia pastoris [J]. Molecular Genetics and Genomics, 2020, 295(5): 1295-1304. |
| 113 | TAKAGI S, TSUTSUMI N, TERUI Y J, et al. Engineering the expression system for Komagataella phaffii (Pichia pastoris): an attempt to develop a methanol-free expression system[J]. FEMS Yeast Research, 2019, 19(6): foz059. |
| 114 | LIAO X H, LI L, JAMEEL A, et al. A versatile toolbox for CRISPR-based genome engineering in Pichia pastoris [J]. Applied Microbiology Biotechnology, 2021, 105(24): 9211-9218. |
| 115 | BAUMSCHABL M, PRIELHOFER R, MATTANOVICH D, et al. Fine-tuning of transcription in Pichia pastoris using dCas9 and RNA scaffolds[J]. ACS Synthetic Biology, 2020, 9(12): 3202-3209. |
| 116 | SHEN W, XUE Y, LIU Y Q, et al. A novel methanol-free Pichia pastoris system for recombinant protein expression[J]. Microbial Cell Factories, 2016, 15(1): 178. |
| 117 | ZHANG P, ZHANG W W, ZHOU X S, et al. Catabolite repression of Aox in Pichia pastoris is dependent on hexose transporter PpHxt1 and pexophagy[J]. Applied and Environmental Microbiology, 2010, 76(18): 6108-6118. |
| 118 | POLUPANOV A S, NAZARKO V Y, SIBIRNY A A. Gss1 protein of the methylotrophic yeast Pichia pastoris is involved in glucose sensing, pexophagy and catabolite repression[J]. The International Journal of Biochemistry & Cell Biology, 2012, 44(11): 1906-1918. |
| 119 | ZHAN C J, WANG S W, SUN Y, et al. The Pichia pastoris transmembrane protein GT1 is a glycerol transporter and relieves the repression of glycerol on AOX1 expression[J]. FEMS Yeast Research, 2016, 16(4): fow033. |
| 120 | XIA P F, LING H, FOO J L, et al. Synthetic genetic circuits for programmable biological functionalities[J]. Biotechnology Advances, 2019, 37(6): 107393. |
| 121 | JENSEN M K, KEASLING J D. Recent applications of synthetic biology tools for yeast metabolic engineering[J]. FEMS Yeast Research, 2015, 15(1): 1-10. |
| 122 | SOWA S W, GELDERMAN G, CONTRERAS L M. Advances in synthetic dynamic circuits design: using novel synthetic parts to engineer new generations of gene oscillations[J]. Current Opinion in Biotechnology, 2015, 36: 161-167. |
| 123 | D'AMBROSIO V, JENSEN M K. Lighting up yeast cell factories by transcription factor-based biosensors[J]. FEMS Yeast Research, 2017, 17(7): fox076. |
| 124 | QI L S, LARSON M H, GILBERT L A, et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression[J]. Cell, 2013, 152(5): 1173-1183. |
| 125 | XU X S, QI L S. A CRISPR-dCas toolbox for genetic engineering and synthetic biology[J]. Journal of Molecular Biology, 2019, 431(1): 34-47. |
| 126 | RUDD P M, ELLIOTT T, CRESSWELL P, et al. Glycosylation and the immune system[J]. Science, 2001, 291(5512): 2370-2376. |
| 127 | SINCLAIR A M, ELLIOTT S. Glycoengineering: the effect of glycosylation on the properties of therapeutic proteins[J]. Journal of Pharmaceutical Sciences, 2005, 94(8): 1626-1635. |
| 128 | SWIECH K, DE FREITAS M C C, COVAS D T, et al. Recombinant glycoprotein production in human cell lines[J]. Methods in Molecular Biology, 2015, 1258: 223-240. |
| 129 | HAMILTON S R, ZHA D X. Progress in yeast glycosylation engineering[J]. Methods in Molecular Biology, 2015, 1321: 73-90. |
| 130 | ANYAOGU D C, MORTENSEN U H. Manipulating the glycosylation pathway in bacterial and lower eukaryotes for production of therapeutic proteins[J]. Current Opinion in Biotechnology, 2015, 36: 122-128. |
| 131 | GE F, ZHU L B, AANG A N, et al. Recent advances in enhanced enzyme activity, thermostability and secretion by N-glycosylation regulation in yeast[J]. Biotechnology Letters, 2018, 40(5): 847-854. |
| 132 | JACOBS P P, GEYSENS S, VERVECKEN W, et al. Engineering complex-type N-glycosylation in Pichia pastoris using GlycoSwitch technology[J]. Nature Protocols, 2009, 4(1): 58-70. |
| 133 | THAK E J, KIM J, LEE D J, et al. Structural analysis of N-/O-glycans assembled on proteins in yeasts[J]. Journal of Microbiology, 2018, 56(1): 11-23. |
| 134 | BICKEL T, LEHLE L, SCHWARZ M, et al. Biosynthesis of lipid-linked oligosaccharides in Saccharomyces cerevisiae: Alg13p and Alg14p form a complex required for the formation of GlcNAc2-PP-dolichol[J]. The Journal of Biological Chemistry, 2005, 280(41): 34500-34506. |
| 135 | CIPOLLO J F, TRIMBLE R B, CHI J H, et al. The yeast ALG11 gene specifies addition of the terminal α-1,2-Man to the Man5GlcNAc2-PP-dolichol N-glycosylation intermediate formed on the cytosolic side of the endoplasmic reticulum[J]. The Journal of Biological Chemistry, 2001, 276(24): 21828-21840. |
| 136 | GAO X D, MORIYAMA S, MIURA N, et al. Interaction between the C termini of Alg13 and Alg14 mediates formation of the active UDP-N-acetylglucosamine transferase complex[J]. The Journal of Biological Chemistry, 2008, 283(47): 32534-32541. |
| 137 | CHOI B K, BOBROWICZ P, DAVIDSON R C, et al. Use of combinatorial genetic libraries to humanize N-linked glycosylation in the yeast Pichia pastoris [J]. Proceedings of the National Academy of Sciences of the United States of America, 2003, 100(9): 5022-5027. |
| 138 | HAMILTON S R, BOBROWICZ P, BOBROWICZ B, et al. Production of complex human glycoproteins in yeast[J]. Science, 2003, 301(5637): 1244-1246. |
| 139 | HAMILTON S R, DAVIDSON R C, SETHURAMAN N, et al. Humanization of yeast to produce complex terminally sialylated glycoproteins[J]. Science, 2006, 313(5792): 1441-1443. |
| 140 | LIU C P, TSAI T I, CHENG T, et al. Glycoengineering of antibody (Herceptin) through yeast expression and in vitro enzymatic glycosylation[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(4): 720-725. |
| 141 | KATLA S, YOGANAND K N R, HINGANE S, et al. Novel glycosylated human interferon alpha 2b expressed in glycoengineered Pichia pastoris and its biological activity: N-linked glycoengineering approach[J]. Enzyme and Microbial Technology, 2019, 128: 49-58. |
| 142 | PARSAIE NASAB F, AEBI M, BERNHARD G, et al. A combined system for engineering glycosylation efficiency and glycan structure in Saccharomyces cerevisiae [J]. Applied and Environmental Microbiology, 2013, 79(3): 997-1007. |
| 143 | CHEON S A, KIM H, OH D B, et al. Remodeling of the glycosylation pathway in the methylotrophic yeast Hansenula polymorpha to produce human hybrid-type N-glycans[J]. Journal of Microbiology, 2012, 50(2): 341-348. |
| 144 | NILSSON I, LARA P, HESSA T, et al. The code for directing proteins for translocation across ER membrane: SRP cotranslationally recognizes specific features of a signal sequence[J]. Journal of Molecular Biology, 2015, 427(6 Pt A): 1191-1201. |
| 145 | CHAHAL S, WEI P, MOUA P C, et al. Structural characterization of the α-mating factor prepro-peptide for secretion of recombinant proteins in Pichia pastoris [J]. Gene, 2017, 598: 50-62. |
| 146 | AHN J, JANG M J, ANG K S, et al. Codon optimization of Saccharomyces cerevisiae mating factor alpha prepro-leader to improve recombinant protein production in Pichia pastoris [J]. Biotechnology Letters, 2016, 38(12): 2137-2143. |
| 147 | BARRERO J J, CASLER J C, VALERO F, et al. An improved secretion signal enhances the secretion of model proteins from Pichia pastoris [J]. Microbial Cell Factories, 2018, 17(1): 161. |
| 148 | MURASUGI A, TOHMA-AIBA Y. Comparison of three signals for secretory expression of recombinant human midkine in Pichia pastoris [J]. Bioscience, Biotechnology, and Biochemistry, 2001, 65(10): 2291-2293. |
| 149 | LIANG S L, LI C, YE Y R, et al. Endogenous signal peptides efficiently mediate the secretion of recombinant proteins in Pichia pastoris [J]. Biotechnology Letters, 2013, 35(1): 97-105. |
| 150 | DUAN G D, DING L M, WEI D S, et al. Screening endogenous signal peptides and protein folding factors to promote the secretory expression of heterologous proteins in Pichia pastoris [J]. Journal of Biotechnology, 2019, 306: 193-202. |
| 151 | SHEN Q, ZHOU X T, GUO Q, et al. Potential of the signal peptide derived from the PAS_chr3_0030 gene product for secretory expression of valuable enzymes in Pichia pastoris [J]. Applied and Environmental Microbiology, 2022, 88(9): e0029622. |
| 152 | MASSAHI A, CALIK P. Endogenous signal peptides in recombinant protein production by Pichia pastoris: from in-silico analysis to fermentation[J]. Journal of Theoretical Biology, 2016, 408: 22-33. |
| 153 | MASSAHI A, CALIK P. In-silico determination of Pichia pastoris signal peptides for extracellular recombinant protein production[J]. Journal of Theoretical Biology, 2015, 364: 179-188. |
| 154 | RASCHMANOVÁ H, WENINGER A, KNEJZLÍK Z, et al. Engineering of the unfolded protein response pathway in Pichia pastoris: enhancing production of secreted recombinant proteins[J]. Applied Microbiology and Biotechnology, 2021, 105(11): 4397-4414. |
| 155 | HAN M H, WANG W X, ZHOU J L, et al. Activation of the unfolded protein response via co-expression of the HAC1 i gene enhances expression of recombinant elastase in Pichia pastoris [J]. Biotechnology and Bioprocess Engineering, 2020, 25(2): 302-307. |
| 156 | LIU J, HAN Q, CHENG Q K, et al. Efficient expression of human lysozyme through the increased gene dosage and co-expression of transcription factor Hac1p in Pichia pastoris [J]. Current Microbiology, 2020, 77(5): 846-854. |
| 157 | SONG W, ZHANG N, YANG M, et al. Multiple strategies to improve the yield of chitinase a from Bacillus licheniformis in Pichia pastoris to obtain plant growth enhancer and GlcNAc[J]. Microbial Cell Factories, 2020, 19(1): 181. |
| 158 | NAVONE L, VOGL T, LUANGTHONGKAM P, et al. Synergistic optimisation of expression, folding, and secretion improves E. coli AppA phytase production in Pichia pastoris [J]. Microbial Cell Factories, 2021, 20(1): 8. |
| 159 | AZOUN S BEN, BELHAJ A E, GÖNGRICH R, et al. Molecular optimization of rabies virus glycoprotein expression in Pichia pastoris [J]. Microbial Biotechnology, 2016, 9(3): 355-368. |
| 160 | LI J D, CAI J, MA M T, et al. Preparation of a Bombyx mori acetylcholinesterase enzyme reagent through chaperone protein disulfide isomerase co-expression strategy in Pichia pastoris for detection of pesticides[J]. Enzyme and Microbial Technology, 2021, 144: 109741. |
| 161 | GUAN B, CHEN F X, SU S, et al. Effects of co-overexpression of secretion helper factors on the secretion of a HSA fusion protein (IL2-HSA) in Pichia pastoris [J]. Yeast, 2016, 33(11): 587-600. |
| 162 | YANG J, LU Z P, CHEN J W, et al. Effect of cooperation of chaperones and gene dosage on the expression of porcine PGLYRP-1 in Pichia pastoris [J]. Applied Microbiology and Biotechnology, 2016, 100(12): 5453-5465. |
| 163 | SALLADA N D, HARKINS L E, BERGER B W. Effect of gene copy number and chaperone coexpression on recombinant hydrophobin HFBI biosurfactant production in Pichia pastoris [J]. Biotechnology and Bioengineering, 2019, 116(8): 2029-2040. |
| 164 | BAUMANN K, ADELANTADO N, LANG C, et al. Protein trafficking, ergosterol biosynthesis and membrane physics impact recombinant protein secretion in Pichia pastoris [J]. Microbial Cell Factories, 2011, 10: 93. |
| 165 | GASSER B, SAUER M, MAURER M, et al. Transcriptomics-based identification of novel factors enhancing heterologous protein secretion in yeasts[J]. Applied and Environmental Microbiology, 2007, 73(20): 6499-6507. |
| 166 | SAKARIKA M, GANIGUÉ R, RABAEY K. Methylotrophs: from C1 compounds to food[J]. Current Opinion in Biotechnology, 2022, 75: 102685. |
| 167 | ZHANG W M, SONG M, YANG Q, et al. Current advance in bioconversion of methanol to chemicals[J]. Biotechnology for Biofuels, 2018, 11: 260. |
| 168 | DUAN X P, GAO J Q, ZHOU Y J J. Advances in engineering methylotrophic yeast for biosynthesis of valuable chemicals from methanol[J]. Chinese Chemical Letters, 2018, 29(5): 681-686. |
| 169 | RUßMAYER H, BUCHETICS M, GRUBER C, et al. Systems-level organization of yeast methylotrophic lifestyle[J]. BMC Biology, 2015, 13: 80. |
| 170 | ANTONIEWICZ M R. Synthetic methylotrophy: Strategies to assimilate methanol for growth and chemicals production[J]. Current Opinion in Biotechnology, 2019, 59: 165-174. |
| 171 | ZHANG T, GE C Y, DENG L, et al. C4-dicarboxylic acid production by overexpressing the reductive TCA pathway[J]. FEMS Microbiology Letters, 2015, 362(9): fnv052. |
| 172 | GUO F, DAI Z X, PENG W F, et al. Metabolic engineering of Pichia pastoris for malic acid production from methanol[J]. Biotechnology and Bioengineering, 2021, 118(1): 357-371. |
| 173 | CAI P, WU X Y, DENG J, et al. Methanol biotransformation toward high-level production of fatty acid derivatives by engineering the industrial yeast Pichia pastoris [J]. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(29): e2201711119. |
| 174 | CAI P, LI Y X, ZHAI X X, et al. Microbial synthesis of long-chain α-alkenes from methanol by engineering Pichia pastoris [J]. Bioresources and Bioprocessing, 2022, 9: 58. |
| 175 | MEESAPYODSUK D, CHEN Y, NG S H, et al. Metabolic engineering of Pichia pastoris to produce ricinoleic acid, a hydroxy fatty acid of industrial importance[J]. Journal of Lipid Research, 2015, 56(11): 2102-2109. |
| 176 | KIM S H, ROH K H, KIM K S, et al. Coexpression of multiple genes reconstitutes two pathways of very long-chain polyunsaturated fatty acid biosynthesis in Pichia pastoris [J]. Biotechnology Letters, 2014, 36(9): 1843-1851. |
| 177 | BHATAYA A, SCHMIDT-DANNERT C, LEE P C. Metabolic engineering of Pichia pastoris X-33 for lycopene production[J]. Process Biochemistry, 2009, 44(10): 1095-1102. |
| 178 | WRIESSNEGGER T, AUGUSTIN P, ENGLEDER M, et al. Production of the sesquiterpenoid (+)-nootkatone by metabolic engineering of Pichia pastoris [J]. Metabolic Engineering, 2014, 24: 18-29. |
| 179 | LIU X B, LIU M, TAO X Y, et al. Metabolic engineering of Pichia pastoris for the production of dammarenediol-II[J]. Journal of Biotechnology, 2015, 216: 47-55. |
| 180 | ZHANG X Y, WANG D G, DUAN Y H, et al. Production of lycopene by metabolically engineered Pichia pastoris [J]. Bioscience, Biotechnology, and Biochemistry, 2020, 84(3): 463-470. |
| 181 | GAO L M, CAI M H, SHEN W, et al. Engineered fungal polyketide biosynthesis in Pichia pastoris: a potential excellent host for polyketide production[J]. Microbial Cell Factories, 2013, 12: 77. |
| 182 | XUE Y, KONG C X, SHEN W, et al. Methylotrophic yeast Pichia pastoris as a chassis organism for polyketide synthesis via the full citrinin biosynthetic pathway[J]. Journal of Biotechnology, 2017, 242: 64-72. |
| 183 | KONG C X, HUANG H Z, XUE Y, et al. Heterologous pathway assembly reveals molecular steps of fungal terreic acid biosynthesis[J]. Scientific Reports, 2018, 8: 2116. |
| 184 | LIU Y Q, TU X H, XU Q, et al. Engineered monoculture and co-culture of methylotrophic yeast for de novo production of monacolin J and lovastatin from methanol[J]. Metabolic Engineering, 2018, 45: 189-199. |
| 185 | CANALES C, ALTAMIRANO C, BERRIOS J. Effect of dilution rate and methanol-glycerol mixed feeding on heterologous Rhizopus oryzae lipase production with Pichia pastoris Mut+ phenotype in continuous culture[J]. Biotechnology Progress, 2015, 31(3): 707-714. |
| 186 | BERRIOS J, FLORES M O, DÍAZ-BARRERA A, et al. A comparative study of glycerol and sorbitol as co-substrates in methanol-induced cultures of Pichia pastoris: temperature effect and scale-up simulation[J]. Journal of Industrial Microbiology and Biotechnology, 2017, 44(3): 407-411 |
| 187 | GU L, ZHANG J, LIU B H, et al. High-level extracellular production of glucose oxidase by recombinant Pichia pastoris using a combined strategy[J]. Applied Biochemistry and Biotechnology, 2015, 175(3): 1429-1447. |
| 188 | NIU H X, JOST L, PIRLOT N, et al. A quantitative study of methanol/sorbitol co-feeding process of a Pichia pastoris Mut+/pAOX1-lacZ strain[J]. Microbial Cell Factories, 2013, 12: 33. |
| 189 | JIA L Q, MPOFU E, TU T Y, et al. Transcriptional analysis for carbon metabolism and kinetic modeling for heterologous proteins productions by Pichia pastoris in induction process with methanol/sorbitol co-feeding[J]. Process Biochemistry, 2017, 59: 159-166. |
| 190 | PRABHU A A, VEERANKI V D. Metabolic engineering of Pichia pastoris GS115 for enhanced pentose phosphate pathway (PPP) flux toward recombinant human interferon gamma (hIFN-ɣ) production[J]. Molecular Biology Reports, 2018, 45(5): 961-972. |
| 191 | XIE J L, ZHOU Q W, DU P, et al. Use of different carbon sources in cultivation of recombinant Pichia pastoris for angiostatin production[J]. Enzyme and Microbial Technology, 2005, 36(2/3): 210-216. |
| 192 | WANG J J, WANG X L, SHI L, et al. Reduced methanol input induces increased protein output by AOX1 promoter in a trans-acting elements engineered Pichia pastoris [J]. Journal of Industrial Microbiology & Biotechnology, 2018, 45(1): 25-30. |
| 193 | GASSLER T, BAUMSCHABL M, SALLABERGER J, et al. Adaptive laboratory evolution and reverse engineering enhances autotrophic growth in Pichia pastoris [J]. Metabolic Engineering, 2022, 69: 112-121. |
| 194 | KRIVORUCHKO A, ZHANG Y M, SIEWERS V, et al. Microbial acetyl-CoA metabolism and metabolic engineering[J]. Metabolic Engineering, 2015, 28: 28-42. |
| 195 | KARAOGLAN M, KARAOGLAN F E, INAN M. Functional analysis of alcohol dehydrogenase (ADH) genes in Pichia pastoris [J]. Biotechnology Letters, 2016, 38(3): 463-469. |
| 196 | ERDEN-KARAOĞLAN F, KARAOĞLAN M, YıLMAZ G, et al. Deletion analysis of Pichia pastoris alcohol dehydrogenase 2 (ADH2) promoter and development of synthetic promoters[J]. Biotechnology Journal, 2022, 17(2): 2100332. |
| 197 | ERGÜN B G, GASSER B, MATTANOVICH D, et al. Engineering of alcohol dehydrogenase 2 hybrid-promoter architectures in Pichia pastoris to enhance recombinant protein expression on ethanol[J]. Biotechnology and Bioengineering, 2019, 116(10): 2674-2686. |
| 198 | BARBAY D, MAČÁKOVÁ M, SÜTZL L, et al. Two homologs of the Cat8 transcription factor are involved in the regulation of ethanol utilization in Komagataella phaffii [J]. Current Genetics, 2021, 67(4): 641-661. |
| 199 | XU Q, BAI C X, LIU Y Q, et al. Modulation of acetate utilization in Komagataella phaffii by metabolic engineering of tolerance and metabolism[J]. Biotechnology for Biofuels, 2019, 12: 61. |
| 200 | PAES B G, STEINDORFF A S, FORMIGHIERI E F, et al. Physiological characterization and transcriptome analysis of Pichia pastoris reveals its response to lignocellulose-derived inhibitors[J]. AMB Express, 2021, 11(1): 2. |
| 201 | LIU Y Q, BAI C X, XU Q, et al. Improved methanol-derived lovastatin production through enhancement of the biosynthetic pathway and intracellular lovastatin efflux in methylotrophic yeast[J]. Bioresources and Bioprocessing, 2018, 5: 22. |
| 202 | ARAYA-GARAY J M, FEIJOO-SIOTA L, ROSA-DOS-SANTOS F, et al. Construction of new Pichia pastoris X-33 strains for production of lycopene and β-carotene[J]. Applied Microbiology and Biotechnology, 2012, 93(6): 2483-2492. |
| 203 | ARAYA-GARAY J M, AGEITOS J M, VALLEJO J A, et al. Construction of a novel Pichia pastoris strain for production of xanthophylls[J]. AMB Express, 2012, 2(1): 24. |
| 204 | PEÑA D A, GASSER B, ZANGHELLINI J, et al. Metabolic engineering of Pichia pastoris [J]. Metabolic Engineering, 2018, 50: 2-15. |
| 205 | REN Y N, LIU Q, LIU H F, et al. Engineering substrate and energy metabolism for living cell production of cytidine-5'-diphosphocholine[J]. Biotechnology and Bioengineering, 2020, 117(5): 1426-1435. |
| 206 | WEN J, TIAN L, LIU Q, et al. Engineered dynamic distribution of malonyl-CoA flux for improving polyketide biosynthesis in Komagataella phaffii [J]. Journal of Biotechnology, 2020, 320: 80-85. |
| 207 | WEN J, TIAN L, XU M Q, et al. A synthetic malonyl-CoA metabolic oscillator in Komagataella phaffii [J]. ACS Synthetic Biology, 2020, 9(5): 1059-1068. |
| 208 | MAN Z W, GUO J, ZHANG Y Y, et al. Regulation of intracellular ATP supply and its application in industrial biotechnology[J]. Critical Reviews in Biotechnology, 2020, 40(8): 1151-1162. |
| 209 | JIA L Q, TU T Y, HUAI Q Q, et al. Enhancing monellin production by Pichia pastoris at low cell induction concentration via effectively regulating methanol metabolism patterns and energy utilization efficiency[J]. PLoS One, 2017, 12(10): e0184602. |
| 210 | GAO Y H, LIU N, ZHU Y X, et al. Improving glutathione production by engineered Pichia pastoris: strain construction and optimal precursor feeding[J]. Applied Microbiology and Biotechnology, 2022, 106(5/6): 1905-1917. |
| 211 | CHEN H X, CHU J, ZHANG S L, et al. Intracellular expression of Vitreoscilla hemoglobin improves S-adenosylmethionine production in a recombinant Pichia pastoris [J]. Applied Microbiology and Biotechnology, 2007, 74(6): 1205-1212. |
| 212 | RAVI KANT H, BALAMURALI M, MEENAKSHISUNDARAM S. Enhancing precursors availability in Pichia pastoris for the overproduction of S-adenosyl-L-methionine employing molecular strategies with process tuning[J]. Journal of Biotechnology, 2014, 188: 112-121. |
| 213 | JAYACHANDRAN C, PALANISAMY ATHIYAMAN B, SANKARANARAYANAN M. Cofactor engineering improved CALB production in Pichia pastoris through heterologous expression of NADH oxidase and adenylate kinase[J]. PLoS One, 2017, 12(7): e0181370. |
| 214 | LIU J H, LI H L, ZHAO G R, et al. Redox cofactor engineering in industrial microorganisms: strategies, recent applications and future directions[J]. Journal of Industrial Microbiology & Biotechnology, 2018, 45(5): 313-327. |
| 215 | SCHROER K, PETER LUEF K, STEFAN HARTNER F, et al. Engineering the Pichia pastoris methanol oxidation pathway for improved NADH regeneration during whole-cell biotransformation[J]. Metabolic Engineering, 2010, 12(1): 8-17. |
| 216 | FINA A, BRÊDA G C, PÉREZ-TRUJILLO M, et al. Benchmarking recombinant Pichia pastoris for 3-hydroxypropionic acid production from glycerol[J]. Microbial Biotechnology, 2021, 14(4): 1671-1682. |
| 217 | RUTH C, ZUELLIG T, MELLITZER A, et al. Variable production windows for porcine trypsinogen employing synthetic inducible promoter variants in Pichia pastoris [J]. Systems and Synthetic Biology, 2010, 4(3): 181-191. |
| 218 | BAGHBAN R, FARAJNIA S, RAJABIBAZL M, et al. Yeast expression systems: overview and recent advances[J]. Molecular Biotechnology, 2019, 61(5): 365-384. |
| [1] | 应汉杰, 柳东, 王振宇, 沈涛, 庄伟, 朱晨杰. 工业生物制造与“碳中和”目标探讨[J]. 合成生物学, 2025, 6(1): 1-7. |
| [2] | 刘夺, 刘培源, 李连月, 王雅欣, 崔钰惠, 薛慧敏, 王汉杰. 工程化细胞外囊泡的设计合成与生物医学应用[J]. 合成生物学, 2024, 5(1): 154-173. |
| [3] | 晏雄鹰, 王振, 娄吉芸, 张皓瑜, 黄星宇, 王霞, 杨世辉. 生物燃料高效生产微生物细胞工厂构建研究进展[J]. 合成生物学, 2023, 4(6): 1082-1121. |
| [4] | 孙梦楚, 陆亮宇, 申晓林, 孙新晓, 王佳, 袁其朋. 基于荧光检测的高通量筛选技术和装备助力细胞工厂构建[J]. 合成生物学, 2023, 4(5): 947-965. |
| [5] | 刁志钿, 王喜先, 孙晴, 徐健, 马波. 单细胞拉曼光谱测试分选装备研制及应用进展[J]. 合成生物学, 2023, 4(5): 1020-1035. |
| [6] | 吴玉洁, 刘欣欣, 刘健慧, 杨开广, 随志刚, 张丽华, 张玉奎. 基于高通量液相色谱质谱技术的菌株筛选与关键分子定量分析研究进展[J]. 合成生物学, 2023, 4(5): 1000-1019. |
| [7] | 陈永灿, 司同, 张建志. 自动化合成生物技术在DNA组装与微生物底盘操作中的应用[J]. 合成生物学, 2023, 4(5): 857-876. |
| [8] | 孙美莉, 王凯峰, 陆然, 纪晓俊. 解脂耶氏酵母底盘细胞的工程改造及应用[J]. 合成生物学, 2023, 4(4): 779-807. |
| [9] | 高纤云, 牛灵雪, 见妮, 管宁子. 微生物合成生物学在疾病诊疗上的应用进展[J]. 合成生物学, 2023, 4(2): 263-282. |
| [10] | 涂然, 李世新, 李昊霓, 王猛. 液滴微流控技术在微生物工程菌株选育中的应用进展[J]. 合成生物学, 2023, 4(1): 165-184. |
| [11] | 王喜先, 孙晴, 刁志钿, 徐健, 马波. 拉曼光谱技术在单细胞表型检测与分选中的应用进展[J]. 合成生物学, 2023, 4(1): 204-224. |
| [12] | 刘家宇, 杨智晗, 杨蕾, 朱丽英, 朱政明, 江凌. 合成生物技术驱动酪丁酸梭菌细胞工厂开发的研究进展[J]. 合成生物学, 2022, 3(6): 1174-1200. |
| [13] | 陶飞, 孙韬, 王钰, 魏婷, 倪俊, 许平. “双碳”背景下聚球藻底盘研究的挑战与机遇[J]. 合成生物学, 2022, 3(5): 932-952. |
| [14] | 董正鑫, 孙韬, 陈磊, 张卫文. 调控工程在光合蓝细菌中的应用[J]. 合成生物学, 2022, 3(5): 966-984. |
| [15] | 崔金玉, 张爱娣, 栾国栋, 吕雪峰. 微藻光驱固碳合成技术的发展现状与未来展望[J]. 合成生物学, 2022, 3(5): 884-900. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||