合成生物学 ›› 2023, Vol. 4 ›› Issue (4): 738-755.DOI: 10.12211/2096-8280.2022-076
CRISPR/Cas基因组编辑技术在丝状真菌次级代谢产物合成中的应用
林继聪1, 邹根2, 刘宏民1, 魏勇军1
- 1.郑州大学药学院,郑州大学药物研究院,郑州大学合成生物学实验室,药物关键制备技术教育部重点实验室,河南 郑州 450001
2.上海市农业科学院食用菌研究所,农业农村部南方食用菌资源利用重点实验室,国家食用菌工程技术研究中心,上海 201403
-
收稿日期:2022-12-28修回日期:2023-02-27出版日期:2023-08-31发布日期:2023-09-14 -
通讯作者:刘宏民,魏勇军 -
作者简介:林继聪 (1999—),男,硕士研究生。研究方向为丝状真菌合成生物学。E-mail:ljc990101@163.com刘宏民 (1960—),男,博士,教授。研究方向为药物设计与合成。E-mail:liuhm@zzu.edu.cn魏勇军 (1986—),男,博士,副教授。研究方向为合成微生物组学。E-mail:yongjunwei@zzu.edu.cn -
基金资助:国家自然科学基金(32111530179)
Application of CRISPR/Cas genome editing technology in the synthesis of secondary metabolites of filamentous fungi
LIN Jicong1, ZOU Gen2, LIU Hongmin1, WEI Yongjun1
- 1.School of Pharmaceutical Sciences,Institute of Drug Discovery and Development,Laboratory of Synthetic Biology,Key Laboratory of Advanced Drug Preparation Technologies,Ministry of Education of China,Zhengzhou University,Zhengzhou 450001,Henan,China
2.Institute of Edible Fungi,Shanghai Academy of Agricultural Sciences,Southern Key Laboratory of Edible Fungus Resource Utilization,Ministry of Agriculture,National Engineering Research Center of Edible Fungi,Shanghai 201403,China
-
Received:2022-12-28Revised:2023-02-27Online:2023-08-31Published:2023-09-14 -
Contact:LIU Hongmin, WEI Yongjun
摘要:
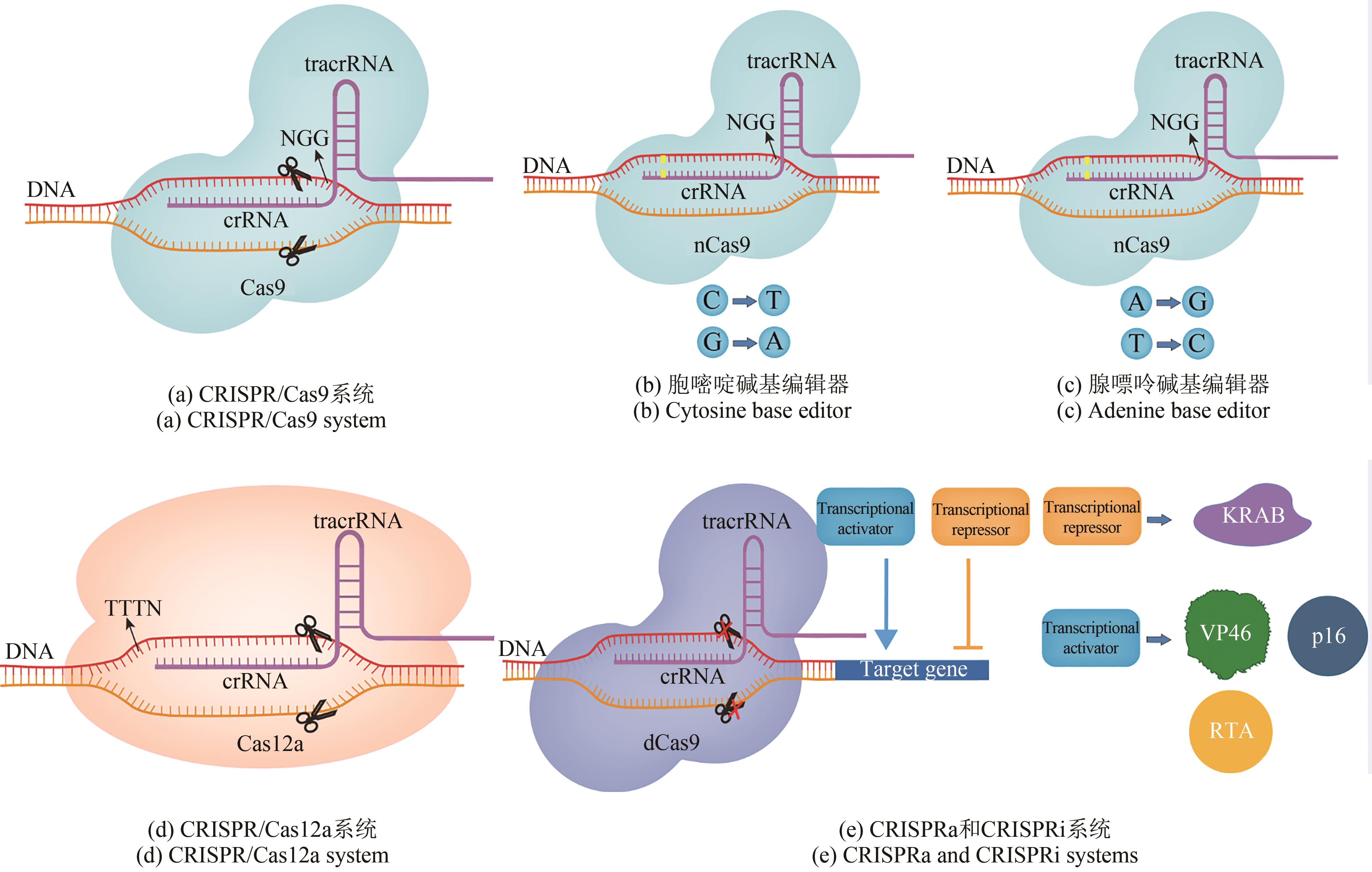
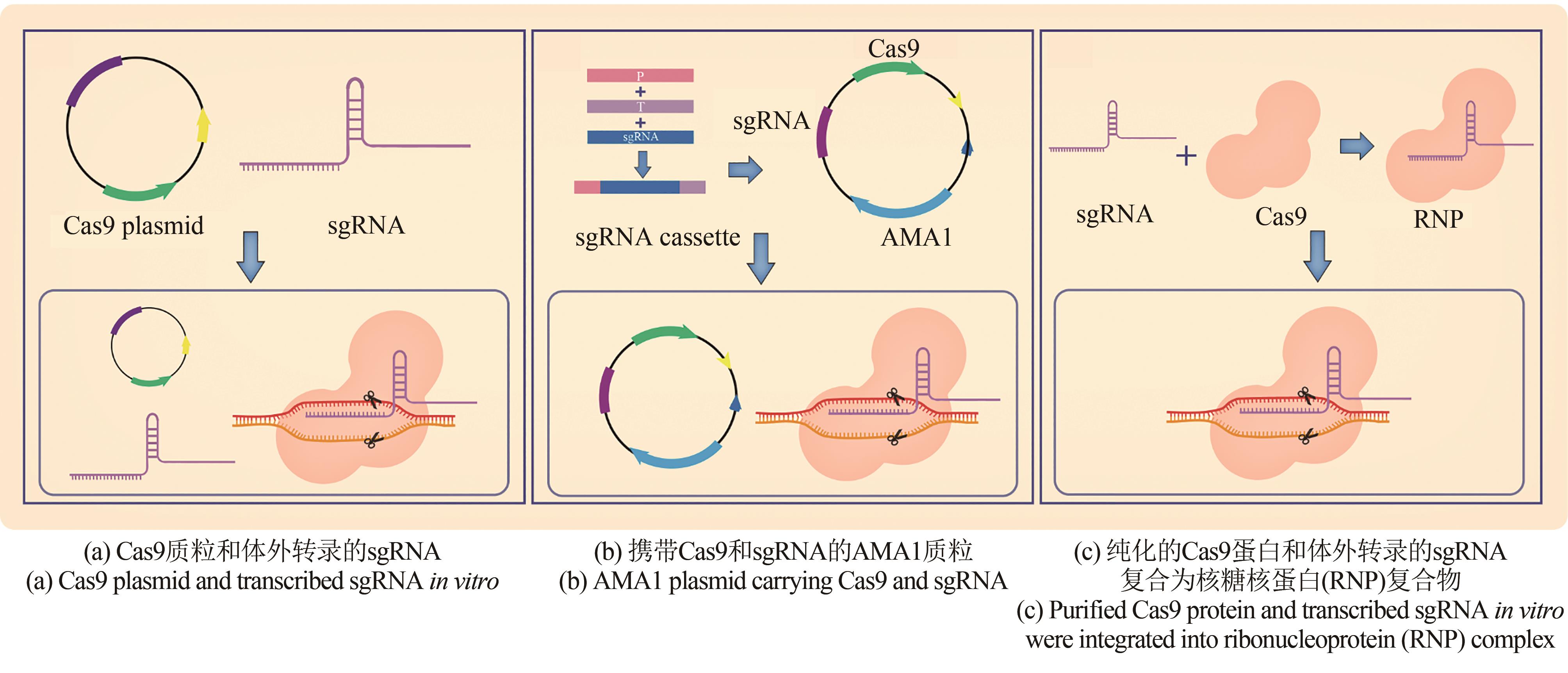
丝状真菌能够合成抗生素、色素、酶制剂、激素等多种天然产物,广泛应用于医药、化工、农业和基础生物学研究等领域。丝状真菌遗传背景复杂,阻碍了对其的进一步开发利用。基因组编辑是基于核酸酶对基因组位点特异性序列进行靶向切割,产生双链断裂,从而通过非同源末端连接或同源重组进行修复的技术。其中,CRISPR(clustered regularly interspaced short palindromic repeats)系统是目前使用最普遍的基因组编辑技术,已在丝状真菌遗传育种、基因改造和多种天然产物合成等方面进行了大量应用。本文总结了丝状真菌CRISPR/Cas的技术原理、元件表达、递送方式及该系统在次级代谢产物等研究中的应用。对于脱靶效应以及转化率低的问题,本文讨论了可能的解决方法。在此基础上,展望了基于CRISPR/Cas的基因组编辑技术在真菌基因功能表征、天然产物合成代谢途径解析与重构、高效丝状真菌底盘细胞构建、天然产物合成等方面的应用。
中图分类号:
引用本文
林继聪, 邹根, 刘宏民, 魏勇军. CRISPR/Cas基因组编辑技术在丝状真菌次级代谢产物合成中的应用[J]. 合成生物学, 2023, 4(4): 738-755.
LIN Jicong, ZOU Gen, LIU Hongmin, WEI Yongjun. Application of CRISPR/Cas genome editing technology in the synthesis of secondary metabolites of filamentous fungi[J]. Synthetic Biology Journal, 2023, 4(4): 738-755.
| 丝状真菌 | sgRNA 启动子 | 表达方式 | 靶标基因 | 编辑效率 | 参考文献 |
|---|---|---|---|---|---|
| 里氏木霉 | 体内表达密码子优化的Cas9和体外转录的sgRNA | ura5, lae1, vib1, clr2 | 单基因93%~100%;多基因4.2%~45% | [ | |
| 里氏木霉 | U6 | 体内表达密码子优化的Cas9和U6启动子驱动sgRNA1的表达 | ura5 | 8%~10% | [ |
| 里氏木霉 | 5S rRNA | 体内表达密码子优化的Cas9和5S rRNA驱动sgRNA的表达 | lae1 | 36.67% | [ |
| 里氏木霉 | 体外组装的核糖核蛋白(RNP)复合物 | ura5, lae1, cbh1, cbh2, eg1 | 56.52%~100% | [ | |
| 草酸青霉 | 5S rRNA | 体内表达密码子优化的Cas9和5S rRNA驱动sgRNA的表达 | bgl2, creA | 30%~80% | [ |
| 鲁本斯青霉 | U6, tRNA, utp25 | 体内表达密码子优化的Cas9与U6,tRNA和utp25启动子驱动sgRNA的表达;体外组装的核糖核蛋白(RNP)复合物 | pks17, roqA, lovF, hcpA, pcbA, penDE, chyA | 60%~100% | [ |
| 黑曲霉 | tRNA | 体内表达密码子优化的Cas9和tRNA驱动sgRNA的表达 | albA, olvA, glaA | 20%~93% | [ |
| 黑曲霉 | 5S rRNA | 体内表达密码子优化的Cas9和tRNA驱动sgRNA的表达 | albA, fum5, fum1 | 33%~100% | [ |
| 米曲霉 | U6 | 体内表达密码子优化的Cas9和U6启动子驱动sgRNA的表达 | wA, pyrG, yA | 10%~100% | [ |
| 米曲霉 | U6 | 携带Cas9和sgRNA的自主复制质粒 | wA, pyrG, yA | 55.6%~100% | [ |
| 烟曲霉 | 体内表达密码子优化的Cas9和体外转录的sgRNA | pksP, cnaA | 95%~100% | [ | |
| 藤仓镰刀菌 | PgpdA, U6, 5S rRNA | 体内表达密码子优化的Cas9与PgpdA 、U6、5S rRNA启动子驱动sgRNA的表达 | fcc1, ura3, ppt1 | 37.5%~79.2% | [ |
| Glarea lozoyensis | 5S rRNA | 体内表达密码子优化的Cas9和5S rRNA驱动sgRNA的表达 | gloA, gloF | 28% | [ |
| 嗜热毁丝霉 | U6 | 体内表达密码子优化的Cas9和U6启动子驱动sgRNA的表达 | cre-1, res-1, gh1-1, alp-1 | 单基因95%; 多基因22%~70% | [ |
| 嗜热毁丝霉 | U6 | 体内表达密码子优化的Cas12a和U6启动子驱动sgRNA的表达 | cre-1, res-1, gh1-1 | 单基因90%; 多基因13%~41% | [ |
| 稻瘟病菌 | 体外组装的Cas12a核糖核蛋白(RNP)复合物 | BUF1, FKBP12, FTR1, BAS4, AVRPI9 | 25%~77% | [ |
表1 丝状真菌中CRISPR/Cas基因组编辑系统的应用
Table 1 Application of CRISPR/Cas genome editing system in filamentous fungi
| 丝状真菌 | sgRNA 启动子 | 表达方式 | 靶标基因 | 编辑效率 | 参考文献 |
|---|---|---|---|---|---|
| 里氏木霉 | 体内表达密码子优化的Cas9和体外转录的sgRNA | ura5, lae1, vib1, clr2 | 单基因93%~100%;多基因4.2%~45% | [ | |
| 里氏木霉 | U6 | 体内表达密码子优化的Cas9和U6启动子驱动sgRNA1的表达 | ura5 | 8%~10% | [ |
| 里氏木霉 | 5S rRNA | 体内表达密码子优化的Cas9和5S rRNA驱动sgRNA的表达 | lae1 | 36.67% | [ |
| 里氏木霉 | 体外组装的核糖核蛋白(RNP)复合物 | ura5, lae1, cbh1, cbh2, eg1 | 56.52%~100% | [ | |
| 草酸青霉 | 5S rRNA | 体内表达密码子优化的Cas9和5S rRNA驱动sgRNA的表达 | bgl2, creA | 30%~80% | [ |
| 鲁本斯青霉 | U6, tRNA, utp25 | 体内表达密码子优化的Cas9与U6,tRNA和utp25启动子驱动sgRNA的表达;体外组装的核糖核蛋白(RNP)复合物 | pks17, roqA, lovF, hcpA, pcbA, penDE, chyA | 60%~100% | [ |
| 黑曲霉 | tRNA | 体内表达密码子优化的Cas9和tRNA驱动sgRNA的表达 | albA, olvA, glaA | 20%~93% | [ |
| 黑曲霉 | 5S rRNA | 体内表达密码子优化的Cas9和tRNA驱动sgRNA的表达 | albA, fum5, fum1 | 33%~100% | [ |
| 米曲霉 | U6 | 体内表达密码子优化的Cas9和U6启动子驱动sgRNA的表达 | wA, pyrG, yA | 10%~100% | [ |
| 米曲霉 | U6 | 携带Cas9和sgRNA的自主复制质粒 | wA, pyrG, yA | 55.6%~100% | [ |
| 烟曲霉 | 体内表达密码子优化的Cas9和体外转录的sgRNA | pksP, cnaA | 95%~100% | [ | |
| 藤仓镰刀菌 | PgpdA, U6, 5S rRNA | 体内表达密码子优化的Cas9与PgpdA 、U6、5S rRNA启动子驱动sgRNA的表达 | fcc1, ura3, ppt1 | 37.5%~79.2% | [ |
| Glarea lozoyensis | 5S rRNA | 体内表达密码子优化的Cas9和5S rRNA驱动sgRNA的表达 | gloA, gloF | 28% | [ |
| 嗜热毁丝霉 | U6 | 体内表达密码子优化的Cas9和U6启动子驱动sgRNA的表达 | cre-1, res-1, gh1-1, alp-1 | 单基因95%; 多基因22%~70% | [ |
| 嗜热毁丝霉 | U6 | 体内表达密码子优化的Cas12a和U6启动子驱动sgRNA的表达 | cre-1, res-1, gh1-1 | 单基因90%; 多基因13%~41% | [ |
| 稻瘟病菌 | 体外组装的Cas12a核糖核蛋白(RNP)复合物 | BUF1, FKBP12, FTR1, BAS4, AVRPI9 | 25%~77% | [ |
| 1 | HÜTTNER S, JOHANSSON A, GONÇALVES TEIXEIRA P, et al. Recent advances in the intellectual property landscape of filamentous fungi[J]. Fungal Biology and Biotechnology, 2020, 7(1): 16. |
| 2 | EVANS D A, BEIGER J J, BURCH J D, et al. Total synthesis of aflastatin A[J]. Journal of the American Chemical Society, 2022, 144(43): 19953-19972. |
| 3 | PÉREZ-PÉREZ W D, CARRASCO-NAVARRO U, GARCÍA-ESTRADA C, et al. bZIP transcription factors PcYap1 and PcRsmA link oxidative stress response to secondary metabolism and development in Penicillium chrysogenum [J]. Microbial Cell Factories, 2022, 21(1): 50. |
| 4 | LIU L, CHEN Z, LIU W Y, et al. Cephalosporin C biosynthesis and fermentation in Acremonium chrysogenum [J]. Applied Microbiology and Biotechnology, 2022, 106(19): 6413-6426. |
| 5 | YAO G S, BAI X F, ZHANG B X, et al. Enhanced production of terrein in marine-derived Aspergillus terreus by refactoring both global and pathway-specific transcription factors[J]. Microbial Cell Factories, 2022, 21(1): 136. |
| 6 | GUO W Z, YANG J H, HUANG T C, et al. Synergistic effects of multiple enzymes from industrial Aspergillus niger strain O1 on starch saccharification[J]. Biotechnology for Biofuels, 2021, 14(1): 225. |
| 7 | AHMED T, RANA M R, ZZAMAN W, et al. Optimization of substrate composition for pectinase production from Satkara (Citrus macroptera) peel using Aspergillus niger-ATCC 1640 in solid-state fermentation[J]. Heliyon, 2021, 7(10): e08133. |
| 8 | WANG Y, CHEN H Y, MA L, et al. Use of CRISPR-Cas tools to engineer Trichoderma species[J]. Microbial Biotechnology, 2022, 15(10): 2521-2532. |
| 9 | SCHALÉN M, ANYAOGU D C, HOOF J B, et al. Effect of secretory pathway gene overexpression on secretion of a fluorescent reporter protein in Aspergillus nidulans [J]. Fungal Biology and Biotechnology, 2016, 3: 3. |
| 10 | SCHÜLLER A, STUDT-REINHOLD L, STRAUSS J. How to completely squeeze a fungus—advanced genome mining tools for novel bioactive substances[J]. Pharmaceutics, 2022, 14(9): 1837. |
| 11 | TANG S, MEN P, ZHANG W, et al. Identification of a polyketide biosynthesis gene cluster by transcriptional regulator activation in Aspergillus terreus [J]. Fungal Genetics and Biology, 2022, 160: 103690. |
| 12 | ZHOU Y, WANG Y L, CHEN K, et al. Near-complete genomes of two Trichoderma species: a resource for biological control of plant pathogens[J]. Molecular Plant-Microbe Interactions, 2020, 33(8): 1036-1039. |
| 13 | ZOU G, NIELSEN J B, WEI Y J. Harnessing synthetic biology for mushroom farming[J]. Trends in Biotechnology, 2023, 41(4): 480-483. |
| 14 | WRIGHT A V, NUÑEZ J K, DOUDNA J A. Biology and applications of CRISPR systems: Harnessing nature's toolbox for genome engineering[J]. Cell, 2016, 164(1/2): 29-44. |
| 15 | HUANG X N, MEN P, TANG S, et al. Aspergillus terreus as an industrial filamentous fungus for pharmaceutical biotechnology[J]. Current Opinion in Biotechnology, 2021, 69: 273-280. |
| 16 | CUI Z F, LIU H, ZHANG H F, et al. The comparison of ZFNs, TALENs, and SpCas9 by GUIDE-seq in HPV-targeted gene therapy[J]. Molecular Therapy Nucleic Acids, 2021, 26: 1466-1478. |
| 17 | BARRANGOU R, DOUDNA J A. Applications of CRISPR technologies in research and beyond[J]. Nature Biotechnology, 2016, 34(9): 933-941. |
| 18 | DOUDNA J A. The promise and challenge of therapeutic genome editing[J]. Nature, 2020, 578(7794): 229-236. |
| 19 | VALENTE S, COMETTO A, PIOMBO E, et al. Elaborated regulation of griseofulvin biosynthesis in Penicillium griseofulvum and its role on conidiation and virulence[J]. International Journal of Food Microbiology, 2020, 328: 108687. |
| 20 | HOFFMEISTER D, KELLER N P. Natural products of filamentous fungi: enzymes, genes, and their regulation[J]. Natural Product Reports, 2007, 24(2): 393-416. |
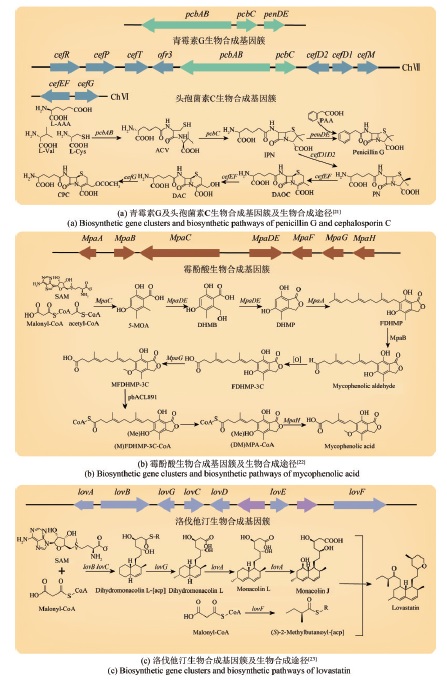
| 21 | 刘佳佳, 刘钢. 头孢菌素C生物合成调控研究进展[J]. 微生物学报, 2016, 56(3): 461-470. |
| LIU J J, LIU G. Advances in the regulation of cephalosporin C biosynthesis[J]. Acta Microbiologica Sinica, 2016, 56(3): 461-470. | |
| 22 | 黄润业, 亓兰达, 陈国参, 等. 免疫抑制剂霉酚酸的研究及产业化进展[J]. 微生物学报, 2021, 61(10): 3010-3025. |
| HUANG R Y, QI L D, CHEN G C, et al. Research and industrialization progress of immunosuppressant mycophenolic acid[J]. Acta Microbiologica Sinica, 2021, 61(10): 3010-3025. | |
| 23 | 黄雪年, 唐慎, 吕雪峰. 工业丝状真菌土曲霉合成生物技术研究进展及展望[J]. 合成生物学, 2020, 1(2): 187-211. |
| HUANG X N, TANG S, LV X F. Progress and prospect for synthetic biology research of the industrial filamentous fungi Aspergillus terreus [J]. Synthetic Biology Journal, 2020, 1(2): 187-211. | |
| 24 | RAJA H A, MILLER A N, PEARCE C J, et al. Fungal identification using molecular tools: a primer for the natural products research community[J]. Journal of Natural Products, 2017, 80(3): 756-770. |
| 25 | WU Q W, LI M Z, BILAL M, et al. Enhanced production of mycophenolic acid from Penicillium brevicompactum via optimized fermentation strategy[J]. Applied Biochemistry and Biotechnology, 2022, 194(7): 3001-3015. |
| 26 | ISTVAN E S, DEISENHOFER J. Structural mechanism for statin inhibition of HMG-CoA reductase[J]. Science, 2001, 292(5519): 1160-1164. |
| 27 | HUANG X N, LIANG Y J, YANG Y, et al. Single-step production of the simvastatin precursor monacolin J by engineering of an industrial strain of Aspergillus terreus [J]. Metabolic Engineering, 2017, 42: 109-114. |
| 28 | HUANG X N, TANG S, ZHENG L H, et al. Construction of an efficient and robust Aspergillus terreus cell factory for monacolin J production[J]. ACS Synthetic Biology, 2019, 8(4): 818-825. |
| 29 | KELLER N P. Fungal secondary metabolism: regulation, function and drug discovery[J]. Nature Reviews Microbiology, 2019, 17(3): 167-180. |
| 30 | LAZARUS C M, WILLIAMS K, BAILEY A M. Reconstructing fungal natural product biosynthetic pathways[J]. Natural Product Reports, 2014, 31(10): 1339-1347. |
| 31 | ZHANG R K, TAN Y S, CUI Y Z, et al. Lignin valorization for protocatechuic acid production in engineered Saccharomyces cerevisiae [J]. Green Chemistry, 2021, 23(17): 6515-6526. |
| 32 | RO D K, PARADISE E M, OUELLET M, et al. Production of the antimalarial drug precursor artemisinic acid in engineered yeast[J]. Nature, 2006, 440(7086): 940-943. |
| 33 | WANG P P, WEI W, YE W, et al. Synthesizing ginsenoside Rh2 in Saccharomyces cerevisiae cell factory at high-efficiency[J]. Cell Discovery, 2019, 5: 5. |
| 34 | ZHANG J, HANSEN L G, GUDICH O, et al. A microbial supply chain for production of the anti-cancer drug vinblastine[J]. Nature, 2022, 609(7926): 341-347. |
| 35 | CONG L, RAN F A, COX D, et al. Multiplex genome engineering using CRISPR/Cas systems[J]. Science, 2013, 339(6121): 819-823. |
| 36 | YANG W, YAN J Q, ZHUANG P Z, et al. Progress of delivery methods for CRISPR-Cas9[J]. Expert Opinion on Drug Delivery, 2022, 19(8): 913-926. |
| 37 | DICARLO J E, NORVILLE J E, MALI P, et al. Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems[J]. Nucleic Acids Research, 2013, 41(7): 4336-4343. |
| 38 | LIU R, CHEN L, JIANG Y P, et al. Efficient genome editing in filamentous fungus Trichoderma reesei using the CRISPR/Cas9 system[J]. Cell Discovery, 2015, 1: 15007. |
| 39 | ZHU Y M. Advances in CRISPR/Cas9[J]. BioMed Research International, 2022, 2022: 9978571. |
| 40 | FAN C, ZHANG W, SU X Y, et al. CRISPR/Cas9-mediated genome editing directed by a 5S rRNA-tRNAGly hybrid promoter in the thermophilic filamentous fungus Humicola insolens [J]. Biotechnology for Biofuels, 2021, 14(1): 206. |
| 41 | GHOSH D, RAGHAVAN S C. Nonhomologous end joining: new accessory factors fine tune the machinery[J]. Trends in Genetics, 2021, 37(6): 582-599. |
| 42 | SUZUKI K, TSUNEKAWA Y, HERNANDEZ-BENITEZ R, et al. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration[J]. Nature, 2016, 540(7631): 144-149. |
| 43 | CLEMMENSEN S E, KROMPHARDT K K, FRANDSEN R N. Marker-free CRISPR-Cas9 based genetic engineering of the phytopathogenic fungus, Penicillium expansum [J]. Fungal Genetics and Biology, 2022, 160: 103689. |
| 44 | NØDVIG C S, NIELSEN J B, KOGLE M E, et al. A CRISPR-Cas9 system for genetic engineering of filamentous fungi[J]. PLoS One, 2015, 10(7): e0133085. |
| 45 | SHOJAEI BAGHINI S, GARDANOVA Z R, ABADI S A H, et al. CRISPR/Cas9 application in cancer therapy: a pioneering genome editing tool[J]. Cellular & Molecular Biology Letters, 2022, 27(1): 35. |
| 46 | LI J, ZHANG L Y, XU Q, et al. CRISPR-Cas9 toolkit for genome editing in an autotrophic CO2-fixing methanogenic archaeon[J]. Microbiology Spectrum, 2022, 10(4): e0116522. |
| 47 | DARMA R, LUTZ A, ELLIOTT C E, et al. Identification of a gene cluster for the synthesis of the plant hormone abscisic acid in the plant pathogen Leptosphaeria maculans [J]. Fungal Genetics and Biology:, 2019, 130: 62-71. |
| 48 | WANG Q, COBINE P A, COLEMAN J J. Efficient genome editing in Fusarium oxysporum based on CRISPR/Cas9 ribonucleoprotein complexes[J]. Fungal Genetics and Biology, 2018, 117: 21-29. |
| 49 | SHI T Q, GAO J, WANG W J, et al. CRISPR/Cas9-based genome editing in the filamentous fungus Fusarium fujikuroi and its application in strain engineering for gibberellic acid production[J]. ACS Synthetic Biology, 2019, 8(2): 445-454. |
| 50 | REN C, LIU Y F, GUO Y C, et al. Optimizing the CRISPR/Cas9 system for genome editing in grape by using grape promoters[J]. Horticulture Research, 2021, 8: 52. |
| 51 | DENG H X, GAO R J, LIAO X R, et al. Genome editing in Shiraia bambusicola using CRISPR-Cas9 system[J]. Journal of Biotechnology, 2017, 259: 228-234. |
| 52 | CHEN J J, LAI Y L, WANG L L, et al. CRISPR/Cas9-mediated efficient genome editing via blastospore-based transformation in entomopathogenic fungus Beauveria bassiana [J]. Scientific Reports, 2017, 7: 45763. |
| 53 | LEYNAUD-KIEFFER L M C, CURRAN S C, KIM I, et al. A new approach to Cas9-based genome editing in Aspergillus niger that is precise, efficient and selectable[J]. PLoS One, 2019, 14(1): e0210243. |
| 54 | TONG S, AN K X, CHEN W X, et al. Evasion of Cas9 toxicity to develop an efficient genome editing system and its application to increase ethanol yield in Fusarium venenatum TB01[J]. Applied Microbiology and Biotechnology, 2022, 106(19): 6583-6593. |
| 55 | GUSTAFSSON O, RÄDLER J, ROUDI S, et al. Efficient peptide-mediated in vitro delivery of Cas9 RNP[J]. Pharmaceutics, 2021, 13(6): 878. |
| 56 | DE VRIES R P, RILEY R, WIEBENGA A, et al. Comparative genomics reveals high biological diversity and specific adaptations in the industrially and medically important fungal genus Aspergillus [J]. Genome Biology, 2017, 18(1): 28. |
| 57 | POHL C, KIEL J W, DRIESSEN A M, et al. CRISPR/Cas9 based genome editing of Penicillium chrysogenum [J]. ACS Synthetic Biology, 2016, 5(7): 754-764. |
| 58 | SONG R J, ZHAI Q, SUN L, et al. CRISPR/Cas9 genome editing technology in filamentous fungi: progress and perspective[J]. Applied Microbiology and Biotechnology, 2019, 103(17): 6919-6932. |
| 59 | LIU Q, GAO R R, LI J G, et al. Development of a genome-editing CRISPR/Cas9 system in thermophilic fungal Myceliophthora species and its application to hyper-cellulase production strain engineering[J]. Biotechnology for Biofuels, 2017, 10: 1. |
| 60 | VAN DEN BERG M A, ALBANG R, ALBERMANN K, et al. Genome sequencing and analysis of the filamentous fungus Penicillium chrysogenum [J]. Nature Biotechnology, 2008, 26(10): 1161-1168. |
| 61 | SONG L T, OUEDRAOGO J P, KOLBUSZ M, et al. Efficient genome editing using tRNA promoter-driven CRISPR/Cas9 gRNA in Aspergillus niger [J]. PLoS One, 2018, 13(8): e0202868. |
| 62 | ZHENG X M, ZHENG P, ZHANG K, et al. 5S rRNA promoter for guide RNA expression enabled highly efficient CRISPR/Cas9 genome editing in Aspergillus niger [J]. ACS Synthetic Biology, 2019, 8(7): 1568-1574. |
| 63 | FULLER K K, CHEN S, LOROS J J, et al. Development of the CRISPR/Cas9 system for targeted gene disruption in Aspergillus fumigatus [J]. Eukaryotic Cell, 2015, 14(11): 1073-1080. |
| 64 | WEBER J, VALIANTE V, NØDVIG C S, et al. Functional reconstitution of a fungal natural product gene cluster by advanced genome editing[J]. ACS Synthetic Biology, 2017, 6(1): 62-68. |
| 65 | ZHANG P, ZHOU S, WANG G, et al. Two transcription factors cooperatively regulate DHN melanin biosynthesis and development in Pestalotiopsis fici [J]. Molecular Microbiology, 2019, 112(2): 649-666. |
| 66 | XIANG B Y, HAO X R, XIE Q H, et al. Deletion of a rare fungal PKS CgPKS11 promotes chaetoglobosin A biosynthesis, yet defers the growth and development of Chaetomium globosum [J]. Journal of Fungi, 2021, 7(9): 750. |
| 67 | ALEKSENKO A, CLUTTERBUCK A J. Autonomous plasmid replication in Aspergillus nidulans: AMA1 and MATE elements[J]. Fungal Genetics and Biology, 1997, 21(3): 373-387. |
| 68 | KATAYAMA T, NAKAMURA H, ZHANG Y, et al. Forced recycling of an AMA1-based genome-editing plasmid allows for efficient multiple gene deletion/integration in the industrial filamentous fungus Aspergillus oryzae [J]. Applied and Environmental Microbiology, 2019, 85(3): e01896-e01818. |
| 69 | NIELSEN M L, ISBRANDT T, RASMUSSEN K B, et al. Genes linked to production of secondary metabolites in Talaromyces atroroseus revealed using CRISPR-Cas9[J]. PLoS One, 2017, 12(1): e0169712. |
| 70 | WENDEROTH M, PINECKER C, VOß B, et al. Establishment of CRISPR/Cas9 in Alternaria alternata [J]. Fungal Genetics and Biology, 2017, 101: 55-60. |
| 71 | SEEKLES S J, TEUNISSE P P P, PUNT M, et al. Preservation stress resistance of melanin deficient conidia from Paecilomyces variotii and Penicillium roqueforti mutants generated via CRISPR/Cas9 genome editing[J]. Fungal Biology and Biotechnology, 2021, 8(1): 4. |
| 72 | VAKULSKAS C A, BEHLKE M A. Evaluation and reduction of CRISPR off-target cleavage events[J]. Nucleic Acid Therapeutics, 2019, 29(4): 167-174. |
| 73 | ZOU G, XIAO M L, CHAI S X, et al. Efficient genome editing in filamentous fungi via an improved CRISPR-Cas9 ribonucleoprotein method facilitated by chemical reagents[J]. Microbial Biotechnology, 2021, 14(6): 2343-2355. |
| 74 | GRIJSEELS S, POHL C, NIELSEN J C, et al. Identification of the decumbenone biosynthetic gene cluster in Penicillium decumbens and the importance for production of calbistrin[J]. Fungal Biology and Biotechnology, 2018, 5: 18. |
| 75 | VALENTE S, PIOMBO E, SCHROECKH V, et al. CRISPR-Cas9-based discovery of the verrucosidin biosynthesis gene cluster in Penicillium polonicum [J]. Frontiers in Microbiology, 2021, 12: 660871. |
| 76 | FERRARA M, HAIDUKOWSKI M, LOGRIECO A F, et al. A CRISPR-Cas9 system for genome editing of Fusarium proliferatum [J]. Scientific Reports, 2019, 9(1): 19836. |
| 77 | MCLEAN K J, HANS M, MEIJRINK B, et al. Single-step fermentative production of the cholesterol-lowering drug pravastatin via reprogramming of Penicillium chrysogenum [J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(9): 2847-2852. |
| 78 | LIANG B, DU X J, LI P, et al. Investigation of citrinin and pigment biosynthesis mechanisms in Monascus purpureus by transcriptomic analysis[J]. Frontiers in Microbiology, 2018, 9: 1374. |
| 79 | LIU W W, AN C Y, SHU X, et al. A dual-plasmid CRISPR/cas system for mycotoxin elimination in polykaryotic industrial fungi[J]. ACS Synthetic Biology, 2020, 9(8): 2087-2095. |
| 80 | SILBERSTEIN S D, SHREWSBURY S B, HOEKMAN J. Dihydroergotamine (DHE) - then and now: a narrative review[J]. Headache, 2020, 60(1): 40-57. |
| 81 | YU L, XIAO M L, ZHU Z H, et al. Efficient genome editing in Claviceps purpurea using a CRISPR/Cas9 ribonucleoprotein method[J]. Synthetic and Systems Biotechnology, 2022, 7(2): 664-670. |
| 82 | CELIA-SANCHEZ B N, MANGUM B, BREWER M, et al. Analysis of Cyp51 protein sequences shows 4 major Cyp51 gene family groups across fungi[J]. G3 Genes|Genomes|Genetics, 2022, 12(11): jkac249. |
| 83 | GUO Z Q, LIU X Y, WANG N, et al. Membrane component ergosterol builds a platform for promoting effector secretion and virulence in Magnaporthe oryzae [J]. The New Phytologist, 2023, 237(3): 930-943. |
| 84 | ASSRESS H A, SELVARAJAN R, NYONI H, et al. Antifungal azoles and azole resistance in the environment: current status and future perspectives—a review[J]. Reviews in Environmental Science and Bio/Technology, 2021, 20(4): 1011-1041. |
| 85 | KHAN A A, FAROOQ F, JAIN S K, et al. Comparative host-pathogen interaction analyses of SARS-CoV2 and Aspergillus fumigatus, and pathogenesis of COVID-19-associated aspergillosis[J]. Microbial Ecology, 2022, 84(4): 1236-1244. |
| 86 | PÉREZ-CANTERO A, MARTIN-VICENTE A, GUARRO J, et al. Analysis of the cyp51 genes contribution to azole resistance in Aspergillus section Nigri with the CRISPR-Cas9 technique[J]. Antimicrobial Agents and Chemotherapy, 2021, 65(5): e01996-20. |
| 87 | ZHANG L H, ZHENG X M, CAIRNS T C, et al. Disruption or reduced expression of the orotidine-5′-decarboxylase gene pyrG increases citric acid production: a new discovery during recyclable genome editing in Aspergillus niger [J]. Microbial Cell Factories, 2020, 19(1): 76. |
| 88 | YANG L, HENRIKSEN M M, HANSEN R S, et al. Metabolic engineering of Aspergillus niger via ribonucleoprotein-based CRISPR-Cas9 system for succinic acid production from renewable biomass[J]. Biotechnology for Biofuels, 2020, 13(1): 206. |
| 89 | BRITO A F, MOREIRA L K S, MENEGATTI R, et al. Piperazine derivatives with central pharmacological activity used as therapeutic tools[J]. Fundamental & Clinical Pharmacology, 2019, 33(1): 13-24. |
| 90 | YUAN B C, LIU D, GUAN X, et al. Piperazine ring formation by a single-module NRPS and cleavage by an α-KG-dependent nonheme iron dioxygenase in brasiliamide biosynthesis[J]. Applied Microbiology and Biotechnology, 2020, 104(14): 6149-6159. |
| 91 | SZYMAŃSKI M, CHMIELEWSKA S, CZYŻEWSKA U, et al. Echinocandins-structure, mechanism of action and use in antifungal therapy[J]. Journal of Enzyme Inhibition and Medicinal Chemistry, 2022, 37(1): 876-894. |
| 92 | ZHANG F, LIU H, ZHANG T, et al. Biochemical and genetic characterization of fungal proline hydroxylase in echinocandin biosynthesis[J]. Applied Microbiology and Biotechnology, 2018, 102(18): 7877-7890. |
| 93 | WEI T Y, WU Y J, XIE Q P, et al. CRISPR/Cas9-based genome editing in the filamentous fungus Glarea lozoyensis and its application in manipulating gloF [J]. ACS Synthetic Biology, 2020, 9(8): 1968-1977. |
| 94 | WOODCRAFT C, CHOOI Y H, ROUX I. The expanding CRISPR toolbox for natural product discovery and engineering in filamentous fungi[J]. Natural Product Reports, 2023, 40(1): 158-173. |
| 95 | OMANAKUTTAN V K, JOHN J, HOPF H. Synthesis of 3(2H)-furanones: a review[J]. European Journal of Organic Chemistry, 2021, 2021(2): 163-201. |
| 96 | WEI X X, MATSUYAMA T, SATO H, et al. Molecular and computational bases for spirofuranone formation in setosusin biosynthesis[J]. Journal of the American Chemical Society, 2021, 143(42): 17708-17715. |
| 97 | NØDVIG C S, HOOF J B, KOGLE M E, et al. Efficient oligo nucleotide mediated CRISPR-Cas9 gene editing in Aspergilli [J]. Fungal Genetics and Biology, 2018, 115: 78-89. |
| 98 | KUIVANEN J, WANG Y M J, RICHARD P. Engineering Aspergillus niger for galactaric acid production: elimination of galactaric acid catabolism by using RNA sequencing and CRISPR/Cas9[J]. Microbial Cell Factories, 2016, 15(1): 210. |
| 99 | DONG L B, LIN X T, YU D, et al. High-level expression of highly active and thermostable trehalase from Myceliophthora thermophila in Aspergillus niger by using the CRISPR/Cas9 tool and its application in ethanol fermentation[J]. Journal of Industrial Microbiology & Biotechnology, 2020, 47(1): 133-144. |
| 100 | WONG G, LIM L R, TAN Y Q, et al. Reconstituting the complete biosynthesis of D-lysergic acid in yeast[J]. Nature Communications, 2022, 13(1): 712. |
| 101 | JASTRZĘBSKI M K, KACZOR A A, WRÓBEL T M. Methods of lysergic acid synthesis-the key ergot alkaloid[J]. Molecules, 2022, 27(21): 7322. |
| 102 | LEADMON C E, SAMPSON J K, MAUST M D, et al. Several Metarhizium species produce ergot alkaloids in a condition-specific manner[J]. Applied and Environmental Microbiology, 2020, 86(14): e00373-e00320. |
| 103 | DAVIS K A, SAMPSON J K, PANACCIONE D G. Genetic reprogramming of the ergot alkaloid pathway of Metarhizium brunneum [J]. Applied and Environmental Microbiology, 2020, 86(19): e01251-e01220. |
| 104 | WU C, CHEN Y M, QIU Y F, et al. A simple approach to mediate genome editing in the filamentous fungus Trichoderma reesei by CRISPR/Cas9-coupled in vivo gRNA transcription[J]. Biotechnology Letters, 2020, 42(7): 1203-1210. |
| 105 | WANG Q, ZHAO Q Q, LIU Q, et al. CRISPR/Cas9-mediated genome editing in Penicillium oxalicum and Trichoderma reesei using 5S rRNA promoter-driven guide RNAs[J]. Biotechnology Letters, 2021, 43(2): 495-502. |
| 106 | KATAYAMA T, TANAKA Y, OKABE T, et al. Development of a genome editing technique using the CRISPR/Cas9 system in the industrial filamentous fungus Aspergillus oryzae [J]. Biotechnology Letters, 2016, 38(4): 637-642. |
| 107 | ZHANG C, MENG X H, WEI X L, et al. Highly efficient CRISPR mutagenesis by microhomology-mediated end joining in Aspergillus fumigatus [J]. Fungal Genetics and Biology, 2016, 86: 47-57. |
| 108 | LIU Q, ZHANG Y L, LI F Y, et al. Upgrading of efficient and scalable CRISPR-Cas-mediated technology for genetic engineering in thermophilic fungus Myceliophthora thermophila [J]. Biotechnology for Biofuels, 2019, 12: 293. |
| 109 | HUANG J, ROWE D, SUBEDI P, et al. CRISPR-Cas12a induced DNA double-strand breaks are repaired by multiple pathways with different mutation profiles in Magnaporthe oryzae [J]. Nature Communications, 2022, 13: 7168. |
| 110 | JIA Y, XU R G, REN X J, et al. Next-generation CRISPR/Cas9 transcriptional activation in Drosophila using flySAM[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(18): 4719-4724. |
| 111 | JANG S, JANG S, JUNG G Y. Toward tunable dynamic repression using CRISPRi[J]. Biotechnology Journal, 2018, 13(9): e1800152. |
| 112 | ROUX I, WOODCRAFT C, HU J Y, et al. CRISPR-mediated activation of biosynthetic gene clusters for bioactive molecule discovery in filamentous fungi[J]. ACS Synthetic Biology, 2020, 9(7): 1843-1854. |
| 113 | LI X J, HUANG L G, PAN L J, et al. CRISPR/dCas9-mediated epigenetic modification reveals differential regulation of histone acetylation on Aspergillus niger secondary metabolite[J]. Microbiological Research, 2021, 245: 126694. |
| 114 | KEYS H R, KNOUSE K A. Genome-scale CRISPR screening in a single mouse liver[J]. Cell Genomics, 2022, 2(12): 100217. |
| 115 | LI Z M, KIM K S. RELATe enables genome-scale engineering in fungal genomics[J]. Science Advances, 2020, 6(38): eabb8783. |
| 116 | LUU X C, SHIDA Y, SUZUKI Y, et al. A novel high-throughput approach for transforming filamentous fungi employing a droplet-based microfluidic platform[J]. New Biotechnology, 2022, 72: 149-158. |
| 117 | HEIGWER F, BOUTROS M. Cloud-based design of short guide RNA (sgRNA) libraries for CRISPR experiments[M]//CRISPR Guide RNA Design, Methods in Molecular Biology. New York, NY: Springer US, 2020: 3-22. |
| 118 | LABUN K, MONTAGUE T G, GAGNON J A, et al. CHOPCHOPv2: a web tool for the next generation of CRASPR genome engineering. Nucleic Acid Res, 2016, 44(W1): W272-276. |
| 119 | HWANG G H, KIM J S, BAE S S. Web-based CRISPR toolkits: Cas-OFFinder, Cas-designer, and Cas-analyzer[M]// CRISPR Guide RNA Design, Methods in Molecular Biology. New York, NY: Springer US, 2020: 23-33. |
| 120 | LEISEN T, BIETZ F, WERNER J, et al. CRISPR/Cas with ribonucleoprotein complexes and transiently selected telomere vectors allows highly efficient marker-free and multiple genome editing in Botrytis cinerea [J]. PLoS Pathogens, 2020, 16(8): e1008326. |
| 121 | ABDULRACHMAN D, EURWILAICHITR L, CHAMPREDA V, et al. Development of a CRISPR/Cpf1 system for targeted gene disruption in Aspergillus aculeatus TBRC 277[J]. BMC Biotechnology, 2021, 21(1): 15. |
| 122 | KIM B, KIM H J, LEE S J. Effective blocking of microbial transcriptional initiation by dCas9-NG-mediated CRISPR interference[J]. Journal of Microbiology and Biotechnology, 2020, 30(12): 1919-1926. |
| 123 | HAN H A, PANG J K S, SOH B S. Mitigating off-target effects in CRISPR/Cas9-mediated in vivo gene editing[J]. Journal of Molecular Medicine, 2020, 98(5): 615-632. |
| 124 | NGUYEN L T, MACALUSO N C, PIZZANO B L M, et al. A thermostable Cas12b from Brevibacillus leverages one-pot discrimination of SARS-CoV-2 variants of concern[J]. eBioMedicine, 2022, 77: 103926. |
| 125 | GHOUNEIMY A, MAHFOUZ M. Streamlined detection of SARS-CoV-2 via Cas13[J]. Nature Biomedical Engineering, 2022, 6(8): 925-927. |
| 126 | ZHOU B, YANG R L, SOHAIL M, et al. CRISPR/Cas14 provides a promising platform in facile and versatile aptasensing with improved sensitivity[J]. Talanta, 2023, 254: 124120. |
| 127 | HAN X, LIU Z B, JO M C, et al. CRISPR-Cas9 delivery to hard-to-transfect cells via membrane deformation[J]. Science Advances, 2015, 1(7): e1500454. |
| 128 | AMALAMOL D, ASHWIN N M R, LAKSHANA K V, et al. A highly efficient stratagem for protoplast isolation and genetic transformation in filamentous fungus Colletotrichum falcatum [J]. Folia Microbiologica, 2022, 67(3): 479-490. |
| 129 | WANG J, TENG Y X, GONG X Y, et al. Exploring and engineering PAM-diverse Streptococci Cas9 for PAM-directed bifunctional and titratable gene control in bacteria[J]. Metabolic Engineering, 2023, 75: 68-77. |
| 130 | FU Y W, DAI X Y, WANG W T, et al. Dynamics and competition of CRISPR-Cas9 ribonucleoproteins and AAV donor-mediated NHEJ, MMEJ and HDR editing[J]. Nucleic Acids Research, 2021, 49(2): 969-985. |
| 131 | ENDO M, IWAKAMI S, TOKI S. Precision genome editing in plants via gene targeting and subsequent break-induced single-strand annealing[J]. Plant Biotechnology Journal, 2021, 19(3): 563-574. |
| 132 | NEUGEBAUER M E, HSU A, ARBAB M, et al. Evolution of an adenine base editor into a small, efficient cytosine base editor with low off-target activity[J/OL]. Nature Biotechnology, 2022[2023-01-03]. . |
| 133 | HUANG L G, DONG H Z, ZHENG J W, et al. Highly efficient single base editing in Aspergillus niger with CRISPR/Cas9 cytidine deaminase fusion[J]. Microbiological Research, 2019, 223/224/225: 44-50. |
| 134 | ZHANG C Y, LI N, RAO L, et al. Development of an efficient C-to-T base-editing system and its application to cellulase transcription factor precise engineering in thermophilic fungus Myceliophthora thermophila [J]. Microbiology Spectrum, 2022, 10(3): e0232121. |
| 135 | JIN S, ZONG Y, GAO Q, et al. Cytosine, but not adenine, base editors induce genome-wide off-target mutations in rice[J]. Science, 2019, 364(6437): 292-295. |
| 136 | 刘倩, 李金根, 张晨阳, 等. 工业丝状真菌基因组编辑技术研究进展[J]. 合成生物学, 2021, 2(2): 256-273. |
| LIU Q, LI J G, ZHANG C Y, et al. Research progress of genome editing technologies for industrial filamentous fungi[J]. Synthetic Biology Journal, 2021, 2(2): 256-273. | |
| 137 | 肖晗, 刘宜欣. CRISPR-Cas系统编辑丝状真菌的进展与挑战[J]. 合成生物学, 2021, 2(2): 274-286. |
| XIAO H, LIU Y X. Progress and challenge of the CRISPR-Cas system in gene editing for filamentous fungi[J]. Synthetic Biology Journal, 2021, 2(2): 274-286. |
| [1] | 惠真, 唐啸宇. CRISPR/Cas9编辑系统在微生物天然产物研究中的应用[J]. 合成生物学, 2024, 5(3): 658-671. |
| [2] | 陈盈盈, 刘扬, 史俊杰, 马俊英, 鞠建华. CRISPR/Cas基因编辑及其新兴技术在丝状真菌研究中的系统应用[J]. 合成生物学, 2024, 5(3): 672-693. |
| [3] | 赖铭元, 韦健, 许建和, 郁惠蕾. 非特异性过加氧酶(UPO)的研究综述[J]. 合成生物学, 2022, 3(6): 1235-1249. |
| [4] | 董佳钰, 李敏, 肖宗华, 胡明, 松田侑大, 汪伟光. 米曲霉异源表达天然产物研究进展[J]. 合成生物学, 2022, 3(6): 1126-1149. |
| [5] | 龚仕涛, 王宇, 陈宇庭. CRISPR/Cas9及其衍生编辑器在衰老研究中的应用进展[J]. 合成生物学, 2022, 3(1): 66-77. |
| [6] | 王凤姣, 徐海洋, 闫建斌, 李伟. 植物天然农药除虫菊酯的生物合成和应用研究进展[J]. 合成生物学, 2021, 2(5): 751-763. |
| [7] | 周海波, 申琪瑶, 陈汉娜, 王宗杰, 李越中, 张友明, 卞小莹. 利用异源表达挖掘纤维堆囊菌So0157-2的新型天然产物[J]. 合成生物学, 2021, 2(5): 837-849. |
| [8] | 肖晗, 刘宜欣. CRISPR-Cas系统编辑丝状真菌的进展与挑战[J]. 合成生物学, 2021, 2(2): 274-286. |
| [9] | 刘倩, 李金根, 张晨阳, 李芳雅, 田朝光. 工业丝状真菌基因组编辑技术研究进展[J]. 合成生物学, 2021, 2(2): 256-273. |
| [10] | 黄雪年, 唐慎, 吕雪峰. 工业丝状真菌土曲霉合成生物技术研究进展及展望[J]. 合成生物学, 2020, 1(2): 187-211. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||