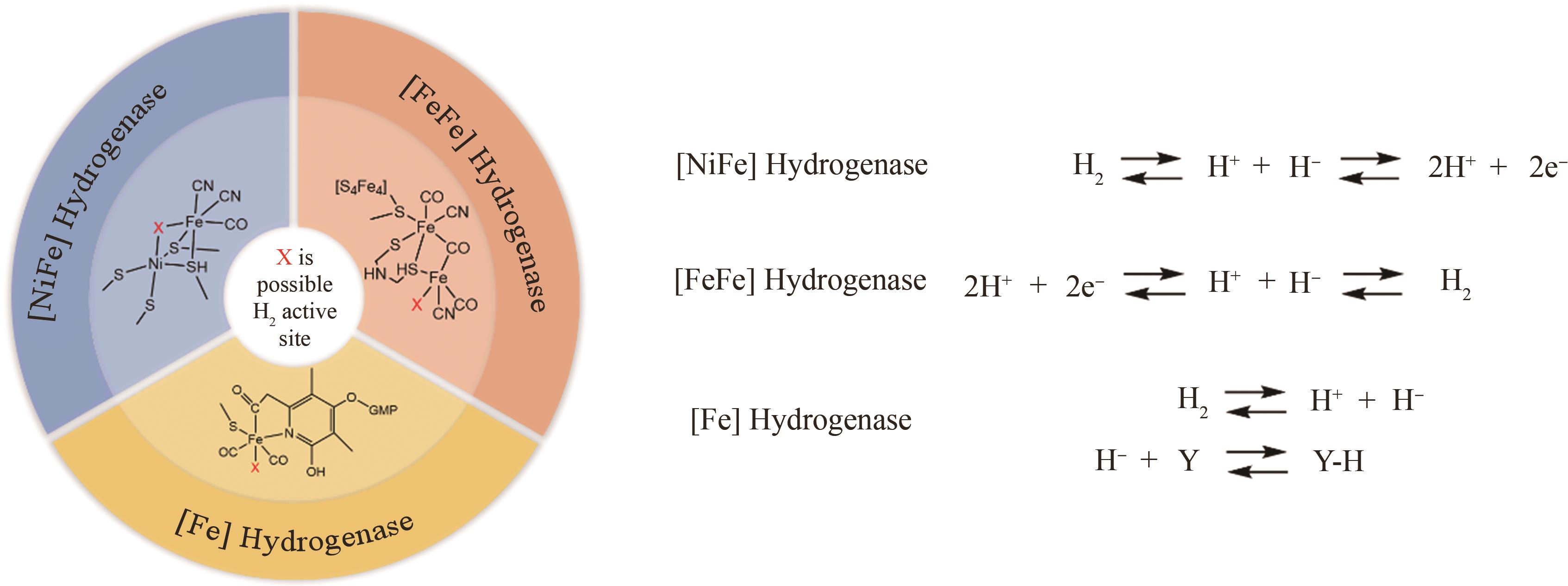
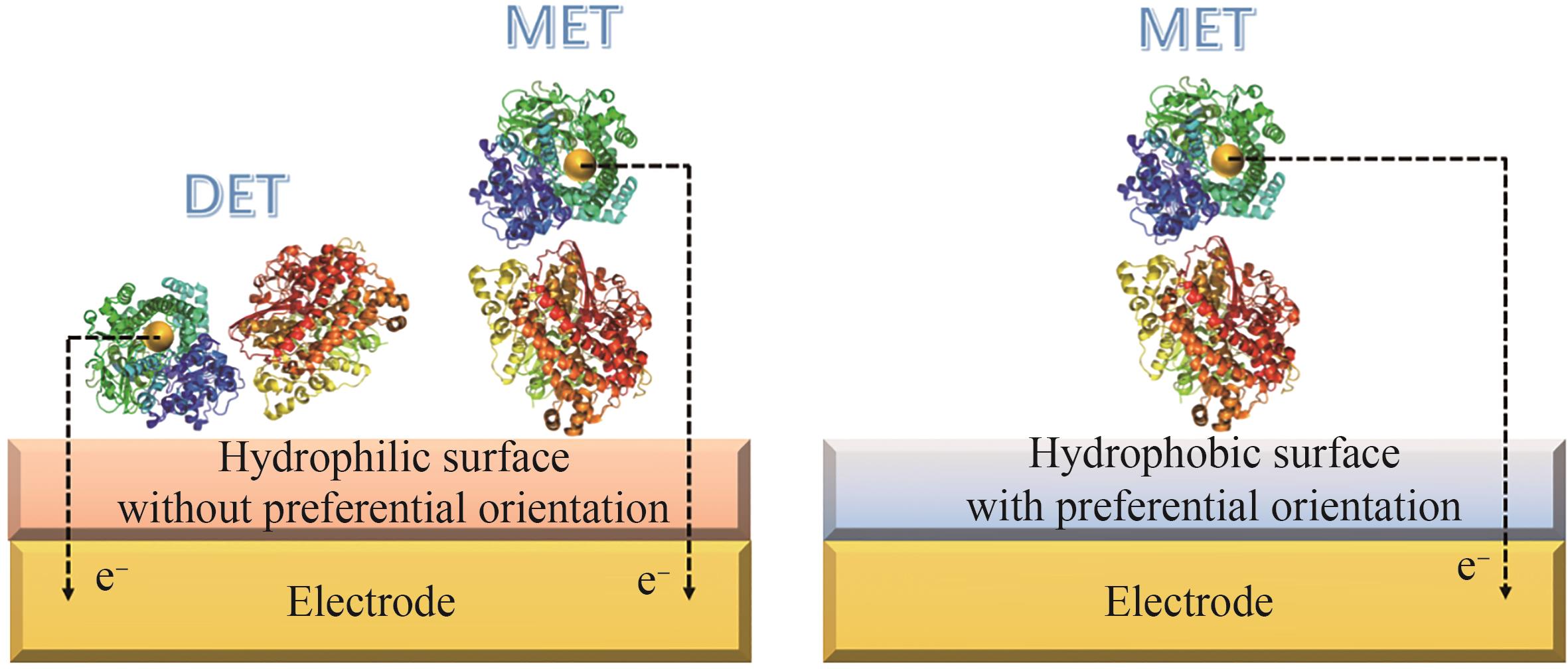
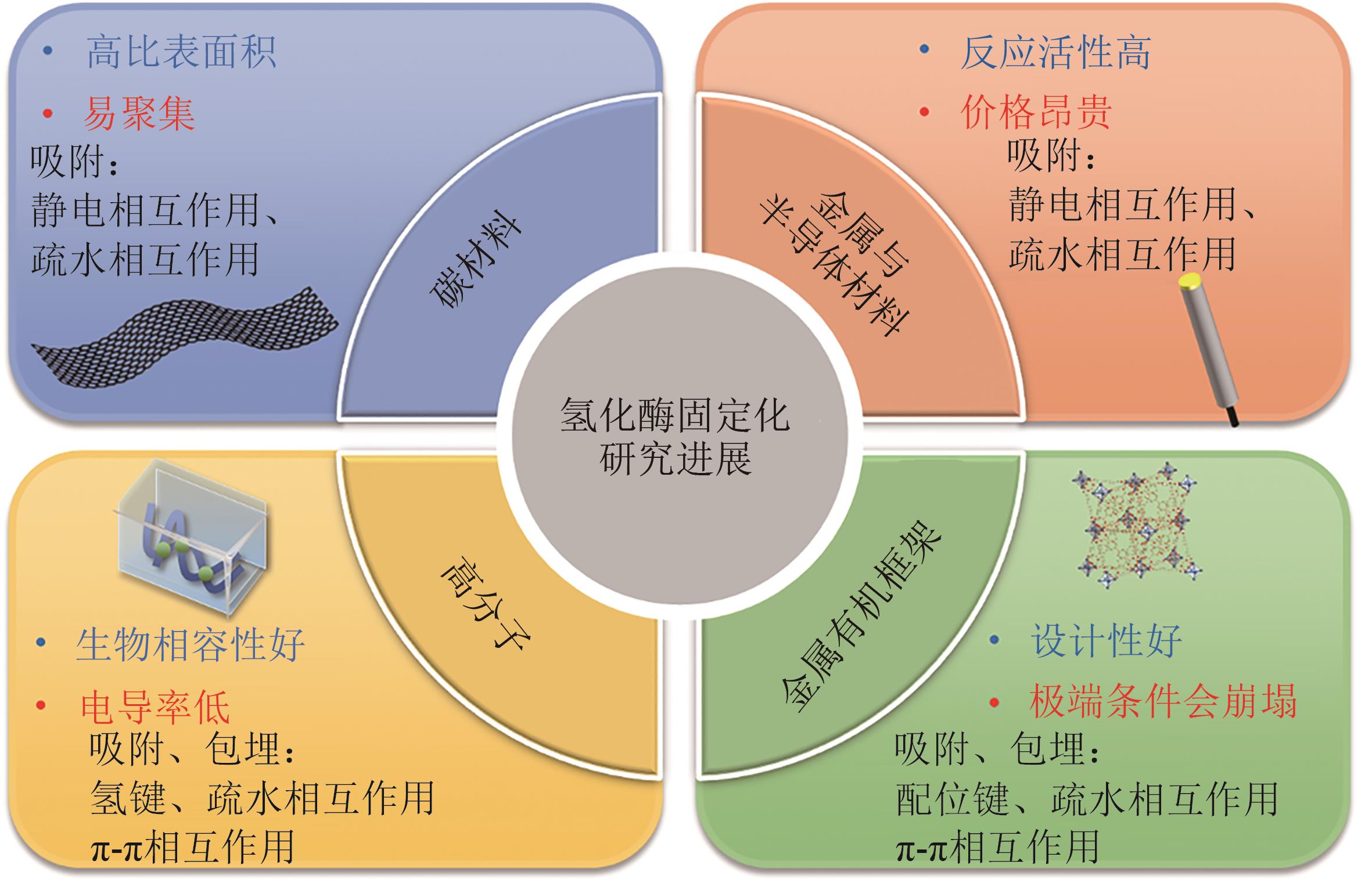
Synthetic Biology Journal ›› 2024, Vol. 5 ›› Issue (6): 1485-1497.DOI: 10.12211/2096-8280.2024-022
• Invited Review • Previous Articles Next Articles
Advance in the immobilization of hydrogenases
LEI Hangbin1, HE Ning1, LI Feixuan1, DONG Lingling1, WANG Shizhen1,2
- 1.Department of Chemical and Biochemical Engineering,College of Chemistry and Chemical Engineering,Xiamen University,Xiamen 361005,Fujian,China
2.The Key Lab for Synthetic Biotechnology of Xiamen City,Xiamen University,Xiamen 361005,Fujian,China
-
Received:2024-03-11Revised:2024-05-17Online:2025-01-10Published:2024-12-31 -
Contact:WANG Shizhen
氢化酶固定化研究进展
雷航彬1, 何宁1, 李斐煊1, 董玲玲1, 王世珍1,2
- 1.厦门大学化学化工学院化学工程与生物工程系,福建 厦门 361005
2.厦门大学厦门市合成生物学重点实验室,福建 厦门 361005
-
通讯作者:王世珍 -
作者简介:雷航彬 (2000—),男,硕士研究生。研究方向为固定化酶。E-mail:lhb20000410@qq.com王世珍 (1982—),女,副教授,硕士生导师。研究方向为合成生物学、生物催化与转化、酶工程等。E-mail:szwang@xmu.edu.cn -
基金资助:国家重点研发计划“合成生物学”重点专项“糖水氢电系统——体外多酶高效产氢及氢电装置的基础及工程研究”(2022YFA0912003)
CLC Number:
Cite this article
LEI Hangbin, HE Ning, LI Feixuan, DONG Lingling, WANG Shizhen. Advance in the immobilization of hydrogenases[J]. Synthetic Biology Journal, 2024, 5(6): 1485-1497.
雷航彬, 何宁, 李斐煊, 董玲玲, 王世珍. 氢化酶固定化研究进展[J]. 合成生物学, 2024, 5(6): 1485-1497.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2024-022
| 材料 | 具体固定化载体 | 氢化酶来源 | 分类 | 应用 | 参考文献 |
|---|---|---|---|---|---|
| 碳材料 | 石墨 | Escherichia coli ([NiFe]) | [NiFe] | 生物电催化 | [ |
| 石墨 | Aquifex aeolicus | [NiFe] | 生物电催化 | [ | |
| 石墨 | Clostridium acetobutylicum | [FeFe] | 生物电催化 | [ | |
| 石墨 | Ralstonia eutropha | [NiFe] | 生物电催化 | [ | |
| 石墨 | Desulfovibrio gigas | [NiFe] | 生物电催化 | [ | |
| 石墨 | Ralstonia metallidurans | [NiFe] | 生物燃料电池 | [ | |
| 石墨 | Allochromatium vinosum | [NiFe] | 动力学研究 | [ | |
| 碳黑 | Ralstonia eutropha | [NiFe] | 光谱电化学研究 | [ | |
| 碳丝 | Thiocapsa roseopersicina | [NiFe] | 生物燃料电池 | [ | |
| 碳丝 | Thiocapsa roseopersicina | [NiFe] | 生物反应器 | [ | |
| 碳纸 | Pyrococcus furiosus | [NiFe] | 生物燃料电池 | [ | |
| 碳毡 | Clostridium acetobutylicum | [NiFe] | 生物燃料电池 | [ | |
| 单壁碳纳米管 | Clostridium acetobutylicum | [FeFe] | 生物电催化 | [ | |
| 单壁碳纳米管 | Clostridium acetobutylicum | [FeFe] | 生物电催化 | [ | |
| 单壁碳纳米管 | Allochromatium vinosum | [FeFe] | 生物电催化 | [ | |
| 多壁碳纳米管 | Aquifex aeolicus | [NiFe] | 电化学传感器 | [ | |
| 多壁碳纳米管 | Ralstonia eutropha Aquifex aeolicus | [NiFe] | 生物燃料电池 | [ |
Table 1 Applications of carbon materials for hydrogenases immobilization
| 材料 | 具体固定化载体 | 氢化酶来源 | 分类 | 应用 | 参考文献 |
|---|---|---|---|---|---|
| 碳材料 | 石墨 | Escherichia coli ([NiFe]) | [NiFe] | 生物电催化 | [ |
| 石墨 | Aquifex aeolicus | [NiFe] | 生物电催化 | [ | |
| 石墨 | Clostridium acetobutylicum | [FeFe] | 生物电催化 | [ | |
| 石墨 | Ralstonia eutropha | [NiFe] | 生物电催化 | [ | |
| 石墨 | Desulfovibrio gigas | [NiFe] | 生物电催化 | [ | |
| 石墨 | Ralstonia metallidurans | [NiFe] | 生物燃料电池 | [ | |
| 石墨 | Allochromatium vinosum | [NiFe] | 动力学研究 | [ | |
| 碳黑 | Ralstonia eutropha | [NiFe] | 光谱电化学研究 | [ | |
| 碳丝 | Thiocapsa roseopersicina | [NiFe] | 生物燃料电池 | [ | |
| 碳丝 | Thiocapsa roseopersicina | [NiFe] | 生物反应器 | [ | |
| 碳纸 | Pyrococcus furiosus | [NiFe] | 生物燃料电池 | [ | |
| 碳毡 | Clostridium acetobutylicum | [NiFe] | 生物燃料电池 | [ | |
| 单壁碳纳米管 | Clostridium acetobutylicum | [FeFe] | 生物电催化 | [ | |
| 单壁碳纳米管 | Clostridium acetobutylicum | [FeFe] | 生物电催化 | [ | |
| 单壁碳纳米管 | Allochromatium vinosum | [FeFe] | 生物电催化 | [ | |
| 多壁碳纳米管 | Aquifex aeolicus | [NiFe] | 电化学传感器 | [ | |
| 多壁碳纳米管 | Ralstonia eutropha Aquifex aeolicus | [NiFe] | 生物燃料电池 | [ |
| 材料 | 具体固定化载体 | 氢化酶来源 | 分类 | 应用 | 参考文献 |
|---|---|---|---|---|---|
| 金属 | 金电极 | Chlamydomonas reinhardtii | [FeFe] | 生物电催化 | [ |
| 金电极 | Desulfovibrio vulgaris | [NiFe] | 电化学研究 | [ | |
| 金电极 | Desulfovibrio vulgaris | [NiFe] | 电化学分析 | [ | |
| 金电极 | Ralstonia eutropha | [NiFe] | 电化学研究 | [ | |
| 金电极 | Desulfovibrio vulgaris | [NiFe] | 生物电催化 | [ | |
| 金电极 | Ralstonia eutropha | [NiFe] | 生物电催化 | [ | |
| 硫醇修饰金电极 | Aquifex aeolicus | [NiFe] | 生物电催化 | [ | |
| 紫精修饰金电极 | Desulfovibrio desulfuricans | [FeFe] | 生物燃料电池 | [ | |
| 碳纳米管修饰金电极 | Desulfovibrio gigas | [NiFe] | 生物燃料电池 | [ | |
| 碳纳米管修饰金电极 | Desulfovibrio fructosovorans | [NiFe] | 生物燃料电池 | [ | |
| 纳米金电极 | Aquifex aeolicus | [NiFe] | 生物燃料电池 | [ | |
| 纳米金电极 | Allochromatium vinosum | [NiFe] | 单酶分子电化学 | [ | |
| 银纳米团簇 | Escherichia coli | [NiFe] | 光电催化 | [ | |
| 半导体 | TiO2 | Thiocapsa roseopersicina | [NiFe] | 光电催化 | [ |
| TiO2 | Desulfomicrobium baculatum | [NiFeSe] | 光电催化 | [ | |
| TiO2 | Desulfomicrobium baculatum | [NiFeSe] | 光电催化 | [ | |
| TiO2 | Clostridium acetobutylicum | [FeFe] | 生物电催化 | [ | |
| PVK|IO-TiO2 | Desulfovibrio vulgaris | [NiFeSe] | 光电化学集成系统 | [ | |
| ITO | Desulfovibrio vulgaris | [NiFe] | 光电催化 | [ | |
| ITO | Desulfomicrobium baculatum | [NiFeSe] | 光电催化 | [ | |
| ITO | Ralstonia eutropha | [NiFe] | 生物电子设备 | [ | |
| CN x (氮化碳) | Desulfomicrobium baculatum | [NiFeSe] | 光电催化 | [ | |
| CdS | Clostridium acetobutylicum | [FeFe] | 光电催化 | [ | |
| CdS | Clostridium acetobutylicum | [FeFe] | 电子转移动力学研究 | [ | |
| CdTe | Clostridium acetobutylicum | [FeFe] | 光电催化 | [ | |
| CdTe | Thiocapsa roseopersicina | [NiFe] | 光电催化 | [ | |
| In2S3 | Desulfovibrio vulgaris | [NiFeSe] | 光电催化 | [ | |
| FTO-NiO-In2S3 | Desulfovibrio vulgaris | [NiFeSe] | 光电催化 | [ |
Table 2 Applications of metals and semiconductors for hydrogenases immobilization
| 材料 | 具体固定化载体 | 氢化酶来源 | 分类 | 应用 | 参考文献 |
|---|---|---|---|---|---|
| 金属 | 金电极 | Chlamydomonas reinhardtii | [FeFe] | 生物电催化 | [ |
| 金电极 | Desulfovibrio vulgaris | [NiFe] | 电化学研究 | [ | |
| 金电极 | Desulfovibrio vulgaris | [NiFe] | 电化学分析 | [ | |
| 金电极 | Ralstonia eutropha | [NiFe] | 电化学研究 | [ | |
| 金电极 | Desulfovibrio vulgaris | [NiFe] | 生物电催化 | [ | |
| 金电极 | Ralstonia eutropha | [NiFe] | 生物电催化 | [ | |
| 硫醇修饰金电极 | Aquifex aeolicus | [NiFe] | 生物电催化 | [ | |
| 紫精修饰金电极 | Desulfovibrio desulfuricans | [FeFe] | 生物燃料电池 | [ | |
| 碳纳米管修饰金电极 | Desulfovibrio gigas | [NiFe] | 生物燃料电池 | [ | |
| 碳纳米管修饰金电极 | Desulfovibrio fructosovorans | [NiFe] | 生物燃料电池 | [ | |
| 纳米金电极 | Aquifex aeolicus | [NiFe] | 生物燃料电池 | [ | |
| 纳米金电极 | Allochromatium vinosum | [NiFe] | 单酶分子电化学 | [ | |
| 银纳米团簇 | Escherichia coli | [NiFe] | 光电催化 | [ | |
| 半导体 | TiO2 | Thiocapsa roseopersicina | [NiFe] | 光电催化 | [ |
| TiO2 | Desulfomicrobium baculatum | [NiFeSe] | 光电催化 | [ | |
| TiO2 | Desulfomicrobium baculatum | [NiFeSe] | 光电催化 | [ | |
| TiO2 | Clostridium acetobutylicum | [FeFe] | 生物电催化 | [ | |
| PVK|IO-TiO2 | Desulfovibrio vulgaris | [NiFeSe] | 光电化学集成系统 | [ | |
| ITO | Desulfovibrio vulgaris | [NiFe] | 光电催化 | [ | |
| ITO | Desulfomicrobium baculatum | [NiFeSe] | 光电催化 | [ | |
| ITO | Ralstonia eutropha | [NiFe] | 生物电子设备 | [ | |
| CN x (氮化碳) | Desulfomicrobium baculatum | [NiFeSe] | 光电催化 | [ | |
| CdS | Clostridium acetobutylicum | [FeFe] | 光电催化 | [ | |
| CdS | Clostridium acetobutylicum | [FeFe] | 电子转移动力学研究 | [ | |
| CdTe | Clostridium acetobutylicum | [FeFe] | 光电催化 | [ | |
| CdTe | Thiocapsa roseopersicina | [NiFe] | 光电催化 | [ | |
| In2S3 | Desulfovibrio vulgaris | [NiFeSe] | 光电催化 | [ | |
| FTO-NiO-In2S3 | Desulfovibrio vulgaris | [NiFeSe] | 光电催化 | [ |
| 固定化载体 | 氢化酶来源 | 分类 | 储存/反应条件及剩余酶活 | 参考文献 |
|---|---|---|---|---|
| 紫精凝胶 | Desulfovibrio vulgaris | [NiFe] | 4 ℃,磷酸盐缓冲液pH=7.0,储存20天保持50%的酶活 | [ |
| 聚合物多孔凝胶 | Clostridium pasteurianum | [Fe] | 室温,厌氧缓冲液pH=8.0,储存28天保持70%的活性 | [ |
| 聚合物多孔凝胶 | Lamprobacter modestogalophilus | [NiFe] | 室温,厌氧缓冲液pH=8.0,储存28天保持50%的活性 | [ |
| 海藻酸钙凝胶 | Desulphovibrio desulphuricans | [NiFe] | 4 ℃,Tris-HCl缓冲液pH=7.5,储存40天保持60%的活性 | [ |
| 海藻酸钙凝胶 | DesuEfouibrio sp. | [NiFe] | 30 ℃,Tris-HCl缓冲液pH=7.6,反应50 h保持40%的活性 | [ |
| 阴离子交换树脂 | Ralstonia eutropha | [NiFe] | 35 ℃,Tris-HCl缓冲液pH=8.0,反应32 h保持50%的活性 | [ |
| 琼脂糖凝胶 | Chromatium vinosum | [NiFe] | 65 ℃,Tris-HCl缓冲液pH=8.0,孵育80 min保持50%的稳定性 | [ |
Table 3 Stability of hydrogenases immobilized by polymers
| 固定化载体 | 氢化酶来源 | 分类 | 储存/反应条件及剩余酶活 | 参考文献 |
|---|---|---|---|---|
| 紫精凝胶 | Desulfovibrio vulgaris | [NiFe] | 4 ℃,磷酸盐缓冲液pH=7.0,储存20天保持50%的酶活 | [ |
| 聚合物多孔凝胶 | Clostridium pasteurianum | [Fe] | 室温,厌氧缓冲液pH=8.0,储存28天保持70%的活性 | [ |
| 聚合物多孔凝胶 | Lamprobacter modestogalophilus | [NiFe] | 室温,厌氧缓冲液pH=8.0,储存28天保持50%的活性 | [ |
| 海藻酸钙凝胶 | Desulphovibrio desulphuricans | [NiFe] | 4 ℃,Tris-HCl缓冲液pH=7.5,储存40天保持60%的活性 | [ |
| 海藻酸钙凝胶 | DesuEfouibrio sp. | [NiFe] | 30 ℃,Tris-HCl缓冲液pH=7.6,反应50 h保持40%的活性 | [ |
| 阴离子交换树脂 | Ralstonia eutropha | [NiFe] | 35 ℃,Tris-HCl缓冲液pH=8.0,反应32 h保持50%的活性 | [ |
| 琼脂糖凝胶 | Chromatium vinosum | [NiFe] | 65 ℃,Tris-HCl缓冲液pH=8.0,孵育80 min保持50%的稳定性 | [ |
| 1 | WANG C, LAI Z L, HUANG G F, et al. Current state of [Fe]-hydrogenase and its biomimetic models[J]. Chemistry, 2022, 28(57): e202201499. |
| 2 | AL-SHAMERI A, SIEBERT D L, SUTIONO S, et al. Hydrogenase-based oxidative biocatalysis without oxygen[J]. Nature Communications, 2023, 14(1): 2693. |
| 3 | JI H S, WAN L, GAO Y X, et al. Hydrogenase as the basis for green hydrogen production and utilization[J]. Journal of Energy Chemistry, 2023, 85: 348-362. |
| 4 | WANG X, FU J Q, LIU Z, et al. Review of researches on important components of hydrogen supply systems and rapid hydrogen refueling processes[J]. International Journal of Hydrogen Energy, 2023, 48(5): 1904-1929. |
| 5 | 肖艳, 刘亚君, 冯银刚, 等. 热纤梭菌在生物质能源开发中的合成生物学研究进展[J]. 合成生物学, 2023, 4(6): 1055-1081. |
| XIAO Y, LIU Y J, FENG Y G, et al. Progress in synthetic biology research of Clostridium thermocellum for biomass energy applications[J]. Synthetic Biology Journal, 2023, 4(6): 1055-1081. | |
| 6 | MARTINO M, RUOCCO C, MELONI E, et al. Main hydrogen production processes: an overview[J]. Catalysts, 2021, 11(5): 547. |
| 7 | OGO S, KISHIMA T, YATABE T, et al. [NiFe], [FeFe], and [Fe] hydrogenase models from isomers[J]. Science Advances, 2020, 6(24): eaaz8181. |
| 8 | OGATA H, LUBITZ W. Bioenergetics theory and components | Hydrogenases structure and function[M/OL]//Encyclopedia of Biological Chemistry Ⅲ. Oxford: Elsevier, 2021, 2: 66-73. (2021-08-02)[2024-02-01]. . |
| 9 | HUANG G F, WAGNER T, WODRICH M D, et al. The atomic-resolution crystal structure of activated [Fe]-hydrogenase[J]. Nature Catalysis, 2019, 2(6): 537-543. |
| 10 | LUBITZ W, OGATA H, RÜDIGER O, et al. Hydrogenases[J]. Chemical Reviews, 2014, 114(8): 4081-4148. |
| 11 | ABOU HAMDAN A, LIEBGOTT P P, FOURMOND V, et al. Relation between anaerobic inactivation and oxygen tolerance in a large series of NiFe hydrogenase mutants[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(49): 19916-19921. |
| 12 | RODRÍGUEZ-MACIÁ P, REIJERSE E J, VAN GASTEL M, et al. Sulfide protects [FeFe] hydrogenases from O2 [J]. Journal of the American Chemical Society, 2018, 140(30): 9346-9350. |
| 13 | YUSHKOVA E D, NAZAROVA E A, MATYUHINA A V, et al. Application of immobilized enzymes in food industry[J]. Journal of Agricultural and Food Chemistry, 2019, 67(42): 11553-11567. |
| 14 | JIANG Q Y, LI T P, YANG J, et al. Synthetic engineering of a new biocatalyst encapsulating [NiFe]-hydrogenases for enhanced hydrogen production[J]. Journal of Materials Chemistry B, 2023, 11(12): 2684-2692. |
| 15 | PAVLIDIS I V, VORHABEN T, TSOUFIS T, et al. Development of effective nanobiocatalytic systems through the immobilization of hydrolases on functionalized carbon-based nanomaterials[J]. Bioresource Technology, 2012, 115: 164-171. |
| 16 | MOROZOV S V, KARYAKINA E E, ZADVORNYI O A, et al. Bioelectrocatalysis by hydrogenase Th. roseopersicina immobilized on carbon materials[J]. Russian Journal of Electrochemistry, 2002, 38(1): 97-102. |
| 17 | QUINSON J, HIDALGO R, ASH P A, et al. Comparison of carbon materials as electrodes for enzyme electrocatalysis: hydrogenase as a case study[J]. Faraday Discussions, 2014, 172: 473-496. |
| 18 | LOJOU É, GIUDICI-ORTICONI M T, BIANCO P. Hydrogenases from the hyperthermophilic bacterium Aquifex aeolicus: electrocatalysis of the hydrogen production/consumption reactions at carbon electrodes[J]. Journal of Electroanalytical Chemistry, 2005, 577(1): 79-86. |
| 19 | BAFFERT C, DEMUEZ M, COURNAC L, et al. Hydrogen-activating enzymes: activity does not correlate with oxygen sensitivity[J]. Angewandte Chemie International Edition, 2008, 47(11): 2052-2054. |
| 20 | GOLDET G, WAIT A F, CRACKNELL J A, et al. Hydrogen production under aerobic conditions by membrane-bound hydrogenases from Ralstonia species[J]. Journal of the American Chemical Society, 2008, 130(33): 11106-11113. |
| 21 | RÜDIGER O, ABAD J M, HATCHIKIAN E C, et al. Oriented immobilization of Desulfovibrio gigas hydrogenase onto carbon electrodes by covalent bonds for nonmediated oxidation of H2 [J]. Journal of the American Chemical Society, 2005, 127(46): 16008-16009. |
| 22 | VINCENT K A, CRACKNELL J A, CLARK J R, et al. Electricity from low-level H2 in still air: an ultimate test for an oxygen tolerant hydrogenase[J]. Chemical Communications, 2006(48): 5033-5035. |
| 23 | LAMLE S E, VINCENT K A, HALLIWELL L M, et al. Hydrogenase on an electrode: a remarkable heterogeneous catalyst[J]. Dalton Transactions, 2003(21): 4152-4157. |
| 24 | HEALY A J, ASH P A, LENZ O, et al. Attenuated total reflectance infrared spectroelectrochemistry at a carbon particle electrode; unmediated redox control of a [NiFe]- hydrogenase solution[J]. Physical Chemistry Chemical Physics, 2013, 15(19): 7055-7059. |
| 25 | KARYAKIN A A, MOROZOV S V, KARYAKINA E E, et al. Hydrogen fuel electrode based on bioelectrocatalysis by the enzyme hydrogenase[J]. Electrochemistry Communications, 2002, 4(5): 417-420. |
| 26 | SHASTIK E S, VOKHMYANINA D V, ZORIN N A, et al. Demonstration of hydrogenase electrode operation in a bioreactor[J]. Enzyme and Microbial Technology, 2011, 49(5): 453-458. |
| 27 | JOHNSTON W, COONEY M J, LIAW B Y, et al. Design and characterization of redox enzyme electrodes: new perspectives on established techniques with application to an extremeophilic hydrogenase[J]. Enzyme and Microbial Technology, 2005, 36(4): 540-549. |
| 28 | HAMBOURGER M, GERVALDO M, SVEDRUZIC D, et al. [FeFe]-hydrogenase-catalyzed H2 production in a photoelectrochemical biofuel cell[J]. Journal of the American Chemical Society, 2008, 130(6): 2015-2022. |
| 29 | SVEDRUŽIĆ D, BLACKBURN J L, TENENT R C, et al. High-performance hydrogen production and oxidation electrodes with hydrogenase supported on metallic single-wall carbon nanotube networks[J]. Journal of the American Chemical Society, 2011, 133(12): 4299-4306. |
| 30 | MCDONALD T J, SVEDRUZIC D, KIM Y H, et al. Wiring-up hydrogenase with single-walled carbon nanotubes[J]. Nano Letters, 2007, 7(11): 3528-3534. |
| 31 | HOEBEN F J M, HELLER I, ALBRACHT S P J, et al. Polymyxin-coated Au and carbon nanotube electrodes for stable [NiFe]-hydrogenase film voltammetry[J]. Langmuir, 2008, 24(11): 5925-5931. |
| 32 | KYRPEL T, SASKA V, DE POULPIQUET A, et al. Hydrogenase-based electrode for hydrogen sensing in a fermentation bioreactor[J]. Biosensors & Bioelectronics, 2023, 225: 115106. |
| 33 | MONSALVE K, MAZURENKO I, GUTIERREZ-SANCHEZ C, et al. Impact of carbon nanotube surface chemistry on hydrogen oxidation by membrane-bound oxygen-tolerant hydrogenases[J]. ChemElectroChem, 2016, 3(12): 2179-2188. |
| 34 | LIU J, WU W J, FANG F, et al. Immobilization of hydrogenase on carbon nanotube polyelectrolytes as heterogeneous catalysts for electrocatalytic interconversion of protons and hydrogen[J]. Journal of Nanoparticle Research, 2016, 18(8): 220. |
| 35 | MAZURENKO I, CLÉMENT R, BYRNE-KODJABACHIAN D, et al. Pore size effect of MgO-templated carbon on enzymatic H2 oxidation by the hyperthermophilic hydrogenase from Aquifex aeolicus [J]. Journal of Electroanalytical Chemistry, 2018, 812: 221-226. |
| 36 | SUN Q, ZORIN N A, CHEN D, et al. Langmuir-Blodgett films of pyridyldithio-modified multiwalled carbon nanotubes as a support to immobilize hydrogenase[J]. Langmuir, 2010, 26(12): 10259-10265. |
| 37 | WANG Y M, SONG Y H, MA C L, et al. Electrochemical characterization of a truncated hydrogenase from Pyrococcus furiosus [J]. Electrochimica Acta, 2021, 387: 138502. |
| 38 | GENTIL S, CHE MANSOR S M, JAMET H, et al. Oriented immobilization of [NiFeSe] hydrogenases on covalently and noncovalently functionalized carbon nanotubes for H2/air enzymatic fuel cells[J]. ACS Catalysis, 2018, 8(5): 3957-3964. |
| 39 | TSYGANKOV A A, ZORIN N A, STARODUBOV A S, et al. A simple method for oriented immobilization of HydSL hydrogenase of Thiocapsa bogorovii on carbon electrodes[J]. International Journal of Hydrogen Energy, 2023, 48(100): 39989-39999. |
| 40 | RUIZ-RODRÍGUEZ M A, COOPER C D, ROCCHIA W, et al. Modeling of the electrostatic interaction and catalytic activity of [NiFe] hydrogenases on a planar electrode[J]. The Journal of Physical Chemistry B, 2022, 126(43): 8777-8790. |
| 41 | WANG Y M, KANG Z P, ZHANG L L, et al. Elucidating the interactions between a [NiFe]-hydrogenase and carbon electrodes for enhanced bioelectrocatalysis[J]. ACS Catalysis, 2022, 12(2): 1415-1427. |
| 42 | KRASSEN H, STRIPP S T, BÖHM N, et al. Tailor-made modification of a gold surface for the chemical binding of a high-activity [FeFe] hydrogenase[J]. European Journal of Inorganic Chemistry, 2011, 2011(7): 1138-1146. |
| 43 | MILLO D, PANDELIA M E, UTESCH T, et al. Spectroelectrochemical study of the [NiFe] hydrogenase from Desulfovibrio vulgaris Miyazaki F in solution and immobilized on biocompatible gold surfaces[J]. The Journal of Physical Chemistry B, 2009, 113(46): 15344-15351. |
| 44 | MILLO D, HILDEBRANDT P, PANDELIA M E, et al. SEIRA spectroscopy of the electrochemical activation of an immobilized [NiFe] hydrogenase under turnover and non-turnover conditions[J]. Angewandte Chemie International Edition, 2011, 50(11): 2632-2634. |
| 45 | HEIDARY N, UTESCH T, ZERBALL M, et al. Orientation-controlled electrocatalytic efficiency of an adsorbed oxygen-tolerant hydrogenase[J]. PLoS One, 2015, 10(11): e0143101. |
| 46 | SHIRAIWA S, SO K, SUGIMOTO Y, et al. Reactivation of standard [NiFe]-hydrogenase and bioelectrochemical catalysis of proton reduction and hydrogen oxidation in a mediated-electron-transfer system[J]. Bioelectrochemistry, 2018, 123: 156-161. |
| 47 | RADU V, FRIELINGSDORF S, EVANS S D, et al. Enhanced oxygen-tolerance of the full heterotrimeric membrane-bound [NiFe]-hydrogenase of Ralstonia eutropha [J]. Journal of the American Chemical Society, 2014, 136(24): 8512-8515. |
| 48 | LUO X J, BRUGNA M, TRON-INFOSSI P, et al. Immobilization of the hyperthermophilic hydrogenase from Aquifex aeolicus bacterium onto gold and carbon nanotube electrodes for efficient H2 oxidation[J]. Journal of Biological Inorganic Chemistry, 2009, 14(8): 1275-1288. |
| 49 | OUGHLI A A, VÉLEZ M, BIRRELL J A, et al. Viologen-modified electrodes for protection of hydrogenases from high potential inactivation while performing H2 oxidation at low overpotential[J]. Dalton Transactions, 2018, 47(31): 10685-10691. |
| 50 | ALONSO-LOMILLO M A, RÜDIGER O, MAROTO-VALIENTE A, et al. Hydrogenase-coated carbon nanotubes for efficient H2 oxidation[J]. Nano Letters, 2007, 7(6): 1603-1608. |
| 51 | LOJOU É, LUO X, BRUGNA M, et al. Biocatalysts for fuel cells: efficient hydrogenase orientation for H2 oxidation at electrodes modified with carbon nanotubes[J]. Journal of Biological Inorganic Chemistry, 2008, 13(7): 1157-1167. |
| 52 | MONSALVE K, ROGER M, GUTIERREZ-SANCHEZ C, et al. Hydrogen bioelectrooxidation on gold nanoparticle-based electrodes modified by Aquifex aeolicus hydrogenase: application to hydrogen/oxygen enzymatic biofuel cells[J]. Bioelectrochemistry, 2015, 106(Pt A): 47-55. |
| 53 | HOEBEN F J M, MEIJER F S, DEKKER C, et al. Toward single-enzyme molecule electrochemistry: [NiFe]-hydrogenase protein film voltammetry at nanoelectrodes[J]. ACS Nano, 2008, 2(12): 2497-2504. |
| 54 | ZHANG L Y, MORELLO G, CARR S B, et al. Aerobic photocatalytic H2 production by a [NiFe] hydrogenase engineered to place a silver nanocluster in the electron relay[J]. Journal of the American Chemical Society, 2020, 142(29): 12699-12707. |
| 55 | NIKANDROV V V, SHLYK M A, ZORIN N A, et al. Efficient photoinduced electron transfer from inorganic semiconductor TiO2 to bacterial hydrogenase[J]. FEBS Letters, 1988, 234(1): 111-114. |
| 56 | CAPUTO C A, WANG L D, BERANEK R, et al. Carbon nitride-TiO2 hybrid modified with hydrogenase for visible light driven hydrogen production[J]. Chemical Science, 2015, 6(10): 5690-5694. |
| 57 | REISNER E, POWELL D J, CAVAZZA C, et al. Visible light-driven H2 production by hydrogenases attached to dye-sensitized TiO2 nanoparticles[J]. Journal of the American Chemical Society, 2009, 131(51): 18457-18466. |
| 58 | MORRA S, VALETTI F, SADEGHI S J, et al. Direct electrochemistry of an [FeFe]-hydrogenase on a TiO2 electrode[J]. Chemical Communications, 2011, 47(38): 10566-10568. |
| 59 | EDWARDES MOORE E, ANDREI V, ZACARIAS S, et al. Integration of a hydrogenase in a lead halide perovskite photoelectrode for tandem solar water splitting[J]. ACS Energy Letters, 2020, 5(1): 232-237. |
| 60 | ASAKURA N. Photoinduced hydrogen evolution with Ni-Fe hydrogenase-viologen-porphyrin immobilized ITO electrode[J]. ECS Meeting Abstracts, 2020, MA2020-02(44): 2807. |
| 61 | MERSCH D, LEE C Y, ZHANG J Z, et al. Wiring of photosystem Ⅱ to hydrogenase for photoelectrochemical water splitting[J]. Journal of the American Chemical Society, 2015, 137(26): 8541-8549. |
| 62 | HARRIS T G A A, HEIDARY N, FRIELINGSDORF S, et al. Electrografted interfaces on metal oxide electrodes for enzyme immobilization and bioelectrocatalysis[J]. ChemElectroChem, 2021, 8(7): 1329-1336. |
| 63 | CAPUTO C A, GROSS M A, LAU V W, et al. Photocatalytic hydrogen production using polymeric carbon nitride with a hydrogenase and a bioinspired synthetic Ni catalyst[J]. Angewandte Chemie, 2014, 53(43): 11538-11542. |
| 64 | BROWN K A, WILKER M B, BOEHM M, et al. Characterization of photochemical processes for H2 production by CdS nanorod-[FeFe] hydrogenase complexes[J]. Journal of the American Chemical Society, 2012, 134(12): 5627-5636. |
| 65 | WILKER M B, SHINOPOULOS K E, BROWN K A, et al. Electron transfer kinetics in CdS nanorod-[FeFe]-hydrogenase complexes and implications for photochemical H2 generation[J]. Journal of the American Chemical Society, 2014, 136(11): 4316-4324. |
| 66 | BROWN K A, DAYAL S, AI X, et al. Controlled assembly of hydrogenase-CdTe nanocrystal hybrids for solar hydrogen production[J]. Journal of the American Chemical Society, 2010, 132(28): 9672-9680. |
| 67 | GREENE B L, JOSEPH C A, MARONEY M J, et al. Direct evidence of active-site reduction and photodriven catalysis in sensitized hydrogenase assemblies[J]. Journal of the American Chemical Society, 2012, 134(27): 11108-11111. |
| 68 | TAPIA C, ZACARIAS S, PEREIRA I A C, et al. In situ determination of photobioproduction of H2 by In2S3-[NiFeSe] hydrogenase from Desulfovibrio vulgaris hildenborough using only visible light[J]. ACS Catalysis, 2016, 6(9): 5691-5698. |
| 69 | LUNA-LÓPEZ G, DEL BARRIO M, FIZE J, et al. Photobio-electrocatalytic production of H2 using fluorine-doped tin oxide (FTO) electrodes covered with a NiO-In2S3 p-n junction and NiFeSe hydrogenase[J]. Bioelectrochemistry, 2023, 150: 108361. |
| 70 | CIACCAFAVA A, INFOSSI P, ILBERT M, et al. Electrochemistry, AFM, and PM-IRRA spectroscopy of immobilized hydrogenase: role of a hydrophobic helix in enzyme orientation for efficient H2 oxidation[J]. Angewandte Chemie International Edition, 2012, 51(4): 953-956. |
| 71 | GUTIÉRREZ-SÁNCHEZ C, OLEA D, MARQUES M, et al. Oriented immobilization of a membrane-bound hydrogenase onto an electrode for direct electron transfer[J]. Langmuir, 2011, 27(10): 6449-6457. |
| 72 | UTESCH T, MILLO D, CASTRO M A, et al. Effect of the protonation degree of a self-assembled monolayer on the immobilization dynamics of a [NiFe] hydrogenase[J]. Langmuir, 2013, 29(2): 673-682. |
| 73 | KRASSEN H, STRIPP S, VON ABENDROTH G, et al. Immobilization of the [FeFe]-hydrogenase CrHydAl on a gold electrode: design of a catalytic surface for the production of molecular hydrogen[J]. Journal of Biotechnology, 2009, 142(1): 3-9. |
| 74 | HARRIS T G A A, HEIDARY N, KOZUCH J, et al. In situ spectroelectrochemical studies into the formation and stability of robust diazonium-derived interfaces on gold electrodes for the immobilization of an oxygen-tolerant hydrogenase[J]. ACS Applied Materials & Interfaces, 2018, 10(27): 23380-23391. |
| 75 | SEZER M, FRIELINGSDORF S, MILLO D, et al. Role of the HoxZ subunit in the electron transfer pathway of the membrane-bound [NiFe]-hydrogenase from Ralstonia eutropha immobilized on electrodes[J]. The Journal of Physical Chemistry B, 2011, 115(34): 10368-10374. |
| 76 | LIU X, RISBAKK S, ALMEIDA CARVALHO P, et al. Immobilization of FeFe-hydrogenase on black TiO2 nanotubes as biocathodes for the hydrogen evolution reaction[J]. Electrochemistry Communications, 2022, 135: 107221. |
| 77 | DAVIS V, HEIDARY N, GUIET A, et al. Immobilization of O2-tolerant [NiFe] hydrogenase from Cupriavidus necator on tin-rich indium oxide alters the catalytic bias from H2 oxidation to proton reduction[J]. ACS Catalysis, 2023, 13(9): 6312-6327. |
| 78 | JANG Y J, LEE J S. Photoelectrochemical water splitting with p-type metal oxide semiconductor photocathodes[J]. ChemSusChem, 2019, 12(9): 1835-1845. |
| 79 | TIAN L, NÉMETH B, BERGGREN G, et al. Hydrogen evolution by a photoelectrochemical cell based on a Cu2O-ZnO-[FeFe] hydrogenase electrode[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2018, 366: 27-33. |
| 80 | LEE C Y, PARK H S, FONTECILLA-CAMPS J C, et al. Photoelectrochemical H2 evolution with a hydrogenase immobilized on a TiO2-protected silicon electrode[J]. Angewandte Chemie International Edition, 2016, 55(20): 5971-5974. |
| 81 | LUNA-LÓPEZ G, SAINZ R, COITO A M, et al. Hybrid biological/inorganic photocathode for H2 production based on a NiFeSe hydrogenase immobilized on electrodeposited CuGaS2[J]. Catalysis Today, 2023, 423: 114281. |
| 82 | LYU X Y, GONZALEZ R, HORTON A, et al. Immobilization of enzymes by polymeric materials[J]. Catalysts, 2021, 11(10): 1211. |
| 83 | NOSAKA Y, KUWABARA A, KOBAYASHI T, et al. Immobilization of hydrogenase in nylon gel containing electron mediator and application to an artificial photosystem[J]. Biotechnology and Bioengineering, 1986, 28(3): 456-460. |
| 84 | ELGREN T E, ZADVORNY O A, BRECHT E, et al. Immobilization of active hydrogenases by encapsulation in polymeric porous gels[J]. Nano Letters, 2005, 5(10): 2085-2087. |
| 85 | ZIOMEK E, MARTIN W G, VELIKY I A, et al. Immobilization of Desulphovibrio desulphuricans: cell-associated hydrogenase in beaded matrices[J]. Enzyme and Microbial Technology, 1982, 4(6): 405-408. |
| 86 | BARRETO M C, CABRAL J M S. Immobilization of Desulfovibrio vulgaris cells with hydrogenase activity[J]. Journal of Chemical Technology & Biotechnology, 1991, 50(4): 563-570. |
| 87 | HERR N, RATZKA J, LAUTERBACH L, et al. Stability enhancement of an O2 - tolerant NAD+-reducing [NiFe]- hydrogenase by a combination of immobilisation and chemical modification[J]. Journal of Molecular Catalysis B: Enzymatic, 2013, 97: 169-174. |
| 88 | KLIBANOV A M, KAPLAN N O, KAMEN M D. Thermal stabilities of membrane-bound, solubilized, and artificially immobilized hydrogenase from Chromatium vinosum [J]. Archives of Biochemistry and Biophysics, 1980, 199(2): 545-549. |
| 89 | LI H G, MÜNCHBERG U, OUGHLI A A, et al. Suppressing hydrogen peroxide generation to achieve oxygen-insensitivity of a [NiFe] hydrogenase in redox active films[J]. Nature Communications, 2020, 11(1): 920. |
| 90 | RUTH J C, MILTON R D, GU W Y, et al. Enhanced electrosynthetic hydrogen evolution by hydrogenases embedded in a redox-active hydrogel[J]. Chemistry, 2020, 26(32): 7323-7329. |
| 91 | TAPIA C, MILTON R D, PANKRATOVA G, et al. Wiring of photosystemⅠand hydrogenase on an electrode for photoelectrochemical H2 production by using redox polymers for relatively positive onset potential[J]. ChemElectroChem, 2017, 4(1): 90-95. |
| 92 | KALMS J, SCHMIDT A, FRIELINGSDORF S, et al. Tracking the route of molecular oxygen in O2-tolerant membrane-bound [NiFe] hydrogenase[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(10): E2229-E2237. |
| 93 | SHAKERI F, ARIAEENEJAD S, GHOLLASI M, et al. Synthesis of two novel bio-based hydrogels using sodium alginate and chitosan and their proficiency in physical immobilization of enzymes[J]. Scientific Reports, 2022, 12(1): 2072. |
| 94 | PLUMERÉ N, RÜDIGER O, OUGHLI A A, et al. A redox hydrogel protects hydrogenase from high-potential deactivation and oxygen damage[J]. Nature Chemistry, 2014, 6(9): 822-827. |
| 95 | RENGARAJ S, HADDAD R, LOJOU E, et al. Interprotein electron transfer between FeS-protein nanowires and oxygen-tolerant NiFe hydrogenase[J]. Angewandte Chemie International Edition, 2017, 56(27): 7774-7778. |
| 96 | OUGHLI A A, HARDT S, RÜDIGER O, et al. Reactivation of sulfide-protected [FeFe] hydrogenase in a redox-active hydrogel[J]. Chemical Communications, 2020, 56(69): 9958-9961. |
| 97 | MUKOYOSHI M, KITAGAWA H. Nanoparticle/metal-organic framework hybrid catalysts: elucidating the role of the MOF[J]. Chemical Communications, 2022, 58(77): 10757-10767. |
| 98 | HU Y L, DAI L M, LIU D H, et al. Progress & prospect of metal-organic frameworks (MOFs) for enzyme immobilization (enzyme/MOFs)[J]. Renewable & Sustainable Energy Reviews, 2018, 91: 793-801. |
| 99 | PULLEN S, ROY S, OTT S. [FeFe] Hydrogenase active site model chemistry in a UiO-66 metal-organic framework[J]. Chemical Communications, 2017, 53(37): 5227-5230. |
| 100 | WANG W J, SONG X W, HONG Z X, et al. Incorporation of iron hydrogenase active sites into a stable photosensitizing metal-organic framework for enhanced hydrogen production[J]. Applied Catalysis B: Environmental, 2019, 258: 117979. |
| 101 | CASTNER A T, JOHNSON B A, COHEN S M, et al. Mimicking the electron transport chain and active site of [FeFe] hydrogenases in one metal-organic framework: factors that influence charge transport[J]. Journal of the American Chemical Society, 2021, 143(21): 7991-7999. |
| 102 | BALESTRI D, ROUX Y, MATTAROZZI M, et al. Heterogenization of a [NiFe] hydrogenase mimic through simple and efficient encapsulation into a mesoporous MOF[J]. Inorganic Chemistry, 2017, 56(24): 14801-14808. |
| 103 | WU X L, GE J, YANG C, et al. Facile synthesis of multiple enzyme-containing metal-organic frameworks in a biomolecule-friendly environment[J]. Chemical Communications, 2015, 51(69): 13408-13411. |
| 104 | FERNANDEZ-BARTOLOME E, SANTOS J, KHODABAKHSHI S, et al. A robust and unique iron(ⅱ) mosaic-like MOF[J]. Chemical Communications, 2018, 54(44): 5526-5529. |
| 105 | AHMED M. Recent advancement in bimetallic metal organic frameworks (M’MOFs): synthetic challenges and applications[J]. Inorganic Chemistry Frontiers, 2022, 9(12): 3003-3033. |
| 106 | SHEN H, SHI H M, YANG Y, et al. Highly efficient synergistic biocatalysis driven by stably loaded enzymes within hierarchically porous iron/cobalt metal-organic framework via biomimetic mineralization[J]. Journal of Materials Chemistry B, 2022, 10(10): 1553-1560. |
| 107 | DU C, ZHOU Y L, LIU L, et al. Bacterial surface-assembled chitinosome for dismantling chitin into N-acetyl glucosamine[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(30): 11239-11247. |
| 108 | ZHOU C, HE N S, LIN X F, et al. Site-directed display of zearalenone lactonase on spilt-intein functionalized nanocarrier for green and efficient detoxification of Zearalenone[J]. Food Chemistry, 2024, 446: 138804. |
| [1] | DONG Lingling, LI Feixuan, LEI Hangbin, SONG Qidi, WANG Shizhen. Biomimetic compartmentalization immobilization of multi-enzyme system [J]. Synthetic Biology Journal, 2024, 5(6): 1518-1529. |
| [2] | CUI Xinyu, WU Ranran, WANG Yuanming, ZHU Zhiguang. Construction and enhancement of enzymatic bioelectrocatalytic systems [J]. Synthetic Biology Journal, 2022, 3(5): 1006-1030. |
| [3] | YOU Zixuan, LI Feng, SONG Hao. Design and construction of electroactive cells by synthetic biology strategies [J]. Synthetic Biology Journal, 2022, 3(5): 1031-1059. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||