Recent advances in genetically encoded fluorescent sensors for disease diagnosis
Li Rui1,2,3, Zuo Fangting1,2,4, Yang Yi1,2
- 1.Interdisciplinary Research Center of Optogenetics and Synthetic Biology,State Key Laboratory of Bioreactor Engineering,East China University of Science and Technology,Shanghai 200237,China
2.Shanghai Advanced Research Base of Cell Metabolism Genetics,School of Pharmacy,East China University of Science and Technology,Shanghai 200237,China
3.Shenzhen Hospital,Southern Medical University,Shenzhen 518110,China
4.Yangpu Hospital,Tongji University,shanghai 200090,China.
-
Received:2025-05-13Revised:2025-08-28Published:2025-08-29 -
Contact:Yang Yi
遗传编码荧光探针在疾病诊断中的最新进展
李睿1,2,3, 左方婷1,2,4, 杨弋1,2
- 1.华东理工大学光遗传学与合成生物学跨学科研究中心,生物反应器工程国家重点实验室,中国 上海 200237
2.华东理工大学药学院,上海市细胞代谢光遗传学技术前沿科学研究基地,中国 上海 200237
3.南方医科大学深圳医院,中国 深圳 518110
4.同济大学附属杨浦医院,中国 上海 200090
-
通讯作者:杨弋 -
作者简介:李睿 (1996—),女,博士研究生。研究方向合成生物技术与生物传感技术结合在监测细胞内实时动态代谢过程。E-mail:y20180020@mail.ecust.edu.cn杨弋 (1973—),男,博士生导师,主要研究对象为利用合成生物技术与光遗传学技术控制与监测细胞内分子过程的前沿技术;癌症及代谢类疾病药理及药物筛选技术;蛋白质特异性标记、翻译后修饰的鉴定、与细胞内原位成像;蛋白质药物生产技术等。E-mail:yiyang@ecust.edu.cn -
基金资助:国家重点研发计划“基因表达时空精准操控技术研究”(2022YFC3400100);国家自然科学基金-创新研究群体项目“细胞代谢监测与调控”(32121005);上海市青年科技英才扬帆计划“近红外荧光RNA的开发与应用研究”(24YF2709300);博士后创新人才支持计划“基于新型胆汁酸生物传感器在肠道菌群中时空动态监测与调控”
CLC Number:
Cite this article
Li Rui, Zuo Fangting, Yang Yi. Recent advances in genetically encoded fluorescent sensors for disease diagnosis[J]. Synthetic Biology Journal, DOI: 10.12211/2096-8280.2025-045.
李睿, 左方婷, 杨弋. 遗传编码荧光探针在疾病诊断中的最新进展[J]. 合成生物学, DOI: 10.12211/2096-8280.2025-045.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2025-045
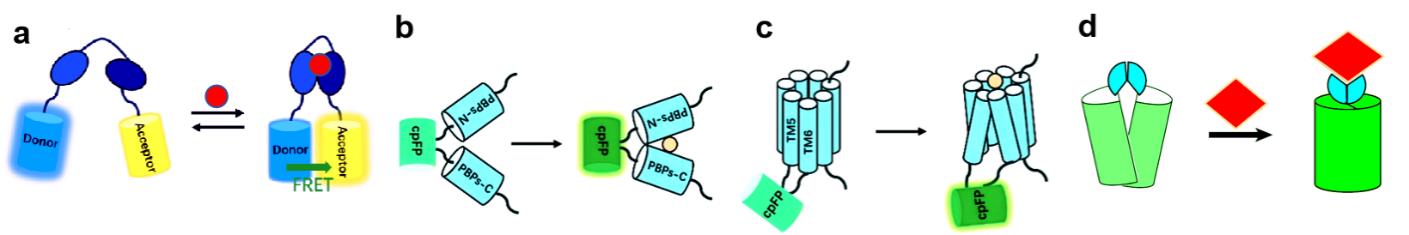
Fig.1 Design and construction of genetically encoded fluorescent sensors(a) Diagram of genetically encoded fluorescent sensor based on FRET principle. (b) PBP-based genetically encoded indicators, following substrate binding, conformational changes of PBPs will change fluorescence of cpFP. (c) GPCRs-based genetically encoded indicators, cpFP is inserted into the intracellular loop of GPCRs between transmembrane domains 5 and 6, following substrate binding, conformational changes of GPCRs will change fluorescence of cpFP[6]. (d) ATOM-based genetically encoded fluorescence indicators, ligand is binded to fluorescence protein domain, restored its natural conformation, and matured the chromophore[5].
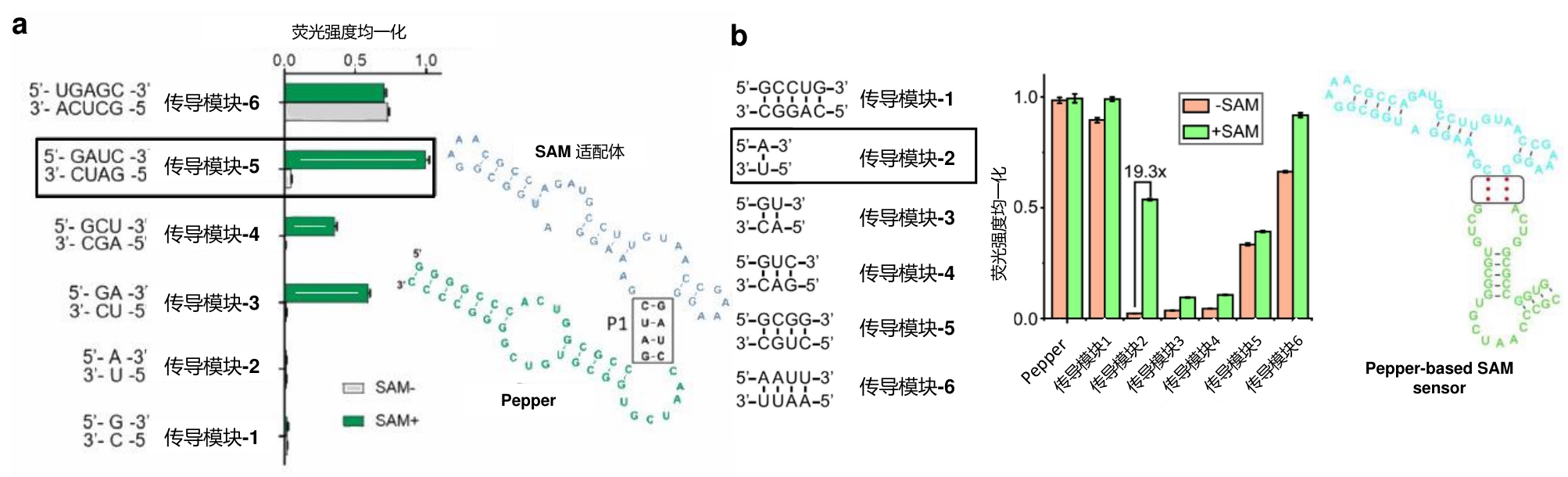
Fig.3 RNA sensors for detecting SAM(a) Design principle of Pepper-SAM-C14U sensor and its response to SAM[17]. (b) Design principle of ratiometric SAM sensor based on Pepper and RhoBAST and its response to SAM[18].
| [1] | WANG X, DING Q, GROLEAU R R, et al. Fluorescent Probes for Disease Diagnosis [J]. Chemical Reviews, 2024, 124(11): 7106-64. |
| [2] | GHOUNEIMY A, MAHAS A, MARSIC T, et al. CRISPR-Based Diagnostics: Challenges and Potential Solutions toward Point-of-Care Applications [J]. ACS Synth Biol, 2023, 12(1): 1-16. |
| [3] | HAN Y, YANG J, LI Y, et al. Bright and sensitive red voltage indicators for imaging action potentials in brain slices and pancreatic islets [J]. Sci Adv, 2023, 9(47): eadi4208. |
| [4] | ZHANG Y, RóZSA M, LIANG Y, et al. Fast and sensitive GCaMP calcium indicators for imaging neural populations [J]. Nature, 2023, 615(7954): 884-91. |
| [5] | SEKHON H, HA J H, PRESTI M F, et al. Adaptable, turn-on maturation (ATOM) fluorescent biosensors for multiplexed detection in cells [J]. Nature Methods, 2023, 20(12): 1920-9. |
| [6] | WU Z, LIN D, LI Y. Pushing the frontiers: tools for monitoring neurotransmitters and neuromodulators [J]. Nat Rev Neurosci, 2022, 23(5): 257-74. |
| [7] | CHANG H, CLEMENS S, GAO P, et al. Fluorogenic Rhodamine-Based Chemigenetic Biosensor for Monitoring Cellular NADPH Dynamics [J]. Journal of the American Chemical Society, 2024, 146(30): 20569-76. |
| [8] | HU L, CAO W, JIANG Y, et al. Designing artificial fluorescent proteins and biosensors by genetically encoding molecular rotor-based amino acids [J]. Nature Chemistry, 2024, 16(12): 1960-71. |
| [9] | LU S, HOU Y, ZHANG X E, et al. Live cell imaging of DNA and RNA with fluorescent signal amplification and background reduction techniques [J]. Front Cell Dev Biol, 2023, 11: 1216232. |
| [10] | SONG Q, TAI X, REN Q, et al. Structure-based insights into fluorogenic RNA aptamers [J]. Acta Biochim Biophys Sin (Shanghai), 2024, 57(1): 108-18. |
| [11] | GAO W K C Z Y, RONG X X, ET AL. . Progress in RNA dynamic imaging technology in live cells [J]. SCIENTIA SINICA Vitae, 2024, 54(4): 651-67. |
| [12] | ZUO F T, ZHANG Y Q, YANG H M, et al. Progress on fluorescent RNA and fluorescent RNA-based biosensing technology [J]. Yi Chuan, 2024, 46(2): 92-108. |
| [13] | LU X, KONG K Y S, UNRAU P J. Harmonizing the growing fluorogenic RNA aptamer toolbox for RNA detection and imaging [J]. Chemical Society Reviews, 2023, 52(12): 4071-98. |
| [14] | HUANG K, CHEN X, LI C, et al. Structure-based investigation of fluorogenic Pepper aptamer [J]. Nature Chemical Biology, 2021, 17(12): 1289-95. |
| [15] | WANG Q, XIAO F, SU H, et al. Inert Pepper aptamer-mediated endogenous mRNA recognition and imaging in living cells [J]. Nucleic Acids Res, 2022, 50(14): e84. |
| [16] | ZHENG H, LIU X, LIU L, et al. Imaging of endogenous RNA in live cells using sequence-activated fluorescent RNA probes [J]. Nucleic Acids Res, 2024. |
| [17] | FANG M, LI H, XIE X, et al. Imaging intracellular metabolite and protein changes in live mammalian cells with bright fluorescent RNA-based genetically encoded sensors [J]. Biosens Bioelectron, 2023, 235: 115411. |
| [18] | CHEN Z, CHEN W, REHEMAN Z, et al. Genetically encoded RNA-based sensors with Pepper fluorogenic aptamer [J]. Nucleic Acids Research, 2023, 51(16): 8322-36. |
| [19] | JIANG L, XIE X, SU N, et al. Large Stokes shift fluorescent RNAs for dual-emission fluorescence and bioluminescence imaging in live cells [J]. Nature Methods, 2023, 20(10): 1563-72. |
| [20] | ZUO F, JIANG L, SU N, et al. Imaging the dynamics of messenger RNA with a bright and stable green fluorescent RNA [J]. Nature Chemical Biology, 2024, 20(10): 1272-81. |
| [21] | JIANG L, ZUO F, PAN Y, et al. Bright and Stable Cyan Fluorescent RNA Enables Multicolor RNA Imaging in Live Escherichia coli [J]. Small, 2025, 21(9): e2405165. |
| [22] | CHEN Z, CHEN W, XU C, et al. Near-infrared fluorogenic RNA for in vivo imaging and sensing [J]. Nature Communications, 2025, 16(1): 518. |
| [23] | YIN P, HUANG C, ZHANG L, et al. Developing Orthogonal Fluorescent RNAs for Photoactive Dual-Color Imaging of RNAs in Live Cells [J]. Angewandte Chemie International Edition, 2025, n/a(n/a): e202424060. |
| [24] | HOU J, GUO P, WANG J, et al. Artificial dynamic structure ensemble-guided rational design of a universal RNA aptamer-based sensing tag [J]. Proceedings of the National Academy of Sciences of the United States of America, 2024, 121(52): e2414793121. |
| [25] | WONG F, HE D, KRISHNAN A, et al. Deep generative design of RNA aptamers using structural predictions [J]. Nature Computational Science, 2024, 4(11): 829-39. |
| [26] | WU Z, CUI Y, WANG H, et al. Neuronal activity-induced, equilibrative nucleoside transporter-dependent, somatodendritic adenosine release revealed by a GRAB sensor [J]. Proceedings of the National Academy of Sciences of the United States of America, 2023, 120(14): e2212387120. |
| [27] | ZHUO Y, LUO B, YI X, et al. Improved green and red GRAB sensors for monitoring dopaminergic activity in vivo [J]. Nature Methods, 2024, 21(4): 680-91. |
| [28] | ZENG J, LI X, ZHANG R, et al. Local 5-HT signaling bi-directionally regulates the coincidence time window for associative learning [J]. Neuron, 2023, 111(7): 1118-35.e5. |
| [29] | DENG F, WAN J, LI G, et al. Improved green and red GRAB sensors for monitoring spatiotemporal serotonin release in vivo [J]. Nature Methods, 2024, 21(4): 692-702. |
| [30] | TOUHARA K K, ROSSEN N D, DENG F, et al. Topological segregation of stress sensors along the gut crypt–villus axis [J]. Nature, 2025. |
| [31] | QIAN T, WANG H, WANG P, et al. A genetically encoded sensor measures temporal oxytocin release from different neuronal compartments [J]. Nature Biotechnology, 2023, 41(7): 944-57. |
| [32] | FENG J, DONG H, LISCHINSKY J E, et al. Monitoring norepinephrine release in vivo using next-generation GRAB(NE) sensors [J]. Neuron, 2024, 112(12): 1930-42.e6. |
| [33] | WANG H, QIAN T, ZHAO Y, et al. A tool kit of highly selective and sensitive genetically encoded neuropeptide sensors [J]. Science, 2023, 382(6672): eabq8173. |
| [34] | XIA X, LI Y. A high-performance GRAB sensor reveals differences in the dynamics and molecular regulation between neuropeptide and neurotransmitter release [J]. Nature Communications, 2025, 16(1): 819. |
| [35] | WU T, KUMAR M, ZHANG J, et al. A genetically encoded far-red fluorescent indicator for imaging synaptically released Zn2+ [J]. Science Advances, 9(9): eadd2058. |
| [36] | YANG L, PATHIRANAGE V, ZHOU S, et al. Genetically Encoded Red Fluorescent Indicators for Imaging Intracellular and Extracellular Potassium Ions [J]. bioRxiv, 2024: 2024.12.20.629597. |
| [37] | LAI C, YANG L, PATHIRANAGE V, et al. Genetically encoded green fluorescent sensor for probing sulfate transport activity of solute carrier family 26 member a2 (Slc26a2) protein [J]. Communications Biology, 2024, 7(1): 1375. |
| [38] | RAHMAN T, PATEL S. Recent developments in probing the levels and flux of selected organellar cations as well as organellar mechanosensitivity [J]. Current Opinion in Chemical Biology, 2025, 87: 102600. |
| [39] | MARVIN J S, KOKOTOS A C, KUMAR M, et al. iATPSnFR2: A high-dynamic-range fluorescent sensor for monitoring intracellular ATP [J]. Proceedings of the National Academy of Sciences, 2024, 121(21): e2314604121. |
| [40] | WANG K, CHEN T-L, ZHANG X-X, et al. Unveiling tryptophan dynamics and functions across model organisms via quantitative imaging [J]. BMC Biology, 2024, 22(1): 258. |
| [41] | LIU B, ZHAO Z, WANG P, et al. GlutaR: A High‐Performance Fluorescent Protein-Based Sensor for Spatiotemporal Monitoring of Glutamine Dynamics In Vivo [J]. Angewandte Chemie International Edition, 2024. |
| [42] | LI X, ZHANG Y, XU L, et al. Ultrasensitive sensors reveal the spatiotemporal landscape of lactate metabolism in physiology and disease [J]. Cell Metab, 2023, 35(1): 200-11 e9. |
| [43] | LI R, LI Y, JIANG K, et al. Lighting up arginine metabolism reveals its functional diversity in physiology and pathology [J]. Cell Metabolism, 2025, 37(1): 291-304.e9. |
| [44] | HUANG D, ZHANG C, XIAO M, et al. Redox metabolism maintains the leukemogenic capacity and drug resistance of AML cells [J]. Proceedings of the National Academy of Sciences of the United States of America, 2023, 120(13): e2210796120. |
| [45] | CHANDRASEKHARAN A, TIWARI S K, MUNIRPASHA H A, et al. Genetically encoded caspase sensor and RFP-LC3 for temporal analysis of apoptosis-autophagy [J]. International Journal of Biological Macromolecules, 2024, 257(Pt 2): 128807. |
| [46] | XU B, WANG Y, BAHRIZ S M F M, et al. Probing spatiotemporal PKA activity at the ryanodine receptor and SERCA2a nanodomains in cardomyocytes [J]. Cell Communication and Signaling, 2022, 20(1): 143. |
| [47] | ROMERO-SUAREZ D, WULFF T, RONG Y, et al. A Reporter System for Cytosolic Protein Aggregates in Yeast [J]. ACS Synth Biol, 2021, 10(3): 466-77. |
| [48] | LIU S, LIU J, FOOTE A, et al. Digital and Tunable Genetically Encoded Tension Sensors Based on Engineered Coiled-Coils [J]. Angewandte Chemie International Edition, 2025, 64(8): e202407359. |
| [49] | MOLNAR K, J-B MANNEVILLE. Emerging mechanobiology techniques to probe intracellular mechanics [J]. npj Biological Physics and Mechanics, 2025, 2(1): 12. |
| [50] | FREI M S, MEHTA S, ZHANG J. Next-Generation Genetically Encoded Fluorescent Biosensors Illuminate Cell Signaling and Metabolism [J]. Annual Review of Biophysics, 2024, 53(Volume 53, 2024): 275-97. |
| [51] | ZHOU L, YANG R, LI X, et al. COF-Coated Microelectrode for Space-Confined Electrochemical Sensing of Dopamine in Parkinson's Disease Model Mouse Brain [J]. Journal of the American Chemical Society, 2023, 145(43): 23727-38. |
| [52] | AGGARWAL A, LIU R, CHEN Y, et al. Glutamate indicators with improved activation kinetics and localization for imaging synaptic transmission [J]. Nature Methods, 2023, 20(6): 925-34. |
| [53] | JI D, WANG B, LO K W, et al. Pre-Defined Stem-Loop Structure Library for the Discovery of L-RNA Aptamers that Target RNA G-Quadruplexes [J]. Angewandte Chemie International Edition in English, 2025, 64(5): e202417247. |
| [54] | GUO S K, LIU C X, XU Y F, et al. Therapeutic application of circular RNA aptamers in a mouse model of psoriasis [J]. Nature Biotechnology, 2025, 43(2): 236-46. |
| [55] | DING D, ZHAO H, WEI D, et al. The First-in-Human Whole-Body Dynamic Pharmacokinetics Study of Aptamer [J]. Research (Wash D C), 2023, 6: 0126. |
| [1] | GAO Xianyun, NIU Lingxue, JIAN Ni, GUAN Ningzi. Applications of microbial synthetic biology in the diagnosis and treatment of diseases [J]. Synthetic Biology Journal, 2023, 4(2): 263-282. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||