Synthetic Biology Journal ›› 2021, Vol. 2 ›› Issue (5): 734-750.DOI: 10.12211/2096-8280.2021-056
• Invited Review • Previous Articles Next Articles
Biosynthesis of alkyne moiety in natural products and application of alkyne biosynthetic machineries
LYU Jianming1, ZHAO Huan2, HU Dan1, GAO Hao1
- 1.Institute of Traditional Chinese Medicine and Natural Products,College of Pharmacy,Jinan University,Guangzhou 510632,Guangdong,China
2.College of Traditional Chinese Medicine,Jinan University,Guangzhou 510632,Guangdong,China
-
Received:2021-05-07Revised:2021-07-25Online:2021-11-19Published:2021-10-31 -
Contact:HU Dan, GAO Hao
天然产物中炔基的生物合成机制研究及其应用
吕建明1, 赵欢2, 胡丹1, 高昊1
- 1.暨南大学药学院,中药及天然药物研究所,广东 广州 510632
2.暨南大学中医学院,广东 广州 510632
-
通讯作者:胡丹,高昊 -
作者简介:吕建明 (1985—),男,博士,副研究员。研究方向为真菌天然产物的生物合成。E-mail:ljm21@jnu.edu.cn胡丹 (1979—),男,博士,研究员。研究方向为天然产物的生物合成。E-mail:thudan@jnu.edu.cn高昊 (1979—),男,博士,教授。研究方向为天然药物化学。E-mail:tghao@jnu.edu.cn -
基金资助:国家重点研发计划(2018YFA0903200/2018YFA0903201)
CLC Number:
Cite this article
LYU Jianming, ZHAO Huan, HU Dan, GAO Hao. Biosynthesis of alkyne moiety in natural products and application of alkyne biosynthetic machineries[J]. Synthetic Biology Journal, 2021, 2(5): 734-750.
吕建明, 赵欢, 胡丹, 高昊. 天然产物中炔基的生物合成机制研究及其应用[J]. 合成生物学, 2021, 2(5): 734-750.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2021-056
| 序号 | 来源 | 乙炔酶名称与NCBI登录号 | 产物 | 文献 | |
|---|---|---|---|---|---|
| 1 | 植物 | Crepis alpina | Crep1 (CAA76158) |  | [ |
| 2 | Petroselinum crispum | PcACET (AAB80697) |  | [ | |
| 3 | Hedera helix | HhACET (AAO38031) |  | [ | |
| 4 | Helianthus annuus | HaACET (AAO38032) |  | [ | |
| 5 | Calendula officinalis | CoACET (CAB64256) |  | [ | |
| 6 | Daucus carota | DCAR_013552 (XP_017247320) |  | [ | |
| 7 | Daucus carota | DCAR_017011 (XP_017253817) |  | [ | |
| 8 | Daucus carota | DCAR_013548 (XP_017246930) |  | [ | |
| 9 | Solanum lycopersicum | ACET1a/b |  | [ | |
| 10 | Ceratodon purpureus | CpAcet6 (Q9LEN0) | 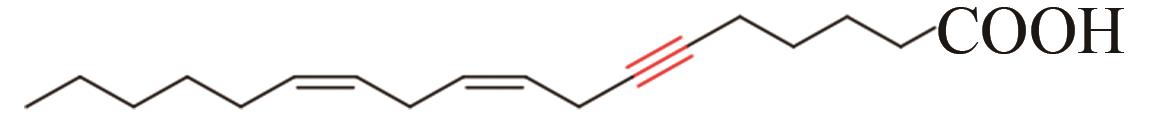 | [ | |
| 11 | Bidens pilosa | BPFAA (MF318525) | 未知 | [ | |
| 12 | 昆虫 | Thaumetopoea pityocampa | Tpi-PGFAD (ABO43722) | 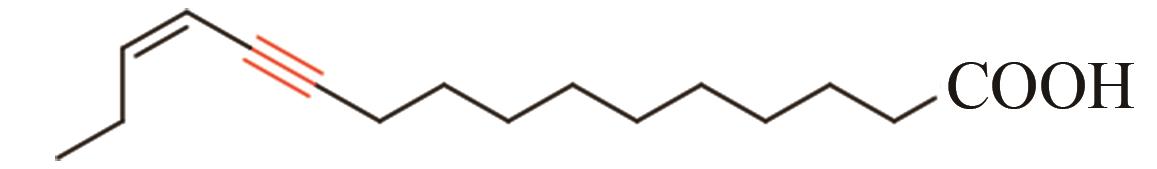 | [ |
| 13 | Chauliognathus lugubris | CL10 (AFJ66832) |  | [ | |
| 14 | Chauliognathus lugubris | CL2-1 (AFJ66828) | 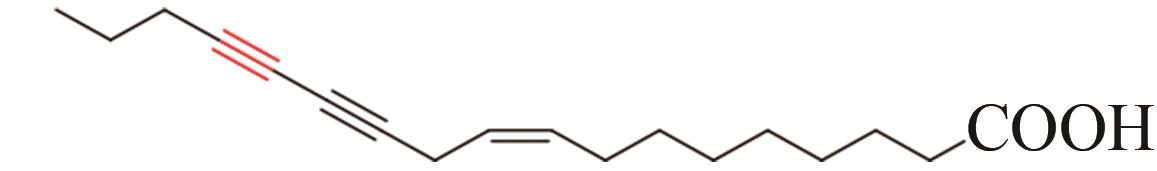 | [ | |
| 15 | Chauliognathus lugubris | CL2-2 (AFJ66829) | 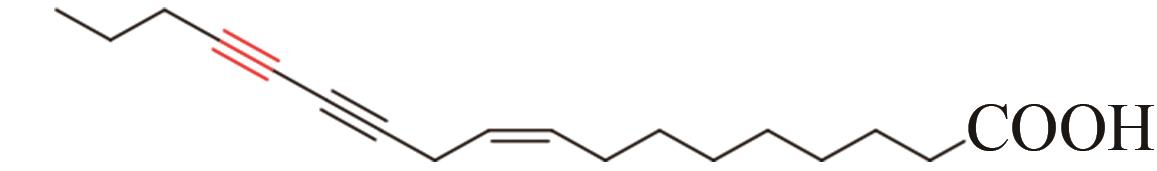 | [ | |
| 16 | 微生物 | Cantharellus formosus | CfACET (ADK24720) |  | [ |
| 17 | Burkholderia caryophylli | CayB (AIG53817) | 未知 | [ | |
| 18 | Burkholderia caryophylli | CayC (AIG53820) | 未知 | [ |
Tab. 1 Acetylenases identified for the biosynthesis of acetylenic fatty acids
| 序号 | 来源 | 乙炔酶名称与NCBI登录号 | 产物 | 文献 | |
|---|---|---|---|---|---|
| 1 | 植物 | Crepis alpina | Crep1 (CAA76158) |  | [ |
| 2 | Petroselinum crispum | PcACET (AAB80697) |  | [ | |
| 3 | Hedera helix | HhACET (AAO38031) |  | [ | |
| 4 | Helianthus annuus | HaACET (AAO38032) |  | [ | |
| 5 | Calendula officinalis | CoACET (CAB64256) |  | [ | |
| 6 | Daucus carota | DCAR_013552 (XP_017247320) |  | [ | |
| 7 | Daucus carota | DCAR_017011 (XP_017253817) |  | [ | |
| 8 | Daucus carota | DCAR_013548 (XP_017246930) |  | [ | |
| 9 | Solanum lycopersicum | ACET1a/b |  | [ | |
| 10 | Ceratodon purpureus | CpAcet6 (Q9LEN0) |  | [ | |
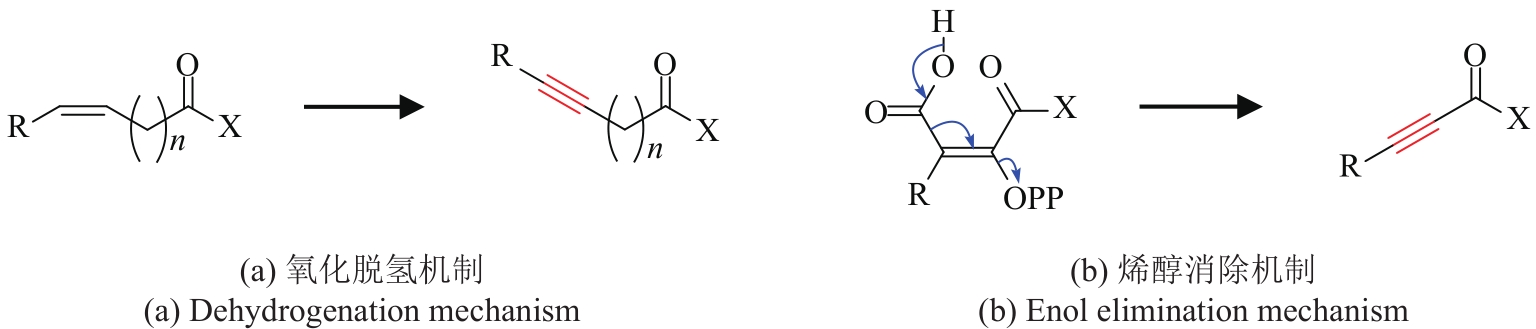
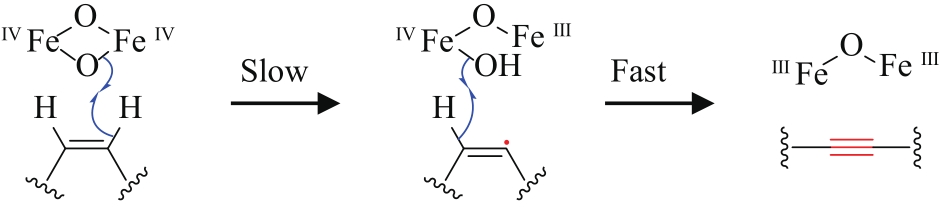
| 11 | Bidens pilosa | BPFAA (MF318525) | 未知 | [ | |
| 12 | 昆虫 | Thaumetopoea pityocampa | Tpi-PGFAD (ABO43722) |  | [ |
| 13 | Chauliognathus lugubris | CL10 (AFJ66832) |  | [ | |
| 14 | Chauliognathus lugubris | CL2-1 (AFJ66828) |  | [ | |
| 15 | Chauliognathus lugubris | CL2-2 (AFJ66829) |  | [ | |
| 16 | 微生物 | Cantharellus formosus | CfACET (ADK24720) |  | [ |
| 17 | Burkholderia caryophylli | CayB (AIG53817) | 未知 | [ | |
| 18 | Burkholderia caryophylli | CayC (AIG53820) | 未知 | [ |
| 1 | TALELE T T. Acetylene group, friend or foe in medicinal chemistry[J]. Journal of Medicinal Chemistry, 2020, 63(11): 5625-5663. |
| 2 | CHRISTIN-MAITRE S. History of oral contraceptive drugs and their use worldwide[J]. Best Practice & Research Clinical Endocrinology & Metabolism, 2013, 27(1): 3-12. |
| 3 | RAKHMANINA N Y, ANKER J N VAN DEN. Efavirenz in the therapy of HIV infection[J]. Expert Opinion on Drug Metabolism & Toxicology, 2010, 6(1): 95-103. |
| 4 | TAN F H, PUTOCZKI T L, STYLLI S S, et al. Ponatinib: a novel multi-tyrosine kinase inhibitor against human malignancies[J]. OncoTargets and Therapy, 2019, 12: 635-645. |
| 5 | JIRSCHELE K, SAND P K. Oxybutynin: past, present, and future[J]. International Urogynecology Journal, 2013, 24(4): 595-604. |
| 6 | SHEAR N H, VILLARS V V, MARSOLAIS C. Terbinafine: an oral and topical antifungal agent[J]. Clinics in Dermatology, 1991, 9(4): 487-495. |
| 7 | CHANDRARATNA R A S. Tazarotene: the first receptor-selective topical retinoid for the treatment of psoriasis[J]. Journal of the American Academy of Dermatology, 1997, 37(2 pt 3): S12-S17. |
| 8 | KUKLEV D V, DOMB A J, DEMBITSKY V M. Bioactive acetylenic metabolites[J]. Phytomedicine, 2013, 20(13): 1145-1159. |
| 9 | ZHOU Z F, MENNA M, CAI Y S, et al. Polyacetylenes of marine origin: Chemistry and bioactivity[J]. Chemical Reviews, 2015, 115(3): 1543-1596. |
| 10 | NEGRI R. Polyacetylenes from terrestrial plants and fungi: Recent phytochemical and biological advances[J]. Fitoterapia, 2015, 106: 92-109. |
| 11 | CHRISTENSEN L P. Bioactive C17 and C18 acetylenic oxylipins from terrestrial plants as potential lead compounds for anticancer drug development[J]. Molecules, 2020, 25(11): 2568. |
| 12 | DALY J W. Thirty years of discovering arthropod alkaloids in amphibian skin[J]. Journal of Natural Products, 1998, 61(1): 162-172. |
| 13 | CHRISTENSEN L P. Aliphatic C17-polyacetylenes of the falcarinol type as potential health promoting compounds in food plants of the Apiaceae family[J]. Recent Patents on Food, Nutrition & Agriculture, 2011, 3(1): 64-77. |
| 14 | DAWID C, DUNEMANN F, SCHWAB W, et al. Bioactive C17-polyacetylenes in carrots (Daucus carota L.): Current knowledge and future perspectives[J]. Journal of Agricultural and Food Chemistry, 2015, 63(42): 9211-9222. |
| 15 | NICOLAOU K C, CHEN J S, DALBY S M. From nature to the laboratory and into the clinic[J]. Bioorganic & Medicinal Chemistry, 2009, 17(6): 2290-2303. |
| 16 | SHAO R G. Pharmacology and therapeutic applications of enediyne antitumor antibiotics[J]. Current Molecular Pharmacology, 2008, 1(1): 50-60. |
| 17 | TYKWINSKI R R. Evolution in the palladium-catalyzed cross-coupling of sp-and sp2-hybridized carbon atoms[J]. Angewandte Chemie International Edition, 2003, 42(14): 1566-1568. |
| 18 | KOSCHKER P, BREIT B. Branching out: Rhodium-catalyzed allylation with alkynes and allenes[J]. Accounts of Chemical Research, 2016, 49(8): 1524-1536. |
| 19 | GODOI B, SCHUMACHER R F, ZENI G. Synthesis of heterocycles via electrophilic cyclization of alkynes containing heteroatom[J]. Chemical Reviews, 2011, 111(4): 2937-2980. |
| 20 | ZENG X M. Recent advances in catalytic sequential reactions involving hydroelement addition to carbon-carbon multiple bonds[J]. Chemical Reviews, 2013, 113(8): 6864-6900. |
| 21 | PATEL M, SAUNTHWAL R K, VERMA A K. Base-mediated hydroamination of alkynes[J]. Accounts of Chemical Research, 2017, 50(2): 240-254. |
| 22 | TROST B M, MASTERS J T. Transition metal-catalyzed couplings of alkynes to 1, 3-enynes: modern methods and synthetic applications[J]. Chemical Society Reviews, 2016, 45(8): 2212-2238. |
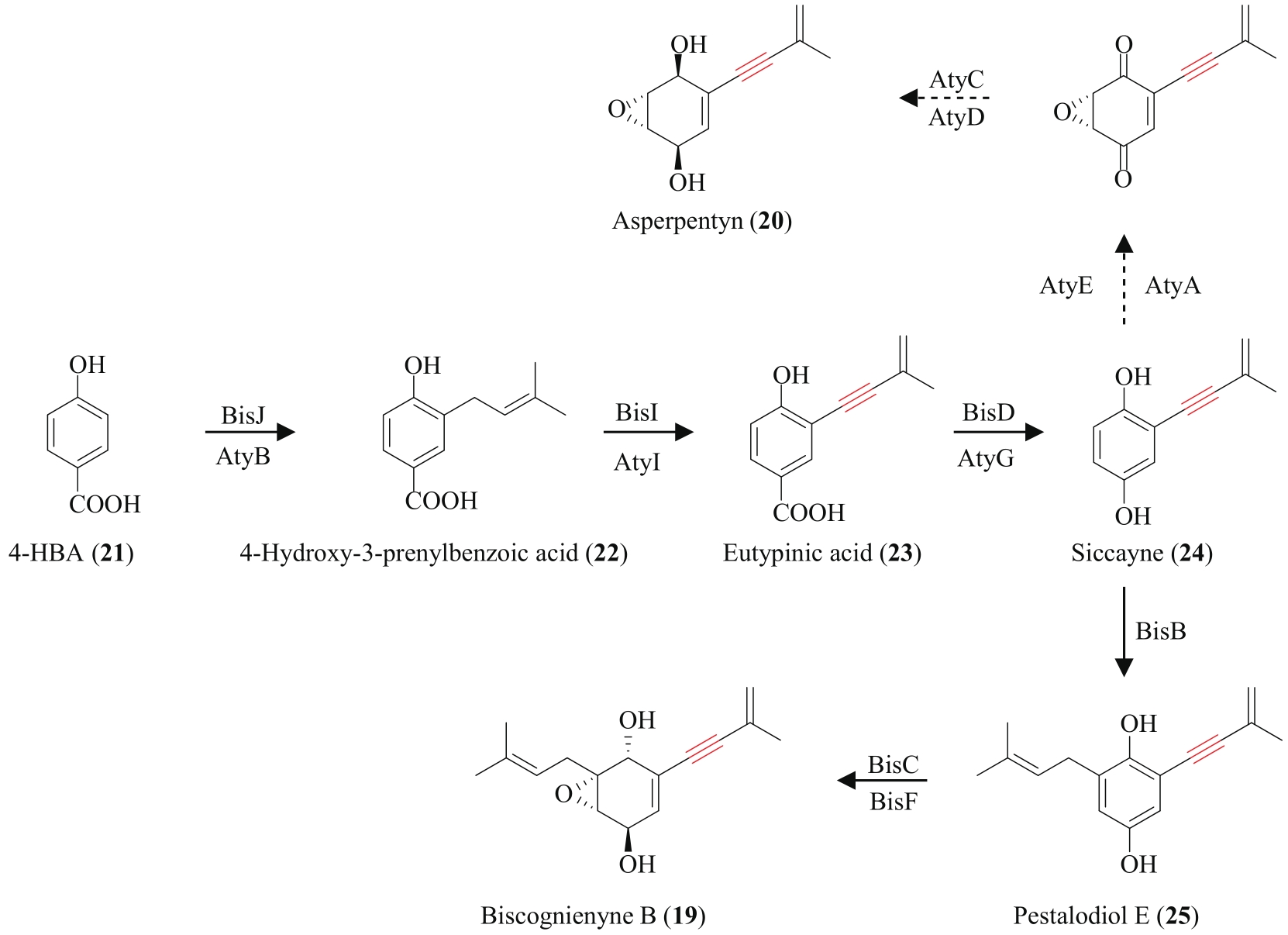
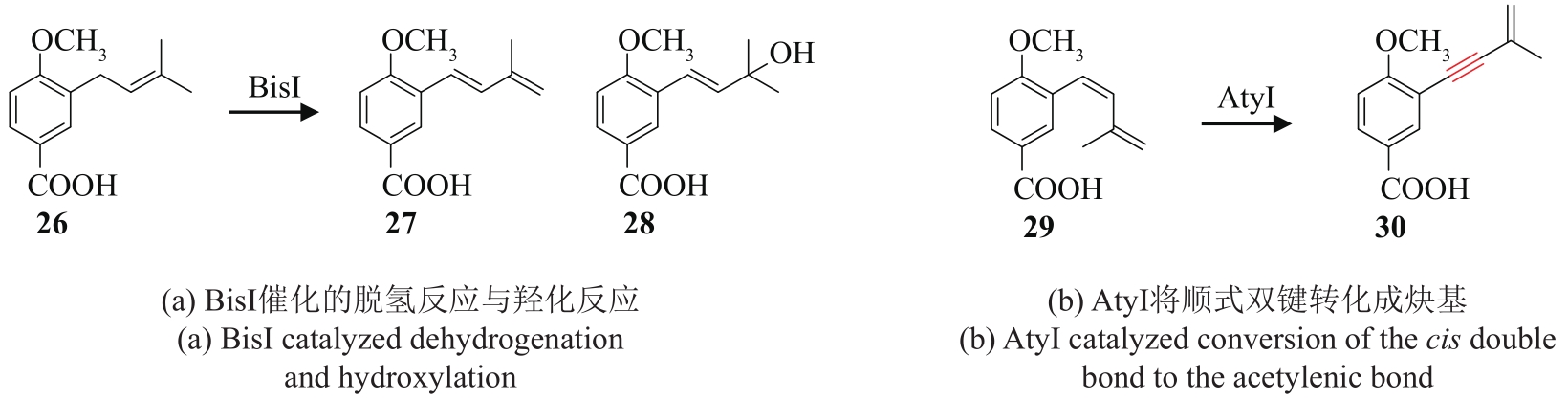
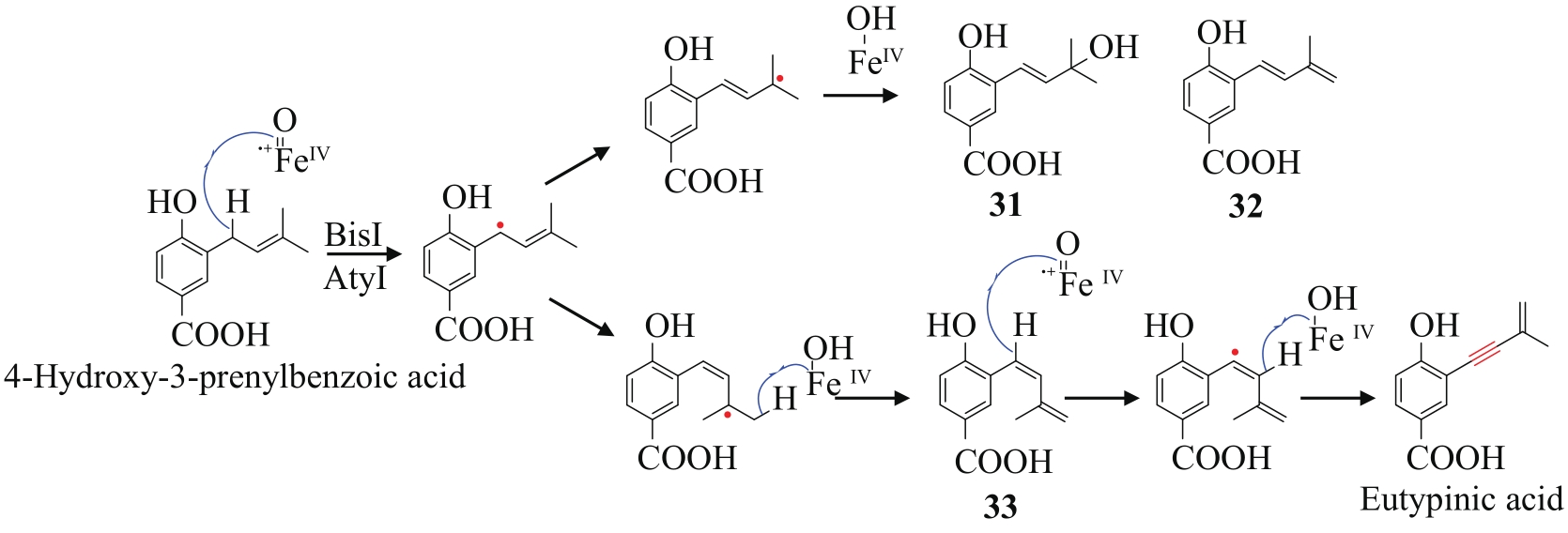
| 23 | TADROSS P M, STOLTZ B M. A comprehensive history of arynes in natural product total synthesis[J]. Chemical Reviews, 2012, 112(6): 3550-3577. |
| 24 | JURÍČEK M, KOUWER P H J, ROWAN A E. Triazole: a unique building block for the construction of functional materials[J]. Chemical Communications, 2011, 47(31): 8740-8749. |
| 25 | THIRUMURUGAN P, MATOSIUK D, JOZWIAK K. Click chemistry for drug development and diverse chemical-biology applications[J]. Chemical Reviews, 2013, 113(7): 4905-4979. |
| 26 | HABRANT D, RAUHALA V, KOSKINEN A M. Conversion of carbonyl compounds to alkynes: general overview and recent developments[J]. Chemical Society Reviews, 2010, 39(6): 2007-2017. |
| 27 | SHAW R, ELAGAMY A, ALTHAGAFI I, et al. Synthesis of alkynes from non-alkyne sources[J]. Organic & Biomolecular Chemistry, 2020, 18(20): 3797-3817. |
| 28 | DUPUIS S N, ROBERTSON A W, VEINOT T, et al. Synthetic diversification of natural products: Semi-synthesis and evaluation of triazole jadomycins[J]. Chemical Science, 2012, 3(5): 1640-1644. |
| 29 | YAN Y, CHEN J, ZHANG L H, et al. Multiplexing of combinatorial chemistry in antimycin biosynthesis: Expansion of molecular diversity and utility[J]. Angewandte Chemie International Edition, 2013, 52(47): 12308-12312. |
| 30 | SANDY M, ZHU X J, RUI Z, et al. Characterization of AntB, a promiscuous acyltransferase involved in antimycin biosynthesis[J]. Organic Letters, 2013, 15(13): 3396-3399. |
| 31 | HARVEY C J, PUGLISI J D, PANDE V S, et al. Precursor directed biosynthesis of an orthogonally functional erythromycin analogue: Selectivity in the ribosome macrolide binding pocket[J]. Journal of the American Chemical Society, 2012, 134(29): 12259-12265. |
| 32 | 林章凛, 张艳, 王胥, 等. 合成生物学研究进展[J]. 化工学报, 2015, 66(8): 2863-2871. |
| LIN Z L, ZHANG Y, WANG X, et al. Recent advances in synthetic biology[J]. CIESC Journal, 2015, 66(8): 2863-2871. | |
| 33 | KEASLING J D. Synthetic biology for synthetic chemistry[J]. ACS Chemical Biology, 2008, 3(1): 64-76. |
| 34 | MINTO R E, BLACKLOCK B J. Biosynthesis and function of polyacetylenes and allied natural products[J]. Progress in Lipid Research, 2008, 47(4): 233-306. |
| 35 | CHAI Q Y, YANG Z, LIN H W, et al. Alkynyl-containing peptides of marine origin: a review[J]. Marine Drugs, 2016, 14(11): 216. |
| 36 | MEINWALD J, MEINWALD Y C, CHALMERS A M, et al. Dihydromatricaria acid: Acetylenic acid secreted by soldier beetle[J]. Science, 1968, 160(3830): 890-892. |
| 37 | BU'LOCK J D, SMITH G N. The origin of naturally-occurring acetylenes[J]. Journal of the Chemical Society C: Organic, 1967, 332-336. |
| 38 | FLEMING I, HARLEY-MASON J. Enol elimination reactions (I): a new synthesis of acetylenic acids[J/OL]. Journal of the Chemical Society, 1963: 4771-4777. |
| CDCAC 75 A932C42E72F36729D947&thid=OIP.5Uql7uEJ1xbUyr_-yZCzyQHaJs&mediaurl=https%3a%2f%2fpubs.rsc.org%2fen%2fImage%2fGet%3fimageInfo.ImageType%3dGA%26imageInfo.ImageIdentifier.ManuscriptID%3dJR9630004771%26imageInfo.ImageIdentifier.Year%3d1963&cdnurl=https%3a%2f%2ftse1-mm.cn.bing.net%2fth%2fid%2fR-C.e54aa5eee109d716d4cabffec990b3c9%3frik%3dR9kpZ%252fNyLsQyqQ%26pid%3dImgRaw%26r%3d0&exph=655&expw=500&q=enol+elimination+reactions+i&simid=607992233410701701&FORM=IRPRST&ck=7D5B10BF8DF7FBE406CF4E280217CA91&selectedIndex=0&qpvt=enol+elimination+reactions+i&ajaxhist=0&ajaxserp=0 | |
| 39 | LEE M, LENMAN M, BANAŚ A, et al. Identification of non-heme diiron proteins that catalyze triple bond and epoxy group formation[J]. Science, 1998, 280(5365): 915-918. |
| 40 | CARLSSON A S, THOMAEUS S, HAMBERG M, et al. Properties of two multifunctional plant fatty acid acetylenase/desaturase enzymes[J]. European Journal of Biochemistry, 2004, 271(14): 2991-2997. |
| 41 | CAHOON E B, SCHNURR J A, HUFFMAN E A, et al. Fungal responsive fatty acid acetylenases occur widely in evolutionarily distant plant families[J]. The Plant Journal, 2003, 34(5): 671-683. |
| 42 | BUSTA L, YIM W C, LABRANT E W, et al. Identification of genes encoding enzymes catalyzing the early steps of carrot polyacetylene biosynthesis[J]. Plant Physiology, 2018, 178(4): 1507-1521. |
| 43 | JEON J E, KIM J G, FISCHER C R, et al. A pathogen-responsive gene cluster for highly modified fatty acids in tomato[J]. Cell, 2020, 180(1): 176-187. |
| 44 | SPERLING P, LEE M, GIRKE T, et al. A bifunctional Δ6-fatty acyl acetylenase/desaturase from the moss Ceratodon purpureus. a new member of the cytochrome b5 superfamily[J]. European Journal of Biochemistry, 2000, 267(12): 3801-3811. |
| 45 | CHEN P Y, HSIEH M J, TSAI Y T, et al. Transformation and characterization of Δ12-fatty acid acetylenase and Δ12-oleate desaturase potentially involved in the polyacetylene biosynthetic pathway from Bidens pilosa [J]. Plants, 2020, 9(11): 1483. |
| 46 | CHUNG H H, TING H M, WANG W H, et al. Elucidation of enzymes involved in the biosynthetic pathway of bioactive polyacetylenes in Bidens pilosa using integrated omics approaches[J]. Journal of Experimental Botany, 2021, 72(2): 525-541. |
| 47 | SERRA M, PIÑA B, ABAD J L, et al. A multifunctional desaturase involved in the biosynthesis of the processionary moth sex pheromone[J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(42): 16444-16449. |
| 48 | HARITOS V S, HORNE I, DAMCEVSKI K, et al. The convergent evolution of defensive polyacetylenic fatty acid biosynthesis genes in soldier beetles[J]. Nature Communications, 2012, 3: 1150. |
| 49 | BLACKLOCK B J, SCHEFFLER B E, SHEPARD M R, et al. Functional diversity in fungal fatty acid synthesis: the first acetylenase from the Pacific golden chanterelle, Cantharellus formosus[J]. The Journal of Biological Chemistry, 2010, 285(37): 28442-28449. |
| 50 | ROSS C, SCHERLACH K, KLOSS F, et al. The molecular basis of conjugated polyyne biosynthesis in phytopathogenic bacteria[J]. Angewandte Chemie International Edition, 2014, 53(30): 7794-7798. |
| 51 | BLOOMFIELD D K, BLOCH K. The formation of Δ9-unsaturated fatty acids[J]. Journal of Biological Chemistry, 1960, 235: 337-345. |
| 52 | SHANKLIN J, CAHOON E B. Desaturation and related modifications of fatty acids[J]. Annual Review of Plant Physiology and Plant Molecular Biology, 1998, 49: 611-641. |
| 53 | BUIST P H. Fatty acid desaturases: Selecting the dehydrogenation channel[J]. Natural Product Reports, 2004, 21(2): 249-262. |
| 54 | SHANKLIN J, GUY J E, MISHRA G, et al. Desaturases: emerging models for understanding functional diversification of diiron-containing enzymes[J]. The Journal of Biological Chemistry, 2009, 284(28): 18559-18563. |
| 55 | BAI Y, MCCOY J G, LEVIN E J, et al. X-ray structure of a mammalian stearoyl-CoA desaturase[J]. Nature, 2015, 524(7564): 252-256. |
| 56 | LINDQVIST Y, HUANG W, SCHNEIDER G, et al. Crystal structure of Δ9 stearoyl-acyl carrier protein desaturase from castor seed and its relationship to other di-iron proteins[J]. The EMBO Journal, 1996, 15(16): 4081-4092. |
| 57 | REED D W, POLICHUK D R, BUIST P H, et al. Mechanistic study of an improbable reaction-alkene dehydrogenation by the Δ12 acetylenase of Crepis alpina [J]. Journal of the American Chemical Society, 2003, 125(35): 10635-10640. |
| 58 | BEHROUZIAN B, FAUCONNOT L, DALIGAULT F, et al. Mechanism of fatty acid desaturation in the green alga Chlorella vulgaris [J]. European Journal of Biochemistry, 2001, 268(12): 3545-3549. |
| 59 | LANEN S G VAN, SHEN B. Biosynthesis of enediyne antitumor antibiotics[J]. Current Topics in Medicinal Chemistry, 2008, 8(6): 448-459. |
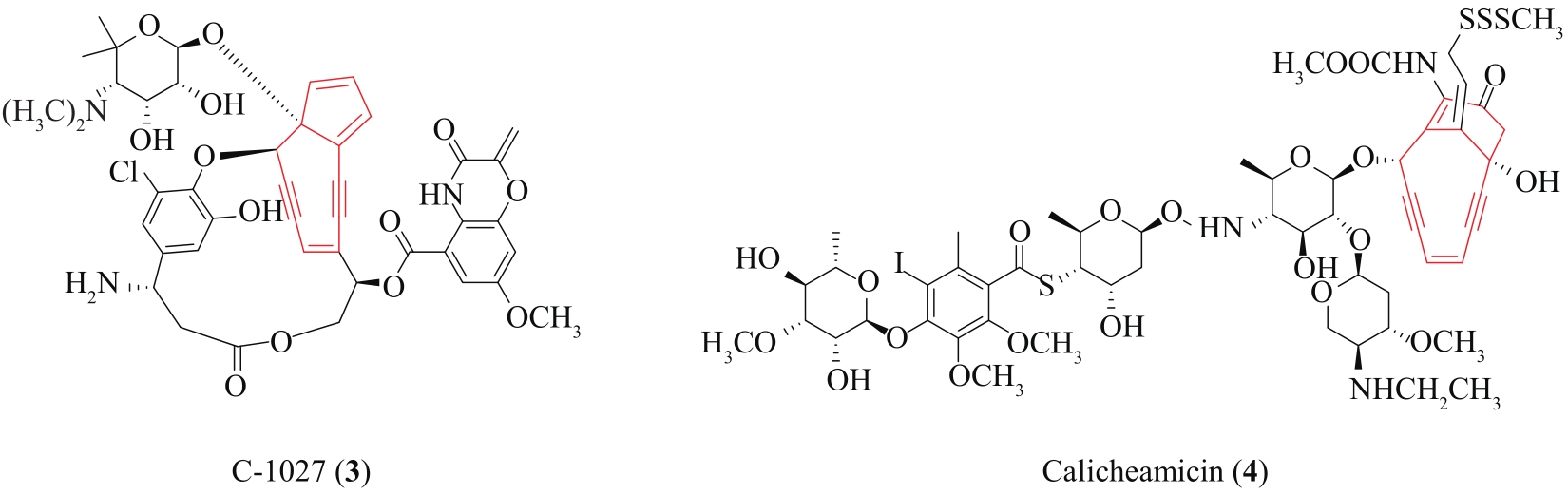
| 60 | LIANG Z X. Complexity and simplicity in the biosynthesis of enediyne natural products[J]. Natural Product Reports, 2010, 27(4): 499-528. |
| 61 | MAEDA H, SAWA T, KONNO T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS[J]. Journal of Controlled Release, 2001, 74(1-3): 47-61. |
| 62 | DAMLE N K, FROST P. Antibody-targeted chemotherapy with immunoconjugates of calicheamicin[J]. Current Opinion in Pharmacology, 2003, 3(4): 386-390. |
| 63 | HENSENS O D, GINER J L, GOLDBERG I H. Biosynthesis of NCS Chrom A, the chromophore of the antitumor antibiotic neocarzinostatin[J]. Journal of the American Chemical Society, 1989, 111(9): 3295-3299. |
| 64 | LAM K S, VEITCH J A, GOLIK J, et al. Biosynthesis of esperamicin A1, an enediyne antitumor antibiotic[J]. Journal of the American Chemical Society, 1993, 115(26): 12340-12345. |
| 65 | THORSON J S, SHEN B, WHITWAM R E, et al. Enediyne biosynthesis and self-resistance: a progress report[J]. Bioorganic Chemistry, 1999, 27(2): 172-188. |
| 66 | LIU W, CHRISTENSON S D, STANDAGE S, et al. Biosynthesis of the enediyne antitumor antibiotic C-1027[J]. Science, 2002, 297(5584): 1170-1173. |
| 67 | AHLERT J, SHEPARD E, LOMOVSKAYA N, et al. The calicheamicin gene cluster and its iterative type I enediyne PKS[J]. Science, 2002, 297(5584): 1173-1176. |
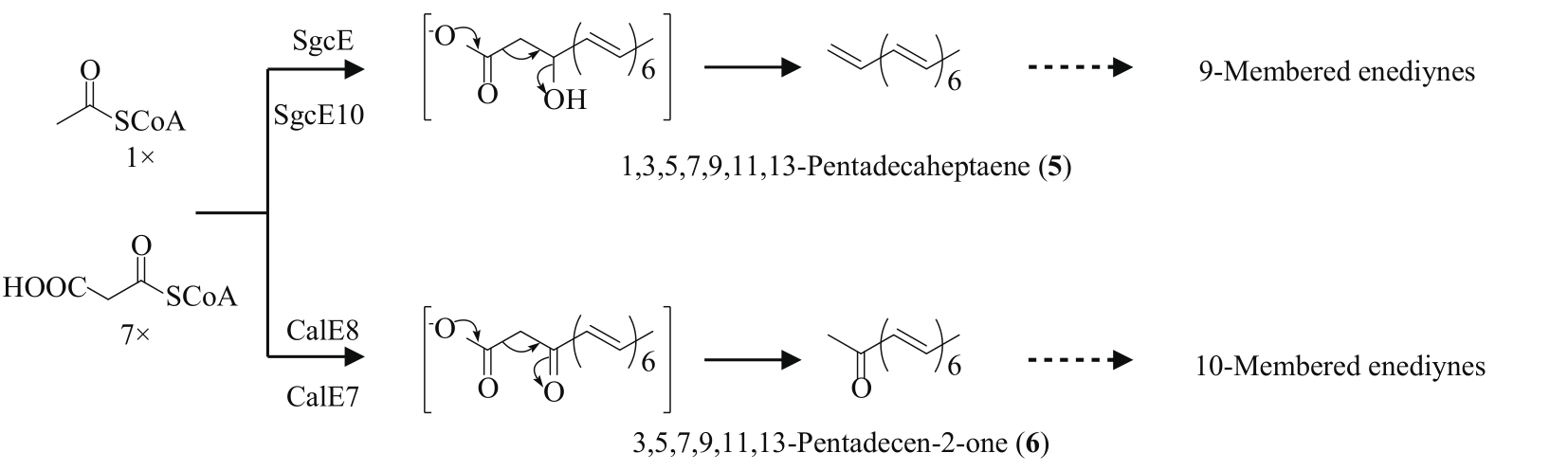
| 68 | ZHANG J, LANEN S G VAN, JU J H, et al. A phosphopantetheinylating polyketide synthase producing a linear polyene to initiate enediyne antitumor antibiotic biosynthesis[J]. Proceeding of the National Academy of Sciences of the United States of America, 2008, 105(5): 1460-1465. |
| 69 | KONG R, GOH L P, LIEW C W, et al. Characterization of a carbonyl-conjugated polyene precursor in 10-membered enediyne biosynthesis[J]. Journal of the American Chemical Society, 2008, 130(26): 8142-8143. |
| 70 | RIVAS L, ROJAS V. Cyanobacterial peptides as a tour de force in the chemical space of antiparasitic agents[J]. Archives of Biochemistry and Biophysics, 2019, 664: 24-39. |
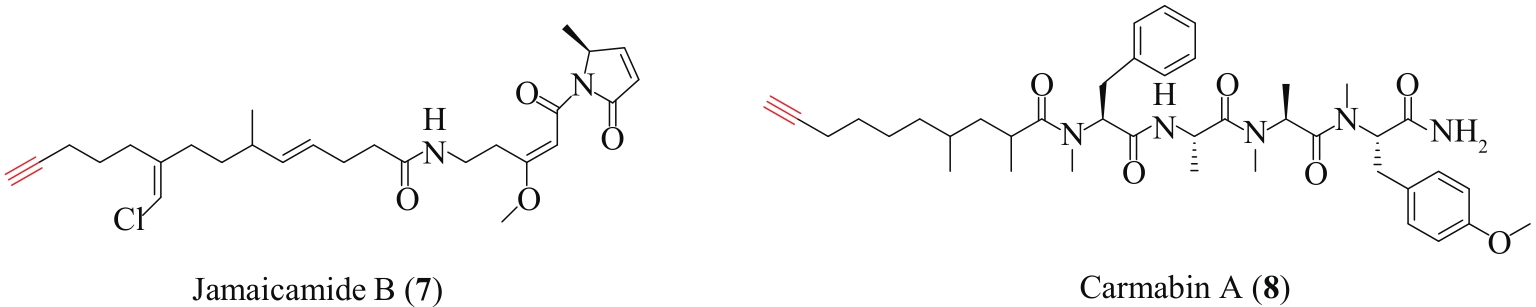
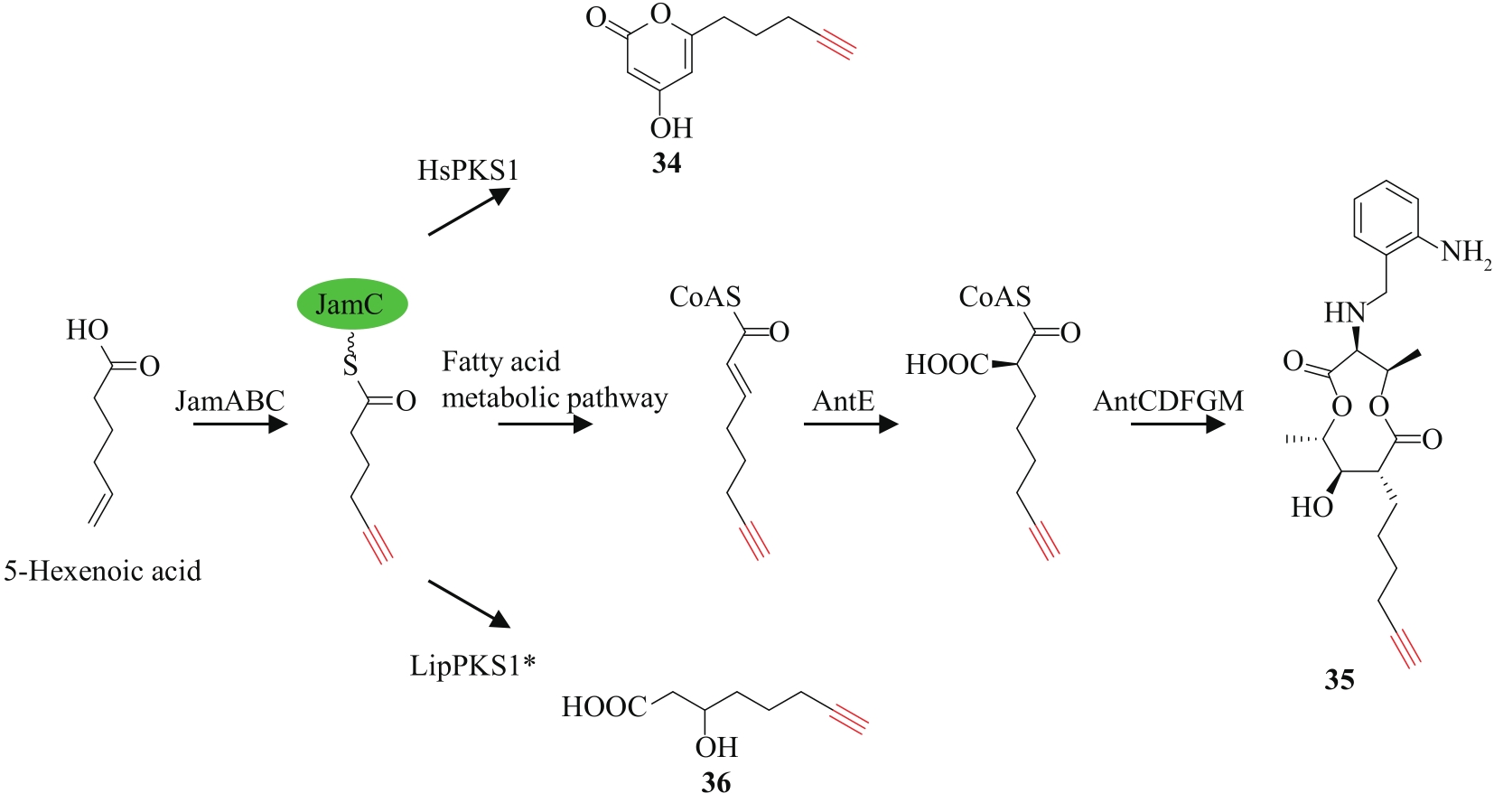
| 71 | EDWARDS D J, MARQUEZ B L, NOGLE L M, et al. Structure and biosynthesis of the jamaicamides, new mixed polyketide-peptide neurotoxins from the marine cyanobacterium Lyngbya majuscula [J]. Chemistry & Biology, 2004, 11(6): 817-833. |
| 72 | JONES A C, MONROE E A, PODELL S, et al. Genomic insights into the physiology and ecology of the marine filamentous cyanobacterium Lyngbya majuscula [J]. Proceeding of the National Academy of Sciences of the United States of America, 2011, 108(21): 8815-8820. |
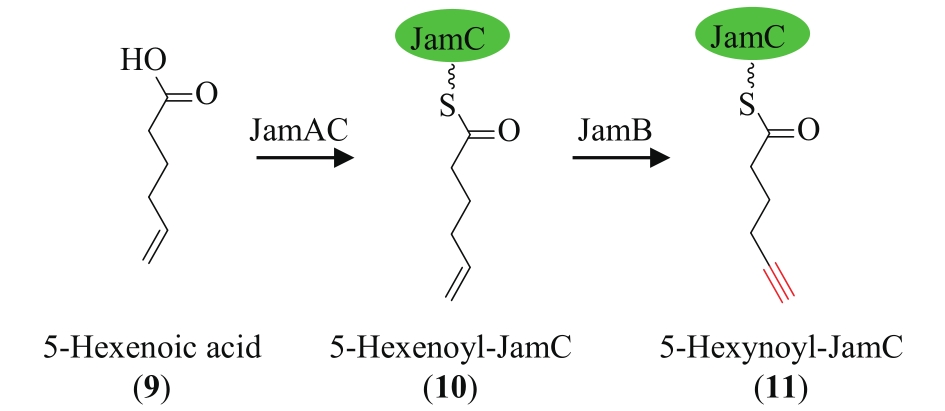
| 73 | ZHU X J, LIU J, ZHANG W J. De novo biosynthesis of terminal alkyne-labeled natural products[J]. Nature Chemical Biology, 2015, 11(2): 115-120. |
| 74 | ZHU X J, SU M, MANICKAM K, et al. Bacterial genome mining of enzymatic tools for alkyne biosynthesis[J]. ACS Chemical Biology, 2015, 10(12): 2785-2793. |
| 75 | POTGIETER H C, VERMEULEN N M J, POTGIETER D J J, et al. A toxic amino acid, 2(S),3(R)-2-amino-3-hydroxypent-4-ynoic acid from the fungus Sclerotium rolfsii [J]. Phytochemistry, 1977, 16(11): 1757-1759. |
| 76 | SANADA M, MIYANO T, IWADARE S. β-Ethynylserine, an antimetabolite of L-threonine, from Streptomyces cattleya [J]. The Journal of Antibiotics, 1986, 39(2): 304-305. |
| 77 | KURODA Y, OKUHARA M, GOTO T, et al. FR-900130, a novel amino acid antibiotic (Ⅱ): Isolation and structure elucidation of the acetyl derivative of FR-900130[J]. The Journal of Antibiotics, 1980, 33(2): 132-136. |
| 78 | HATANAKA S I. Amino acids from mushrooms[J]. Progress in the Chemistry of Organic Natural Products, 1992, 59: 1-140. |
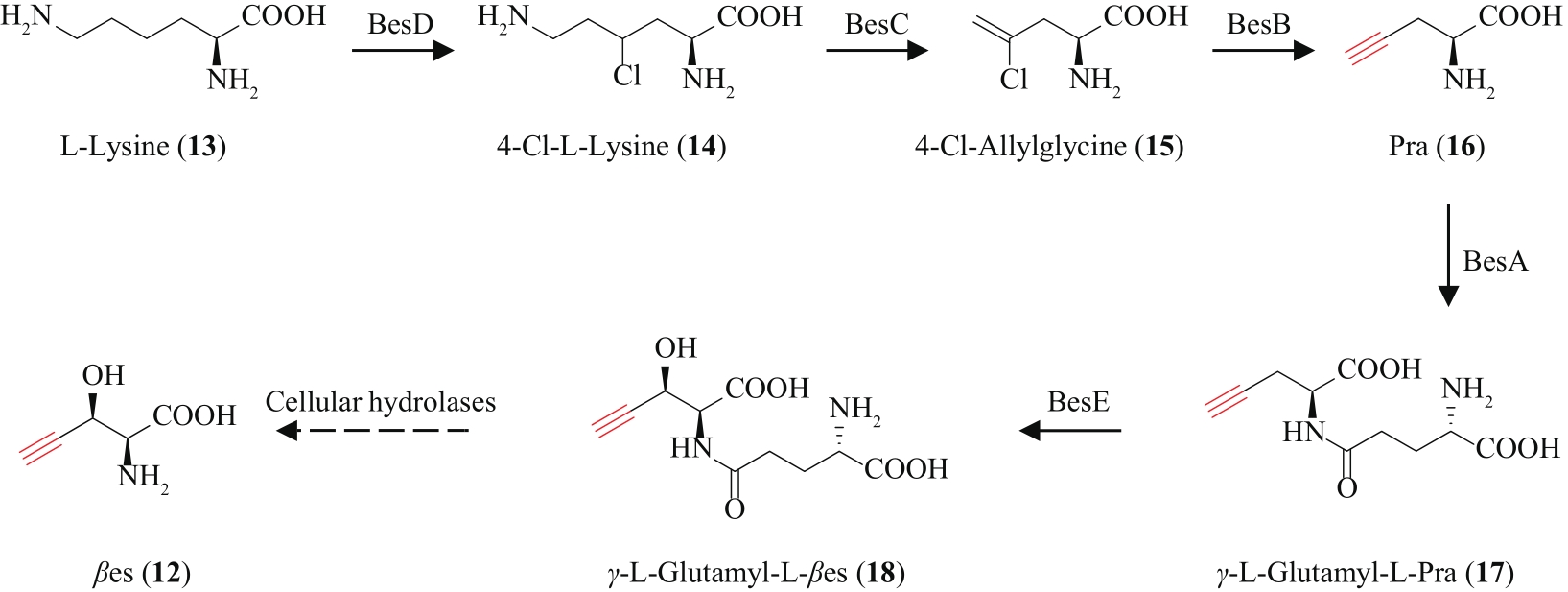
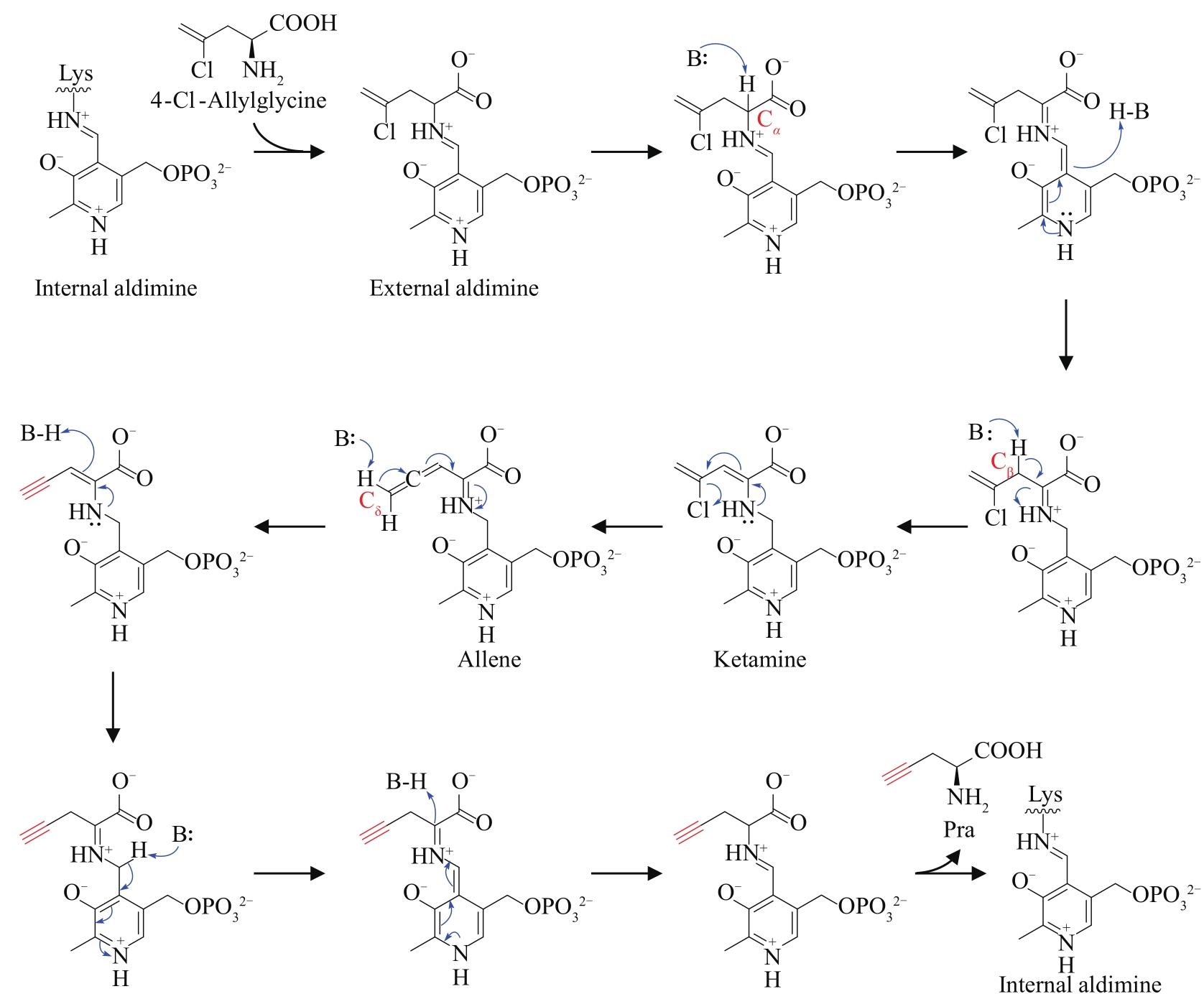
| 79 | MARCHAND J A, NEUGEBAUER M E, ING M C, et al. Discovery of a pathway for terminal-alkyne amino acid biosynthesis[J]. Nature, 2019, 567(7748): 420-424. |
| 80 | SUN Q X, COLLINS R, HUANG S F, et al. Structural basis for the inhibition mechanism of human cystathionine γ-lyase, an enzyme responsible for the production of H2S[J]. Journal of Biological Chemistry, 2009, 284(5): 3076-3085. |
| 81 | ABELES R H, WALSH C T. Acetylenic enzyme inactivators. Inactivation of γ-cystathionase, in vitro and in vivo, by propargylglycine[J]. Journal of the American Chemical Society, 1973, 95(18): 6124-6125. |
| 82 | ZHAO H, CHEN G D, ZOU J, et al. Dimericbiscognienyne A: A meroterpenoid dimer from Biscogniauxia sp. with new skeleton and its activity[J]. Organic Letters, 2017, 19(1): 38-41. |
| 83 | ZHAO H, WANG M Z, CHEN G D, et al. Dimericbiscognienynes B and C: new diisoprenyl-cyclohexene-type meroterpenoid dimers from Biscogniauxia sp.[J]. Chinese Chemical Letters, 2019, 30(1): 51-54. |
| 84 | PAN Y Y, LIU L, GUAN F F, et al. Characterization of a prenyltransferase for iso-A82775C biosynthesis and generation of new congeners of chloropestolides[J]. ACS Chemical Biology, 2018, 13(3): 703-711. |
| 85 | KIM G, KIM M J, CHUNG G, et al. (+)-Dimericbiscognienyne A: total synthesis and mechanistic investigations of the key heterodimerization[J]. Organic Letters, 2018, 20(21): 6886-6890. |
| 86 | SIEBERTZ R, PROKSCH P, WITTE L. Accumulation and biosynthesis of the chromenes precocene I and II in Ageratum houstonianum [J]. Phytochemistry, 1990, 29(7): 2135-2138. |
| 87 | LÜ J M, GAO Y H, ZHAO H, et al. Biosynthesis of biscognienyne B involving a cytochrome P450-dependent alkynylation[J]. Angewandte Chemie International Edition, 2020, 59(32): 13531-13536. |
| 88 | CHEN Y R, NARESH A, LIANG S Y, et al. Discovery of a dual function cytochrome P450 that catalyzes enyne formation in cyclohexanoid terpenoid biosynthesis[J]. Angewandte Chemie International Edition, 2020, 59(32): 13537-13541. |
| 89 | MORITA H, YAMASHITA M, SHI S P, et al. Synthesis of unnatural alkaloid scaffolds by exploiting plant polyketide synthase[J]. Proceeding of the National Academy of Sciences of the United States of America, 2011, 108(33): 13504-13509. |
| 90 | PORTERFIELD W B, POENATEETAI N, ZHANG W J. Engineered biosynthesis of alkyne-tagged polyketides by type I PKSs[J]. iScience, 2020, 23(3): 100938. |
| 91 | HUANG L S, COBESSI D, TUNG E Y, et al. Binding of the respiratory chain inhibitor antimycin to the mitochondrial bc1 complex: A new crystal structure reveals an altered intramolecular hydrogen-bonding pattern[J]. Journal of Molecular Biology, 2005, 351(3): 573-597. |
| 92 | SCHWARTZ P S, MANION M K, EMERSON C B, et al. 2-Methoxy antimycin reveals a unique mechanism for Bcl-xL inhibition[J]. Molecular Cancer Therapeutics, 2007, 6(7): 2073-2080. |
| 93 | BIHLMAIER C, WELLE E, HOFMANN C, et al. Biosynthetic gene cluster for the polyenoyltetramic acid α-lipomycin[J]. Antimicrobial Agents and Chemotherapy, 2006, 50(6): 2113-2121. |
| 94 | YUZAWA S, DENG K, WANG G, et al. Comprehensive in vitro analysis of acyltransferase domain exchanges in modular polyketide synthases and its application for short-chain ketone production[J]. ACS Synthetic Biology, 2017, 6(1): 139-147. |
| 95 | ZHU X J, SHIEH P, SU M, et al. A fluorogenic screening platform enables directed evolution of an alkyne biosynthetic tool[J]. Chemical Communications, 2016, 52(75): 11239-11242. |
| 96 | 谢泽雄, 陈祥荣, 肖文海, 等. 基因组再造与重排构建细胞工厂[J]. 化工学报, 2019, 70(10): 3712-3721. |
| XIE Z X, CHEN X R, XIAO W H, et al. Cell factory construction accelerated by genome synthesis and rearrangement[J]. CIESC Journal, 2019, 70(10): 3712-3721. | |
| 97 | LANG K, CHIN J W. Cellular incorporation of unnatural amino acids and bioorthogonal labeling of proteins[J]. Chemical Reviews, 2014, 114(9): 4764-4806. |
| 98 | BOUTUREIRA O, BERNARDES G J L. Advances in chemical protein modification[J]. Chemical Reviews, 2015, 115(5): 2174-2195. |
| 99 | SAKAMOTO S, HAMACHI I. Recent progress in chemical modification of proteins[J]. Analytical Sciences, 2019, 35(1): 5-27. |
| 100 | 付宪, 林涛, 张帆, 等. 基因密码子拓展技术的方法原理和前沿应用研究进展[J]. 合成生物学, 2020, 1(1): 103-119. |
| FU X, LIN T, ZHANG F, et al. Progress in the study of genetic code expansion related methods, principles and applications[J]. Synthetic Biology Journal, 2020, 1(1): 103-119. | |
| 101 | LIU C C, SCHULTZ P G. Adding new chemistries to the genetic code[J]. Annual Review of Biochemistry, 2010, 79: 413-444. |
| 102 | TRUONG F, YOO T H, LAMPO T J, et al. Two-strain, cell-selective protein labeling in mixed bacterial cultures[J]. Journal of the American Chemical Society, 2012, 134(20): 8551-8556. |
| [1] | XIONG Liangbin, SONG Lu, ZHAO Yunqiu, LIU Kun, LIU Yongjun, WANG Fengqing, WEI Dongzhi. Green biomanufacturing of steroids: from biotransformation to de novo synthesis by microorganisms [J]. Synthetic Biology Journal, 2021, 2(6): 942-963. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||