Synthetic Biology Journal ›› 2021, Vol. 2 ›› Issue (5): 716-733.DOI: 10.12211/2096-8280.2021-058
• Invited Review • Previous Articles Next Articles
Advances and challenges in microbial production of benzylisoquinoline alkaloids
LIN Zhi, HU Zhiwei, QU Xudong, LIN Shuangjun
- State Key Laboratory of Microbial Metabolism,School of Life Science and Biotechnology & Joint International Research Laboratory of Metabolic and Developmental Sciences,Shanghai Jiao Tong University,Shanghai 200240,China
-
Received:2021-05-07Revised:2021-06-10Online:2021-11-19Published:2021-10-31 -
Contact:LIN Shuangjun
苄基异喹啉类生物碱的微生物合成研究进展及挑战
林芝, 胡致伟, 瞿旭东, 林双君
- 上海交通大学生命科学技术学院,微生物代谢国家重点实验室,教育部代谢与发育科学国际合作联合实验室,上海 200240
-
通讯作者:林双君 -
作者简介:林芝 (1989—),女,博士,助理研究员。研究方向为重要手性化合物的绿色制造,活性天然产物的生物合成和合成生物学等。E-mail:linz@sjtu.edu.cn林双君 (1972—),男,博士,教授,博士生导师。研究方向为生物活性天然产物的发现及结构鉴定,天然产物的生物合成与组合生物合成,酶催化反应的机理及应用等。E-mail:linsj@sjtu.edu.cn -
基金资助:国家自然科学基金(21632007);国家重点研发计划(2018YFA0901900);上海交通大学新进教师启动计划(21X010500698)
CLC Number:
Cite this article
LIN Zhi, HU Zhiwei, QU Xudong, LIN Shuangjun. Advances and challenges in microbial production of benzylisoquinoline alkaloids[J]. Synthetic Biology Journal, 2021, 2(5): 716-733.
林芝, 胡致伟, 瞿旭东, 林双君. 苄基异喹啉类生物碱的微生物合成研究进展及挑战[J]. 合成生物学, 2021, 2(5): 716-733.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2021-058
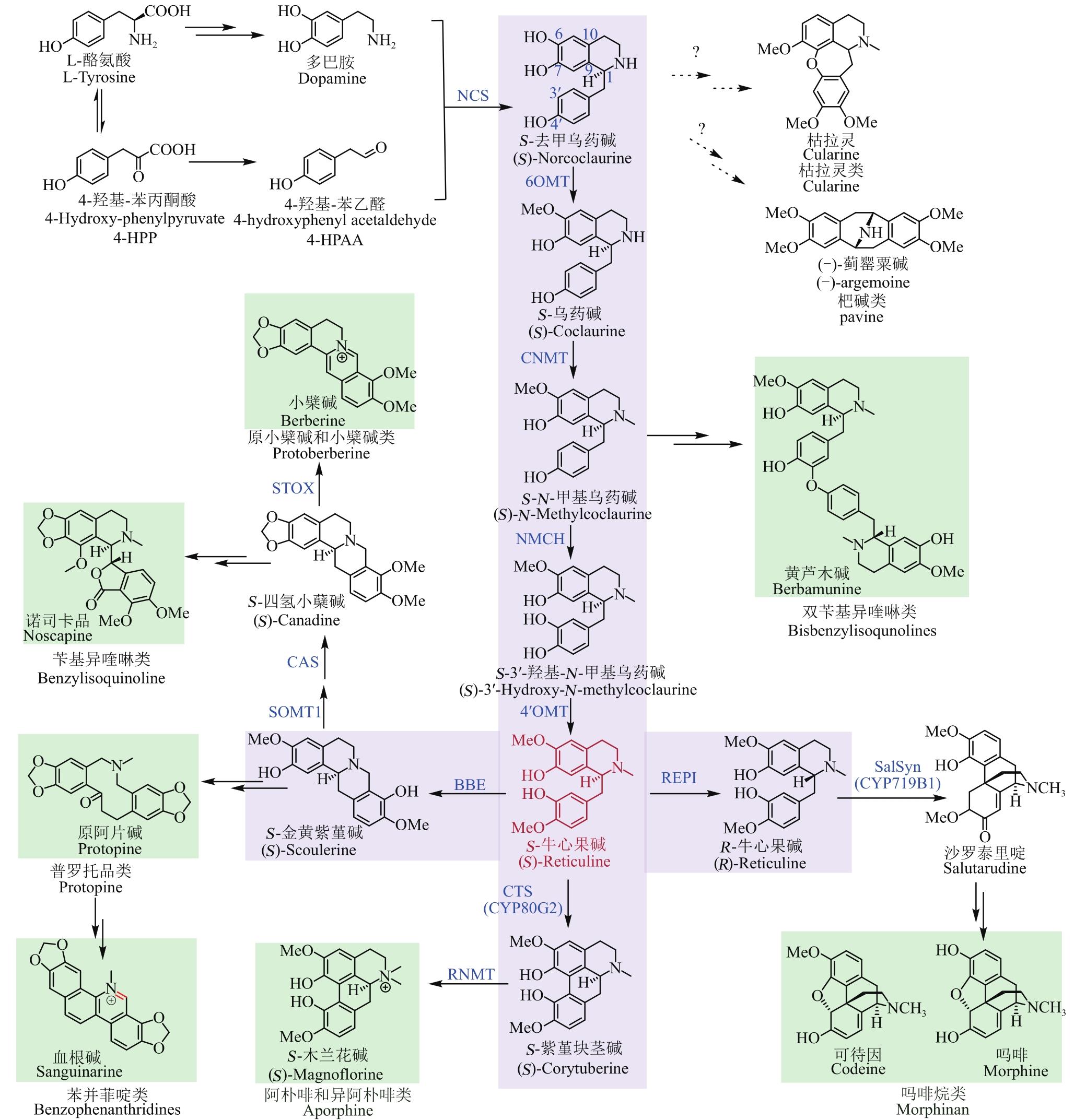
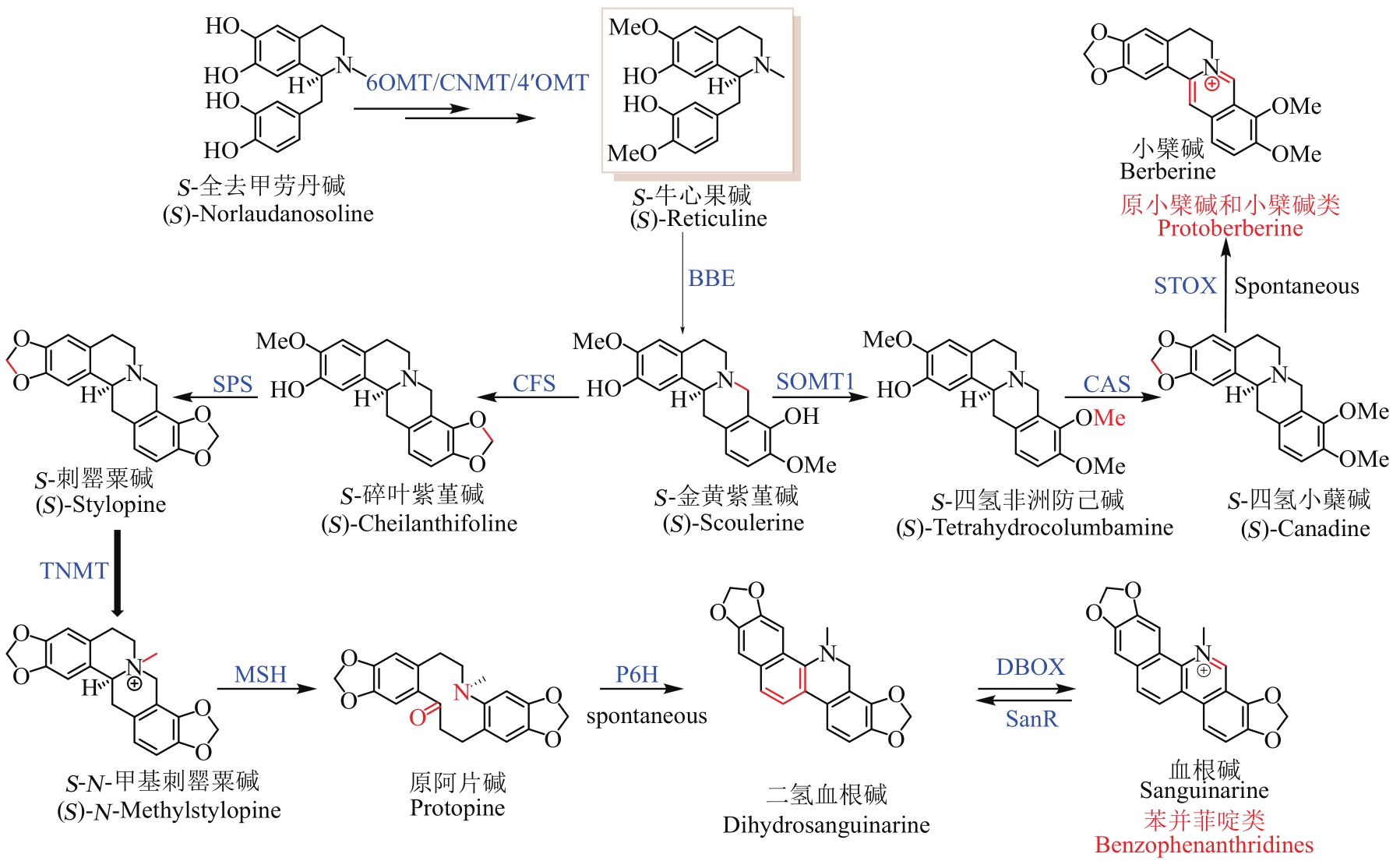
Fig. 1 Skeletons of BIAs and the proposed pathways for their biosynthesis(The green box represents the seven skeletons of BIAs, and the purple box represents the shared-upstream synthetic pathway and key branch points for synthesizing BIAs. Red color indicates the key intermediate (S)-Reticuline. The solid and dashed lines show known and unknown pathways for the synthesis of BIAs, respectively)

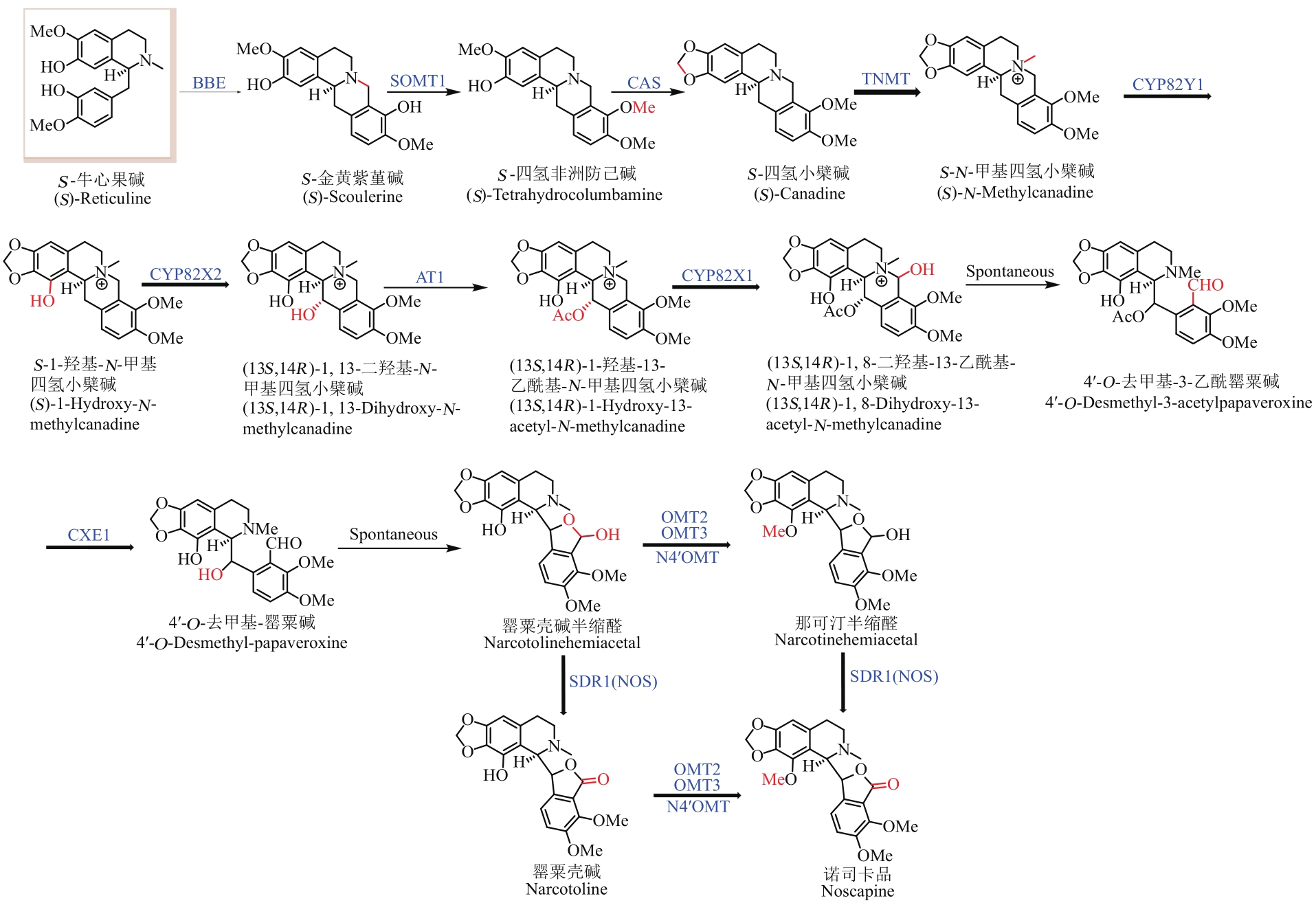
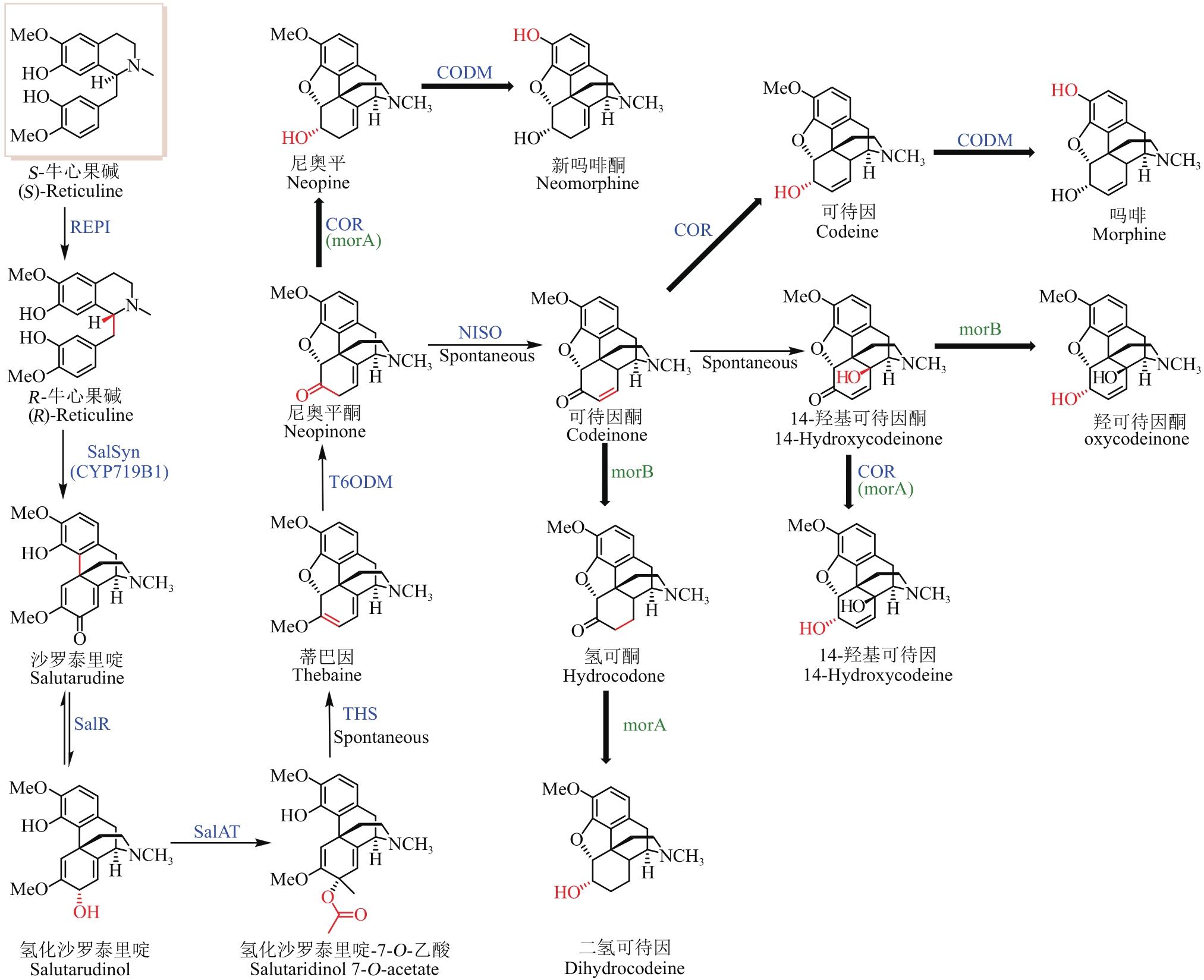
Fig. 2 Pathway for the microbial synthesis of benzylisoquinoline alkaloids(The microbial synthesis pathway of BIAs with enzymes highlighted by blue color is a mimic of the natural biosynthetic pathway, while enzymes and hydroxyl highlighted with red color represent the artificially designed pathway for microbial synthesis of BIAs. The bold and thin arrows indicate that the substrate recognition of the enzyme is relatively broad and specific, respectively)
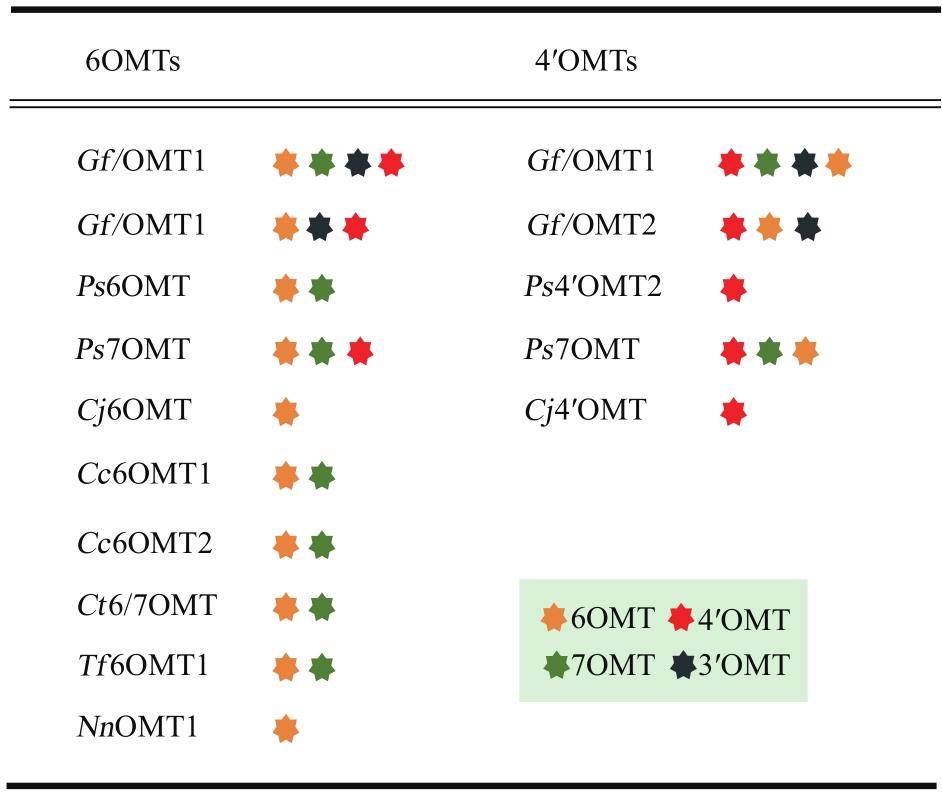
Fig. 3 Cross-regioselectivity of 6OMT and 4′OMT to 3′, 4′, 6 and 7 hydroxyl groups(The stars with different colors represent the methylation function at different positions)
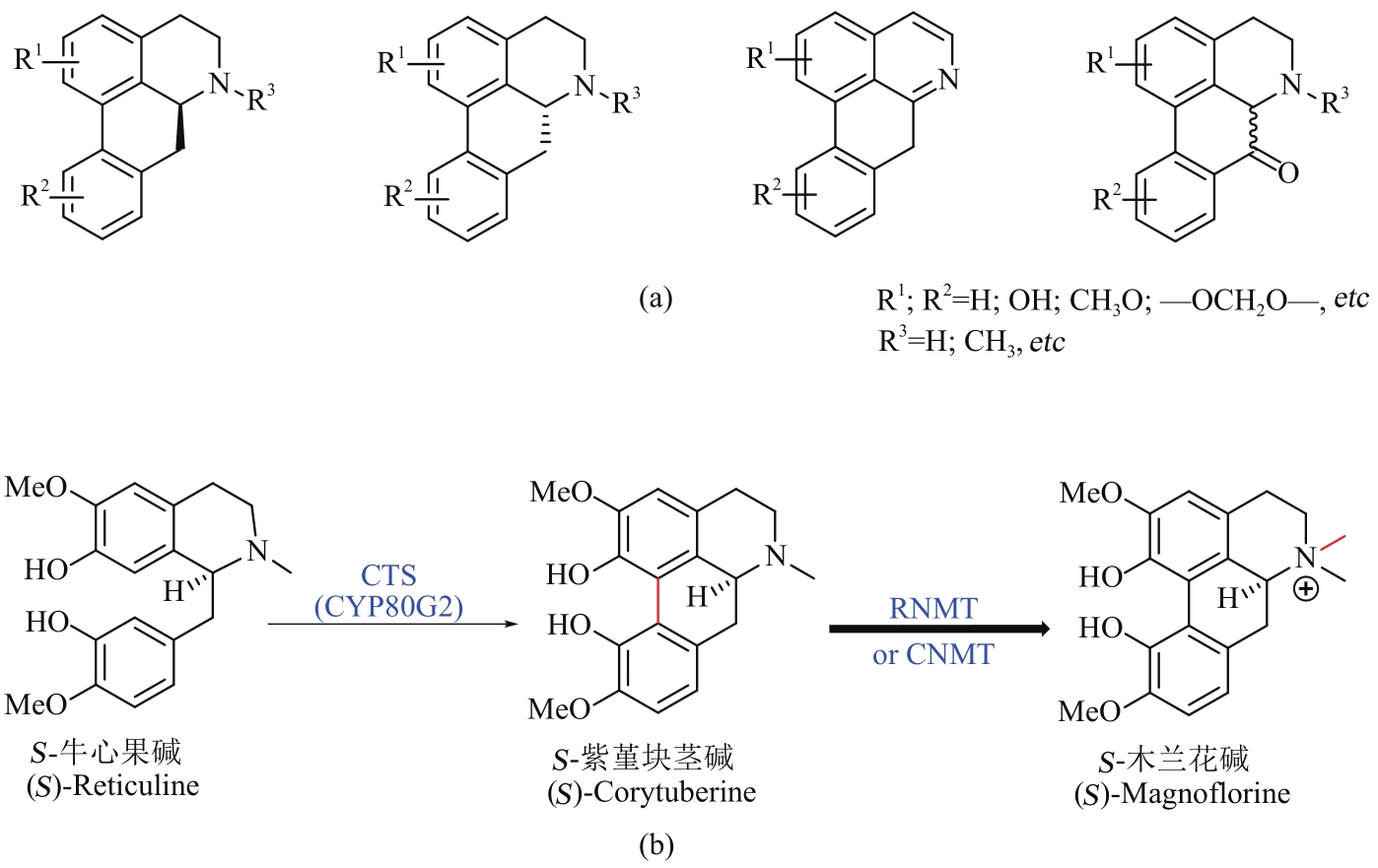
Fig. 4 Skeletons of aporphines (a) and their microbial synthesis (b)(The red color on the compound indicates the bond formed in each step of the enzyme reaction)
| 1 | LI J W, VEDERAS J C. Drug discovery and natural products: end of an era or an endless frontier?[J]. Science, 2009, 325(5937):161-165. |
| 2 | HARVEY A L. Natural products as a screening resource[J]. Current Opinion in Chemical Biology, 2007, 11(5):480-484. |
| 3 | LEONARD E, RUNGUPHAN W, O′CONNOR S, et al. Opportunities in metabolic engineering to facilitate scalable alkaloid production[J]. Nature Chemical Biology, 2009, 5(5):292-300. |
| 4 | PADDON C J, KEASLING J D. Semi-synthetic artemisinin: a model for the use of synthetic biology in pharmaceutical development[J]. Nature Reviews Microbiology, 2014, 12(5):355-367. |
| 5 | WEBER C, OPATZ T. Bisbenzylisoquinoline alkaloids[J]. The Alkaloids: Chemistry and Biology, 2019, 81:1-114. |
| 6 | HAGEL J M, FACCHINI P J. Benzylisoquinoline alkaloid metabolism: a century of discovery and a brave new world[J]. Plant and Cell Physiology, 2013, 54(5):647-672. |
| 7 | QING Z X, HUANG J L, YANG X Y, et al. Anticancer and reversing multidrug resistance activities of natural isoquinoline alkaloids and their structure-activity relationship[J]. Current Medicinal Chemistry, 2018, 25(38):5088-5114. |
| 8 | MOHAMED S M, HASSAN E M, IBRAHIM N A. Cytotoxic and antiviral activities of aporphine alkaloids of Magnolia grandiflora L[J]. Natural Product Research, 2010, 24(15):1395-1402. |
| 9 | MUÑOZ V, SAUVAIN M, MOLLINEDO P, et al. Antimalarial activity and cytotoxicity of (-)-roemrefidine isolated from the stem bark of Sparattanthelium amazonum [J]. Planta Medica, 1999, 65(5):448-449. |
| 10 | GUO Y G, DING Y H, WU G J, et al. Three new alkaloids from Xylopia vielana and their antiinflammatory activities[J]. Fitoterapia, 2018, 127:96-100. |
| 11 | CHIA Y C, CHEN K S, CHANG Y L, et al. Antiplatelet actions of aporphinoids from Formosan plants[J]. Bioorganic & Medicinal Chemistry Letters, 1999, 9(23):3295-3300. |
| 12 | CHEN K S, KO F N, TENG C M, et al. Antiplatelet and vasorelaxing actions of some aporphinoids[J]. Planta Medica, 1996, 62(2):133-136. |
| 13 | DASTMALCHI M, PARK M R, MORRIS J S, et al. Family portraits: the enzymes behind benzylisoquinoline alkaloid diversity[J]. Phytochemistry Reviews, 2018, 17(2):249-277. |
| 14 | NARCROSS L, FOSSATI E, BOURGEOIS L, et al. Microbial factories for the production of benzylisoquinoline alkaloids[J]. Trends in Biotechnology, 2016, 34(3):228-241. |
| 15 | FUJII N, INUI T, IWASA K, et al. Knockdown of berberine bridge enzyme by RNAi accumulates (S)-reticuline and activates a silent pathway in cultured California poppy cells[J]. Transgenic Research, 2007, 16(3):363-375. |
| 16 | LISCOMBE D K, FACCHINI P J. Molecular cloning and characterization of tetrahydroprotoberberine cis-N-methyltransferase, an enzyme involved in alkaloid biosynthesis in opium poppy[J]. The Journal of Biological Chemistry, 2007, 282(20):14741-14751. |
| 17 | TAKEMURA T, IKEZAWA N, IWASA K, et al. Molecular cloning and characterization of a cytochrome P450 in sanguinarine biosynthesis from Eschscholzia californica cells[J]. Phytochemistry, 2013, 91:100-108. |
| 18 | WINKLER A, LYSKOWSKI A, RIEDL S, et al. A concerted mechanism for berberine bridge enzyme[J]. Nature Chemical Biology, 2008, 4(12):739-741. |
| 19 | HAGEL J M, BEAUDOIN G A, FOSSATI E, et al. Characterization of a flavoprotein oxidase from opium poppy catalyzing the final steps in sanguinarine and papaverine biosynthesis[J]. The Journal of Biological Chemistry, 2012, 287(51):42972-42983. |
| 20 | AMANN M, NAGAKURA N, ZENK M H. Purification and properties of (S)-tetrahydroprotoberberine oxidase from suspension-cultured cells of Berberis wilsoniae [J]. European Journal of Biochemistry, 1988, 175(1):17-25. |
| 21 | GALANIE S, THODEY K, TRENCHARD I J, et al. Complete biosynthesis of opioids in yeast[J]. Science, 2015, 349(6252):1095-1100. |
| 22 | WINZER T, KERN M, KING A J, et al. Plant science. Morphinan biosynthesis in opium poppy requires a P450-oxidoreductase fusion protein[J]. Science, 2015, 349(6245):309-312. |
| 23 | FARROW S C, HAGEL J M, BEAUDOIN G A, et al. Stereochemical inversion of (S)-reticuline by a cytochrome P450 fusion in opium poppy[J]. Nature Chemical Biology, 2015, 11(9):728-732. |
| 24 | HAGEL J M, FACCHINI P J. Dioxygenases catalyze the O-demethylation steps of morphine biosynthesis in opium poppy[J]. Nature Chemical Biology, 2010, 6(4):273-275. |
| 25 | IKEZAWA N, IWASA K, SATO F. Molecular cloning and characterization of CYP80G2, a cytochrome P450 that catalyzes an intramolecular C-C phenol coupling of (S)-reticuline in magnoflorine biosynthesis, from cultured Coptis japonica cells[J]. The Journal of Biological Chemistry, 2008, 283(14):8810-8821. |
| 26 | MINAMI H, KIM J S, IKEZAWA N, et al. Microbial production of plant benzylisoquinoline alkaloids[J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(21):7393-7398. |
| 27 | KIM J S, NAKAGAWA A, YAMAZAKI Y, et al. Improvement of reticuline productivity from dopamine by using engineered Escherichia coli [J]. Bioscience, Biotechnology, and Biochemistry, 2013, 77(10):2166-2168. |
| 28 | NAKAGAWA A, MINAMI H, KIM J S, et al. A bacterial platform for fermentative production of plant alkaloids[J]. Nature Communications, 2011, 2:326. |
| 29 | NAKAGAWA A, MATSUZAKI C, MATSUMURA E, et al. (R,S)-tetrahydropapaveroline production by stepwise fermentation using engineered Escherichia coli [J]. Scientific Reports, 2014, 4:6695. |
| 30 | LÜTKE-EVERSLOH T, STEPHANOPOULOS G. L-Tyrosine production by deregulated strains of Escherichia coli [J]. Applied Microbiology and Biotechnology, 2007, 75(1):103-110. |
| 31 | LICHMAN B R, GERSHATER M C, LAMMING E D, et al. "Dopamine-first" mechanism enables the rational engineering of the norcoclaurine synthase aldehyde activity profile[J]. The FEBS Journal, 2015, 282(6):1137-1151. |
| 32 | HAWKINS K M, SMOLKE C D. Production of benzylisoquinoline alkaloids in Saccharomyces cerevisiae [J]. Nature Chemical Biology, 2008, 4(9):564-573. |
| 33 | FOSSATI E, EKINS A, NARCROSS L, et al. Reconstitution of a 10-gene pathway for synthesis of the plant alkaloid dihydrosanguinarine in Saccharomyces cerevisiae [J]. Nature Communications, 2014, 5:3283. |
| 34 | FITZPATRICK P F. Tetrahydropterin-dependent amino acid hydroxylases[J]. Annual Review of Biochemistry, 1999, 68:355-381. |
| 35 | CLAUS H, DECKER H. Bacterial tyrosinases[J]. Systematic and Applied Microbiology, 2006, 29(1):3-14. |
| 36 | HALAOULI S, ASTHER M, SIGOILLOT J C, et al. Fungal tyrosinases: new prospects in molecular characteristics, bioengineering and biotechnological applications[J]. Journal of Applied Microbiology, 2006, 100(2):219-232. |
| 37 | DELOACHE W C, RUSS Z N, NARCROSS L, et al. An enzyme-coupled biosensor enables (S)-reticuline production in yeast from glucose[J]. Nature Chemical Biology, 2015, 11(7):465-471. |
| 38 | PYNE M E, KEVVAI K, GREWAL P S, et al. A yeast platform for high-level synthesis of tetrahydroisoquinoline alkaloids[J]. Nature Communications, 2020, 11(1):3337. |
| 39 | BOURGEOIS L, PYNE M E, MARTIN V J J. A highly characterized synthetic landing pad system for precise multicopy gene integration in yeast[J]. ACS Synthetic Biology, 2018, 7(11):2675-2685. |
| 40 | LI Y, LI S, THODEY K, et al. Complete biosynthesis of noscapine and halogenated alkaloids in yeast[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(17):E3922-E3931. |
| 41 | NISHIHACHIJO M, HIRAI Y, KAWANO S, et al. Asymmetric synthesis of tetrahydroisoquinolines by enzymatic Pictet-Spengler reaction[J]. Bioscience, Biotechnology, and Biochemistry, 2014, 78(4):701-707. |
| 42 | PESNOT T, GERSHATER M C, WARD J M, et al. The catalytic potential of Coptis japonica NCS2 revealed-development and utilisation of a fluorescamine-based assay[J]. Advanced Synthesis & Catalysis, 2012, 354(16):2997-3008. |
| 43 | MINAMI H, DUBOUZET E, IWASA K, et al. Functional analysis of norcoclaurine synthase in Coptis japonica [J]. The Journal of Biological Chemistry, 2007, 282(9):6274-6282. |
| 44 | RODDAN R, WARD J M, KEEP N H, et al. Pictet-Spenglerases in alkaloid biosynthesis: future applications in biocatalysis[J]. Current Opinion in Chemical Biology, 2020, 55:69-76. |
| 45 | ILARI A, FRANCESCHINI S, BONAMORE A, et al. Structural basis of enzymatic (S)-norcoclaurine biosynthesis[J]. The Journal of Biological Chemistry, 2009, 284(2):897-904. |
| 46 | ZHAO J X, MÉNDEZ-SÁNCHEZ D, RODDAN R, et al. Norcoclaurine synthase-mediated stereoselective synthesis of 1,1′-disubstituted, spiro- and bis-tetrahydroisoquinoline alkaloids[J]. ACS Catalysis, 2021, 11(1):131-138. |
| 47 | BONAMORE A, CALISTI L, CALCATERRA A, et al. A novel enzymatic strategy for the synthesis of substituted tetrahydroisoquinolines[J]. Chemistry Select, 2016, 1(8):1525-1528. |
| 48 | LICHMAN B R, LAMMING E D, PESNOT T, et al. One-pot triangular chemoenzymatic cascades for the syntheses of chiral alkaloids from dopamine[J]. Green Chemistry, 2015, 17(2):852-855. |
| 49 | RODDAN R, GYGLI G, SULA A, et al. Acceptance and kinetic resolution of α-methyl-substituted aldehydes by norcoclaurine synthases[J]. ACS Catalysis, 2019, 9(10):9640-9649. |
| 50 | LICHMAN B R, ZHAO J, HAILES H C, et al. Enzyme catalysed Pictet-Spengler formation of chiral 1,1'-disubstituted- and spiro-tetrahydroisoquinolines[J]. Nature Communications, 2017, 8:14883. |
| 51 | ZHAO J, LICHMAN B R, WARD J M, et al. One-pot chemoenzymatic synthesis of trolline and tetrahydroisoquinoline analogues[J]. Chemical Communications, 2018, 54(11):1323-1326. |
| 52 | GREWAL P S, SAMSON J A, BAKER J J, et al. Peroxisome compartmentalization of a toxic enzyme improves alkaloid production[J]. Nature Chemical Biology, 2021, 17(1):96-103. |
| 53 | MORRIS J S, FACCHINI P J. Molecular origins of functional diversity in benzylisoquinoline alkaloid methyltransferases[J]. Frontiers in Plant Science, 2019, 10:1058. |
| 54 | MORISHIGE T, TSUJITA T, YAMADA Y, et al. Molecular characterization of the S-adenosyl-L-methionine:3′-hydroxy-N-methylcoclaurine 4′-O-methyltransferase involved in isoquinoline alkaloid biosynthesis in Coptis japonica [J]. The Journal of Biological Chemistry, 2000, 275(30):23398-23405. |
| 55 | OUNAROON A, DECKER G, SCHMIDT J, et al. (R,S)-Reticuline 7-O-methyltransferase and (R,S)-norcoclaurine 6-O-methyltransferase of Papaver somniferum-cDNA cloning and characterization of methyl transfer enzymes of alkaloid biosynthesis in opium poppy[J]. The Plant Journal, 2003, 36(6):808-819. |
| 56 | FACCHINI P J, PARK S U. Developmental and inducible accumulation of gene transcripts involved in alkaloid biosynthesis in opium poppy[J]. Phytochemistry, 2003, 64(1):177-186. |
| 57 | SAMANANI N, PARK S U, FACCHINI P J. Cell type-specific localization of transcripts encoding nine consecutive enzymes involved in protoberberine alkaloid biosynthesis[J]. The Plant Cell, 2005, 17(3):915-926. |
| 58 | INUI T, TAMURA K, FUJII N, et al. Overexpression of Coptis japonica norcoclaurine 6-O-methyltransferase overcomes the rate-limiting step in benzylisoquinoline alkaloid biosynthesis in cultured Eschscholzia californica [J]. Plant and Cell Physiology, 2007, 48(2):252-262. |
| 59 | DESGAGNÉ-PENIX I, FACCHINI P J. Systematic silencing of benzylisoquinoline alkaloid biosynthetic genes reveals the major route to papaverine in opium poppy[J]. The Plant Journal, 2012, 72(2):331-344. |
| 60 | CHANG L M, HAGEL J M, FACCHINI P J. Isolation and characterization of O-methyltransferases involved in the biosynthesis of glaucine in Glaucium flavum [J]. Plant Physiology, 2015, 169(2):1127-1140. |
| 61 | ROBIN A Y, GIUSTINI C, GRAINDORGE M, et al. Crystal structure of norcoclaurine-6-O-methyltransferase, a key rate-limiting step in the synthesis of benzylisoquinoline alkaloids[J]. The Plant Journal, 2016, 87(6):641-653. |
| 62 | GUO L, WINZER T, YANG X, et al. The opium poppy genome and morphinan production[J]. Science, 2018, 362(6412):343-347. |
| 63 | MENÉNDEZ-PERDOMO I M, FACCHINI P J. Isolation and characterization of two O-methyltransferases involved in benzylisoquinoline alkaloid biosynthesis in sacred lotus (Nelumbo nucifera)[J]. The Journal of Biological Chemistry, 2020, 295(6): 1598-1612. |
| 64 | CHOI K B, MORISHIGE T, SHITAN N, et al. Molecular cloning and characterization of coclaurine N-methyltransferase from cultured cells of Coptis japonica [J]. The Journal of Biological Chemistry, 2002, 277(1):830-835. |
| 65 | LISCOMBE D K, ZIEGLER J, SCHMIDT J, et al. Targeted metabolite and transcript profiling for elucidating enzyme function: isolation of novel N-methyltransferases from three benzylisoquinoline alkaloid-producing species[J]. The Plant Journal, 2009, 60(4):729-743. |
| 66 | HAGEL J M, MORRIS J S, LEE E J, et al. Transcriptome analysis of 20 taxonomically related benzylisoquinoline alkaloid-producing plants[J]. BMC Plant Biology, 2015, 15(1):227. |
| 67 | ZIEGLER J, DIAZ-CHÁVEZ M L, KRAMELL R, et al. Comparative macroarray analysis of morphine containing Papaver somniferum and eight morphine free Papaver species identifies an O-methyltransferase involved in benzylisoquinoline biosynthesis[J]. Planta, 2005, 222(3):458-471. |
| 68 | PAULI H H, KUTCHAN T M. Molecular cloning and functional heterologous expression of two alleles encoding (S)-N-methylcoclaurine 3'-hydroxylase (CYP80B1), a new methyl jasmonate-inducible cytochrome P-450-dependent mono-oxygenase of benzylisoquinoline alkaloid biosynthesis[J]. The Plant Journal, 1998, 13(6):793-801. |
| 69 | HUANG F C, KUTCHAN T M. Distribution of morphinan and benzo[c]phenanthridine alkaloid gene transcript accumulation in Papaver somniferum [J]. Phytochemistry, 2000, 53(5):555-564. |
| 70 | GUINAUDEAU H, LEBŒUF M, CAVÉ A. Aporphinoid alkaloids, V[J]. Journal of Natural Products, 1994, 57(8):1033-1135. |
| 71 | MORRIS J S, FACCHINI P J. Isolation and characterization of reticuline N-methyltransferase involved in biosynthesis of the aporphine alkaloid magnoflorine in opium poppy[J]. The Journal of Biological Chemistry, 2016, 291(45):23416-23427. |
| 72 | GRYCOVÁ L, DOSTÁL J, MAREK R. Quaternary protoberberine alkaloids[J]. Phytochemistry, 2007, 68(2):150-175. |
| 73 | WINKLER A, HARTNER F, KUTCHAN T M, et al. Biochemical evidence that berberine bridge enzyme belongs to a novel family of flavoproteins containing a bi-covalently attached FAD cofactor[J]. The Journal of Biological Chemistry, 2006, 281(30):21276-21285. |
| 74 | KUTCHAN T M, DITTRICH H. Characterization and mechanism of the berberine bridge enzyme, a covalently flavinylated oxidase of benzophenanthridine alkaloid biosynthesis in plants[J]. The Journal of Biological Chemistry, 1995, 270(41):24475-24481. |
| 75 | WINKLER A, PUHL M, WEBER H, et al. Berberine bridge enzyme catalyzes the six electron oxidation of (S)-reticuline to dehydroscoulerine[J]. Phytochemistry, 2009, 70(9):1092-1097. |
| 76 | DANG T-T T, FACCHINI P J. Characterization of three O-methyltransferases involved in noscapine biosynthesis in opium poppy[J]. Plant Physiology, 2012, 159(2):618-631. |
| 77 | IKEZAWA N, TANAKA M, NAGAYOSHI M, et al. Molecular cloning and characterization of CYP719, a methylenedioxy bridge-forming enzyme that belongs to a novel P450 family, from cultured Coptis japonica cells[J]. The Journal of Biological Chemistry, 2003, 278(40):38557-38565. |
| 78 | DÍAZ CHÁVEZ M L, ROLF M, GESELL A, et al. Characterization of two methylenedioxy bridge-forming cytochrome P450-dependent enzymes of alkaloid formation in the Mexican prickly poppy Argemone mexicana[J]. Archives of Biochemistry and Biophysics, 2011, 507(1):186-193. |
| 79 | GESELL A, DÍAZ CHÁVEZ M L, KRAMELL R, et al. Heterologous expression of two FAD-dependent oxidases with (S)-tetrahydroprotoberberine oxidase activity from Arge mone mexicana and Berberis wilsoniae in insect cells[J]. Planta, 2011, 233(6):1185-1197. |
| 80 | BEAUDOIN G A, FACCHINI P J. Isolation and characterization of a cDNA encoding (S)-cis-N-methylstylopine 14-hydroxylase from opium poppy, a key enzyme in sanguinarine biosynthesis[J]. Biochemical and Biophysical Reserach Communications, 2013, 431(3):597-603. |
| 81 | GALANIE S, SMOLKE C D. Optimization of yeast-based production of medicinal protoberberine alkaloids[J]. Microbial Cell Factories, 2015, 14:144. |
| 82 | TRENCHARD I J, SMOLKE C D. Engineering strategies for the fermentative production of plant alkaloids in yeast[J]. Metabolic Engineering, 2015, 30:96-104. |
| 83 | HORI K, OKANO S, SATO F. Efficient microbial production of stylopine using a Pichia pastoris expression system[J]. Scientific Reports, 2016, 6:22201. |
| 84 | KONZETT H, ROTHLIN E. [The effect of narcotine on cough reflex and on bronchial musculature[J]. Experientia, 1954, 10(11):472-473. |
| 85 | KARLSSON M O, DAHLSTRÖM B, ECKERNÄS S A, et al. Pharmacokinetics of oral noscapine[J]. European Journal of Clinical Pharmacology, 1990, 39(3):275-279. |
| 86 | YE K, KE Y, KESHAVA N, et al. Opium alkaloid noscapine is an antitumor agent that arrests metaphase and induces apoptosis in dividing cells[J]. Proceedings of the National Academy of Sciences of the United States of America, 1998, 95(4):1601-1606. |
| 87 | KE Y, YE K Q, GROSSNIKLAUS H E, et al. Noscapine inhibits tumor growth with little toxicity to normal tissues or inhibition of immune responses[J]. Cancer Immunology, Immunotherapy, 2000, 49(4/5):217-225. |
| 88 | ZHOU J, GUPTA K, YAO J, et al. Paclitaxel-resistant human ovarian cancer cells undergo c-Jun NH2-terminal kinase-mediated apoptosis in response to noscapine[J]. The Journal of Biological Chemistry, 2002, 277(42):39777-39785. |
| 89 | MAHMOUDIAN M, RAHIMI-MOGHADDAM P. The anti-cancer activity of noscapine: a review[J]. Recent Patents on Anti-Cancer Drug Discovery, 2009, 4(1):92-97. |
| 90 | WINZER T, GAZDA V, HE Z, et al. A Papaver somniferum 10-gene cluster for synthesis of the anticancer alkaloid noscapine[J]. Science, 2012, 336(6089):1704-1708. |
| 91 | DANG T T, CHEN X, FACCHINI P J. Acetylation serves as a protective group in noscapine biosynthesis in opium poppy[J]. Nature Chemical Biology, 2015, 11(2):104-106. |
| 92 | DANG T T, FACCHINI P J. CYP82Y1 is N-methylcanadine 1-hydroxylase, a key noscapine biosynthetic enzyme in opium poppy[J]. The Journal of Biological Chemistry, 2014, 289(4):2013-2026. |
| 93 | LI Y, SMOLKE C D. Engineering biosynthesis of the anticancer alkaloid noscapine in yeast[J]. Nature Communications, 2016, 7:12137. |
| 94 | KIRBY G W. Biosynthesis of the morphine alkaloids[J]. Science, 1967, 155(3759):170-173. |
| 95 | THODEY K, GALANIE S, SMOLKE C D. A microbial biomanufacturing platform for natural and semisynthetic opioids[J]. Nature Chemical Biology, 2014, 10(10):837-844. |
| 96 | FOSSATI E, NARCROSS L, EKINS A, et al. Synthesis of morphinan alkaloids in Saccharomyces cerevisiae [J]. PLoS One, 2015, 10(4):e0124459. |
| 97 | NAKAGAWA A, MATSUMURA E, KOYANAGI T, et al. Total biosynthesis of opiates by stepwise fermentation using engineered Escherichia coli [J]. Nature Communications, 2016, 7:10390. |
| 98 | GESELL A, ROLF M, ZIEGLER J, et al. CYP719B1 is salutaridine synthase, the C-C phenol-coupling enzyme of morphine biosynthesis in opium poppy[J]. The Journal of Biological Chemistry, 2009, 284(36):24432-24442. |
| 99 | ZIEGLER J, VOIGTLÄNDER S, SCHMIDT J, et al. Comparative transcript and alkaloid profiling in Papaver species identifies a short chain dehydrogenase/reductase involved in morphine biosynthesis[J]. The Plant Journal, 2006, 48(2):177-192. |
| 100 | LENZ R, ZENK M H. Acetyl coenzyme A:salutaridinol-7-O-acetyltransferase from Papaver somniferum plant cell cultures. The enzyme catalyzing the formation of thebaine in morphine biosynthesis[J]. The Journal of Biological Chemistry, 1995, 270(52):31091-31096. |
| 101 | GROTHE T, LENZ R, KUTCHAN T M. Molecular characterization of the salutaridinol 7-O-acetyltransferase involved in morphine biosynthesis in opium poppy Papaver somniferum [J]. The Journal of Biological Chemistry, 2001, 276(33):30717-30723. |
| 102 | THEUNS H G, B.M.LENTING H, A.SALEMINK C, et al. Neodihydrothebaine and bractazonine, two dibenz[d,f]azonine alkaloids of Papaver bracteatum [J]. Phytochemistry, 1984, 23:1929-1932. |
| 103 | CHEN X, HAGEL J M, CHANG L, et al. A pathogenesis-related 10 protein catalyzes the final step in thebaine biosynthesis[J]. Nature Chemical Biology, 2018, 14(7):738-743. |
| 104 | FARROW S C, FACCHINI P J. Dioxygenases catalyze O-demethylation and O,O-demethylenation with widespread roles in benzylisoquinoline alkaloid metabolism in opium poppy[J]. The Journal of Biological Chemistry, 2013, 288(40):28997-29012. |
| 105 | UNTERLINNER B, LENZ R, KUTCHAN T M. Molecular cloning and functional expression of codeinone reductase: the penultimate enzyme in morphine biosynthesis in the opium poppy Papaver somniferum [J]. The Plant Journal, 1999, 18(5):465-475. |
| 106 | DASTMALCHI M, CHEN X, HAGEL J M, et al. Neopinone isomerase is involved in codeine and morphine biosynthesis in opium poppy[J]. Nature Chemical Biology, 2019, 15(4):384-390. |
| 107 | DASTMALCHI M, CHANG L, TORRES M A, et al. Codeinone reductase isoforms with differential stability, efficiency and product selectivity in opium poppy[J]. The Plant Journal, 2018. |
| 108 | ZHU J M, TAN H Q, YANG L, et al. Enantioselective synthesis of 1-aryl-substituted tetrahydroisoquinolines employing imine reductase[J]. ACS Catalysis, 2017, 7(10):7003-7007. |
| 109 | YANG L, ZHU J, SUN C, et al. Biosynthesis of plant tetrahydroisoquinoline alkaloids through an imine reductase route[J]. Chemical Science, 2020, 11(2):364-371. |
| [1] | GUO Shuyuan, ZHANG Qiannan, Gulikezi· MAIMAITIREXIATI, YANG Yiqun, YU Tao. Advances in microbial production of liquid biofuels [J]. Synthetic Biology Journal, 2025, 6(1): 18-44. |
| [2] | SHAO Mingwei, SUN Simian, YANG Shimao, CHEN Guoqiang. Bioproduction based on extremophiles [J]. Synthetic Biology Journal, 2024, 5(6): 1419-1436. |
| [3] | ZHAO Liang, LI Zhenshuai, FU Liping, LYU Ming, WANG Shi’an, ZHANG Quan, LIU Licheng, LI Fuli, LIU Ziyong. Progress in biomanufacturing of lipids and single cell protein from one-carbon compounds [J]. Synthetic Biology Journal, 2024, 5(6): 1300-1318. |
| [4] | ZHU Fanghuan, CEN Xuecong, CHEN Zhen. Research progress of diols production by microbes [J]. Synthetic Biology Journal, 2024, 5(6): 1367-1385. |
| [5] | YU Wei, GAO Jiaoqi, ZHOU Yongjin. Bioconversion of one carbon feedstocks for producing organic acids [J]. Synthetic Biology Journal, 2024, 5(5): 1169-1188. |
| [6] | CHEN Xiwei, ZHANG Huaran, ZOU Yi. Biosynthesis and metabolic engineering of fungal non-ribosomal peptides [J]. Synthetic Biology Journal, 2024, 5(3): 571-592. |
| [7] | XIE Huang, ZHENG Yilei, SU Yiting, RUAN Jingyi, LI Yongquan. An overview on reconstructing the biosynthetic system of actinomycetes for polyketides production [J]. Synthetic Biology Journal, 2024, 5(3): 612-630. |
| [8] | ZHAO Jingyu, ZHANG Jian, QI Qingsheng, WANG Qian. Research progress in biosensors based on bacterial two-component systems [J]. Synthetic Biology Journal, 2024, 5(1): 38-52. |
| [9] | SUN Huili, CUI Jinyu, LUAN Guodong, LYU Xuefeng. Progress of cyanobacterial synthetic biotechnology for efficient light-driven carbon fixation and ethanol production [J]. Synthetic Biology Journal, 2023, 4(6): 1161-1177. |
| [10] | YAN Xiongying, WANG Zhen, LOU Jiyun, ZHANG Haoyu, HUANG Xingyu, WANG Xia, YANG Shihui. Progress in the construction of microbial cell factories for efficient biofuel production [J]. Synthetic Biology Journal, 2023, 4(6): 1082-1121. |
| [11] | CHENG Zhenzhen, ZHANG Jian, GAO Cong, LIU Liming, CHEN Xiulai. Progress in metabolic engineering of microorganisms for the utilization of formate [J]. Synthetic Biology Journal, 2023, 4(4): 756-778. |
| [12] | LIU Jiayu, YANG Zhihan, YANG Lei, ZHU Liying, ZHU Zhengming, JIANG Ling. Advances in the development of Clostridium tyrobutyricum cell factories driven by synthetic biotechnology [J]. Synthetic Biology Journal, 2022, 3(6): 1174-1200. |
| [13] | GUO Shuyuan, WU Lianghuan, LIU Xiangjian, WANG Bo, YU Tao. Developing C1-based metabolic network in methylotrophy for biotransformation [J]. Synthetic Biology Journal, 2022, 3(1): 116-137. |
| [14] | CHEN Jiuzhou, WANG Yu, PU Wei, ZHENG Ping, SUN Jibin. Advances and perspective on bioproduction of 5-aminolevulinic acid [J]. Synthetic Biology Journal, 2021, 2(6): 1000-1016. |
| [15] | WANG Qingzhuo, SONG Ping, HUANG He. Synthetic biotechnology drives the development of natural eukaryotic lipid cell factories [J]. Synthetic Biology Journal, 2021, 2(6): 920-941. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||