Synthetic Biology Journal ›› 2024, Vol. 5 ›› Issue (6): 1419-1436.DOI: 10.12211/2096-8280.2024-016
• Invited Review • Previous Articles Next Articles
Bioproduction based on extremophiles
SHAO Mingwei1, SUN Simian1, YANG Shimao1, CHEN Guoqiang1,2,3
- 1.School of Life Sciences,Tsinghua University,Beijing 100084,China
2.Center for Synthetic and Systems Biology,Tsinghua University,Beijing 100084,China
3.MOE Key Lab of Industrial Biocatalysis,Department of Chemical Engineering,Tsinghua University,Beijing 100084,China
-
Received:2024-02-04Revised:2024-04-27Online:2025-01-10Published:2024-12-31 -
Contact:CHEN Guoqiang
基于极端微生物的生物制造
邵明威1, 孙思勉1, 杨时茂1, 陈国强1,2,3
- 1.清华大学生命科学学院,北京 100084
2.清华大学合成与系统生物学中心,北京 100084
3.清华大学化学工程系,教育部工业生物催化重点实验室,北京 100084
-
通讯作者:陈国强 -
作者简介:邵明威 (2001—),男,博士研究生。研究方向为盐单胞菌进化系统构建与应用。E-mail:shaomingwei@phalab.org陈国强 (1963—),男,博士,教授。研究方向为微生物聚羟基脂肪酸酯(PHA)的合成、代谢和应用。E-mail:chengq@mail.tsinghua.edu.cn -
基金资助:国家自然科学基金(32130001)
CLC Number:
Cite this article
SHAO Mingwei, SUN Simian, YANG Shimao, CHEN Guoqiang. Bioproduction based on extremophiles[J]. Synthetic Biology Journal, 2024, 5(6): 1419-1436.
邵明威, 孙思勉, 杨时茂, 陈国强. 基于极端微生物的生物制造[J]. 合成生物学, 2024, 5(6): 1419-1436.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2024-016
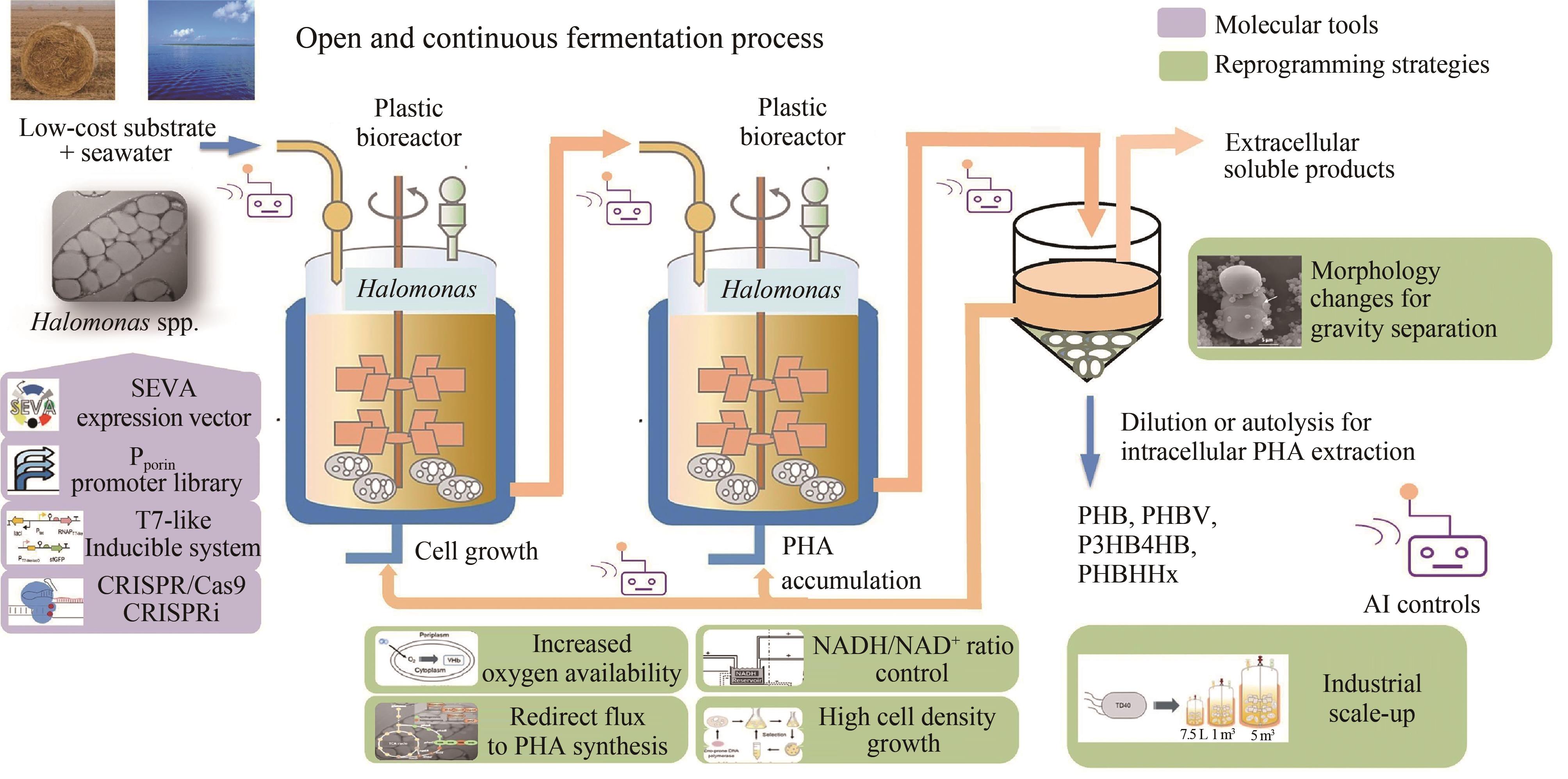
Fig. 1 Next generation industrial biotechnology based on extremophiles represented by Halomonas spp. [12](Production of intracellular PHA products or proteins and extracelullar small molecules by recombinant Halomonas grown on low-cost substrates in seawater under open unsterile and continuous processes conducted in plastic or other low cost bioreactors)
| 类型 | 生长环境 | 代表微生物 | 生产应用 | 参考文献 |
|---|---|---|---|---|
| 嗜盐菌 | 高盐高碱(NaCl>30 g/L, pH 8~10) | 盐单胞菌:H. bluephagenesis TD01,H.campaniensis LS21,H. smyrnensis AAD6 | 生产PHA(以H. bluephagenesis为例:细胞干重大于80 g/L,PHB质量分数大于90%),四氢嘧啶[以H. bluephagenesis为例,生产滴度达85 g/L,生产速率达1 g/(L·h)]等 | [ |
| 嗜热菌 | 高温(>45 ℃) | 热细菌:Fervidobacterium thermophilum,Thermoanaerobacterium saccharolyticum | 生产生物燃料[以Thermoanaerobacter sp.X514为例,正丁醇生产滴度达357 mg/L,生产速率达2.975 g/(L·h)],分离热稳定酶(纤维素酶,角质溶解酶)等 | [ |
| 嗜酸/碱菌 | 极酸或极碱(pH<3,pH>10) | 嗜酸氧化亚铁硫杆菌Acidithiobacillus ferrooxidans,嗜碱细菌Clostridium alkalicellulosi | 生产有机酸(以Issatchenkia orientalis SD108为例,琥珀酸生产滴度达到11.63 g/L) | [ |
Table 1 Characteristics and production applications of various types of extremophiles
| 类型 | 生长环境 | 代表微生物 | 生产应用 | 参考文献 |
|---|---|---|---|---|
| 嗜盐菌 | 高盐高碱(NaCl>30 g/L, pH 8~10) | 盐单胞菌:H. bluephagenesis TD01,H.campaniensis LS21,H. smyrnensis AAD6 | 生产PHA(以H. bluephagenesis为例:细胞干重大于80 g/L,PHB质量分数大于90%),四氢嘧啶[以H. bluephagenesis为例,生产滴度达85 g/L,生产速率达1 g/(L·h)]等 | [ |
| 嗜热菌 | 高温(>45 ℃) | 热细菌:Fervidobacterium thermophilum,Thermoanaerobacterium saccharolyticum | 生产生物燃料[以Thermoanaerobacter sp.X514为例,正丁醇生产滴度达357 mg/L,生产速率达2.975 g/(L·h)],分离热稳定酶(纤维素酶,角质溶解酶)等 | [ |
| 嗜酸/碱菌 | 极酸或极碱(pH<3,pH>10) | 嗜酸氧化亚铁硫杆菌Acidithiobacillus ferrooxidans,嗜碱细菌Clostridium alkalicellulosi | 生产有机酸(以Issatchenkia orientalis SD108为例,琥珀酸生产滴度达到11.63 g/L) | [ |
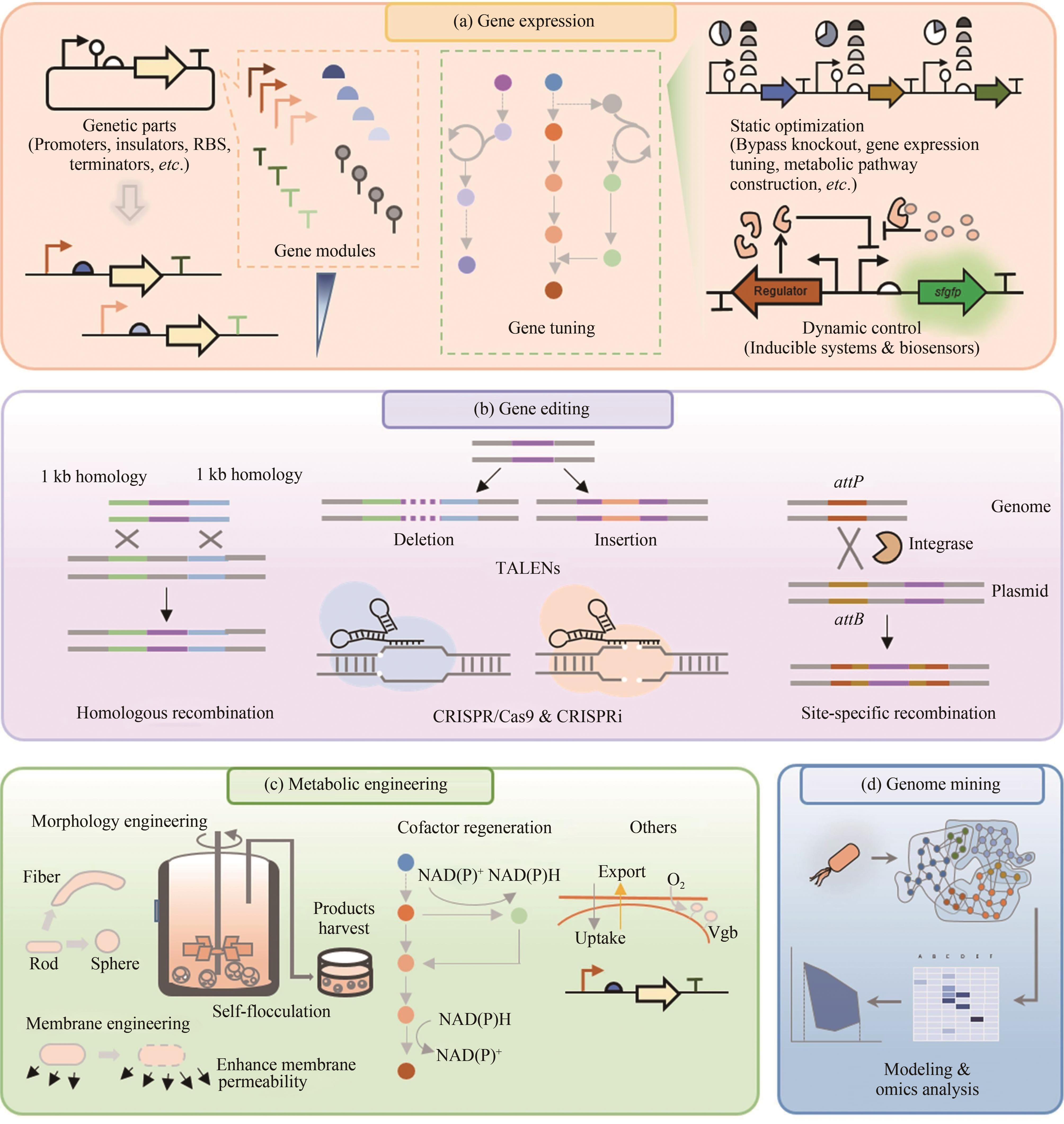
Fig. 2 Genetic and metabolic engineering of extremophiles[51](a) Gene expression regulatory elements and tools. Genetic elements include promoters, insulators, ribosome binding sites, and terminators. Gene regulation includes static regulation such as bypass knockout, pathway overexpression, and dynamic regulation such as chemical-induction system, biosensors; (b) Gene editing tools include homologous recombination, TALENs, CRISPR/Cas9 and specific-site recombination; (c) Metabolic engineering includes morphological engineering, membrane engineering, regulation of NAD(P)+/NAD(P)H ratio, and expression of hemoglobin; (D) Genome mining. The applications of omics data to build metabolic models for prediction of new elements, enzymes, and pathways
| 工程化改造思路 | 具体策略 | 参考文献 |
|---|---|---|
| 基因表达元件工程 | porin启动子,类T7表达系统,响应十种小分子诱导物的多重诱导系统 | [ |
| 基因编辑技术 | CRISPR/Cas9系统,CRISPRi系统,CRISPR/AID系统,sRNA调控系统 | [ |
| 生物传感器与动态调控 | 感知油酸、群体感应信号分子AHL调控系统,荧光定量PHA含量(qPHA) | [ |
| 命运共同体策略 | 将PHA合成基因插入必需基因ompW启动子后共表达 | [ |
| 形态学工程 | 抑制细胞骨架基因mreB,ftsZ的表达 | [ |
| 稳定质粒表达载体 | 基于盐单胞菌内源性质粒中的hbpB/hbpC毒素-抗毒素系统 | [ |
Table 2 Genetic engineering of Halomonas spp.
| 工程化改造思路 | 具体策略 | 参考文献 |
|---|---|---|
| 基因表达元件工程 | porin启动子,类T7表达系统,响应十种小分子诱导物的多重诱导系统 | [ |
| 基因编辑技术 | CRISPR/Cas9系统,CRISPRi系统,CRISPR/AID系统,sRNA调控系统 | [ |
| 生物传感器与动态调控 | 感知油酸、群体感应信号分子AHL调控系统,荧光定量PHA含量(qPHA) | [ |
| 命运共同体策略 | 将PHA合成基因插入必需基因ompW启动子后共表达 | [ |
| 形态学工程 | 抑制细胞骨架基因mreB,ftsZ的表达 | [ |
| 稳定质粒表达载体 | 基于盐单胞菌内源性质粒中的hbpB/hbpC毒素-抗毒素系统 | [ |
| 产物 | 碳源/底物 | 改造策略 | 产量 | 参考文献 |
|---|---|---|---|---|
| PHB | 葡萄糖,淀粉水解物,厨余垃圾混合碳源 | 限氧,限氮;平衡NADH+/NAD比例,补充乙酸 | 细胞干重大于80 g/L,PHB质量分数大于90% | [ |
| 3-羟基丁酸与4-羟基丁酸共聚物P(3HB-co-4HB) | 葡萄糖,葡萄糖酸盐废弃物,废弃玉米浆,γ-丁内酯 | 构建两条相互关联的4-羟基丁酸(4HB)生物合成途径,表达4-羟基丁酸转移酶基因orfZ;敲除琥珀酸半醛脱氢酶基因gadD;构建数学模型与理性计算辅助设计;敲除外膜相关基因lpxL和lpxM | 7 L发酵罐产生26.3 g/L细胞干重,包含质量分数60.5%的P(3HB-co-4HB),其中4HB的摩尔分数为17.04%;5 L发酵罐中产生100 g/L细胞干重包含质量分数60.4%的 P(3HB-co-4HB),其中4HB的摩尔分数为13.5%;敲除外膜菌H. bluephagenesis WZY254在7 L发酵罐产生84 g/L细胞干重包含质量分数81%的P(3HB-co-4HB),其中4HB的摩尔分数为26% | [ |
| 3-羟基丁酸与3-羟基戊酸共聚物(PHBV) | 葡萄糖,葡萄糖酸钠 | 敲低或敲除2-甲基柠檬酸合成酶基因prpC;敲除TCA循环相关基因sdhE和icl;在染色体上表达编码磷酸烯醇式丙酮酸羧化酶基因ppc | 摇瓶中6.3 g/L细胞干重,包含质量分数65%的PHBV,其中3HV摩尔分数达到35% | [ |
| 3-羟基丁酸与3-羟基己酸共聚物(PHBHHx) | 葡萄糖,己酸钠 | 表达来自Aeromonas caviae FA440的PHA合成酶基因phaCac和烯酰辅酶-A水合酶基因phaJac | 7 L发酵罐产生33.1 g/L细胞干重,包含质量分数50.32%的P(3HB-co-37.23% 3HHx) ,3HHx摩尔比例可以在0%~37%范围调控 | [ |
| PHA颗粒相蛋白(PhaP) | 葡萄糖 | 敲除phaC基因,在基因组上过表达phaP基因 | PhaP累积量占比19%,产量为1.86 g/L | [ |
| 淀粉酶,葡萄糖苷酶,PHA,小分子氨基酸(L-苏氨酸,L-赖氨酸) | 淀粉 | 筛选合适的信号肽和连接子将过表达的α-淀粉酶和葡萄糖苷酶分泌到胞外,异源表达5个L-苏氨酸合成基因和外排转运蛋白,敲除内运转运蛋白和L-苏氨酸脱氢酶;过表达L-赖氨酸合成相关基因,解除底物抑制效应,增强L-赖氨酸外排能力 | 以淀粉作为唯一碳源生产PHA、四氢嘧啶、苏氨酸等多种产品 | [ |
| 葡萄糖 | 7 L发酵罐生产苏氨酸,产量33 g/L;7 L发酵罐生产赖氨酸,产量22.59 g/L | [ | ||
| 戊二胺 | 赖氨酸 | 异源表达赖氨酸脱羧酶基因CadA, LdcC | 7 L发酵罐生产戊二胺,产量118 g/L | [ |
| 四氢嘧啶 | 葡萄糖 | 理性调控和四氢嘧啶合成相关的ectABC、lysC和asd三个基因簇,提高前体供应,增强产物转运系统,优化培养条件 | 7 L发酵罐生产四氢嘧啶,产量85 g/L | [ |
| 5-氨基戊酸 | 赖氨酸 | 敲除gabT基因,在基因组上表达三个拷贝的dvaBA基因 | 7 L发酵罐生产5-氨基戊酸,产量67.4 g/L | [ |
| 3-羟基丙酸 | 葡萄糖,1,3-丙二醇 | 理性调控3-羟基丙酸合成相关的AldDHb和AdhP基因,敲除降解基因DddA | 7 L发酵罐生产3-羟基丙酸,产量154 g/L | [ |
| 乙偶姻 | 丙酮酸 | 异源表达枯草芽孢杆菌α-乙酰乳酸合酶基因alsS和α-乙酰乳酸脱羧酶基因alsD | 全细胞催化生产乙偶姻,产量85.84 g/L | [ |
| 衣康酸 | 柠檬酸 | 表达顺乌头酸脱羧酶编码基因cadA和顺乌头酸酶编码基因acn;表达分子伴侣GroESL;增加编码限速酶基因acn的拷贝数以及弱化竞争途径 | 摇瓶中生产衣康酸,产量63.60 g/L | [ |
| 甲羟戊酸 | 葡萄糖 | 敲除phaB和phaC基因;异源表达甲羟戊酸合成基因HMG-CoA合成酶和HMG-CoA-还原酶;CIRSPRi技术敲低50个候选基因;引入非氧糖酵解通路(NOG通路)减少碳损失 | 5 L发酵罐中生产甲羟戊酸,产量121 g/L | [ |
Table 3 Various products based on Halomonas spp.
| 产物 | 碳源/底物 | 改造策略 | 产量 | 参考文献 |
|---|---|---|---|---|
| PHB | 葡萄糖,淀粉水解物,厨余垃圾混合碳源 | 限氧,限氮;平衡NADH+/NAD比例,补充乙酸 | 细胞干重大于80 g/L,PHB质量分数大于90% | [ |
| 3-羟基丁酸与4-羟基丁酸共聚物P(3HB-co-4HB) | 葡萄糖,葡萄糖酸盐废弃物,废弃玉米浆,γ-丁内酯 | 构建两条相互关联的4-羟基丁酸(4HB)生物合成途径,表达4-羟基丁酸转移酶基因orfZ;敲除琥珀酸半醛脱氢酶基因gadD;构建数学模型与理性计算辅助设计;敲除外膜相关基因lpxL和lpxM | 7 L发酵罐产生26.3 g/L细胞干重,包含质量分数60.5%的P(3HB-co-4HB),其中4HB的摩尔分数为17.04%;5 L发酵罐中产生100 g/L细胞干重包含质量分数60.4%的 P(3HB-co-4HB),其中4HB的摩尔分数为13.5%;敲除外膜菌H. bluephagenesis WZY254在7 L发酵罐产生84 g/L细胞干重包含质量分数81%的P(3HB-co-4HB),其中4HB的摩尔分数为26% | [ |
| 3-羟基丁酸与3-羟基戊酸共聚物(PHBV) | 葡萄糖,葡萄糖酸钠 | 敲低或敲除2-甲基柠檬酸合成酶基因prpC;敲除TCA循环相关基因sdhE和icl;在染色体上表达编码磷酸烯醇式丙酮酸羧化酶基因ppc | 摇瓶中6.3 g/L细胞干重,包含质量分数65%的PHBV,其中3HV摩尔分数达到35% | [ |
| 3-羟基丁酸与3-羟基己酸共聚物(PHBHHx) | 葡萄糖,己酸钠 | 表达来自Aeromonas caviae FA440的PHA合成酶基因phaCac和烯酰辅酶-A水合酶基因phaJac | 7 L发酵罐产生33.1 g/L细胞干重,包含质量分数50.32%的P(3HB-co-37.23% 3HHx) ,3HHx摩尔比例可以在0%~37%范围调控 | [ |
| PHA颗粒相蛋白(PhaP) | 葡萄糖 | 敲除phaC基因,在基因组上过表达phaP基因 | PhaP累积量占比19%,产量为1.86 g/L | [ |
| 淀粉酶,葡萄糖苷酶,PHA,小分子氨基酸(L-苏氨酸,L-赖氨酸) | 淀粉 | 筛选合适的信号肽和连接子将过表达的α-淀粉酶和葡萄糖苷酶分泌到胞外,异源表达5个L-苏氨酸合成基因和外排转运蛋白,敲除内运转运蛋白和L-苏氨酸脱氢酶;过表达L-赖氨酸合成相关基因,解除底物抑制效应,增强L-赖氨酸外排能力 | 以淀粉作为唯一碳源生产PHA、四氢嘧啶、苏氨酸等多种产品 | [ |
| 葡萄糖 | 7 L发酵罐生产苏氨酸,产量33 g/L;7 L发酵罐生产赖氨酸,产量22.59 g/L | [ | ||
| 戊二胺 | 赖氨酸 | 异源表达赖氨酸脱羧酶基因CadA, LdcC | 7 L发酵罐生产戊二胺,产量118 g/L | [ |
| 四氢嘧啶 | 葡萄糖 | 理性调控和四氢嘧啶合成相关的ectABC、lysC和asd三个基因簇,提高前体供应,增强产物转运系统,优化培养条件 | 7 L发酵罐生产四氢嘧啶,产量85 g/L | [ |
| 5-氨基戊酸 | 赖氨酸 | 敲除gabT基因,在基因组上表达三个拷贝的dvaBA基因 | 7 L发酵罐生产5-氨基戊酸,产量67.4 g/L | [ |
| 3-羟基丙酸 | 葡萄糖,1,3-丙二醇 | 理性调控3-羟基丙酸合成相关的AldDHb和AdhP基因,敲除降解基因DddA | 7 L发酵罐生产3-羟基丙酸,产量154 g/L | [ |
| 乙偶姻 | 丙酮酸 | 异源表达枯草芽孢杆菌α-乙酰乳酸合酶基因alsS和α-乙酰乳酸脱羧酶基因alsD | 全细胞催化生产乙偶姻,产量85.84 g/L | [ |
| 衣康酸 | 柠檬酸 | 表达顺乌头酸脱羧酶编码基因cadA和顺乌头酸酶编码基因acn;表达分子伴侣GroESL;增加编码限速酶基因acn的拷贝数以及弱化竞争途径 | 摇瓶中生产衣康酸,产量63.60 g/L | [ |
| 甲羟戊酸 | 葡萄糖 | 敲除phaB和phaC基因;异源表达甲羟戊酸合成基因HMG-CoA合成酶和HMG-CoA-还原酶;CIRSPRi技术敲低50个候选基因;引入非氧糖酵解通路(NOG通路)减少碳损失 | 5 L发酵罐中生产甲羟戊酸,产量121 g/L | [ |
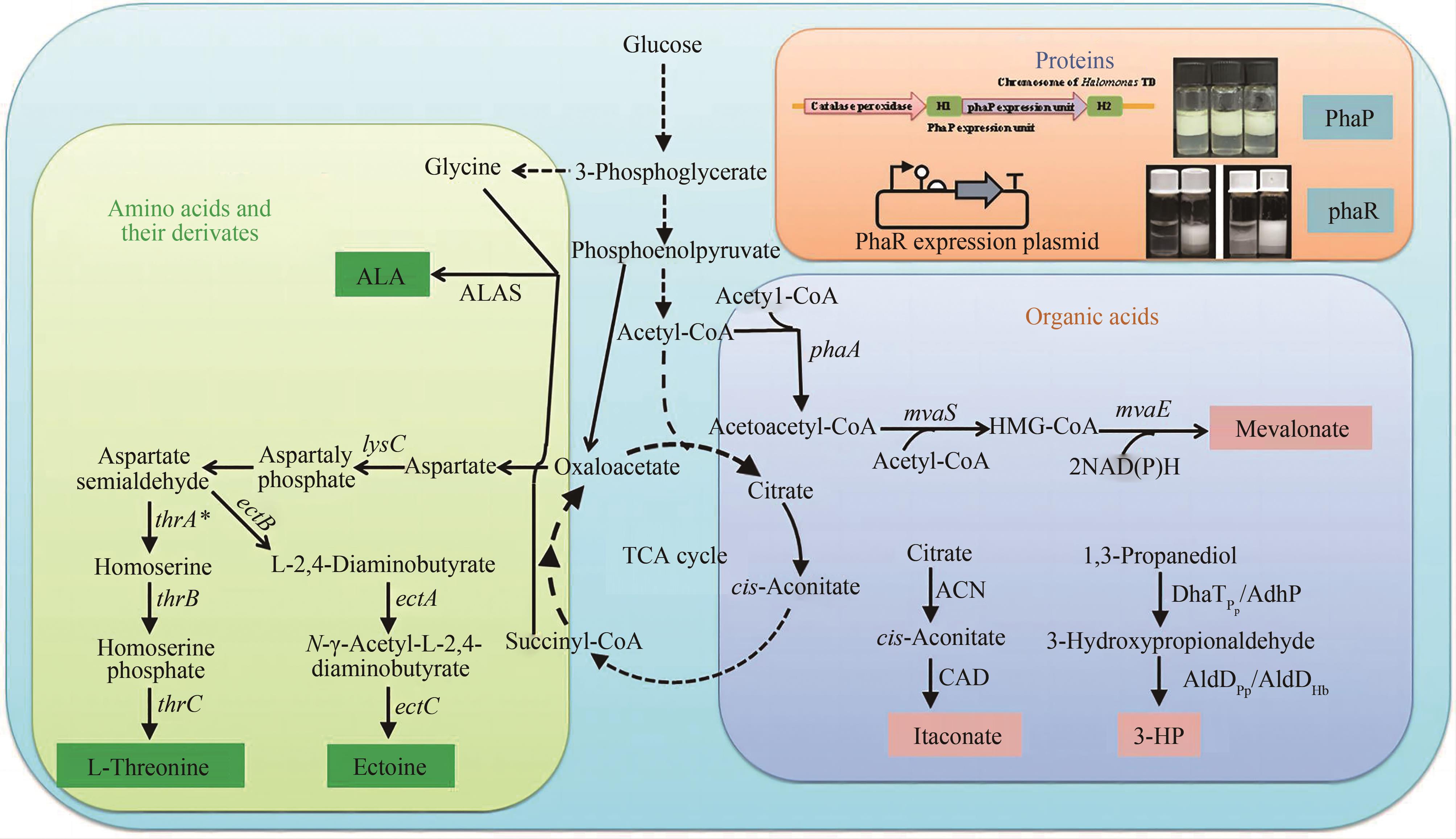
Fig. 3 Diverse chemical compounds produced by Halomonas spp. [107](H. bluephagenesis has been engineered to produce non-PHA chemicals including proteins, amino acids, and their derivates, organic acids.) ALAS—5-aminolevulinic acid synthase; lysC—gene of aspartokinase; thrA*—gene of homoserine dehydrogenase mutant at G433R from Escherichia coli MG1655; thrB—gene of homoserine kinase; thrC—gene of L-threonine synthase; ectA—encoding L-2,4-diaminobutyrate acetyltransferase; ectB—encoding L-2,4-diaminobutyrate transaminase; ectC—encoding ectoine synthase; ACN—aconitase; CAD—cis-aconitate decarboxylase; DhaTPp and AdhP—alcohol dehydrogenase and aldehyde dehydrogenase; AldDPp—aldehyde dehydrogenase from Pseudomonas putida KT2440; AldDHb—aldehyde dehydrogenase from Halomonas bluephagenesis; phaA—encoding 3-ketothiolase; mvaS—encoding HMG-CoA synthase; mvaE—encoding HMG-CoA reductase
| 类型 | 未来产品 | 优势 |
|---|---|---|
| 嗜冷菌 | 蛋白质或酶 | 在胞内不容易形成包涵体 |
| 嗜热菌 | 挥发性小分子化合物 | 直接蒸馏提纯产品,简化处理工艺 |
| 嗜酸菌 | 酸性化合物(例如有机酸) | 耐受高浓度酸性产物 |
| 嗜碱菌 | 碱性化合物(例如赖氨酸) | 耐受高浓度碱性产物 |
| 嗜盐菌 | 饲料蛋白与酸性小分子化合物 | 开放式发酵,无需灭菌,耐渗透压 |
Table 4 Products based on extremophiles in the future
| 类型 | 未来产品 | 优势 |
|---|---|---|
| 嗜冷菌 | 蛋白质或酶 | 在胞内不容易形成包涵体 |
| 嗜热菌 | 挥发性小分子化合物 | 直接蒸馏提纯产品,简化处理工艺 |
| 嗜酸菌 | 酸性化合物(例如有机酸) | 耐受高浓度酸性产物 |
| 嗜碱菌 | 碱性化合物(例如赖氨酸) | 耐受高浓度碱性产物 |
| 嗜盐菌 | 饲料蛋白与酸性小分子化合物 | 开放式发酵,无需灭菌,耐渗透压 |
| 1 | GAVRILESCU M, CHISTI Y. Biotechnology - a sustainable alternative for chemical industry[J]. Biotechnology Advances, 2005, 23(7/8): 471-499. |
| 2 | KIRCHER M. Bioeconomy - present status and future needs of industrial value chains[J]. New Biotechnology, 2021, 60: 96-104. |
| 3 | RITTMANN B E. Opportunities for renewable bioenergy using microorganisms[J]. Biotechnology and Bioengineering, 2008, 100(2): 203-212. |
| 4 | LEVI P G, CULLEN J M. Mapping global flows of chemicals: from fossil fuel feedstocks to chemical products[J]. Environmental Science & Technology, 2018, 52(4): 1725-1734. |
| 5 | SHELDON R A, BRADY D. Green chemistry, biocatalysis, and the chemical industry of the future[J]. ChemSusChem, 2022, 15(9): e202102628. |
| 6 | GARTLAND K M, BRUSCHI F, DUNDAR M, et al. Progress towards the ‘golden age’ of biotechnology[J]. Current Opinion in Biotechnology, 2013, 24(): S6-S13. |
| 7 | CHEN G Q, JIANG X R. Next generation industrial biotechnology based on extremophilic bacteria[J]. Current Opinion in Biotechnology, 2018, 50: 94-100. |
| 8 | ROBINSON C J, CARBONELL P, JERVIS A J, et al. Rapid prototyping of microbial production strains for the biomanufacture of potential materials monomers[J]. Metabolic Engineering, 2020, 60: 168-182. |
| 9 | DE LORENZO V, KRASNOGOR N, SCHMIDT M. For the sake of the bioeconomy: define what a Synthetic Biology Chassis is![J]. New Biotechnology, 2021, 60: 44-51. |
| 10 | ZHANG X, LIN Y N, CHEN G Q. Halophiles as chassis for bioproduction[J]. Advanced Biosystems, 2018, 2(11): 1800088. |
| 11 | YU L P, WU F Q, CHEN G Q. Next-generation industrial biotechnology-transforming the current industrial biotechnology into competitive processes[J]. Biotechnology Journal, 2019, 14(9): e1800437. |
| 12 | TAN D, WANG Y, TONG Y, et al. Grand challenges for industrializing polyhydroxyalkanoates (PHAs)[J]. Trends in Biotechnology, 2021, 39(9): 953-963. |
| 13 | ORELLANA R, MACAYA C, BRAVO G, et al. Living at the frontiers of life: extremophiles in Chile and their potential for bioremediation[J]. Frontiers in Microbiology, 2018, 9: 2309. |
| 14 | DALMASSO C, OGER P, SELVA G, et al. Thermococcus piezophilus sp. nov., a novel hyperthermophilic and piezophilic archaeon with a broad pressure range for growth, isolated from a deepest hydrothermal vent at the Mid-Cayman Rise[J]. Systematic and Applied Microbiology, 2016, 39(7): 440-444. |
| 15 | FELLER G. Protein folding at extreme temperatures: current issues[J]. Seminars in Cell & Developmental Biology, 2018, 84: 129-137. |
| 16 | RAMPELOTTO P H. Extremophiles and extreme environments[J]. Life, 2013, 3(3): 482-485. |
| 17 | REKADWAD B, GONZALEZ J M. Multidisciplinary involvement and potential of thermophiles[J]. Folia Microbiologica, 2019, 64(3): 389-406. |
| 18 | REKADWAD B N, LI W J, GONZALEZ J M, et al. Extremophiles: the species that evolve and survive under hostile conditions[J]. 3 Biotech, 2023, 13(9): 316. |
| 19 | MESBAH N M, WIEGEL J. Life under multiple extreme conditions: diversity and physiology of the halophilic alkalithermophiles[J]. Applied and Environmental Microbiology, 2012, 78(12): 4074-4082. |
| 20 | CHEN X B, YIN J, YE J W, et al. Engineering Halomonas bluephagenesis TD01 for non-sterile production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate)[J]. Bioresource Technology, 2017, 244(Pt 1): 534-541. |
| 21 | OBULISAMY P K, MEHARIYA S. Polyhydroxyalkanoates from extremophiles: a review[J]. Bioresource Technology, 2021, 325: 124653. |
| 22 | QUILLAGUAMÁN J, GUZMÁN H, VAN-THUOC D, et al. Synthesis and production of polyhydroxyalkanoates by halophiles: current potential and future prospects[J]. Applied Microbiology and Biotechnology, 2010, 85(6): 1687-1696. |
| 23 | EDBEIB M F, WAHAB R A, HUYOP F. Halophiles: biology, adaptation, and their role in decontamination of hypersaline environments[J]. World Journal of Microbiology & Biotechnology, 2016, 32(8): 135. |
| 24 | MARGESIN R, SCHINNER F. Bioremediation (natural attenuation and biostimulation) of diesel-oil-contaminated soil in an alpine glacier skiing area[J]. Applied and Environmental Microbiology, 2001, 67(7): 3127-3133. |
| 25 | MA H, ZHAO Y Q, HUANG W Z, et al. Rational flux-tuning of Halomonas bluephagenesis for co-production of bioplastic PHB and ectoine[J]. Nature Communications, 2020, 11(1): 3313. |
| 26 | DELGADO-GARCÍA M, VALDIVIA-URDIALES B, AGUILAR-GONZÁLEZ C N, et al. Halophilic hydrolases as a new tool for the biotechnological industries[J]. Journal of the Science of Food and Agriculture, 2012, 92(13): 2575-2580. |
| 27 | YIN J, CHEN J C, WU Q, et al. Halophiles, coming stars for industrial biotechnology[J]. Biotechnology Advances, 2015, 33(7): 1433-1442. |
| 28 | TAN D, WU Q, CHEN J C, et al. Engineering Halomonas TD01 for the low-cost production of polyhydroxyalkanoates[J]. Metabolic Engineering, 2014, 26: 34-47. |
| 29 | TAO W, LV L, CHEN G Q. Engineering Halomonas species TD01 for enhanced polyhydroxyalkanoates synthesis via CRISPRi[J]. Microbial Cell Factories, 2017, 16(1): 48. |
| 30 | YE J W, HUANG W Z, WANG D S, et al. Pilot scale-up of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) production by Halomonas bluephagenesis via cell growth adapted optimization process[J]. Biotechnology Journal, 2018, 13(5): e1800074. |
| 31 | TAN D, XUE Y S, AIBAIDULA G, et al. Unsterile and continuous production of polyhydroxybutyrate by Halomonas TD01[J]. Bioresource Technology, 2011, 102(17): 8130-8136. |
| 32 | YUE H T, LING C, YANG T, et al. A seawater-based open and continuous process for polyhydroxyalkanoates production by recombinant Halomonas campaniensis LS21 grown in mixed substrates[J]. Biotechnology for Biofuels, 2014, 7(1): 108. |
| 33 | SARILMISER H K, ATES O, OZDEMIR G, et al. Effective stimulating factors for microbial levan production by Halomonas smyrnensis AAD6T[J]. Journal of Bioscience and Bioengineering, 2015, 119(4): 455-463. |
| 34 | SOMAYAJI A, DHANJAL C R, LINGAMSETTY R, et al. An insight into the mechanisms of homeostasis in extremophiles[J]. Microbiological Research, 2022, 263: 127115. |
| 35 | NARSING RAO M P, LUO Z H, DONG Z Y, et al. Metagenomic analysis further extends the role of Chloroflexi in fundamental biogeochemical cycles[J]. Environmental Research, 2022, 209: 112888. |
| 36 | ZHU D C, ADEBISI W A, AHMAD F, et al. Recent development of extremophilic bacteria and their application in biorefinery[J]. Frontiers in Bioengineering and Biotechnology, 2020, 8: 483. |
| 37 | SAXENA R, DHAKAN D B, MITTAL P, et al. Metagenomic analysis of hot springs in central India reveals hydrocarbon degrading thermophiles and pathways essential for survival in extreme environments[J]. Frontiers in Microbiology, 2017, 7: 2123. |
| 38 | DAMER B, DEAMER D. The hot spring hypothesis for an origin of life[J]. Astrobiology, 2020, 20(4): 429-452. |
| 39 | LV Z B, DING J X, WANG H, et al. Isolation of a novel thermophilic methanogen and the evolutionary history of the class Methanobacteria [J]. Biology, 2022, 11(10): 1514. |
| 40 | KIM J S, KLUSKENS L D, DE VOS W M, et al. Crystal structure of fervidolysin from Fervidobacterium pennivorans, a keratinolytic enzyme related to subtilisin[J]. Journal of Molecular Biology, 2004, 335(3): 787-797. |
| 41 | ATALAH J, CÁCERES-MORENO P, ESPINA G, et al. Thermophiles and the applications of their enzymes as new biocatalysts[J]. Bioresource Technology, 2019, 280: 478-488. |
| 42 | KHONGTO B, LAOTENG K, TONGTA A. Fermentation process development of recombinant Hansenula polymorpha for gamma-linolenic acid production[J]. Journal of Microbiology and Biotechnology, 2010, 20(11): 1555-1562. |
| 43 | BHANDIWAD A, SHAW A J, GUSS A, et al. Metabolic engineering of Thermoanaerobacterium saccharolyticum for n-butanol production[J]. Metabolic Engineering, 2014, 21: 17-25. |
| 44 | TIAN L, CONWAY P M, CERVENKA N D, et al. Metabolic engineering of Clostridium thermocellum for n-butanol production from cellulose[J]. Biotechnology for Biofuels, 2019, 12: 186. |
| 45 | KRULWICH T A, SACHS G, PADAN E. Molecular aspects of bacterial pH sensing and homeostasis[J]. Nature Reviews Microbiology, 2011, 9(5): 330-343. |
| 46 | LEIGH M B, WU W M, CARDENAS E, et al. Microbial communities biostimulated by ethanol during uranium (Ⅵ) bioremediation in contaminated sediment as shown by stable isotope probing[J]. Frontiers of Environmental Science & Engineering, 2015, 9(3): 453-464. |
| 47 | ARULAZHAGAN P, AL-SHEKRI K, HUDA Q, et al. Biodegradation of polycyclic aromatic hydrocarbons by an acidophilic Stenotrophomonas maltophilia strain AJH1 isolated from a mineral mining site in Saudi Arabia[J]. Extremophiles, 2017, 21(1): 163-174. |
| 48 | XIAO H, SHAO Z Y, JIANG Y, et al. Exploiting Issatchenkia orientalis SD108 for succinic acid production[J]. Microbial Cell Factories, 2014, 13: 121. |
| 49 | JUNG H, INABA Y, BANTA S. Genetic engineering of the acidophilic chemolithoautotroph Acidithiobacillus ferrooxidans [J]. Trends in Biotechnology, 2022, 40(6): 677-692. |
| 50 | SOUSA J A, SOROKIN D Y, BIJMANS M F, et al. Ecology and application of haloalkaliphilic anaerobic microbial communities[J]. Applied Microbiology and Biotechnology, 2015, 99(22): 9331-9336. |
| 51 | YE J W, LIN Y N, YI X Q, et al. Synthetic biology of extremophiles: a new wave of biomanufacturing[J]. Trends in Biotechnology, 2023, 41(3): 342-357. |
| 52 | LI T T, LI T, JI W Y, et al. Engineering of core promoter regions enables the construction of constitutive and inducible promoters in Halomonas sp[J]. Biotechnology Journal, 2016, 11(2): 219-227. |
| 53 | SHEN R, YIN J, YE J W, et al. Promoter engineering for enhanced P(3HB-co-4HB) production by Halomonas bluephagenesis [J]. ACS Synthetic Biology, 2018, 7(8): 1897-1906. |
| 54 | OLSON D G, MALONEY M, LANAHAN A A, et al. Identifying promoters for gene expression in Clostridium thermocellum [J]. Metabolic Engineering Communications, 2015, 2: 23-29. |
| 55 | ZHANG Y T, LIU H L, LIU Y J, et al. A promoter engineering-based strategy enhances polyhydroxyalkanoate production in Pseudomonas putida KT2440[J]. International Journal of Biological Macromolecules, 2021, 191: 608-617. |
| 56 | SUN W H, JIANG B, ZHAO D Y, et al. Integration of metabolic pathway manipulation and promoter engineering for the fine-tuned biosynthesis of malic acid in Bacillus coagulans [J]. Biotechnology and Bioengineering, 2021, 118(7): 2597-2608. |
| 57 | KERNAN T, MAJUMDAR S, LI X Z, et al. Engineering the iron-oxidizing chemolithoautotroph Acidithiobacillus ferrooxidans for biochemical production[J]. Biotechnology and Bioengineering, 2016, 113(1): 189-197. |
| 58 | WERNICK D G, PONTRELLI S P, POLLOCK A W, et al. Sustainable biorefining in wastewater by engineered extreme alkaliphile Bacillus marmarensis [J]. Scientific Reports, 2016, 6: 20224. |
| 59 | MA Y Y, YE J W, LIN Y N, et al. Flux optimization using multiple promoters in Halomonas bluephagenesis as a model chassis of the next generation industrial biotechnology[J]. Metabolic Engineering, 2024, 81: 249-261. |
| 60 | WANG L J, JIANG X R, HOU J, et al. Engineering Halomonas bluephagenesis via small regulatory RNAs[J]. Metabolic Engineering, 2022, 73: 58-69. |
| 61 | LIU X, LI D J, YAN X, et al. Rapid quantification of polyhydroxyalkanoates accumulated in living cells based on green fluorescence protein-labeled phasins: the qPHA method[J]. Biomacromolecules, 2022, 23(10): 4153-4166. |
| 62 | ZHAO D H, CAI L, WU J H, et al. Improving polyhydroxyalkanoate production by knocking out the genes involved in exopolysaccharide biosynthesis in Haloferax mediterranei [J]. Applied Microbiology and Biotechnology, 2013, 97(7): 3027-3036. |
| 63 | MARX C J, LIDSTROM M E. Broad-host-range cre-lox system for antibiotic marker recycling in gram-negative bacteria[J]. BioTechniques, 2002, 33(5): 1062-1067. |
| 64 | XU J Q, YANG S, YANG L R. Vibrio natriegens as a host for rapid biotechnology[J]. Trends in Biotechnology, 2022, 40(4): 381-384. |
| 65 | INABA Y, BANERJEE I, KERNAN T, et al. Transposase-mediated chromosomal integration of exogenous genes in Acidithiobacillus ferrooxidans [J]. Applied and Environmental Microbiology, 2018, 84(21): e01381-18. |
| 66 | ZHAO C H, ZHENG T R, FENG Y H, et al. Engineered Halomonas spp. for production of L-lysine and cadaverine[J]. Bioresource Technology, 2022, 349: 126865. |
| 67 | XU T, CHEN J Y, MITRA R, et al. Deficiency of exopolysaccharides and O-antigen makes Halomonas bluephagenesis self-flocculating and amenable to electrotransformation[J]. Communications Biology, 2022, 5(1): 623. |
| 68 | WANG J T, WEI J W, LI H J, et al. High-efficiency genome editing of an extreme thermophile Thermus thermophilus using endogenous type Ⅰ and type Ⅲ CRISPR-Cas systems[J]. mLife, 2022, 1(4): 412-427. |
| 69 | BOST J, RECALDE A, WAßMER B, et al. Application of the endogenous CRISPR-Cas type Ⅰ-D system for genetic engineering in the thermoacidophilic archaeon Sulfolobus acidocaldarius [J]. Frontiers in Microbiology, 2023, 14: 1254891. |
| 70 | LIN L, CHEN J Y, MITRA R, et al. Optimising PHBV biopolymer production in haloarchaea via CRISPRi-mediated redirection of carbon flux[J]. Communications Biology, 2021, 4(1): 1007. |
| 71 | LAN L H, ZHAO H, CHEN J C, et al. Engineering Halomonas spp. as a low-cost production host for production of bio-surfactant protein PhaP[J]. Biotechnology Journal, 2016, 11(12): 1595-1604. |
| 72 | YASUI K, KANO Y, TANAKA K, et al. Improvement of bacterial transformation efficiency using plasmid artificial modification[J]. Nucleic Acids Research, 2009, 37(1): e3. |
| 73 | SUZUKI T, YASUI K. Plasmid artificial modification: a novel method for efficient DNA transfer into bacteria[J]. Methods in Molecular Biology, 2011, 765: 309-326. |
| 74 | JI M K, ZHENG T R, WANG Z Y, et al. PHB production from food waste hydrolysates by Halomonas bluephagenesis Harboring PHB operon linked with an essential gene[J]. Metabolic Engineering, 2023, 77: 12-20. |
| 75 | JIANG X R, YAO Z H, CHEN G Q. Controlling cell volume for efficient PHB production by Halomonas [J]. Metabolic Engineering, 2017, 44: 30-37. |
| 76 | WANG Z Y, QIN Q, ZHENG Y F, et al. Engineering the permeability of Halomonas bluephagenesis enhanced its chassis properties[J]. Metabolic Engineering, 2021, 67: 53-66. |
| 77 | REN K, ZHAO Y Q, CHEN G Q, et al. Construction of a stable expression system based on the endogenous hbpB/hbpC toxin-antitoxin system of Halomonas bluephagenesis [J]. ACS Synthetic Biology, 2024, 13(1): 61-67. |
| 78 | QIN Q, LING C, ZHAO Y Q, et al. CRISPR/Cas9 editing genome of extremophile Halomonas spp[J]. Metabolic Engineering, 2018, 47: 219-229. |
| 79 | MA Y Y, ZHENG X R, LIN Y N, et al. Engineering an oleic acid-induced system for Halomonas, E. coli and Pseudomonas [J]. Metabolic Engineering, 2022, 72: 325-336. |
| 80 | DE FOUCHÉCOUR F, SÁNCHEZ-CASTAÑEDA A K, SAULOU-BÉRION C, et al. Process engineering for microbial production of 3-hydroxypropionic acid[J]. Biotechnology Advances, 2018, 36(4): 1207-1222. |
| 81 | JIANG X R, YAN X, YU L P, et al. Hyperproduction of 3-hydroxypropionate by Halomonas bluephagenesis [J]. Nature Communications, 2021, 12(1): 1513. |
| 82 | LIN Y N, GUAN Y Y, DONG X, et al. Engineering Halomonas bluephagenesis as a chassis for bioproduction from starch[J]. Metabolic Engineering, 2021, 64: 134-145. |
| 83 | ZHANG L Z, LIN Y N, YI X Q, et al. Engineering low-salt growth Halomonas bluephagenesis for cost-effective bioproduction combined with adaptive evolution[J]. Metabolic Engineering, 2023, 79: 146-158. |
| 84 | CHEN J, LI W, ZHANG Z Z, et al. Metabolic engineering of Escherichia coli for the synthesis of polyhydroxyalkanoates using acetate as a main carbon source[J]. Microbial Cell Factories, 2018, 17(1): 102. |
| 85 | CHEN Y, CHEN X Y, DU H T, et al. Chromosome engineering of the TCA cycle in Halomonas bluephagenesis for production of copolymers of 3-hydroxybutyrate and 3-hydroxyvalerate (PHBV)[J]. Metabolic Engineering, 2019, 54: 69-82. |
| 86 | SHAW A J, PODKAMINER K K, DESAI S G, et al. Metabolic engineering of a thermophilic bacterium to produce ethanol at high yield[J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(37): 13769-13774. |
| 87 | DU H T, ZHAO Y Q, WU F Q, et al. Engineering Halomonas bluephagenesis for L-threonine production[J]. Metabolic Engineering, 2020, 60: 119-127. |
| 88 | BELL S C, TURNER J M. Bacterial catabolism of threonine. Threonine degradation initiated by L-threonine-NAD+ oxidoreductase[J]. Biochemical Journal, 1976, 156(2): 449-458. |
| 89 | MICHETTI D, BRANDSDAL B O, BON D, et al. A comparative study of cold- and warm-adapted endonucleases A using sequence analyses and molecular dynamics simulations[J]. PLoS One, 2017, 12(2): e0169586. |
| 90 | JOSHI S, SATYANARAYANA T. Biotechnology of cold-active proteases[J]. Biology, 2013, 2(2): 755-783. |
| 91 | MESBAH N M. Industrial biotechnology based on enzymes from extreme environments[J]. Frontiers in Bioengineering and Biotechnology, 2022, 10: 870083. |
| 92 | ELLEUCHE S, SCHRÖDER C, SAHM K, et al. Extremozymes: biocatalysts with unique properties from extremophilic microorganisms[J]. Current Opinion in Biotechnology, 2014, 29: 116-123. |
| 93 | XIAN L, WANG F, LUO X, et al. Purification and characterization of a highly efficient calcium-independent α-amylase from Talaromyces pinophilus 1-95[J]. PLoS One, 2015, 10(3): e0121531. |
| 94 | SHARMA A, KAWARABAYASI Y, SATYANARAYANA T. Acidophilic bacteria and Archaea: acid stable biocatalysts and their potential applications[J]. Extremophiles, 2012, 16(1): 1-19. |
| 95 | VESTER J K, GLARING M A, STOUGAARD P. Discovery of novel enzymes with industrial potential from a cold and alkaline environment by a combination of functional metagenomics and culturing[J]. Microbial Cell Factories, 2014, 13: 72. |
| 96 | FU X Z, TAN D, AIBAIDULA G, et al. Development of Halomonas TD01 as a host for open production of chemicals[J]. Metabolic Engineering, 2014, 23: 78-91. |
| 97 | LING C, QIAO G Q, SHUAI B W, et al. Engineering NADH/NAD+ ratio in Halomonas bluephagenesis for enhanced production of polyhydroxyalkanoates (PHA)[J]. Metabolic Engineering, 2018, 49: 275-286. |
| 98 | YE J W, HU D K, CHE X M, et al. Engineering of Halomonas bluephagenesis for low cost production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) from glucose[J]. Metabolic Engineering, 2018, 47: 143-152. |
| 99 | WANG Z Y, ZHENG Y F, JI M K, et al. Hyperproduction of PHA copolymers containing high fractions of 4-hydroxybutyrate (4HB) by outer membrane-defected Halomonas bluephagenesis grown in bioreactors[J]. Microbial Biotechnology, 2022, 15(5): 1586-1597. |
| 100 | WANG H, YE J W, CHEN X Y, et al. Production of PHA copolymers consisting of 3-hydroxybutyrate and 3-hydroxyhexanoate (PHBHHx) by recombinant Halomonas bluephagenesis [J]. Chemical Engineering Journal, 2023, 466: 143261. |
| 101 | ZHANG J, ZHANG X, MAO Y F, et al. Substrate profiling and tolerance testing of Halomonas TD01 suggest its potential application in sustainable manufacturing of chemicals[J]. Journal of Biotechnology, 2020, 316: 1-5. |
| 102 | ZHANG J, JIN B, HONG K Q, et al. Cell catalysis of citrate to itaconate by engineered Halomonas bluephagenesis [J]. ACS Synthetic Biology, 2021, 10(11): 3017-3027. |
| 103 | ZHANG J, YUAN Y, WANG Z W, et al. Metabolic engineering of Halomonas bluephagenesis for high-level mevalonate production from glucose and acetate mixture[J]. Metabolic Engineering, 2023, 79: 203-213. |
| 104 | ZHENG M Y, CUI Z Z, ZHANG J, et al. Efficient acetoin production from pyruvate by engineered Halomonas bluephagenesis whole-cell biocatalysis[J]. Frontiers of Chemical Science and Engineering, 2023, 17(4): 425-436. |
| 105 | HU Q T, SUN S M, ZHANG Z N, et al. Ectoine hyperproduction by engineered Halomonas bluephagenesis [J]. Metabolic Engineering, 2024, 82: 238-249. |
| 106 | YANG F, WANG H, ZHAO C H, et al. Metabolic engineering of Halomonas bluephagenesis for production of five carbon molecular chemicals derived from L-lysine[J]. Metabolic Engineering, 2024, 81: 227-237. |
| 107 | CHEN G Q, ZHANG X, LIU X, et al. Halomonas spp., as chassis for low-cost production of chemicals[J]. Applied Microbiology and Biotechnology, 2022, 106(21): 6977-6992. |
| 108 | MARTÍNEZ-GARCÍA E, FRAILE S, ALGAR E, et al. SEVA 4.0: an update of the Standard European Vector Architecture database for advanced analysis and programming of bacterial phenotypes[J]. Nucleic Acids Research, 2023, 51(D1): D1558-D1567. |
| 109 | GURDO N, VOLKE D C, NIKEL P I. Merging automation and fundamental discovery into the design-build-test-learn cycle of nontraditional microbes[J]. Trends in Biotechnology, 2022, 40(10): 1148-1159. |
| 110 | JOHNSTON C, MARTIN B, FICHANT G, et al. Bacterial transformation: distribution, shared mechanisms and divergent control[J]. Nature Reviews Microbiology, 2014, 12(3): 181-196. |
| 111 | VAN BELJOUW S P B, SANDERS J, RODRÍGUEZ-MOLINA A, et al. RNA-targeting CRISPR-Cas systems[J]. Nature Reviews Microbiology, 2023, 21(1): 21-34. |
| 112 | VOLKE D C, ORSI E, NIKEL P I. Emergent CRISPR-Cas-based technologies for engineering non-model bacteria[J]. Current Opinion in Microbiology, 2023, 75: 102353. |
| 113 | ARROYO-OLARTE R D, BRAVO RODRÍGUEZ R, MORALES-RÍOS E. Genome editing in bacteria: CRISPR-Cas and beyond[J]. Microorganisms, 2021, 9(4): 844. |
| 114 | ZHANG L, ZHAO R, JIA D C, et al. Engineering Clostridium ljungdahlii as the gas-fermenting cell factory for the production of biofuels and biochemicals[J]. Current Opinion in Chemical Biology, 2020, 59: 54-61. |
| 115 | NORMAN R O J, MILLAT T, WINZER K, et al. Progress towards platform chemical production using Clostridium autoethanogenum [J]. Biochemical Society Transactions, 2018, 46(3): 523-535. |
| 116 | BRAUTASET T, JAKOBSEN Ø M, DEGNES K F, et al. Bacillus methanolicus pyruvate carboxylase and homoserine dehydrogenase Ⅰ and Ⅱ and their roles for L-lysine production from methanol at 50 ℃[J]. Applied Microbiology and Biotechnology, 2010, 87(3): 951-964. |
| 117 | CUI L Y, WANG S S, GUAN C G, et al. Breeding of methanol-tolerant Methylobacterium extorquens AM1 by atmospheric and room temperature plasma mutagenesis combined with adaptive laboratory evolution[J]. Biotechnology Journal, 2018, 13(6): e1700679. |
| [1] | GUO Shuyuan, ZHANG Qiannan, Gulikezi· MAIMAITIREXIATI, YANG Yiqun, YU Tao. Advances in microbial production of liquid biofuels [J]. Synthetic Biology Journal, 2025, 6(1): 18-44. |
| [2] | GAO Ge, BIAN Qi, WANG Baojun. Synthetic genetic circuit engineering: principles, advances and prospects [J]. Synthetic Biology Journal, 2025, 6(1): 45-64. |
| [3] | LI Jiyuan, WU Guosheng. Two hypothesises for the origins of organisms from the synthetic biology perspective [J]. Synthetic Biology Journal, 2025, 6(1): 190-202. |
| [4] | LIU Xiaoyue, WANG Pandi, WU Gang, LIU Fang. Efficient biosynthesis of glucoraphanin in Brassicaceae crops by genetic engineering [J]. Synthetic Biology Journal, 2025, 6(1): 136-156. |
| [5] | JIAO Hongtao, QI Meng, SHAO Bin, JIANG Jinsong. Legal issues for the storage of DNA data [J]. Synthetic Biology Journal, 2025, 6(1): 177-189. |
| [6] | TANG Xinghua, LU Qianneng, HU Yilin. Philosophical reflections on synthetic biology in the Anthropocene [J]. Synthetic Biology Journal, 2025, 6(1): 203-212. |
| [7] | XU Huaisheng, SHI Xiaolong, LIU Xiaoguang, XU Miaomiao. Key technologies for DNA storage: encoding, error correction, random access, and security [J]. Synthetic Biology Journal, 2025, 6(1): 157-176. |
| [8] | LI Yifei, CHEN Ai, SUN Junsong, ZHANG Yi-Heng P. Job. Studies on hydrogenases for hydrogen production using in vitro synthetic enzymatic biosystems [J]. Synthetic Biology Journal, 2024, 5(6): 1461-1484. |
| [9] | ZHU Fanghuan, CEN Xuecong, CHEN Zhen. Research progress of diols production by microbes [J]. Synthetic Biology Journal, 2024, 5(6): 1367-1385. |
| [10] | SHI Ting, SONG Zhan, SONG Shiyi, ZHANG Yi-Heng P. Job. In vitro BioTransformation (ivBT): a new frontier of industrial biomanufacturing [J]. Synthetic Biology Journal, 2024, 5(6): 1437-1460. |
| [11] | CHAI Meng, WANG Fengqing, WEI Dongzhi. Synthesis of organic acids from lignocellulose by biotransformation [J]. Synthetic Biology Journal, 2024, 5(6): 1242-1263. |
| [12] | ZHAO Liang, LI Zhenshuai, FU Liping, LYU Ming, WANG Shi’an, ZHANG Quan, LIU Licheng, LI Fuli, LIU Ziyong. Progress in biomanufacturing of lipids and single cell protein from one-carbon compounds [J]. Synthetic Biology Journal, 2024, 5(6): 1300-1318. |
| [13] | CHEN Yu, ZHANG Kang, QIU Yijing, CHENG Caiyun, YIN Jingjing, SONG Tianshun, XIE Jingjing. Progress of microbial electrosynthesis for conversion of CO2 [J]. Synthetic Biology Journal, 2024, 5(5): 1142-1168. |
| [14] | ZHENG Haotian, LI Chaofeng, LIU Liangxu, WANG Jiawei, LI Hengrun, NI Jun. Design, optimization and application of synthetic carbon-negative phototrophic community [J]. Synthetic Biology Journal, 2024, 5(5): 1189-1210. |
| [15] | CHEN Guo-Qiang, TAN Dan. Reprogramming microbial chassis for low-cost bioprodcution of tailor-made polyhydroxyalkanoates [J]. Synthetic Biology Journal, 2024, 5(5): 1211-1226. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||