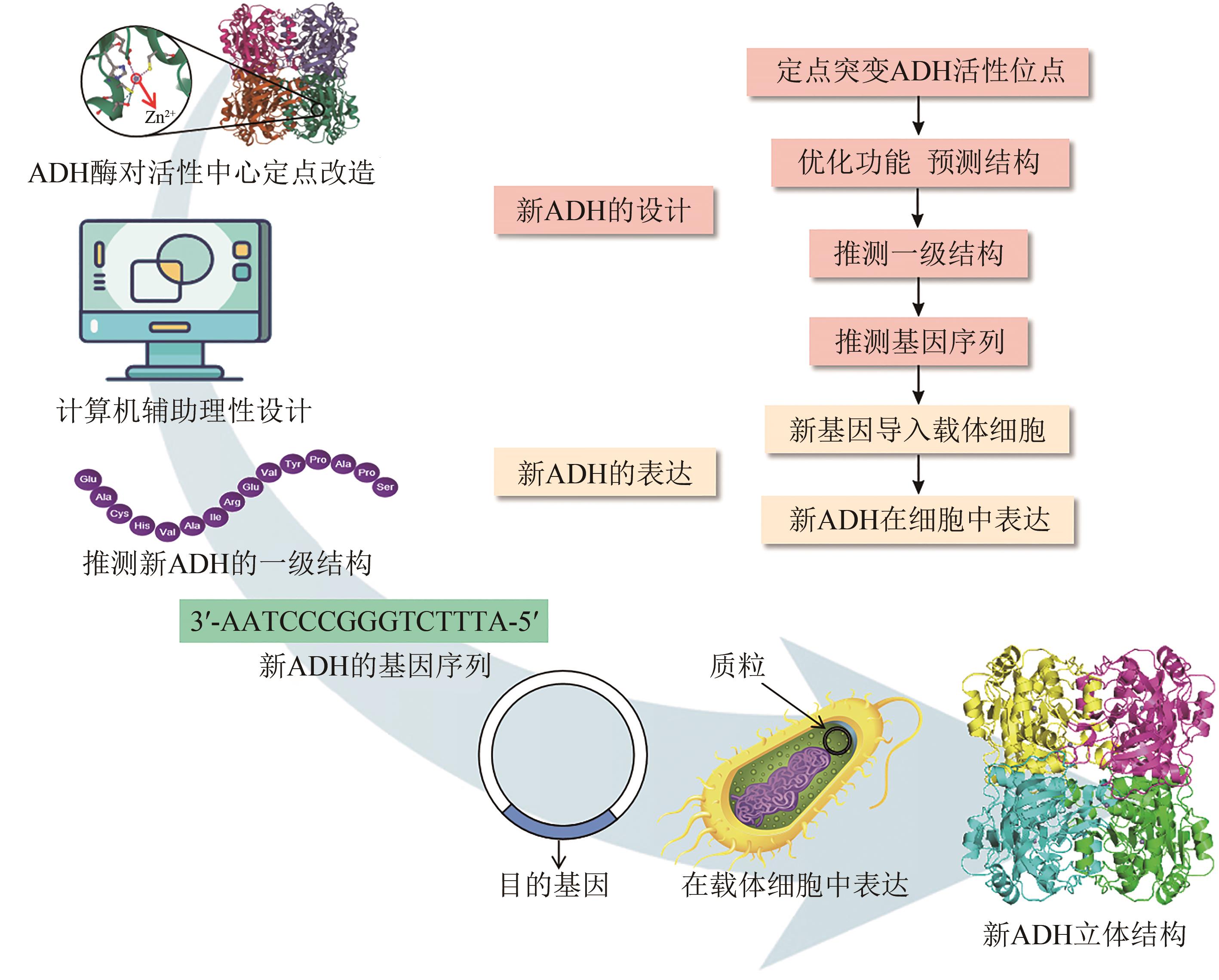
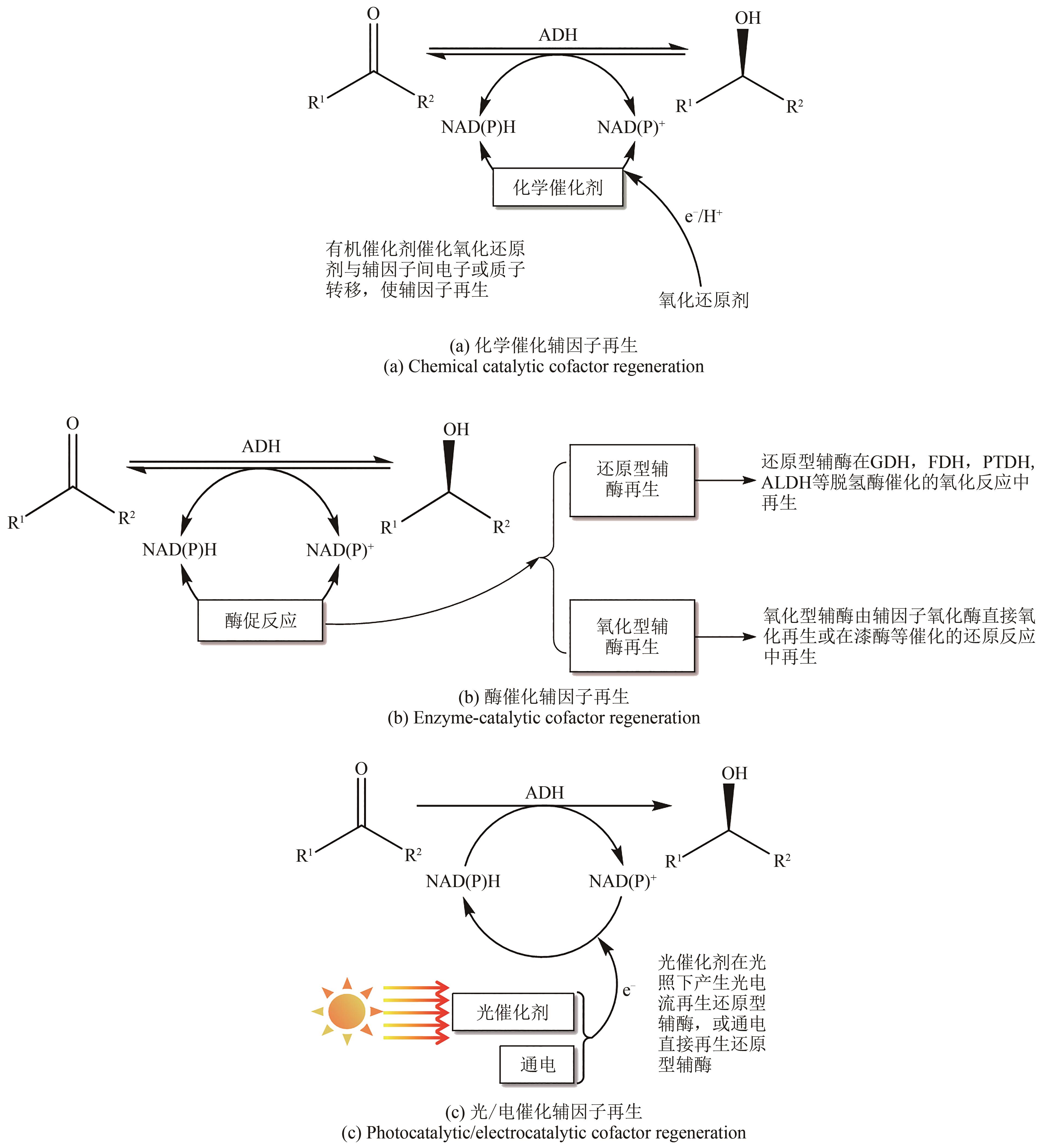
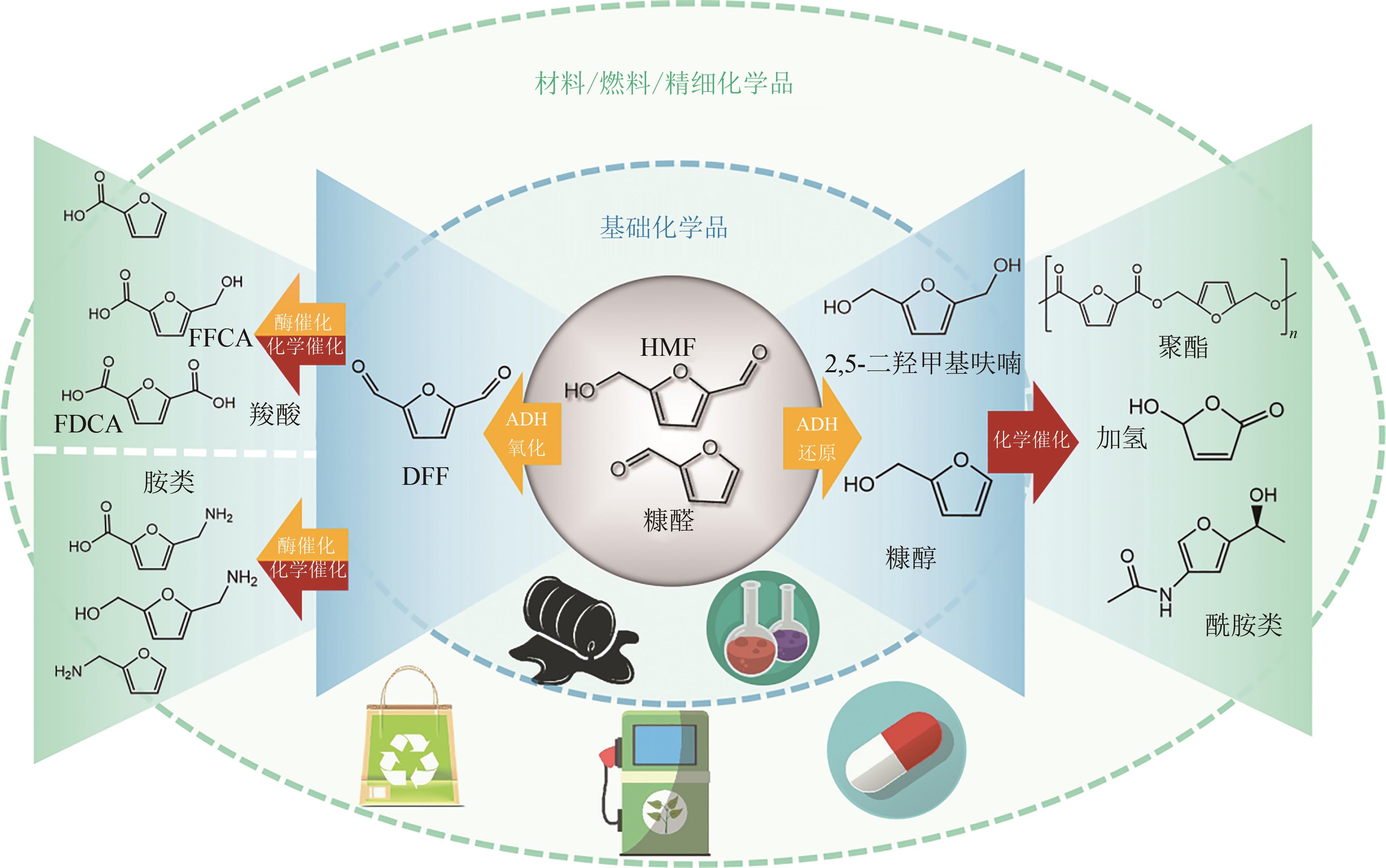
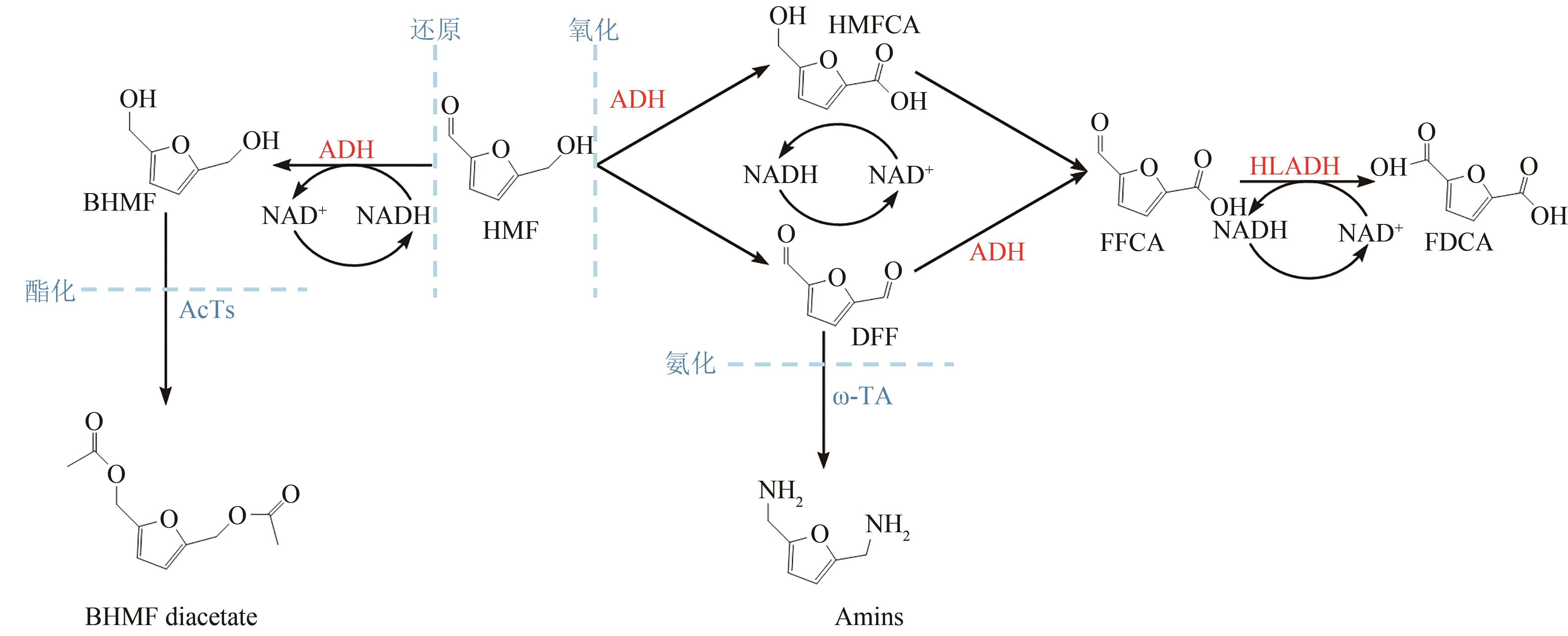
Synthetic Biology Journal ›› 2023, Vol. 4 ›› Issue (6): 1122-1139.DOI: 10.12211/2096-8280.2023-059
• Invited Review • Previous Articles Next Articles
State-of-the-art for alcohol dehydrogenase development and the prospect of its applications in bio-based furan compounds valorization
LIU Xiangshi1, WU Yilu1, ZHAN Peng1, HUANG Tianhao2, CAI Di1, QIN Peiyong2
- 1.National Energy R&D Center for Biorefinery,Beijing University of Chemical Technology,Beijing 100029,China
2.Collage of Life Science and Technology,Beijing University of Chemical Technology,Beijing 100029,China
-
Received:2023-08-21Revised:2023-10-17Online:2024-01-19Published:2023-12-31 -
Contact:CAI Di, QIN Peiyong
醇脱氢酶的研究进展及其催化增值生物基呋喃化合物前景展望
刘庠诗1, 吴奕禄1, 詹鹏1, 黄天灏2, 蔡的1, 秦培勇2
- 1.北京化工大学,国家能源生物炼制研发中心,北京 100029
2.北京化工大学,生命科学与技术学院,北京 100029
-
通讯作者:蔡的,秦培勇 -
作者简介:刘庠诗 (2000—),女,硕士研究生。研究方向为光/电-生物催化生物基平台化合物合成。E-mail:lxs20000417@163.com蔡的 (1989—),男,副教授。研究方向主要为绿色生物制造。E-mail:caidibuct@163.com秦培勇 (1976—),男,教授。研究方向主要为生物化工和膜分离等。E-mail:qinpy@mail.buct.edu.cn -
基金资助:国家自然科学基金(22078018);北京自然科学基金(2222016)
CLC Number:
Cite this article
LIU Xiangshi, WU Yilu, ZHAN Peng, HUANG Tianhao, CAI Di, QIN Peiyong. State-of-the-art for alcohol dehydrogenase development and the prospect of its applications in bio-based furan compounds valorization[J]. Synthetic Biology Journal, 2023, 4(6): 1122-1139.
刘庠诗, 吴奕禄, 詹鹏, 黄天灏, 蔡的, 秦培勇. 醇脱氢酶的研究进展及其催化增值生物基呋喃化合物前景展望[J]. 合成生物学, 2023, 4(6): 1122-1139.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2023-059
| 酶的来源 | 底盘菌株 | 改造方法 | 改造结果 | 参考文献 |
|---|---|---|---|---|
| Clostridium beijerinckii | Escherichia coli | 利用PROSS对来源于Clostridium beijerinckii的乙醇脱氢酶基因中Ser24Pro、Gly182Ala、Gly196Ala、His222Asp、Ser250Glu和Ser254Arg六个位点进行定点诱变 | 催化活性增大到野生型9倍 | [ |
| Thermoanaerobacter ethanolicus | Escherichia coli | 使用两步PCR法对来源于Thermoanaerobacter ethanolicus的乙醇脱氢酶基因中Ile86Ala等底物结合位点附近残基进行定点诱变 | 底物结合活性位点增大 | [ |
| Thermoanaerobacter ethanolicus | Escherichia coli | 通过PCR技术对来源于Thermoanaerobacter ethanolicus 39E的乙醇脱氢酶基因的Trp110Ala残基发生定点突变,并在Escherichia coli中过表达 | ADH的活性位点增大 提升对映体选择性 热稳定性提升 | [ |
| Bacillus stearothermophilus | Escherichia coli | 定点诱变来源于Bacillus stearothermophilus LLD-R的乙醇脱氢酶基因的Glu11Lys/Pro242Ala残基,并在Escherichia coli中过表达 | 重组菌株ADH表达量增加 该ADH耐热性进一步提升 | [ |
| Thermoanaerobacter brockii | Escherichia coli | 定点诱变来源于Thermoanaerobacter brockii的乙醇脱氢酶基因的Asp275Pro残基,并在Escherichia coli过表达 | 提高ADH分子的热稳定性 | [ |
| Escherichia coli BL21(ED3) | Escherichia coli | 使Escherichia coli共表达GDH与EcYjgB | 重组菌株HMF耐受性提升 HMF到BHMF催化效率提升 | [ |
| Thermoanaerobacter ethanolicus | — | 定点诱变来源于Thermoanaerobacter ethanolicus的乙醇脱氢酶基因的Ser39Tyr和Cys295Ala两个残基 | 对映体选择性提高 | [ |
| Pyrococcus furiosus | Escherichia coli | 定点诱变来源于Pyrococcus furiosus的乙醇脱氢酶基因的Lys249Gly/His255Arg两个残基,并在Escherichia coli中过表达 | ADH由NADH依赖性变为多种辅因子依赖型 | [ |
| Deinococcus geothermalis | — | 定点诱变来源于Deinococcus geothermalis的乙醇脱氢酶基因的Asp55Asn等位于辅因子结合位点的残基 | ADH由NADH依赖型变成NADPH依赖型 | [ |
| Rana perezi | — | 定点诱变来源于Rana perezi的乙醇脱氢酶基因的Gly223Asp/Thr224Ile/His225Asn三个连续残基 | ADH由NADPH依赖型变成NADH依赖型 | [ |
Table 1 Current advances in ADH modification and expression
| 酶的来源 | 底盘菌株 | 改造方法 | 改造结果 | 参考文献 |
|---|---|---|---|---|
| Clostridium beijerinckii | Escherichia coli | 利用PROSS对来源于Clostridium beijerinckii的乙醇脱氢酶基因中Ser24Pro、Gly182Ala、Gly196Ala、His222Asp、Ser250Glu和Ser254Arg六个位点进行定点诱变 | 催化活性增大到野生型9倍 | [ |
| Thermoanaerobacter ethanolicus | Escherichia coli | 使用两步PCR法对来源于Thermoanaerobacter ethanolicus的乙醇脱氢酶基因中Ile86Ala等底物结合位点附近残基进行定点诱变 | 底物结合活性位点增大 | [ |
| Thermoanaerobacter ethanolicus | Escherichia coli | 通过PCR技术对来源于Thermoanaerobacter ethanolicus 39E的乙醇脱氢酶基因的Trp110Ala残基发生定点突变,并在Escherichia coli中过表达 | ADH的活性位点增大 提升对映体选择性 热稳定性提升 | [ |
| Bacillus stearothermophilus | Escherichia coli | 定点诱变来源于Bacillus stearothermophilus LLD-R的乙醇脱氢酶基因的Glu11Lys/Pro242Ala残基,并在Escherichia coli中过表达 | 重组菌株ADH表达量增加 该ADH耐热性进一步提升 | [ |
| Thermoanaerobacter brockii | Escherichia coli | 定点诱变来源于Thermoanaerobacter brockii的乙醇脱氢酶基因的Asp275Pro残基,并在Escherichia coli过表达 | 提高ADH分子的热稳定性 | [ |
| Escherichia coli BL21(ED3) | Escherichia coli | 使Escherichia coli共表达GDH与EcYjgB | 重组菌株HMF耐受性提升 HMF到BHMF催化效率提升 | [ |
| Thermoanaerobacter ethanolicus | — | 定点诱变来源于Thermoanaerobacter ethanolicus的乙醇脱氢酶基因的Ser39Tyr和Cys295Ala两个残基 | 对映体选择性提高 | [ |
| Pyrococcus furiosus | Escherichia coli | 定点诱变来源于Pyrococcus furiosus的乙醇脱氢酶基因的Lys249Gly/His255Arg两个残基,并在Escherichia coli中过表达 | ADH由NADH依赖性变为多种辅因子依赖型 | [ |
| Deinococcus geothermalis | — | 定点诱变来源于Deinococcus geothermalis的乙醇脱氢酶基因的Asp55Asn等位于辅因子结合位点的残基 | ADH由NADH依赖型变成NADPH依赖型 | [ |
| Rana perezi | — | 定点诱变来源于Rana perezi的乙醇脱氢酶基因的Gly223Asp/Thr224Ile/His225Asn三个连续残基 | ADH由NADPH依赖型变成NADH依赖型 | [ |
| 再生方法 | 优点 | 缺点 |
|---|---|---|
| 化学法再生 | 反应速率快 成本低 | 反应体系中成分复杂分离困难 反应条件苛刻 环境不友好 易导致酶活性降低 |
| 酶法再生 | 反应条件温和 反应选择性高 | 成本高 酶易失活系统稳定性差 酶难回收 体系成分复杂分离困难 |
| 光/电再生 | 反应体系成分简单 反应条件温和环保 | 反应速率慢 反应体系小 技术成熟度低 |
Table 2 Advantages and disadvantages of different cofactor regeneration systems[8-12, 17]
| 再生方法 | 优点 | 缺点 |
|---|---|---|
| 化学法再生 | 反应速率快 成本低 | 反应体系中成分复杂分离困难 反应条件苛刻 环境不友好 易导致酶活性降低 |
| 酶法再生 | 反应条件温和 反应选择性高 | 成本高 酶易失活系统稳定性差 酶难回收 体系成分复杂分离困难 |
| 光/电再生 | 反应体系成分简单 反应条件温和环保 | 反应速率慢 反应体系小 技术成熟度低 |
| 类型 | 酶/菌种名称 | 底物 | 产物 | 特点 | 参考文献 |
|---|---|---|---|---|---|
| 单酶 催化 | HLADH | FAL | FOL | 使用肌红蛋白作为催化剂使辅因子再生 证明人造辅因子BNAH可用于替代呋喃合成过程中的NAD(P)H等 48 h FOL产率高达93% | [ |
| HLADH | FAL | FCA | 使用血红蛋白作为催化剂使辅因子再生 48 h FAL转化率100%,FCA产率达到98% | [ | |
| HMF | HMFCA | 使用血红蛋白作为催化剂使辅因子再生 60 h HMF转化率100%,HMFCA产率81% | |||
| FFCA | FDCA | 使用血红蛋白作为催化剂使辅因子再生 108 h FFCA转化率79%,FDCA产率54% | |||
| DFF | FDCA | 使用血红蛋白作为催化剂使辅因子再生 60 h DFF转化率100%,FDCA产率96% | |||
| ADH | FAL | FOL | 通过构筑Rh电子介体-固定化ADH复合电极,电化学方式辅助辅因子再生 FAL还原为FOL的选择性达96.4%,产率90.0% | [ [ | |
| ADH | FAL | FOL | 使用附着有CdSe/ZnS纳米颗粒的C3N4作为光催化剂驱动辅因子再生 达到近100%的FAL转化率,产物FOL浓度为0.6 mmol/L | [ | |
| BovADH | FAL | FCA | 对羰基氧化成羧基有高反应活性 FAL、HMF转化率达99% 在pH8.5情况下活性温度在40 ℃以上 | [ | |
| HMF | HMFCA | ||||
| EcADH | FAL | FCA | 对羰基氧化成羧基有高反应活性 FAL、HMF转化率达99% 在pH8.5情况下活性温度在40 ℃以上 | ||
| HMF | HMFCA | ||||
| PpADH | FAL | FCA | 在pH8.5下活性温度在40 ℃以上 对羰基氧化成羧基有高反应活性,FAL、HMF转化率达99% | ||
| HMF | HMFCA | ||||
| 多酶级 联催化 | GDH + ADH + AcTs | HMF | BHMF | 设计了一种共表达GDH与EcYjgB的大肠杆菌重组菌株,实现了HMF的还原与辅因子的再生,BHMF产率高达15 g/(L·h),HMF收率大于99% 使用AcTs催化BHMF酯化反应,HMF生产BHMF双酯总产率达到88% | [ |
| AlaDH + ADH + ω-TA | BHMF | 呋喃氨 基醇 | 可通过控制级联中助溶剂的种类和比例控制氨基醇和二胺的选择性 辅因子与氨供体在级联体系中可循环使用 在10% DME,20 ℃下BHMF的转化率和二胺的产率均达到了99% | [ | |
| ADH + L-AlaDH + ω-TA | HMF | 呋喃二 甲胺 | 设计了一种用于固定化酶的多孔载体,使固定化酶活性下降不超过10%, ADH回收利用率达84% HMF转化率达80% | [ | |
| GOase + ADH | HMF | FDCA | 通过控制不同的底物浓度、反应时间、CaCO3的添加量、两种酶的浓度和比例,实现FDCA、FFCA可控合成 10 mmol/L HMF,1.6 µmol/L GOase与36 µmol/L HLADH反应60h时的HMF转化率达到99%,FDCA产率95% | [ | |
| HMF | FFCA | 通过控制不同的底物浓度、反应时间、CaCO3的添加量、两种酶的浓度和比例,实现FDCA、FFCA可控合成 100 mmol/L HMF,3.2 µmol/L GOase与66 µmol/L SADH反应48h时的HMF转化率达到99%,FFCA产率97% | |||
| 全细胞催化 | Comamonas testosteroni SC1588 | HMF | HMFCA | 对HMF耐受性好,低浓度FOL可提高菌体活性,组氨酸可进一步提高菌体耐受性并控制pH HMF转化率达100%,且对HMFCA的选择性为87%~88% | [ |
| Saccharomyces cerevisiae NL22 | FAL | FOL | 对FAL耐受性好,在100 mmol/L FAL中仍保持高催化活性 8 h FAL转化率达到98%,还原为FOL选择性高达87.9% | [ | |
| Escherichia coli TS | FAL | FA | 优化含HLADH的Escherichia coli TS菌体催化活性 72 h将25 mmol/L FAL完全转化为FA | [ |
Table 3 Summary of catalytic reactions of bio-based furan derivatives
| 类型 | 酶/菌种名称 | 底物 | 产物 | 特点 | 参考文献 |
|---|---|---|---|---|---|
| 单酶 催化 | HLADH | FAL | FOL | 使用肌红蛋白作为催化剂使辅因子再生 证明人造辅因子BNAH可用于替代呋喃合成过程中的NAD(P)H等 48 h FOL产率高达93% | [ |
| HLADH | FAL | FCA | 使用血红蛋白作为催化剂使辅因子再生 48 h FAL转化率100%,FCA产率达到98% | [ | |
| HMF | HMFCA | 使用血红蛋白作为催化剂使辅因子再生 60 h HMF转化率100%,HMFCA产率81% | |||
| FFCA | FDCA | 使用血红蛋白作为催化剂使辅因子再生 108 h FFCA转化率79%,FDCA产率54% | |||
| DFF | FDCA | 使用血红蛋白作为催化剂使辅因子再生 60 h DFF转化率100%,FDCA产率96% | |||
| ADH | FAL | FOL | 通过构筑Rh电子介体-固定化ADH复合电极,电化学方式辅助辅因子再生 FAL还原为FOL的选择性达96.4%,产率90.0% | [ [ | |
| ADH | FAL | FOL | 使用附着有CdSe/ZnS纳米颗粒的C3N4作为光催化剂驱动辅因子再生 达到近100%的FAL转化率,产物FOL浓度为0.6 mmol/L | [ | |
| BovADH | FAL | FCA | 对羰基氧化成羧基有高反应活性 FAL、HMF转化率达99% 在pH8.5情况下活性温度在40 ℃以上 | [ | |
| HMF | HMFCA | ||||
| EcADH | FAL | FCA | 对羰基氧化成羧基有高反应活性 FAL、HMF转化率达99% 在pH8.5情况下活性温度在40 ℃以上 | ||
| HMF | HMFCA | ||||
| PpADH | FAL | FCA | 在pH8.5下活性温度在40 ℃以上 对羰基氧化成羧基有高反应活性,FAL、HMF转化率达99% | ||
| HMF | HMFCA | ||||
| 多酶级 联催化 | GDH + ADH + AcTs | HMF | BHMF | 设计了一种共表达GDH与EcYjgB的大肠杆菌重组菌株,实现了HMF的还原与辅因子的再生,BHMF产率高达15 g/(L·h),HMF收率大于99% 使用AcTs催化BHMF酯化反应,HMF生产BHMF双酯总产率达到88% | [ |
| AlaDH + ADH + ω-TA | BHMF | 呋喃氨 基醇 | 可通过控制级联中助溶剂的种类和比例控制氨基醇和二胺的选择性 辅因子与氨供体在级联体系中可循环使用 在10% DME,20 ℃下BHMF的转化率和二胺的产率均达到了99% | [ | |
| ADH + L-AlaDH + ω-TA | HMF | 呋喃二 甲胺 | 设计了一种用于固定化酶的多孔载体,使固定化酶活性下降不超过10%, ADH回收利用率达84% HMF转化率达80% | [ | |
| GOase + ADH | HMF | FDCA | 通过控制不同的底物浓度、反应时间、CaCO3的添加量、两种酶的浓度和比例,实现FDCA、FFCA可控合成 10 mmol/L HMF,1.6 µmol/L GOase与36 µmol/L HLADH反应60h时的HMF转化率达到99%,FDCA产率95% | [ | |
| HMF | FFCA | 通过控制不同的底物浓度、反应时间、CaCO3的添加量、两种酶的浓度和比例,实现FDCA、FFCA可控合成 100 mmol/L HMF,3.2 µmol/L GOase与66 µmol/L SADH反应48h时的HMF转化率达到99%,FFCA产率97% | |||
| 全细胞催化 | Comamonas testosteroni SC1588 | HMF | HMFCA | 对HMF耐受性好,低浓度FOL可提高菌体活性,组氨酸可进一步提高菌体耐受性并控制pH HMF转化率达100%,且对HMFCA的选择性为87%~88% | [ |
| Saccharomyces cerevisiae NL22 | FAL | FOL | 对FAL耐受性好,在100 mmol/L FAL中仍保持高催化活性 8 h FAL转化率达到98%,还原为FOL选择性高达87.9% | [ | |
| Escherichia coli TS | FAL | FA | 优化含HLADH的Escherichia coli TS菌体催化活性 72 h将25 mmol/L FAL完全转化为FA | [ |
| 1 | DONG J J, FERNÁNDEZ-FUEYO E, HOLLMANN F, et al. Biocatalytic oxidation reactions: a chemist's perspective[J]. Angewandte Chemie International Edition, 2018, 57(30): 9238-9261. |
| 2 | MAGOMEDOVA Z, GRECU A, SENSEN C W, et al. Characterization of two novel alcohol short-chain dehydrogenases/reductases from Ralstonia eutropha H16 capable of stereoselective conversion of bulky substrates[J]. Journal of Biotechnology, 2016, 221: 78-90. |
| 3 | SHANMUGANATHAN S, NATALIA D, GREINER L, et al. Oxidation-hydroxymethylation-reduction: a one-pot three-step biocatalytic synthesis of optically active α-aryl vicinal diols[J]. Green Chemistry, 2012, 14(1): 94-97. |
| 4 | STAMPFER W, KOSJEK B, MOITZI C, et al. Biocatalytic asymmetric hydrogen transfer[J]. Angewandte Chemie International Edition, 2002, 41(6): 1014-1017. |
| 5 | VELASCO-LOZANO S, ROCHA-MARTIN J, FAVELA-TORRES E, et al. Hydrolysis and oxidation of racemic esters into prochiral ketones catalyzed by a consortium of immobilized enzymes[J]. Biochemical Engineering Journal, 2016, 112: 136-142. |
| 6 | VOSS C V, GRUBER C C, FABER K, et al. Orchestration of concurrent oxidation and reduction cycles for stereoinversion and deracemisation of sec-alcohols[J]. Journal of the American Chemical Society, 2008, 130(42): 13969-13972. |
| 7 | VOSS C V, GRUBER C C, KROUTIL W. Deracemization of secondary alcohols through a concurrent tandem biocatalytic oxidation and reduction[J]. Angewandte Chemie International Edition, 2008, 47(4): 741-745. |
| 8 | KOCHIUS S, MAGNUSSON A O, HOLLMANN F, et al. Immobilized redox mediators for electrochemical NAD(P)+ regeneration[J]. Applied Microbiology and Biotechnology, 2012, 93(6): 2251-2264. |
| 9 | BURNETT J W H, CHEN H, LI J W, et al. Supported Pt enabled proton-driven NAD(P)+ regeneration for biocatalytic oxidation[J]. ACS Applied Materials & Interfaces, 2022, 14(18): 20943-20952. |
| 10 | LIU W F, WANG P. Cofactor regeneration for sustainable enzymatic biosynthesis[J]. Biotechnology Advances, 2007, 25(4): 369-384. |
| 11 | NISHIGAKI J I, ISHIDA T, HONMA T, et al. Oxidation of β-nicotinamide adenine dinucleotide (NADH) by Au cluster and nanoparticle catalysts aiming for coenzyme regeneration in enzymatic glucose oxidation[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(28): 10413-10422. |
| 12 | WU H, TIAN C Y, SONG X K, et al. Methods for the regeneration of nicotinamidecoenzymes[J]. Green Chemistry, 2013, 15(7): 1773-1789. |
| 13 | 许松伟, 姜忠义, 吴洪. 醇脱氢酶结构和作用机理研究进展[J]. 有机化学, 2005, 25(6): 629-633. |
| XU S W, JIANG Z Y, WU H. Progress in structure and kinetic mechanism of alcohol dehydrogenase[J]. Chinese Journal of Organic Chemistry, 2005, 25(6): 629-633. | |
| 14 | RADIANINGTYAS H, WRIGHT P C. Alcohol dehydrogenases from thermophilic and hyperthermophilic archaea and bacteria[J]. FEMS Microbiology Reviews, 2003, 27(5): 593-616. |
| 15 | FABER K. Biotransformations in Organic Chemistry[M/OL]. Berlin, Heidelberg: Springer Berlin Heidelberg, 2004[2023-07-01]. . |
| 16 | ZHANG Z B, MUSCHIOL J, HUANG Y H, et al. Efficient ionic liquid-based platform for multi-enzymatic conversion of carbon dioxide to methanol[J]. Green Chemistry, 2018, 20(18): 4339-4348. |
| 17 | 李慧敏, 李宁宁, 翁建清, 等. 酶促还原过程中辅因子的再生研究[J]. 杭州师范大学学报(自然科学版), 2019, 18(6): 605-609, 668. |
| LI H M, LI N N, WENG J Q, et al. Regeneration of cofactors in enzymatic reduction[J]. Journal of Hangzhou Normal University (Natural Science Edition), 2019, 18(6): 605-609, 668. | |
| 18 | VAN DER DONK W A, ZHAO H M. Recent developments in pyridine nucleotide regeneration[J]. Current Opinion in Biotechnology, 2003, 14(4): 421-426. |
| 19 | 吉爱国, 高培基. 烟酰胺类辅因子的保留和再生研究进展[J]. 药物生物技术, 1997, 4(2): 122-128. |
| JI A G, GAO P J. Advances in retention and regeneration of nicotinamide cofactors[J]. Pharmaceutical Biotechnology, 1997, 4(2): 122-128. | |
| 20 | YEH A H W, NORN C, KIPNIS Y, et al. De novo design of luciferases using deep learning[J]. Nature, 2023, 614(7949): 774-780. |
| 21 | 周柱, 查凡, 张琴, 等. Klebsiella sp.WL1316乙醇脱氢酶基因过表达提高乙醇生产效率[J]. 化学工程, 2022, 50(10): 1-7. |
| ZHOU Z, ZHA F, ZHANG Q, et al. Overexpression of alcohol dehydrogenase in Klebsiella sp. WL1316 to improve hydrogen production efficiency[J]. Chemical Engineering (China), 2022, 50(10): 1-7. | |
| 22 | HOLLMANN F, ARENDS I W C E, BUEHLER K. Biocatalytic redox reactions for organic synthesis: nonconventional regeneration methods[J]. ChemCatChem, 2010, 2(7): 762-782. |
| 23 | BORNSCHEUER U T, HUISMAN G W, KAZLAUSKAS R J, et al. Engineering the third wave of biocatalysis[J]. Nature, 2012, 485(7397): 185-194. |
| 24 | BÖTTCHER D, BORNSCHEUER U T. Protein engineering of microbial enzymes[J]. Current Opinion in Microbiology, 2010, 13(3): 274-282. |
| 25 | 李寅. 合成生物制造2022[J]. 生物工程学报, 2023, 39(3): 807-841. |
| LI Y. Biomanufacturing driven by engineered organisms (2022)[J]. Chinese Journal of Biotechnology, 2023, 39(3): 807-841. | |
| 26 | 曲戈, 袁波, 孙周通. 工业蛋白质理性设计与应用[J]. 生物工程学报, 2022, 38(11): 4068-4080. |
| QU G, YUAN B, SUN Z T. Rational design and applications of industrial proteins[J]. Chinese Journal of Biotechnology, 2022, 38(11): 4068-4080. | |
| 27 | 韩旭, 李倩, 韦泓丽, 等. 工业应用导向的蛋白质结构与功能研究进展[J]. 生物工程学报, 2022, 38(11): 4050-4067. |
| HAN X, LI Q, WEI H L, et al. Application-oriented structure and function study of proteins: a review[J]. Chinese Journal of Biotechnology, 2022, 38(11): 4050-4067. | |
| 28 | XU J L, ZHOU H S, YU H R, et al. Computational design of highly stable and soluble alcohol dehydrogenase for NADPH regeneration[J]. Bioresources and Bioprocessing, 2021, 8: 12. |
| 29 | MUSA M M, LOTT N, LAIVENIEKS M, et al. A single point mutation reverses the enantiopreference of Thermoanaerobacter ethanolicus secondary alcohol dehydrogenase[J]. ChemCatChem, 2009, 1(1): 89-93. |
| 30 | ZIEGELMANN-FJELD K I, MUSA M M, PHILLIPS R S, et al. A Thermoanaerobacter ethanolicus secondary alcohol dehydrogenase mutant derivative highly active and stereoselective on phenylacetone and benzylacetone[J]. Protein Engineering, Design & Selection, 2007, 20(2): 47-55. |
| 31 | FIORENTINO G, CANNIO R, ROSSI M, et al. Decreasing the stability and changing the substrate specificity of the Bacillus stearothermophilus alcohol dehydrogenase by single amino acid replacements[J]. Protein Engineering, Design and Selection, 1998, 11(10): 925-930. |
| 32 | GOIHBERG E, DYM O, TEL-OR S, et al. Thermal stabilization of the protozoan Entamoeba histolytica alcohol dehydrogenase by a single proline substitution[J]. Proteins: Structure, Function, and Bioinformatics, 2008, 72(2): 711-719. |
| 33 | WU Q A, ZONG M H, LI N. One-pot chemobiocatalytic production of 2,5-bis(hydroxymethyl)furan and its diester from biomass in aqueous media[J]. ACS Catalysis, 2023, 13(14): 9404-9414. |
| 34 | PHILLIPS R S. Tailoring the substrate specificity of secondary alcohol dehydrogenase[J]. Canadian Journal of Chemistry, 2002, 80(6): 680-685. |
| 35 | CAMPBELL E, WHEELDON I R, BANTA S. Broadening the cofactor specificity of a thermostable alcohol dehydrogenase using rational protein design introduces novel kinetic transient behavior[J]. Biotechnology and Bioengineering, 2010, 107(5): 763-774. |
| 36 | WULF H, MALLIN H, BORNSCHEUER U T. Protein engineering of a thermostable polyol dehydrogenase[J]. Enzyme and Microbial Technology, 2012, 51(4): 217-224. |
| 37 | ROSELL A, VALENCIA E, OCHOA W F, et al. Complete reversal of coenzyme specificity by concerted mutation of three consecutive residues in alcohol dehydrogenase[J]. Journal of Biological Chemistry, 2003, 278(42): 40573-40580. |
| 38 | GOIHBERG E, PERETZ M, TEL-OR S, et al. Biochemical and structural properties of chimeras constructed by exchange of cofactor-binding domains in alcohol dehydrogenases from thermophilic and mesophilic microorganisms[J]. Biochemistry, 2010, 49(9): 1943-1953. |
| 39 | PIETRICOLA G, CHAMORRO L, CASTELLINO M, et al. Covalent immobilization of dehydrogenases on carbon felt for reusable anodes with effective electrochemical cofactor regeneration[J]. ChemistryOpen, 2022, 11(11): e202200102. |
| 40 | THOMPSON M P, TURNER N J. Two-enzyme hydrogen-borrowing amination of alcohols enabled by a cofactor-switched alcohol dehydrogenase[J]. ChemCatChem, 2017, 9(20): 3833-3836. |
| 41 | POIZAT M, ARENDS I W C E, HOLLMANN F. On the nature of mutual inactivation between[Cp*Rh(bpy)(H2O)]2+ and enzymes-analysis and potential remedies[J]. Journal of Molecular Catalysis B: Enzymatic, 2010, 63(3/4): 149-156. |
| 42 | CANIVET J, SÜSS-FINK G, ŠTĚPNIČKA P. Water-soluble phenanthroline complexes of rhodium, iridium and ruthenium for the regeneration of NADH in the enzymatic reduction of ketones[J]. European Journal of Inorganic Chemistry, 2007, 2007(30): 4736-4742. |
| 43 | 曹礼梅, 邱兆富, 张巍, 等. 化工废催化剂污染特征及资源化途径[J]. 化工进展, 2021, 40(10): 5293-5301. |
| CAO L M, QIU Z F, ZHANG W, et al. Pollution and utilization of chemical industry spent catalysts[J]. Chemical Industry and Engineering Progress, 2021, 40(10): 5293-5301. | |
| 44 | CHENAULT H K, WHITESIDES G M. Regeneration of nicotinamide cofactors for use in organic synthesis[J]. Applied Biochemistry and Biotechnology, 1987, 14(2): 147-197. |
| 45 | KOSJEK B, STAMPFER W, POGOREVC M, et al. Purification and characterization of a chemotolerant alcohol dehydrogenase applicable to coupled redox reactions[J]. Biotechnology and Bioengineering, 2004, 86(1): 55-62. |
| 46 | CAZELLES R, DRONE J, FAJULA F, et al. Reduction of CO2 to methanol by a polyenzymatic system encapsulated in phospholipids-silica nanocapsules[J]. New Journal of Chemistry, 2013, 37(11): 3721-3730. |
| 47 | AKSU S, ARENDS I W C E, HOLLMANN F. A new regeneration system for oxidized nicotinamide cofactors[J]. Advanced Synthesis & Catalysis, 2009, 351(9): 1211-1216. |
| 48 | SINGH R K, SINGH R, SIVAKUMAR D, et al. Insights into cell-free conversion of CO2 to chemicals by a multienzyme cascade reaction[J]. ACS Catalysis, 2018, 8(12): 11085-11093. |
| 49 | WANG Z J, CLARY K N, BERGMAN R G, et al. A supramolecular approach to combining enzymatic and transition metal catalysis[J]. Nature Chemistry, 2013, 5(2): 100-103. |
| 50 | TISHKOV V I, POPOV V O. Protein engineering of formate dehydrogenase[J]. Biomolecular Engineering, 2006, 23(2/3): 89-110. |
| 51 | JOHANNES T W, WOODYER R D, ZHAO H M. Efficient regeneration of NADPH using an engineered phosphite dehydrogenase[J]. Biotechnology and Bioengineering, 2007, 96(1): 18-26. |
| 52 | LOPEZ DE FELIPE F, KLEEREBEZEM M, DE VOS W M, et al. Cofactor engineering: a novel approach to metabolic engineering in Lactococcus lactis by controlled expression of NADH oxidase[J]. Journal of Bacteriology, 1998, 180(15): 3804-3808. |
| 53 | WECKBECKER A, HUMMEL W. Glucose dehydrogenase for the regeneration of NADPH and NADH[M/OL]//BARREDO J L. Microbial enzymes and biotransformations. Totowa, NJ: Humana Press, 2005: 225-238 [2023-07-01]. . |
| 54 | RIEBEL B, GIBBS P, WELLBORN W, et al. Cofactor regeneration of NAD+ from NADH: novel water-forming NADH oxidases[J]. Advanced Synthesis & Catalysis, 2002, 344(10): 1156-1168. |
| 55 | RIEBEL B, GIBBS P, WELLBORN W, et al. Cofactor regeneration of both NAD+ from NADH and NADP+ from NADPH: NADH oxidase from Lactobacillus sanfranciscensis [J]. Advanced Synthesis & Catalysis, 2003, 345(6/7): 707-712. |
| 56 | HUMMEL W, RIEBEL B. Isolation and biochemical characterization of a new NADH oxidase from Lactobacillus brevis [J]. Biotechnology Letters, 2003, 25(1): 51-54. |
| 57 | HUMMEL W, KUZU M, GEUEKE B. An efficient and selective enzymatic oxidation system for the synthesis of enantiomerically pured-tert-leucine[J]. Organic Letters, 2003, 5(20): 3649-3650. |
| 58 | GEUEKE B, RIEBEL B, HUMMEL W. NADH oxidase from Lactobacillus brevis: a new catalyst for the regeneration of NAD[J]. Enzyme and Microbial Technology, 2003, 32(2): 205-211. |
| 59 | JIANG R R, BOMMARIUS A S. Hydrogen peroxide-producing NADH oxidase (nox-1) from Lactococcus lactis [J]. Tetrahedron: Asymmetry, 2004, 15(18): 2939-2944. |
| 60 | HIRANO J I, MIYAMOTO K, OHTA H. Purification and characterization of thermostable H2O2-forming NADH oxidase from 2-phenylethanol-assimilating Brevibacterium sp. KU1309[J]. Applied Microbiology and Biotechnology, 2008, 80(1): 71-78. |
| 61 | HUANG L, SAYOGA G V, HOLLMANN F, et al. Horse liver alcohol dehydrogenase-catalyzed oxidative lactamization of amino alcohols[J]. ACS Catalysis, 2018, 8(9): 8680-8684. |
| 62 | NOWAK C, BEER B, PICK A, et al. A water-forming NADH oxidase from Lactobacillus pentosus suitable for the regeneration of synthetic biomimetic cofactors[J]. Frontiers in Microbiology, 2015, 6: 957. |
| 63 | JIA H Y, ZONG M H, ZHENG G W, et al. Myoglobin-catalyzed efficient in situ regeneration of NAD(P)+ and their synthetic biomimetic for dehydrogenase-mediated oxidations[J]. ACS Catalysis, 2019, 9(3): 2196-2202. |
| 64 | JIA H Y, ZONG M H, YU H L, et al. Dehydrogenase-catalyzed oxidation of furanics: exploitation of hemoglobin catalytic promiscuity[J]. ChemSusChem, 2017, 10(18): 3524-3528. |
| 65 | LEE S H, CHOI D S, KUK S K, et al. Photobiocatalysis: activating redox enzymes by direct or indirect transfer of photoinduced electrons[J]. Angewandte Chemie International Edition, 2018, 57(27): 7958-7985. |
| 66 | MACIÁ-AGULLÓ J A, CORMA A, GARCIA H. Photobiocatalysis: the power of combining photocatalysis and enzymes[J]. Chemistry-A European Journal, 2015, 21(31): 10940-10959. |
| 67 | YANG D, ZOU H J, WU Y Z, et al. Constructing quantum Dots@Flake graphitic carbon nitride isotype heterojunctions for enhanced visible-light-driven NADH regeneration and enzymatic hydrogenation[J]. Industrial & Engineering Chemistry Research, 2017, 56(21): 6247-6255. |
| 68 | 吕陈秋, 姜忠义, 王姣. 烟酰型辅酶NAD(P)+和NAD(P)H再生的研究进展[J]. 有机化学, 2004, 24(11): 1366-1379. |
| LÜ C Q, JIANG Z Y, WANG J. Progress in regeneration of NAD(P)+ and NAD(P)H[J]. Chinese Journal of Organic Chemistry, 2004, 24(11): 1366-1379. | |
| 69 | 周一诺, 杨楠, 田瑶, 等. 基于辅因子的光驱动酶催化复合体系[J]. 科学通报, 2020, 65(36): 4213-4222. |
| ZHOU Y N, YANG N, TIAN Y, et al. Cofactor-based solar-driven enzymatic catalysis systems[J]. Chinese Science Bulletin, 2020, 65(36): 4213-4222. | |
| 70 | LI S H, SHI J F, LIU S S, et al. Molecule-electron-proton transfer in enzyme-photo-coupled catalytic system[J]. Chinese Journal of Catalysis, 2023, 44: 96-110. |
| 71 | SÁNCHEZ-IGLESIAS A, CHUVILIN A, GRZELCZAK M. Plasmon-driven photoregeneration of cofactor molecules[J]. Chemical Communications, 2015, 51(25): 5330-5333. |
| 72 | YU S S, ZHANG S D, LI K N, et al. Furfuryl alcohol production with high selectivity by a novel visible-light driven biocatalysis process[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(42): 15980-15988. |
| 73 | ZHAO H Q, QI Y N, ZHAN P, et al. Artificial photoenzymatic reduction of carbon dioxide to methanol by using electron mediator and co-factorassembled ZnIn2S4 nanoflowers[J]. ChemSusChem, 2023, 16(12): e202300061. |
| 74 | JARVIS A G. Designer metalloenzymes for synthetic biology: enzyme hybrids for catalysis[J]. Current Opinion in Chemical Biology, 2020, 58: 63-71. |
| 75 | WANG Y Z, SUN J, ZHANG H H, et al. Tetra (4-carboxyphenyl) porphyrin for efficient cofactor regeneration under visible light and its immobilization[J]. Catalysis Science & Technology, 2018, 8(10): 2578-2587. |
| 76 | ZHU Q, ZHUANG Y, ZHAO H Q, et al. 2,5-Diformylfuran production by photocatalytic selective oxidation of 5-hydroxymethylfurfural in water using MoS2/CdIn2S4 flower-like heterojunctions[J]. Chinese Journal of Chemical Engineering, 2023, 54: 180-191. |
| 77 | ZHUANG Y, ZHU Q, LI G Z, et al. Photocatalytic degradation of organic dyes using covalent triazine-based framework[J]. Materials Research Bulletin, 2022, 146: 111619. |
| 78 | WU Y Z, SHI J F, LI D L, et al. Synergy of electron transfer and electron utilization via metal-organic frameworks as an electron buffer tank for nicotinamide regeneration[J]. ACS Catalysis, 2020, 10(5): 2894-2905. |
| 79 | JI X Y, LIU C C, WANG J, et al. Integration of functionalized two-dimensional TaS2 nanosheets and an electron mediator for more efficient biocatalyzed artificial photosynthesis[J]. Journal of Materials Chemistry A, 2017, 5(11): 5511-5522. |
| 80 | BROWN K A, WILKER M B, BOEHM M, et al. Photocatalytic regeneration of nicotinamide cofactors by quantum dot-enzyme biohybrid complexes[J]. ACS Catalysis, 2016, 6(4): 2201-2204. |
| 81 | HAFENSTINE G R, HARRIS A W, MA K, et al. Conversion of ethanol to 2-ethylhexenal at ambient conditions using tandem, biphasic catalysis[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(11): 10483-10489. |
| 82 | TEOH W Y, SCOTT J A, AMAL R. Progress in heterogeneous photocatalysis: from classical radical chemistry to engineering nanomaterials and solar reactors[J]. The Journal of Physical Chemistry Letters, 2012, 3(5): 629-639. |
| 83 | LUTZ J, MOZHAEV V V, KHMELNITSKY Y L, et al. Preparative application of 2-hydroxybiphenyl 3-monooxygenase with enzymatic cofactor regeneration in organic-aqueous reaction media[J]. Journal of Molecular Catalysis B: Enzymatic, 2002, 19/20: 177-187. |
| 84 | DÉLÉCOULS-SERVAT K, BASSÉGUY R, BERGEL A. Membrane electrochemical reactor (MER): application to NADH regeneration for ADH-catalysed synthesis[J]. Chemical Engineering Science, 2002, 57(21): 4633-4642. |
| 85 | RUINATSCHA R, BUEHLER K, SCHMID A. Development of a high performance electrochemical cofactor regeneration module and its application to the continuous reduction of FAD[J]. Journal of Molecular Catalysis B: Enzymatic, 2014, 103: 100-105. |
| 86 | KIWI J. Photochemical generation of reduced β-nicotinamide-adenine dinucleotide (induced by visible light)[J]. Journal of Photochemistry, 1981, 16(2): 193-202. |
| 87 | WIENKAMP R, STECKHAN E. Selective generation of NADH by visible light[J]. Angewandte Chemie International Edition, 1983, 22(6): 497. |
| 88 | RODRÍGUEZ-HINESTROZA R A, LÓPEZ C, LÓPEZ-SANTÍN J, et al. HLADH-catalyzed synthesis of β-amino acids, assisted by continuous electrochemical regeneration of NAD+ in a filter press microreactor[J]. Chemical Engineering Science, 2017, 158: 196-207. |
| 89 | ZHAN P, LIU X S, ZHU Q A, et al. Selective furfuryl alcohol production from furfural via bio-electrocatalysis[J]. Catalysts, 2023, 13(1): 101. |
| 90 | TOSSTORFF A, KRONER C, OPPERMAN D J, et al. Towards electroenzymatic processes involving old yellow enzymes and mediated cofactor regeneration[J]. Engineering in Life Sciences, 2017, 17(1): 71-76. |
| 91 | WANG X D, SABA T, YIU H H P, et al. Cofactor NAD(P)H regeneration inspired by heterogeneous pathways[J]. Chem, 2017, 2(5): 621-654. |
| 92 | CAHN J K B, WERLANG C A, BAUMSCHLAGER A, et al. A general tool for engineering the NAD/NADP cofactor preference of oxidoreductases[J]. ACS Synthetic Biology, 2017, 6(2): 326-333. |
| 93 | ZHAN P, LIU X S, ZHANG S D, et al. Electroenzymatic reduction of furfural to furfuryl alcohol by an electron mediator and enzyme orderly assembled biocathode[J]. ACS Applied Materials & Interfaces, 2023, 15(10): 12855-12863. |
| 94 | SON E J, LEE S H, KUK S K, et al. Carbon nanotube–graphitic carbon nitride hybrid films for flavoenzyme-catalyzed photoelectrochemical cells[J]. Advanced Functional Materials, 2018, 28(24): 1705232. |
| 95 | ZHANG J Z, CAI D, QIN Y L, et al. High value-added monomer chemicals and functional bio‐based materials derived from polymeric components of lignocellulose by organosolv fractionation[J]. Biofuels, Bioproducts and Biorefining, 2020, 14(2): 371-401. |
| 96 | JIA Q Q, TENG X N, YU S S, et al. Production of furfural from xylose and hemicelluloses using tin-loaded sulfonated diatomite as solid acid catalyst in biphasic system[J]. Bioresource Technology Reports, 2019, 6: 145-151. |
| 97 | LI N, ZONG M H. (Chemo)biocatalytic upgrading of biobased furanic platforms to chemicals, fuels, and materials: a comprehensive review[J]. ACS Catalysis, 2022, 12(16): 10080-10114. |
| 98 | BOZELL J J, PETERSEN G R. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy's "Top 10" revisited[J]. Green Chemistry, 2010, 12(4): 539-554. |
| 99 | VAN PUTTEN R J, VAN DER WAAL J C, DE JONG E, et al. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources[J]. Chemical Reviews, 2013, 113(3): 1499-1597. |
| 100 | CARRO J, FERNÁNDEZ-FUEYO E, FERNÁNDEZ-ALONSO C, et al. Self-sustained enzymatic cascade for the production of 2,5-furandicarboxylic acid from 5-methoxymethylfurfural[J]. Biotechnology for Biofuels, 2018, 11: 86. |
| 101 | KUMAR H, FRAAIJE M W. Conversion of furans by baeyer-villiger monooxygenases[J]. Catalysts, 2017, 7(6): 179. |
| 102 | KNAUS T, TSELIOU V, HUMPHREYS L D, et al. A biocatalytic method for the chemoselective aerobic oxidation of aldehydes to carboxylic acids[J]. Green Chemistry, 2018, 20(17): 3931-3943. |
| 103 | SATTLER J H, FUCHS M, TAUBER K, et al. Redox self-sufficient biocatalyst network for the amination of primary alcohols[J]. Angewandte Chemie International Edition, 2012, 51(36): 9156-9159. |
| 104 | VELASCO-LOZANO S, SANTIAGO-ARCOS J, MAYORAL J A, et al. Co-immobilization and colocalization of multi-enzyme systems for the cell-free biosynthesis of aminoalcohols[J]. ChemCatChem, 2020, 12(11): 3030-3041. |
| 105 | JIA H Y, ZONG M H, ZHENG G W, et al. One-pot enzyme cascade for controlled synthesis of furancarboxylic acids from 5-hydroxymethylfurfural by H2O2 internal recycling[J]. ChemSusChem, 2019, 12(21): 4764-4768. |
| 106 | ZHANG X Y, ZONG M H, LI N. Whole-cell biocatalytic selective oxidation of 5-hydroxymethylfurfural to 5-hydroxymethyl-2-furancarboxylic acid[J]. Green Chemistry, 2017, 19(19): 4544-4551. |
| 107 | PENG B, MA C L, ZHANG P Q, et al. An effective hybrid strategy for converting rice straw to furoic acid by tandem catalysis via Sn-sepiolite combined with recombinant E. coli whole cells harboring horse liver alcohol dehydrogenase[J]. Green Chemistry, 2019, 21(21): 5914-5923. |
| 108 | LIAO H X, JIA H Y, DAI J R, et al. Bioinspired cooperative photobiocatalytic regeneration of oxidized nicotinamide cofactors for catalytic oxidations[J]. ChemSusChem, 2021, 14(7): 1687-1691. |
| 109 | 吴淑可, 周颐, 王文, 等. 从单酶催化到多酶级联催化——从王义翘教授在酶技术领域的贡献说开去[J]. 合成生物学, 2021, 2(4): 543-558. |
| WU S K, ZHOU Y, WANG W, et al. From single-enzyme catalysis to multienzyme cascade: inspired from Professor Daniel I.C. Wang's pioneer work in enzyme technology[J]. Synthetic Biology Journal, 2021, 2(4): 543-558. | |
| 110 | 汤恒, 韩鑫, 邹树平, 等. 多酶催化体系在医药化学品合成中的应用[J]. 合成生物学, 2021, 2(4): 559-576. |
| TANG H, HAN X, ZOU S P, et al. Application of multi-enzyme catalytic system in the synthesis of pharmaceutical chemicals[J]. Synthetic Biology Journal, 2021, 2(4): 559-576. | |
| 111 | CARBALLEIRA J D, QUEZADA M A, HOYOS P, et al. Microbial cells as catalysts for stereoselective red-ox reactions[J]. Biotechnology Advances, 2009, 27(6): 686-714. |
| 112 | WACHTMEISTER J, ROTHER D. Recent advances in whole cell biocatalysis techniques bridging from investigative to industrial scale[J]. Current Opinion in Biotechnology, 2016, 42: 169-177. |
| 113 | ZHANG D X, ONG Y L, LI Z, et al. Biological detoxification of furfural and 5-hydroxyl methyl furfural in hydrolysate of oil palm empty fruit bunch by Enterobacter sp. FDS8[J]. Biochemical Engineering Journal, 2013, 72: 77-82. |
| 114 | RAN H, ZHANG J, GAO Q Q, et al. Analysis of biodegradation performance of furfural and 5-hydroxymethylfurfural by Amorphotheca resinae ZN1 [J]. Biotechnology for Biofuels, 2014, 7(1): 51. |
| 115 | YAN Y X, BU C Y, HUANG X, et al. Efficient whole‐cell biotransformation of furfural to furfuryl alcohol by Saccharomyces cerevisiae NL22[J]. Journal of Chemical Technology & Biotechnology, 2019, 94(12): 3825-3831. |
| 116 | PUETZ H, PUCHĽOVÁ E, VRANKOVÁ K, et al. Biocatalytic oxidation of alcohols[J]. Catalysts, 2020, 10(9): 952. |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||