Synthetic Biology Journal ›› 2023, Vol. 4 ›› Issue (6): 1082-1121.DOI: 10.12211/2096-8280.2023-047
• Invited Review • Previous Articles Next Articles
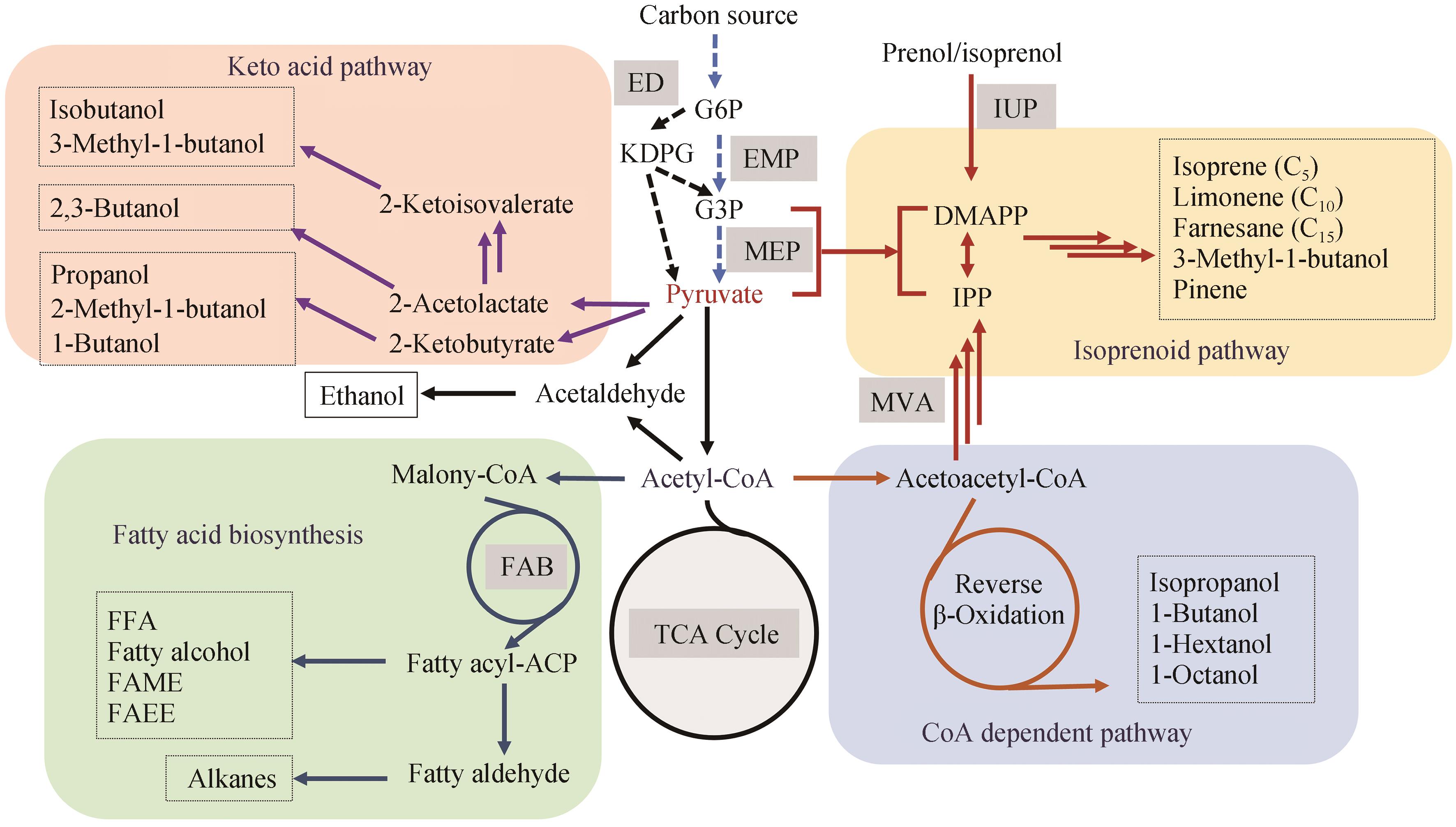
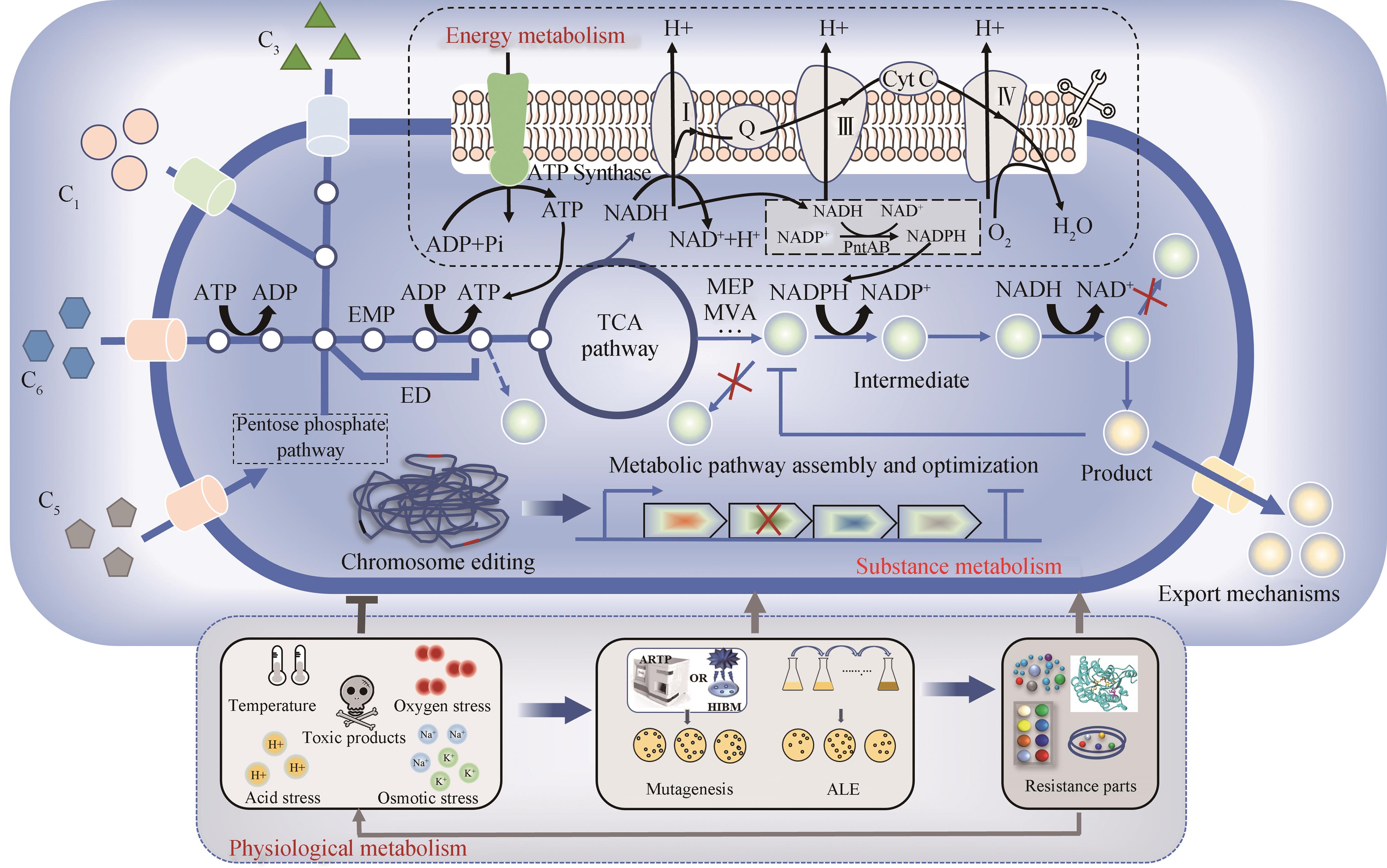
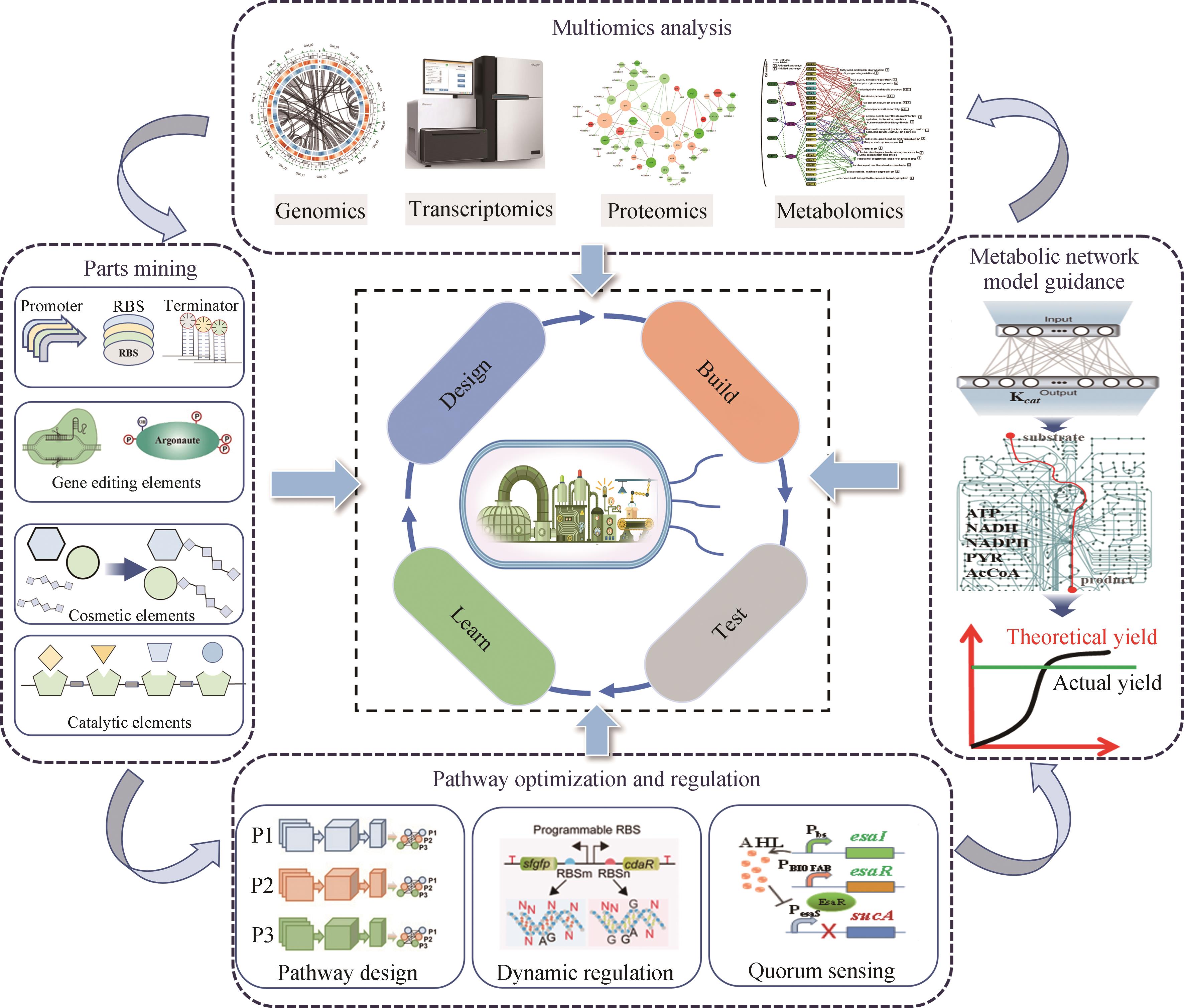
Progress in the construction of microbial cell factories for efficient biofuel production
YAN Xiongying, WANG Zhen, LOU Jiyun, ZHANG Haoyu, HUANG Xingyu, WANG Xia, YANG Shihui
- State Key Laboratory of Biocatalysis and Enzyme Engineering,School of Life Sciences,Hubei University,Wuhan 430062,Hubei,China
-
Received:2023-07-02Revised:2023-08-30Online:2024-01-19Published:2023-12-31 -
Contact:WANG Xia, YANG Shihui
生物燃料高效生产微生物细胞工厂构建研究进展
晏雄鹰, 王振, 娄吉芸, 张皓瑜, 黄星宇, 王霞, 杨世辉
- 湖北大学生命科学学院,省部共建生物催化与酶工程国家重点实验室,湖北 武汉 430062
-
通讯作者:王霞,杨世辉 -
作者简介:晏雄鹰 (1998—),男,博士研究生。研究方向为微生物代谢工程与合成生物学。E-mail:xiongying.Yan@stu.hubu.edu.cn王霞 (1988—),女,博士,讲师。研究方向为合成生物学与微生物代谢工程。E-mail:xxwang@hubu.edu.cn杨世辉 (1971—),男,博士,教授,“省部共建生物催化与酶工程国家重点实验室”副主任。研究方向为微生物代谢工程、合成生物学以及生物能源与绿色生物制造等。E-mail:Shihui.Yang@hubu.edu.cn -
基金资助:国家重点研发计划(2022YFA0911800);国家自然科学基金(22108064);湖北省科技厅重大科技创新计划(2021BAD001)
CLC Number:
Cite this article
YAN Xiongying, WANG Zhen, LOU Jiyun, ZHANG Haoyu, HUANG Xingyu, WANG Xia, YANG Shihui. Progress in the construction of microbial cell factories for efficient biofuel production[J]. Synthetic Biology Journal, 2023, 4(6): 1082-1121.
晏雄鹰, 王振, 娄吉芸, 张皓瑜, 黄星宇, 王霞, 杨世辉. 生物燃料高效生产微生物细胞工厂构建研究进展[J]. 合成生物学, 2023, 4(6): 1082-1121.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2023-047
产物 Product | 价格 Price /(元/t) | 宿主 Host | 发酵方式 Fermentation | 原料 Substrate | 滴度 Titer /(g/L) | 参考文献 Reference |
|---|---|---|---|---|---|---|
| Propanol | 7000~7400 | E. coli | Fed-batch | Glucose or glycerol | 10.3 | [ |
| E. coli | Shake flask | Glucose | 3.5 | [ | ||
| Isopropanol | 6500~7500 | E. coli | Fed-batch with gas stripping | Glucose | 143 | [ |
| E. coli | Shake flask | Glucose | 13.6 | [ | ||
| 1-Butanol | 8550 | C. acetobutylicum | Bioreactor | Glucose | 20.3 | [ |
| E. coli | Bioreactor with gas stripping | Glucose | 30 | [ | ||
| C. tyrobutyricum | Bioreactor | Mannitol | 20.5 | [ | ||
| Isobutanol | 8100 | E. coli | Capped flask | Glucose | 22 | [ |
| E. coli | Bioreactor | Glucose | 56 | [ | ||
| Z. mobilis | Shake flask | Glucose | 4 | [ | ||
| C. thermocellum | Consolidated bioprocessing | Cellulose | 5.4 | [ | ||
| C. glutamicum | Shake flask | Glucose | 20.8 | [ | ||
| S. cerevisiae | NA | Glucose | 5.8 | [ | ||
| 2,3-Butanediol | 10 000 | S. marcescens | Shake flask | Glucose | 42.5 | [ |
| S. marcescens | Fed-batch | Sucrose | 152 | [ | ||
| Z. mobilis | Shake flask | Glucose | 13.3 | [ | ||
| 2-Methy-1-butanol | 15 500 | B. flavum | Shake flash | Glucose; duckweed | 19.5/17.5 | [ |
| E. coli | Shake flask | Glucose | 1.25 | [ | ||
| C. crenatum | Shake flask | Glucose | 5.26 | [ | ||
| 3-Methy-1-butanol | 22 000 | E. coli | Shake flask; two-phase fermentation | Glucose | 9.5 | [ |
| B. flavum | Shake flask | Glucose; duckweed | 0.79/0.78 | [ | ||
| C. crenatum | Shake flask | Glucose | 3.78 | [ |
Table 1 Summary of microbial production of higher carbon chain alcohols
产物 Product | 价格 Price /(元/t) | 宿主 Host | 发酵方式 Fermentation | 原料 Substrate | 滴度 Titer /(g/L) | 参考文献 Reference |
|---|---|---|---|---|---|---|
| Propanol | 7000~7400 | E. coli | Fed-batch | Glucose or glycerol | 10.3 | [ |
| E. coli | Shake flask | Glucose | 3.5 | [ | ||
| Isopropanol | 6500~7500 | E. coli | Fed-batch with gas stripping | Glucose | 143 | [ |
| E. coli | Shake flask | Glucose | 13.6 | [ | ||
| 1-Butanol | 8550 | C. acetobutylicum | Bioreactor | Glucose | 20.3 | [ |
| E. coli | Bioreactor with gas stripping | Glucose | 30 | [ | ||
| C. tyrobutyricum | Bioreactor | Mannitol | 20.5 | [ | ||
| Isobutanol | 8100 | E. coli | Capped flask | Glucose | 22 | [ |
| E. coli | Bioreactor | Glucose | 56 | [ | ||
| Z. mobilis | Shake flask | Glucose | 4 | [ | ||
| C. thermocellum | Consolidated bioprocessing | Cellulose | 5.4 | [ | ||
| C. glutamicum | Shake flask | Glucose | 20.8 | [ | ||
| S. cerevisiae | NA | Glucose | 5.8 | [ | ||
| 2,3-Butanediol | 10 000 | S. marcescens | Shake flask | Glucose | 42.5 | [ |
| S. marcescens | Fed-batch | Sucrose | 152 | [ | ||
| Z. mobilis | Shake flask | Glucose | 13.3 | [ | ||
| 2-Methy-1-butanol | 15 500 | B. flavum | Shake flash | Glucose; duckweed | 19.5/17.5 | [ |
| E. coli | Shake flask | Glucose | 1.25 | [ | ||
| C. crenatum | Shake flask | Glucose | 5.26 | [ | ||
| 3-Methy-1-butanol | 22 000 | E. coli | Shake flask; two-phase fermentation | Glucose | 9.5 | [ |
| B. flavum | Shake flask | Glucose; duckweed | 0.79/0.78 | [ | ||
| C. crenatum | Shake flask | Glucose | 3.78 | [ |
类别 Class | 产物 Product | 宿主 Host | 发酵方式 Fermentation | 底物 Substrate | 产量 Titer/(g/L) | 参考文献 Reference |
|---|---|---|---|---|---|---|
| Fatty acids | Lipid | Y. lipolytica | 3-L bioreactor | Glucose | 90.00 | [ |
| Shake flask | Fructose | 5.51 | [ | |||
| Shake flask | Sucrose | 9.15 | [ | |||
| 2-L bioreactor | Galactose | 3.22 | [ | |||
| 3-L, fed-batch | Hydrolysate | 16.50 | [ | |||
| E. coli | 0.45-L, fed-batch | Glucose | 21.50 | [ | ||
| L. starkeyi | Shake flask | Glucose and xylose | 12.60 | [ | ||
| Thraustochytrid T18 | 7-L, fed-batch | Glucose and xylose | 87.00 | [ | ||
| Fatty acid | S. cerevisiae | 1-L bioreactor, fed-batch | Glucose | 33.40 | [ | |
| R. opacus | 6.6-L, fed-batch | Glucose | 50.20 | [ | ||
| C. glutamicum | Shake flask | Glucose | 1.07 | [ | ||
| Fatty acid ethyl esters | Y. lipolytica | Shake flash | Glucose | 0.137 | [ | |
| R. toruloides | 1-L, fed-batch | Glucose | 9.97 | [ | ||
| Wax ester | A. baylyi | Shake flask | Glucose | 1.82 | [ | |
| Methyl ketone | E. coli | 2-L, fed-batch | Glucose | 3.40 | [ | |
| P. putida | Test tube | Glucose | 1.10 | [ | ||
| Heavy oils | A. melanogenum | 10-L bioreactor | Glucose | 43.00 | [ | |
| Alkanes | Short chain alkane (C2~C5) | E. coli | Bioreactor | Glycerol | 0.11~0.14 | [ |
Medium chain alkane (C6~C12) | Synechocystis sp. PCC6803 | Bioreactor | CO2 | 0.026 | [ | |
| E. coli | 5-L, fed-batch | Glucose | 1.01 | [ | ||
| 6.6-L, fed-batch | Glucose | 0.58 | [ | |||
| 5-L, fed-batch | Glucose | 2.5 | [ | |||
| Shake flask | Glucose | 0.26 | [ | |||
Long chain alkane (C13~C22) | R. opacus | 6.6-L, fed-batch | Glucose | 5.2 | [ | |
| A. melanogenum | 10-L bioreactor | Glucose | 32.5 | [ | ||
| S. cerevisiae | NA | Glucose | 86 μg/g | [ |
Table 2 Summary of microbial production of fatty acids and alkanes
类别 Class | 产物 Product | 宿主 Host | 发酵方式 Fermentation | 底物 Substrate | 产量 Titer/(g/L) | 参考文献 Reference |
|---|---|---|---|---|---|---|
| Fatty acids | Lipid | Y. lipolytica | 3-L bioreactor | Glucose | 90.00 | [ |
| Shake flask | Fructose | 5.51 | [ | |||
| Shake flask | Sucrose | 9.15 | [ | |||
| 2-L bioreactor | Galactose | 3.22 | [ | |||
| 3-L, fed-batch | Hydrolysate | 16.50 | [ | |||
| E. coli | 0.45-L, fed-batch | Glucose | 21.50 | [ | ||
| L. starkeyi | Shake flask | Glucose and xylose | 12.60 | [ | ||
| Thraustochytrid T18 | 7-L, fed-batch | Glucose and xylose | 87.00 | [ | ||
| Fatty acid | S. cerevisiae | 1-L bioreactor, fed-batch | Glucose | 33.40 | [ | |
| R. opacus | 6.6-L, fed-batch | Glucose | 50.20 | [ | ||
| C. glutamicum | Shake flask | Glucose | 1.07 | [ | ||
| Fatty acid ethyl esters | Y. lipolytica | Shake flash | Glucose | 0.137 | [ | |
| R. toruloides | 1-L, fed-batch | Glucose | 9.97 | [ | ||
| Wax ester | A. baylyi | Shake flask | Glucose | 1.82 | [ | |
| Methyl ketone | E. coli | 2-L, fed-batch | Glucose | 3.40 | [ | |
| P. putida | Test tube | Glucose | 1.10 | [ | ||
| Heavy oils | A. melanogenum | 10-L bioreactor | Glucose | 43.00 | [ | |
| Alkanes | Short chain alkane (C2~C5) | E. coli | Bioreactor | Glycerol | 0.11~0.14 | [ |
Medium chain alkane (C6~C12) | Synechocystis sp. PCC6803 | Bioreactor | CO2 | 0.026 | [ | |
| E. coli | 5-L, fed-batch | Glucose | 1.01 | [ | ||
| 6.6-L, fed-batch | Glucose | 0.58 | [ | |||
| 5-L, fed-batch | Glucose | 2.5 | [ | |||
| Shake flask | Glucose | 0.26 | [ | |||
Long chain alkane (C13~C22) | R. opacus | 6.6-L, fed-batch | Glucose | 5.2 | [ | |
| A. melanogenum | 10-L bioreactor | Glucose | 32.5 | [ | ||
| S. cerevisiae | NA | Glucose | 86 μg/g | [ |
产物 Product | 宿主 Host | 发酵方式 Fermentation | 底物 Substrate | 产量 Titer/(g/L) | 参考文献 Reference |
|---|---|---|---|---|---|
| Pinene | E. coli | Shake flask | Glucose | 0.14 | [ |
| Y. lipolytica | Shake flask | Hydrolysate | 0.036 | [ | |
| C. glutamicum | Shake flask | Glucose | 27 μg/g | [ | |
| R. sphaeroides | Shake flask | CO2 | 0.54 mg/L | [ | |
| Sabinene | E. coli | 5-L bioreactor | Glycerol | 2.65 | [ |
| S. cerevisiae | Shake flask | Glucose | 0.018 | [ | |
| Limonene | E. coli | Shake flask | Glucose | 1.29 | [ |
| 3.1-L,two-phase | Glycerol | 3.6 | [ | ||
| Y. lipolytica | 1.5-L;fed-batch | Glycerol | 0.17 | [ | |
| S. cerevisiae | Shake flask | Glucose | 0.92 | [ | |
| Farnesene | E. coli | Shake flask | Glycerol | 8.74 | [ |
| S. cerevisiae | NA | NA | 104.3 | Amyris | |
| P. pastoris | Shake flask | Oleic acid; sorbitol | 2.56 | [ | |
| Y. lipolytica | 1-L;fed-batch | Glucose | 2.56 | [ | |
| 200 t;fed-batch | Cane syrup | 130 | [ | ||
| Bisabolene | E. coli | Shake flask | Glucose | 0.91 | [ |
| S. cerevisiae | Shake flask | Mannose; glucose | 0.99 | [ | |
| R. capsulatus | Shake flask | Glucose | 1.08 | [ |
Table 3 Summary of microbial production of isoprenoid-derived fuels
产物 Product | 宿主 Host | 发酵方式 Fermentation | 底物 Substrate | 产量 Titer/(g/L) | 参考文献 Reference |
|---|---|---|---|---|---|
| Pinene | E. coli | Shake flask | Glucose | 0.14 | [ |
| Y. lipolytica | Shake flask | Hydrolysate | 0.036 | [ | |
| C. glutamicum | Shake flask | Glucose | 27 μg/g | [ | |
| R. sphaeroides | Shake flask | CO2 | 0.54 mg/L | [ | |
| Sabinene | E. coli | 5-L bioreactor | Glycerol | 2.65 | [ |
| S. cerevisiae | Shake flask | Glucose | 0.018 | [ | |
| Limonene | E. coli | Shake flask | Glucose | 1.29 | [ |
| 3.1-L,two-phase | Glycerol | 3.6 | [ | ||
| Y. lipolytica | 1.5-L;fed-batch | Glycerol | 0.17 | [ | |
| S. cerevisiae | Shake flask | Glucose | 0.92 | [ | |
| Farnesene | E. coli | Shake flask | Glycerol | 8.74 | [ |
| S. cerevisiae | NA | NA | 104.3 | Amyris | |
| P. pastoris | Shake flask | Oleic acid; sorbitol | 2.56 | [ | |
| Y. lipolytica | 1-L;fed-batch | Glucose | 2.56 | [ | |
| 200 t;fed-batch | Cane syrup | 130 | [ | ||
| Bisabolene | E. coli | Shake flask | Glucose | 0.91 | [ |
| S. cerevisiae | Shake flask | Mannose; glucose | 0.99 | [ | |
| R. capsulatus | Shake flask | Glucose | 1.08 | [ |
类别 Class | 菌株 Strain | 生长条件 Growth condition | 安全性 Safety status | 基因组大小 Genome size /Mb | 底物 Substrates | 基因组修饰工具 Genome manipulation tools | 产物 Products |
|---|---|---|---|---|---|---|---|
| Model microbes | E. coli | Facultative aerobic | Not GRAS | 4.64 | Pentose, hexose, glycerol, starch | Various tools | Alcohols, fatty acids and terpenoids |
| S. cerevisiae | Facultative aerobic | GRAS | 11.8 16 chromosomes | Starch, sucrose, hexose | Various tools | Terpenoids, nature products | |
| C. glutamicum | Facultative aerobic | GRAS | 3.28 | Sugars, alcohols, organic acid | HR, CRISPR-Cas9 CRISPR-Cpf1/dCpf1 | Alcohols, aminol acid | |
| Non-model microbes | Y. lipolytica | Facultative aerobic | GRAS | 20.5 6 chromosomes | Glucose, glycerol, sucrose, starch, inulin, cellobiose | NHEJ, ZFN, TALEN CRISPR-Cas9 (CRISPRi/CRISPRa) | Lipid, FAAE, terpenoids, Alkanes |
| Z. mobilis | Facultative anaerobic | GRAS | 2.2 4 plasmids | Glucose, sucrose, fructose | HR, CRISPR-Cas9, CRISPR-Cas 12a, Endogenous Type-Ⅰ-F CRISPR-Cas system | Ethanol, isobutanol, 2,3-butanediol, PHB | |
| C. thermocellum | Strictly anaerobic | Not GRAS | 3.56 | Hydrolysate | Endogenous Ⅰ-B CRISPR system; Heterologous Ⅱ CRISPR system | Ethanol, isobutanol | |
| C. acetobutylicum | Strictly anaerobic | Not GRAS | 4.1 | Glucose | CRISPR-Cas9/dCas9 | Acetone, ethanol, butanol |
Table 4 Characteristics of partial model and non-model microbial chassis cell
类别 Class | 菌株 Strain | 生长条件 Growth condition | 安全性 Safety status | 基因组大小 Genome size /Mb | 底物 Substrates | 基因组修饰工具 Genome manipulation tools | 产物 Products |
|---|---|---|---|---|---|---|---|
| Model microbes | E. coli | Facultative aerobic | Not GRAS | 4.64 | Pentose, hexose, glycerol, starch | Various tools | Alcohols, fatty acids and terpenoids |
| S. cerevisiae | Facultative aerobic | GRAS | 11.8 16 chromosomes | Starch, sucrose, hexose | Various tools | Terpenoids, nature products | |
| C. glutamicum | Facultative aerobic | GRAS | 3.28 | Sugars, alcohols, organic acid | HR, CRISPR-Cas9 CRISPR-Cpf1/dCpf1 | Alcohols, aminol acid | |
| Non-model microbes | Y. lipolytica | Facultative aerobic | GRAS | 20.5 6 chromosomes | Glucose, glycerol, sucrose, starch, inulin, cellobiose | NHEJ, ZFN, TALEN CRISPR-Cas9 (CRISPRi/CRISPRa) | Lipid, FAAE, terpenoids, Alkanes |
| Z. mobilis | Facultative anaerobic | GRAS | 2.2 4 plasmids | Glucose, sucrose, fructose | HR, CRISPR-Cas9, CRISPR-Cas 12a, Endogenous Type-Ⅰ-F CRISPR-Cas system | Ethanol, isobutanol, 2,3-butanediol, PHB | |
| C. thermocellum | Strictly anaerobic | Not GRAS | 3.56 | Hydrolysate | Endogenous Ⅰ-B CRISPR system; Heterologous Ⅱ CRISPR system | Ethanol, isobutanol | |
| C. acetobutylicum | Strictly anaerobic | Not GRAS | 4.1 | Glucose | CRISPR-Cas9/dCas9 | Acetone, ethanol, butanol |
菌株 Strain | 模型 Model | 基因、反应与代谢物 Genes, reactions and metabolites | 时间 Time | 应用 Applications | 参考文献 Reference |
|---|---|---|---|---|---|
| E. coli | iJE660 | 660、627、438 | 2000 | NR | [ |
| iJR904 | 904、931、625 | 2003 | 1,4-BDO production | [ | |
| iAF1260 | 1260、2077、1039 | 2007 | Fatty acid production | [ | |
| iJO1366 | 1366、2251、1136 | 2011 | NR | [ | |
| iML1515 | 1515、2719、1192 | 2017 | NR | [ | |
| B. subtilis | iBsu1103 | 1103、1437、1138 | 2009 | NR | [ |
| iBsu1103V2 | 1147、1742、1456 | 2013 | NR | [ | |
| iBsu1147 | 1147、1742、1456 | 2013 | Riboflavin, cellulase, 2,3-butanediol and isobutanol production | [ | |
| iBsu1144 | 1144、1955、1103 | 2017 | Serine alkaline protease production | [ | |
| ec-iY0844 | 844、1020、988 | 2019 | Poly-γ-glutamic acid production | [ | |
| C. glutamicum | iCW773 | 773、1207、950 | 2017 | L-lysine and hyaluronic acid production | [ |
| iJM658 | 658、1065、984 | 2016 | NR | [ | |
| S. cerevisiae | iFF708 | 708、1175、584 | 2003 | Ethanol production | [ |
| iND750 | 750、1149、646 | 2004 | NR | [ | |
| iLL672 | 672、1038、636 | 2005 | NR | [ | |
| iLN800 | 800、1446、1013 | 2008 | NR | [ | |
| iMM904 | 904、1412、1228 | 2009 | 2,3-BDO production | [ | |
| Yeast1 | 832、962、813 | 2008 | NR | [ | |
| Yeast8 | 1133、3949、2680 | 2019 | NR | [ |
Table 5 Summary of typical microbial metabolic network models and their application
菌株 Strain | 模型 Model | 基因、反应与代谢物 Genes, reactions and metabolites | 时间 Time | 应用 Applications | 参考文献 Reference |
|---|---|---|---|---|---|
| E. coli | iJE660 | 660、627、438 | 2000 | NR | [ |
| iJR904 | 904、931、625 | 2003 | 1,4-BDO production | [ | |
| iAF1260 | 1260、2077、1039 | 2007 | Fatty acid production | [ | |
| iJO1366 | 1366、2251、1136 | 2011 | NR | [ | |
| iML1515 | 1515、2719、1192 | 2017 | NR | [ | |
| B. subtilis | iBsu1103 | 1103、1437、1138 | 2009 | NR | [ |
| iBsu1103V2 | 1147、1742、1456 | 2013 | NR | [ | |
| iBsu1147 | 1147、1742、1456 | 2013 | Riboflavin, cellulase, 2,3-butanediol and isobutanol production | [ | |
| iBsu1144 | 1144、1955、1103 | 2017 | Serine alkaline protease production | [ | |
| ec-iY0844 | 844、1020、988 | 2019 | Poly-γ-glutamic acid production | [ | |
| C. glutamicum | iCW773 | 773、1207、950 | 2017 | L-lysine and hyaluronic acid production | [ |
| iJM658 | 658、1065、984 | 2016 | NR | [ | |
| S. cerevisiae | iFF708 | 708、1175、584 | 2003 | Ethanol production | [ |
| iND750 | 750、1149、646 | 2004 | NR | [ | |
| iLL672 | 672、1038、636 | 2005 | NR | [ | |
| iLN800 | 800、1446、1013 | 2008 | NR | [ | |
| iMM904 | 904、1412、1228 | 2009 | 2,3-BDO production | [ | |
| Yeast1 | 832、962、813 | 2008 | NR | [ | |
| Yeast8 | 1133、3949、2680 | 2019 | NR | [ |
| 174 | KABIR M M, SHIMIZU K. Fermentation characteristics and protein expression patterns in a recombinant Escherichia coli mutant lacking phosphoglucose isomerase for poly(3-hydroxybutyrate) production[J]. Applied Microbiology and Biotechnology, 2003, 62(2): 244-255. |
| 175 | KIM Y M, CHO H S, JUNG G Y, et al. Engineering the pentose phosphate pathway to improve hydrogen yield in recombinant Escherichia coli [J]. Biotechnology and Bioengineering, 2011, 108(12): 2941-2946. |
| 176 | LIU Y, GHOSH I N, MARTIEN J, et al. Regulated redirection of central carbon flux enhances anaerobic production of bioproducts in Zymomonas mobilis [J]. Metabolic Engineering, 2020, 61: 261-274. |
| 177 | ATSUMI S, CANN A F, CONNOR M R, et al. Metabolic engineering of Escherichia coli for 1-butanol production[J]. Metabolic Engineering, 2008, 10(6): 305-311. |
| 178 | TROTTER C L, BABU G S, WALLACE S. Engineering biology for sustainable 1,4-butanediol synthesis[J]. Trends in Biotechnology, 2023, 41(3): 286-288. |
| 179 | BAEK J M, MAZUMDAR S, LEE S W, et al. Butyrate production in engineered Escherichia coli with synthetic scaffolds[J]. Biotechnology and Bioengineering, 2013, 110(10): 2790-2794. |
| 180 | LI Y, WANG Y, WANG R X, et al. Metabolic engineering of Zymomonas mobilis for continuous co-production of bioethanol and poly-3-hydroxybutyrate (PHB)[J]. Green Chemistry, 2022, 24(6): 2588-2601. |
| 181 | TIPPMANN S, FERREIRA R, SIEWERS V, et al. Effects of acetoacetyl-CoA synthase expression on production of farnesene in Saccharomyces cerevisiae [J]. Journal of Industrial Microbiology & Biotechnology, 2017, 44(6): 911-922. |
| 182 | KIM Y, INGRAM L O, SHANMUGAM K T. Dihydrolipoamide dehydrogenase mutation alters the NADH sensitivity of pyruvate dehydrogenase complex of Escherichia coli K-12[J]. Journal of Bacteriology, 2008, 190(11): 3851-3858. |
| 183 | YIM H, HASELBECK R, NIU W, et al. Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol[J]. Nature Chemical Biology, 2011, 7(7): 445-452. |
| 184 | BOND-WATTS B B, BELLEROSE R J, CHANG M C Y. Enzyme mechanism as a kinetic control element for designing synthetic biofuel pathways[J]. Nature Chemical Biology, 2011, 7(4): 222-227. |
| 185 | XU P, GU Q, WANG W Y, et al. Modular optimization of multi-gene pathways for fatty acids production in E. coli [J]. Nature Communications, 2013, 4: 1409. |
| 186 | FARMER W R, LIAO J C. Reduction of aerobic acetate production by Escherichia coli [J]. Applied and Environmental Microbiology, 1997, 63(8): 3205-3210. |
| 187 | ZHA W J, RUBIN-PITEL S B, SHAO Z Y, et al. Improving cellular malonyl-CoA level in Escherichia coli via metabolic engineering[J]. Metabolic Engineering, 2009, 11(3): 192-198. |
| 188 | XIAO Y, RUAN Z H, LIU Z G, et al. Engineering Escherichia coli to convert acetic acid to free fatty acids[J]. Biochemical Engineering Journal, 2013, 76: 60-69. |
| 189 | BATT C A, CARYALLO S, EASSON D D JR, et al. Direct evidence for a xylose metabolic pathway in Saccharomyces cerevisiae [J]. Biotechnology and Bioengineering, 1986, 28(4): 549-553. |
| 190 | BRUINENBERG P M, DE BOT P H M, VAN DIJKEN J P, et al. NADH-linked aldose reductase: the key to anaerobic alcoholic fermentation of xylose by yeasts[J]. Applied Microbiology and Biotechnology, 1984, 19(4): 256-260. |
| 191 | WENGER J W, SCHWARTZ K, SHERLOCK G. Bulk segregant analysis by high-throughput sequencing reveals a novel xylose utilization gene from Saccharomyces cerevisiae [J]. PLoS Genetics, 2010, 6(5): e1000942. |
| 192 | LEE S M, JELLISON T, ALPER H S. Systematic and evolutionary engineering of a xylose isomerase-based pathway in Saccharomyces cerevisiae for efficient conversion yields[J]. Biotechnology for Biofuels, 2014, 7(1): 122. |
| 193 | CAO L M, TANG X L, ZHANG X Y, et al. Two-stage transcriptional reprogramming in Saccharomyces cerevisiae for optimizing ethanol production from xylose[J]. Metabolic Engineering, 2014, 24: 150-159. |
| 194 | CADETE R M, DE LAS HERAS A M, SANDSTRÖM A G, et al. Exploring xylose metabolism in Spathaspora species: XYL1.2 from Spathaspora passalidarum as the key for efficient anaerobic xylose fermentation in metabolic engineered Saccharomyces cerevisiae [J]. Biotechnology for Biofuels, 2016, 9: 167. |
| 195 | ZHANG G C, KONG I I, WEI N, et al. Optimization of an acetate reduction pathway for producing cellulosic ethanol by engineered yeast[J]. Biotechnology and Bioengineering, 2016, 113(12): 2587-2596. |
| 196 | FARWICK A, BRUDER S, SCHADEWEG V, et al. Engineering of yeast hexose transporters to transport D-xylose without inhibition by D-glucose[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(14): 5159-5164. |
| 197 | WANG C Q, BAO X M, LI Y W, et al. Cloning and characterization of heterologous transporters in Saccharomyces cerevisiae and identification of important amino acids for xylose utilization[J]. Metabolic Engineering, 2015, 30: 79-88. |
| 198 | LI H B, SCHMITZ O, ALPER H S. Enabling glucose/xylose co-transport in yeast through the directed evolution of a sugar transporter[J]. Applied Microbiology and Biotechnology, 2016, 100(23): 10215-10223. |
| 199 | REIDER APEL A, OUELLET M, SZMIDT-MIDDLETON H, et al. Evolved hexose transporter enhances xylose uptake and glucose/xylose co-utilization in Saccharomyces cerevisiae [J]. Scientific Reports, 2016, 6: 19512. |
| 1 | 马晓焉, 王雪芹, 马炼杰, 等. 高级醇的微生物绿色制造[J]. 生物工程学报, 2021, 37(5): 1721-1736. |
| MA X Y, WANG X Q, MA L J, et al. Microbial green manufacturing of higher alcohols[J]. Chinese Journal of Biotechnology, 2021, 37(5): 1721-1736. | |
| 2 | LIU Y Z, CRUZ-MORALES P, ZARGAR A, et al. Biofuels for a sustainable future[J]. Cell, 2021, 184(6): 1636-1647. |
| 3 | ZHANG J Z, CHEN Y C, FU L H, et al. Accelerating strain engineering in biofuel research via build and test automation of synthetic biology[J]. Current Opinion in Biotechnology, 2021, 67: 88-98. |
| 4 | PERALTA-YAHYA P P, ZHANG F Z, DEL CARDAYRE S B, et al. Microbial engineering for the production of advanced biofuels[J]. Nature, 2012, 488(7411): 320-328. |
| 5 | WU B, WANG Y W, DAI Y H, et al. Current status and future prospective of bio-ethanol industry in China[J]. Renewable and Sustainable Energy Reviews, 2021, 145: 111079. |
| 6 | PANESAR P S, MARWAHA S S, KENNEDY J F. Zymomonas mobilis: an alternative ethanol producer[J]. Journal of Chemical Technology & Biotechnology, 2006, 81(4): 623-635. |
| 7 | ROGERS P L, LEE K J, SKOTNICKI M L, et al. Ethanol production by Zymomonas mobilis [M/OL]//Advances in biochemical engineering/biotechnology: microbial reactions. Berlin, Heidelberg: Springer Berlin Heidelberg, 1982: 37-84 [2023-06-01]. . |
| 8 | BHATIA S K, JAGTAP S S, BEDEKAR A A, et al. Recent developments in pretreatment technologies on lignocellulosic biomass: effect of key parameters, technological improvements, and challenges[J]. Bioresource Technology, 2020, 300: 122724. |
| 9 | YANG Q, YANG Y F, TANG Y, et al. Development and characterization of acidic-pH-tolerant mutants of Zymomonas mobilis through adaptation and next-generation sequencing-based genome resequencing and RNA-Seq[J]. Biotechnology for Biofuels, 2020, 13: 144. |
| 10 | YANG S H, PELLETIER D A, LU T Y S, et al. The Zymomonas mobilis regulator hfq contributes to tolerance against multiple lignocellulosic pretreatment inhibitors[J]. BMC Microbiology, 2010, 10: 135. |
| 11 | TANG Y, WANG Y, YANG Q, et al. Molecular mechanism of enhanced ethanol tolerance associated with hfq overexpression in Zymomonas mobilis [J]. Frontiers in Bioengineering and Biotechnology, 2022, 10: 1098021. |
| 200 | ZHANG M, EDDY C, DEANDA K, et al. Metabolic engineering of a pentose metabolism pathway in ethanologenic Zymomonas mobilis [J]. Science, 1995, 267(5195): 240-243. |
| 201 | DUNN K L, RAO C V. Expression of a xylose-specific transporter improves ethanol production by metabolically engineered Zymomonas mobilis [J]. Applied Microbiology and Biotechnology, 2014, 98(15): 6897-6905. |
| 202 | DUNN K L, RAO C V. High-throughput sequencing reveals adaptation-induced mutations in pentose-fermenting strains of Zymomonas mobilis [J]. Biotechnology and Bioengineering, 2015, 112(11): 2228-2240. |
| 203 | DEANDA K, ZHANG M, EDDY C, et al. Development of an Arabinose-fermenting Zymomonas mobilis strain by metabolic pathway engineering[J]. Applied and Environmental Microbiology, 1996, 62(12): 4465-4470. |
| 204 | KAWAGUCHI H, VERTÈS A A, OKINO S, et al. Engineering of a xylose metabolic pathway in Corynebacterium glutamicum [J]. Applied and Environmental Microbiology, 2006, 72(5): 3418-3428. |
| 205 | MEISWINKEL T M, GOPINATH V, LINDNER S N, et al. Accelerated pentose utilization by Corynebacterium glutamicum for accelerated production of lysine, glutamate, ornithine and putrescine[J]. Microbial Biotechnology, 2013, 6(2): 131-140. |
| 206 | EBERHARDT D, JENSEN J V K, WENDISCH V F. L-citrulline production by metabolically engineered Corynebacterium glutamicum from glucose and alternative carbon sources[J]. AMB Express, 2014, 4(1): 85. |
| 207 | SASAKI M, JOJIMA T, KAWAGUCHI H, et al. Engineering of pentose transport in Corynebacterium glutamicum to improve simultaneous utilization of mixed sugars[J]. Applied Microbiology and Biotechnology, 2009, 85(1): 105-115. |
| 208 | SASAKI M, JOJIMA T, INUI M, et al. Simultaneous utilization of D-cellobiose, D-glucose, and D-xylose by recombinant Corynebacterium glutamicum under oxygen-deprived conditions[J]. Applied Microbiology and Biotechnology, 2008, 81(4): 691-699. |
| 209 | KAWAGUCHI H, SASAKI M, VERTÈS A A, et al. Engineering of an L-arabinose metabolic pathway in Corynebacterium glutamicum [J]. Applied Microbiology and Biotechnology, 2008, 77(5): 1053-1062. |
| 210 | JOJIMA T, NOBURYU R, SASAKI M, et al. Metabolic engineering for improved production of ethanol by Corynebacterium glutamicum [J]. Applied Microbiology and Biotechnology, 2015, 99(3): 1165-1172. |
| 211 | DONG C, QIAO J, WANG X P, et al. Engineering Pichia pastoris with surface-display minicellulosomes for carboxymethyl cellulose hydrolysis and ethanol production[J]. Biotechnology for Biofuels, 2020, 13: 108. |
| 212 | SIRIPONG W, WOLF P, KUSUMOPUTRI T P, et al. Metabolic engineering of Pichia pastoris for production of isobutanol and isobutyl acetate[J]. Biotechnology for Biofuels, 2018, 11: 1. |
| 12 | GENG B N, LIU S Y, CHEN Y H, et al. A plasmid-free Zymomonas mobilis mutant strain reducing reactive oxygen species for efficient bioethanol production using industrial effluent of xylose mother liquor[J]. Frontiers in Bioengineering and Biotechnology, 2022, 10: 1110513. |
| 13 | INGRAM L O, CONWAY T, CLARK D P, et al. Genetic engineering of ethanol production in Escherichia coli [J]. Applied and Environmental Microbiology, 1987, 53(10): 2420-2425. |
| 14 | OHTA K, BEALL D S, MEJIA J P, et al. Genetic improvement of Escherichia coli for ethanol production: chromosomal integration of Zymomonas mobilis genes encoding pyruvate decarboxylase and alcohol dehydrogenase Ⅱ[J]. Applied and Environmental Microbiology, 1991, 57(4): 893-900. |
| 15 | INGRAM L O, GOMEZ P F, LAI X, et al. Metabolic engineering of bacteria for ethanol production[J]. Biotechnology and Bioengineering, 1998, 58(2/3): 204-214. |
| 16 | CHOI Y J, PARK J H, KIM T Y, et al. Metabolic engineering of Escherichia coli for the production of 1-propanol[J]. Metabolic Engineering, 2012, 14(5): 477-486. |
| 17 | ATSUMI S, LIAO J C. Directed evolution of Methanococcus jannaschii citramalate synthase for biosynthesis of 1-propanol and 1-butanol by Escherichia coli [J]. Applied and Environmental Microbiology, 2008, 74(24): 7802-7808. |
| 18 | INOKUMA K, LIAO J C, OKAMOTO M, et al. Improvement of isopropanol production by metabolically engineered Escherichia coli using gas stripping[J]. Journal of Bioscience and Bioengineering, 2010, 110(6): 696-701. |
| 19 | JOJIMA T, INUI M, YUKAWA H. Production of isopropanol by metabolically engineered Escherichia coli [J]. Applied Microbiology and Biotechnology, 2008, 77(6): 1219-1224. |
| 20 | XU M M, ZHAO J B, YU L, et al. Engineering Clostridium acetobutylicum with a histidine kinase knockout for enhanced n-butanol tolerance and production[J]. Applied Microbiology and Biotechnology, 2015, 99(2): 1011-1022. |
| 21 | SHEN C R, LAN E I, DEKISHIMA Y, et al. Driving forces enable high-titer anaerobic 1-butanol synthesis in Escherichia coli [J]. Applied and Environmental Microbiology, 2011, 77(9): 2905-2915. |
| 22 | YU M R, DU Y M, JIANG W Y, et al. Effects of different replicons in conjugative plasmids on transformation efficiency, plasmid stability, gene expression and n-butanol biosynthesis in Clostridium tyrobutyricum [J]. Applied Microbiology and Biotechnology, 2012, 93(2): 881-889. |
| 23 | ATSUMI S, HANAI T, LIAO J C. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels[J]. Nature, 2008, 451(7174): 86-89. |
| 24 | YU H, WANG N, HUO W B, et al. Establishment of BmoR-based biosensor to screen isobutanol overproducer[J]. Microbial Cell Factories, 2019, 18(1): 30. |
| 25 | QIU M Y, SHEN W, YAN X Y, et al. Metabolic engineering of Zymomonas mobilis for anaerobic isobutanol production[J]. Biotechnology for Biofuels, 2020, 13: 15. |
| 26 | LIN P P, MI L, MORIOKA A H, et al. Consolidated bioprocessing of cellulose to isobutanol using Clostridium thermocellum [J]. Metabolic Engineering, 2015, 31: 44-52. |
| 27 | HASEGAWA S, JOJIMA T, SUDA M, et al. Isobutanol production in Corynebacterium glutamicum: suppressed succinate by-production by pckA inactivation and enhanced productivity via the Entner-Doudoroff pathway[J]. Metabolic Engineering, 2020, 59: 24-35. |
| 28 | DUNDON C A, ARISTIDOU A, HAWKINS A, et al. Methods of increasing dihydroxy acid dehydratase activity to improve production of fuels, chemicals, and amino acids: US20120015417[P]. 2012-01-19. |
| 29 | RAO B, ZHANG L Y, SUN J A, et al. Characterization and regulation of the 2,3-butanediol pathway in Serratia marcescens [J]. Applied Microbiology and Biotechnology, 2012, 93(5): 2147-2159. |
| 30 | ZHANG L Y, SUN J A, HAO Y L, et al. Microbial production of 2,3-butanediol by a surfactant (serrawettin)-deficient mutant of Serratia marcescens H30[J]. Journal of Industrial Microbiology & Biotechnology, 2010, 37(8): 857-862. |
| 31 | YANG S H, MOHAGHEGHI A, FRANDEN M A, et al. Metabolic engineering of Zymomonas mobilis for 2,3-butanediol production from lignocellulosic biomass sugars[J]. Biotechnology for Biofuels, 2016, 9(1): 189. |
| 32 | SU H F, LIN J F, WANG Y H, et al. Engineering Brevibacterium flavum for the production of renewable bioenergy: C4-C5 advanced alcohols[J]. Biotechnology and Bioengineering, 2017, 114(9): 1946-1958. |
| 33 | CANN A F, LIAO J C. Production of 2-methyl-1-butanol in engineered Escherichia coli [J]. Applied Microbiology and Biotechnology, 2008, 81(1): 89-98. |
| 34 | SU H F, CHEN H, LIN J F. Enriching the production of 2-methyl-1-butanol in fermentation process using Corynebacterium crenatum [J]. Current Microbiology, 2020, 77(8): 1699-1706. |
| 35 | CONNOR M R, CANN A F, LIAO J C. 3-Methyl-1-butanol production in Escherichia coli: random mutagenesis and two-phase fermentation[J]. Applied Microbiology and Biotechnology, 2010, 86(4): 1155-1164. |
| 213 | YANG Z L, ZHANG Z S. Production of (2R,3R)-2,3-butanediol using engineered Pichia pastoris: strain construction, characterization and fermentation[J]. Biotechnology for Biofuels, 2018, 11: 35. |
| 214 | MEESAPYODSUK D, CHEN Y, NG S H, et al. Metabolic engineering of Pichia pastoris to produce ricinoleic acid, a hydroxy fatty acid of industrial importance[J]. Journal of Lipid Research, 2015, 56(11): 2102-2109. |
| 215 | GAO J Q, LI Y X, YU W, et al. Rescuing yeast from cell death enables overproduction of fatty acids from sole methanol[J]. Nature Metabolism, 2022, 4(7): 932-943. |
| 216 | WEGAT V, FABARIUS J T, SIEBER V. Synthetic methylotrophic yeasts for the sustainable fuel and chemical production[J]. Biotechnology for Biofuels and Bioproducts, 2022, 15(1): 113. |
| 217 | JIANG W, HERNÁNDEZ VILLAMOR D, PENG H D, et al. Metabolic engineering strategies to enable microbial utilization of C1 feedstocks[J]. Nature Chemical Biology, 2021, 17(8): 845-855. |
| 218 | KELLER P, REITER M A, KIEFER P, et al. Generation of an Escherichia coli strain growing on methanol via the ribulose monophosphate cycle[J]. Nature Communications, 2022, 13: 5243. |
| 219 | YU H, LIAO J C. A modified serine cycle in Escherichia coli coverts methanol and CO2 to two-carbon compounds[J]. Nature Communications, 2018, 9: 3992. |
| 220 | LIU J M, ZHANG H, XU Y Y, et al. Turn air-captured CO2 with methanol into amino acid and pyruvate in an ATP/NAD(P)H-free chemoenzymatic system[J]. Nature Communications, 2023, 14: 2772. |
| 221 | SANTOS CORREA S, SCHULTZ J, LAUERSEN K J, et al. Natural carbon fixation and advances in synthetic engineering for redesigning and creating new fixation pathways[J]. Journal of Advanced Research, 2023, 47: 75-92. |
| 222 | GLEIZER S, BEN-NISSAN R, BAR-ON Y M, et al. Conversion of Escherichia coli to generate all biomass carbon from CO2 [J]. Cell, 2019, 179(6): 1255-1263.e12. |
| 223 | GASSLER T, SAUER M, GASSER B, et al. The industrial yeast Pichia pastoris is converted from a heterotroph into an autotroph capable of growth on CO2 [J]. Nature Biotechnology, 2020, 38(2): 210-216. |
| 224 | ZHENG T T, ZHANG M L, WU L H, et al. Upcycling CO2 into energy-rich long-chain compounds via electrochemical and metabolic engineering[J]. Nature Catalysis, 2022, 5(5): 388-396. |
| 225 | CAI T, SUN H B, QIAO J, et al. Cell-free chemoenzymatic starch synthesis from carbon dioxide[J]. Science, 2021, 373(6562): 1523-1527. |
| 36 | NAWAB S, WANG N, MA X Y, et al. Genetic engineering of non-native hosts for 1-butanol production and its challenges: a review[J]. Microbial Cell Factories, 2020, 19(1): 79. |
| 37 | NIELSEN D R, LEONARD E, YOON S H, et al. Engineering alternative butanol production platforms in heterologous bacteria[J]. Metabolic Engineering, 2009, 11(4/5): 262-273. |
| 38 | BEREZINA O V, ZAKHAROVA N V, BRANDT A, et al. Reconstructing the clostridial n-butanol metabolic pathway in Lactobacillus brevis [J]. Applied Microbiology and Biotechnology, 2010, 87(2): 635-646. |
| 39 | YU A Q, ZHAO Y K, PANG Y R, et al. An oleaginous yeast platform for renewable 1-butanol synthesis based on a heterologous CoA-dependent pathway and an endogenous pathway[J]. Microbial Cell Factories, 2018, 17(1): 166. |
| 40 | WANG M M, HU L J, FAN L H, et al. Enhanced 1-butanol production in engineered Klebsiella pneumoniae by NADH regeneration[J]. Energy & Fuels, 2015, 29(3): 1823-1829. |
| 41 | WANG M M, FAN L H, TAN T W. 1-Butanol production from glycerol by engineered Klebsiella pneumoniae [J]. RSC Advances, 2014, 4(101): 57791-57798. |
| 42 | LAN E I, LIAO J C. ATP drives direct photosynthetic production of 1-butanol in cyanobacteria[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(16): 6018-6023. |
| 43 | LAN E I, RO S Y, LIAO J C. Oxygen-tolerant coenzyme A-acylating aldehyde dehydrogenase facilitates efficient photosynthetic n-butanol biosynthesis in cyanobacteria[J]. Energy & Environmental Science, 2013, 6(9): 2672. |
| 44 | BRANDUARDI P, LONGO V, BERTERAME N M, et al. A novel pathway to produce butanol and isobutanol in Saccharomyces cerevisiae [J]. Biotechnology for Biofuels, 2013, 6(1): 68. |
| 45 | LIAN J Z, SI T, NAIR N U, et al. Design and construction of acetyl-CoA overproducing Saccharomyces cerevisiae strains[J]. Metabolic Engineering, 2014, 24: 139-149. |
| 46 | SI T, LUO Y Z, XIAO H, et al. Utilizing an endogenous pathway for 1-butanol production in Saccharomyces cerevisiae [J]. Metabolic Engineering, 2014, 22: 60-68. |
| 47 | FELPETO-SANTERO C, ROJAS A, TORTAJADA M, et al. Engineering alternative isobutanol production platforms[J]. AMB Express, 2015, 5: 32. |
| 226 | DE KOK S, KOZAK B U, PRONK J T, et al. Energy coupling in Saccharomyces cerevisiae: selected opportunities for metabolic engineering[J]. FEMS Yeast Research, 2012, 12(4): 387-397. |
| 227 | HARA K Y, KONDO A. ATP regulation in bioproduction[J]. Microbial Cell Factories, 2015, 14: 198. |
| 228 | YOON S H, DO J H, LEE S Y, et al. Production of poly-γ- glutamic acid by fed-batch culture of Bacillus licheniformis [J]. Biotechnology Letters, 2000, 22(7): 585-588. |
| 229 | ZHANG X X, LIU S K, TAKANO T. Overexpression of a mitochondrial ATP synthase small subunit gene (AtMtATP6) confers tolerance to several abiotic stresses in Saccharomyces cerevisiae and Arabidopsis thaliana [J]. Biotechnology Letters, 2008, 30(7): 1289-1294. |
| 230 | SINGH A, SOH K C, HATZIMANIKATIS V, et al. Manipulating redox and ATP balancing for improved production of succinate in E. coli [J]. Metabolic Engineering, 2011, 13(1): 76-81. |
| 231 | QI H S, LI S S, ZHAO S M, et al. Model-driven redox pathway manipulation for improved isobutanol production in Bacillus subtilis complemented with experimental validation and metabolic profiling analysis[J]. PLoS One, 2014, 9(4): e93815. |
| 232 | SHI A Q, ZHU X N, LU J, et al. Activating transhydrogenase and NAD kinase in combination for improving isobutanol production[J]. Metabolic Engineering, 2013, 16: 1-10. |
| 233 | ZHAN Y Y, XU Y, LU X C, et al. Metabolic engineering of Bacillus licheniformis for sustainable production of isobutanol[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(51): 17254-17265. |
| 234 | ATSUMI S, WU T Y, ECKL E M, et al. Engineering the isobutanol biosynthetic pathway in Escherichia coli by comparison of three aldehyde reductase/alcohol dehydrogenase genes[J]. Applied Microbiology and Biotechnology, 2010, 85(3): 651-657. |
| 235 | BASTIAN S, LIU X, MEYEROWITZ J T, et al. Engineered ketol-acid reductoisomerase and alcohol dehydrogenase enable anaerobic 2-methylpropan-1-ol production at theoretical yield in Escherichia coli [J]. Metabolic Engineering, 2011, 13(3): 345-352. |
| 236 | HAO Y N, MA Q, LIU X Q, et al. High-yield production of L-valine in engineered Escherichia coli by a novel two-stage fermentation[J]. Metabolic Engineering, 2020, 62: 198-206. |
| 237 | GUO X J, LIU Y X, WANG Q A, et al. Non-natural cofactor and formate-driven reductive carboxylation of pyruvate[J]. Angewandte Chemie International Edition, 2020, 59(8): 3143-3146. |
| 48 | HAO T, HAN B B, MA H W, et al. In silico metabolic engineering of Bacillus subtilis for improved production of riboflavin, Egl-237, (R,R)-2,3-butanediol and isobutanol[J]. Molecular BioSystems, 2013, 9(8): 2034-2044. |
| 49 | BUIJS N A, SIEWERS V, NIELSEN J. Advanced biofuel production by the yeast Saccharomyces cerevisiae [J]. Current Opinion in Chemical Biology, 2013, 17(3): 480-488. |
| 50 | SMITH K M, CHO K M, LIAO J C. Engineering Corynebacterium glutamicum for isobutanol production[J]. Applied Microbiology and Biotechnology, 2010, 87(3): 1045-1055. |
| 51 | MA C Q, WANG A L, QIN J Y, et al. Enhanced 2,3-butanediol production by Klebsiella pneumoniae SDM[J]. Applied Microbiology and Biotechnology, 2009, 82(1): 49-57. |
| 52 | CELIŃSKA E, GRAJEK W. Biotechnological production of 2,3-butanediol—current state and prospects[J]. Biotechnology Advances, 2009, 27(6): 715-725. |
| 53 | KAY J E, JEWETT M C. Lysate of engineered Escherichia coli supports high-level conversion of glucose to 2,3-butanediol[J]. Metabolic Engineering, 2015, 32: 133-142. |
| 54 | SHIN H D, YOON S H, WU J R, et al. High-yield production of meso-2,3-butanediol from cellodextrin by engineered E. coli biocatalysts[J]. Bioresource Technology, 2012, 118: 367-373. |
| 55 | NOZZI N E, ATSUMI S. Genome engineering of the 2,3-butanediol biosynthetic pathway for tight regulation in cyanobacteria[J]. ACS Synthetic Biology, 2015, 4(11): 1197-1204. |
| 56 | CONNOR M R, ATSUMI S. Synthetic biology guides biofuel production[J]. Journal of Biomedicine & Biotechnology, 2010, 2010: 541698. |
| 57 | FORTMAN J L, CHHABRA S, MUKHOPADHYAY A, et al. Biofuel alternatives to ethanol: pumping the microbial well[J]. Trends in Biotechnology, 2008, 26(7): 375-381. |
| 58 | ISSARIYAKUL T, DALAI A K. Biodiesel from vegetable oils[J]. Renewable and Sustainable Energy Reviews, 2014, 31: 446-471. |
| 59 | KAMARAJ R, RAO Y K S S, BALAKRISHNA B. Biodiesel blends: a comprehensive systematic review on various constraints[J]. Environmental Science and Pollution Research, 2022, 29(29): 43770-43785. |
| 60 | VERMA S, KUILA A. Involvement of green technology in microalgal biodiesel production[J]. Reviews on Environmental Health, 2020, 35(2): 173-188. |
| 61 | YADAV A K, KUILA A, GARLAPATI V K. Biodiesel production from Brassica juncea using oleaginous yeast[J]. Applied Biochemistry and Biotechnology, 2022, 194(9): 4066-4080. |
| 62 | BUDIN I, DE ROND T, CHEN Y, et al. Viscous control of cellular respiration by membrane lipid composition[J]. Science, 2018, 362(6419): 1186-1189. |
| 63 | CHO H, CRONAN J E JR. Defective export of a periplasmic enzyme disrupts regulation of fatty acid synthesis (∗)[J]. Journal of Biological Chemistry, 1995, 270(9): 4216-4219. |
| 64 | LU X F, VORA H, KHOSLA C. Overproduction of free fatty acids in E. coli: implications for biodiesel production[J]. Metabolic Engineering, 2008, 10(6): 333-339. |
| 65 | JIANG P, CRONAN J E JR. Inhibition of fatty acid synthesis in Escherichia coli in the absence of phospholipid synthesis and release of inhibition by thioesterase action[J]. Journal of Bacteriology, 1994, 176(10): 2814-2821. |
| 66 | QIAO K J, WASYLENKO T M, ZHOU K, et al. Lipid production in Yarrowia lipolytica is maximized by engineering cytosolic redox metabolism[J]. Nature Biotechnology, 2017, 35(2): 173-177. |
| 67 | LAZAR Z, DULERMO T, NEUVÉGLISE C, et al. Hexokinase—a limiting factor in lipid production from fructose in Yarrowia lipolytica [J]. Metabolic Engineering, 2014, 26: 89-99. |
| 68 | LAZAR Z, GAMBOA-MELÉNDEZ H, LE COQ A M C, et al. Awakening the endogenous Leloir pathway for efficient galactose utilization by Yarrowia lipolytica [J]. Biotechnology for Biofuels, 2015, 8: 185. |
| 69 | NIEHUS X, CRUTZ-LE COQ A M, SANDOVAL G, et al. Engineering Yarrowia lipolytica to enhance lipid production from lignocellulosic materials[J]. Biotechnology for Biofuels, 2018, 11: 11. |
| 70 | XIAO Y, BOWEN C H, LIU D, et al. Exploiting nongenetic cell-to-cell variation for enhanced biosynthesis[J]. Nature Chemical Biology, 2016, 12(5): 339-344. |
| 71 | ZHAO X, KONG X L, HUA Y Y, et al. Medium optimization for lipid production through co-fermentation of glucose and xylose by the oleaginous yeast Lipomyces starkeyi [J]. European Journal of Lipid Science and Technology, 2008, 110(5): 405-412. |
| 238 | WANG X Y, FENG Y B, GUO X J, et al. Creating enzymes and self-sufficient cells for biosynthesis of the non-natural cofactor nicotinamide cytosine dinucleotide[J]. Nature Communications, 2021, 12: 2116. |
| 239 | QURESHI A S, ZHANG J, BAO J. High ethanol fermentation performance of the dry dilute acid pretreated corn stover by an evolutionarily adapted Saccharomyces cerevisiae strain[J]. Bioresource Technology, 2015, 189: 399-404. |
| 240 | ROYCE L A, YOON J M, CHEN Y X, et al. Evolution for exogenous octanoic acid tolerance improves carboxylic acid production and membrane integrity[J]. Metabolic Engineering, 2015, 29: 180-188. |
| 241 | TAN F R, DAI L C, WU B, et al. Improving furfural tolerance of Zymomonas mobilis by rewiring a sigma factor RpoD protein[J]. Applied Microbiology and Biotechnology, 2015, 99(12): 5363-5371. |
| 242 | WU B, QIN H, YANG Y W, et al. Engineered Zymomonas mobilis tolerant to acetic acid and low pH via multiplex atmospheric and room temperature plasma mutagenesis[J]. Biotechnology for Biofuels, 2019, 12: 10. |
| 243 | YANG Y F, HU M M, TANG Y, et al. Progress and perspective on lignocellulosic hydrolysate inhibitor tolerance improvement in Zymomonas mobilis [J]. Bioresources and Bioprocessing, 2018, 5(1): 6. |
| 244 | XU K, QIN L, BAI W X, et al. Multilevel defense system (MDS) relieves multiple stresses for economically boosting ethanol production of industrial Saccharomyces cerevisiae [J]. ACS Energy Letters, 2020, 5(2): 572-582. |
| 245 | 常瀚文, 郑鑫铃, 骆健美, 等. 抗逆元件及其在高效微生物细胞工厂构建中的应用进展[J]. 生物技术通报, 2020, 36(6): 13-34. |
| CHANG H W, ZHENG X L, LUO J M, et al. Tolerance elements and their application progress on the construction of highly-efficient microbial cell factory[J]. Biotechnology Bulletin, 2020, 36(6): 13-34. | |
| 246 | YUAN Y B, BI C H, NICOLAOU S A, et al. Overexpression of the Lactobacillus plantarum peptidoglycan biosynthesis murA2 gene increases the tolerance of Escherichia coli to alcohols and enhances ethanol production[J]. Applied Microbiology and Biotechnology, 2014, 98(19): 8399-8411. |
| 247 | TAN Z G, KHAKBAZ P, CHEN Y X, et al. Engineering Escherichia coli membrane phospholipid head distribution improves tolerance and production of biorenewables[J]. Metabolic Engineering, 2017, 44: 1-12. |
| 248 | TAN Z G, YOON J M, NIELSEN D R, et al. Membrane engineering via trans unsaturated fatty acids production improves Escherichia coli robustness and production of biorenewables[J]. Metabolic Engineering, 2016, 35: 105-113. |
| 72 | MERKX-JACQUES A, RASMUSSEN H, MUISE D M, et al. Engineering xylose metabolism in thraustochytrid T18[J]. Biotechnology for Biofuels, 2018, 11: 248. |
| 73 | YU T, ZHOU Y J, HUANG M T, et al. Reprogramming yeast metabolism from alcoholic fermentation to lipogenesis[J]. Cell, 2018, 174(6): 1549-1558.e14. |
| 74 | KIM H M, CHAE T U, CHOI S Y, et al. Engineering of an oleaginous bacterium for the production of fatty acids and fuels[J]. Nature Chemical Biology, 2019, 15(7): 721-729. |
| 75 | IKEDA M, TAKAHASHI K, OHTAKE T, et al. A futile metabolic cycle of fatty acyl coenzyme A (acyl-CoA) hydrolysis and resynthesis in Corynebacterium glutamicum and its disruption leading to fatty acid production[J]. Applied and Environmental Microbiology, 2021, 87(4): e02469-20. |
| 76 | XU P, QIAO K J, AHN W S, et al. Engineering Yarrowia lipolyticaas a platform for synthesis of drop-in transportation fuels and oleochemicals[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(39): 10848-10853. |
| 77 | ZHANG Y, PENG J, ZHAO H M, et al. Engineering oleaginous yeast Rhodotorula toruloides for overproduction of fatty acid ethyl esters[J]. Biotechnology for Biofuels, 2021, 14(1): 115. |
| 78 | LUO J, EFIMOVA E, LOSOI P, et al. Wax ester production in nitrogen-rich conditions by metabolically engineered Acinetobacter baylyi ADP1[J]. Metabolic Engineering Communications, 2020, 10: e00128. |
| 79 | GOH E B, BAIDOO E E K, BURD H, et al. Substantial improvements in methyl ketone production in E. coli and insights on the pathway from in vitro studies[J]. Metabolic Engineering, 2014, 26: 67-76. |
| 80 | DONG J E, CHEN Y, BENITES V T, et al. Methyl ketone production by Pseudomonas putida is enhanced by plant-derived amino acids[J]. Biotechnology and Bioengineering, 2019, 116(8): 1909-1922. |
| 81 | XIN F H, ZHANG Y, XUE S J, et al. Heavy oils (mainly alkanes) over-production from inulin by Aureobasidium melanogenum 9-1 and its transformant 88 carrying an inulinase gene[J]. Renewable Energy, 2017, 105: 561-568. |
| 82 | AMER M, TOOGOOD H, SCRUTTON N S. Engineering nature for gaseous hydrocarbon production[J]. Microbial Cell Factories, 2020, 19(1): 209. |
| 83 | WANG W H, LIU X F, LU X F. Engineering cyanobacteria to improve photosynthetic production of alka(e)nes[J]. Biotechnology for Biofuels, 2013, 6(1): 69. |
| 249 | FOO J L, JENSEN H M, DAHL R H, et al. Improving microbial biogasoline production in Escherichia coli using tolerance engineering[J]. mBio, 2014, 5(6): e01932. |
| 250 | SUO Y K, LUO S, ZHANG Y N, et al. Enhanced butyric acid tolerance and production by Class Ⅰ heat shock protein-overproducing Clostridium tyrobutyricum ATCC 25755[J]. Journal of Industrial Microbiology & Biotechnology, 2017, 44(8): 1145-1156. |
| 251 | ABDULLAH-AL-MAHIN, SUGIMOTO S, HIGASHI C, et al. Improvement of multiple-stress tolerance and lactic acid production in Lactococcus lactis NZ9000 under conditions of thermal stress by heterologous expression of Escherichia coli DnaK[J]. Applied and Environmental Microbiology, 2010, 76(13): 4277-4285. |
| 252 | WU C D, ZHANG J, DU G C, et al. Heterologous expression of Lactobacillus casei RecO improved the multiple-stress tolerance and lactic acid production in Lactococcus lactis NZ9000 during salt stress[J]. Bioresource Technology, 2013, 143: 238-241. |
| 253 | LUO J M, SONG Z Y, NING J, et al. The ethanol-induced global alteration in Arthrobacter simplex and its mutants with enhanced ethanol tolerance[J]. Applied Microbiology and Biotechnology, 2018, 102(21): 9331-9350. |
| 254 | YAN X Y, WANG X, YANG Y F, et al. Cysteine supplementation enhanced inhibitor tolerance of Zymomonas mobilis for economic lignocellulosic bioethanol production[J]. Bioresource Technology, 2022, 349: 126878. |
| 255 | YANG S H, FRANDEN M A, WANG X A, et al. Transcriptomic profiles of Zymomonas mobilis 8b to furfural acute and long-term stress in both glucose and xylose conditions[J]. Frontiers in Microbiology, 2020, 11: 13. |
| 256 | KIM D, HAHN J S. Roles of the Yap1 transcription factor and antioxidants in Saccharomyces cerevisiae's tolerance to furfural and 5-hydroxymethylfurfural, which function as thiol-reactive electrophiles generating oxidative stress[J]. Applied and Environmental Microbiology, 2013, 79(16): 5069-5077. |
| 257 | SHATALIN K, SHATALINA E, MIRONOV A, et al. H2S: a universal defense against antibiotics in bacteria[J]. Science, 2011, 334(6058): 986-990. |
| 258 | AROCA A, BENITO J M, GOTOR C, et al. Persulfidation proteome reveals the regulation of protein function by hydrogen sulfide in diverse biological processes in Arabidopsis [J]. Journal of Experimental Botany, 2017, 68(17): 4915-4927. |
| 259 | MIRONOV A, SEREGINA T, NAGORNYKH M, et al. Mechanism of H2S-mediated protection against oxidative stress in Escherichia coli [J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(23): 6022-6027. |
| 260 | GAO X, JIANG L, ZHU L Y, et al. Tailoring of global transcription sigma D factor by random mutagenesis to improve Escherichia coli tolerance towards low-pHs[J]. Journal of Biotechnology, 2016, 224: 55-63. |
| 84 | WANG J L, YU H Y, SONG X J, et al. The influence of fatty acid supply and aldehyde reductase deletion on cyanobacteria alkane generating pathway in Escherichia coli [J]. Journal of Industrial Microbiology and Biotechnology, 2018, 45(5): 329-334. |
| 85 | CHOI Y J, LEE S Y. Microbial production of short-chain alkanes[J]. Nature, 2013, 502(7472): 571-574. |
| 86 | FATMA Z, HARTMAN H, POOLMAN M G, et al. Model-assisted metabolic engineering of Escherichia coli for long chain alkane and alcohol production[J]. Metabolic Engineering, 2018, 46: 1-12. |
| 87 | SONG X J, YU H Y, ZHU K. Improving alkane synthesis in Escherichia coli via metabolic engineering[J]. Applied Microbiology and Biotechnology, 2016, 100(2): 757-767. |
| 88 | LIU Y Y, CHI Z, WANG Z P, et al. Heavy oils, principally long-chain n-alkanes secreted by Aureobasidium pullulans var. melanogenum strain P5 isolated from mangrove system[J]. Journal of Industrial Microbiology & Biotechnology, 2014, 41(9): 1329-1337. |
| 89 | BERNARD A, DOMERGUE F, PASCAL S, et al. Reconstitution of plant alkane biosynthesis in yeast demonstrates that Arabidopsis ECERIFERUM1 and ECERIFERUM3 are core components of a very-long-chain alkane synthesis complex[J]. The Plant Cell, 2012, 24(7): 3106-3118. |
| 90 | DELLOMONACO C, CLOMBURG J M, MILLER E N, et al. Engineered reversal of the β-oxidation cycle for the synthesis of fuels and chemicals[J]. Nature, 2011, 476(7360): 355-359. |
| 91 | LIAN J Z, ZHAO H M. Reversal of the β-oxidation cycle in Saccharomyces cerevisiae for production of fuels and chemicals[J]. ACS Synthetic Biology, 2015, 4(3): 332-341. |
| 92 | LAZAR Z, LIU N, STEPHANOPOULOS G. Holistic approaches in lipid production by Yarrowia lipolytica [J]. Trends in Biotechnology, 2018, 36(11): 1157-1170. |
| 93 | CHATTERJEE S, MOHAN S V. Microbial lipid production by Cryptococcus curvatus from vegetable waste hydrolysate[J]. Bioresource Technology, 2018, 254: 284-289. |
| 94 | DEEBA F, PRUTHI V, NEGI Y S. Converting paper mill sludge into neutral lipids by oleaginous yeast Cryptococcus vishniaccii for biodiesel production[J]. Bioresource Technology, 2016, 213: 96-102. |
| 95 | XAVIER M C A, CORADINI A L V, DECKMANN A C, et al. Lipid production from hemicellulose hydrolysate and acetic acid by Lipomyces starkeyi and the ability of yeast to metabolize inhibitors[J]. Biochemical Engineering Journal, 2017, 118: 11-19. |
| 261 | LAL A, KRISHNA S, SESHASAYEE A S N. Regulation of global transcription in Escherichia coli by Rsd and 6S RNA[J]. G3 Genes|Genomes|Genetics, 2018, 8(6): 2079-2089. |
| 262 | ADHIKARI S, CURTIS P D. DNA methyltransferases and epigenetic regulation in bacteria[J]. FEMS Microbiology Reviews, 2016, 40(5): 575-591. |
| 263 | XU Y, ZHAO Z, TONG W H, et al. An acid-tolerance response system protecting exponentially growing Escherichia coli [J]. Nature Communications, 2020, 11: 1496. |
| 264 | GUARNIERI M T, LEVERING J, HENARD C A, et al. Genome sequence of the oleaginous green alga, Chlorella vulgaris UTEX 395[J]. Frontiers in Bioengineering and Biotechnology, 2018, 6: 37. |
| 265 | SHEN Q, CHEN Y, JIN D F, et al. Comparative genome analysis of the oleaginous yeast Trichosporon fermentans reveals its potential applications in lipid accumulation[J]. Microbiological Research, 2016, 192: 203-210. |
| 266 | CHO S H, LEI R, HENNINGER T D, et al. Discovery of ethanol-responsive small RNAs in Zymomonas mobilis [J]. Applied and Environmental Microbiology, 2014, 80(14): 4189-4198. |
| 267 | CHO S H, HANING K T, SHEN W, et al. Identification and characterization of 5′ untranslated regions (5′UTRs) in Zymomonas mobilis as regulatory biological parts[J]. Frontiers in Microbiology, 2017, 8: 2432. |
| 268 | XU N, LV H F, WEI L, et al. Impaired oxidative stress and sulfur assimilation contribute to acid tolerance of Corynebacterium glutamicum [J]. Applied Microbiology and Biotechnology, 2019, 103(4): 1877-1891. |
| 269 | YANG Y F, SHEN W, HUANG J, et al. Prediction and characterization of promoters and ribosomal binding sites of Zymomonas mobilis in system biology era[J]. Biotechnology for Biofuels, 2019, 12: 52. |
| 270 | YANG Y F, RONG Z Y, SONG H Y, et al. Identification and characterization of ethanol-inducible promoters of Zymomonas mobilis based on omics data and dual reporter-gene system[J]. Biotechnology and Applied Biochemistry, 2020, 67(1): 158-165. |
| 271 | IRLA M, HAKVÅG S, BRAUTASET T. Developing a riboswitch-mediated regulatory system for metabolic flux control in thermophilic Bacillus methanolicus [J]. International Journal of Molecular Sciences, 2021, 22(9): 4686. |
| 272 | ZHOU S H, DING R P, CHEN J, et al. Obtaining a panel of cascade promoter-5′-UTR complexes in Escherichia coli [J]. ACS Synthetic Biology, 2017, 6(6): 1065-1075. |
| 96 | CASTAÑEDA M T, NUÑEZ S, GARELLI F, et al. Comprehensive analysis of a metabolic model for lipid production in Rhodosporidium toruloides [J]. Journal of Biotechnology, 2018, 280: 11-18. |
| 97 | LIU Y T, WANG Y P, LIU H J, et al. Enhanced lipid production with undetoxified corncob hydrolysate by Rhodotorula glutinis using a high cell density culture strategy[J]. Bioresource Technology, 2015, 180: 32-39. |
| 98 | POONTAWEE R, YONGMANITCHAI W, LIMTONG S. Lipid production from a mixture of sugarcane top hydrolysate and biodiesel-derived crude glycerol by the oleaginous red yeast, Rhodosporidiobolus fluvialis [J]. Process Biochemistry, 2018, 66: 150-161. |
| 99 | FEI Q, CHANG H N, SHANG L A, et al. The effect of volatile fatty acids as a sole carbon source on lipid accumulation by Cryptococcus albidus for biodiesel production[J]. Bioresource Technology, 2011, 102(3): 2695-2701. |
| 100 | QIN L, LIU L, ZENG A P, et al. From low-cost substrates to single cell oils synthesized by oleaginous yeasts[J]. Bioresource Technology, 2017, 245(Pt B): 1507-1519. |
| 101 | TAI M, STEPHANOPOULOS G. Engineering the push and pull of lipid biosynthesis in oleaginous yeast Yarrowia lipolytica for biofuel production[J]. Metabolic Engineering, 2013, 15: 1-9. |
| 102 | RAKICKA M, LAZAR Z, DULERMO T, et al. Lipid production by the oleaginous yeast Yarrowia lipolytica using industrial by-products under different culture conditions[J]. Biotechnology for Biofuels, 2015, 8: 104. |
| 103 | DAVIS M S, SOLBIATI J, CRONAN J E JR. Overproduction of acetyl-CoA carboxylase activity increases the rate of fatty acid biosynthesis in Escherichia coli [J]. Journal of Biological Chemistry, 2000, 275(37): 28593-28598. |
| 104 | BORRELLI G M, TRONO D. Recombinant lipases and phospholipases and their use as biocatalysts for industrial applications[J]. International Journal of Molecular Sciences, 2015, 16(9): 20774-20840. |
| 105 | KYOTANI S, NAKASHIMA T, IZUMOTO E, et al. Continuous interesterification of oils and fats using dried fungus immobilized in biomass support particles[J]. Journal of Fermentation and Bioengineering, 1991, 71(4): 286-288. |
| 106 | MATSUMOTO T, FUKUDA H, UEDA M, et al. Construction of yeast strains with high cell surface lipase activity by using novel display systems based on the Flo1p flocculation functional domain[J]. Applied and Environmental Microbiology, 2002, 68(9): 4517-4522. |
| 107 | KALSCHEUER R, STÖVEKEN T, LUFTMANN H, et al. Neutral lipid biosynthesis in engineered Escherichia coli: jojoba oil-like wax esters and fatty acid butyl esters[J]. Applied and Environmental Microbiology, 2006, 72(2): 1373-1379. |
| 273 | HOLTZ W J, KEASLING J D. Engineering static and dynamic control of synthetic pathways[J]. Cell, 2010, 140(1): 19-23. |
| 274 | ZHUANG K, YANG L, CLUETT W R, et al. Dynamic strain scanning optimization: an efficient strain design strategy for balanced yield, titer, and productivity. DySScO strategy for strain design[J]. BMC Biotechnology, 2013, 13: 8. |
| 275 | FARMER W R, LIAO J C. Improving lycopene production in Escherichia coli by engineering metabolic control[J]. Nature Biotechnology, 2000, 18(5): 533-537. |
| 276 | SKERRA A. Use of the tetracycline promoter for the tightly regulated production of a murine antibody fragment in Escherichia coli [J]. Gene, 1994, 151(1/2): 131-135. |
| 277 | YIN X, SHIN H D, LI J H, et al. Pgas, a low-pH-induced promoter, as a tool for dynamic control of gene expression for metabolic engineering of Aspergillus niger [J]. Applied and Environmental Microbiology, 2017, 83(6): e03222-16. |
| 278 | ZHOU L, NIU D D, TIAN K M, et al. Genetically switched D-lactate production in Escherichia coli [J]. Metabolic Engineering, 2012, 14(5): 560-568. |
| 279 | HWANG H J, KIM J W, JU S Y, et al. Application of an oxygen-inducible nar promoter system in metabolic engineering for production of biochemicals in Escherichia coli [J]. Biotechnology and Bioengineering, 2017, 114(2): 468-473. |
| 280 | ZHAO E M, ZHANG Y F, MEHL J, et al. Optogenetic regulation of engineered cellular metabolism for microbial chemical production[J]. Nature, 2018, 555(7698): 683-687. |
| 281 | ROMANO E, BAUMSCHLAGER A, AKMERIÇ E B, et al. Engineering AraC to make it responsive to light instead of arabinose[J]. Nature Chemical Biology, 2021, 17(7): 817-827. |
| 282 | BAÑARES A B, VALDEHUESA K N G, RAMOS K R M, et al. A pH-responsive genetic sensor for the dynamic regulation of D-xylonic acid accumulation in Escherichia coli [J]. Applied Microbiology and Biotechnology, 2020, 104(5): 2097-2108. |
| 283 | LIU D, XIAO Y, EVANS B S, et al. Negative feedback regulation of fatty acid production based on a malonyl-CoA sensor-actuator[J]. ACS Synthetic Biology, 2015, 4(2): 132-140. |
| 284 | XU P, LI L Y, ZHANG F M, et al. Improving fatty acids production by engineering dynamic pathway regulation and metabolic control[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(31): 11299-11304. |
| 108 | STEEN E J, KANG Y S, BOKINSKY G, et al. Microbial production of fatty-acid-derived fuels and chemicals from plant biomass [J]. Nature, 2010, 463(7280): 559-562. |
| 109 | KANG M K, ZHOU Y J, BUIJS N A, et al. Functional screening of aldehyde decarbonylases for long-chain alkane production by Saccharomyces cerevisiae [J]. Microbial Cell Factories, 2017, 16: 74. |
| 110 | SCHIRMER A, RUDE M A, LI X Z, et al. Microbial biosynthesis of alkanes[J]. Science, 2010, 329(5991): 559-562. |
| 111 | LADYGINA N, DEDYUKHINA E G, VAINSHTEIN M B. A review on microbial synthesis of hydrocarbons[J]. Process Biochemistry, 2006, 41(5): 1001-1014. |
| 112 | JAROENSUK J, INTASIAN P, WATTANASUEPSIN W, et al. Enzymatic reactions and pathway engineering for the production of renewable hydrocarbons[J]. Journal of Biotechnology, 2020, 309: 1-19. |
| 113 | RUI Z, HARRIS N C, ZHU X J, et al. Discovery of a family of desaturase-like enzymes for 1-alkene biosynthesis[J]. ACS Catalysis, 2015, 5(12): 7091-7094. |
| 114 | RUI Z, LI X, ZHU X J, et al. Microbial biosynthesis of medium-chain 1-alkenes by a nonheme iron oxidase[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(51): 18237-18242. |
| 115 | LIU Y, WANG C, YAN J Y, et al. Hydrogen peroxide-independent production of α-alkenes by OleTJE P450 fatty acid decarboxylase[J]. Biotechnology for Biofuels, 2014, 7(1): 28. |
| 116 | ZHOU Y J, HU Y T, ZHU Z W, et al. Engineering 1-alkene biosynthesis and secretion by dynamic regulation in yeast[J]. ACS Synthetic Biology, 2018, 7(2): 584-590. |
| 117 | FRIAS J A, GOBLIRSCH B R, WACKETT L P, et al. Cloning, purification, crystallization and preliminary X-ray diffraction of the OleC protein from Stenotrophomonas maltophilia involved in head-to-head hydrocarbon biosynthesis[J]. Acta Crystallographica Section F: Structural Biology and Crystallization Communications, 2010, 66(Pt 9): 1108-1110. |
| 118 | FRIAS J A, RICHMAN J E, ERICKSON J S, et al. Purification and characterization of OleA from Xanthomonas campestris and demonstration of a non-decarboxylative Claisen condensation reaction[J]. The Journal of Biological Chemistry, 2011, 286(13): 10930-10938. |
| 119 | BELLER H R, GOH E B, KEASLING J D. Genes involved in long-chain alkene biosynthesis in Micrococcus luteus [J]. Applied and Environmental Microbiology, 2010, 76(4): 1212-1223. |
| 285 | ZHANG F Z, CAROTHERS J M, KEASLING J D. Design of a dynamic sensor-regulator system for production of chemicals and fuels derived from fatty acids[J]. Nature Biotechnology, 2012, 30(4): 354-359. |
| 286 | LANDICK R. Active-site dynamics in RNA polymerases[J]. Cell, 2004, 116(3): 351-353. |
| 287 | STUDIER F W, MOFFATT B A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes[J]. Journal of Molecular Biology, 1986, 189(1): 113-130. |
| 288 | DU F, LIU Y Q, XU Y S, et al. Regulating the T7 RNA polymerase expression in E. coli BL21 (DE3) to provide more host options for recombinant protein production[J]. Microbial Cell Factories, 2021, 20(1): 189. |
| 289 | LIU R M, LIANG L Y, FREED E F, et al. Engineering regulatory networks for complex phenotypes in E. coli [J]. Nature Communications, 2020, 11: 4050. |
| 290 | WANG T M, ZHENG X, JI H N, et al. Dynamics of transcription-translation coordination tune bacterial indole signaling[J]. Nature Chemical Biology, 2020, 16(4): 440-449. |
| 291 | SOMA Y, HANAI T. Self-induced metabolic state switching by a tunable cell density sensor for microbial isopropanol production[J]. Metabolic Engineering, 2015, 30: 7-15. |
| 292 | LIU H W, LU T. Autonomous production of 1, 4-butanediol via a de novo biosynthesis pathway in engineered Escherichia coli [J]. Metabolic Engineering, 2015, 29: 135-141. |
| 293 | KIM E M, WOO H M, TIAN T, et al. Autonomous control of metabolic state by a quorum sensing (QS)-mediated regulator for bisabolene production in engineered E. coli [J]. Metabolic Engineering, 2017, 44: 325-336. |
| 294 | LANDON S, REES-GARBUTT J, MARUCCI L, et al. Genome-driven cell engineering review: in vivo and in silico metabolic and genome engineering[J]. Essays in Biochemistry, 2019, 63(2): 267-284. |
| 295 | EDWARDS J S, PALSSON B O. The Escherichia coli MG1655 in silico metabolic genotype: its definition, characteristics, and capabilities[J]. Proceedings of the National Academy of Sciences of the United States of America, 2000, 97(10): 5528-5533. |
| 296 | REED J L, VO T D, SCHILLING C H, et al. An expanded genome-scale model of Escherichia coli K-12 (iJR904 GSM/GPR)[J]. Genome Biology, 2003, 4(9): R54. |
| 120 | TAN X M, YAO L, GAO Q Q, et al. Photosynthesis driven conversion of carbon dioxide to fatty alcohols and hydrocarbons in cyanobacteria[J]. Metabolic Engineering, 2011, 13(2): 169-176. |
| 121 | ANTHONY J R, ANTHONY L C, NOWROOZI F, et al. Optimization of the mevalonate-based isoprenoid biosynthetic pathway in Escherichia coli for production of the anti-malarial drug precursor amorpha-4,11-diene[J]. Metabolic Engineering, 2009, 11(1): 13-19. |
| 122 | YOON S H, LEE S H, DAS A, et al. Combinatorial expression of bacterial whole mevalonate pathway for the production of β-carotene in E. coli [J]. Journal of Biotechnology, 2009, 140(3/4): 218-226. |
| 123 | YOON S H, LEE Y M, KIM J E, et al. Enhanced lycopene production in Escherichia coli engineered to synthesize isopentenyl diphosphate and dimethylallyl diphosphate from mevalonate[J]. Biotechnology and Bioengineering, 2006, 94(6): 1025-1032. |
| 124 | MARTIN V J J, PITERA D J, WITHERS S T, et al. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids[J]. Nature Biotechnology, 2003, 21(7): 796-802. |
| 125 | LEAVELL M D, MCPHEE D J, PADDON C J. Developing fermentative terpenoid production for commercial usage[J]. Current Opinion in Biotechnology, 2016, 37: 114-119. |
| 126 | YE Z L, SHI B, HUANG Y L, et al. Revolution of vitamin E production by starting from microbial fermented farnesene to isophytol[J]. The Innovation, 2022, 3(3): 100228. |
| 127 | LI M J, HOU F F, WU T, et al. Recent advances of metabolic engineering strategies in natural isoprenoid production using cell factories[J]. Natural Product Reports, 2020, 37(1): 80-99. |
| 128 | CHATZIVASILEIOU A O, WARD V, EDGAR S M, et al. Two-step pathway for isoprenoid synthesis[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(2): 506-511. |
| 129 | TASHIRO M, KIYOTA H, KAWAI-NOMA S, et al. Bacterial production of pinene by a laboratory-evolved pinene-synthase[J]. ACS Synthetic Biology, 2016, 5(9): 1011-1020. |
| 130 | WEI L J, ZHONG Y T, NIE M Y, et al. Biosynthesis of α-pinene by genetically engineered Yarrowia lipolytica from low-cost renewable feedstocks[J]. Journal of Agricultural and Food Chemistry, 2021, 69(1): 275-285. |
| 131 | KANG M K, EOM J H, KIM Y, et al. Biosynthesis of pinene from glucose using metabolically-engineered Corynebacterium glutamicum [J]. Biotechnology Letters, 2014, 36(10): 2069-2077. |
| 132 | WU X M, MA G, LIU C Y, et al. Biosynthesis of pinene in purple non-sulfur photosynthetic bacteria[J]. Microbial Cell Factories, 2021, 20(1): 101. |
| 297 | FEIST A M, HENRY C S, REED J L, et al. A genome-scale metabolic reconstruction for Escherichia coli K-12 MG1655 that accounts for 1260 ORFs and thermodynamic information[J]. Molecular Systems Biology, 2007, 3: 121. |
| 298 | ORTH J D, CONRAD T M, NA J, et al. A comprehensive genome-scale reconstruction of Escherichia coli metabolism—2011[J]. Molecular Systems Biology, 2011, 7: 535. |
| 299 | MONK J M, LLOYD C J, BRUNK E, et al. iML1515, a knowledgebase that computes Escherichia coli traits[J]. Nature Biotechnology, 2017, 35(10): 904-908. |
| 300 | HENRY C S, ZINNER J F, COHOON M P, et al. iBsu1103: a new genome-scale metabolic model of Bacillus subtilis based on SEED annotations[J]. Genome Biology, 2009, 10(6): R69. |
| 301 | TANAKA K, HENRY C S, ZINNER J F, et al. Building the repertoire of dispensable chromosome regions in Bacillus subtilis entails major refinement of cognate large-scale metabolic model[J]. Nucleic Acids Research, 2013, 41(1): 687-699. |
| 302 | KOCABAŞ P, ÇALıK P, ÇALıK G, et al. Analyses of extracellular protein production in Bacillus subtilis-Ⅰ: genome-scale metabolic model reconstruction based on updated gene-enzyme-reaction data[J]. Biochemical Engineering Journal, 2017, 127: 229-241. |
| 303 | MASSAIU I, PASOTTI L, SONNENSCHEIN N, et al. Integration of enzymatic data in Bacillus subtilis genome-scale metabolic model improves phenotype predictions and enables in silico design of poly-γ-glutamic acid production strains[J]. Microbial Cell Factories, 2019, 18(1): 3. |
| 304 | ZHANG Y, CAI J Y, SHANG X L, et al. A new genome-scale metabolic model of Corynebacterium glutamicum and its application[J]. Biotechnology for Biofuels, 2017, 10: 169. |
| 305 | CHENG F Y, YU H M, STEPHANOPOULOS G. Engineering Corynebacterium glutamicum for high-titer biosynthesis of hyaluronic acid[J]. Metabolic Engineering, 2019, 55: 276-289. |
| 306 | MEI J, XU N, YE C, et al. Reconstruction and analysis of a genome-scale metabolic network of Corynebacterium glutamicum S9114[J]. Gene, 2016, 575(2 Pt 3): 615-622. |
| 307 | FÖRSTER J, FAMILI I, FU P, et al. Genome-scale reconstruction of the Saccharomyces cerevisiae metabolic network[J]. Genome Research, 2003, 13(2): 244-253. |
| 308 | DUARTE N C, HERRGÅRD M J, PALSSON B Ø. Reconstruction and validation of Saccharomyces cerevisiae iND750, a fully compartmentalized genome-scale metabolic model[J]. Genome Research, 2004, 14(7): 1298-1309. |
| 133 | ZHANG H B, LIU Q, CAO Y J, et al. Microbial production of sabinene—a new terpene-based precursor of advanced biofuel[J]. Microbial Cell Factories, 2014, 13: 20. |
| 134 | IGNEA C, PONTINI M, MAFFEI M E, et al. Engineering monoterpene production in yeast using a synthetic dominant negative geranyl diphosphate synthase[J]. ACS Synthetic Biology, 2014, 3(5): 298-306. |
| 135 | WU J H, CHENG S, CAO J Y, et al. Systematic optimization of limonene production in engineered Escherichia coli [J]. Journal of Agricultural and Food Chemistry, 2019, 67(25): 7087-7097. |
| 136 | ROLF J, JULSING M K, ROSENTHAL K, et al. A gram-scale limonene production process with engineered Escherichia coli [J]. Molecules, 2020, 25(8): 1881. |
| 137 | CHENG B Q, WEI L J, LV Y B, et al. Elevating limonene production in oleaginous yeast Yarrowia lipolytica via genetic engineering of limonene biosynthesis pathway and optimization of medium composition[J]. Biotechnology and Bioprocess Engineering, 2019, 24(3): 500-506. |
| 138 | CHENG S, LIU X, JIANG G Z, et al. Orthogonal engineering of biosynthetic pathway for efficient production of limonene in Saccharomyces cerevisiae [J]. ACS Synthetic Biology, 2019, 8(5): 968-975. |
| 139 | YOU S P, YIN Q D, ZHANG J Y, et al. Utilization of biodiesel by-product as substrate for high-production of β-farnesene via relatively balanced mevalonate pathway in Escherichia coli [J]. Bioresource Technology, 2017, 243: 228-236. |
| 140 | LIU Y H, JIANG X, CUI Z Y, et al. Engineering the oleaginous yeast Yarrowia lipolytica for production of α-farnesene[J]. Biotechnology for Biofuels, 2019, 12: 296. |
| 141 | LIU H, CHEN S L, XU J Z, et al. Dual regulation of cytoplasm and peroxisomes for improved Α-farnesene production in recombinant Pichia pastoris [J]. ACS Synthetic Biology, 2021, 10(6): 1563-1573. |
| 142 | MEADOWS A L, HAWKINS K M, TSEGAYE Y, et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production[J]. Nature, 2016, 537(7622): 694-697. |
| 143 | PERALTA-YAHYA P P, OUELLET M, CHAN R, et al. Identification and microbial production of a terpene-based advanced biofuel[J]. Nature Communications, 2011, 2: 483. |
| 144 | ZHANG Y, SONG X H, LAI Y M, et al. High-yielding terpene-based biofuel production in Rhodobacter capsulatus [J]. ACS Synthetic Biology, 2021, 10(6): 1545-1552. |
| 309 | KUEPFER L, SAUER U, BLANK L M. Metabolic functions of duplicate genes in Saccharomyces cerevisiae [J]. Genome Research, 2005, 15(10): 1421-1430. |
| 310 | NOOKAEW I, JEWETT M, MEECHAI A, et al. The genome-scale metabolic model iIN800 of Saccharomyces cerevisiae and its validation: a scaffold to query lipid metabolism[J]. BMC System Biology, 2008, 2: 71. |
| 311 | MO M L, PALSSON B O, HERRGÅRD M J. Connecting extracellular metabolomic measurements to intracellular flux states in yeast[J]. BMC Systems Biology, 2009, 3: 37. |
| 312 | HERRGÅRD M J, SWAINSTON N, DOBSON P, et al. A consensus yeast metabolic network reconstruction obtained from a community approach to systems biology[J]. Nature Biotechnology, 2008, 26(10): 1155-1160. |
| 313 | LU H, LI F, SÁNCHEZ B J, et al. A consensus S. cerevisiae metabolic model Yeast8 and its ecosystem for comprehensively probing cellular metabolism [J]. Nature Communications, 2019, 10: 3586. |
| 314 | LEE S J, LEE D Y, KIM T Y, et al. Metabolic engineering of Escherichia coli for enhanced production of succinic acid, based on genome comparison and in silico gene knockout simulation[J]. Applied and Environmental Microbiology, 2005, 71(12): 7880-7887. |
| 315 | BODOR Z, TOMPOS L, NECHIFOR A C, et al. In silico analysis of 1,4-butanediol heterologous pathway impact on Escherichia coli metabolism[J]. Revista De Chimie, 2019, 70(10): 3448-3455. |
| 316 | BRO C, REGENBERG B, FÖRSTER J, et al. In silico aided metabolic engineering of Saccharomyces cerevisiae for improved bioethanol production[J]. Metabolic Engineering, 2006, 8(2): 102-111. |
| 317 | FONG S S, BURGARD A P, HERRING C D, et al. In silico design and adaptive evolution of Escherichia coli for production of lactic acid[J]. Biotechnology and Bioengineering, 2005, 91(5): 643-648. |
| 318 | NG C Y, JUNG M Y, LEE J, et al. Production of 2,3-butanediol in Saccharomyces cerevisiae by in silico aided metabolic engineering[J]. Microbial Cell Factories, 2012, 11: 68. |
| 319 | RANGANATHAN S, TEE T W, CHOWDHURY A, et al. An integrated computational and experimental study for overproducing fatty acids in Escherichia coli [J]. Metabolic Engineering, 2012, 14(6): 687-704. |
| 320 | 杨永富, 耿碧男, 宋皓月, 等. 运动发酵单胞菌底盘细胞研究现状及展望[J]. 合成生物学, 2021, 2(1): 59-90. |
| 145 | SARRIA S, WONG B, GARCÍA MARTÍN H, et al. Microbial synthesis of pinene[J]. ACS Synthetic Biology, 2014, 3(7): 466-475. |
| 146 | ZADA B, WANG C L, PARK J B, et al. Metabolic engineering of Escherichia coli for production of mixed isoprenoid alcohols and their derivatives[J]. Biotechnology for Biofuels, 2018, 11: 210. |
| 147 | LIU H W, WANG Y, TANG Q, et al. MEP pathway-mediated isopentenol production in metabolically engineered Escherichia coli [J]. Microbial Cell Factories, 2014, 13: 135. |
| 148 | HUANG Y L, YE Z L, WAN X K, et al. Systematic mining and evaluation of the sesquiterpene skeletons as high energy aviation fuel molecules[J]. Advanced Science, 2023, 10(23): e2300889. |
| 149 | TAO H, LAUTERBACH L, BIAN G K, et al. Discovery of non-squalene triterpenes[J]. Nature, 2022, 606(7913): 414-419. |
| 150 | 杨永富, 耿碧男, 宋皓月, 等. 合成生物学时代基于非模式细菌的工业底盘细胞研究现状与展望[J]. 生物工程学报, 2021, 37(3): 874-910. |
| YANG Y F, GENG B N, SONG H Y, et al. Progress and perspective on development of non-model industrial bacteria as chassis cells for biochemical production in the synthetic biology era[J]. Chinese Journal of Biotechnology, 2021, 37(3): 874-910. | |
| 151 | WANG C L, PFLEGER B F, KIM S W. Reassessing Escherichia coli as a cell factory for biofuel production[J]. Current Opinion in Biotechnology, 2017, 45: 92-103. |
| 152 | BECKER J, ROHLES C M, WITTMANN C. Metabolically engineered Corynebacterium glutamicum for bio-based production of chemicals, fuels, materials, and healthcare products[J]. Metabolic Engineering, 2018, 50: 122-141. |
| 153 | TSIGIE Y A, WANG C Y, TRUONG C T, et al. Lipid production from Yarrowia lipolytica Po1g grown in sugarcane bagasse hydrolysate[J]. Bioresource Technology, 2011, 102(19): 9216-9222. |
| 154 | ZHU Q, JACKSON E N. Metabolic engineering of Yarrowia lipolytica for industrial applications[J]. Current Opinion in Biotechnology, 2015, 36: 65-72. |
| 155 | LI H B, ALPER H S. Enabling xylose utilization in Yarrowia lipolytica for lipid production[J]. Biotechnology Journal, 2016, 11(9): 1230-1240. |
| 320 | YANG Y F, GENG B N, SONG H Y, et al. Progress and perspectives on developing Zymomonas mobilis as a chassis cell[J]. Synthetic Biology Journal, 2021, 2(1): 59-90. |
| 321 | LI M D, ZHANG Y, LI J C, et al. Biosynthesis of 1,3-propanediol via a new pathway from glucose in Escherichia coli [J]. ACS Synthetic Biology, 2023, 12(7): 2083-2093. |
| 322 | HAEUSSLER M, CONCORDET J P. Genome editing with CRISPR-Cas9: can it get any better?[J]. Journal of Genetics and Genomics, 2016, 43(5): 239-250. |
| 323 | KLEINSTIVER B P, PATTANAYAK V, PREW M S, et al. High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects[J]. Nature, 2016, 529(7587): 490-495. |
| 324 | ZHANG Y P, WANG J, WANG Z B, et al. A gRNA-tRNA array for CRISPR-Cas9 based rapid multiplexed genome editing in Saccharomyces cerevisiae [J]. Nature Communications, 2019, 10: 1053. |
| 325 | O'CONNELL MOTHERWAY M, O'DRISCOLL J, FITZGERALD G F, et al. Overcoming the restriction barrier to plasmid transformation and targeted mutagenesis in Bifidobacterium breve UCC2003[J]. Microbial Biotechnology, 2009, 2(3): 321-332. |
| 326 | WESSELS H H, STIRN A, MÉNDEZ-MANCILLA A, et al. Prediction of on-target and off-target activity of CRISPR-Cas13d guide RNAs using deep learning[J/OL]. Nature Biotechnology, 2023[2023-07-10]. . |
| 327 | PÓSFAI G, PLUNKETT G 3 RD, FEHÉR T,et al. Emergent properties of reduced-genome Escherichia coli[J]. Science, 2006, 312(5776): 1044-1046. |
| 328 | FAN X, ZHANG Y T, ZHAO F J, et al. Genome reduction enhances production of polyhydroxyalkanoate and alginate oligosaccharide in Pseudomonas mendocina [J]. International Journal of Biological Macromolecules, 2020, 163: 2023-2031. |
| 329 | HILLSON N, CADDICK M, CAI Y Z, et al. Building a global alliance of biofoundries[J]. Nature Communications, 2019, 10: 2040. |
| 330 | CHAO R, MISHRA S, SI T, et al. Engineering biological systems using automated biofoundries[J]. Metabolic Engineering, 2017, 42: 98-108. |
| 331 | HUGHES S R, BUTT T R, BARTOLETT S, et al. Design and construction of a first-generation high-throughput integrated robotic molecular biology platform for bioenergy applications[J]. Journal of the Association for Laboratory Automation, 2011, 16(4): 292-307. |
| 156 | LARROUDE M, ROSSIGNOL T, NICAUD J M, et al. Synthetic biology tools for engineering Yarrowia lipolytica [J]. Biotechnology Advances, 2018, 36(8): 2150-2164. |
| 157 | WANG X, HE Q N, YANG Y F, et al. Advances and prospects in metabolic engineering of Zymomonas mobilis [J]. Metabolic Engineering, 2018, 50: 57-73. |
| 158 | SHEN W, ZHANG J, GENG B N, et al. Establishment and application of a CRISPR-Cas12a assisted genome-editing system in Zymomonas mobilis [J]. Microbial Cell Factories, 2019, 18(1): 162. |
| 159 | ZHENG Y L, HAN J M, WANG B Y, et al. Characterization and repurposing of the endogenous Type Ⅰ-F CRISPR-Cas system of Zymomonas mobilis for genome engineering[J]. Nucleic Acids Research, 2019, 47(21): 11461-11475. |
| 160 | YANG S H, FEI Q, ZHANG Y P, et al. Zymomonas mobilis as a model system for production of biofuels and biochemicals[J]. Microbial Biotechnology, 2016, 9(6): 699-717. |
| 161 | LI Q, CHEN J, MINTON N P, et al. CRISPR-based genome editing and expression control systems in Clostridium acetobutylicum and Clostridium beijerinckii [J]. Biotechnology Journal, 2016, 11(7): 961-972. |
| 162 | TAFUR RANGEL A E, CROFT T, GONZÁLEZ BARRIOS A F, et al. Transcriptomic analysis of a Clostridium thermocellum strain engineered to utilize xylose: responses to xylose versus cellobiose feeding[J]. Scientific Reports, 2020, 10: 14517. |
| 163 | LIU Y J, LI B, FENG Y G, et al. Consolidated bio-saccharification: leading lignocellulose bioconversion into the real world[J]. Biotechnology Advances, 2020, 40: 107535. |
| 164 | ZHANG X N, LIU C L, DAI J B, et al. Enabling technology and core theory of synthetic biology[J]. Science China Life Sciences, 2023, 66(8): 1742-1785. |
| 165 | GALINIER A, DEUTSCHER J. Sophisticated regulation of transcriptional factors by the bacterial phosphoenolpyruvate: sugar phosphotransferase system[J]. Journal of Molecular Biology, 2017, 429(6): 773-789. |
| 166 | WANG Q Z, WU C Y, CHEN T, et al. Expression of galactose permease and pyruvate carboxylase in Escherichia coli ptsG mutant increases the growth rate and succinate yield under anaerobic conditions[J]. Biotechnology Letters, 2006, 28(2): 89-93. |
| 167 | HERNÁNDEZ-MONTALVO V, MARTÍNEZ A, HERNÁNDEZ-CHAVEZ G, et al. Expression of galP and glk in a Escherichia coli PTS mutant restores glucose transport and increases glycolytic flux to fermentation products[J]. Biotechnology and Bioengineering, 2003, 83(6): 687-694. |
| 332 | YUAN Y, DU J, ZHAO H. Customized optimization of metabolic pathways by combinatorial transcriptional engineering[M/OL]//Methods in molecular biology, 2013, 985: 177-209 [2023-06-01]. . |
| 333 | SI T, CHAO R, MIN Y H, et al. Automated multiplex genome-scale engineering in yeast[J]. Nature Communications, 2017, 8: 15187. |
| 168 | SNOEP J L, ARFMAN N, YOMANO L P, et al. Reconstruction of glucose uptake and phosphorylation in a glucose-negative mutant of Escherichia coli by using Zymomonas mobilis genes encoding the glucose facilitator protein and glucokinase[J]. Journal of Bacteriology, 1994, 176(7): 2133-2135. |
| 169 | MIAO R, XIE H, HO F M, et al. Protein engineering of α-ketoisovalerate decarboxylase for improved isobutanol production in Synechocystis PCC 6803[J]. Metabolic Engineering, 2018, 47: 42-48. |
| 170 | PASTOR J M, BORGES N, PAGÁN J P, et al. Fructose metabolism in Chromohalobacter salexigens: interplay between the Embden-Meyerhof-Parnas and Entner-Doudoroff pathways[J]. Microbial Cell Factories, 2019, 18: 134. |
| 171 | NG C Y, FARASAT I, MARANAS C D, et al. Rational design of a synthetic Entner-Doudoroff pathway for improved and controllable NADPH regeneration[J]. Metabolic Engineering, 2015, 29: 86-96. |
| 172 | LIANG S X, CHEN H, LIU J, et al. Rational design of a synthetic Entner-Doudoroff pathway for enhancing glucose transformation to isobutanol in Escherichia coli [J]. Journal of Industrial Microbiology & Biotechnology, 2018, 45(3): 187-199. |
| 173 | NODA S, MORI Y, OYAMA S, et al. Reconstruction of metabolic pathway for isobutanol production in Escherichia coli [J]. Microbial Cell Factories, 2019, 18(1): 124. |
| [1] | GUO Shuyuan, ZHANG Qiannan, Gulikezi· MAIMAITIREXIATI, YANG Yiqun, YU Tao. Advances in microbial production of liquid biofuels [J]. Synthetic Biology Journal, 2025, 6(1): 18-44. |
| [2] | GAO Ge, BIAN Qi, WANG Baojun. Synthetic genetic circuit engineering: principles, advances and prospects [J]. Synthetic Biology Journal, 2025, 6(1): 45-64. |
| [3] | LI Jiyuan, WU Guosheng. Two hypothesises for the origins of organisms from the synthetic biology perspective [J]. Synthetic Biology Journal, 2025, 6(1): 190-202. |
| [4] | JIAO Hongtao, QI Meng, SHAO Bin, JIANG Jinsong. Legal issues for the storage of DNA data [J]. Synthetic Biology Journal, 2025, 6(1): 177-189. |
| [5] | TANG Xinghua, LU Qianneng, HU Yilin. Philosophical reflections on synthetic biology in the Anthropocene [J]. Synthetic Biology Journal, 2025, 6(1): 203-212. |
| [6] | XU Huaisheng, SHI Xiaolong, LIU Xiaoguang, XU Miaomiao. Key technologies for DNA storage: encoding, error correction, random access, and security [J]. Synthetic Biology Journal, 2025, 6(1): 157-176. |
| [7] | SHI Ting, SONG Zhan, SONG Shiyi, ZHANG Yi-Heng P. Job. In vitro BioTransformation (ivBT): a new frontier of industrial biomanufacturing [J]. Synthetic Biology Journal, 2024, 5(6): 1437-1460. |
| [8] | CHAI Meng, WANG Fengqing, WEI Dongzhi. Synthesis of organic acids from lignocellulose by biotransformation [J]. Synthetic Biology Journal, 2024, 5(6): 1242-1263. |
| [9] | SHAO Mingwei, SUN Simian, YANG Shimao, CHEN Guoqiang. Bioproduction based on extremophiles [J]. Synthetic Biology Journal, 2024, 5(6): 1419-1436. |
| [10] | ZHAO Liang, LI Zhenshuai, FU Liping, LYU Ming, WANG Shi’an, ZHANG Quan, LIU Licheng, LI Fuli, LIU Ziyong. Progress in biomanufacturing of lipids and single cell protein from one-carbon compounds [J]. Synthetic Biology Journal, 2024, 5(6): 1300-1318. |
| [11] | ZHU Fanghuan, CEN Xuecong, CHEN Zhen. Research progress of diols production by microbes [J]. Synthetic Biology Journal, 2024, 5(6): 1367-1385. |
| [12] | CHEN Yu, ZHANG Kang, QIU Yijing, CHENG Caiyun, YIN Jingjing, SONG Tianshun, XIE Jingjing. Progress of microbial electrosynthesis for conversion of CO2 [J]. Synthetic Biology Journal, 2024, 5(5): 1142-1168. |
| [13] | ZHENG Haotian, LI Chaofeng, LIU Liangxu, WANG Jiawei, LI Hengrun, NI Jun. Design, optimization and application of synthetic carbon-negative phototrophic community [J]. Synthetic Biology Journal, 2024, 5(5): 1189-1210. |
| [14] | YU Wei, GAO Jiaoqi, ZHOU Yongjin. Bioconversion of one carbon feedstocks for producing organic acids [J]. Synthetic Biology Journal, 2024, 5(5): 1169-1188. |
| [15] | CHEN Ziling, XIANG Yangfei. Integrated development of organoid technology and synthetic biology [J]. Synthetic Biology Journal, 2024, 5(4): 795-812. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||