Synthetic Biology Journal ›› 2024, Vol. 5 ›› Issue (2): 221-238.DOI: 10.12211/2096-8280.2023-079
• Invited Review • Previous Articles Next Articles
Applications of synthetic biology in developing microbial-vectored cancer vaccines
TAN Zibin, LIANG Kang, CHEN Youhai
- Center for Cancer Immunology,Faculty of Pharmaceutical Sciences,Shenzhen Institute of Advanced Technology,Shenzhen University of Advanced Technology,Chinese Academy of Sciences (CAS),Shenzhen 518055,Guangdong,China
-
Received:2023-11-20Revised:2024-02-05Online:2024-04-28Published:2024-04-30 -
Contact:CHEN Youhai
合成生物学在基于微生物载体肿瘤疫苗设计中的应用
谭子斌, 梁康, 陈有海
- 深圳理工大学药学院,中国科学院深圳先进技术研究院癌症免疫中心,广东 深圳 518055
-
通讯作者:陈有海 -
作者简介:谭子斌 (1990—),男,助理研究员。研究方向为肿瘤疫苗与免疫治疗。E-mail:tanzibin108@gmail.com陈有海 (1963—),博士生导师,欧洲科学院(Academia Europaea)院士,美国医学与生物工程院(AIMBE)Fellow,国家特聘教授,教育部长江学者,深圳理工大学药学院讲席教授、院长。研究方向为肿瘤免疫治疗。E-mail:yh.chen@siat.ac.cn -
基金资助:国家重点研发计划(2022YFA0912400);深圳市科技计划(JCYJ20220818100806015);国家自然科学基金(32130040);深圳市医学科研基金(B2301006)
CLC Number:
Cite this article
TAN Zibin, LIANG Kang, CHEN Youhai. Applications of synthetic biology in developing microbial-vectored cancer vaccines[J]. Synthetic Biology Journal, 2024, 5(2): 221-238.
谭子斌, 梁康, 陈有海. 合成生物学在基于微生物载体肿瘤疫苗设计中的应用[J]. 合成生物学, 2024, 5(2): 221-238.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2023-079
| 疫苗名称 | 载体 类型 | 来源 | 临床状态 | 肿瘤类型 | 临床试验编号 | 方法 免疫 | 肿瘤特异性抗原 | 结合疗法 | 参考 文献 |
|---|---|---|---|---|---|---|---|---|---|
| BCG | 减毒 活细菌 | 牛结核菌 | 临床使用 | 膀胱癌 | — | 瘤内 | 无 | 手术 | [ |
| T-VEC | 工程化病毒 | 单纯疱疹 病毒1型 | 临床使用 | 无法切除的转移性ⅢB/C-ⅣM1a期黑色素瘤 | — | 瘤内 | 无 | 无 | [ |
| 工程化病毒 | 单纯疱疹 病毒1型 | 临床使用 | 复发性神经胶质瘤 | UMIN000002661 UMIN000015995 | 瘤内 | 无 | 无 | [ | |
| REOLYSIN | 工程化病毒 | 呼肠孤病毒Dearing type 3 | Ⅰb | 高级神经胶质瘤、脑转移 | EudraCT 2011-005635-10 | 静脉 | 无 | 手术 | [ |
| Delta-24-RGD | 工程化病毒 | 腺病毒Ad5 | Ⅰ | 儿童弥散内生型脑桥胶质瘤(DIPG) | 瘤内 | 无 | 标准放疗+/化疗 | [ | |
| T-VEC | 工程化病毒 | 单纯疱疹 病毒1型 | Ⅱ | 可手术的ⅢB/C-ⅣM1a期黑色素瘤 | NCT02211131 | 瘤内 | 无 | 手术 | [ |
| NOUS-209 | 工程化病毒 | GAd、MVA | Ⅰ/Ⅱ | 一/二线转移性dMMR/MSI-H结直肠癌、胃癌、胃食管交界腺癌 | NCT04041310 | 瘤内 | 209个dMMR 移码肽 | PD-1单抗帕博利珠 | [ |
| GRANITE | 工程化病毒 | 猩猩腺病毒ChAd68、 委内瑞拉 马脑炎病毒 | Ⅰ/Ⅱ | 多种转移性实体瘤,包括非小细胞肺癌、结直肠癌、胃食管交界腺癌、泌尿上皮癌 | 肌肉 | 个性化 新生抗原 | 标准化疗,PD-1单抗纳武利尤,CTLA-4单抗易普利姆玛 | [ | |
| 工程化病毒 | 腺病毒 | Ⅰ | 多种晚期上皮瘤,包括肺癌、乳癌、卵巢癌、前列腺癌、肠癌 | 皮下 | 分泌型MUC-1-CD40L 融合蛋白 | 标准化疗 | [ | ||
| CAN-3110 | 工程化病毒 | 单纯疱疹 病毒1型 | Ⅰ | 恶性胶质母细胞瘤,恶性星形细胞瘤,少突胶质细胞瘤 | 瘤内 | 无 | 化疗 | [ | |
| Delta-24-RGD | 工程化病毒 | 腺病毒Ad5 | Ⅰ/Ⅱ | 神经胶质瘤,神经内分泌瘤 | 瘤内 | 无 | PD-1单抗帕博利珠 | [ | |
| T-VEC | 工程化病毒 | 单纯疱疹 病毒1型 | Ⅱ | 二-三期三阴性乳腺癌 | NCT02779855 | 瘤内 | 无 | 新辅助化疗,手术 | [ |
Table 1 A summary of current clinical microbial-vectored cancer vaccines and recently reported studies on clinical trials
| 疫苗名称 | 载体 类型 | 来源 | 临床状态 | 肿瘤类型 | 临床试验编号 | 方法 免疫 | 肿瘤特异性抗原 | 结合疗法 | 参考 文献 |
|---|---|---|---|---|---|---|---|---|---|
| BCG | 减毒 活细菌 | 牛结核菌 | 临床使用 | 膀胱癌 | — | 瘤内 | 无 | 手术 | [ |
| T-VEC | 工程化病毒 | 单纯疱疹 病毒1型 | 临床使用 | 无法切除的转移性ⅢB/C-ⅣM1a期黑色素瘤 | — | 瘤内 | 无 | 无 | [ |
| 工程化病毒 | 单纯疱疹 病毒1型 | 临床使用 | 复发性神经胶质瘤 | UMIN000002661 UMIN000015995 | 瘤内 | 无 | 无 | [ | |
| REOLYSIN | 工程化病毒 | 呼肠孤病毒Dearing type 3 | Ⅰb | 高级神经胶质瘤、脑转移 | EudraCT 2011-005635-10 | 静脉 | 无 | 手术 | [ |
| Delta-24-RGD | 工程化病毒 | 腺病毒Ad5 | Ⅰ | 儿童弥散内生型脑桥胶质瘤(DIPG) | 瘤内 | 无 | 标准放疗+/化疗 | [ | |
| T-VEC | 工程化病毒 | 单纯疱疹 病毒1型 | Ⅱ | 可手术的ⅢB/C-ⅣM1a期黑色素瘤 | NCT02211131 | 瘤内 | 无 | 手术 | [ |
| NOUS-209 | 工程化病毒 | GAd、MVA | Ⅰ/Ⅱ | 一/二线转移性dMMR/MSI-H结直肠癌、胃癌、胃食管交界腺癌 | NCT04041310 | 瘤内 | 209个dMMR 移码肽 | PD-1单抗帕博利珠 | [ |
| GRANITE | 工程化病毒 | 猩猩腺病毒ChAd68、 委内瑞拉 马脑炎病毒 | Ⅰ/Ⅱ | 多种转移性实体瘤,包括非小细胞肺癌、结直肠癌、胃食管交界腺癌、泌尿上皮癌 | 肌肉 | 个性化 新生抗原 | 标准化疗,PD-1单抗纳武利尤,CTLA-4单抗易普利姆玛 | [ | |
| 工程化病毒 | 腺病毒 | Ⅰ | 多种晚期上皮瘤,包括肺癌、乳癌、卵巢癌、前列腺癌、肠癌 | 皮下 | 分泌型MUC-1-CD40L 融合蛋白 | 标准化疗 | [ | ||
| CAN-3110 | 工程化病毒 | 单纯疱疹 病毒1型 | Ⅰ | 恶性胶质母细胞瘤,恶性星形细胞瘤,少突胶质细胞瘤 | 瘤内 | 无 | 化疗 | [ | |
| Delta-24-RGD | 工程化病毒 | 腺病毒Ad5 | Ⅰ/Ⅱ | 神经胶质瘤,神经内分泌瘤 | 瘤内 | 无 | PD-1单抗帕博利珠 | [ | |
| T-VEC | 工程化病毒 | 单纯疱疹 病毒1型 | Ⅱ | 二-三期三阴性乳腺癌 | NCT02779855 | 瘤内 | 无 | 新辅助化疗,手术 | [ |
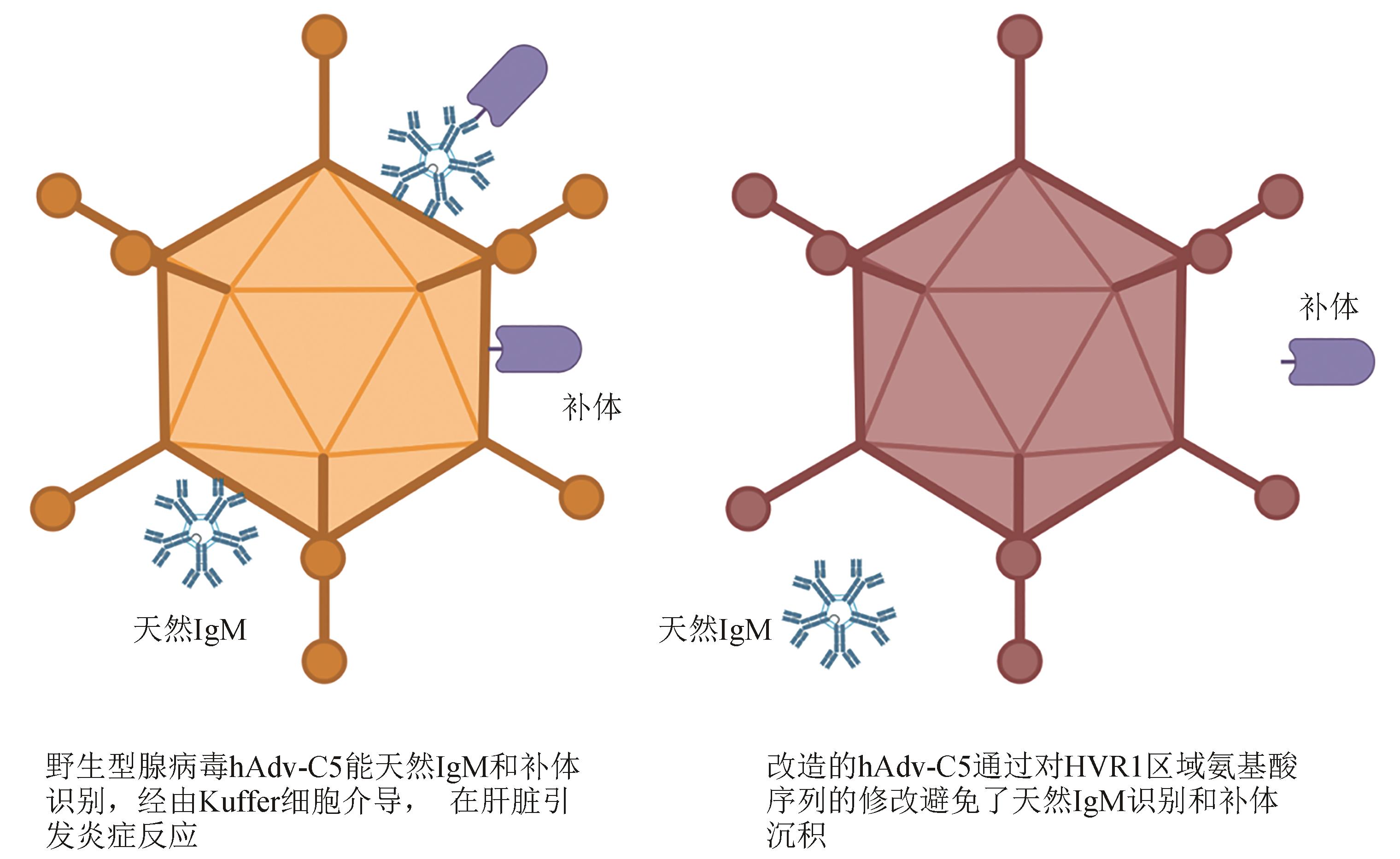
Fig. 3 Surface antigen site-specific mutagenesis for reducing the risk of systemic inflammation caused by the intravenous administration of adenoviral vectored vaccines
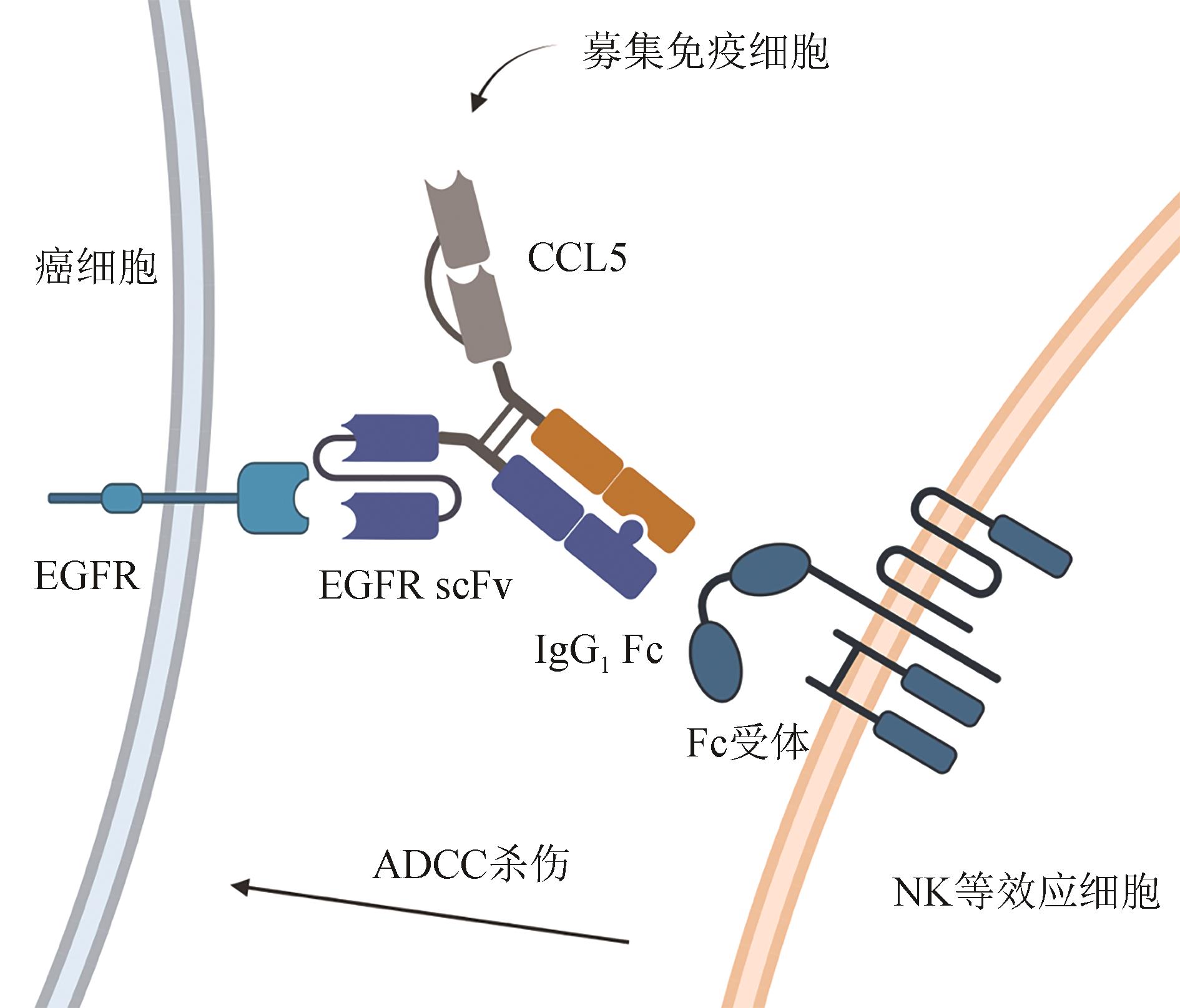
Fig. 4 A bispecific antibody-like protein with multiple function domains for multi-purpose TME modifications to bind EGFR on cancer cell surface, recruiting immune cells to mediate ADCC killing against cancer cells
| 1 | JU W, ZHENG R S, ZHANG S W, et al. Cancer statistics in Chinese older people, 2022: current burden, time trends, and comparisons with the US, Japan, and the Republic of Korea[J]. Science China Life Sciences, 2023, 66(5): 1079-1091. |
| 2 | ROJAS L A, SETHNA Z, SOARES K C, et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer[J]. Nature, 2023, 618(7963): 144-150. |
| 3 | Vaccins anti-hépatite B: note de synthèse de l’OMS-juillet 2017[J/OL]. (2017-07-07)[2023-08-01]. Relevé épidémiologique hebdomadaire, 2017, 92(27): 369-392. [J/OL]. (2017-07-07)[2023-08-01]. Weekly epidemiological record, 2017, 92(27): 369-392. . |
| 4 | RODEN R, WU T C. How will HPV vaccines affect cervical cancer?[J]. Nature Reviews Cancer, 2006, 6(10): 753-763. |
| 5 | PLOTKIN S. History of vaccination[J]. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(34): 12283-12287. |
| 6 | MCCANN N, O’CONNOR D, LAMBE T, et al. Viral vector vaccines[J]. Current Opinion in Immunology, 2022, 77: 102210. |
| 7 | LIN I, VAN T, SMOOKER P. Live-attenuated bacterial vectors: tools for vaccine and therapeutic agent delivery[J]. Vaccines, 2015, 3(4): 940-972. |
| 8 | TOUSSAINT B, CHAUCHET X, WANG Y, et al. Live-attenuated bacteria as a cancer vaccine vector[J]. Expert Review of Vaccines, 2013, 12(10): 1139-1154. |
| 9 | SASSO E, D’ALISE A M, ZAMBRANO N, et al. New viral vectors for infectious diseases and cancer[J]. Seminars in Immunology, 2020, 50: 101430. |
| 10 | D’ALISE A M, BRASU N, INTINIS C D, et al. Adenoviral-based vaccine promotes neoantigen-specific CD8+ T cell stemness and tumor rejection[J]. Science Translational Medicine, 2022, 14(657): eabo7604. |
| 11 | DUMMER R, GYORKI D E, HYNGSTROM J, et al. Neoadjuvant talimogene laherparepvec plus surgery versus surgery alone for resectable stage ⅢB-ⅣM1a melanoma: a randomized, open-label, phase 2 trial[J]. Nature Medicine, 2021, 27(10): 1789-1796. |
| 12 | FERRUCCI P F, PALA L, CONFORTI F, et al. Talimogene laherparepvec (T-VEC): an intralesional cancer immunotherapy for advanced melanoma[J]. Cancers, 2021, 13(6): 1383. |
| 13 | LING A L, SOLOMON I H, LANDIVAR A M, et al. Clinical trial links oncolytic immunoactivation to survival in glioblastoma[J]. Nature, 2023, 623(7985): 157-166. |
| 14 | MARTÍNEZ-VÉLEZ N, GARCIA-MOURE M, MARIGIL M, et al. The oncolytic virus Delta-24-RGD elicits an antitumor effect in pediatric glioma and DIPG mouse models[J]. Nature Communications, 2019, 10(1): 2235. |
| 15 | NASSIRI F, PATIL V, YEFET L S, et al. Oncolytic DNX-2401 virotherapy plus pembrolizumab in recurrent glioblastoma: a phase 1/2 trial[J]. Nature Medicine, 2023, 29(6): 1370-1378. |
| 16 | OVERMAN M, FAKIH M, LE D, et al. 410 Phase Ⅰ interim study results of Nous-209, an off-the-shelf immunotherapy, with pembrolizumab, for the treatment of tumors with a deficiency in mismatch repair/microsatellite instability (dMMR/MSI)[J]. Journal for ImmunoTherapy of Cancer, 2021, 9(): A441. |
| 17 | OVERMAN M J, MAUREL J, OBERSTEIN P E, et al. Results of phase Ⅰ-Ⅱ bridging study for Nous-209, a neoantigen cancer immunotherapy, in combination with pembrolizumab as first line treatment in patients with advanced dMMR/MSI-h colorectal cancer[J]. Journal of Clinical Oncology, 2023, 41(): e14665. |
| 18 | PALMER C D, RAPPAPORT A R, DAVIS M J, et al. Individualized, heterologous chimpanzee adenovirus and self-amplifying mRNA neoantigen vaccine for advanced metastatic solid tumors: phase 1 trial interim results[J]. Nature Medicine, 2022, 28(8): 1619-1629. |
| 19 | SAMSON A, SCOTT K J, TAGGART D, et al. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade[J]. Science Translational Medicine, 2018, 10(422): eaam7577. |
| 20 | SOLIMAN H, HOGUE D, HAN H, et al. Oncolytic T-VEC virotherapy plus neoadjuvant chemotherapy in nonmetastatic triple-negative breast cancer: a phase 2 trial[J]. Nature Medicine, 2023, 29(2): 450-457. |
| 21 | TAN T J, GLADYS ANG W X G, WANG W W, et al. A phase Ⅰ study of an adenoviral vector delivering a MUC1/CD40-ligand fusion protein in patients with advanced adenocarcinoma[J]. Nature Communications, 2022, 13(1): 6453. |
| 22 | TODO T, INO Y, OHTSU H, et al. A phase Ⅰ/Ⅱ study of triple-mutated oncolytic herpes virus G47∆ in patients with progressive glioblastoma[J]. Nature Communications, 2022, 13(1): 4119. |
| 23 | TODO T, ITO H, INO Y, et al. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: a phase 2 trial[J]. Nature Medicine, 2022, 28(8): 1630-1639. |
| 24 | REDELMAN-SIDI G, GLICKMAN M S, BOCHNER B H. The mechanism of action of BCG therapy for bladder cancer: a current perspective[J]. Nature Reviews Urology, 2014, 11(3): 153-162. |
| 25 | MCCARTHY E F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas[J]. The Iowa Orthopaedic Journal, 2006, 26: 154-158. |
| 26 | NAGHAVIAN R, FAIGLE W, OLDRATI P, et al. Microbial peptides activate tumour-infiltrating lymphocytes in glioblastoma[J]. Nature, 2023, 617(7962): 807-817. |
| 27 | LUO X, LI Z, LIN S, et al. Antitumor effect of VNP20009, an attenuated Salmonella, in murine tumor models[J]. Oncology Research, 2001, 12(11-12): 501-508. |
| 28 | ZHENG J H, NGUYEN V H, JIANG S N, et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin[J]. Science Translational Medicine, 2017, 9(376): eaak9537. |
| 29 | SU L, ZHANG Y W, ZHANG X, et al. Combination immunotherapy with two attenuated Listeria strains carrying shuffled HPV-16 E6E7 protein causes tumor regression in a mouse tumor model[J]. Scientific Reports, 2021, 11(1): 13404. |
| 30 | SELVANESAN B C, CHANDRA D, QUISPE-TINTAYA W, et al. Listeria delivers tetanus toxoid protein to pancreatic tumors and induces cancer cell death in mice[J]. Science Translational Medicine, 2022, 14(637): eabc1600. |
| 31 | JAWALAGATTI V, KIRTHIKA P, LEE J H. Targeting primary and metastatic tumor growth in an aggressive breast cancer by engineered tryptophan auxotrophic Salmonella typhimurium [J]. Molecular Therapy Oncolytics, 2022, 25: 350-363. |
| 32 | YUE Y L, XU J Q, LI Y, et al. Antigen-bearing outer membrane vesicles as tumour vaccines produced in situ by ingested genetically engineered bacteria[J]. Nature Biomedical Engineering, 2022, 6(7): 898-909. |
| 33 | SAVAGE T M, VINCENT R L, RAE S S, et al. Chemokines expressed by engineered bacteria recruit and orchestrate antitumor immunity[J]. Science Advances, 2023, 9(10): eadc9436. |
| 34 | CHENG K M, ZHAO R F, LI Y, et al. Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via plug-and-display technology[J]. Nature Communications, 2021, 12(1): 2041. |
| 35 | ZHU J M, KE Y H, LIU Q, et al. Engineered Lactococcus lactis secreting Flt3L and OX40 ligand for in situ vaccination-based cancer immunotherapy[J]. Nature Communications, 2022, 13(1): 7466. |
| 36 | CHEN Y E, BOUSBAINE D, VEINBACHS A, et al. Engineered skin bacteria induce antitumor T cell responses against melanoma[J]. Science, 2023, 380(6641): 203-210. |
| 37 | KITAGAWA K, GONOI R, TATSUMI M, et al. Preclinical development of a WT1 oral cancer vaccine using a bacterial vector to treat castration-resistant prostate cancer[J]. Molecular Cancer Therapeutics, 2019, 18(5): 980-990. |
| 38 | KITAGAWA K, TATSUMI M, KATO M, et al. An oral cancer vaccine using a Bifidobacterium vector suppresses tumor growth in a syngeneic mouse bladder cancer model[J]. Molecular Therapy Oncolytics, 2021, 22: 592-603. |
| 39 | UEKI H, KITAGAWA K, KATO M, et al. An oral cancer vaccine using Bifidobacterium vector augments combination of anti-PD-1 and anti-CTLA-4 antibodies in mouse renal cell carcinoma model[J]. Scientific Reports, 2023, 13(1): 9994. |
| 40 | WEYANT K B, OLOYEDE A, PAL S, et al. A modular vaccine platform enabled by decoration of bacterial outer membrane vesicles with biotinylated antigens[J]. Nature Communications, 2023, 14(1): 464. |
| 41 | KROEMER G, GALASSI C, ZITVOGEL L, et al. Immunogenic cell stress and death[J]. Nature Immunology, 2022, 23(4): 487-500. |
| 42 | WANG W G, XU H H, YE Q S, et al. Systemic immune responses to irradiated tumours via the transport of antigens to the tumour periphery by injected flagellate bacteria[J]. Nature Biomedical Engineering, 2022, 6(1): 44-53. |
| 43 | WIELAND A, PATEL M R, CARDENAS M A, et al. Defining HPV-specific B cell responses in patients with head and neck cancer[J]. Nature, 2021, 597(7875): 274-278. |
| 44 | FERREIRO-IGLESIAS A, MCKAY J D, BRENNER N, et al. Germline determinants of humoral immune response to HPV-16 protect against oropharyngeal cancer[J]. Nature Communications, 2021, 12(1): 5945. |
| 45 | EBERHARDT C S, KISSICK H T, PATEL M R, et al. Functional HPV-specific PD-1+ stem-like CD8 T cells in head and neck cancer[J]. Nature, 2021, 597(7875): 279-284. |
| 46 | ROSATO P C, WIJEYESINGHE S, STOLLEY J M, et al. Virus-specific memory T cells populate tumors and can be repurposed for tumor immunotherapy[J]. Nature Communications, 2019, 10(1): 567. |
| 47 | STRICKLEY J D, MESSERSCHMIDT J L, AWAD M E, et al. Immunity to commensal papillomaviruses protects against skin cancer[J]. Nature, 2019, 575(7783): 519-522. |
| 48 | RESTREPO J, HERRERA T, SAMAKOSES R, et al. Ten-year follow-up of 9-valent human papillomavirus vaccine: immunogenicity, effectiveness, and safety[J]. Pediatrics, 2023, 152(4): e2022060993. |
| 49 | CLARK K T, TRIMBLE C L. Current status of therapeutic HPV vaccines[J]. Gynecologic Oncology, 2020, 156(2): 503-510. |
| 50 | BANASZYNSKI L A, LIU C W, WANDLESS T J. Characterization of the FKBP.rapamycin.FRB ternary complex[J]. Journal of the American Chemical Society, 2005, 127(13): 4715-4721. |
| 51 | AZAD T, REZAEI R, SINGARAVELU R, et al. Synthetic virology approaches to improve the safety and efficacy of oncolytic virus therapies[J]. Nature Communications, 2023, 14(1): 3035. |
| 52 | HEILMANN E, KIMPEL J, HOFER B, et al. Chemogenetic ON and OFF switches for RNA virus replication[J]. Nature Communications, 2021, 12(1): 1362. |
| 53 | KELLY E J, HADAC E M, GREINER S, et al. Engineering microRNA responsiveness to decrease virus pathogenicity[J]. Nature Medicine, 2008, 14(11): 1278-1283. |
| 54 | HUANG H Y, LIU Y Q, LIAO W X, et al. Oncolytic adenovirus programmed by synthetic gene circuit for cancer immunotherapy[J]. Nature Communications, 2019, 10(1): 4801. |
| 55 | GUO L, HU C, LIU Y, et al. Directed natural evolution generates a next-generation oncolytic virus with a high potency and safety profile[J]. Nature Communications, 2023, 14(1): 3410. |
| 56 | DAS K, BELNOUE E, ROSSI M, et al. A modular self-adjuvanting cancer vaccine combined with an oncolytic vaccine induces potent antitumor immunity[J]. Nature Communications, 2021, 12(1): 5195. |
| 57 | MEDINA-ECHEVERZ J, HINTERBERGER M, TESTORI M, et al. Synergistic cancer immunotherapy combines MVA-CD40L induced innate and adaptive immunity with tumor targeting antibodies[J]. Nature Communications, 2019, 10(1): 5041. |
| 58 | ATASHEVA S, EMERSON C C, YAO J, et al. Systemic cancer therapy with engineered adenovirus that evades innate immunity[J]. Science Translational Medicine, 2020, 12(571): eabc6659. |
| 59 | EVGIN L, KOTTKE T, TONNE J, et al. Oncolytic virus-mediated expansion of dual-specific CAR T cells improves efficacy against solid tumors in mice[J]. Science Translational Medicine, 2022, 14(640): eabn2231. |
| 60 | KENNEDY E M, DENSLOW A, HEWETT J, et al. Development of intravenously administered synthetic RNA virus immunotherapy for the treatment of cancer[J]. Nature Communications, 2022, 13(1): 5907. |
| 61 | NIEMANN J, WOLLER N, BROOKS J, et al. Molecular retargeting of antibodies converts immune defense against oncolytic viruses into cancer immunotherapy[J]. Nature Communications, 2019, 10(1): 3236. |
| 62 | SVENSSON-ARVELUND J, CUADRADO-CASTANO S, PANTSULAIA G, et al. Expanding cross-presenting dendritic cells enhances oncolytic virotherapy and is critical for long-term anti-tumor immunity[J]. Nature Communications, 2022, 13(1): 7149. |
| 63 | XU B, TIAN L, CHEN J, et al. An oncolytic virus expressing a full-length antibody enhances antitumor innate immune response to glioblastoma[J]. Nature Communications, 2021, 12(1): 5908. |
| 64 | WANG S Q, YAN W, KONG L K, et al. Oncolytic viruses engineered to enforce cholesterol efflux restore tumor-associated macrophage phagocytosis and anti-tumor immunity in glioblastoma[J]. Nature Communications, 2023, 14(1): 4367. |
| 65 | NAKAO S, ARAI Y, TASAKI M, et al. Intratumoral expression of IL-7 and IL-12 using an oncolytic virus increases systemic sensitivity to immune checkpoint blockade[J]. Science Translational Medicine, 2020, 12(526): eaax7992. |
| 66 | LIU Z Q, GE Y, WANG H Y, et al. Modifying the cancer-immune set point using vaccinia virus expressing re-designed interleukin-2[J]. Nature Communications, 2018, 9(1): 4682. |
| 67 | WANG G, KANG X, CHEN K S, et al. An engineered oncolytic virus expressing PD-L1 inhibitors activates tumor neoantigen-specific T cell responses[J]. Nature Communications, 2020, 11: 1395. |
| 68 | SHEKARIAN T, SIVADO E, JALLAS A C, et al. Repurposing rotavirus vaccines for intratumoral immunotherapy can overcome resistance to immune checkpoint blockade[J]. Science Translational Medicine, 2019, 11(515): eaat5025. |
| 69 | RIVADENEIRA D B, DEPEAUX K, WANG Y Y, et al. Oncolytic viruses engineered to enforce leptin expression reprogram tumor-infiltrating T cell metabolism and promote tumor clearance[J]. Immunity, 2019, 51(3): 548-560.e4. |
| 70 | RUSSELL L, SWANNER J, JAIME-RAMIREZ A C, et al. PTEN expression by an oncolytic herpesvirus directs T-cell mediated tumor clearance[J]. Nature Communications, 2018, 9(1): 5006. |
| 71 | LU Y, HE W B, HUANG X, et al. Strategies to package recombinant Adeno-Associated Virus expressing the N-terminal gasdermin domain for tumor treatment[J]. Nature Communications, 2021, 12: 7155. |
| 72 | LIN J, SUN S H, ZHAO K, et al. Oncolytic Parapoxvirus induces Gasdermin E-mediated pyroptosis and activates antitumor immunity[J]. Nature Communications, 2023, 14(1): 224. |
| 73 | WU A L, LI Z Y, WANG Y L, et al. Recombinant measles virus vaccine rMV-Hu191 exerts an oncolytic effect on esophageal squamous cell carcinoma via caspase-3/GSDME-mediated pyroptosis[J]. Cell Death Discovery, 2023, 9(1): 171. |
| 74 | TIAN L, XU B, CHEN Y Q, et al. Specific targeting of glioblastoma with an oncolytic virus expressing a cetuximab-CCL5 fusion protein via innate and adaptive immunity[J]. Nature Cancer, 2022, 3(11): 1318-1335. |
| 75 | JI D Z, ZHANG Y J, SUN J Q, et al. An engineered influenza virus to deliver antigens for lung cancer vaccination[J/OL]. Nature Biotechnology, 2023[2023-08-01]. . |
| 76 | FUSCIELLO M, FONTANA F, TÄHTINEN S, et al. Artificially cloaked viral nanovaccine for cancer immunotherapy[J]. Nature Communications, 2019, 10(1): 5747. |
| 77 | ROY D G, GEOFFROY K, MARGUERIE M, et al. Adjuvant oncolytic virotherapy for personalized anti-cancer vaccination[J]. Nature Communications, 2021, 12(1): 2626. |
| 78 | D’ALISE A M, LEONI G, COTUGNO G, et al. Adenoviral vaccine targeting multiple neoantigens as strategy to eradicate large tumors combined with checkpoint blockade[J]. Nature Communications, 2019, 10(1): 2688. |
| 79 | RING S S, CUPOVIC J, ONDER L, et al. Viral vector-mediated reprogramming of the fibroblastic tumor stroma sustains curative melanoma treatment[J]. Nature Communications, 2021, 12(1): 4734. |
| 80 | SMITH R, WAFA E I, GEARY S M, et al. Cationic nanoparticles enhance T cell tumor infiltration and antitumor immune responses to a melanoma vaccine[J]. Science Advances, 2022, 8(29): eabk3150. |
| 81 | MOSAHEB M M, DOBRIKOVA E Y, BROWN M C, et al. Genetically stable poliovirus vectors activate dendritic cells and prime antitumor CD8 T cell immunity[J]. Nature Communications, 2020, 11(1): 524. |
| 82 | NATH S, MUKHERJEE P. MUC1: a multifaceted oncoprotein with a key role in cancer progression[J]. Trends in Molecular Medicine, 2014, 20(6): 332-342. |
| 83 | GRECO B, MALACARNE V, GIRARDI F D, et al. Disrupting N-glycan expression on tumor cells boosts chimeric antigen receptor T cell efficacy against solid malignancies[J]. Science Translational Medicine, 2022, 14(628): eabg3072. |
| 84 | RASKA M, CZERNEKOVA L, MOLDOVEANU Z, et al. Differential glycosylation of envelope gp120 is associated with differential recognition of HIV-1 by virus-specific antibodies and cell infection[J]. AIDS Research and Therapy, 2014, 11: 23. |
| 85 | DOORES K J. The HIV glycan shield as a target for broadly neutralizing antibodies[J]. The FEBS Journal, 2015, 282(24): 4679-4691. |
| 86 | LEK A, WONG B, KEELER A, et al. Death after high-dose rAAV9 gene therapy in a patient with duchenne’s muscular dystrophy[J]. New England Journal of Medicine, 2023, 389(13): 1203-1210. |
| 87 | GANESHAN K, NIKKANEN J, MAN K, et al. Energetic trade-offs and hypometabolic states promote disease tolerance[J]. Cell, 2019, 177(2): 399-413.e12. |
| 88 | SAXENA M, VAN T T H, BAIRD F J, et al. Pre-existing immunity against vaccine vectors-friend or foe?[J]. Microbiology, 2013, 159(Pt_1): 1-11. |
| 89 | WANG W C, SAYEDAHMED E E, MITTAL S K. Significance of preexisting vector immunity and activation of innate responses for adenoviral vector-based therapy[J]. Viruses, 2022, 14(12): 2727. |
| 90 | GLORIOSO J C, COHEN J B, GOINS W F, et al. Oncolytic HSV vectors and anti-tumor immunity[J]. Current Issues in Molecular Biology, 2021, 41: 381-468. |
| 91 | SHAW A R, SUZUKI M. Immunology of adenoviral vectors in cancer therapy[J]. Molecular Therapy Methods & Clinical Development, 2019, 15: 418-429. |
| 92 | AUGUSTO D G, MURDOLO L D, CHATZILEONTIADOU D S M, et al. A common allele of HLA is associated with asymptomatic SARS-CoV-2 infection[J]. Nature, 2023, 620(7972): 128-136. |
| [1] | TU Huiyang, HAN Weidong, ZHANG Bin. Strategies for the design and optimization of tumor neoantigen vaccines [J]. Synthetic Biology Journal, 2024, 5(2): 254-266. |
| [2] | Junhong XIE, Jingjing HE, Penghui ZHOU. Synthetic biology and engineered T cell therapy [J]. Synthetic Biology Journal, 2023, 4(2): 373-393. |
| [3] | Sisi LIN, Chao PAN, Yifan ZHANG, Jinyao LIU. Coated probiotic-based drug carriers for oral delivery of tumor antigens [J]. Synthetic Biology Journal, 2022, 3(4): 810-820. |
| [4] | Shilin XU, Haiyan XU. Progress of bispecific antibodies and nanotechnology in tumor immunotherapies [J]. Synthetic Biology Journal, 2022, 3(2): 352-368. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||