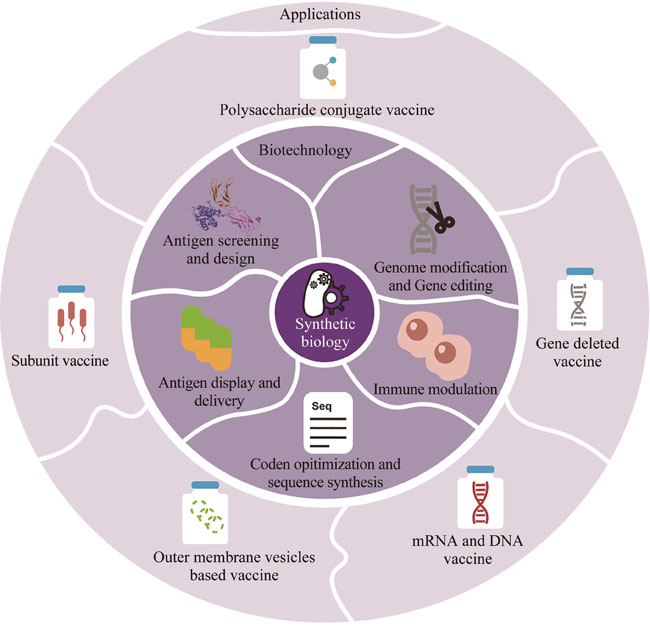
In recent years, bacterial infections have emerged as the second leading cause of death globally, posing a serious threat to public health and demanding prioritized intervention from the healthcare community worldwide. While antibiotics have conventionally been used as the primary strategy to combat bacterial infections, their efficacy is increasingly compromised due to the emergence of drug-resistant bacteria, especially multi-drug-resistant and even pan-drug-resistant superbacteria. Vaccines are thus considered as one of the most scientific, economical, safe, and effective means to prevent infectious diseases and improve public health, which are estimated to save 2 to 3 million lives annually, and can serve as a critical tool in the battle against antimicrobial resistance. However, the complexity of bacterial structure and pathogenic mechanism has hindered the development of vaccines. Challenges include screening and rationally design of effective antigens, ensuring compatibility of various antigen combinations, establishing animal models for preclinical evaluation, and defining reliable endpoints for clinical efficacy assessment. As a result, only a small number of bacteria vaccines have been successfully developed so far, and none of them has been licensed to combat the most prevalent drug-resistant infections, such as Staphylococcus aureus, Acinetobacter baumannii, Pseudomonas aeruginosa and Klebsiella pneumoniae. Synthetic biology is a brand-new multidisciplinary focusing on repurposing natural biological systems and inventing innovative biological tools, technologies, devices, and systems for practical applications, and its concepts, principles and technologies have been extensively employed to facilitate vaccine development, including rational design, screening, and optimization of antigen, carrier, adjuvant and delivery system as well as the modulation of bacterial pathogenicity and immune responses. Herein, we outline the current status of the development of bacterial vaccines and the advancement of clinical trials for drug-resistant bacterial vaccines. Then, we summarize the application of synthetic biology technology in the development of major bacterial vaccines. Finally, we prospect the potential of synthetic biology in creating novel bacterial vaccines. Researchers have access to a greater variety of design possibilities for bacterial vaccines through synthetic biology. To maximize these benefits, we should employ synthetic biology and related technologies more efficiently in developing bacterial vaccines. Meanwhile, we should develop a scientific, reasonable, effective, and feasible management system, as well as regulatory measures, to expedite the development of efficient bacterial vaccines, therefore addressing the problem of antibiotic resistance to protect human health.