Synthetic Biology Journal ›› 2024, Vol. 5 ›› Issue (2): 321-337.DOI: 10.12211/2096-8280.2023-070
• Invited Review • Previous Articles Next Articles
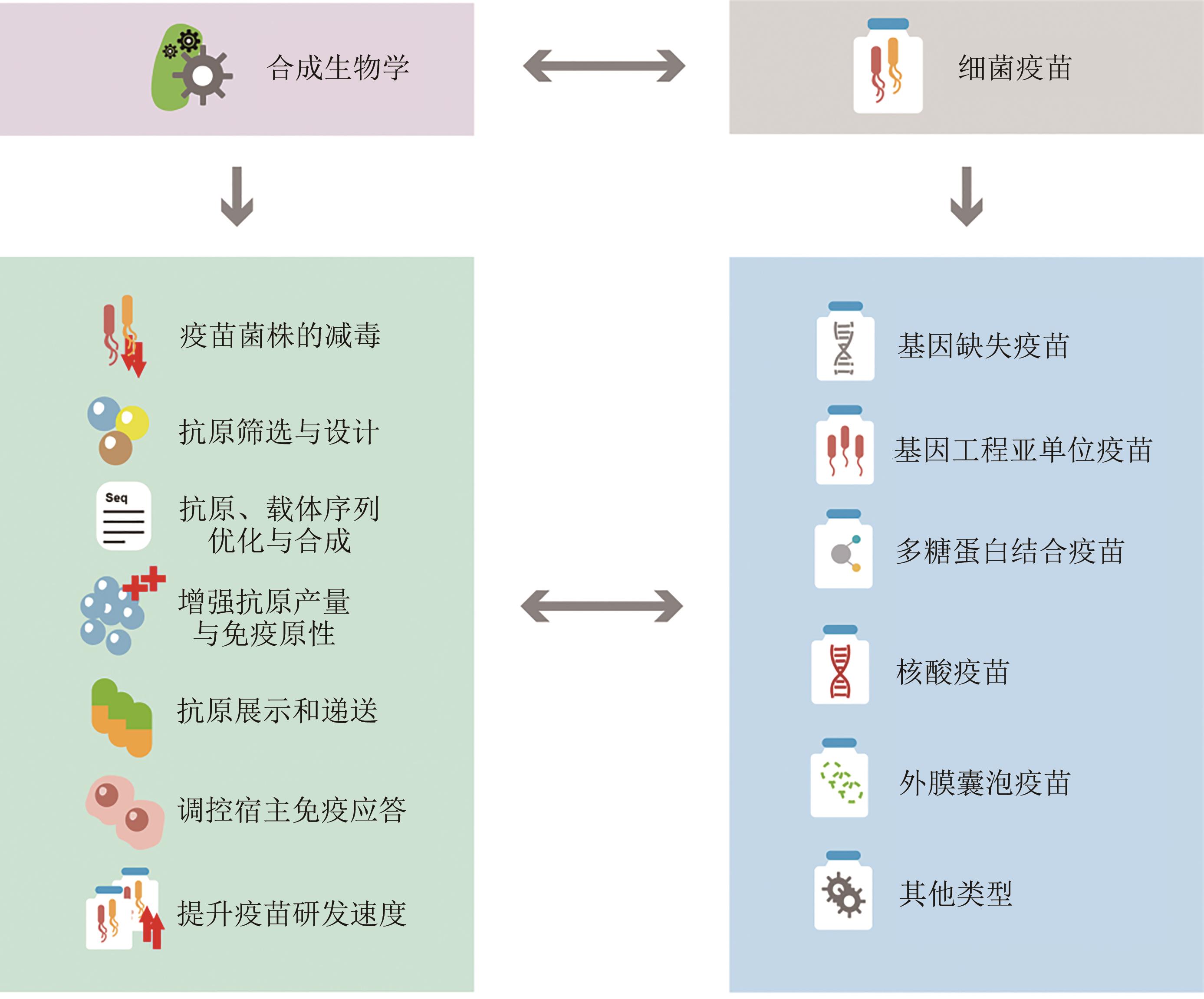
Synthetic biology promotes the development of bacterial vaccines
ZHANG Jinyong, GU Jiang, GUAN Shan, LI Haibo, ZENG Hao, ZOU Quanming
- National Engineering Research Center of Immunological Products,Department of Microbiology and Biochemical Pharmacy,Institute of Pharmacy and Laboratory Medicine,Military Medical University,Chongqing 400037,China
-
Received:2023-10-07Revised:2023-12-05Online:2024-04-28Published:2024-04-30 -
Contact:ZOU Quanming
合成生物学助力细菌疫苗研发
章金勇, 顾江, 关山, 李海波, 曾浩, 邹全明
- 陆军军医大学国家免疫生物制品工程技术研究中心,陆军军医大学药学与检验医学系微生物与生化药学教研室,重庆 400037
-
通讯作者:邹全明 -
作者简介:章金勇 (1982—),男,博士,教授。研究方向为病原体致病机制与免疫防治。E-mail:zhangjy198217@126.com邹全明 (1963—),男,博士,教授。研究方向为超级细菌感染与创新疫苗研发。E-mail:qmzou2007@163.com -
基金资助:国家自然科学基金青年科学基金(32170938)
CLC Number:
Cite this article
ZHANG Jinyong, GU Jiang, GUAN Shan, LI Haibo, ZENG Hao, ZOU Quanming. Synthetic biology promotes the development of bacterial vaccines[J]. Synthetic Biology Journal, 2024, 5(2): 321-337.
章金勇, 顾江, 关山, 李海波, 曾浩, 邹全明. 合成生物学助力细菌疫苗研发[J]. 合成生物学, 2024, 5(2): 321-337.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2023-070
| 靶标细菌 | 研发机构 | 疫苗名称 | 抗原成分 | 试验阶段 | 参考文献 |
|---|---|---|---|---|---|
| 金黄色葡萄球菌 | Pfizer | SA4Ag | CP5、CP8、ClfA、Ag3 | Ⅰ、Ⅱ期 | [ |
| Nabi | rLukS-PV/rAT | rLukS-PV、rAT | Ⅰ、Ⅱ期 | [ | |
| TMMU&Olymvax | rFSAV | Hla、IsdB、SeB、MntC、SpA5 | Ⅰ、Ⅱ期 | [ | |
| 肺炎链球菌 | Sanofi Pasteur | PPrV | PcpA、PhtD、PylD1 | Ⅰ期 | [ |
| Genocea | GEN-004 | SP-2108、SP-0148、SP-1912 | Ⅰ、Ⅱ期 | [ | |
| Intercell AG | IC47 | PcsB、StkP、PsaA | Ⅰ期 | [ | |
| 结核杆菌 | Statens Serum Institut | H56 | Ag85B、ESAT-6、Rv2660c | Ⅰ、Ⅱ期 | [ |
| Quratis Inc | ID93 | Rv2608、RV3619、Rv3620、Rv1813 | Ⅰ、Ⅱ期 | [ | |
| MRF | GamTBvac | Ag85a、ESAT6、CFP10 | Ⅰ、Ⅱ期 | [ | |
| 铜绿假单胞菌 | GmbH | IC43 | OprF、OprI | Ⅲ期 | [ |
Table 1 Major drug-resistant bacterial vaccines under clinical trial evaluation
| 靶标细菌 | 研发机构 | 疫苗名称 | 抗原成分 | 试验阶段 | 参考文献 |
|---|---|---|---|---|---|
| 金黄色葡萄球菌 | Pfizer | SA4Ag | CP5、CP8、ClfA、Ag3 | Ⅰ、Ⅱ期 | [ |
| Nabi | rLukS-PV/rAT | rLukS-PV、rAT | Ⅰ、Ⅱ期 | [ | |
| TMMU&Olymvax | rFSAV | Hla、IsdB、SeB、MntC、SpA5 | Ⅰ、Ⅱ期 | [ | |
| 肺炎链球菌 | Sanofi Pasteur | PPrV | PcpA、PhtD、PylD1 | Ⅰ期 | [ |
| Genocea | GEN-004 | SP-2108、SP-0148、SP-1912 | Ⅰ、Ⅱ期 | [ | |
| Intercell AG | IC47 | PcsB、StkP、PsaA | Ⅰ期 | [ | |
| 结核杆菌 | Statens Serum Institut | H56 | Ag85B、ESAT-6、Rv2660c | Ⅰ、Ⅱ期 | [ |
| Quratis Inc | ID93 | Rv2608、RV3619、Rv3620、Rv1813 | Ⅰ、Ⅱ期 | [ | |
| MRF | GamTBvac | Ag85a、ESAT6、CFP10 | Ⅰ、Ⅱ期 | [ | |
| 铜绿假单胞菌 | GmbH | IC43 | OprF、OprI | Ⅲ期 | [ |
| 1 | COOK M A, WRIGHT G D. The past, present, and future of antibiotics[J]. Science Translational Medicine, 2022, 14(657): eabo7793. |
| 2 | GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990—2019: a systematic analysis for the Global Burden of Disease Study 2019[J]. The Lancet, 2020, 396(10258): 1204-1222. |
| 3 | SULIS G, SAYOOD S, KATUKOORI S, et al. Exposure to World Health Organization’s aware antibiotics and isolation of multidrug resistant bacteria: a systematic review and meta-analysis[J]. Clinical Microbiology and Infection, 2022, 28(9): 1193-1202. |
| 4 | GBD 2019 Antimicrobial Resistance Collaborators. Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019[J]. The Lancet, 2022, 400(10369): 2221-2248. |
| 5 | Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis [J]. The Lancet, 2022, 399(10325): 629-655. |
| 6 | FERRI M, RANUCCI E, ROMAGNOLI P, et al. Antimicrobial resistance: a global emerging threat to public health systems[J]. Critical Reviews in Food Science and Nutrition, 2017, 57(13): 2857-2876. |
| 7 | ZHANG Y D, LI M, DU G S, et al. Advancedoral vaccine delivery strategies for improving the immunity[J]. Advanced Drug Delivery Reviews, 2021, 177: 113928. |
| 8 | MICOLI F, BAGNOLI F, RAPPUOLI R, et al. The role of vaccines in combatting antimicrobial resistance[J]. Nature Reviews Microbiology, 2021, 19(5): 287-302. |
| 9 | 赵国屏. 合成生物学: 从“造物致用”到产业转化[J]. 生物工程学报, 2022, 38(11): 4001-4011. |
| ZHAO G P. Synthetic biology: from “build-for-use” to commercialization[J]. Chinese Journal of Biotechnology, 2022, 38(11): 4001-4011. | |
| 10 | 宋斐, 蔡志明, 黄卫人. 合成生物开启生物医药崭新篇章: 预防、诊断与治疗[J]. 合成生物学, 2023, 4(2): 241-243. |
| SONG F, CAI Z M, HUANG W R. Synthetic biology opens a new chapter in biomedicine: prevention, diagnosis and treatment[J]. Synthetic Biology Journal, 2023, 4(2): 241-243. | |
| 11 | 邹全明, 曾浩. 超级细菌疫苗研究的挑战与策略[J]. 第三军医大学学报, 2019, 41(19): 1823-1825, 1827. |
| ZOU Q M, ZENG H. Development of vaccines against superbugs: challenges and strategies[J]. Journal of Third Military Medical University, 2019, 41(19): 1823-1825, 1827. | |
| 12 | FROST I, SATI H, GARCIA-VELLO P, et al. The role of bacterial vaccines in the fight against antimicrobial resistance: an analysis of the preclinical and clinical development pipeline[J]. The Lancet Microbe, 2023, 4(2): e113-e125. |
| 13 | JIANG X Y, GONG M Q, ZHANG H J, et al. The safety and immunogenicity of a recombinant five-antigen Staphylococcus aureus vaccine among patients undergoing elective surgery for closed fractures: a randomized, double-blind, placebo-controlled, multicenter phase 2 clinical trial[J]. Vaccine, 2023, 41(38): 5562-5571. |
| 14 | HASSANZADEH H, BABER J, BEGIER E, et al. Efficacy of a 4-antigen Staphylococcus aureus vaccine in spinal surgery: the Staphylococcus aureus surgical inpatient vaccine efficacy (STRIVE) randomized clinical trial[J]. Clinical Infectious Diseases, 2023, 77(2): 312-320. |
| 15 | LANDRUM M L, LALANI T, NIKNIAN M, et al. Safety and immunogenicity of a recombinant Staphylococcus aureus α-toxoid and a recombinant Panton-Valentine leukocidin subunit, in healthy adults[J]. Human Vaccines & Immunotherapeutics, 2017, 13(4): 791-801. |
| 16 | XU Q F, PRYHARSKI K, PICHICHERO M E. Trivalent pneumococcal protein vaccine protects against experimental acute otitis media caused by Streptococcus pneumoniae in an infant murine model[J]. Vaccine, 2017, 35(2): 337-344. |
| 17 | MOFFITT K, MALLEY R. Rationale and prospects for novel pneumococcal vaccines[J]. Human Vaccines & Immunotherapeutics, 2016, 12(2): 383-392. |
| 18 | SCHMID P, SELAK S, KELLER M, et al. Th17/Th1 biased immunity to the pneumococcal proteins PcsB, StkP and PsaA in adults of different age[J]. Vaccine, 2011, 29(23): 3982-3989. |
| 19 | SULIMAN S, LUABEYA A K K, GELDENHUYS H, et al. Dose optimization of H56: IC31 vaccine for tuberculosis-endemic populations. A double-blind, placebo-controlled, dose-selection trial[J]. American Journal of Respiratory and Critical Care Medicine, 2019, 199(2): 220-231. |
| 20 | PENN-NICHOLSON A, TAMERIS M, SMIT E, et al. Safety and immunogenicity of the novel tuberculosis vaccine ID93 + GLA-SE in BCG-vaccinated healthy adults in South Africa: a randomised, double-blind, placebo-controlled phase 1 trial[J]. The Lancet Respiratory Medicine, 2018, 6(4): 287-298. |
| 21 | TKACHUK A P, BYKONIA E N, POPOVA L I, et al. Safety and immunogenicity of the GamTBvac, the recombinant subunit tuberculosis vaccine candidate: a phase Ⅱ, multi-center, double-blind, randomized, placebo-controlled study[J]. Vaccines, 2020, 8(4): 652. |
| 22 | ADLBRECHT C, WURM R, DEPUYDT P, et al. Efficacy, immunogenicity, and safety of IC43 recombinant Pseudomonas aeruginosa vaccine in mechanically ventilated intensive care patients-a randomized clinical trial[J]. Critical Care, 2020, 24(1): 74. |
| 23 | BREWER S M, BRUBAKER S W, MONACK D M. Host inflammasome defense mechanisms and bacterial pathogen evasion strategies[J]. Current Opinion in Immunology, 2019, 60: 63-70. |
| 24 | MASKELL D, FRANKEL G, DOUGAN G. Phase and antigenic variation—the impact on strategies for bacterial vaccine design[J]. Trends in Biotechnology, 1993, 11(12): 506-510. |
| 25 | LAMBERTI Y, SURMANN K. The intracellular phase of extracellular respiratory tract bacterial pathogens and its role on pathogen-host interactions during infection[J]. Current Opinion in Infectious Diseases, 2021, 34(3): 197-205. |
| 26 | BJÖRKSTÉN B. Diverse microbial exposure - consequences for vaccine development[J]. Vaccine, 2012, 30(29): 4336-4340. |
| 27 | 邹全明, 石云. 超级细菌疫苗研究进展[J]. 第三军医大学学报, 2016, 38(7): 663-668. |
| ZOU Q M, SHI Y. Prospect for vaccines to prevent superbug infection[J]. Journal of Third Military Medical University, 2016, 38(7): 663-668. | |
| 28 | SAKURAI F, TACHIBANA M, MIZUGUCHI H. Adenovirus vector-based vaccine for infectious diseases[J]. Drug Metabolism and Pharmacokinetics, 2022, 42: 100432. |
| 29 | TAN X, LETENDRE J H, COLLINS J J, et al. Synthetic biology in the clinic: engineering vaccines, diagnostics, and therapeutics[J]. Cell, 2021, 184(4): 881-898. |
| 30 | VOLPEDO G, BHATTACHARYA P, GANNAVARAM S, et al. The history of live attenuated Centrin gene-deleted Leishmania vaccine candidates[J]. Pathogens, 2022, 11(4): 431. |
| 31 | 冯生, 刘宝山, 陈泽良, 等. 布鲁菌基因缺失疫苗侯选株研究进展[J]. 动物医学进展, 2020, 41(3): 110-113. |
| FENG S, LIU B S, CHEN Z L, et al. Progress on Brucella gene deletion vaccine strains[J]. Progress in Veterinary Medicine, 2020, 41(3): 110-113. | |
| 32 | PRIEBE G P, BRINIG M M, HATANO K, et al. Construction and characterization of a live, attenuated aroA deletion mutant of Pseudomonas aeruginosa as a candidate intranasal vaccine[J]. Infection and Immunity, 2002, 70(3): 1507-1517. |
| 33 | JINEK M, CHYLINSKI K, FONFARA I, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity[J]. Science, 2012, 337(6096): 816-821. |
| 34 | BHUJBAL S, BHUJBAL R, GIRAM P. An overview: CRISPR/Cas-based gene editing for viral vaccine development[J]. Expert Review of Vaccines, 2022, 21(11): 1581-1593. |
| 35 | ZHANG W W, KARMAKAR S, GANNAVARAM S, et al. A second generation leishmanization vaccine with a markerless attenuated Leishmania major strain using CRISPR gene editing[J]. Nature Communications, 2020, 11: 3461. |
| 36 | ATASOY M O, ROHAIM M A, MUNIR M. Simultaneous deletion of virulence factors and insertion of antigens into the infectious laryngotracheitis virus using NHEJ-CRISPR/Cas9 and Cre-Lox system for construction of a stable vaccine vector[J]. Vaccines, 2019, 7(4): 207. |
| 37 | TONG X L, FU M Y, CHEN P, et al. Ultrabithorax and abdominal-A specify the abdominal appendage in a dosage-dependent manner in silkworm, Bombyx mori [J]. Heredity, 2017, 118(6): 578-584. |
| 38 | JEONG S H, LEE H J, LEE S J. Recent advances in CRISPR-Cas technologies for synthetic biology[J]. Journal of Microbiology, 2023, 61(1): 13-36. |
| 39 | GARST A D, BASSALO M C, PINES G, et al. Genome-wide mapping of mutations at single-nucleotide resolution for protein, metabolic and genome engineering[J]. Nature Biotechnology, 2017, 35(1): 48-55. |
| 40 | PACHECO A R, LAZARUS J E, SIT B, et al. CRISPR screen reveals that EHEC′s T3SS and shiga toxin rely on shared host factors for infection[J]. mBio, 2018, 9(3): e01003-e01018. |
| 41 | GUTIÉRREZ-ORTEGA A, MORENO D A, FERRARI S A, et al. High-yield production of major T-cell ESAT6-CFP10 fusion antigen of M. tuberculosis complex employing codon-optimized synthetic gene[J]. International Journal of Biological Macromolecules, 2021, 171: 82-88. |
| 42 | 孙鹏, 朱爱臣, 梁天, 等. 鸡IL-17基因的克隆、序列分析及密码子优化提高表达水平[J]. 农业生物技术学报, 2023, 31(7): 1464-1476. |
| SUN P, ZHU A C, LIANG T, et al. Cloning, sequence analysis and codon optimization of chicken (gallus gallus) IL-17 gene to improve the expression level[J]. Journal of Agricultural Biotechnology, 2023, 31(7): 1464-1476. | |
| 43 | CHEN Z F, GOU Q, XIONG Q S, et al. Immunodominance of epitopes and protective efficacy of HI antigen are differentially altered using different adjuvants in a mouse model of Staphylococcus aureus bacteremia[J]. Frontiers in Immunology, 2021, 12: 684823. |
| 44 | YANG F, GU J, YANG L Y, et al. Protective efficacy of the trivalent Pseudomonas aeruginosa vaccine candidate PcrV-OprI-Hcp1 in murine pneumonia and burn models[J]. Scientific Reports, 2017, 7: 3957. |
| 45 | CARADONNA T M, SCHMIDT A G. Protein engineering strategies for rational immunogen design[J]. NPJ Vaccines, 2021, 6: 154. |
| 46 | BENNE N, VAN DUIJN J, KUIPER J, et al. Orchestrating immune responses: how size, shape and rigidity affect the immunogenicity of particulate vaccines[J]. Journal of Controlled Release, 2016, 234: 124-134. |
| 47 | CHARLTON HUME H K, VIDIGAL J, CARRONDO M J T, et al. Synthetic biology for bioengineering virus-like particle vaccines[J]. Biotechnology and Bioengineering, 2019, 116(4): 919-935. |
| 48 | JOYNER J A, DALY S M, PEABODY J, et al. Vaccination with VLPs presenting a linear neutralizing domain of S. aureus Hla elicits protective immunity[J]. Toxins, 2020, 12(7): 450. |
| 49 | JING H M, ZHANG X L, ZOU J T, et al. Oligomerization of IC43 resulted in improved immunogenicity and protective efficacy against Pseudomonas aeruginosa lung infection[J]. International Journal of Biological Macromolecules, 2020, 159: 174-182. |
| 50 | LI Y H, PU R X, ZHANG Y, et al. Self-assembled ferritin nanoparticles displaying PcrV and OprI as an adjuvant-free Pseudomonas aeruginosa vaccine[J]. Frontiers in Immunology, 2023, 14: 1184863. |
| 51 | ZOU J T, JING H M, YUAN Y, et al. Pore-forming alpha-hemolysin efficiently improves the immunogenicity and protective efficacy of protein antigens[J]. PLoS Pathogens, 2021, 17(7): e1009752. |
| 52 | MEHTA N K, PRADHAN R V, SOLEIMANY A P, et al. Pharmacokinetic tuning of protein-antigen fusions enhances the immunogenicity of T-cell vaccines[J]. Nature Biomedical Engineering, 2020, 4(6): 636-648. |
| 53 | ASSONI L, GIRARDELLO R, CONVERSO T R, et al. Current stage in the development of Klebsiella pneumoniae vaccines[J]. Infectious Diseases and Therapy, 2021, 10(4): 2157-2175. |
| 54 | VAN DER PUT R M F, METZ B, PIETERS R J. Carriers and antigens: new developments in glycoconjugate vaccines[J]. Vaccines, 2023, 11(2): 219. |
| 55 | 程亚慧, 沈荣, 乔瑞洁. 细菌性多糖蛋白结合疫苗免疫应答机制的研究进展[J]. 微生物学免疫学进展, 2018, 46(4): 81-86. |
| CHENG Y H, SHEN R, QIAO R J. Advances on immune response mechanisms of bacterial glyco-conjugate vaccines[J]. Progress in Microbiology and Immunology, 2018, 46(4): 81-86. | |
| 56 | 叶精勤, 黄文华, 潘超, 等. 合成生物学在多糖结合疫苗研发中的应用[J]. 合成生物学, 2024, 5(2):338-352. |
| YE J Q, HUANG W H, PAN C, et al. Application of synthetic biology in the development of polysaccharide conjugate vaccines[J]. Synthetic Biology Journal, 2024, 5(2):338-352. | |
| 57 | KAY E, CUCCUI J, WREN B W. Recent advances in the production of recombinant glycoconjugate vaccines[J]. NPJ Vaccines, 2019, 4: 16. |
| 58 | HARDING C M, NASR M A, SCOTT N E, et al. A platform for glycoengineering a polyvalent pneumococcal bioconjugate vaccine using E. coli as a host[J]. Nature Communications, 2019, 10: 891. |
| 59 | FELDMAN M F, MAYER BRIDWELL A E, SCOTT N E, et al. A promising bioconjugate vaccine against hypervirulent Klebsiella pneumoniae [J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(37): 18655-18663. |
| 60 | 彭哲慧, 潘超, 孙鹏, 等. 生物法合成伤寒O-糖蛋白结合疫苗及其免疫原性评估[J]. 遗传, 2015, 37(5): 473-479. |
| PENG Z H, PAN C, SUN P, et al. Preparation and immunogenicity-evaluation of typhoid O-specific polysaccharides bio-conjugate vaccines[J]. Hereditas, 2015, 37(5): 473-479. | |
| 61 | SU H L, LIU Q, BIAN X P, et al. Synthesis and delivery of Streptococcus pneumoniae capsular polysaccharides by recombinant attenuated Salmonella vaccines[J]. Proceedings of the National Academy of Sciences of the United States of America, 2021, 118(2): e2013350118. |
| 62 | RIDDLE M S, KAMINSKI R W, DI PAOLO C, et al. Safety and immunogenicity of a candidate bioconjugate vaccine against Shigella flexneri 2a administered to healthy adults: a single-blind, randomized phase I study[J]. Clinical and Vaccine Immunology: CVI, 2016, 23(12): 908-917. |
| 63 | HUTTNER A, HATZ C, VAN DEN DOBBELSTEEN G, et al. Safety, immunogenicity, and preliminary clinical efficacy of a vaccine against extraintestinal pathogenic Escherichia coli in women with a history of recurrent urinary tract infection: a randomised, single-blind, placebo-controlled phase 1b trial[J]. The Lancet Infectious Diseases, 2017, 17(5): 528-537. |
| 64 | PARDI N, HOGAN M J, PORTER F W, et al. mRNA vaccines — a new era in vaccinology[J]. Nature Reviews Drug Discovery, 2018, 17(4): 261-279. |
| 65 | 龚方苑. DNA疫苗与mRNA疫苗: 对抗人类疾病的两把利器[J]. 张江科技评论, 2023(3): 37-39. |
| GONG F Y. DNA vaccine and mRNA vaccine: two powerful tools against human diseases[J]. Zhangjiang Technology Review, 2023(3): 37-39. | |
| 66 | 刘畅, 邹全明, 李海波. 新冠病毒DNA疫苗的研究现状及展望[J]. 免疫学杂志, 2022, 38(8): 726-732. |
| LIU C, ZOU Q M, LI H B. Research status and prospects of SARS-CoV-2 DNA vaccines[J]. Immunological Journal, 2022, 38(8): 726-732. | |
| 67 | 顾江, 曾浩, 邹全明. 创新细菌疫苗的研究进展及挑战[J]. 中国生物制品学杂志, 2021, 34(9): 1017-1022. |
| GU J, ZENG H, ZOU Q M. Advances and challenges in research on innovative bacterial vaccines[J]. Chinese Journal of Biologicals, 2021, 34(9): 1017-1022. | |
| 68 | NIE X, ZHANG Z, WANG C H, et al. Interactions in DNA condensation: an important factor for improving the efficacy of gene transfection[J]. Bioconjugate Chemistry, 2019, 30(2): 284-292. |
| 69 | SUN S, LI E T, ZHAO G, et al. Respiratory mucosal vaccination of peptide-poloxamine-DNA nanoparticles provides complete protection against lethal SARS-CoV-2 challenge[J]. Biomaterials, 2023, 292: 121907. |
| 70 | LIU S L, JIANG Q A, ZHAO X A, et al. A DNA nanodevice-based vaccine for cancer immunotherapy[J]. Nature Materials, 2021, 20(3): 421-430. |
| 71 | 章德广, 王正敏, 徐江红, 等. 小鼠肺炎链球菌psaA核酸疫苗的制备及其初免-蛋白加强免疫策略的研究[J]. 中华耳鼻咽喉头颈外科杂志, 2009, 44(9): 762-766. |
| ZHANG D G, WANG Z M, XU J H, et al. Improvement of immunogenicity of Streptococcus pneumoniae psaA DNA vaccine by prime and boost strategy[J]. Chinese Journal of Otorhinolaryngology Head and Neck Surgery, 2009, 44(9): 762-766. | |
| 72 | GAUDREAU M C, LACASSE P, TALBOT B G. Protective immune responses to a multi-gene DNA vaccine against Staphylococcus aureus [J]. Vaccine, 2007, 25(5): 814-824. |
| 73 | GONG Q A, RUAN M D, NIU M F, et al. Immune efficacy of different immunization doses of divalent combination DNA vaccine pOPRL+pOPRF of Pseudomonas aeruginosa [J]. Journal of Veterinary Medical Science, 2021, 83(12): 1959-1964. |
| 74 | JIANG M Z, YAO J, FENG G Z. Protective effect of DNA vaccine encoding Pseudomonas exotoxin A and PcrV against acute pulmonary P. aeruginosa infection[J]. PLoS One, 2014, 9(5): e96609. |
| 75 | 赵轩, 江晓烽, 秦江雷, 等. 载铜绿假单胞菌OprF和PcrV基因联合DNA疫苗的水凝胶缓释系统的构建及免疫效力评价[J]. 解放军医学杂志, 2022, 47(9): 871-878. |
| ZHAO X, JIANG X F, QIN J L, et al. Construction and immune efficacy evaluation of a hydrogel sustained-release system containing a combined DNA vaccine of Pseudomonas aeruginosa OprF and PcrV genes[J]. Medical Journal of Chinese People’s Liberation Army, 2022, 47(9): 871-878. | |
| 76 | FAYEZ N AL, NASSAR M S, ALSHEHRI A A, et al. Recent advancement in mRNA vaccine development and applications[J]. Pharmaceutics, 2023, 15(7): 1972. |
| 77 | JIA L F, MAO Y H, JI Q Q, et al. Decoding mRNA translatability and stability from the 5′ UTR[J]. Nature Structural & Molecular Biology, 2020, 27(9): 814-821. |
| 78 | KARIKÓ K, BUCKSTEIN M, NI H P, et al. Suppression of RNA recognition by toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA[J]. Immunity, 2005, 23(2): 165-175. |
| 79 | KARIKÓ K, MURAMATSU H, WELSH F A, et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability[J]. Molecular Therapy, 2008, 16(11): 1833-1840. |
| 80 | ANDRIES O, MCCAFFERTY S, DE SMEDT S C, et al. N 1-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice[J]. Journal of Controlled Release, 2015, 217: 337-344. |
| 81 | LI C Y, LIANG Z H, HU Y X, et al. Cytidine-containing tails robustly enhance and prolong protein production of synthetic mRNA in cell and in vivo [J]. Molecular Therapy - Nucleic Acids, 2022, 30: 300-310. |
| 82 | ZHANG H, ZHANG L, LIN A, et al. Algorithm for optimized mRNA design improves stability and immunogenicity[J]. Nature, 2023, 621(7978): 396-403. |
| 83 | PARDI N, HOGAN M J, WEISSMAN D. Recent advances in mRNA vaccine technology[J]. Current Opinion in Immunology, 2020, 65: 14-20. |
| 84 | MATARAZZO L, BETTENCOURT P J G. mRNA vaccines: a new opportunity for malaria, tuberculosis and HIV[J]. Frontiers in Immunology, 2023, 14: 1172691. |
| 85 | TERAN-NAVARRO H, SALCINES-CUEVAS D, CALDERON-GONZALEZ R, et al. A comparison between recombinant listeria GAPDH proteins and GAPDH encoding mRNA conjugated to lipids as cross-reactive vaccines for listeria, mycobacterium, and streptococcus[J]. Frontiers in Immunology, 2021, 12: 632304. |
| 86 | MARUGGI G, CHIAROT E, GIOVANI C, et al. Immunogenicity and protective efficacy induced by self-amplifying mRNA vaccines encoding bacterial antigens[J]. Vaccine, 2017, 35(2): 361-368. |
| 87 | WANG X Y, LIU C, RCHEULISHVILI N, et al. Strong immune responses and protection of PcrV and OprF-I mRNA vaccine candidates against Pseudomonas aeruginosa [J]. NPJ Vaccines, 2023, 8: 76. |
| 88 | KAWAGUCHI K, KINOSHITA M, SUDO K, et al. mRNA vaccine induces protective immunity against the type Ⅲ secretory virulence of Pseudomonas aeruginosa [EB/OL]. BioRxiv, 2023: 544431[2023-09-01]. . |
| 89 | KON E, LEVY Y, ELIA U, et al. A single-dose F1-based mRNA-LNP vaccine provides protection against the lethal plague bacterium[J]. Science Advances, 2023, 9(10): eadg1036. |
| 90 | SAJID A, MATIAS J, ARORA G, et al. mRNA vaccination induces tick resistance and prevents transmission of the Lyme disease agent[J]. Science Translational Medicine, 2021, 13(620): eabj9827. |
| 91 | PINE M, ARORA G, HART T M, et al. Development of an mRNA-lipid nanoparticle vaccine against Lyme disease[J]. Molecular Therapy, 2023, 31(9): 2702-2714. |
| 92 | KAPARAKIS-LIASKOS M, FERRERO R L. Immune modulation by bacterial outer membrane vesicles[J]. Nature Reviews Immunology, 2015, 15(6): 375-387. |
| 93 | ABITBOL V, SOHN W Y, HORN M, et al. Safety and immunogenicity of co-administered meningococcal serogroup B (4CMenB) vaccine: a literature review[J]. Human Vaccines & Immunotherapeutics, 2023, 19(2): 2245705. |
| 94 | MARSHALL G S, ABBING-KARAHAGOPIAN V, MARSHALL H S, et al. A comprehensive review of clinical and real-world safety data for the four-component serogroup B meningococcal vaccine (4CMenB)[J]. Expert Review of Vaccines, 2023, 22(1): 530-544. |
| 95 | SCHWECHHEIMER C, KUEHN M J. Outer-membrane vesicles from gram-negative bacteria: biogenesis and functions[J]. Nature Reviews Microbiology, 2015, 13(10): 605-619. |
| 96 | 姚崧源, 孙述学. 细菌外膜囊泡在疫苗领域的研究进展[J]. 微生物学免疫学进展, 2021, 49(1): 78-82. |
| YAO S Y, SUN S X. Advances in research on bacterial outer membrane vesicles in vaccine[J]. Progress in Microbiology and Immunology, 2021, 49(1): 78-82. | |
| 97 | AASS H C D, HELLUM M, TRØSEID A M S, et al. Whole-blood incubation with the Neisseria meningitidis lpxL1 mutant induces less pro-inflammatory cytokines than the wild type, and IL-10 reduces the MyD88-dependent cytokines[J]. Innate Immunity, 2018, 24(2): 101-111. |
| 98 | VAN DER LEY P, VAN DEN DOBBELSTEEN G. Next-generation outer membrane vesicle vaccines against Neisseria meningitidis based on nontoxic LPS mutants[J]. Human Vaccines, 2011, 7(8): 886-890. |
| 99 | FRIRDICH E, WHITFIELD C. Review: Lipopolysaccharide inner core oligosaccharide structure and outer membrane stability in human pathogens belonging to the Enterobacteriaceae[J]. Journal of Endotoxin Research, 2005, 11(3): 133-144. |
| 100 | LI P, WANG X R, SUN X W, et al. Recombinant Pseudomonas bionanoparticles induce protection against pneumonic Pseudomonas aeruginosa infection[J]. Infection and Immunity, 2021, 89(11): e00396-21. |
| 101 | 易洁, 刘青, 孔庆科. 革兰氏阴性菌外膜囊泡作为亚单位疫苗的研究进展[J]. 微生物学报, 2016, 56(6): 911-921. |
| YI J, LIU Q, KONG Q K. Advances in outer membrane vesicles of gram-negative bacteria as sub-unit vaccines - a review[J]. Acta Microbiologica Sinica, 2016, 56(6): 911-921. | |
| 102 | DEATHERAGE B L, LARA J C, BERGSBAKEN T, et al. Biogenesis of bacterial membrane vesicles[J]. Molecular Microbiology, 2009, 72(6): 1395-1407. |
| 103 | ARIGITA C, JISKOOT W, WESTDIJK J, et al. Stability of mono- and trivalent meningococcal outer membrane vesicle vaccines[J]. Vaccine, 2004, 22(5/6): 629-642. |
| 104 | SCHWECHHEIMER C, RODRIGUEZ D L, KUEHN M J. NlpI-mediated modulation of outer membrane vesicle production through peptidoglycan dynamics in Escherichia coli [J]. MicrobiologyOpen, 2015, 4(3): 375-389. |
| 105 | GNOPO Y M D, WATKINS H C, STEVENSON T C, et al. Designer outer membrane vesicles as immunomodulatory systems - reprogramming bacteria for vaccine delivery[J]. Advanced Drug Delivery Reviews, 2017, 114: 132-142. |
| 106 | CHEN D J, OSTERRIEDER N, METZGER S M, et al. Delivery of foreign antigens by engineered outer membrane vesicle vaccines[J]. Proceedings of the National Academy of Sciences of the United States of America, 2010, 107(7): 3099-3104. |
| 107 | HUANG W W, WANG S J, YAO Y F, et al. Employing Escherichia coli-derived outer membrane vesicles as an antigen delivery platform elicits protective immunity against Acinetobacter baumannii infection[J]. Scientific Reports, 2016, 6: 37242. |
| 108 | CHEN L X, VALENTINE J L, HUANG C J, et al. Outer membrane vesicles displaying engineered glycotopes elicit protective antibodies[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(26): E3609-E3618. |
| 109 | SUZUKI H, NOGUCHI T, OGAWA K, et al. Fusion of parvovirus B19 receptor-binding domain and pneumococcal surface protein A induces protective immunity against parvovirus B19 and Streptococcus pneumoniae [J]. Vaccine, 2021, 39(36): 5146-5152. |
| 110 | MURALINATH M, KUEHN M J, ROLAND K L, et al. Immunization with Salmonella enterica serovar typhimurium-derived outer membrane vesicles delivering the pneumococcal protein PspA confers protection against challenge with Streptococcus pneumoniae [J]. Infection and Immunity, 2011, 79(2): 887-894. |
| 111 | MATTHIAS K A, REVEILLE A, CONNOLLY K L, et al. Deletion of major porins from meningococcal outer membrane vesicle vaccines enhances reactivity against heterologous serogroup B Neisseria meningitidis strains[J]. Vaccine, 2020, 38(10): 2396-2405. |
| 112 | GERRITZEN M J H, MAAS R H W, VAN DEN IJSSEL J, et al. High dissolved oxygen tension triggers outer membrane vesicle formation by Neisseria meningitidis [J]. Microbial Cell Factories, 2018, 17(1): 157. |
| 113 | ROSENBERG G, RIQUELME S, PRINCE A, et al. Immunometabolic crosstalk during bacterial infection[J]. Nature Microbiology, 2022, 7(4): 497-507. |
| 114 | EICHELBERGER K R, CASSAT J E. Metabolic adaptations during Staphylococcus aureus and Candida albicans co-infection[J]. Frontiers in Immunology, 2021, 12: 797550. |
| 115 | PULENDRAN B, ARUNACHALAM P S, O′HAGAN D T. Emerging concepts in the science of vaccine adjuvants[J]. Nature Reviews Drug Discovery, 2021, 20(6): 454-475. |
| 116 | QIN S G, TANG X S, CHEN Y T, et al. mRNA-based therapeutics: powerful and versatile tools to combat diseases[J]. Signal Transduction and Targeted Therapy, 2022, 7: 166. |
| 117 | NGUYEN B, TOLIA N H. Protein-based antigen presentation platforms for nanoparticle vaccines[J]. NPJ Vaccines, 2021, 6: 70. |
| 118 | NAKAHASHI-OUCHIDA R, FUJIHASHI K, KURASHIMA Y, et al. Nasal vaccines: solutions for respiratory infectious diseases[J]. Trends in Molecular Medicine, 2023, 29(2): 124-140. |
| 119 | 申赵铃, 吴艳玲, 应天雷. 合成生物学与病毒疫苗研发[J]. 合成生物学, 2023, 4(2): 333-346. |
| SHEN Z L, WU Y L, YING T L. Synthetic biology and viral vaccine development[J]. Synthetic Biology Journal, 2023, 4(2): 333-346. | |
| 120 | ZHAO W J. A forum on synthetic biology: meet the great challenges with new technology[J]. National Science Review, 2021, 8(1): nwaa252. |
| [1] | GAO Ge, BIAN Qi, WANG Baojun. Synthetic genetic circuit engineering: principles, advances and prospects [J]. Synthetic Biology Journal, 2025, 6(1): 45-64. |
| [2] | LI Jiyuan, WU Guosheng. Two hypothesises for the origins of organisms from the synthetic biology perspective [J]. Synthetic Biology Journal, 2025, 6(1): 190-202. |
| [3] | JIAO Hongtao, QI Meng, SHAO Bin, JIANG Jinsong. Legal issues for the storage of DNA data [J]. Synthetic Biology Journal, 2025, 6(1): 177-189. |
| [4] | TANG Xinghua, LU Qianneng, HU Yilin. Philosophical reflections on synthetic biology in the Anthropocene [J]. Synthetic Biology Journal, 2025, 6(1): 203-212. |
| [5] | XU Huaisheng, SHI Xiaolong, LIU Xiaoguang, XU Miaomiao. Key technologies for DNA storage: encoding, error correction, random access, and security [J]. Synthetic Biology Journal, 2025, 6(1): 157-176. |
| [6] | SHI Ting, SONG Zhan, SONG Shiyi, ZHANG Yi-Heng P. Job. In vitro BioTransformation (ivBT): a new frontier of industrial biomanufacturing [J]. Synthetic Biology Journal, 2024, 5(6): 1437-1460. |
| [7] | CHAI Meng, WANG Fengqing, WEI Dongzhi. Synthesis of organic acids from lignocellulose by biotransformation [J]. Synthetic Biology Journal, 2024, 5(6): 1242-1263. |
| [8] | SHAO Mingwei, SUN Simian, YANG Shimao, CHEN Guoqiang. Bioproduction based on extremophiles [J]. Synthetic Biology Journal, 2024, 5(6): 1419-1436. |
| [9] | CHEN Yu, ZHANG Kang, QIU Yijing, CHENG Caiyun, YIN Jingjing, SONG Tianshun, XIE Jingjing. Progress of microbial electrosynthesis for conversion of CO2 [J]. Synthetic Biology Journal, 2024, 5(5): 1142-1168. |
| [10] | ZHENG Haotian, LI Chaofeng, LIU Liangxu, WANG Jiawei, LI Hengrun, NI Jun. Design, optimization and application of synthetic carbon-negative phototrophic community [J]. Synthetic Biology Journal, 2024, 5(5): 1189-1210. |
| [11] | CHEN Ziling, XIANG Yangfei. Integrated development of organoid technology and synthetic biology [J]. Synthetic Biology Journal, 2024, 5(4): 795-812. |
| [12] | CAI Bingyu, TAN Xiangtian, LI Wei. Advances in synthetic biology for engineering stem cell [J]. Synthetic Biology Journal, 2024, 5(4): 782-794. |
| [13] | XIE Huang, ZHENG Yilei, SU Yiting, RUAN Jingyi, LI Yongquan. An overview on reconstructing the biosynthetic system of actinomycetes for polyketides production [J]. Synthetic Biology Journal, 2024, 5(3): 612-630. |
| [14] | ZHA Wenlong, BU Lan, ZI Jiachen. Advances in synthetic biology for producing potent pharmaceutical ingredients of traditional Chinese medicine [J]. Synthetic Biology Journal, 2024, 5(3): 631-657. |
| [15] | HUI Zhen, TANG Xiaoyu. Applications of the CRISPR/Cas9 editing system in the study of microbial natural products [J]. Synthetic Biology Journal, 2024, 5(3): 658-671. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||