Synthetic Biology Journal ›› 2024, Vol. 5 ›› Issue (2): 239-253.DOI: 10.12211/2096-8280.2023-061
• Invited Review • Previous Articles Next Articles
Progress with the application of synthetic biology in designing of cancer vaccines
FANG Chao1, HUANG Weiren1,2,3
- 1.Institute of Synthetic Biology,Shenzhen Institute of Advanced Technology,Chinese Academy of Sciences,Shenzhen 518035,Guangdong,China
2.Department of Urology,The First Affiliated Hospital of Shenzhen University,Shenzhen Second People’s Hospital,Shenzhen Institute of Translational Medicine,Shenzhen 518035,Guangdong,China
3.Guangdong Provincial Key Laboratory of Systems Biology and Synthetic Biology for Urogenital Tumors,Shenzhen 518035,Guangdong,China
-
Received:2023-08-25Revised:2024-02-29Online:2024-04-28Published:2024-04-30 -
Contact:HUANG Weiren
合成生物学在肿瘤疫苗设计中的应用进展
方超1, 黄卫人1,2,3
- 1.中国科学院深圳先进技术研究院,合成生物学研究所,广东 深圳 518035
2.深圳大学第一附属医院,深圳市第二人民医院,泌尿外科,深圳转化医学研究院,广东 深圳 518035
3.广东省泌尿生殖肿瘤系统生物学与合成生物学重点实验室,广东 深圳 518035
-
通讯作者:黄卫人 -
作者简介:方超 (1990—),男,博士后。研究方向:(1)肿瘤合成生物学;(2)肿瘤表观遗传学。E-mail:c.fang@siat.ac.cn黄卫人 (1980—),男,研究员,博士生导师,深圳市转化医学研究院副院长。研究方向:(1)肿瘤基因组学,应用多组学手段鉴定肿瘤及微环境诊疗标志物,开发相关临床应用;(2)肿瘤类器官,利用体外培养系统还原肿瘤体内生长,药物筛选及耐药机制研究;(3)医学合成生物学,创新肿瘤治疗新方法。E-mail:pony8980@163.com -
基金资助:国家重点研发计划(2019YFA0906003);国家自然科学基金(81972368);广东省培养高层次人才特殊支持计划(2021JC06Y578)
CLC Number:
Cite this article
FANG Chao, HUANG Weiren. Progress with the application of synthetic biology in designing of cancer vaccines[J]. Synthetic Biology Journal, 2024, 5(2): 239-253.
方超, 黄卫人. 合成生物学在肿瘤疫苗设计中的应用进展[J]. 合成生物学, 2024, 5(2): 239-253.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://synbioj.cip.com.cn/EN/10.12211/2096-8280.2023-061
| 肿瘤抗原 | 抗原类型 | 表达位置 | 高丰度表达位置 |
|---|---|---|---|
| 肿瘤相关性抗原 | 高表达蛋白或者多肽 | 肿瘤或正常细胞 | 肿瘤 |
| 肿瘤种系抗原 | 肿瘤,生殖细胞 | 肿瘤,生殖细胞 | |
| 肿瘤特异性抗原 | 肿瘤病毒 | 病毒性肿瘤 | 病毒性肿瘤 |
| 肿瘤新抗原 | 肿瘤 | 肿瘤 |
Table 1 Classification of cancer antigens
| 肿瘤抗原 | 抗原类型 | 表达位置 | 高丰度表达位置 |
|---|---|---|---|
| 肿瘤相关性抗原 | 高表达蛋白或者多肽 | 肿瘤或正常细胞 | 肿瘤 |
| 肿瘤种系抗原 | 肿瘤,生殖细胞 | 肿瘤,生殖细胞 | |
| 肿瘤特异性抗原 | 肿瘤病毒 | 病毒性肿瘤 | 病毒性肿瘤 |
| 肿瘤新抗原 | 肿瘤 | 肿瘤 |
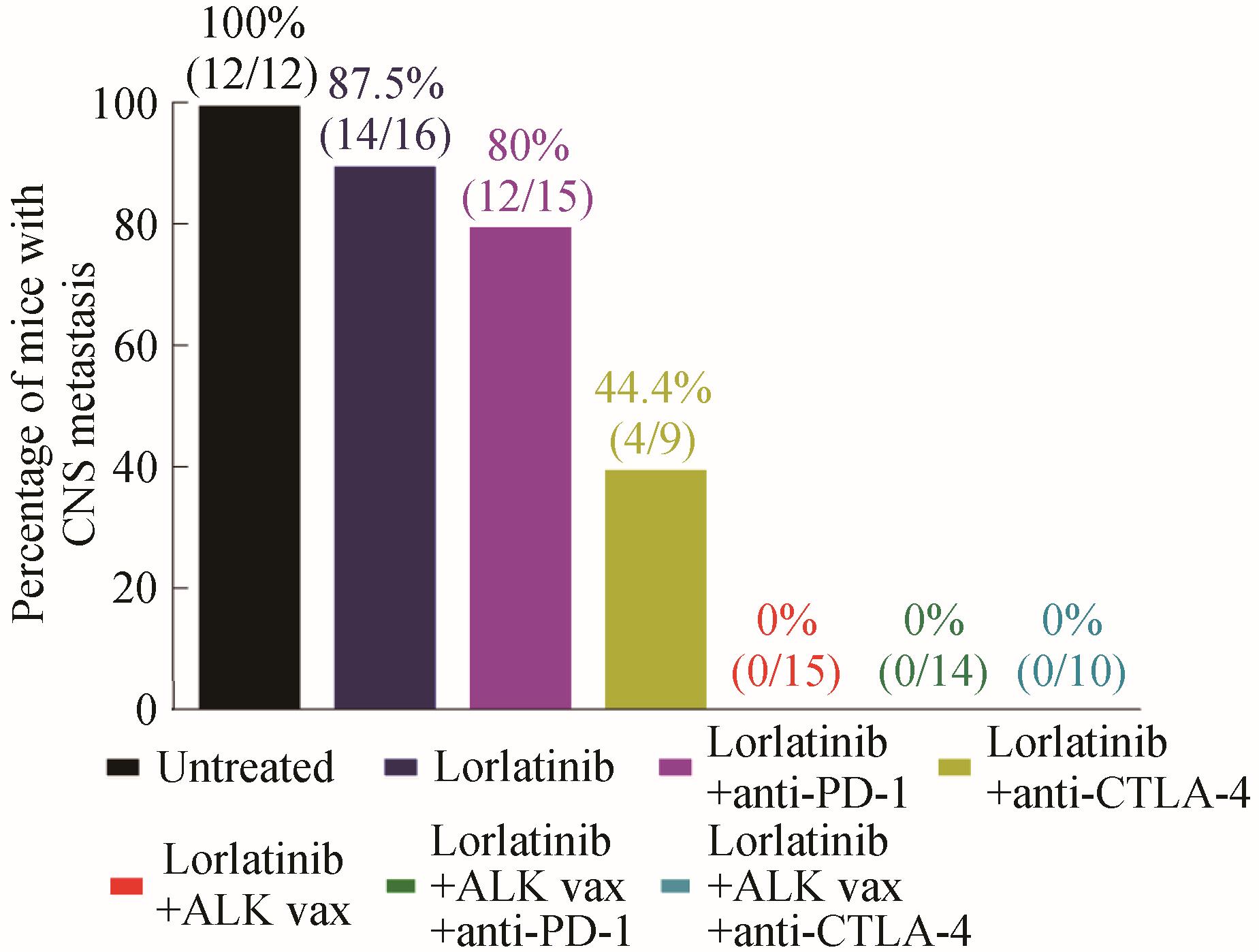
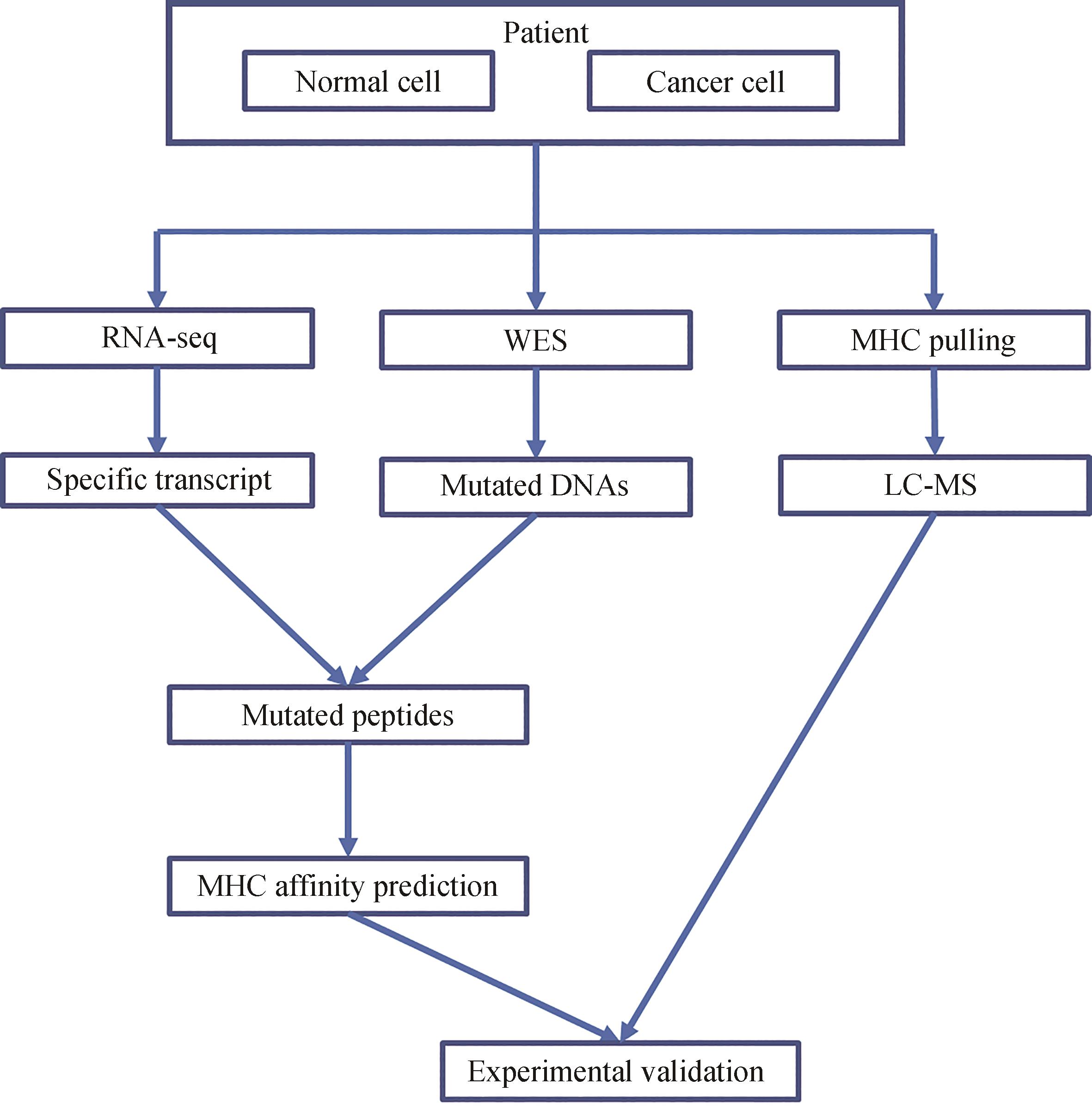
Fig. 2 Inhibition of tumor metastasis by ALK peptide vaccine characterized by the number of tested mice with metastatic tumors in their central nervous systems[72]
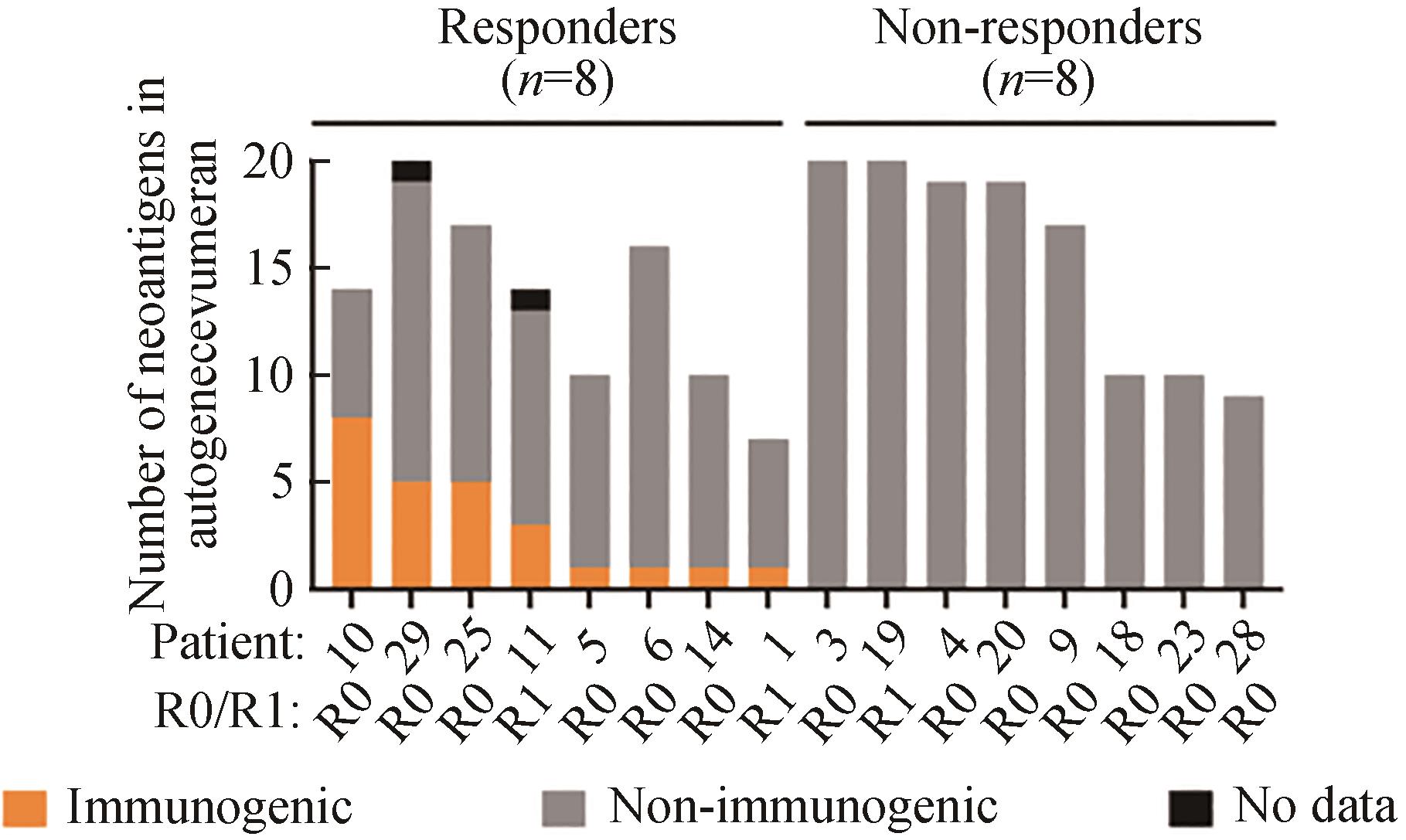
Fig. 3 Immune response of the patients to the autogene cevumera vaccine[88](The number of vaccine-induced IFNγ+ T cells in PBMC collected from the patients after vaccination with new vaccine antigens. R0/R1 indicates surgical resection margin status. Adapted with permission from reference.)
| 1 | BOYLSTON A. The origins of inoculation[J]. Journal of the Royal Society of Medicine, 2012, 105(7): 309-313. |
| 2 | DEMARIA P J, BILUSIC M. Cancer vaccines[J]. Hematology/Oncology Clinics of North America, 2019, 33(2): 199-214. |
| 3 | GARY E N, WEINER D B. DNA vaccines: prime time is now[J]. Current Opinion in Immunology, 2020, 65: 21-27. |
| 4 | BECK J D, REIDENBACH D, SALOMON N, et al. mRNA therapeutics in cancer immunotherapy[J]. Molecular Cancer, 2021, 20(1): 69. |
| 5 | GARG A D, COULIE P G, VAN DEN EYNDE B J, et al. Integrating next-generation dendritic cell vaccines into the current cancer immunotherapy landscape[J]. Trends in Immunology, 2017, 38(8): 577-593. |
| 6 | FAN T, ZHANG M N, YANG J X, et al. Therapeutic cancer vaccines: advancements, challenges, and prospects[J]. Signal Transduction and Targeted Therapy, 2023, 8(1): 450. |
| 7 | GEBRE M S, BRITO L A, TOSTANOSKI L H, et al. Novel approaches for vaccine development[J]. Cell, 2021, 184(6): 1589-1603. |
| 8 | MINATI R, PERREAULT C, THIBAULT P. A roadmap toward the definition of actionable tumor-specific antigens[J]. Frontiers in Immunology, 2020, 11: 583287. |
| 9 | CHEN G, HUANG A C, ZHANG W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response[J]. Nature, 2018, 560(7718): 382-386. |
| 10 | KALAORA S, NAGLER A, WARGO J A, et al. Mechanisms of immune activation and regulation: lessons from melanoma[J]. Nature Reviews Cancer, 2022, 22(4): 195-207. |
| 11 | CHIA W K, WANG W W, TEO M, et al. A phase Ⅱ study evaluating the safety and efficacy of an adenovirus-ΔLMP1-LMP2 transduced dendritic cell vaccine in patients with advanced metastatic nasopharyngeal carcinoma[J]. Annals of Oncology, 2012, 23(4): 997-1005. |
| 12 | KENTER G G, WELTERS M J P, VALENTIJN A R P M, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia[J]. The New England Journal of Medicine, 2009, 361(19): 1838-1847. |
| 13 | TRIMBLE C L, MORROW M P, KRAYNYAK K A, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial[J]. The Lancet, 2015, 386(10008): 2078-2088. |
| 14 | HARPER D M, NIEMINEN P, DONDERS G, et al. The efficacy and safety of Tipapkinogen Sovacivec therapeutic HPV vaccine in cervical intraepithelial neoplasia grades 2 and 3: randomized controlled phase Ⅱ trial with 2.5 years of follow-up[J]. Gynecologic Oncology, 2019, 153(3): 521-529. |
| 15 | KIM T J, JIN H T, HUR S Y, et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients[J]. Nature Communications, 2014, 5: 5317. |
| 16 | PIPERNO-NEUMANN S, HASSEL J C, RUTKOWSKI P, et al. Abstract CT002: phase 3 randomized trial comparing tebentafusp with investigator’s choice in first line metastatic uveal melanoma[J]. Cancer Research, 2021, 81(): CT002. |
| 17 | ROSENBERG S A, YANG J C, SCHWARTZENTRUBER D J, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma[J]. Nature Medicine, 1998, 4(3): 321-327. |
| 18 | CUNHA A C, WEIGLE B, KIESSLING A, et al. Tissue-specificity of prostate specific antigens: comparative analysis of transcript levels in prostate and non-prostatic tissues[J]. Cancer Letters, 2006, 236(2): 229-238. |
| 19 | KANTOFF P W, HIGANO C S, SHORE N D, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer[J]. The New England Journal of Medicine, 2010, 363(5): 411-422. |
| 20 | O’ROURKE D M, NASRALLAH M P, DESAI A, et al. A single dose of peripherally infused EGFRvⅢ-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma[J]. Science Translational Medicine, 2017, 9(399): eaaa0984. |
| 21 | ZAHEDIPOUR F, ZAMANI P, MASHREGHI M, et al. Nanoliposomal VEGF-R2 peptide vaccine acts as an effective therapeutic vaccine in a murine B16F10 model of melanoma[J]. Cancer Nanotechnology, 2023, 14(1): 62. |
| 22 | SCHUSTER J, LAI R K, RECHT L D, et al. A phase Ⅱ, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT Ⅲ study[J]. Neuro-oncology, 2015, 17(6): 854-861. |
| 23 | REARDON D A, DESJARDINS A, VREDENBURGH J J, et al. Rindopepimut with bevacizumab for patients with relapsed EGFRvⅢ-expressing glioblastoma (ReACT): results of a double-blind randomized phase Ⅱ trial[J]. Clinical Cancer Research, 2020, 26(7): 1586-1594. |
| 24 | WELLER M, BUTOWSKI N, TRAN D D, et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvⅢ-expressing glioblastoma (ACT Ⅳ): a randomised, double-blind, international phase 3 trial[J]. The Lancet Oncology, 2017, 18(10): 1373-1385. |
| 25 | LIU C, YE D Y, YANG H L, et al. RAS-targeted cancer therapy: advances in drugging specific mutations[J]. MedComm, 2023, 4(3): e285. |
| 26 | BANNOURA S F, UDDIN M H, NAGASAKA M, et al. Targeting KRAS in pancreatic cancer: new drugs on the horizon[J]. Cancer Metastasis Reviews, 2021, 40(3): 819-835. |
| 27 | LIU X Q, HUANG P, YANG R S, et al. mRNA cancer vaccines: construction and boosting strategies[J]. ACS Nano, 2023, 17(20): 19550-19580. |
| 28 | CHEEVER M A, ALLISON J P, FERRIS A S, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research[J]. Clinical Cancer Research, 2009, 15(17): 5323-5337. |
| 29 | QI X W, ZHANG F, WU H, et al. Wilms’ tumor 1 (WT1) expression and prognosis in solid cancer patients: a systematic review and meta-analysis[J]. Scientific Reports, 2015, 5: 8924. |
| 30 | OKA Y, TSUBOI A, TAGUCHI T, et al. Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression[J]. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(38): 13885-13890. |
| 31 | MASLAK P G, TAO D, BERNAL Y, et al. Phase 2 trial of a multivalent WT1 peptide vaccine (galinpepimut-S) in acute myeloid leukemia[J]. Blood Advances, 2018, 2(3): 224-234. |
| 32 | KEILHOLZ U, LETSCH A, BUSSE A, et al. A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS[J]. Blood, 2009, 113(26): 6541-6548. |
| 33 | ANGUILLE S, VAN DE VELDE A L, SMITS E L, et al. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia[J]. Blood, 2017, 130(15): 1713-1721. |
| 34 | VANSTEENKISTE J, ZIELINSKI M, LINDER A, et al. Adjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: phase Ⅱ randomized study results[J]. Journal of Clinical Oncology, 2013, 31(19): 2396-2403. |
| 35 | KRUIT W H J, SUCIU S, DRENO B, et al. Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: results of a randomized phase Ⅱ study of the European Organisation for Research and Treatment of Cancer Melanoma Group in Metastatic Melanoma[J]. Journal of Clinical Oncology, 2013, 31(19): 2413-2420. |
| 36 | SLINGLUFF C L, LEWIS K D, ANDTBACKA R, et al. Multicenter, double-blind, placebo-controlled trial of seviprotimut-L polyvalent melanoma vaccine in patients with post-resection melanoma at high risk of recurrence[J]. Journal for Immunotherapy of Cancer, 2021, 9(10): e003272. |
| 37 | THOMAS R, AL-KHADAIRI G, ROELANDS J, et al. NY-ESO-1 based immunotherapy of cancer: current perspectives[J]. Frontiers in Immunology, 2018, 9: 947. |
| 38 | DHODAPKAR M V, SZNOL M, ZHAO B W, et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205[J]. Science Translational Medicine, 2014, 6(232): 232ra51. |
| 39 | GASSER O, SHARPLES K J, BARROW C, et al. A phase Ⅰ vaccination study with dendritic cells loaded with NY-ESO-1 and α-galactosylceramide: induction of polyfunctional T cells in high-risk melanoma patients[J]. Cancer Immunology, Immunotherapy, 2018, 67(2): 285-298. |
| 40 | ODUNSI K, QIAN F, MATSUZAKI J, et al. Vaccination with an NY-ESO-1 peptide of HLA class Ⅰ/Ⅱ specificities induces integrated humoral and T cell responses in ovarian cancer[J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(31): 12837-12842. |
| 41 | MITTENDORF E A, ARDAVANIS A, SYMANOWSKI J, et al. Primary analysis of a prospective, randomized, single-blinded phase Ⅱ trial evaluating the HER2 peptide AE37 vaccine in breast cancer patients to prevent recurrence[J]. Annals of Oncology, 2016, 27(7): 1241-1248. |
| 42 | MITTENDORF E A, LU B, MELISKO M, et al. Efficacy and safety analysis of nelipepimut-S vaccine to prevent breast cancer recurrence: a randomized, multicenter, phase Ⅲ clinical trial[J]. Clinical Cancer Research, 2019, 25(14): 4248-4254. |
| 43 | LIN M H, SHEN K Y, LIU B S, et al. Immunological evaluation of a novel HLA-A2 restricted phosphopeptide of tumor associated antigen, TRAP1, on cancer therapy[J]. Vaccine: Ⅹ, 2019, 1: 100017. |
| 44 | CLIFTON G T, HALE D, VREELAND T J, et al. Results of a randomized phase Ⅱb trial of nelipepimut-S+trastuzumab versus trastuzumab to prevent recurrences in patients with high-risk HER2 low-expressing breast cancer[J]. Clinical Cancer Research, 2020, 26(11): 2515-2523. |
| 45 | COX K E, LIU S L, LWIN T M, et al. The mucin family of proteins: candidates as potential biomarkers for colon cancer[J]. Cancers, 2023, 15(5): 1491. |
| 46 | PACILIO C, ROSATI G, CRISPO A, et al. An overview of the roles of CDK4/6 inhibitors in metastatic breast cancer elderly patients[J]. In Vivo, 2023, 37(4): 1445-1449. |
| 47 | CHUNG V M, KOS F, HARDWICK N, et al. A phase 1 study of p53MVA vaccine in combination with pembrolizumab[J]. Journal of Clinical Oncology, 2018, 36(): 206. |
| 48 | KIM C, LIU S V, SUBRAMANIAM D S, et al. Phase Ⅰ study of the 177Lu-DOTA0-Tyr3-Octreotate (lutathera) in combination with nivolumab in patients with neuroendocrine tumors of the lung[J]. Journal for Immunotherapy of Cancer, 2020, 8(2): e000980. |
| 49 | ANTONIA S J, MIRZA N, FRICKE I, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer[J]. Clinical Cancer Research, 2006, 12(3 Pt 1): 878-887. |
| 50 | HARDWICK N R, FRANKEL P, RUEL C, et al. p53-Reactive T cells are associated with clinical benefit in patients with platinum-resistant epithelial ovarian cancer after treatment with a p53 vaccine and gemcitabine chemotherapy[J]. Clinical Cancer Research, 2018, 24(6): 1315-1325. |
| 51 | SPEETJENS F M, KUPPEN P J K, WELTERS M J P, et al. Induction of p53-specific immunity by a p53 synthetic long peptide vaccine in patients treated for metastatic colorectal cancer[J]. Clinical Cancer Research, 2009, 15(3): 1086-1095. |
| 52 | CHUNG V, KOS F J, HARDWICK N, et al. Evaluation of safety and efficacy of p53MVA vaccine combined with pembrolizumab in patients with advanced solid cancers[J]. Clinical & Translational Oncology, 2019, 21(3): 363-372. |
| 53 | QUANDT J, SCHLUDE C, BARTOSCHEK M, et al. Long-peptide vaccination with driver gene mutations in p53 and Kras induces cancer mutation-specific effector as well as regulatory T cell responses[J]. Oncoimmunology, 2018, 7(12): e1500671. |
| 54 | KJELDSEN J W, LORENTZEN C L, MARTINENAITE E, et al. A phase 1/2 trial of an immune-modulatory vaccine against IDO/PD-L1 in combination with nivolumab in metastatic melanoma[J]. Nature Medicine, 2021, 27(12): 2212-2223. |
| 55 | SCHUMACHER T N, SCHREIBER R D. Neoantigens in cancer immunotherapy[J]. Science, 2015, 348(6230): 69-74. |
| 56 | XIE N, SHEN G B, GAO W, et al. Neoantigens: promising targets for cancer therapy[J]. Signal Transduction and Targeted Therapy, 2023, 8(1): 9. |
| 57 | WANG L, SHAMARDANI K, BABIKIR H, et al. The evolution of alternative splicing in glioblastoma under therapy[J]. Genome Biology, 2021, 22(1): 48. |
| 58 | JUHARI W K W, AHMAD AMIN NOORDIN K B, ZAKARIA A D, et al. Whole-genome profiles of Malay colorectal cancer patients with intact MMR proteins[J]. Genes, 2021, 12(9): 1448. |
| 59 | HANSEN U K, RAMSKOV S, BJERREGAARD A M, et al. Tumor-infiltrating T cells from clear cell renal cell carcinoma patients recognize neoepitopes derived from point and frameshift mutations[J]. Frontiers in Immunology, 2020, 11: 373. |
| 60 | LU S X, NEEF E D, THOMAS J D, et al. Pharmacologic modulation of RNA splicing enhances anti-tumor immunity[J]. Cell, 2021, 184(15): 4032-4047.e31. |
| 61 | DAO T, MUN S S, MOLVI Z, et al. A TCR mimic monoclonal antibody reactive with the “public” phospho-neoantigen pIRS2/HLA-A*02: 01 complex[J]. JCI Insight, 2022, 7(5): e151624. |
| 62 | KRUMP N A, YOU J X. From merkel cell polyomavirus infection to merkel cell carcinoma oncogenesis[J]. Frontiers in Microbiology, 2021, 12: 739695. |
| 63 | ZHANG W T, ZHU G L, XU W Q, et al. Association of PD-1/PD-L1 expression and Epstein: Barr virus infection in patients with invasive breast cancer[J]. Diagnostic Pathology, 2022, 17(1): 61. |
| 64 | CHAN C K, AIMAGAMBETOVA G, UKYBASSOVA T, et al. Human papillomavirus infection and cervical cancer: epidemiology, screening, and vaccination-review of current perspectives[J]. Journal of Oncology, 2019, 2019: 3257939. |
| 65 | PURCELL A W, RAMARATHINAM S H, TERNETTE N. Mass spectrometry-based identification of MHC-bound peptides for immunopeptidomics[J]. Nature Protocols, 2019, 14: 1687-1707. |
| 66 | KRISTENSEN N P, HEEKE C, TVINGSHOLM S A, et al. Neoantigen-reactive CD8+ T cells affect clinical outcome of adoptive cell therapy with tumor-infiltrating lymphocytes in melanoma[J]. The Journal of Clinical Investigation, 2022, 132(2): e150535. |
| 67 | HOLM J S, FUNT S A, BORCH A, et al. Neoantigen-specific CD8 T cell responses in the peripheral blood following PD-L1 blockade might predict therapy outcome in metastatic urothelial carcinoma[J]. Nature Communications, 2022, 13(1): 1935. |
| 68 | BISWAS N, CHAKRABARTI S, PADUL V, et al. Designing neoantigen cancer vaccines, trials, and outcomes[J]. Frontiers in Immunology, 2023, 14: 1105420. |
| 69 | VITA R, MAHAJAN S, OVERTON J A, et al. The Immune Epitope Database (IEDB): 2018 update[J]. Nucleic Acids Research, 2019, 47(D1): D339-D343. |
| 70 | ZHOU W J, QU Z, SONG C Y, et al. NeoPeptide: an immunoinformatic database of T-cell-defined neoantigens[J]. Database, 2019, 2019: baz128. |
| 71 | CHAROENTONG P, FINOTELLO F, ANGELOVA M, et al. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade[J]. Cell Reports, 2017, 18(1): 248-262. |
| 72 | WU J C, ZHAO W Y, ZHOU B B, et al. TSNAdb: a database for tumor-specific neoantigens from immunogenomics data analysis[J]. Genomics, Proteomics & Bioinformatics, 2018, 16(4): 276-282. |
| 73 | SHAW A T, BAUER T M, MARINIS F D, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer[J]. The New England Journal of Medicine, 2020, 383(21): 2018-2029. |
| 74 | PETERS S, CAMIDGE D R, SHAW A T, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer[J]. The New England Journal of Medicine, 2017, 377(9): 829-838. |
| 75 | CAMIDGE D R, KIM H R, AHN M J, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer[J]. The New England Journal of Medicine, 2018, 379(21): 2027-2039. |
| 76 | KWAK E L, BANG Y J, CAMIDGE D R, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer[J]. The New England Journal of Medicine, 2010, 363(18): 1693-1703. |
| 77 | SHAW A T, KIM T M, CRINÒ L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial[J]. The Lancet Oncology, 2017, 18(7): 874-886. |
| 78 | ZHANG I, ZAORSKY N G, PALMER J D, et al. Targeting brain metastases in ALK-rearranged non-small-cell lung cancer[J]. The Lancet Oncology, 2015, 16(13): e510-e521. |
| 79 | JOHUNG K L, YEH N, DESAI N B, et al. Extended survival and prognostic factors for patients with ALK-rearranged non-small-cell lung cancer and brain metastasis[J]. Journal of Clinical Oncology, 2016, 34(2): 123-129. |
| 80 | MOTA I, PATRUCCO E, MASTINI C, et al. ALK peptide vaccination restores the immunogenicity of ALK-rearranged non-small cell lung cancer[J]. Nature Cancer, 2023, 4(7): 1016-1035. |
| 81 | CHOI Y M, KIM D H, JANG J, et al. A hepatitis B virus-derived peptide combined with HBsAg exerts an anti-HBV effect in an HBV transgenic mouse model as a therapeutic vaccine[J]. Frontiers in Immunology, 2023, 14: 1155637. |
| 82 | SURI S, DAKSHANAMURTHY S. IntegralVac: a machine learning-based comprehensive multivalent epitope vaccine design method[J]. Vaccines, 2022, 10(10): 1678. |
| 83 | HU Z T, LEET D E, ALLESØE R L, et al. Personal neoantigen vaccines induce persistent memory T cell responses and epitope spreading in patients with melanoma[J]. Nature Medicine, 2021, 27(3): 515-525. |
| 84 | WIEDERMANN U, GARNER-SPITZER E, CHAO Y E, et al. Clinical and immunologic responses to a B-cell epitope vaccine in patients with HER2/neu-overexpressing advanced gastric cancer-results from phase ib trial IMU.ACS.001[J]. Clinical Cancer Research, 2021, 27(13): 3649-3660. |
| 85 | PANDYA A, SHAH Y, KOTHARI N, et al. The future of cancer immunotherapy: DNA vaccines leading the way[J]. Medical Oncology, 2023, 40(7): 200. |
| 86 | STRIOGA M M, DARINSKAS A, PASUKONIENE V, et al. Xenogeneic therapeutic cancer vaccines as breakers of immune tolerance for clinical application: to use or not to use?[J]. Vaccine, 2014, 32(32): 4015-4024. |
| 87 | RICCARDO F, BOLLI E, MACAGNO M, et al. Chimeric DNA vaccines: an effective way to overcome immune tolerance[M/OL]//SAVELYEVA N, OTTENSMEIER C. Current topics in microbiology and immunology: cancer vaccines. Cham: Springer International Publishing, 2014: 99-122 [2023-12-01]. . |
| 88 | SAFAVI A, KEFAYAT A, ABIRI A, et al. In silico analysis of transmembrane protein 31 (TMEM31) antigen to design novel multiepitope peptide and DNA cancer vaccines against melanoma[J]. Molecular Immunology, 2019, 112: 93-102. |
| 89 | LI L J, ZHANG X L, WANG X L, et al. Optimized polyepitope neoantigen DNA vaccines elicit neoantigen-specific immune responses in preclinical models and in clinical translation[J]. Genome Medicine, 2021, 13(1): 56. |
| 90 | DURÁNTEZ M, LÓPEZ-VÁZQUEZ A B, DE CERIO A L D, et al. Induction of multiepitopic and long-lasting immune responses against tumour antigens by immunization with peptides, DNA and recombinant adenoviruses expressing minigenes[J]. Scandinavian Journal of Immunology, 2009, 69(2): 80-89. |
| 91 | KESKIN D B, ANANDAPPA A J, SUN J, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial[J]. Nature, 2019, 565(7738): 234-239. |
| 92 | MIAO L, ZHANG Y, HUANG L. mRNA vaccine for cancer immunotherapy[J]. Molecular Cancer, 2021, 20(1): 41. |
| 93 | TEWS B A, MEYERS G. Self-replicating RNA[M/OL]//KRAMPS T, ELBERS K. Methods in molecular Biology: RNA vaccines. New York, NY: Springer New York, 2017, 1499: 15-35 [2023-12-01]. . |
| 94 | SIEGEL R L, MILLER K D, WAGLE N S, et al. Cancer statistics, 2023[J]. CA: A Cancer Journal for Clinicians, 2023, 73(1): 17-48. |
| 95 | BAILEY P, CHANG D K, FORGET M A, et al. Exploiting the neoantigen landscape for immunotherapy of pancreatic ductal adenocarcinoma[J]. Scientific Reports, 2016, 6: 35848. |
| 96 | BALACHANDRAN V P, ŁUKSZA M, ZHAO J N, et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer[J]. Nature, 2017, 551(7681): 512-516. |
| 97 | ŁUKSZA M, SETHNA Z M, ROJAS L A, et al. Neoantigen quality predicts immunoediting in survivors of pancreatic cancer[J]. Nature, 2022, 606(7913): 389-395. |
| 98 | ROJAS L A, SETHNA Z, SOARES K C, et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer[J]. Nature, 2023, 618(7963): 144-150. |
| 99 | KUAI R, OCHYL L J, BAHJAT K S, et al. Designer vaccine nanodiscs for personalized cancer immunotherapy[J]. Nature Materials, 2017, 16(4): 489-496. |
| 100 | SCHLOSSER E, MUELLER M, FISCHER S, et al. TLR ligands and antigen need to be coencapsulated into the same biodegradable microsphere for the generation of potent cytotoxic T lymphocyte responses[J]. Vaccine, 2008, 26(13): 1626-1637. |
| 101 | FISCHER N O, RASLEY A, CORZETT M, et al. Colocalized delivery of adjuvant and antigen using nanolipoprotein particles enhances the immune response to recombinant antigens[J]. Journal of the American Chemical Society, 2013, 135(6): 2044-2047. |
| 102 | KOERNER J, HORVATH D, HERRMANN V L, et al. PLGA-particle vaccine carrying TLR3/RIG-I ligand Riboxxim synergizes with immune checkpoint blockade for effective anti-cancer immunotherapy[J]. Nature Communications, 2021, 12(1): 2935. |
| 103 | HEIDEGGER S, KREPPEL D, BSCHEIDER M, et al. RIG-I activating immunostimulatory RNA boosts the efficacy of anticancer vaccines and synergizes with immune checkpoint blockade[J]. EBioMedicine, 2019, 41: 146-155. |
| 104 | LI W Z, LIU J Q, CHEN M, et al. Circular RNA in cancer development and immune regulation[J]. Journal of Cellular and Molecular Medicine, 2022, 26(6): 1785-1798. |
| 105 | YU L L, XIAO Q, YU B, et al. CircRNAs in tumor immunity and immunotherapy: perspectives from innate and adaptive immunity[J]. Cancer Letters, 2023, 564: 216219. |
| 106 | BALAN S, SAXENA M, BHARDWAJ N. Dendritic cell subsets and locations[M/OL]//International review of cell and molecular biology. Amsterdam: Elsevier, 2019, 348: 1-68 [2023-12-01]. . |
| 107 | SALLUSTO F, LANZAVECCHIA A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha[J]. The Journal of Experimental Medicine, 1994, 179(4): 1109-1118. |
| 108 | LIAU L M, ASHKAN K, BREM S, et al. Association of autologous tumor lysate-loaded dendritic cell vaccination with extension of survival among patients with newly diagnosed and recurrent glioblastoma: a phase 3 prospective externally controlled cohort trial[J]. JAMA Oncology, 2023, 9(1): 112-121. |
| 109 | GABBA A, ATTARIYA R, BEHREN S, et al. MUC1 glycopeptide vaccine modified with a GalNAc glycocluster targets the macrophage galactose C-type lectin on dendritic cells to elicit an improved humoral response[J]. Journal of the American Chemical Society, 2023, 145(24): 13027-13037. |
| 110 | LIU C, LIU X, XIANG X C, et al. A nanovaccine for antigen self-presentation and immunosuppression reversal as a personalized cancer immunotherapy strategy[J]. Nature Nanotechnology, 2022, 17(5): 531-540. |
| 111 | SOLIMAN H, HOGUE D, HAN H, et al. Oncolytic T-VEC virotherapy plus neoadjuvant chemotherapy in nonmetastatic triple-negative breast cancer: a phase 2 trial[J]. Nature Medicine, 2023, 29(2): 450-457. |
| 112 | ZHU J M, KE Y H, LIU Q, et al. Engineered Lactococcus lactis secreting Flt3L and OX40 ligand for in situ vaccination-based cancer immunotherapy[J]. Nature Communications, 2022, 13(1): 7466. |
| 113 | WANG W G, XU H H, YE Q S, et al. Systemic immune responses to irradiated tumours via the transport of antigens to the tumour periphery by injected flagellate bacteria[J]. Nature Biomedical Engineering, 2022, 6(1): 44-53. |
| [1] | ZHONG Quanzhou, SHAN Yiyi, PEI Qingyun, JIN Yanyun, WANG Yihan, MENG Luyuan, WANG Xinyun, ZHANG Yuxin, LIU Kunyuan, WANG Huizhong, FENG Shangguo. Research progress in the production of α-arbutin through biosynthesis [J]. Synthetic Biology Journal, 2025, 6(1): 118-135. |
| [2] | ZHU Fanghuan, CEN Xuecong, CHEN Zhen. Research progress of diols production by microbes [J]. Synthetic Biology Journal, 2024, 5(6): 1367-1385. |
| [3] | LIU Yining, PU Wei, YANG Jinxing, WANG Yu. Recent advances in the biosynthesis of ω-amino acids and lactams [J]. Synthetic Biology Journal, 2024, 5(6): 1350-1366. |
| [4] | ZHENG Haotian, LI Chaofeng, LIU Liangxu, WANG Jiawei, LI Hengrun, NI Jun. Design, optimization and application of synthetic carbon-negative phototrophic community [J]. Synthetic Biology Journal, 2024, 5(5): 1189-1210. |
| [5] | CHENG Xiaolei, LIU Tiangang, TAO Hui. Recent research progress in non-canonical biosynthesis of terpenoids [J]. Synthetic Biology Journal, 2024, 5(5): 1050-1071. |
| [6] | LIU Zijian, MU Baiyang, DUAN Zhiqiang, WANG Xuan, LU Xiaojie. Advances in the development of DNA-compatible chemistries [J]. Synthetic Biology Journal, 2024, 5(5): 1102-1124. |
| [7] | ZHANG Shouqi, WANG Tao, KONG Yao, ZOU Jiasheng, LIU Yuanning, XU Zhengren. Chemoenzymatic synthesis of natural products: evolution of synthetic methodology and strategy [J]. Synthetic Biology Journal, 2024, 5(5): 913-940. |
| [8] | XIE Xiangqian, GUO Wen, WANG Huan, LI Jin. Biosynthesis and chemical synthesis of ribosomally synthesized and post-translationally modified peptides containing aminovinyl cysteine [J]. Synthetic Biology Journal, 2024, 5(5): 981-996. |
| [9] | TANG Zhijun, HU Youcai, LIU Wen. Enzymatic (4+2)- and (2+2)-cycloaddition reactions: fundamentals and applications of regio- and stereoselectivity [J]. Synthetic Biology Journal, 2024, 5(3): 401-407. |
| [10] | ZHANG Jun, JIN Shixue, YUN Qian, QU Xudong. Biosynthesis of the unnatural extender units with polyketides and their structural modifications for applications in medicines [J]. Synthetic Biology Journal, 2024, 5(3): 561-570. |
| [11] | CHEN Xiwei, ZHANG Huaran, ZOU Yi. Biosynthesis and metabolic engineering of fungal non-ribosomal peptides [J]. Synthetic Biology Journal, 2024, 5(3): 571-592. |
| [12] | FENG Jin, PAN Haixue, TANG Gongli. Research advances in biosynthesis of natural product drugs within the past decade [J]. Synthetic Biology Journal, 2024, 5(3): 408-446. |
| [13] | XI Mengyu, HU Yiling, GU Yucheng, GE Huiming. Genome mining-directed discovery for natural medicinal products [J]. Synthetic Biology Journal, 2024, 5(3): 447-473. |
| [14] | SHI Xinjie, DU Yiling. Research advances in the biosynthesis of nonribosomal peptides within the bisintercalator family as anticancer drugs [J]. Synthetic Biology Journal, 2024, 5(3): 593-611. |
| [15] | SONG Yongxiang, ZHANG Xiufeng, LI Yanqin, XIAO Hua, YAN Yan. Resistance-gene directed discovery of bioactive natural products [J]. Synthetic Biology Journal, 2024, 5(3): 474-491. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||